Abstract
Heart failure (HF) fundamentally reflects an inability of the heart to provide adequate blood flow to the body without incurring the cost of elevated cardiac filling pressures. This failure occurs first during the stressed state but progresses until hemodynamic derangements become apparent at rest. As such, the measurement and interpretation of both resting and stressed hemodynamics serve an integral role in the practice of the HF clinician. In this review, we discuss conceptual and technical best-practices in the performance and interpretation of both resting and invasive exercise hemodynamic catheterization, relate important pathophysiologic concepts to clinical care, and discuss updated, evidence-based applications of hemodynamics as they pertain to the full spectrum of HF conditions.
INTRODUCTION
In 1929, Dr. Werner Forssmann conducted the first cardiac catheterization procedure—on himself—by advancing a urological tube from an antecubital vein to the right atrium, and in doing so, inaugurated a new era in cardiology. Forty years later Swan and Ganz innovated a new pulmonary artery (PA) catheter feasible for use at the bedside, expanding use of hemodynamic assessments in diagnosis and care of patients with cardiac disease. In subsequent years advances in echocardiography and shifts in the cardiac catheterization laboratory from a diagnostic to therapeutic emphasis led to reduced use of invasive hemodynamics. However, the past decade has witnessed a resurgence in the use of resting and exercise invasive hemodynamic assessments, which provide a powerful tool to identify and care for patients with heart failure (HF).1,2 In this review we summarize concepts and best practices in the performance and interpretation of hemodynamic assessments across the HF disease spectrum (Table 1).
Table 1.
Summary of Uses of Resting and Invasive Exercise Hemodynamics.
| Clinical Entity | Use of Resting RHC | Use of Invasive Exercise RHC |
|---|---|---|
| 1. Exertional dyspnea | Evaluate for HF or PH; provocative maneuvers (e.g. fluid) can improve sensitivity | Gold standard evaluation of exertional dyspnea |
| 2. HF with reduced EF | Assess hemodynamics when volume and perfusion status are unclear; guide therapy | Assess CO limitation to exertion |
| 3. Cardiogenic Shock | Diagnosis and assessment; guide medical and mechanical circulatory support therapy | — |
| 4. Advanced HF Evaluation | Evaluate candidacy; vasodilator therapy to assess pre-capillary PH prior to transplant; assess risk of RHF post-LVAD | Assess CO limitation to exertion; differentiate from poor peripheral reserve |
| 5. Heart Transplantation | Concurrent with endomyocardial biopsies to rule out allograft rejection | — |
| 6. LVAD Management | Assess for pre-capillary PH; help diagnose LVAD complications; optimize perfusion and filling pressures via ramp studies | May be a role for assessing adequacy of LVAD pump speed |
| 7. HF with preserved EF | Diagnosis; rule out cardiac amyloidosis via endomyocardial biopsy | Diagnosis when resting assessments remain unclear; assess CO limitation |
| 8. Pulmonary Hypertension | Gold standard evaluation; PH subtyping; vasodilator provocation in PAH | May be a role for diagnosis of exercise-induced PH |
| 9. Valvular Disease | Reconcile differences between clinical and non-invasive assessments | Assess for exertional exacerbation of valvular lesions |
| 10. Congenital Heart Disease | Diagnose left-to-right shunts; assess PH; assess hemodynamics of complex lesions | Potential role in Fontan palliation to evaluate pulmonary vascular reserve |
| 11. High-Output HF | Diagnosis; ascertain shunt lesions | — |
| 11. Preload Reserve and Peripheral Limitations | — | Diagnosis |
| 12. Pericardial Disease | Diagnose constrictive versus restrictive process (with concomitant LHC) | Volume loading of exercise can sometimes help identify occult constriction |
CONCEPTUAL PRINCIPLES
Pressure and Pressure-Volume Relationships
Pressure defines force applied to an area: one mmHg represents the vertical force of 133.3 Newtons applied to an area of one square meter. Cardiac contractions expose the cardiovascular system to cyclic variations in pressure—greatest in the ventricles and lowest in the capillaries. Blood flow to support life is driven by the pressure gradients generated by the heart. During systole, ventricular and atrial pressures rise, increasing pressure to propel blood forward. During relaxation, pressure decays rapidly so that cardiac filling may occur as pressure falls. During diastole, increases in chamber volume stretch elastic elements within the heart and pericardium to increase pressure. The magnitude of this pressure increase is related to the volume of blood distending the chamber, the viscoelastic properties of myocardial tissue, the velocity and extent of relaxation, and the amount of external restraint on the heart. Understanding pressure and pressure-volume concepts helps to contextualize the hemodynamic assessment, since hemodynamics measure pressure and flow but fail to capture volume.
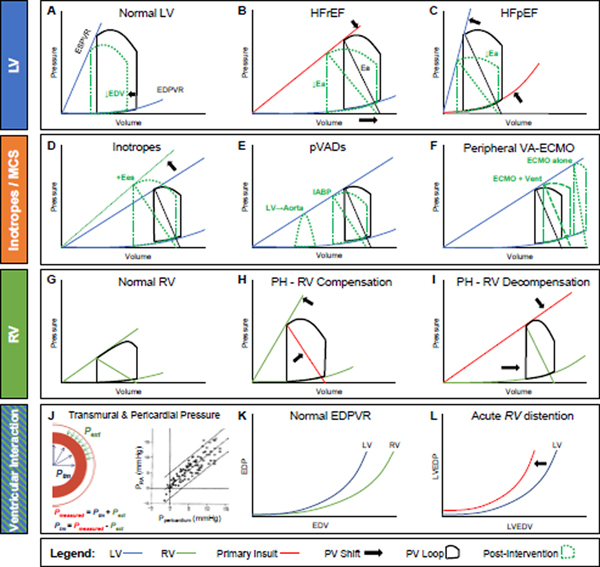
The pressure-volume loop of the left ventricle (LV) operates within the space subtended by the end-systolic pressure-volume relationship (ESPVR; also known as end-systolic elastance, Ees) and the end-diastolic pressure-volume relationship (EDPVR), with the differences between end-diastolic volume (EDV) (right) and end-systolic volume (left) defining the stroke volume (SV) (Figure 1A). The ESPVR and EDPVR characterize, in a load-independent manner, ventricular contractile and diastolic compliance properties, respectively. In a patient with well-compensated HF with reduced ejection fraction (HFrEF), contractility is depressed, leading to a shallower ESPVR (Figure 1B, top arrow), but the LV can still supply a near-normal SV at rest—and even maintain acceptable LV end-diastolic pressure (EDP)—because of an increase in LV volumes (bottom arrow).3 Note that ejection fraction (EF) is depressed because EDV increased, not because SV decreased.
Figure 1.
Pressure-volume physiology of the LV, inotropes and select mechanical circulatory support (MCS) devices, the RV, and ventricular-pericardial interactions. Key principles and all abbreviations thoroughly outlined in text.
Afterload is indexed by effective arterial elastance (Ea), defined in the pressure-volume plane by the quotient of end-systolic pressure and SV (black diagonal). Ea reflects the lumped “stiffness” of the vasculature and is directly related to systemic vascular resistance and heart rate, and inversely related to arterial compliance.3 The shallow ESPVR in HFrEF results in an increase in afterload-sensitivity.4 Diuresis lowers LV EDV (Figure 1A) which, in a hypervolemic HFrEF patient with steeper EDPVR, also lowers pressure and congestive symptoms. With afterload reduction, Ea decreases (Figure 1B, shallower slope, green), leading to a slight reduction in end-systolic pressure but marked improvement in SV. The opposite occurs with a vasoconstrictor (not shown), which can markedly impair SV.
Inotropes and temporary mechanical circulatory support (MCS) devices, used in low output and cardiogenic shock states, also have distinct effects on the HFrEF LV pressure-volume loop.3,5 Inotropes (e.g. dobutamine and milrinone) augment ESPVR while reducing systemic vascular resistance; the net effect is increased SV, reduced Ea, and decreased EDP (Figure 1D).3 These benefits, however, come at cost of increased myocardial oxygen demand. Peripheral ventricular assist devices (pVADs) augment flow from LV to the systemic circulation. Intra-aortic balloon pump (IABP) counterpulsation does so indirectly by reducing systemic afterload during systole. For the LV with sufficient contractile reserve,6 this can reduce Ea, augment SV, and reduce LV EDV and EDP (Figure 1E). Percutaneous micro-axial flow pumps, which actively draw blood from the LV into the aorta, significantly reduce LV filling pressures and myocardial demand (Figure 1E) while dramatically augmenting systemic flow and mean arterial pressure (not shown). Here, the triangular LV pressure-volume loop results from continuous LV emptying which interrupts isovolumic contraction and relaxation. Of note, a durable left ventricular assist device (LVAD) has a nearly identical effect on LV hemodynamics. Lastly, in extreme cases of cardiogenic shock, venoarterial-extracorporeal membrane oxygenation (VA-ECMO) may be necessary to restore adequate flow and perfusion to the body. When done via peripheral cannulation, there is a significant afterload imposed against the ailing LV (Figure 1F). In such cases, LV venting strategies, whether by pharmacologic or mechanical means (latter depicted in Figure 1F), may be needed to mitigate the increased afterload, which in turn reduces LV EDP to prevent pulmonary edema.
In patients with HF with preserved ejection fraction (HFpEF) there is commonly an EDPVR shift up and to the left, due to increases in viscoelastic chamber stiffness (Figure 1C). This results in higher filling pressures relative to chamber volume (bottom arrow), an effect that becomes amplified at higher volumes. In addition to active stiffening (i.e. contractility), ESPVR is increased through passive chamber stiffening, such that ESPVR is elevated in HFpEF even as other measures of chamber and myocardial contractility are depressed7 (upper arrow). Heightened systolic and diastolic stiffening render HFpEF patients highly sensitive to diuretics and vasodilators,4,8 complicating management of both volume status and blood pressure (BP). With the same degree of afterload reduction (green) there is much greater decrease in BP with less increase in SV in HFpEF as compared to HFrEF (Figure 1C). Similarly, the increase in the EDPVR renders them more sensitive to LV preload reduction with venodilators, which can reduce SV.4
The pulmonary circulation accepts the same cardiac output (CO) as the systemic circuit, but at one-fifth of the pressure. The RV pressure-volume loop differs from the LV in the amplitude of pressure change and generally takes a more trapezoidal or triangular shape (Figure 1G). Like in the LV, the RV ESPVR slope (also termed end-systolic elastance, or Ees) should match Ea, resulting in an Ees/Ea ratio of >1.0 in health.9 In pulmonary hypertension (PH), Ea rises as PA pressure increases. When the RV is compensated, there is an accompanying increase in Ees, maintaining the Ees/Ea ratio and EF (Figure 1H). However, over time, patients with PH often progress to RV decompensation (Figure 1I), where RV Ees decreases relative to Ea, the RV dilates to maintain SV, and RV EF declines. With progressive decompensation, RV contractility and diastolic compliance worsen, RV Ees/Ea falls, CO declines, and RV EDP and right atrial pressure (RAP) increase out of proportion to left-sided pressure. It can be difficult to ascertain when the compensated RV may be masking occult RV disease. In such patients, stressing the RV to reveal RV reserve may uncover RV pathology unapparent at rest.10–12
Finally, the pericardium also plays an important role in HF and PH. The RV and LV are connected in series, as the former provides flow to the latter. But they may also influence one another in parallel, through the phenomenon of ventricular interaction, if the restraining effects of the pericardium amplify competition between the right and left heart for volume. Chamber or vascular pressure measured with a catheter (Pmeasured) represents the sum of transmural filling pressure (Ptm, the forces from within favoring distention) together with the external pressure applied on the epicardium (Pext, which when positive, opposes distention, Figure 1J). In the heart, external pressure is equal to pericardial pressure, which can be accurately estimated by RA pressure.13 Under normal conditions, owing to its thicker walls, the LV has a steeper EDPVR than the RV (Figure 1K), and pericardial restraint on the heart is minimal.13 However, with acute increases in cardiac volume, as may accompany exercise, pulmonary embolism, or acute valve insufficiency, increases in pericardial restraint are augmented. The resulting increases in surface contact pressures on the epicardium (Pext) transmit to increase intracavitary pressure (Pmeasured), even as cardiac muscle properties remain unchanged. In other words, the intracavitary pressure is increased even if the transmural distending pressure is normal or even low, as often noted during acute decompensated HF (Figure 1L). RV distention due to central venous congestion shifts the LV EDPVR leftward by way of pericardial restraint. This in turn reduces LV Ptm, reducing LV preload and thus CO (Figure 1L).13 In this situation, decongesting the RV with diuretics and venodilators are crucial to restoring LV transmural filling pressure and raising LV end diastolic volume (true “preload”).13,14
Hemodynamic Principles of Exercise Physiology
Resting hemodynamic abnormalities may be subtle or even absent during early stages of HF, or when patients are well diuresed. In such cases, HF may only become evident during the stressed state, when the heart fails to respond to the heightened physiologic demands of exercise. According to the Fick principle, oxygen consumption (VO2) is equal to the product of CO and the arteriovenous oxygen difference (AVO2-diff). With physical exertion there is an increase in VO2 that is achievable through combined increases in O2 delivery (CO) and enhanced distribution and extraction of O2 in skeletal muscle (increase in AVO2-diff). In health, CO increases at a ratio of 6 ml/min for every 1 ml/min increase in VO2.15 This is achieved through increases in heart rate (HR) and SV. The latter increases due to enhanced venous return to augment preload, along with increases in contractility and reduction in vascular resistances in both the lungs and systemic circulation to facilitate more complete ventricular ejection. The increase in venous return during exercise is related to the combined actions of skeletal muscle pumps combined with venoconstriction in the large capacitance veins of the abdomen, increasing the stressed blood volume (the volume that contributes to increasing vascular pressure).16
At steady state, venous return must equal CO. During exercise, the heart must accommodate marked increases in blood return and flow without increasing filling pressures. This is achieved through biventricular lusitropic reserve, which allows both ventricles to fill to larger volumes despite a shorter diastolic time interval.17 These adaptations enable a minimal rise in left and right atrial pressures during exercise in healthy individuals,18 which allows pulmonary capillary and PA hydrostatic pressures to remain in the normal range.
Patients with HF may display limitations in each of these adaptations. Increases in arterial stiffness and inadequate vasodilation amplify LV afterload, contributing to pulmonary capillary wedge pressure (PCWP) increase.19 LV and RV systolic reserve are impaired, limiting forward SV.20 This combines with abnormalities in diastolic relaxation and compliance that further increase PCWP,10,21 which then leads to increased fluid filtration across the pulmonary capillary-alveolar interface, resulting in extravascular lung water.22 Chronotropic incompetence further limits CO reserve.23,24 Depression in peak VO2 is also related to blunted augmentation of the AVO2-diff.25 Recent studies have further shown that greater increases in stressed blood volume during exercise further tax the heart-lung unit during exercise to increase PCWP in HFpEF, due to reduction in venous capacitance.26 Thus multiple highly integrated mechanisms contribute to the hemodynamic derangements that develop during exercise in HF.
HEMODYNAMIC ASSESSMENT IN THE CATHETERIZATION LABORATORY
Resting Hemodynamic Assessment
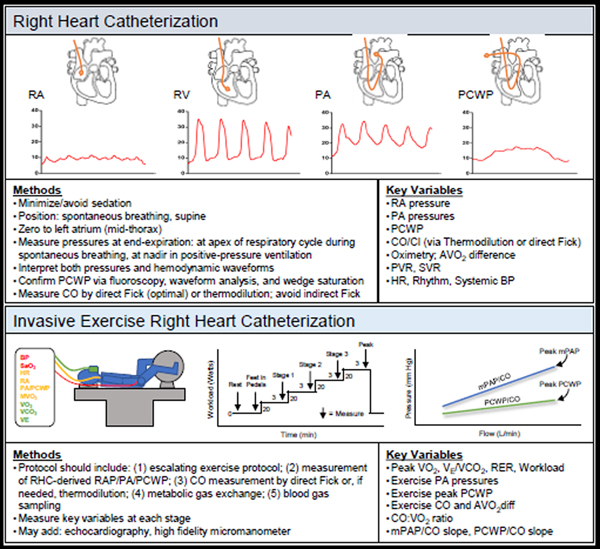
Right heart catheterization (RHC) is ideally performed with minimal conscious sedation in supine patients, often without interrupting anticoagulation, which is important in vulnerable populations such as in LVAD patients. The pressure transducer should be zeroed at mid-thorax (level of the left atrium).27 Over- or under-dampening of pressure tracings should be addressed through good technique including aspiration and catheter flushing. Pressure measurements in each chamber are determined at end-expiration in the spontaneously breathing, quiet patient, taking the mean of ≥3 beats (Figure 2). Note that at end-expiration, pressure is measured at the maximum value in the spontaneously breathing patient, which avoids the impact of negative pleural pressures; by contrast, pressure is measured at the minimum value in the mechanically ventilated patient to account for the impact of positive pleural pressure.28 Care should be devoted to confirming PCWP using fluoroscopy, waveform analysis, and pulmonary capillary wedge saturation.29 Mean right and left atrial pressures are taken at mid a-wave. The PCWP tracing provides an estimate of left atrial pressure, which may differ from LVEDP in patients with mitral valve disease or increased atrial stiffening. In addition to pressure amplitude, characteristics of hemodynamic waveforms reveal important diagnostic findings (Figure 3 and Table 2). CO is most accurately measured via the direct Fick method, where VO2 is directly measured and divided by the difference in arterial and mixed venous (PA) oxygen content (AVO2-diff). If VO2 cannot be measured, thermodilution is preferred because of inaccuracies in estimating indirect VO2. 30 Although thermodilution is perceived to be inaccurate in cases of low output or tricuspid regurgitation, reasonable measurements are observed even in these settings.31
Figure 2.
Resting and Invasive Exercise Right Heart Catheterization. Typical RHC tracings, exercise protocol schematics, key methods and variables outlined for both.
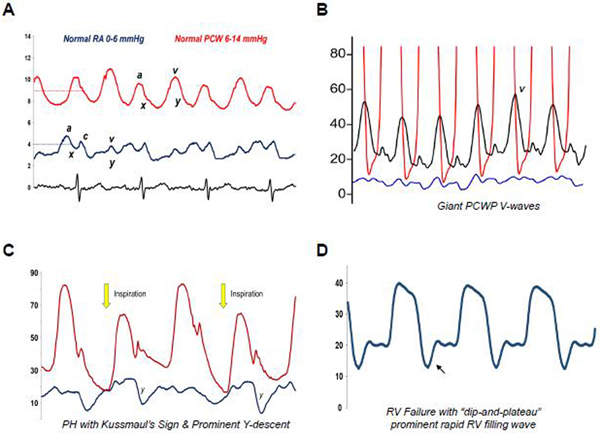
Figure 3.
Example hemodynamic tracings. Key points highlighted alongside tracings. (A) Normal right atrial (RA) and pulmonary capillary wedge (PCW) tracings and values. (B) Giant V-waves on PCW tracing due to reduced left atrial compliance. Care should go towards identifying mean PCW pressure by bisecting the A-wave (and not the V-wave) at end-expiration. (C) Pulmonary hypertension with prominent Y-descents and Kussmaul’s sign (rise in RA pressure) with inspiration, collectively indicative of poor pulmonary vascular and RV compliance. (D) RV failure with a dip-and-plateau sign during diastole, indicative of severe RV dysfunction and diastolic pressure overload. The same can also be seen in constrictive pericarditis and restrictive cardiomyopathies when ventricular filling must occur early and rapidly due to poor ventricular compliance.
Table 2.
Causes of Prominent or Blunted Hemodynamic Tracing Features
| Tracing | Prominent | Blunted/Absent |
|---|---|---|
| V wave | Valve insufficiency; ↓Atrial compliance; ↑Atrial Volume | — |
| A wave | Atrioventricular dyssynchrony; Strong atrial contraction plus Stiff ventricle or valve stenosis | Atrial fibrillation |
| Y descent | Valve insufficiency; Restrictive/constrictive disease | Tamponade; Valve stenosis |
Oximetry plays a vital diagnostic role. A significant “step-up” in oxyhemoglobin saturation (≥8%) between the superior vena cava and PA should prompt a full saturation run (with sampling from the superior and inferior vena cava, RA, RV, PA, and PA wedge position) to characterize a potential left-to-right shunt. The precise location of the step-up can help localize the shunt. Values also help quantify shunt severity by calculating Qp/Qs, or the ratio of pulmonary-to-systemic flow. According to the Fick principle, Qp/Qs = (SAO2 - Mixed Venous O2)/(PCW O2 - PA O2), where mixed venous O2 is taken prior to the step-up (estimated as =0.75(SVC O2) + 0.25(IVC O2) if the step up is at the level of the RA). The Qp/Qs ratio is normally 1:1. A Qp/Qs ratio >1:1 indicates net left-to-right shunting, with ratios >1.5–2.0 considered significant.32
Invasive Hemodynamic Exercise Testing
Invasive hemodynamic exercise testing affords the ability to identify each of the aforementioned hemodynamic abnormalities of exercise, making it the gold standard to differentiate various causes of exertional dyspnea, including HFpEF, exercise-induced PH, dynamic valvular insufficiency, preload failure, and peripheral limitations/mitochondrial disease (Table 1). Protocols differ slightly across centers11,29,33 but should include: (1) an escalating exercise workload in the supine or upright position, (2) simultaneous measurements of RAP, PA, and PCWP, (3) CO measurement using either direct Fick or thermodilution, (4) measurement of gas exchange using a metabolic cart (if available), and (5) assessment of arterial and venous O2 content (Figure 2).11,29,33 Controversies exist as to whether pressures should be measured at end-expiration or using the mean of respiratory cycle; clearly reporting both values is probably ideal. Additional evaluation may be added depending upon the clinical question, including concomitant echocardiographic assessment (e.g. valvular disease). Some laboratories utilize a high-fidelity micromanometer advanced through the fluid-filled catheter to provide more precise pressure waveform data during exercise, where fluid-filled catheters are less accurate.29
SPECIFIC DISEASE STATES
Heart Failure with Reduced Ejection Fraction
Most clinical decisions in HFrEF can be made based on history, physical exam, and laboratory findings. In fact, Heart Failure Society of America (HFSA) guidelines recommend against the routine assessment of invasive hemodynamics in HF.34 These guidelines are supported by the seminal Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness (ESCAPE) trial,35 which found no mortality benefit to routine pulmonary artery catheter (PAC) use in acute decompensated HF.
Hemodynamic assessments, however, do provide actionable data in more challenging HF patients in whom either volume or perfusion status remains unclear (Table 3).34 This is congruent with more recent data suggesting that PAC utilization improves outcomes in advanced HF or cardiogenic shock.36,37 Figure 4 outlines the evolution of hemodynamic indices in the context of HFrEF disease progression. Invasive hemodynamic assessment may be more helpful in the more advanced stages of HFrEF, including patients in whom an occult low output state may be present, when advanced therapies are being considered, and in acutely ill patients with hypotension (Figure 4).
Table 3.
Invasive Hemodynamic Guidance in Common Clinical Scenarios
| Scenarios | BP | HR | RAP | PCWP | CO | SVR | Potential Action |
|---|---|---|---|---|---|---|---|
| Dyspnea and unclear volume status | - | - | - | - | - | - | Workup lung disease, non-cardiac dyspnea |
| - | - | ↑ | ↑↑ | - | - | Diuresis | |
| - | - | ↑↑ | -/↓ | -/↓ | -/↑ | Workup for PH, PE, Pericardial disease, RV MI | |
| Unclear volume status, perfusion, vascular resistance; Worsening renal function during decongestion | - | -/↑ | -/↓ | -/↓ | -/↓ | - | Stop acute decongestion |
| -/↑ | -/↑ | ↑↑ | ↑↑ | ↓ | ↑↑ | Diuresis + Vasodilator*
Diurese to goal RAP (<8 mmHg), PCWP (<15 mmHg); vasodilate to reduce SVR (1000–1200 dynes/s/cm−5) while maintaining MAP (65–70 mmHg) - sacubitril/valsartan oral - hydralazine-isosorbide dinitrate oral - nitroprusside IV (0.25–5.0 mcg/kg/min** |
|
| ↓ | ↑ | ↑↑ | ↑↑ | ↓ | -/↑ | Inotrope*
Inotropes to increase cardiac index (>2.0 L/min/m2) and MAP (>65 mmHg): - dobutamine (2.5–10 mcg/kg/min) - milrinone (0.125–0.500 mcg/kg/min) |
|
| Hypotension of unclear etiology | ↓↓ | ↑ | -/↓ | -/↓ | ↓ | ↑↑ | Hypovolemic shock likely. Treat with IV fluid, or blood, depending on cause. |
| ↓↓ | ↑ | - | - | -/↑ | ↓↓ | Distributive shock likely. Treat with IV fluid + vasopressor, workup etiology. Vasopressors to consider: - norepinephrine (0.01–2 mcg/kg/min) - vasopressin (0.04 U/min) |
|
| ↓↓ | ↑ | ↑↑ | ↑↑ | ↓↓ | ↓/↑ | Cardiogenic shock likely. Inotrope/vasopressor ± temporary MCS, workup etiology. If hypotension severe, consider vasopressors: - epinephrine (0.01–0.1 mcg/kg/min) - norepinephrine (0.01–2 mcg/kg/min) |
In general, vasodilator-based therapy is preferred if BP allows (e.g. MAP >65 mmHg) given the increases in myocardial O2 demand and proarrhythmia associated with inotropes
IV nitroprusside is monitored with invasive BP monitoring, then gradually transitioned to oral vasodilator therapy once desired hemodynamic effects have been achieved
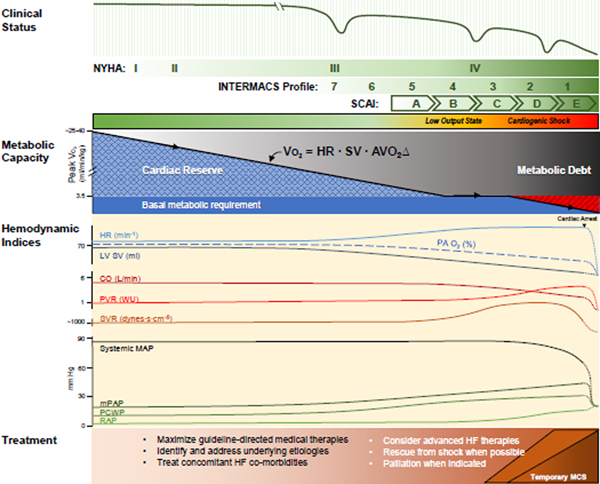
Figure 4.
Hemodynamic indices in HF and CS. As HF progresses, metabolic capacity gradually wanes. Patients first suffer loss of cardiac reserve but maintain normal rest perfusion. Multiple hemodynamic indices continuously adapt to gradually waning SV in order to maintain CO and BP. Cardiac pressures rise as the heart fails to maintain normal filling; pulmonary pressures rise too. As reserve fades, small perturbations in the metabolic supply-demand balance can precipitate exacerbation. In later disease, multiple compensatory mechanisms fail, leading to low output and cardiogenic shock states. Upon cardiac arrest, flow stops and pressures converge upon the mean circulatory filling pressure. Treatments throughout are directed towards the clinical and hemodynamic state. NYHA, New York Heart Association; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support Profiles; SCAI, Society for Cardiovascular Angiography & Interventions Classification of Cardiogenic Shock.
Cardiogenic Shock
Cardiogenic shock (CS) occurs when there is a primary myocardial insult that reduces CO to a level insufficient to maintain end-organ perfusion and results in pulmonary and/or venous congestion from increased cardiac filling pressures.17 The rapidity by which CO declines is important since clinical manifestations may not be apparent if the decline is gradual. Sudden impairment of CO is usually poorly tolerated and results in pallor, cold extremities, and mental status changes; congestion is manifest by tachypnea and hypoxemia. By contrast, the same magnitude of hemodynamic abnormalities that evolves over weeks to months in chronic HF may not be clinically apparent until a tipping point is reached.
Hypotension is the predominant hemodynamic manifestation of CS; in fact, the diagnosis is rarely considered unless hypotension is present. Most clinical investigations have used 90 mmHg for systolic BP (SBP) and 60 mmHg for mean arterial pressure (MAP) for at least 30 minutes. Concomitant presence of hypoperfusion is required, assessed by exam38 or simple measures of hypoperfusion, such as decreased urine output or increased lactate. It should be recognized that tissue hypoperfusion can be present without hypotension if CO has significantly fallen but endogenous vasoconstrictor responses are maintaining BP.39
In 2020, the Society for Cardiovascular Angiography & Interventions (SCAI) Clinical Expert Consensus Statement on the Classification of CS40 leveraged an integrated diagnostic approach using both clinical assessment and invasive hemodynamics to define the CS syndrome as well as its severity across five temporal stages (Table 4). Recent data from the Cardiogenic Shock Working Group (CSWG) registry have revealed that this scheme has prognostic value in CS—particularly increases in central venous pressure, which reflect pericardial restraint and biventricular HF.41 Other indices have been used to provide a more composite quantitative picture of shock. LV cardiac power output (CPO) is the product of CO and MAP divided by 451. Reduction in cardiac power may be a consideration for the use of intra-aortic balloon pump counter-pulsation (IABP), as low LV CPO is associated with poor hemodynamic stabilization in advanced HF patients treated with IABP.6 However, neither CPO nor cardiac index were predictive of shock severity or mortality in the CSWG registry.41 A limitation of the commonly calculated CPO is the absence of the RAP in the formula, which had been part of its original derivation.42
Table 4.
SCAI Grading System of Cardiogenic Shock.
| Stage | Description | Bedside Findings | Biochemistry | Hemodynamics |
|---|---|---|---|---|
|
A
At risk |
At risk for development of CS; includes MI and HF patients | Normal JVP; lungs clear; warm and well perfused | Normal renal function; Normal lactate | Normotensive; CI > 2.5, CVP <10, PA sat > 65% |
|
B
Beginning CS |
Clinical evidence of relative hypotension or tachycardia, without hypoperfusion | Elevated JVP; rales; warm and well perfused | Normal lactate; mild renal impairment; elevated BNP | SBP <90 or MAP <60 or >30 mmHg drop, Pulse >100; CI >2.2, PA sat >65% |
|
C
Classic CS |
Hypotension and hypoperfusion requiring an intervention (inotrope, pressor, or MCS) | May look unwell, ashen; rales, assisted ventilation; cold, clammy, low urine output | Elevated lactate; Creatinine worsening; Increased liver chemistries; Elevated BNP | SBP <90 or MAP <60 or >30 mmHg drop; CI <2.2, PCWP >15, RAP/PCWP >0.8, PAPi <1.85, CPO <0.6 |
|
D
Deteriorating |
A category C patient who is getting worse; failing first interventions | Any of Stage C | Any of Stage C and deteriorating | Any of Stage C despite multiple pressors or additional MCS devices |
|
E Extremis |
Cardiac arrest with ongoing CPR and/or ECMO; requiring multiple interventions | Near pulselessness Cardiac collapse; Mechanical ventilation; Defibrillator use | Any of above and pH <7.2 Lactate >5 | No SBP without resuscitation; PEA or VT/VF; hypotension despite max support |
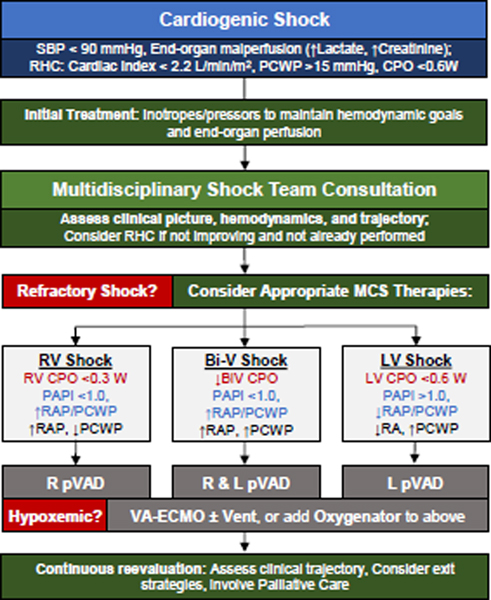
Hemodynamic phenotyping of CS has value in guiding therapeutic strategies to support the circulation43–45 until either recovery or more durable circulatory support (e.g. transplantation or LVAD). Proposed phenotyping algorithms43–45 generally rely on CPO, PAPi (to assess the right heart), and filling pressures to guide management (Figure 5). However, the criteria for escalation to temporary MCS despite pharmacologic support for CS are not clear and, in general, are circumstantially driven by patient characteristics as well as center and provider expertise. Introducing MCS to pharmacologic support may be able to prevent the hemo-metabolic consequences of CS.46 Hemo-metabolic shock constitutes the final and most mortal phase of shock; once manifest alongside congestion and malperfusion, falling systemic vascular resistance due to vascular exhaustion leads to profound hypotension (Figure 4) and particularly poor outcomes. Although lactate is often measured and is associated with outcomes, it has been difficult to show that lowering lactate through either pharmacologic or mechanical means improves outcomes.
Figure 5.
A proposed team-based approach to the management of refractory Cardiogenic Shock. LV CPO, LV cardiac power output (MAP·CO/451); RV CPO (mPAP·CO/451); PAPi, pulmonary artery pulsatility index ((sPAP-dPAP)/RAP); RAP/PCWP, right atrial pressure-to-pulmonary capillary wedge pressure ratio; pVAD, percutaneous ventricular assist device; VA-ECMO, venoarterial-extracorporeal membrane oxygenation.
The authors recommend prompt confirmation of the putative diagnosis of CS with invasive hemodynamic data, followed by team-based management in an appropriate intensive care setting. Several large observational series suggest that PA catheterization use may be associated with improved outcomes in CS patients,28,36,37,41 but prospective randomized data do not yet exist and several practical issues limit widespread adoption in routine clinical practice.
Hemodynamics in Heart Transplant and LVAD
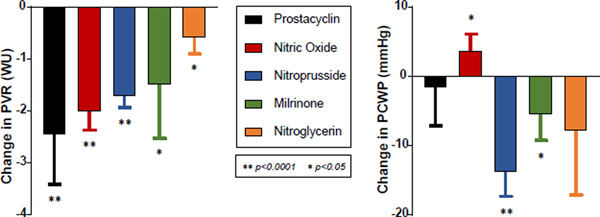
Invasive hemodynamics play an integral role in HFrEF patients being considered for advanced surgical therapies. When considering heart transplantation, fixed pre-capillary PH (pulmonary vascular resistance (PVR) >3.5–5 Wood units (WU)) is a relative contraindication, since transplanting an allograft naïve to high pulmonary pressures can precipitate acute right heart failure and death.47 In these patients, provocative challenge with intravenous sodium nitroprusside can identify lungs with “reversible” pre-capillary PH still amenable to heart transplantation. If patients with baseline PVR >2.5 WU treated with nitroprusside drop to ≤2.5 WU without systemic hypotension, their post-transplant risk profile is similar to those with baseline PVR ≤2.5 WU.48 Other provocative agents such as milrinone and prostacyclin, each with their own anticipated effect on PVR and PCWP (Figure 6), have also been prospectively studied.49
Figure 6.
Average effect on PVR and PCWP of select provocative agents useful for vasodilator testing of pre-capillary pulmonary hypertension.49
While infrequently used for this purpose, invasive exercise testing can also help evaluate transplant appropriateness. By measuring both supply (CO) and demand (VO2) in tandem, one can directly assess the ability of the heart to match metabolic requirements during exercise. CO reserve limitation is defined by supply-demand ratio that is <80% of expected (ΔCO:ΔVO2 <4.8:1). Non-invasive cardiopulmonary exercise testing (CPET) is more often used to assess transplant candidacy and estimate CO reserve, but it must be remembered that 50% of the variability in peak VO2 is explained by the AVO2-diff, which is not measured during non-invasive CPET. Patients with low peak VO2 but normal CO reserve may respond better to exercise training to improve peripheral reserve, rather than undergoing transplant or LVAD. Indeed, Chomsky and colleagues showed that impairments in CO reserve were far more prognostic than peak VO2 in patients with advanced HFrEF undergoing transplant evaluation.50 Additionally, some patients with HF may have relatively normal CO at rest but virtually no CO reserve with exertion; these patients may also benefit from transplant.
Hemodynamics are equally important when considering durable LVADs. Early right-sided HF (RHF) following LVAD remains a relatively common cause of post-LVAD morbidity and mortality, occurring in 9–42% of cases.51 Numerous models have been developed to predict post-operative early RHF, but none has proven to have high discrimination, Some hemodynamic indices have emerged to be more useful: Kussmaul’s sign,52 pre-operative RAP (>15 mmHg), elevated RAP/PCWP ratio (>0.54–0.63), reduced RV stroke work index (<0.25 mmHg·L/m2), tachycardia, and low cardiac index (<2.2 L/min/m2) have predicted RHF risk across multiple models.51 Reduced pulmonary artery pulsatility index (PAPi=(PASP-PADP)/RAP) has also shown predictive capability.53,54 These hemodynamic predictors are complemented by clinical, echocardiographic, and laboratory risk markers of RHF, such as patient acuity, severe RV contractile dysfunction, and elevated blood urea nitrogen.51 An integrated right heart assessment—incorporating clinical, imaging, and hemodynamic predictors of RHF—along with careful monitoring and early treatment of RHF, are well advised in the consideration and execution of LVAD surgery.
Hemodynamics also play a crucial role in managing post-LVAD patients. When needed, hemodynamics enable precise assessments of CO, filling pressures, and afterload, which all significantly affect the very afterload-sensitive flow of blood through an LVAD (in particular the centrifugal continuous-flow LVAD). Hemodynamics, when added to echocardiography, also improve diagnostic accuracy when considering LVAD complications such as pump thrombosis, since pump compromise may manifest in LV pressure and CO changes not apparent on imaging.55 PA catheterization also plays an important role in LVAD speed optimization, since as many as ~40% of LVAD patients have suboptimal RAP and PCWP, even after clinical/echocardiographic-based optimization.56 Hemodynamic-guided ramp studies, during which both RHC and echo measurements are repeated after successive LVAD speed increases, can improve filling pressure optimization,56 direct subsequent medical interventions, and potentially reduce HF hospitalizations,57 though further prospective study is required. PA catheterization also monitors for pre-capillary PH in bridge-to-transplant LVAD patient. Lastly, invasive hemodynamic exercise testing has also been utilized in LVAD recipients. Current generation LVADs maintain a static speed, which limits CO reserve58 and peak VO2,59 and invasive exercise testing may allow for more refined estimates of the ideal LVAD speed that optimizes both resting and exercise flow.
Heart Failure with Preserved Ejection Fraction
More than half of patients with HF suffer from HFpEF.60 Overt HFpEF can be diagnosed when the clinical syndrome occurs in conjunction with preserved LV ejection fraction and clear noninvasive evidence of congestion and diastolic dysfunction.61 Natriuretic peptide levels are helpful if elevated, but often are not.60 Thus, non-invasive diagnostic criteria applied at rest miss a large proportion of HFpEF patients who develop elevations in cardiac filling pressures exclusively during exercise.1 This is because long before abnormalities manifest at rest, the left heart in HFpEF loses its ability to maintain low filling pressures while trying to meet the increased demands of exercise.
Due to the poor sensitivity of clinical and non-invasive diagnostics, invasive hemodynamic testing plays a major role in the optimal evaluation of HFpEF.60 For the patient with overt HFpEF, RHC can readily diagnose elevated left-sided filling pressures at rest while concurrently ruling out World Society of Pulmonary Hypertension (WSPH) Group I PH. If indicated, endomyocardial biopsy performed at time of RHC can rule out cardiac amyloidosis. Hemodynamics are even more useful during exercise testing in HFpEF, because the variables that define HF are directly measured. HFpEF is defined invasively during supine exercise as a peak exercise PCWP ≥25 mmHg,1 which is the current definition endorsed by diagnostic guidelines.62 A complementary hemodynamic pattern indicative of HFpEF involves measuring PCWP as a function of CO at regular intervals during exercise. A PCWP/CO slope >2 mmHg·min·L−1 has been shown to be predictive of outcomes.33 Invasive exercise testing also allows assessment of CO reserve, which as outlined earlier, can help confirm cardiac limitation as the cause of exertional dyspnea.
Invasive exercise testing requires operator expertise, time, and capital investment. Alternative approaches include saline loading, leg raise hemodynamics, and exercise echocardiography. Saline loading is simpler to perform,63 but still involves catheterization and its sensitivity is inferior to exercise testing.64 An increase in PCWP with passive leg raise has been shown to provide good discrimination of HFpEF from non-cardiac dyspnea, but provides less physiologic information.1 Exercise echocardiography shows promise as a non-invasive method, but while some have shown its diagnostic accuracy,65 others have not.66
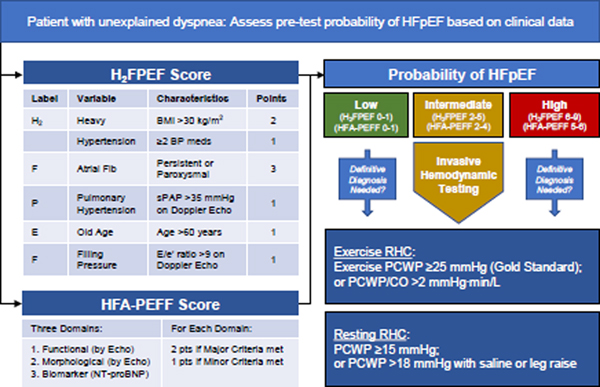
To help inform decision-making regarding invasive testing, we advocate for an integrated, stepped diagnostic approach to the work-up of HFpEF (Figure 7). Recently, Reddy and colleagues derived and then validated a simple diagnostic model to estimate the pretest probability of HFpEF, termed the H2FPEF score (Figure 7).67 The H2FPEF score exhibits robust discrimination of HFpEF from non-cardiac etiologies of dyspnea (area under the curve 0.841, P <0.0001).67 Utilization of this clinical score allows for a Bayesian approach to the HFpEF diagnosis, whereby those with low and high probability can be ruled out and in, respectively, while those with intermediate probability (score 2–5) can go on to invasive hemodynamic exercise testing (Figure 7).60 An alternative score put forth by the European Society of Cardiology called the HFA-PEFF score is somewhat more complicated but can be used as an alternative or complementary approach (Figure 7).62
Figure 7.
A diagnostic approach to HFpEF. Patients with unexplained dyspnea can be assessed by the H2FPEF score, or an alternative HFA-PEFF score. Those with intermediate probability, or others if needed, can be further evaluated using invasive hemodynamic exercise testing (gold standard), or PA catheterization at rest with provocative maneuvers.
Pulmonary Hypertension
PH was defined for nearly 50 years as mPAP ≥25 mmHg,68 but recent studies have shown that normal mPAP is actually 14±3.3 mmHg,69 and several studies have revealed that mPAP in the 21–24 mmHg range confers increased mortality.68,70 Accordingly, the latest WSPH proposed lowering the threshold of PH to mPAP >20 mmHg.68 However, to diagnose Group 1 PH (pre-capillary or pulmonary arterial hypertension, PAH), PVR ≥3 WU and PCWP <15 mmHg must also be present.68 Once PAH is diagnosed, vasoreactivity testing should be performed in patients with idiopathic, heritable, or anorexigen-associated PAH, since in these cases vasoreactivity influences prognosis and treatment.71 Testing is performed by administering inhaled nitric oxide (or alternatives like intravenous epoprostenol), with vasoreactivity present if mPAP falls by >10 mmHg to <40 mmHg without a fall in CO.72
Group 2 PH is diagnosed when mPAP >20 and PCWP ≥15 mmHg and is seen in at least 50–80% of patients.73 Group 2 PH may be subcategorized as isolated post-capillary PH (Ipc-PH) when PVR <3 WU, or combined pre- and post-capillary PH (Cpc-PH) when PVR ≥3.0 WU, where mPAP is elevated “out-of-proportion” to left heart disease.73 Patients in the latter group display a distinct hemodynamic profile characterized by greater RV dysfunction, ventricular interdependence, more severe exercise limitation, and greater risk of death.74–76 Not surprisingly, hemodynamic indices that reflect RHF—including elevated RAP, reduced SV,77 and Kussmaul’s sign78—have been shown to predict poor functional capacity and outcomes, both in Group 2 as well as Group 1 PH. PVR is related to PA compliance (SV/PA pulse pressure) in a hyperbolic fashion, wherein patients with early stages of PA vascular disease (slight increases in PVR) develop marked reductions in compliance with further progression. Increases in PCWP further depress PA compliance at any given PVR.79 Like increases in PVR, reductions in PA compliance are highly prognostic in Group 2 PH.75
Distinguishing Group 1 from Group 2 PH is of crucial therapeutic importance, as there are multiple effective treatments for the former and no proven direct therapies for the latter.80 The lynchpin for this distinction relies on accurate assessment of PCWP. Here, the wedge saturation may be particularly useful. Indeed, a recent study showed routine wedge saturation measurements reclassified WSPH grouping in 11.8% of patients when compared to diagnoses based on PCWP measured without saturation assessment.81
Invasive hemodynamic exercise testing may also play a role in PH. Exercise-induced PH (EiPH) was formerly defined by an exercise mPAP >30 mmHg, but in 2008 this criterion was removed from the guidelines.68 Ongoing efforts exist to define EiPH because earlier identification and treatment of PH hold promise to improve outcomes, though further study is required. Two criteria have been proposed. One defines EiPH as an exercise mPAP/CO slope >3 mmHg·min·L−1,82 while another defines EiPH when both mPAP >30 mmHg and total pulmonary resistance (mPAP/CO) >3 WU at exercise peak.83 In order to distinguish exercise-induced pulmonary vascular disease from HFpEF, measurements of PCWP during exercise are necessary.1,33 Recent studies have revealed that patients with EiPH display poorer clinical outcomes,33,84 and emerging data suggest it may represent a therapeutic target. One recent trial showed that the β-agonist albuterol improved pulmonary vascular reserve with exercise (lower PVR and mPAP/CO slope) as compared with placebo.85 Further study is required with longer durations of treatment in multicenter trials to determine if these hemodynamic improvements translate to clinical improvement.
Other Indications
Invasive hemodynamic assessments are often helpful in patients with valvular heart disease whenever discrepancies exist between the clinical picture and non-invasive assessment of valve severity (e.g. low-flow, low-gradient aortic stenosis or combined mitral stenosis and HFpEF).86
They may also be useful in adult congenital heart disease to diagnose shunts or assess PH, or in more complex diseases, including Fontan physiology. For example, in adults with the Fontan palliation, increased mPAP/CO slope with exercise is associated with more end-organ dysfunction and abnormal flow-mediated vasodilation, suggesting this may provide means to stratify patients for vasodilator administration.87
Invasive assessment is often helpful to make the diagnosis of high output heart failure and may also allow for determination of etiologies, such as intracardiac or systemic shunts.88 Exercise RHC testing can also diagnose preload insufficiency, where peak VO2 is depressed due to low depressed ΔCO/ΔVO2 in tandem with low to normal filling pressures, as well as peripheral limitations, where it is depressed because of low AVO2-diff, with normal CO reserve.16,29
Lastly, invasive hemodynamics play a crucial role distinguishing constrictive pericarditis from primary myocardial disease. Both disorders are characterized by elevated right and left-sided filling pressures with steep Y-descents, a frequently positive Kussmaul sign, and a dip-and- plateau sign in the ventricles. However, simultaneous comparison of RV-LV and LV-PCWP pressure tracings can distinguish myocardial from pericardial disease.89 In constriction, LV and RV systolic pressure changes during respiration are discordant, or 180° out of phase with one another, due to enhanced ventricular interdependence. In restrictive myocardial disease, LV and RV pressures change in phase. In constriction, there is also intrathoracic-intracardiac dissociation, where the mean pressure gradient between PCWP and LV during diastole decreases by 5 mmHg or more.
CONCLUSIONS
After years of dormancy, the value of invasive hemodynamics has re-emerged front and center, playing a crucial role in the scope of the HF practice. Thorough understanding of the principles and practice of the hemodynamic assessment, along with a thoughtful application across the broad range of HF disease states are of vital importance to HF clinicians serving their patients. Future advances in the understanding and application of hemodynamics will only further cement their central role in the care of patients with HF.
Highlights.
This article reviews the role of invasive hemodynamics in the care of patients across the entire spectrum of human heart failure.
Conceptual principles of ventricular function, ventricular-arterial interaction, load response, and ventricular interaction in the right and left heart are reviewed.
Principles and practice of invasive exercise testing are provided, along with detailed discussions on the role of invasive hemodynamics in the evaluation and management of advanced heart failure, shock, mechanical circulatory support, and pulmonary hypertension.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early HFpEF. Circ Heart Fail. 2010;3(5):588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obokata M, Kane GC, Reddy YNV, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for HFpEF: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135(9):825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borlaug BA, Kass DA. Invasive Hemodynamic Assessment in Heart Failure. Heart Fail Clin. 2009;5(2):217–228. doi: 10.1016/j.hfc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in PH with preserved or reduced ejection fraction. J Am Coll Cardiol. 2012;59(5):442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 5.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol. 2015;66(23):2663–2674. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Hsu S, Kambhampati S, Sciortino CM, Russell SD, Schulman SP. Predictors of intra-aortic balloon pump hemodynamic failure in non-acute myocardial infarction cardiogenic shock. Am Heart J. 2018;199:181–191. doi: 10.1016/j.ahj.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease: insights into the pathogenesis of HFpEF. J Am Coll Cardiol. 2009;54(5):410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with HFpEF: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 9.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in PH: an update. Eur Respir J. 2019;53(1):1801900. doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in HFpEF. Eur Heart J. 2016;37(43):3293–3302. doi: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu S, Houston BA, Tampakakis E, et al. Right Ventricular Functional Reserve in Pulmonary Arterial Hypertension. Circulation. 2016;133(24):2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland CG, Damico RL, Kolb TM, et al. Exercise RV ejection fraction predicts RV contractile reserve. J Heart Lung Transplant. 2021;40(6):504–512. doi: 10.1016/j.healun.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlaug BA, Reddy YNV. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail. 2019;7(7):574–585. doi: 10.1016/j.jchf.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349(9067):1720–1724. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- 15.Astrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac Output during Submaximal and Maximal Work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- 16.Fudim M, Sobotka PA, Dunlap ME. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in HFpEF, Autonomic Dysfunction, and Liver Disease. Circ Heart Fail. 2021;14(1):e007308. doi: 10.1161/CIRCHEARTFAILURE.120.007308. [DOI] [PubMed] [Google Scholar]
- 17.Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation. 2020;142(10):998–1012. doi: 10.1161/CIRCULATIONAHA.119.045409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolsk E, Bakkestrøm R, Thomsen JH, et al. The Influence of Age on Hemodynamic Parameters During Rest and Exercise in Healthy Individuals. JACC Heart Fail. 2017;5(5):337–346. doi: 10.1016/j.jchf.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Reddy YNV, Andersen MJ, Obokata M, et al. Arterial Stiffening With Exercise in Patients With HFpEF. J Am Coll Cardiol. 2017;70(2):136–148. doi: 10.1016/j.jacc.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borlaug BA, Olson TP, Lam CSP, et al. Global cardiovascular reserve dysfunction in HFpEF. J Am Coll Cardiol. 2010;56(11):845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borlaug BA, Jaber WA, Ommen SR, Lam CSP, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in HFpEF. Heart. 2011;97(12):964–969. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy YNV, Obokata M, Wiley B, et al. The haemodynamic basis of lung congestion during exercise in HFpEF. Eur Heart J. 2019;40(45):3721–3730. doi: 10.1093/eurheartj/ehz713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with HFpEF. Circulation. 2006;114(20):2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 24.Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Circulation. 1989;80(2):314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 25.Houstis NE, Eisman AS, Pappagianopoulos PP, et al. Exercise Intolerance in HFpEF: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation. 2018;137(2):148–161. doi: 10.1161/CIRCULATIONAHA.117.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorimachi H, Burkhoff D, Verbrugge FH, et al. Obesity, Venous Capacitance, and Venous Compliance in HFpEF. Eur J Heart Fail. May 2021. doi: 10.1002/ejhf.2254. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs G, Avian A, Olschewski A, Olschewski H. Zero reference level for right heart catheterisation. Eur Respir J. 2013;42(6):1586–1594. doi: 10.1183/09031936.00050713. [DOI] [PubMed] [Google Scholar]
- 28.Saxena A, Garan AR, Kapur NK, et al. Value of Hemodynamic Monitoring in Patients With Cardiogenic Shock Undergoing Mechanical Circulatory Support. Circulation. 2020;141(14):1184–1197. doi: 10.1161/CIRCULATIONAHA.119.043080. [DOI] [PubMed] [Google Scholar]
- 29.Jain CC, Borlaug BA. Performance and Interpretation of Invasive Hemodynamic Exercise Testing. Chest. 2020;158(5):2119–2129. doi: 10.1016/j.chest.2020.05.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narang N, Thibodeau JT, Levine BD, et al. Inaccuracy of estimated resting oxygen uptake in the clinical setting. Circulation. 2014;129(2):203–210. doi: 10.1161/CIRCULATIONAHA.113.003334. [DOI] [PubMed] [Google Scholar]
- 31.Hoeper MM, Maier R, Tongers J, et al. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in PH. Am J Respir Crit Care Med. 1999;160(2):535–541. doi: 10.1164/ajrccm.160.2.9811062. [DOI] [PubMed] [Google Scholar]
- 32.Sommer RJ, Hijazi ZM, Rhodes JF. Pathophysiology of congenital heart disease in the adult: part I: Shunt lesions. Circulation. 2008;117(8):1090–1099. doi: 10.1161/CIRCULATIONAHA.107.714402. [DOI] [PubMed] [Google Scholar]
- 33.Eisman AS, Shah RV, Dhakal BP, et al. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail. 2018;11(5):e004750. doi: 10.1161/CIRCHEARTFAILURE.117.004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of Cardiac Failure. 2010;16(6):e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez GA, Lemor A, Blumer V, et al. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in PH With and Without Cardiogenic Shock. Journal of Cardiac Failure. 2019;25(5):364–371. doi: 10.1016/j.cardfail.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Garan AR, Kanwar M, Thayer KL, et al. Complete Hemodynamic Profiling With Pulmonary Artery Catheters in Cardiogenic Shock Is Associated With Lower In-Hospital Mortality. JACC Heart Fail. 2020;8(11):903–913. doi: 10.1016/j.jchf.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Rossello X, Bueno H, Gil V, et al. Synergistic Impact of Systolic Blood Pressure and Perfusion Status on Mortality in Acute Heart Failure. Circ Heart Fail. 2021;14(3):e007347. doi: 10.1161/CIRCHEARTFAILURE.120.007347. [DOI] [PubMed] [Google Scholar]
- 39.Jentzer JC, Burstein B, Van Diepen S, et al. Defining Shock and Preshock for Mortality Risk Stratification in Cardiac Intensive Care Unit Patients. Circ Heart Fail. 2021;14(1):e007678. doi: 10.1161/CIRCHEARTFAILURE.120.007678. [DOI] [PubMed] [Google Scholar]
- 40.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheterization and Cardiovascular Interventions. 2019;94(1):29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 41.Thayer KL, Zweck E, Ayouty M, et al. Invasive Hemodynamic Assessment and Classification of In-Hospital Mortality Risk Among Patients With Cardiogenic Shock. Circ Heart Fail. 2020;13(9):e007099. doi: 10.1161/CIRCHEARTFAILURE.120.007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim HS. Cardiac Power Output Revisited. Circ Heart Fail. 2020;13(10):e007393. doi: 10.1161/CIRCHEARTFAILURE.120.007393. [DOI] [PubMed] [Google Scholar]
- 43.Basir MB, Schreiber T, Dixon S, et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheterization and Cardiovascular Interventions. 2018;91(3):454–461. doi: 10.1002/ccd.27427. [DOI] [PubMed] [Google Scholar]
- 44.Taleb I, Koliopoulou AG, Tandar A, et al. Shock Team Approach in Refractory Cardiogenic Shock Requiring Short-Term Mechanical Circulatory Support: A Proof of Concept. Circulation. 2019;140(1):98–100. doi: 10.1161/CIRCULATIONAHA.119.040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized Team-Based Care for Cardiogenic Shock. J Am Coll Cardiol. 2019;73(13):1659–1669. doi: 10.1016/j.jacc.2018.12.084. [DOI] [PubMed] [Google Scholar]
- 46.Tongers J, Sieweke J-T, Kühn C, et al. Early Escalation of Mechanical Circulatory Support Stabilizes and Potentially Rescues Patients in Refractory Cardiogenic Shock. Circ Heart Fail. 2020;13(3):e005853. doi: 10.1161/CIRCHEARTFAILURE.118.005853. [DOI] [PubMed] [Google Scholar]
- 47.Griepp RB, Stinson EB, Dong E, Clark DA, Shumway NE. Determinants of operative risk in human heart transplantation. Am J Surg. 1971;122(2):192–197. doi: 10.1016/0002-9610(71)90316-3. [DOI] [PubMed] [Google Scholar]
- 48.Costard-Jäckle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation. J Am Coll Cardiol. 1992;19(1):48–54. doi: 10.1016/0735-1097(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 49.Guglin M, Mehra S, Mason TJ. Comparison of drugs for PH reversibility testing: A meta-analysis. Pulm Circ. 2013;3(2):406–413. doi: 10.4103/2045-8932.113180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chomsky DB, Lang CC, Rayos GH, et al. Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation. 1996;94(12):3176–3183. doi: 10.1161/01.cir.94.12.3176. [DOI] [PubMed] [Google Scholar]
- 51.Frankfurter C, Molinero M, Vishram-Nielsen JKK, et al. Predicting the Risk of Right Ventricular Failure in Patients Undergoing LVAD Implantation: A Systematic Review. Circ Heart Fail. 2020;13(10):e006994. doi: 10.1161/CIRCHEARTFAILURE.120.006994. [DOI] [PubMed] [Google Scholar]
- 52.Nadir AM, Beadle R, Lim HS. Kussmaul physiology in patients with heart failure. Circ Heart Fail. 2014;7(3):440–447. doi: 10.1161/CIRCHEARTFAILURE.113.000830. [DOI] [PubMed] [Google Scholar]
- 53.Morine KJ, Kiernan MS, Pham DT, Paruchuri V, Denofrio D, Kapur NK. Pulmonary Artery Pulsatility Index Is Associated With Right Ventricular Failure After LVAD Surgery. Journal of Cardiac Failure. 2016;22(2):110–116. doi: 10.1016/j.cardfail.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after LVAD implantation. J Heart Lung Transplant. 2016;35(1):67–73. doi: 10.1016/j.healun.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Uriel N, Morrison KA, Garan AR, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow LVADs: the Columbia ramp study. J Am Coll Cardiol. 2012;60(18):1764–1775. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uriel N, Sayer G, Addetia K, et al. Hemodynamic Ramp Tests in Patients With LVADs. JACC Heart Fail. 2016;4(3):208–217. doi: 10.1016/j.jchf.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Imamura T, Jeevanandam V, Kim G, et al. Optimal Hemodynamics During LVAD Support Are Associated With Reduced Readmission Rates. Circ Heart Fail. 2019;12(2):e005094. doi: 10.1161/CIRCHEARTFAILURE.118.005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dridi NP, Vishram-Nielsen JKK, Gustafsson F. Exercise Tolerance in Patients Treated With a Durable LVAD: Importance of Myocardial Recovery. Journal of Cardiac Failure. 2021;27(4):486–493. doi: 10.1016/j.cardfail.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Dunlay SM, Allison TG, Pereira NL. Changes in cardiopulmonary exercise testing parameters following continuous flow LVAD implantation and heart transplantation. Journal of Cardiac Failure. 2014;20(8):548–554. doi: 10.1016/j.cardfail.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borlaug BA. Evaluation and management of HFpEF. Nat Rev Cardiol. 2020;17(9):559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 61.Obokata M, Reddy YNV, Borlaug BA. Diastolic Dysfunction and HFpEF: Understanding Mechanisms by Using Noninvasive Methods. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):245–257. doi: 10.1016/j.jcmg.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose HFpEF: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 63.D’Alto M, Badesch D, Bossone E, et al. A Fluid Challenge Test for the Diagnosis of Occult Heart Failure. Chest. 2021;159(2):791–797. doi: 10.1016/j.chest.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 64.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without PH. Circ Heart Fail. 2015;8(1):41–48. doi: 10.1161/CIRCHEARTFAILURE.114.001731. [DOI] [PubMed] [Google Scholar]
- 65.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47(9):1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 66.Obokata M, Borlaug BA. The strengths and limitations of E/e’ in HFpEF. Eur J Heart Fail. 2018;20(9):1312–1314. doi: 10.1002/ejhf.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of HFpEF. Circulation. 2018;138(9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of PH. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 70.Maron BA, Hess E, Maddox TM, et al. Association of Borderline PH With Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation. 2016;133(13):1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1). doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sitbon O, Humbert M, Jaïs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 73.Reddy YNV, Borlaug BA. PH in Left Heart Disease. Clin Chest Med. 2021;42(1):39–58. doi: 10.1016/j.ccm.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in HFpEF and pulmonary vascular disease. Eur Heart J. 2018;39(30):2825–2835. doi: 10.1093/eurheartj/ehy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of PH due to chronic heart failure with reduced ejection fraction: PH and heart failure. JACC Heart Fail. 2013;1(4):290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association Between Hemodynamic Markers of PH and Outcomes in HFpEF. JAMA Cardiol. 2018;3(4):298–306. doi: 10.1001/jamacardio.2018.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weatherald J, Boucly A, Chemla D, et al. Prognostic Value of Follow-Up Hemodynamic Variables After Initial Management in Pulmonary Arterial Hypertension. Circulation. 2018;137(7):693–704. doi: 10.1161/CIRCULATIONAHA.117.029254. [DOI] [PubMed] [Google Scholar]
- 78.Alkhunaizi FA, Harowicz MR, Ireland CG, et al. Kussmaul’s Sign in PH Corresponds With Severe Pulmonary Vascular Pathology Rather Than Right Ventricular Diastolic Dysfunction. Circ Heart Fail. 2021;14(1):e007461. doi: 10.1161/CIRCHEARTFAILURE.120.007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tedford RJ, Hassoun PM, Mathai SC, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125(2):289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vachiery J-L, Tedford RJ, Rosenkranz S, et al. PH due to left heart disease. Eur Respir J. 2019;53(1):1801897. doi: 10.1183/13993003.01897-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Viray MC, Bonno EL, Gabrielle ND, et al. Role of Pulmonary Artery Wedge Pressure Saturation During Right Heart Catheterization: A Prospective Study. Circ Heart Fail. 2020;13(11):e007981. doi: 10.1161/CIRCHEARTFAILURE.120.007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naeije R, Vanderpool R, Dhakal BP, et al. Exercise-induced PH: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187(6):576–583. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hervé P, Lau EM, Sitbon O, et al. Criteria for diagnosis of exercise PH. Eur Respir J. 2015;46(3):728–737. doi: 10.1183/09031936.00021915. [DOI] [PubMed] [Google Scholar]
- 84.Ho JE, Zern EK, Lau ES, et al. Exercise PH Predicts Clinical Outcomes in Patients With Dyspnea on Effort. J Am Coll Cardiol. 2020;75(1):17–26. doi: 10.1016/j.jacc.2019.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reddy YNV, Obokata M, Koepp KE, Egbe AC, Wiley B, Borlaug BA. The β-Adrenergic Agonist Albuterol Improves Pulmonary Vascular Reserve in HFpEF. Circ Res. 2019;124(2):306–314. doi: 10.1161/CIRCRESAHA.118.313832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. In: Vol 129. 2014:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 87.Egbe AC, Miranda WR, Anderson JH, Borlaug BA. Hemodynamic and Clinical Implications of Impaired Pulmonary Vascular Reserve in the Fontan Circulation. J Am Coll Cardiol. 2020;76(23):2755–2763. doi: 10.1016/j.jacc.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reddy YNV, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-Output Heart Failure: A 15-Year Experience. J Am Coll Cardiol. 2016;68(5):473–482. doi: 10.1016/j.jacc.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 89.Givertz MM, Fang JC, Sorajja P, et al. Executive Summary of the SCAI/HFSA Clinical Expert Consensus Document on the Use of Invasive Hemodynamics for the Diagnosis and Management of Cardiovascular Disease. Journal of Cardiac Failure. 2017;23(6):487–491. doi: 10.1016/j.cardfail.2017.04.013. [DOI] [PubMed] [Google Scholar]