Abstract
The aryl hydrocarbon receptor (AHR) pathway modulates the immune system in response to kynurenine, an endogenous tryptophan metabolite. IDO1 and TDO2 catalyze kynurenine production, which promotes cancer progression by compromising host immunosurveillance. However, it is unclear whether the AHR activation regulates the malignant traits of cancer such as metastatic capability or cancer stemness. Here, we carried out systematic analyses of metabolites in patient‐derived colorectal cancer spheroids and identified high levels of kynurenine and TDO2 that were positively associated with liver metastasis. In a mouse colon cancer model, TDO2 expression substantially enhanced liver metastasis, induced AHR‐mediated PD‐L1 transactivation, and dampened immune responses; these changes were all abolished by PD‐L1 knockout. In patient‐derived cancer spheroids, TDO2 or AHR activity was required for not only the expression of PD‐L1, but also for cancer stem cell (CSC)‐related characteristics and Wnt signaling. TDO2 was coexpressed with both PD‐L1 and nuclear β‐catenin in colon xenograft tumors, and the coexpression of TDO2 and PD‐L1 was observed in clinical colon cancer specimens. Thus, our data indicate that the activation of the TDO2‐kynurenine‐AHR pathway facilitates liver metastasis of colon cancer via PD‐L1–mediated immune evasion and maintenance of stemness.
Keywords: aryl hydrocarbon receptor, cancer stem cells, kynurenine, liver metastasis, TDO2
We carried out systematic analyses of metabolites in patient‐derived colorectal cancer spheroids, and identified high levels of kynurenine and TDO2 that were positively associated with liver metastasis. Our data indicate that the activation of the TDO2‐kynurenine‐AHR pathway may lead to emergence of immune‐evasive cancer stem cells (CSCs) promoting liver metastasis.
1. INTRODUCTION
Colorectal cancer is the third leading cause of cancer‐related death worldwide (https://gco.iarc.fr/today/fact‐sheets‐cancers). The presence of distant metastatic lesions leads to a dismal prognosis with the 5‐year survival rate at 14% (https://www.cancer.net/cancer‐types/colorectal‐cancer/statistics) due to chemoresistance to standard chemotherapy and unfavorable responses to immunotherapy. 1 Among the types of distant metastasis in colorectal cancer, liver metastasis is the most frequently observed type and directly linked to the high mortality rate. Hence, understanding how liver metastasis develops is a pressing medical issue.
It was reported that liver metastasis was effectively blocked by the elimination of colon cancer cells that express LGR5, an established functional marker of colon cancer stem cells (CSCs), 2 , 3 suggesting that cancer cells with enhanced stemness play an important role in the liver metastasis of colon cancer. Effective inhibition of liver metastasis by the elimination of LGR5‐positive cells 3 indicate that targeting LGR5‐positive CSCs may be a promising strategy to effectively treat liver metastasis.
In our previous studies, we established spheroid cultures from clinical specimens of primary colorectal cancer. 4 The established spheroids were mainly composed of cells with CSC‐related characteristics including self‐propagating capability and expression of CSC‐related markers (eg, CD44 and activated β‐catenin) and capable of forming xenograft tumors with pathological features similar to those of the original cancers. 5
Here, to gain insight into the biological features of colorectal CSCs that are associated with metastatic capability, we attempted to identify cellular metabolites that were upregulated in spheroids derived from primary tumors of patients with liver metastasis as well as from metastatic lesions of the liver. One of metabolites significantly upregulated in the metastasis‐associated spheroids was kynurenine, a major tryptophan metabolite produced mainly by its catalyzing enzymes, indoleamine‐2,3‐dioxygenase 1 (IDO1) and tryptophan‐2,3‐dioxygenase (TDO2).
It was demonstrated that elevation of kynurenine suppresses antitumor immunity through activation of aryl hydrocarbon receptor (AHR). 6 AHR is a ligand‐dependent transcription factor that responds to various exogenous and endogenous compounds. 7 , 8 Upon binding to these diverse forms of ligands, activated AHR can modulate immune functions, thus playing a major role in fine‐tuning the immune system. 7 , 8
Because of documented roles of kynurenine in cancer progression, we further examined the significance of upregulation of kynurenine in metastatic spheroids. Our study revealed the functional importance of the kynurenine‐regulated TDO2‐AHR pathways in maintenance of stemness and PD‐L1–mediated immunomodulation, which may translate into generation of immune‐evasive CSCs with metastatic capability.
2. MATERIALS AND METHODS
2.1. Primary human colon cancer specimens
All human colon cancer samples were resected from patients who provided informed consent at the National Cancer Center Hospital, and all procedures were conducted under a protocol approved by the ethics committee of the National Cancer Center. Clinical information of each case for the examined spheroids were provided in Figure S1A.
2.2. Animal experiments
All mouse procedures were approved by the Animal Care and Use Committees of the National Cancer Center and conducted in accordance with institutional policies and the Guidelines for Animal Experiments.
3. RESULTS
3.1. Elevated kynurenine and TDO2 levels in patient‐derived colorectal cancer spheroids are associated with liver metastasis
We established patient‐derived spheroids from metastatic liver lesions by using previously described protocol. 4 To identify metabolites with elevated expression levels in metastatic cancer, we cultivated one set of spheroids from the primary tumors of patients without metastasis (CRC‐9, 17, 19, and 20, hereafter called nonmetastatic spheroids) and another set of the newly established spheroids from liver metastases (CRC‐29 M, 31 M, 24 M, 26 M, hereafter called metastatic spheroids) (Figure S1A). After extracting metabolites from the lysates of those spheroids, we compared the levels of metabolites by systematically quantifying the charged metabolites by capillary electrophoresis time‐of‐flight mass spectrometry (CE‐TOF‐MS) (Figure 1A). 9 The 88 cationic metabolites (Table S1) and 126 anionic metabolites (Table S2) that were detected in any of the sample lysates were then compared; we found that the levels of two metabolites in the metastatic spheroids, ie, kynurenine (a cationic metabolite) and octanoate (an anionic metabolite), were significantly higher in lysates in the metastatic spheroids than those in the nonmetastatic ones (Figures 1B and S1B; Tables S3 and S4).
FIGURE 1.
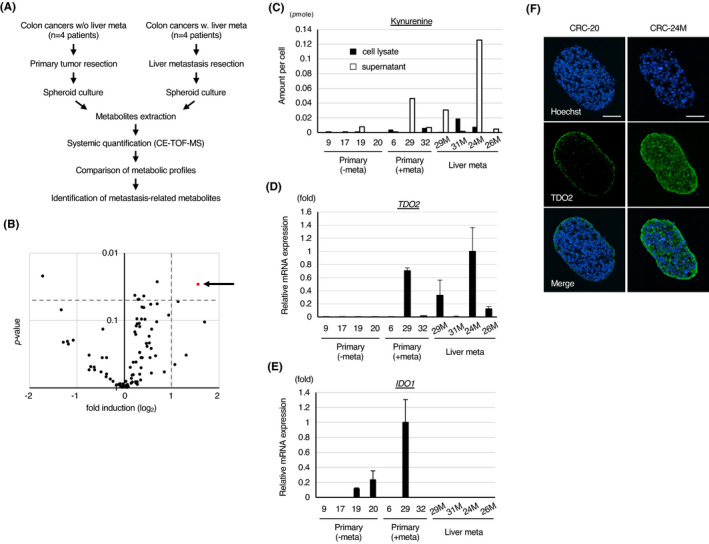
Elevated TDO2‐kynurenine pathway activity in patient‐derived colon cancer spheroids is associated with liver metastasis. A, Experimental scheme. B, Volcano plot of the quantified concentration for cationic metabolites through the comparison of nonmetastatic spheroids vs. metastatic spheroids. L‐kynurenine is indicated by the arrow. C, Levels of kynurenine per cell measured by chromatography tandem mass spectrometry (GC‐MS/MS). D, E, qPCR analyses of (D) TDO2 and (E) IDO1 in the indicated spheroids. F, Representative immunostaining for TDO2 in spheroids with a low (CRC‐20) or high (CRC‐24 M) level of TDO2. Nuclei were counterstained by Hoechst 33 342. Scale bar: 50 µm. Values represent the mean ± SD
We then confirmed the elevation in kynurenine levels in the metastatic spheroids by performing mass spectrometry with a different separation technique, gas chromatography tandem mass spectrometry (GC‐MS/MS). This technique was used because it does not require nonisotonic washing, and we found significantly reduced spheroid viability in CE‐TOF‐MS (see Supplemental Methods section). We measured kynurenine levels by GC‐MS/MS in samples from spheroids derived from the primary tumors of patients with liver metastasis (CRC‐6, 29, 32) (Figure S1A) in addition to samples from nonmetastatic and metastatic spheroids. Kynurenine levels were evaluated in both culture supernatant and cell lysate extracts, as kynurenine can be secreted from cells. 10 The data obtained by GC‐MS/MS confirmed that the kynurenine levels tended to be higher in the metastasis‐associated spheroids than in the nonmetastatic spheroids (Figure 1C). Remarkably, we found that kynurenine levels were generally higher in the supernatants than in the cell lysates, suggesting that a majority of the produced kynurenine was secreted from the cells (Figure 1C).
Kynurenine is produced mainly by two catalytic enzymes, IDO1 and TDO2 (Figure S1C). 6 The expression of TDO2 but not IDO1 was upregulated in metastasis‐associated spheroids with high levels of kynurenine production, suggesting that induction of TDO2 is responsible for the elevated kynurenine levels in the metastatic spheroids (Figures 1D, E and S1D). Consistently, immunostaining studies showed that TDO2 protein was expressed at higher levels in metastatic spheroids (CRC‐24 M) than in nonmetastatic spheroids (CRC‐20) (Figure 1F).
We investigated the mutation status of 114 major cancer‐associated genes by using an in‐house gene panel test 11 (Figure S1E). We did not observe a clear association between high levels of kynurenine and mutations in major genes involved in colorectal cancer (ie, APC, KRAS, and TP53) nor genes associated with high‐frequency microsatellite instability (MSI‐H) (MLH1, MSH2, and POLE). Clustering analyses based on RNA‐seq data indicated that the expression profiles of the spheroids were more similar to colon cancer cells in the CMS‐2 and CMS‐3 category than those in the MSI‐H–related CMS‐1 (Figure S1F), suggesting that all the examined spheroids were associated with microsatellite‐stable phenotype.
3.2. Tdo2 expression promotes liver metastasis in an Ahr‐dependent manner
Next, we evaluated whether Tdo2 overexpression affects colon cancer progression in highly immunogenic colon cancer cells, CT26. 12 Flag‐tagged mouse Tdo2 was introduced into the CT26 cells expressing GFP (CT26/GFP) via lentiviral gene transfer (Figure S2A). Tdo2 overexpression induced an increase in the supernatant kynurenine level that was comparable to that in supernatants of human U87 cells, which express high levels of endogenous TDO2 and kynurenine (Figure S2B). 13 Tdo2 overexpression did not significantly affect Ido1 expression (Figure S2C) and did not significantly promote cell growth (Figure S2D), migration (Figure S2E, upper panel), or cell invasion (Figure S2E, lower panel). Because the activation of AHR signaling has been shown to promote EMT phenotype, 14 we also examined the expression of representative EMT markers. However, Tdo2 overexpression did not significantly enhance their expression (Figure S2F).
Next, we generated liver metastasis by injectingTdo2‐expressing CT26 cells into the spleen of Balb/c syngenic mice (Figure 2A). In vivo imaging revealed that Tdo2 expression significantly enhanced liver metastasis (Figure 2B). The enhancement of metastasis by Tdo2 was abolished by a specific inhibitor of TDO2 (680C91) 13 (Figure 2C). In addition, suppression of Ahr with a specific inhibitor (CH‐223191) 15 completely blocked Tdo2‐mediated enhancement of liver metastasis (Figure 2D). These data collectively indicate that Ahr activation by Tdo2‐produced kynurenine is responsible for the enhancement of liver metastasis of colon cancer cells.
FIGURE 2.
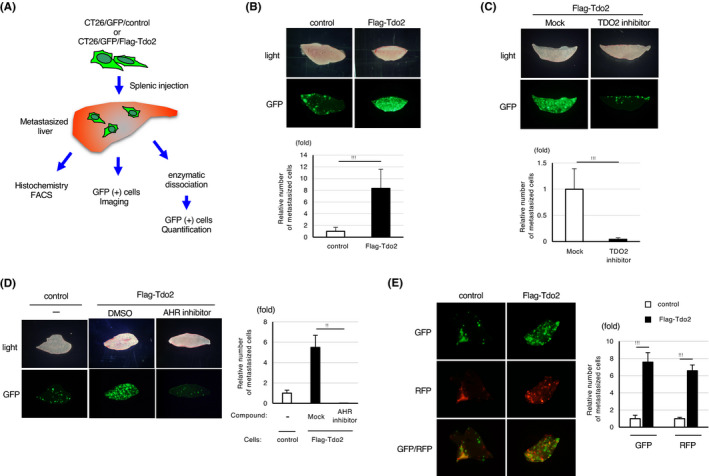
Tdo2 expression promotes liver metastasis in an Ahr‐dependent manner. A, An experimental scheme of the liver metastasis analyses. B, Promotion of liver metastasis by colon cancer cells induced by Tdo2 expression. Top: Representative stereo microscopy and GFP images of the liver at 7 d after splenic injection of CT26‐derived cells. Bottom: Relative numbers of liver‐metastasized GFP‐positive cells. C, Inhibition of Tdo2‐induced liver metastasis by daily intraperitoneal injection of a Tdo2 inhibitor. Top: Representative GFP images of liver metastases. Bottom: Relative numbers of liver‐metastasized GFP‐positive cells. D, Inhibition of Tdo2‐mediated liver metastasis by daily intraperitoneal injection of an AHR inhibitor. Left: Representative GFP images of liver metastases. Right: Relative numbers of metastasized GFP‐positive cells. E, Promotion of liver metastasis by coinjected Tdo2‐expressing cells. Left: Representative fluorescent images of liver metastases. Right: Relative numbers of liver‐metastasized cells. Values represent the mean ± SD; **P < .01, ***P < .001 (two‐sided Student's t‐test)
Remarkably, splenic coinjection of RFP‐labeled CT26 cells (CT26/RFP) together with CT26/Tdo2/GFP cells at 1:1 cell ratio enhanced liver metastasis by the CT26/RFP cells, indicating that Tdo2‐expressing cells affect the metastatic capability of surrounding cancer cells, presumably due to elevated levels of extracellular kynurenine secreted by CT26/Tdo2/GFP (Figure 2E).
3.3. Tdo2‐enhanced liver metastasis is associated with compromised immune responses
Enhanced liver metastasis by Tdo2 overexpression was not observed if the metastasis assay was performed with nude mice (Figure S3A), suggesting that the host immune response is involved in Tdo2‐dependent liver metastasis. Hence, we examined whether Tdo2 overexpression affects immunoenvironment in metastasized liver. The metastatic tumors formed after splenic injection of CT26/GFP cells were infiltrated by both CD4+ and CD8+ lymphocytes (Figure 3A), presumably due to an immunogenic response induced by the retroviral envelope expressed in parental CT26 cells. 16 Notably, the accumulation of both CD4+ and CD8+ lymphocytes was significantly decreased in stroma surrounding Tdo2‐expressing metastatic tumors (Figure 3A, B). In contrast, there were few infiltrating macrophages regardless of whether Tdo2 was expressed (Figure S3B). Consistent with the immunostaining results, FACS analyses indicated that Tdo2 overexpression decreased the proportions of major T lymphocyte subgroups (CD3+, CD4+, CD8+, Treg, and IFN‐γ+ CD8+ cells) (Figure S3C). Compromised host immune responses by Tdo2 expression may be clinically relevant, as the presence of a large number of tumor‐infiltrating T lymphocytes has been shown to be a predictor of good prognosis in multiple solid tumors 17 and liver metastasis. 18
FIGURE 3.
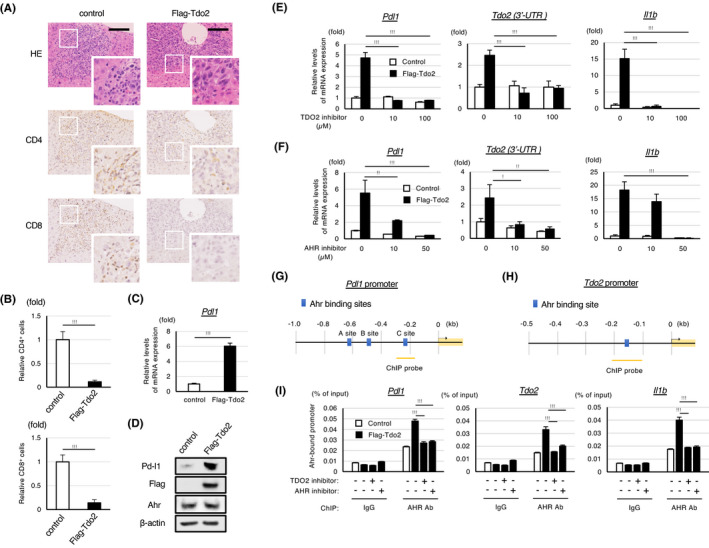
The induction of the TDO2‐kynurenine‐AHR pathway transactivates Pd‐l1 and compromises immune responses in metastatic liver. A, H&E staining and immunohistochemical analyses of metastasized livers at 7 d after the splenic injection of the CT26/GFP cells expressing the indicated construct. Images acquired at a higher magnification are also shown. Scale bar: 100 µm. B, Relative numbers of infiltrating CD4+ and CD8+ cells in the indicated tumors shown in (A). C, qPCR analysis of Pdl1 expression in CT26 cells expressing Flag‐Tdo2 and control cells. D, Western blot analyses of the expression of the indicated proteins in the cells shown in (C). E, F, qPCR analyses of the expression of the indicated genes in the cells shown in (C). The cells were treated with the indicated amounts of a TDO2 or AHR inhibitor for 48 h before the analyses. For quantification of endogenous Tdo2 expression, 3’‐UTR of Tdo2 was evaluated. G, H, Schematic representations of the predicted Ahr binding sites in the Pdl1 and Tdo2 promotors. I, ChIP analyses of AHR binding to the indicated promotor region in CT26/GFP/Flag‐Tdo2 cells or control cells. The cells were treated with the TDO2 or AHR inhibitor for 48 h or mock treated. Enrichment over input (% input) was measured by qPCR (n = 3). As for the ChIP analyses of the Pdl1 promoter, the results of the AHR binding for the C site shown in (G) are shown (the binding to the other sites was not detected). Values represent the mean ± SD; *P < .05, **P < .01, ***P < .001
3.4. The Tdo2‐kynurenine‐Ahr pathway mediates the transactivation of Pd‐l1 and Tdo2 in liver metastasis–competent colon cancer cells
It was demonstrated that Ahr transactivates PD‐L1 in carcinogen‐treated lung epithelial cells. 19 Therefore, we next examined whether the Tdo2‐dependent enhancement of liver metastasis is mediated by Ahr‐dependent Pd‐l1 induction. Indeed, the expression of Pd‐l1 was upregulated in Tdo2‐expressing CT26 cells (Figure 3C, D). Treatment with an inhibitor of Tdo2 or Ahr abolished the induction of Pd‐l1 and Il‐1b, known targets of Ahr 20 (Figure 3E, F). Inhibition of Pd‐l1 expression by the inhibitor of Tdo2 or Ahr was also shown by Western blot analyses (Figure S3E, F).
Interestingly, the examination of the expression of the Tdo2 gene's 3’UTR sequence revealed that endogenous Tdo2 expression was also induced by ectopic Tdo2 expression and that the induction was compromised by the inhibitor of Tdo2 or Ahr (Figure 3E, F). These results suggest that Ahr activates the Tdo2 gene, forming a positive feedback loop. As expected, in silico analyses of genomic sequences showed that the promoter regions of the Pd‐l1 and Tdo2 genes harbor potential binding sites for Ahr (Figures 3G, H and S3G). Additionally, chromatin immunoprecipitation (ChIP) analyses revealed that Tdo2 overexpression markedly enhanced the binding of Ahr to the promoter sequences of Pd‐l1, Tdo2, and Il‐1b, and that the enhanced interactions were largely abolished by the inhibitor of Tdo2 or Ahr (Figure 3I). Collectively, these data indicate that the activation of the kynurenine‐Ahr pathway by Tdo2 induces the transactivation of Pd‐l1 and Tdo2 in colon cancer cells.
3.5. Pd‐l1 is required for the Tdo2‐mediated promotion of liver metastasis and suppression of immune responses
Next, we investigated the functional importance of the induction of Pd‐l1 in liver metastasis. We performed CRISPR/Cas9‐mediated knockout of Pd‐l1 in CT26/GFP/Tdo2 cells (Figure 4A). Knocking out Pd‐l1 almost completely abolished the Tdo2‐mediated enhancement of liver metastasis (Figure 4B, C) and restored the T cell–mediated immune response in liver metastases (Figure 4D‐F), while the knockout did not significantly affect the proliferation of TDO2‐expressing tumor cells in vitro (Figure S4). Tdo2‐mediated liver metastasis could also be abolished by intraperitoneal injection of a neutralizing anti‐PD‐L1 antibody (Figure 4G, H). Thus, Pd‐l1 transactivation is required for the inhibition of antitumor immunity and for liver metastasis stimulated by the activated Tdo2‐kynurenine‐Ahr pathway.
FIGURE 4.
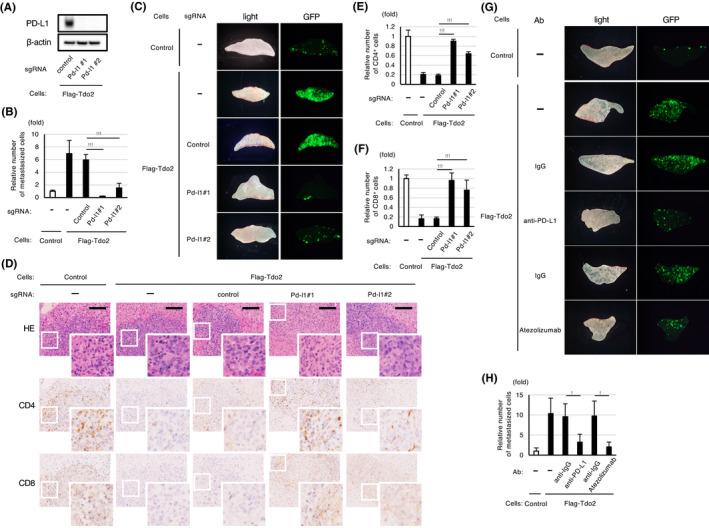
Pd‐l1 is required for the Tdo2‐mediated promotion of liver metastasis and suppression of immune responses. A, Western blot analyses of Pd‐l1 expression in CT26 cells expressing Flag‐Tdo2 subjected to Pd‐l1 (Pd‐l1#1 and #2) or control knockout. B, Abolishment of Tdo2‐mediated liver metastasis by knocking out Pd‐l1. C, Representative GFP images of the liver after splenic injection of the CT26 cells shown in (B). D, Restoration of T cell accumulation in liver metastases generated by Pd‐l1–knockout cells. H&E staining and immunohistochemical analyses for the indicated lymphocytes. Scale bar: 100 μm. E, F, Relative numbers of tumor‐infiltrating CD4‐ and CD8‐positive cells in the indicated tumors. Quantification of relative cell number was performed as described in Figure 3B (n = 3). G, H, Inhibition of Tdo2‐mediated liver metastasis with neutralizing anti‐PD‐L1 antibodies after splenic injection of the indicated CT26 cells. CT26/Flag‐Tdo2 cells were incubated with the indicated antibodies for 48 h and then used for splenic injection. G, Representative GFP images of the liver after splenic injection. H, The extent of inhibition determined by measuring the relative numbers of liver‐metastasized CT26 cells. Values represent the mean ± SD; *P < .05, **P < .01, ***P < .001
3.6. The TDO2‐AHR pathway mediates transactivation of PD‐L1 and TDO2 in colon cancer spheroids
We next examined whether TDO2 or AHR was also required for the expression of these genes in the colon cancer spheroids that express high levels of TDO2 and kynurenine (CRC‐24 M and CRC‐29 M). Treatment with the inhibitors of TDO2 or AHR strongly suppressed the expression of PD‐L1 and TDO2 as well as IL‐1b (Figures 5A, B and S5A, B). It was previously shown that the AHR‐responsive elements are located near the PD‐L1 promoter (Figure S5C), 19 and ChIP analyses indeed showed that AHR was bound to the promoters of PD‐L1 and IL‐1b (Figure S5D). In addition, in silico analyses of genomic sequences indicated that the promoters of TDO2 harbor a potential binding site for AHR (Figure 5C), and ChIP analyses confirmed the binding of AHR to the predicted site of the TDO2 gene, which was abrogated in the presence of the inhibitors for TDO2 or AHR (Figure 5D). Thus, the kynurenine‐AHR pathway mediates the transactivation of PD‐L1 and TDO2 in both mouse and human colon cancer cells.
FIGURE 5.
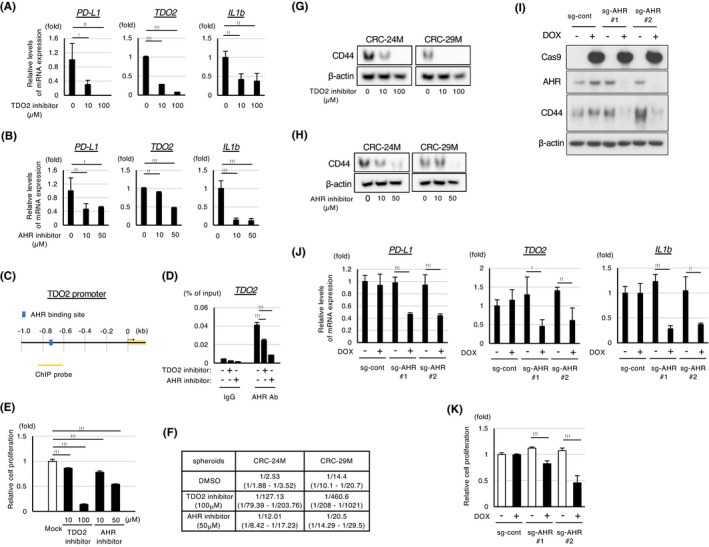
The TDO2‐AHR pathway is required for the transactivation of PD‐L1 and TDO2 and for the maintenance of stemness in colon cancer spheroids. A, B, qPCR analyses of PD‐L1, TDO2, and IL‐1b expression in cancer spheroids (CRC‐29 M) treated with the indicated concentrations of an inhibitor of TDO2 (A) or AHR (B) for 96 h. C, Schematic representation of predicted AHR‐binding sites in the promoters of TDO2. D, ChIP analyses of the AHR‐bound promotors of the TDO2 gene in spheroid cells (CRC‐29 M) treated with the TDO2 inhibitor (100 μmol/L) or AHR inhibitor (50 μmol/L) for 96 h. E, Relative proliferation of spheroid cells (CRC‐29 M) measured by CellTiter‐Glo assays (n = 4). The spheroids were treated with the indicated concentrations of the TDO2 or AHR inhibitor for 7 d. F, Limiting dilution assay with FACS‐sorted spheroid cells. An average frequency of self‐renewing cells (upper lanes) and confidence interval (lower lanes) are shown. G, H, Western blot analyses of CD44 protein expression in the indicated spheroid cells treated with the inhibitor of TDO2 (G) or AHR (H) for 96 h. I, Western blot analyses in spheroid cells (CRC‐29 M) introduced with doxycycline‐inducible Cas9 and the indicated sg‐RNA in the presence or absence of 1 μmol/L doxycycline for 5 d. J, qPCR analyses of the indicated genes in the spheroids shown in (I). K, Relative proliferation of spheroid cells shown in (I). Values represent the mean ± SD; *P < .05, **P < .01, ***P < .001
3.7. The TDO2‐AHR pathway is required for the maintenance of CSC‐related characteristics in colon cancer spheroids
Remarkably, treatment with an inhibitor of TDO2 or AHR significantly inhibited the cell proliferation of the studied cancer spheroids (Figure 5E). Because the established spheroids are enriched in cells with CSC‐related properties, 4 we examined whether TDO2 and AHR inhibitors suppressed the maintenance of stemness in the spheroids. Limiting dilution assays revealed that spheroid formation efficiency was substantially reduced by the inhibitors (Figure 5F). In addition, the expression of the colon CSC marker CD44 21 was suppressed by the inhibition of TDO2 or AHR (Figure 5G, H). qPCR analyses indicated that the suppression of CD44 by the TDO2/AHR inhibitor was at least in part mediated at RNA levels (Figure S5E). Because it was reported that CD44 is one of the major target genes of Wnt signaling, 22 we also examined whether the inhibition of Wnt signaling also suppressed CD44 expression. Of note, treatment of the spheroids with inhibitors of Wnt signaling (XAV 23 and IWR1 24 ) caused suppression of CD44 expression at levels comparable to those observed after the treatment with the TDO2/AHR inhibitors (Figure S5E), suggesting that TDO2 and AHR are involved in CD44 regulation via the Wnt pathway.
To confirm the role of the TDO2‐AHR pathway, we generated a cancer spheroid (CRC‐29 M) in which the AHR gene was targeted for CRISPR‐mediated knockout in a doxycycline‐dependent manner (Figure 5I). As expected, the induction of AHR knockout inhibited the expression of PD‐L1 and TDO2 as well as IL‐1b (Figure 5J), inhibited cell proliferation (Figure 5K), and suppressed CD44 expression (Figure 5I), thus phenocopying the effects of the AHR/TDO2 inhibitors.
3.8. The TDO2‐AHR pathway directly activates LGR5 and mediates the maintenance of Wnt signaling in colon cancer spheroids
Because the Wnt signaling pathway plays a pivotal role in colon cancer stemness, 25 we examined the effect of the TDO2/AHR inhibitors on the expression of representative Wnt target genes. Inhibition of TDO2 or AHR suppressed the expression of all the Wnt target genes we evaluated (Figures 6A and S6A) and blocked nuclear localization of β‐catenin (Figures 6B and S6B). In accordance, the inducible knockout of AHR suppressed the expression of the Wnt target genes and nuclear localization of β‐catenin (Figure 6C, D). Collectively, the TDO2‐kynurenine‐AHR pathway mediates the activation of the Wnt signaling pathway and cancer stemness.
FIGURE 6.
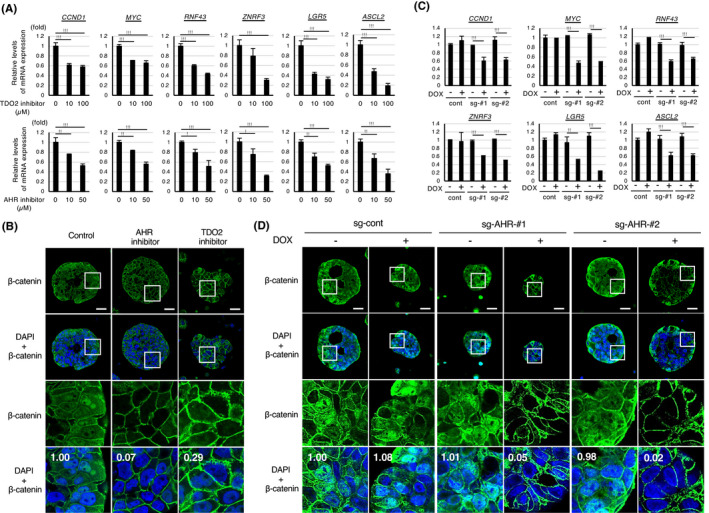
The TDO2‐AHR pathway mediates the maintenance of Wnt signaling in colon cancer spheroids. A, qPCR analyses of representative Wnt target genes in the metastatic spheroids (CRC‐24 M) after treatment with the indicated concentrations of the inhibitor of TDO2 or AHR for 96 h. B, Immunostaining of the spheroids (CRC‐24 M) with β‐catenin in the presence of the indicated inhibitor for 96 h. Nuclei were counterstained with DAPI. Relative quantification values of nuclear β‐catenin were shown in the bottom columns. Scale bar: 25 μm. C, qPCR analyses of representative Wnt target genes in the AHR‐ or TDO2‐inhibited spheroids shown in Figure 5G (100 μmol/L). D, Immunostaining of the spheroids shown in Figure 5I. Relative quantification values of nuclear β‐catenin were shown in the bottom columns. Scale bar: 25 μm. Values represent the mean ± SD; *P < .05, **P < .01, ***P < .001
Of the six Wnt target genes regulated by TDO2/AHR, we further investigated whether LGR5 is directly regulated by AHR. In silico analyses showed that the promoter regions of the LGR5 gene harbors potential binding sites for AHR (Figures S3G and S6C). ChIP analyses confirmed that AHR was bound to the LGR5 promoter, and the binding was abolished in the presence of the inhibitors of AHR or TDO2 (Figure S6D). Thus, the TDO2‐AHR pathway directly regulates LGR5 expression, which is likely to contribute to Wnt regulation and the stemness of colon CSCs.
3.9. TDO2 is coexpressed with PD‐L1 and LGR5 in human colon tumors in vivo
To examine whether TDO2 expression is associated with cancer stemness and PD‐L1 expression in vivo, we investigated the coexpression of TDO2 with LGR5 and PD‐L1 in a mouse xenograft model. Metastatic spheroid cells that express relatively high levels of TDO2 (CRC‐24 M or CRC‐29 M) or nonmetastatic spheroid cells that express TDO2 at marginal levels (CRC‐19 or CRC‐20) were subcutaneously injected into NOD/SCID mice to generate xenograft tumors, and colocalization of TDO2 with LGR5 or PD‐L1 in the formed tumors was examined by in situ hybridization. As expected, the proportion of cancer cells with high TDO2 levels (≧ 2 dots) was higher in the xenograft tumors expressing detectable TDO2 (CRC‐24 M or CRC‐29 M) than in those not (CRC‐19 or CRC‐20) (Figures 7A, B and S7A, B). The TDO2 expression levels in the former xenografted tumor cells were positively correlated with the LGR5 levels (Figures 7C and S7C). Likewise, TDO2 expression in tumor cells was positively correlated with PD‐L1 expression (Figures 7D and S7D).
FIGURE 7.
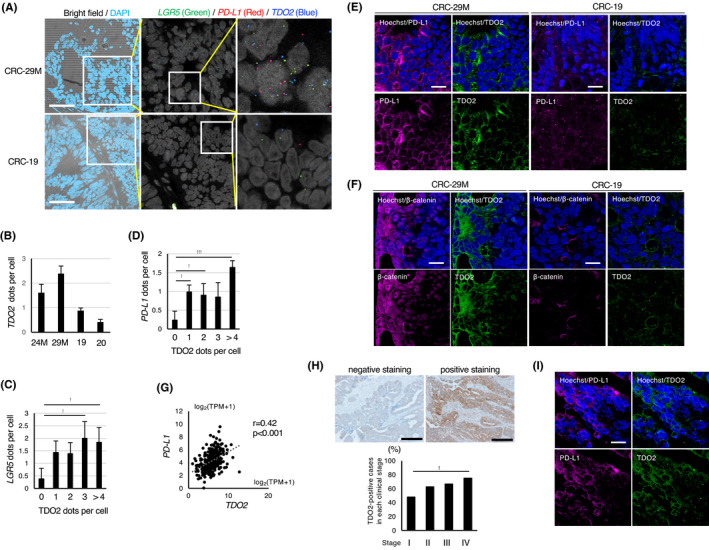
TDO2 is coexpressed with LGR5 and PD‐L1 in colon tumors and positively associated with PD‐L1 expression in clinical specimens. A, In situ RNA hybridization of xenograft tumors derived from cancer spheroids. Left: Merged images of bright‐field microscopy and DAPI staining of the tumors derived from CRC‐29 M and CRC‐19. Scale bar: 50 µm. Middle and right: In situ RNA hybridization with LGR5 (green), PD‐L1 (red), and TDO2 (blue) probes. Images acquired at a higher magnification are shown on the right. B, Average numbers of TDO2‐hybridizing dots per cells in the indicated xenograft tumors. C, D, Correlation of the average number of hybridizing dots for (C) LGR5 or (D) PD‐L1 with the indicated number of dots for TDO2 in xenografted tumors (CRC‐29 M). E, Representative coimmunostaining of xenograft tumors (CRC‐29 M and CRC‐19) for PD‐L1 and TDO2. Scale bar: 10 µm. F, Representative coimmunostaining of xenograft tumors (CRC‐29 M and CRC‐19) for β‐catenin and TDO2. Scale bar: 10 µm. G, Positive association between PD‐L1 and TDO2 mRNA expression in clinical specimens of colon cancer from The Cancer Genome Atlas (TCGA) database. H, Immunohistochemical analyses of TDO2 in clinical specimens of human colon cancers. Top: Representative images of negative and positive staining. Scale bar: 400 μm. Bottom: Proportion of TDO2‐positive cases in each clinical stage is shown. I, Representative coimmunostaining of surgical specimens of primary colon cancer of patients with liver metastasis. Scale bar: 10 µm. Values represent the mean ± SD; *P < .05, **P < .01, ***P < .001
We also examined the coexpression of TDO2 with PD‐L1 or activated β‐catenin in the xenografted tumor. In accordance with the results of the in situ hybridization, TDO2 and PD‐L1 were coexpressed in metastatic spheroid‐derived xenograft tumors, and the expression levels of these proteins in the metastatic spheroid‐derived tumors were significantly higher than those in the nonmetastatic ones (Figures 7E and S7E). In addition, nuclear β‐catenin staining was observed in the metastatic spheroid‐derived tumors, and coexpressed with TDO2 (Figures 7F and S7E). Collectively, these data demonstrate that TDO2 was coexpressed with LGR5, PD‐L1, and nuclear β‐catenin in xenografted tumors derived from metastatic spheroids.
3.10. TDO2 expression is positively associated with PD‐L1 expression in clinical specimens
We next examined whether TDO2 expression is associated with PD‐L1 expression using surgical specimens of colon cancers. A positive correlation was observed between the expression levels of TDO2 and PD‐L1 in clinical colon cancer samples from The Cancer Genome Atlas (TCGA) database (Figure 7G). Further, immunohistological analysis of the samples for each stage of colon cancer showed that TDO2 expression levels were increased in advanced stages of colon cancer (Figure 7H). Remarkably, coimmunostaining studies of the clinical specimens revealed that TDO2 and PD‐L1 were coexpressed in metastatic colon cancer (Figures 7I and S7G), supporting the positive role of TDO2‐induced PD‐L1 expression during metastatic processes.
4. DISCUSSION
It was previously reported that the kynurenine‐AHR pathway plays prometastatic roles in several cancers including breast cancer 26 , 27 and squamous cancers. 28 , 29 The AHR activation may play roles in evasion from immunosurveillance, as the AHR‐mediated transactivation of PD‐L1 is induced by benzo(a)pyrene, an exogenous tobacco carcinogen. 19 In this paper, we extended the prometastatic roles of the AHR pathway by showing that the TDO2‐induced kynurenine production and the resulting activation of the AHR pathway facilitate liver metastasis by stimulating PD‐L1–mediated inhibition of anticancer immunity and Wnt signaling–associated cancer stemness. Thus, kynurenine production by TDO2, together with various endogenous and exogenous ligands, may converge on AHR‐mediated activation of PD‐L1, possibly affecting cancer immunosurveillance. We also revealed LGR5, TDO2, and PD‐L1 as direct AHR targets that are likely to mediate these phenotypes.
Overall, our data suggest that TDO2‐mediated AHR activation facilitates liver metastasis via generation of immune‐evasive CSCs (Figure S7H). Consistent with our model, accumulating reports suggested that CSCs can evade host immunosurveillance. 30 , 31 , 32 , 33 , 34 , 35 , 36 The proposed model suggests that targeting the TDO2‐AHR pathway in the metastatic CSCs could be a therapeutic option to block liver metastasis. By blocking of this pathway, both cancer stemness and immune‐evasive capability might be targeted, giving an advantage over other CSC‐targeted therapies.
While our data indicate that the TDO2‐AHR pathway facilitates liver metastasis, induction of stemness‐related genes may be context dependent. The regulation of PD‐L1, TDO2, and IL1b by the TDO2‐AHR pathway was observed in both CT26 cells and human cancer spheroids, whereas Lgr5 expression was not detectable in CT26 cells (data not shown). Presently, in CT26 cells, it is not clear whether the TDO2‐AHR pathway regulates stemness‐related characteristics, including activation of the Wnt signaling or CD44‐related pathway. Thus, the promotion of cancer stemness by the TDO2‐AHR may be cell type dependent.
Because the AHR pathway is known to play positive roles in cancer progression, IDO1/TDO2‐mediated kynurenine production and the resulting activation of AHR are regarded as potential therapeutic targets. 37 , 38 Especially, IDO1 has been a major focus of therapeutic intervention due to its widespread expression in cancer. 39 , 40 Although recent clinical trials against IDO1 did not result in clear inhibition of primary cancers, 41 the lack of response may be caused by resilient AHR activity induced by TDO2‐derived kynurenine, suggesting that TDO2 may need to be simultaneously targeted for effective therapy.
Our data revealed that TDO2‐activated AHR targets the TDO2 gene for transactivation, suggesting that transient activation of AHR can trigger the formation of a positive feedback loop with TDO2. This suggests that, once temporally activated by a variety of ligands or tryptophan derivatives, AHR may initiate the positive feedback loop between AHR and TDO2. Interestingly, AHR has been reported to transactivate IDO1 and AHR itself in undifferentiated ES cells, 10 suggesting the existence of multiple feedback loops activating AHR.
While some metastatic spheroids expressed high levels of TDO2 and kynurenine, others expressed them at relatively low levels (Figure 1C), suggesting that other unknown mechanisms may substitute the activation of the TDO2‐kynrenine pathway in metastatic colon cancers that express low levels of TDO2‐kynurenine. It may be compensated by kynurenine‐independent mechanisms of AHR activation via other endogenous and exogenous ligands. Alternatively, the dependence on the TDO2‐kynurenine pathway for CSC maintenance may be influenced by the extent of pre‐existing activation of the Wnt signaling pathway, and the cells totally depend on the activation of the TDO2‐kynurenine‐AHR pathway for Wnt activation in the absence of the mutation in the Wnt‐related pathway. In any case, future investigation warrants a global picture of Wnt activation in metastatic colon cancers.
DISCLOSURE
The authors declare no competing interests.
ACCESSION NUMBERS
RNA‐seq data in this publication were deposited in the NCBI Gene Expression Omnibus under accession number GSE167395.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Appendix S1
ACKNOWLEDGEMENTS
We thank the National Cancer Center Research Institute Core Facility for preparation of the samples used in immunohistochemistry and the National Cancer Center Biobank for human cancer specimens. This research was supported by AMED under grant number JP19cm0106563h0001 and 19ak0101043h0105 (KO), the National Cancer Center Research and Development Fund (31‐A‐3, 31‐A‐4, 31‐A‐8) (KO), and the Japan Society for the Promotion of Science (16K10560 to TM).
Miyazaki T, Chung S, Sakai H, et al. Stemness and immune evasion conferred by the TDO2‐AHR pathway are associated with liver metastasis of colon cancer. Cancer Sci.2022;113:170–181. 10.1111/cas.15182
REFERENCES
- 1. Le DT, Uram JN, Wang H, et al. PD‐1 Blockade in Tumors with Mismatch‐Repair Deficiency. N Engl J Med. 2015;372:2509‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leung C, Tan SH, Barker N. Recent advances in Lgr5(+) stem cell research. Trends Cell Biol. 2018;28(5):380‐391. [DOI] [PubMed] [Google Scholar]
- 3. de Sousa e Melo Felipe, Kurtova Antonina V, Harnoss Jonathan M, et al. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature. 2017;543:676‐680. [DOI] [PubMed] [Google Scholar]
- 4. Ohata H, Ishiguro T, Aihara Y, et al. Induction of the stem‐like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer‐initiating cells. Cancer Res. 2012;72:5101‐5110. [DOI] [PubMed] [Google Scholar]
- 5. Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor‐derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017;108:283‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Platten M, Nollen EAA, Rohrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379‐401. [DOI] [PubMed] [Google Scholar]
- 7. Shinde R, McGaha TL. The Aryl Hydrocarbon Receptor: Connecting Immunity to the Microenvironment. Trends Immunol. 2018;39:1005‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gutierrez‐Vazquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satoh K, Yachida S, Sugimoto M, et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc Natl Acad Sci USA. 2017;114:E7697‐E7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamamoto T, Hatabayashi K, Arita M, et al. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci Signal. 2019;12:eaaw3306. [DOI] [PubMed] [Google Scholar]
- 11. Sunami K, Ichikawa H, Kubo T, et al. Feasibility and utility of a panel testing for 114 cancer‐associated genes in a clinical setting: A hospital‐based study. Cancer Sci. 2019;110:1480‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lechner MG, Karimi SS, Barry‐Holson K, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother. 2013;36:477‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour‐promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197‐203. [DOI] [PubMed] [Google Scholar]
- 14. Li L, Wang T, Li S, et al. TDO2 Promotes the EMT of Hepatocellular Carcinoma Through Kyn‐AhR Pathway. Front Oncol. 2020;10:562823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gramatzki D, Pantazis G, Schittenhelm J, et al. Aryl hydrocarbon receptor inhibition downregulates the TGF‐beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28:2593‐2605. [DOI] [PubMed] [Google Scholar]
- 16. Huang AY, Gulden PH, Woods AS, et al. The immunodominant major histocompatibility complex class I‐restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730‐9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fridman WH, Pages F, Sautes‐Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298‐306. [DOI] [PubMed] [Google Scholar]
- 18. Van den Eynde M, Mlecnik B, Bindea G, et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell. 2018;34:1012–1026. [DOI] [PubMed] [Google Scholar]
- 19. Wang GZ, Zhang L, Zhao XC, et al. The Aryl hydrocarbon receptor mediates tobacco‐induced PD‐L1 expression and is associated with response to immunotherapy. Nat Commun. 2019;10:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Memari B, Bouttier M, Dimitrov V, et al. Engagement of the aryl hydrocarbon receptor in mycobacterium tuberculosis‐infected macrophages has pleiotropic effects on innate immune signaling. J Immunol. 2015;195:4479‐4491. [DOI] [PubMed] [Google Scholar]
- 21. Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151‐2162. [DOI] [PubMed] [Google Scholar]
- 22. Wielenga VJ, Smits R, Korinek V, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masuda M, Uno Y, Ohbayashi N, et al. TNIK inhibition abrogates colorectal cancer stemness. Nat Commun. 2016;7:12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng Y, Xue X, Shao Y, et al. Controlled modelling of human epiblast and amnion development using stem cells. Nature. 2019;573:421‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843‐850. [DOI] [PubMed] [Google Scholar]
- 26. D'Amato NC, Rogers TJ, Gordon MA, et al. A TDO2‐AhR signaling axis facilitates anoikis resistance and metastasis in triple‐negative breast cancer. Cancer Res. 2015;75:4651‐4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goode GD, Ballard BR, Manning HC, Freeman ML, Kang Y, Eltom SE. Knockdown of aberrantly upregulated aryl hydrocarbon receptor reduces tumor growth and metastasis of MDA‐MB‐231 human breast cancer cell line. Int J Cancer. 2013;133:2769‐2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanford EA, Ramirez‐Cardenas A, Wang Z, et al. Role for the aryl hydrocarbon receptor and diverse ligands in oral squamous cell carcinoma migration and tumorigenesis. Mol Cancer Res. 2016;14:696‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DiNatale BC, Smith K, John K, Krishnegowda G, Amin SG, Perdew GH. Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol Cancer Res. 2012;10:1369‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castagnoli L, Cancila V, Cordoba‐Romero SL, et al. WNT signaling modulates PD‐L1 expression in the stem cell compartment of triple‐negative breast cancer. Oncogene. 2019;38:4047‐4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee Y, Shin JH, Longmire M, et al. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T‐Cell‐Mediated Immunity by Selective Constitutive and Inducible Expression of PD‐L1. Clin Cancer Res. 2016;22:3571‐3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei F, Zhang T, Deng SC, et al. PD‐L1 promotes colorectal cancer stem cell expansion by activating HMGA1‐dependent signaling pathways. Cancer Lett. 2019;450:1‐13. [DOI] [PubMed] [Google Scholar]
- 33. Terzuoli E, Bellan C, Aversa S, et al. ALDH3A1 overexpression in melanoma and lung tumors drives cancer stem cell expansion, impairing immune surveillance through enhanced PD‐L1 output. Cancers (Basel). 2019;11(12):1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miao Y, Yang H, Levorse J, et al. Adaptive Immune Resistance Emerges from Tumor‐Initiating Stem Cells. Cell. 2019;177:1172‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alvarado AG, Thiagarajan PS, Mulkearns‐Hubert EE, et al. Glioblastoma cancer stem cells evade innate immune suppression of self‐renewal through reduced TLR4 expression. Cell Stem Cell. 2017;20:450‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prager BC, Xie Q, Bao S, Rich JN. Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell. 2019;24:41‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheong JE, Sun L. Targeting the IDO1/TDO2‐KYN‐AhR pathway for cancer immunotherapy ‐ challenges and opportunities. Trends Pharmacol Sci. 2018;39:307‐325. [DOI] [PubMed] [Google Scholar]
- 38. Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan‐kynurenine‐aryl hydrocarbon axis. Clin Cancer Res. 2019;25:1462‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhai L, Ladomersky E, Lenzen A, et al. IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol. 2018;15:447‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu M, Wang X, Wang L, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018;11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO‐301 trial and beyond. Semin Immunopathol. 2019;41:41‐48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Fig S7
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Table S7
Appendix S1


