Abstract
Background:
Ambient to ultraviolet (UV) radiation has been increasing due to climate change. While this may result in adverse health consequences such as an increased incidence of skin cancer, UV radiation is also a source of vitamin D, which has been hypothesized to be protective for breast cancer risk.
Methods:
Using a spatiotemporal kriging model, we estimated residential UV exposure levels for the enrollment addresses (2003–2009) of breast cancer-free women aged 35–74 years participating in the Sister Study and living in the contiguous United States (N=48,450). Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the risk associated with UV exposure levels (mW/m2) categorized in quintiles. We examined the association for breast cancer overall (invasive and ductal carcinoma in situ) and by estrogen receptor (ER) status of the tumor. We considered effect modification by regular (≥ 4 times/week) vitamin D supplement use.
Results:
Over a median of 10.5 years of follow up, 3,510 incident breast cancer diagnoses were reported. We found no evidence of an association between living in areas with higher levels of UV radiation and overall breast cancer risk (HRQ5 vs. Q1=1.00, 95% CI: 0.90, 1.11). Higher UV levels were inversely associated with the risk of ER- breast cancer (HRQ5 vs. Q1=0.73, 95% CI: 0.55–0.99), but not ER+ (HR Q5 vs. Q1=1.04, 95% CI: 0.92–1.18). For ER- breast cancer, the inverse association was only evident in women who did not regularly take vitamin D supplements (HRQ5 vs. Q1= 0.52, 95% CI: 0.33–0.81) compared with those who did regularly take vitamin D supplements (HRQ5 vs. Q1=1.02, 95% CI: 0.68–1.54; p-for-heterogeneity=0.12).
Conclusions:
The findings from this study support a role for UV exposure and vitamin D in the etiology of ER- breast cancer.
Keywords: ultraviolet radiation, breast cancer, ER- breast cancer, vitamin D, cohort studies, sun exposure
Graphical Abstract
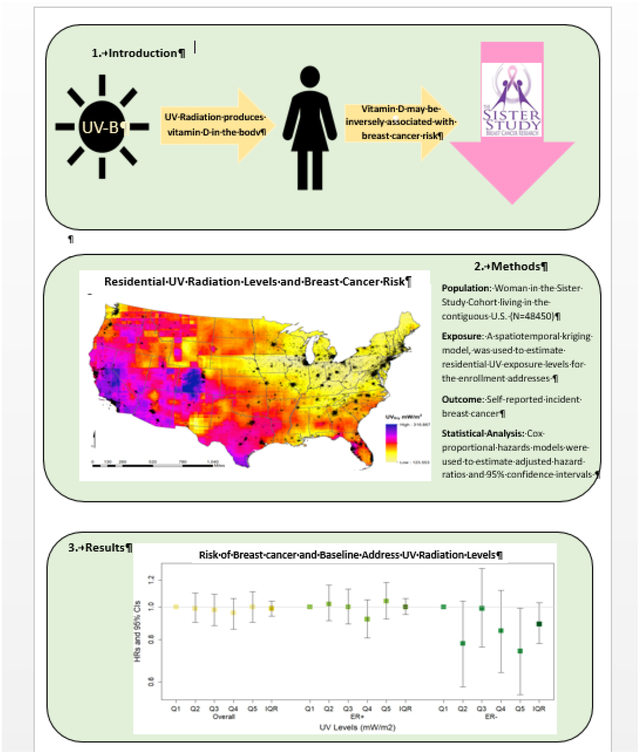
1. Introduction
Climate change has been associated with profound environmental impacts including a potential increase in exposure to ultraviolet (UV) radiation (Hiatt and Beyeler, 2020). Erosion of the ozone layer has reduced the protection that humans have historically had from UV wavelengths (Hiatt and Beyeler, 2020). Potential health effects associated with this change are not fully understood. Although it is established that increased exposure to UV radiation is associated with a higher risk of both melanoma and non-melanoma skin cancers (NMSC), an inverse relationship between UV exposure and breast cancer risk is scientifically plausible (D’Orazio et al., 2013; Hiatt and Beyeler, 2020). Breast cancer is the most commonly diagnosed cancer among women (excluding NMSC) in the United States (U.S.) and worldwide (Siegel et al., 2020). As such, it is critical to identify factors associated with breast cancer incidence.
Recent meta-analyses have reported inverse associations between breast cancer risk and time spent in the sun and separately with ambient UV (Hiller et al., 2020; Li and Ma, 2020). However, few studies have utilized more sophisticated UV exposure assessment. In the prospective Nurses’ Health Study II (NHSII), the authors employed a validated spatiotemporal UV model to assess residential UV exposure and observed that women who lived in areas of higher UV levels at age 30 had a slightly lower risk of developing estrogen receptor-negative breast cancer (VoPham et al., 2019).
The association between UV and breast cancer risk is hypothesized to be due to circulating vitamin D concentrations. Studies suggest that higher circulating concentrations of 25-hydroxyvitamin D (25(OH)D), a precursor to the active form of vitamin D, may be associated with decreased postmenopausal breast cancer risk (Bauer et al., 2013; O’Brien et al., 2017). This is biologically plausible as vitamin D has anticarcinogenic properties including anti-proliferation activity and induction of apoptosis (Feldman et al., 2014; Welsh, 2012; Welsh et al., 2002). Among the U.S. population, exposure to UV radiation is a major contributor to vitamin D levels (Holick, 2007) with an estimated 30% increase in vitamin D levels in the summer compared to winter months (Kroll et al., 2015). Dietary intake and use of supplements also importantly contribute to circulating vitamin D levels (Cashman, 2020; Crowe et al., 2011).
In this study, we aimed to evaluate the association between UV exposure and breast cancer risk in a large prospective U.S.-wide cohort with consideration of whether risk varies by vitamin D supplement use. Additionally, we considered whether associations varied by estrogen receptor status of the tumor or menopausal status.
2. Methods
2.1. Study Population.
The Sister Study cohort is a nationwide prospective cohort that aims to evaluate the role of the environment and lifestyle factors in breast cancer incidence. The Sister Study has been described previously in detail (Sandler et al., 2017). Briefly, women were recruited between 2003–2009 and were eligible if they were between the ages of 35–74 years, lived in the U.S., including Puerto Rico, and if they had at least one sister who had been diagnosed with breast cancer, but no history of breast cancer themselves. At the time of enrollment in the study, women completed detailed computer-assisted telephone questionnaires on demographics, lifestyle factors, environmental exposures, dietary habits, and existing health conditions. A detailed residential history covered enrollment address, longest-lived adult address, and longest-lived address prior to age 14 years.
Study participants complete annual health updates and detailed questionnaires every 2–3 years. Follow-up questionnaires inquire about updated menopausal status and specific health conditions including any breast cancer diagnoses. Response rates have been close to 90% throughout follow-up (National Institute of Environmental Health Sciences, 2020). The Sister Study was approved by the institutional review board of the National Institute of Health (NIH). Data for this analysis includes follow up through September 23, 2018 (Data Release 8.0).
2.2. Exposure assessment.
Residential UV exposure levels were estimated for individuals based on geocoded addresses for their enrollment residence and longest-lived adult residence. UV exposure levels were estimated using a validated exposure model, which has been described previously (VoPham et al., 2016). Briefly, this model, stratified by 9 U.S. regions, incorporates satellite data from the National Aeronautics and Space Administration (NASA) Total Ozone Mapping Spectrometer (TOMS) and Ozone Monitoring Instrument (OMI) and considered 17 predictors associated with UV (including cloud cover, ozone, dew point, elevation, and latitude among others) using an area-to-point residual kriging method at a spatial resolution of 1 km to predict average July noon-time UV estimates for a specific location. Estimates were limited to individuals who lived in the contiguous 48 states at the time of enrollment. For the enrollment address (2003–2009), we used UV estimates for 2006, as it is the midpoint of the enrollment period. For the longest-lived adult address, we used the estimate for the last calendar year lived at that residence unless that year preceded 1980, for which data are not available, in which case we used the measurement from 1980 (1% of participants).
2.3. Covariate assessment.
Study participants reported their race and ethnicity from a list of provided options and answered questions on a range of demographics and lifestyle factors including obtained education, parity, regular physical activity, and dietary supplement use. Each participant had an enrollment home visit with a trained examiner to measure height and weight, which was used to calculate body mass index (BMI) (kg/m2).
2.4. Outcome Assessment.
Incident breast cancer cases (invasive and ductal carcinoma in situ (DCIS)) were identified via self-report. If diagnosed with breast cancer, women were asked to provide pathology reports and access to medical records to confirm the diagnosis and obtain additional details on the tumor. Medical records were available for >80% of cases; we have previously reported a high level of agreement between self-reported case information and medical records and therefore all cases are included in our analyses. (D’Aloisio et al., 2017) Estrogen receptor (ER) status was available for ~80% of cases. In addition to ER status of the tumor, we also considered whether observed associations varied by tumor extent (invasive vs. DCIS) or by menopausal status (pre vs. post).
Statistical Analysis.
We conducted a descriptive analysis comparing demographic characteristics and covariates by quintile of UV exposure estimated for the enrollment address.
2.5 Covariates were identified a priori using a directed acyclic graph (DAG) (Shrier and Platt, 2008). A DAG is a diagram designed to map out the relationship between an exposure and outcome of interest and the corresponding factors that may affect one or both of these entities which can then be used to identify potential confounders of interest (Shrier and Platt, 2008). For UV exposures based on adult residences, we adjusted for race/ethnicity (non-Hispanic White, Black/African-American, other), education (≤ high school diploma, associate’s degree/technical degree/some college, bachelor’s degree or higher), parity (nulliparous, 1 birth, 2–3 births, ≥4 births), BMI (continuous), and total hours of leisure-time physical activity per week (continuous). We excluded women who had a pre-enrollment diagnosis (N=57) and cases who contributed zero person time(N=15), or non-cases who did not contribute any person time (N=322) and those with missing covariate information (N=491). Exclusion of women missing exposure information for enrollment (N=1,549) and longest-lived adult (N=5,106) addresses, resulted in 48,450 (95.2%) and 44,879 (88.2%) participants, respectively, included in the analyses.
We estimated hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the association between UV exposure levels and breast cancer risk using Cox proportional hazards models. We primarily considered UV exposure levels at the enrollment address, but also evaluated associations with UV exposure at the longest-lived adult address. UV exposure levels were examined using quintiles (Q1: 124–155 mW/m2, Q2: 155–168 mW/m2, Q3: 168–183 mW/m2, Q4: 183–208 mW/m2, Q5: 208–290 mW/m2 for enrollment address; Q1: 76.9–158 mW/m2, Q2: 158–170 mW/m2, Q3: 170–185 mW/m2, Q4: 185–213 mW/m2, Q5: 213–305 mW/m2 for longest-lived adult address) and by evaluating an interquartile range (IQR) increase in exposure (42.9 mW/m2 for enrollment address; 43.8 mW/m2 for longest-lived adult address). Age was the timescale of the Cox models; women were followed from their age at enrollment to their age at breast cancer diagnosis, end of follow-up or loss to follow-up. For analyses considering associations by ER status of the tumor, if ER+ breast cancer was the outcome of interest, women were censored if they were diagnosed with ER- breast cancer and vice versa. For analyses considering associations by menopausal status, women were additionally censored in premenopausal breast cancer models when they became postmenopausal. For postmenopausal breast cancer, women entered the Cox model at either their age at enrollment or their age at menopause, whichever was later. We tested statistical heterogeneity by ER status and tumor extent with joint Cox models, an approach used to compare exposure-disease associations across multiple disease subtypes (Xue et al., 2013). Heterogeneity by menopausal status was measured via a Wald test of time-varying menopause by UV status interaction terms.
Effect measure modification of the association between UV and breast cancer risk was evaluated for potential modifiers by including models with and without interaction terms and evaluating likelihood ratio tests. For modifiers, we considered (1) baseline regular supplement use (yes/no taking a vitamin D-containing supplement ≥ 4 times/week) to determine if the association varied based on baseline vitamin D level; (2) race/ethnicity (non-Hispanic White, Black/African-American) to evaluate if there were differences in the association based on UV absorption; and, (3) census region of residence (Northeast, South, Midwest, West) to assess the presence of any regional differences.
We conducted a sensitivity analysis to evaluate the impact of recent exposure by limiting the analysis to cases diagnosed within 5 years of baseline, consistent with prior findings for vitamin D in the Sister Study (O’Brien et al., 2017). We also conducted a sensitivity analysis with further adjustment for residential criteria air pollution (PM2.5, PM10, and NO2), annual household income (<$50,000, $50,000–100,000, ≥$100,000), census region of residence (Northeast, South, Midwest, West), and residence type (Urban, Rural/Small Town, Suburban).
3. Results
Our study population was predominantly composed of non-Hispanic White women (84%), with at least a bachelor’s degree (51%) and who had at least two children (67%) (Table 1). The average age at enrollment was 55 years and participants had a mean BMI of 28 kg/m2. UV exposure varied across U.S. (Figure 1), with 0% of women who lived in the Western US being in the lowest quintile of exposure and 0% of women who lived in the Northeastern or Midwestern U.S. being in the highest quintile of exposure.
Table 1.
Baseline population characteristics of the U.S.-wide prospective Sister Study cohort by quintiles of UV exposure (2003–2009).
| UV exposure level at baseline residence | ||||||
|---|---|---|---|---|---|---|
| Overall | Quintile1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Age at baseline (mean, sd) | 55.6 (9.0) | 55.1 (9.0) | 55.4 (8.9) | 55.3 (8.9) | 56 (8.9) | 56.3 (9.1) |
| BMI (mean, SD) | 27.8 (6.2) | 28 (6.3) | 27.7 (6.2) | 28.2 (6.3) | 27.9 (6.4) | 27.3 (6.0) |
| Physical activity (hours per week) (mean, SD) | 13.9 (8.0) | 13.7 (7.9) | 13.8 (7.9) | 13.7 (7.9) | 13.9 (8.1) | 14.1 (8.1) |
| Race | ||||||
| Non-Hispanic White | 41939 (83.9%) | 8378 (86.5%) | 8654 (89.3%) | 8118 (83.8%) | 7944 (82.0%) | 8215 (84.8%) |
| Black | 4470 (8.9%) | 935 (9.6%) | 704 (7.3%) | 1267 (13.1%) | 1054 (10.9%) | 416 (4.3%) |
| other | 3590 (7.2%) | 378 (3.9%) | 331 (3.4%) | 305 (3.1%) | 692 (7.1%) | 1059 (10.9%) |
| Census Region | ||||||
| Northeast | 8384 (16.8%) | 3024 (31.2%) | 3926 (40.5%) | 1304 (13.5%) | 23 (0.2%) | 0 (0.0%) |
| Midwest | 13494 (27.0%) | 6016 (62.1%) | 2645 (27.3%) | 3281 (33.9%) | 1473 (15.2%) | 3 (0.0%) |
| South | 16429 (32.9%) | 651 (6.7%) | 2689 (27.8%) | 4352 (44.9%) | 6420 (66.3%) | 2116 (21.8%) |
| West | 10831 (21.7%) | 0 (0.0%) | 429 (4.4%) | 753 (7.8%) | 1774 (18.3%) | 7571 (78.1%) |
| Education Level | ||||||
| ≤ High School | 7628 (15.3%) | 1625 (16.8%) | 1442 (14.9%) | 1556 (16.1%) | 1459 (15.1%) | 1252 (12.9%) |
| Associate’s Degree/Technical Degree/Some College | 16869 (33.7%) | 3041 (31.4%) | 3076 (31.7%) | 3390 (35.0%) | 3448 (35.6%) | 3462 (35.7%) |
| Bachelor’s Degree or Higher | 25502 (51.0%) | 5025 (51.9%) | 5171 (53.4%) | 4744 (49.0%) | 4783 (49.4%) | 4976 (51.4%) |
| Parity | ||||||
| Nulliparous | 9061 (18.1%) | 1677 (17.3%) | 1839 (19.0%) | 1680 (17.3%) | 1630 (16.8%) | 1969 (20.3%) |
| 1 | 7234 (14.5%) | 1339 (13.8%) | 1337 (13.8%) | 1428 (14.7%) | 1463 (15.1%) | 1458 (15.1%) |
| 2–3 | 28447 (56.9%) | 5631 (58.1%) | 5513 (56.9%) | 5552 (57.3%) | 5627 (58.1%) | 5225 (53.9%) |
| ≥4 | 5257 (10.5%) | 1044 (10.8%) | 1000 (10.3%) | 1030 (10.6%) | 970 (10.0%) | 1038 (10.7%) |
| Menopausal Status at Baseline | ||||||
| Premenopausal | 16763 (33.5%) | 3517 (36.3%) | 3388 (35.0%) | 3328 (34.3%) | 2981 (30.8%) | 3071 (31.7%) |
| Postmenopausal | 33219 (66.4%) | 6172 (63.7%) | 6300 (65.0%) | 6358 (65.6%) | 6702 (69.2%) | 6617 (68.3%) |
Figure 1.
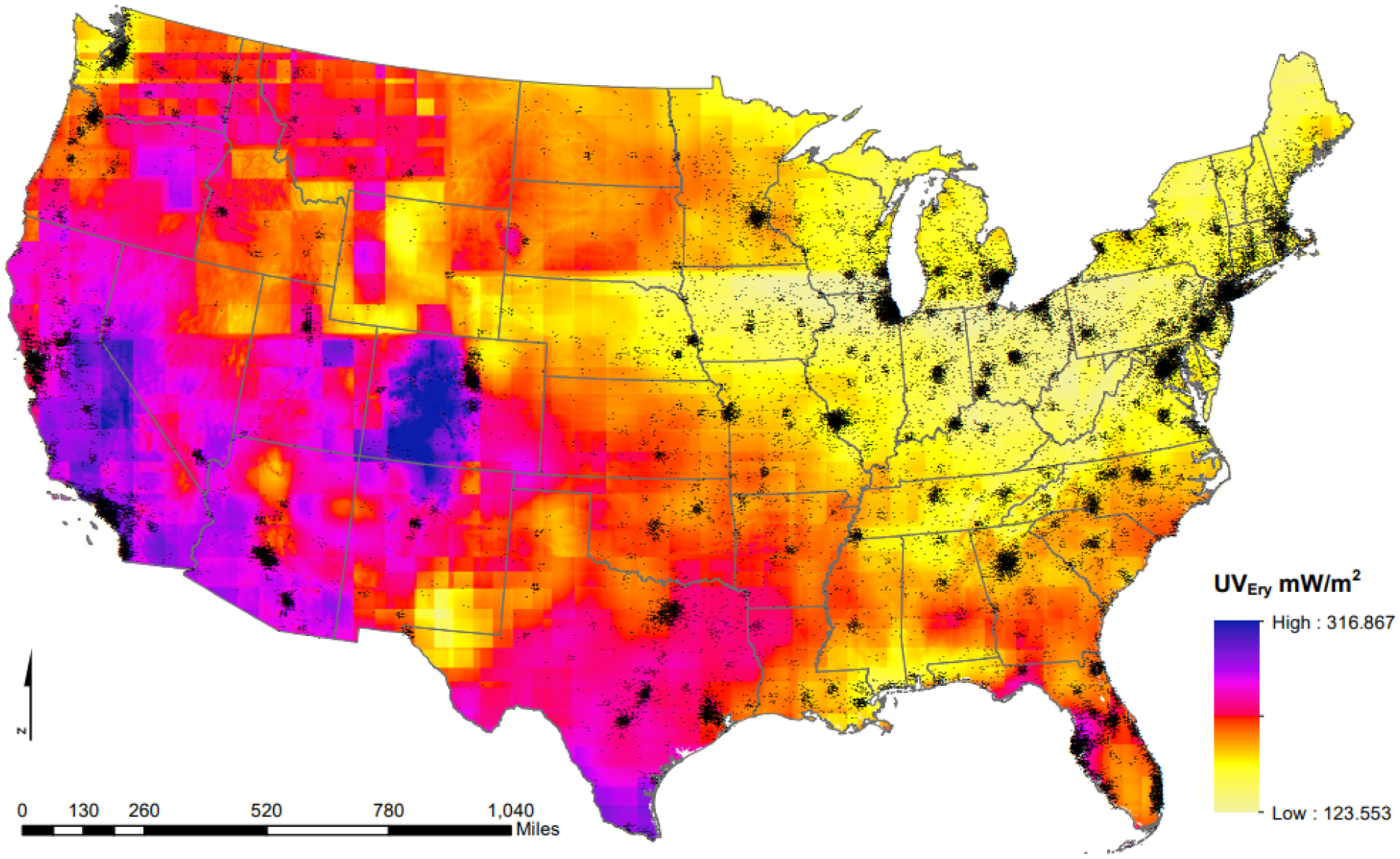
Estimated erythemal ultraviolet radiation levels from spatiotemporal kriging models using data from the NASA Total Ozone Mapping Spectrometer and the Ozone Monitoring Instrument, across the contiguous United States (average July noon-time estimates, 2006) and Sister Study participant residential locations, jiggered for participant privacy (represented by individual black dots).
We did not observe an association between UV exposure and overall breast cancer risk when we considered exposure characterized in quintiles (HRQ5 vs Q1=1.00, 95% CI: 0.90, 1.11) or an IQR increase (HRIQR = 0.99, 95% CI: 0.95, 1.04) (Table 2). Similarly, we saw no association between UV exposure and ER+ breast cancer (HRQ5 vs Q1=1.04, 95% CI: 0.92, 1.18; HRIQR=1.00, 95% CI: 0.94, 1.06). However, higher UV exposure was inversely associated with risk of ER- breast cancer (HRQ5 vs Q1=0.73, 95% CI: 0.55, 0.99; HRIQR = 0.89, 95% CI: 0.78, 1.02). There were similar, but slightly attenuated, patterns of association observed between UV exposure estimated for the longest-lived adult address and breast cancer risk with a similar inverse association observed for ER- breast cancer (HR Q5 vs Q1=0.76, 95% CI: 0.56, 1.03) (Table S1). The longest-lived adult address and the enrollment address were the same for 54.4% of individuals.
Table 2.
The association between UV exposure at the baseline address and breast cancer risk overall and by ER status, Sister Study (2003–2018).
| Overall | ER Positive | ER Negative4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | N Cases | Age-Adjusted | Fully Adjusted1 | N Cases | Age-Adjusted | Fully Adjusted1 | N Cases | Age-Adjusted | Fully Adjusted1 | |
| Quintile 1 2 | 9691 | 713 | 1.00 (ref) | 1.00 (ref) | 512 | 1.00 (ref) | 1.00 (ref) | 104 | 1.00 (ref) | 1.00 (ref) |
| Quintile 2 | 9689 | 709 | 0.99 (0.89,1.10) | 0.99 (0.89,1.10) | 531 | 1.03 (0.91,1.16) | 1.02 (0.91,1.16) | 81 | 0.77 (0.58,1.04) | 0.78 (0.58,1.04) |
| Quintile 3 | 9690 | 700 | 0.98 (0.88,1.09) | 0.98 (0.88,1.09) | 510 | 0.99 (0.88,1.12) | 1.00 (0.89,1.13) | 104 | 1.00 (0.76,1.31) | 0.99 (0.76,1.30) |
| Quintile 4 | 9690 | 685 | 0.95 (0.86,1.06) | 0.96 (0.86,1.06) | 470 | 0.91 (0.80,1.03) | 0.92 (0.81,1.04) | 88 | 0.85 (0.64,1.12) | 0.84 (0.63,1.12) |
| Quintile 5 | 9690 | 727 | 0.99 (0.89,1.10) | 1.00 (0.90,1.11) | 545 | 1.04 (0.92,1.17) | 1.04 (0.92,1.18) | 77 | 0.73 (0.55,0.98) | 0.73 (0.55,0.99) |
| IQR 3 | 48450 | 3534 | 0.99 (0.94,1.04) | 0.99 (0.95,1.04) | 256 8 | 0.99 (0.94,1.05) | 1.00 (0.94,1.06) | 454 | 0.89 (0.78,1.02) | 0.89 (0.78,1.02) |
Adjusted for race (non-Hispanic White, Black, other) education(≤ high school, associate’s degree/technical degree/some college, ≥ bachelor’s degree), parity (nulliparous, 1 birth, 2–3 births, ≥ 4 births), BMI, and total hours of physical activity a week
Q1: 124–155 mW/m2, Q2: 155–168 mW/m2, Q3: 168–183 mW/m2, Q4: 183–208 mW/m2, Q5: 208–290 mW/m2
Interquartile range (IQR) is 42.9 mW/m2 for baseline address
p for heterogeneity tests the null hypothesis that there is no difference in association by ER receptor status, IQR: p = 0.14, Quintiles: p =0.12
The association between UV exposure and breast cancer risk did not vary significantly by whether the tumor was invasive compared to DCIS (IQR p for heterogeneity=0.10), although estimates did appear stronger for DCIS compared to invasive cancer (Table S2). Additionally, there was no evidence of an association between UV exposure and overall or ER+ breast cancer risk when stratifying by menopausal status (Table S3 & S4). However, reduced risk of ER- breast cancer was present only among postmenopausal (HRQ5 vs Q1 = 0.70, 95% CI: 0.51, 0.97) and not among premenopausal women in our study population (HRQ5 vs Q1 = 0.98, 95% CI: 0.44, 2.17), although case numbers were limited (Table S4).
The inverse association we observed between enrollment residential UV exposure and ER- breast cancer was only evident among women who reported not regularly taking supplements (HR Q5 vs Q1=0.52, 95% CI: 0.33, 0.81; HRIQR=0.77, 95% CI: 0.62, 0.94), compared to those women who did report regular supplement use (HR Q5 vs Q1=1.02, 95% CI: 0.68, 1.54; HRIQR=1.03, 95% CI: 0.85, 1.24) (IQR p for heterogeneity =0.04) (Table 3). Risk of ER+ breast cancer did not vary by supplement use status and remained null (Table S5).
Table 3.
The association between UV exposure at baseline address and ER negative breast cancer stratified by regular supplement use, Sister Study (2003–2018).
| No Regular Supplement Use | Regular Supplement Use4 | |||||
|---|---|---|---|---|---|---|
| N | N Cases | Fully-Adjusted1 | N | N Cases | Fully-Adjusted1 | |
| Quintile 1 2 | 4322 | 56 | 1.00 (ref) | 5061 | 45 | 1.00 (ref) |
| Quintile 2 | 4217 | 30 | 0.55 (0.35,0.85) | 5209 | 47 | 1.02 (0.68,1.53) |
| Quintile 3 | 4365 | 51 | 0.90 (0.61,1.31) | 5014 | 51 | 1.14 (0.76,1.69) |
| Quintile 4 | 4367 | 45 | 0.79 (0.53,1.17) | 5005 | 42 | 0.94 (0.62,1.43) |
| Quintile 5 | 4243 | 29 | 0.52 (0.33,0.81) | 5145 | 47 | 1.02 (0.68,1.54) |
| IQR 3 | 21514 | 211 | 0.77 (0.62,0.94) | 25434 | 232 | 1.03 (0.85,1.24) |
Adjusted for race (non-Hispanic White, Black, other) education(≤ high school, associate’s degree/technical degree/some college, ≥ bachelor’s degree), parity (nulliparous, 1 birth, 2–3 births, ≥ 4 births), BMI, and total hours of physical activity a week
Q1: 124–155 mW/m2, Q2: 155–168 mW/m2, Q3: 168–183 mW/m2, Q4: 183–208 mW/m2, Q5: 208–290 mW/m2
Interquartile range (IQR) is 42.9 mW/m2 for baseline address
p for heterogeneity tests the null hypothesis that there is no difference in association by supplement use, IQR: p = 0.04, Quintiles: p =0.12
There was no evidence of effect measure modification by census region (Table S6). The association between UV exposure, when categorized in quintiles or an IQR increase, and overall breast cancer risk did not vary by race/ethnicity (IQR p for heterogeneity=0.59) (Table S7). Although there was limited statistical power when further limiting to ER- cases, the observed inverse association was driven by the non-Hispanic White participants (non-Hispanic White women: HRIQR = 0.87, 95% CI: 0.75, 1.02, Black women/African-American women HRIQR= 1.15, 95% CI: 0.72, 1.84) (Table S7). When limiting our analysis to cases diagnosed within the first five years after baseline, our estimates remained largely unchanged (e.g., ER- HR Q5 vs Q1= 0.71, 95% CI: 0.48, 1.05) (Table S8). After further adjustment for residential criteria air pollution (PM2.5, PM10, and NO2), annual household income (<$50,000, $50,000–100,000, ≥$100,000), census region of residence (Northeast, South, Midwest, West), and residence type (Urban, Rural/Small Town, Suburban), our estimates remained similar (data not shown).
4. Discussion
In this large prospective U.S. cohort of women, we observed that living in areas of higher residential UV was associated with an 11% lower risk of developing ER- breast cancer per interquartile range increase in UV. This inverse association was limited to women who reported not regularly taking vitamin D-containing supplements. However, UV exposure was not associated with risk of overall breast cancer or ER+ breast cancer. These findings provide support for a role of vitamin D in the etiology of ER- breast cancer. The reduction in risk for ER- breast cancer and the established higher risks associated with melanoma and non-melanoma skin cancers suggests that the public health implications of increasing UV exposure in the general population are complex.
Our findings that higher UV exposure at the enrollment and the longest-lived adult residence was inversely related to ER- breast cancer are similar to those reported in a prior study in the Nurses’ Health Study II, which used the same UV exposure model to assign residential UV exposure levels (VoPham et al., 2019). VoPham et al., observed a suggestive inverse association between modeled residential UV levels at age 30 and ER- breast cancers (~10% lower risk for an interquartile range increase in UV), although they did not observe associations with residential UV exposure assessed at other ages (VoPham et al., 2019). In line with our results in the Sister Study, they also saw no evidence of an association between residential UV exposure and overall breast cancer risk (VoPham et al., 2019).
Other epidemiologic studies that have considered the relationship between UV and breast cancer have largely employed questionnaire data, crude regional UV data, or latitude of residence to assess UV exposure. Ecological studies have tended to support an inverse relationship between UV radiation, assessed using regional UV satellite estimates, UV ground estimates and ambient sunlight estimates, and breast cancer (Gorham et al., 1990; Grant, 2002; Mohr et al., 2008). Our exposure estimates differ from those shown in Grant, 2002 likely due to differences in the exposure year considered and due to the fact that we employed a geostatistical exposure modeling approach with adjustment from the ozone monitoring instrument and incorporated predictors of UV (cloud cover, dew point, and latitude among others) (Grant, 2002; VoPham et al., 2016). Most, but not all, case-control studies have similarly observed inverse relationships between breast cancer risk and proxy variables for sun exposure (Anderson et al., 2011; Bidgoli and Azarshab, 2014; Cauchi et al., 2016; Engel et al., 2014; Fuhrman et al., 2013; John et al., 2007; Knight et al., 2007). However, results from cohort studies examining proxies for UV exposure, such as time spent outdoors or sun exposure, have been inconsistent, with some finding no associations and others inverse associations with overall breast cancer risk (Edvardsen et al., 2011; Engel et al., 2011; John et al., 1999.; Kuper et al., 2009; Lin et al., 2012; Yang et al., 2011; Zamoiski et al., 2016). The few studies that have been able to consider risk stratified by ER status, observed a reduced risk for ER+ cancers, in contrast to the null findings for ER+ cancers reported here and in the NHSII (Blackmore et al., 2008; Engel et al., 2014; Qin et al., 2020; VoPham et al., 2019). However, Qin et al. also observed a reduced risk of ER- cancers among Black/African-American women who reported a higher weekly average of daylight hours spent outside (Qin et al., 2020). The other two studies, using either daylight hours spent outside and sun exposure behavioral practices and time spend outside reported null results for an association with ER- cases, but it is important to note that their power may have been limited due to small sample size (N=105–223 ER- cases) (Blackmore et al., 2008; Engel et al., 2014).
The proposed biologic mechanism underlying the relationship between UV levels and breast cancer risk is related to UV-induced levels of circulating vitamin D. Specifically, UV-B wavelengths (280–315 nm) convert cutaneous 7-dehydrocholesterol to vitamin D3 (Holick, 2007). Vitamin D3 is hydroxylated in the liver to form 25(OH)D3, and then converted to the active form of 1,25(OH)D3 by an enzyme in the cytochrome P450 family. This can occur either directly in the tissues, such as mammary tissue, for local effects, or in the kidneys for systemic effects (Feldman et al., 2014). Consistent with a hypothesized biologic mechanism of increased vitamin D, we found that the inverse association between residential UV exposure and ER- breast cancer was only apparent among women not taking vitamin D supplements. Vitamin D supplementation is an important contributor to circulating vitamin D levels (Holick, 2007). A study conducted in a French population observed that those residing in the lowest latitude, corresponding to higher residential UV levels, had a reduced risk of breast cancer if they were also taking a vitamin D supplement or were in the highest tertile of dietary vitamin D (Engel et al., 2011).
Two noteworthy randomized control trials from the Women’s Health Initiative (WHI) and the VITAL study reported null results for the association between vitamin D supplementation and breast cancer risk (Cauley et al., 2013; Manson et al., 2019), but a reanalysis of the WHI results that excluded women who reported any outside supplementation of calcium or vitamin D, observed a decrease in risk of breast cancer (HR = 0.82, 95% CI: 0.60, 0.97) among the women randomized to the calcium and vitamin D treatment group compared to the women randomized to the placebo group (Bolland et al., 2015).
Recent meta-analyses of serum 25(OH)D levels and breast cancer risk supported an inverse relationship between vitamin D levels and breast cancer risk (Estébanez et al., 2018; Song et al., 2019). However, prospective studies have found conflicting results between vitamin D levels and breast cancer risk. A previous study conducted in the Sister Study reported an inverse association between serum 25(OH)D concentrations at baseline and risk of breast cancer within the first 5 years of follow-up (HR = 0.79, 95% CI: 0.63,0.98), while two meta-analyses restricted to prospective studies concluded overall null results (Gandini et al., 2011; Kim and Je, 2014; O’Brien et al., 2017). Prospective studies have additionally failed to observe a consistent association between breast cancer risk and vitamin D status that differs by ER status (Eliassen et al., 2011; Kühn et al., 2013; McCullough et al., 2009; Rejnmark et al., 2009), in contrast to our observed association between UV and ER- breast cancer. Laboratory studies have indicated differing pathways through which vitamin D may alter tumor growth which could explain in part our observed inverse association with ER- breast cancer as opposed to ER+ breast cancer (Davoodi et al., 1995; Yao and Ambrosone, 2013). However, it is possible that our observed inverse association between residential UV and ER- breast cancer may be operating through another biologic mechanism and not vitamin D as hypothesized.
The current study builds on a large, prospective and well-characterized U.S.-wide cohort of women. The prospective nature of the cohort allowed us to consider UV exposure years prior to a breast cancer diagnosis. As our study population resided throughout a large geographic area, there was substantial variability in UV to detect associations. The extensive covariate information available in the Sister Study allowed us to evaluate potential confounding factors and modifiers, including supplement intake.
We used a validated spatiotemporal kriging model used for estimating UV levels at residential addresses, which is a substantial improvement over using proxy questionnaire-based measures that have been used in most of the prior studies. However, this model estimates residential UV exposure, which does not capture the personal exposure each participant receives. Despite the limitations of geographic-based exposure assessment, relying on residential UV exposure as a proxy for vitamin D levels is a useful approach as UV is a major source of vitamin D exposure in the population and allows us to estimate exposure in our entire population, without having to limit our sample size, due to cost, in order to perform biomarker assays (Holick, 2007). We are also able to consider residential UV exposure in the past by considering exposure at the longest-lived adult residence, which may be more relevant etiologically given the long latency period for breast cancer development (Terry et al., 2019). Considering past exposure levels would not be possible using a biomarker of vitamin D. We were also able to evaluate UV levels jointly with vitamin D-related supplement use, another important source of vitamin D exposure, to more accurately define women who were most likely to benefit from exposure to UV radiation (Cashman, 2020; Kantor et al., 2016).
Our study was adequately powered to consider associations stratified by ER status of the tumor. This is important as there are differences in breast cancer etiology by subtype (Gaudet et al., 2018). ER- breast cancer has a worse prognosis than ER+ breast cancer, yet risk factors for ER- cancers are less well understood than for the more common ER+ breast cancers (American Cancer Society., 2019). For these reasons, identifying a risk factor for ER- breast cancer could have a substantial public health impact. Black/African-American women are more likely to be diagnosed with ER receptor-negative breast cancer (Carey et al., 2006). Additionally, it is well-documented that the amount of UV-B exposure required to produce vitamin D increases with increasing melanin present in the skin which affects the absorption of UV-B wavelengths, indicating that Black/African-American women require greater sun exposure time compared to White women to produce the same amount of vitamin D in the same environment (D’Orazio et al., 2013; Webb and Engelsen, 2006). This is consistent with research showing that Black/African-American women generally have lower circulating vitamin D levels (Forrest and Stuhldreher, 2011; Ginde et al., 2009). Although we had limited statistical power when we jointly stratified by both ER status and race/ethnicity, these results do suggest that the inverse association between UV and breast cancer is only evident for non-Hispanic White women. This finding is consistent with established differences in UV-absorption by skin melanin (D’Orazio et al., 2013; Webb and Engelsen, 2006). Future studies in more racially and ethnically diverse cohorts or using pooling efforts are necessary to further investigate the association between UV levels and breast cancer risk among non-white populations.
In this large prospective U.S. cohort, we observed that women who lived in areas of higher exposure to UV during adulthood had a lower risk of ER- breast cancer and that this association was most evident among women who did not report taking a regular vitamin D-containing supplements. Given that UV exposure increases the risk of melanoma and non-melanoma skin cancers, future efforts directed towards elucidating underlying biologic mechanisms and the possible impact of vitamin D supplementation on ER- breast cancer risk are warranted. In addition, more research is necessary characterize this association in non-white populations.
Supplementary Material
Highlights:
In a prospective cohort, UV exposure was inversely related to ER- breast cancer
Inverse association strongest for those who did not use vitamin D supplementation
A validated spatiotemporal kriging model was used to estimate UV
No observed association between UV exposure and overall or ER+ breast cancer
Funding.
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005, Z1AES103332-01)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Institutional review board approval: The Sister Study was approved by the Institutional Review Boards of the National Institute Health. Written informed consent was obtained from all participants.
References
- American Cancer Society., n.d. Breast Cancer Facts & Figures 2019–2020. 2019. 44. [Google Scholar]
- Anderson LN, Cotterchio M, Kirsh VA, Knight JA, 2011. Ultraviolet Sunlight Exposure During Adolescence and Adulthood and Breast Cancer Risk: A Population-based Case-Control Study Among Ontario Women. Am. J. Epidemiol 174, 293–304. 10.1093/aje/kwr091 [DOI] [PubMed] [Google Scholar]
- Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL, 2013. Plasma Vitamin D Levels, Menopause, and Risk of Breast Cancer: Dose-Response Meta-Analysis of Prospective Studies. Medicine (Baltimore) 92, 123–131. 10.1097/MD.0b013e3182943bc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidgoli SA, Azarshab H, 2014. Role of Vitamin D Deficiency and Lack of Sun Exposure in the Incidence of Premenopausal Breast Cancer: a Case Control Study in Sabzevar, Iran. Asian Pac. J. Cancer Prev 15, 3391–3396. 10.7314/APJCP.2014.15.8.3391 [DOI] [PubMed] [Google Scholar]
- Blackmore KM, Lesosky M, Barnett H, Raboud JM, Vieth R, Knight JA, 2008. Vitamin D From Dietary Intake and Sunlight Exposure and the Risk of Hormone-Receptor-Defined Breast Cancer. Am. J. Epidemiol 168, 915–924. 10.1093/aje/kwn198 [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Grey A, Gamble GD, Reid IR, 2015. Concordance of Results from Randomized and Observational Analyses within the Same Study: A Re-Analysis of the Women’s Health Initiative Limited-Access Dataset. PLOS ONE 10, e0139975. 10.1371/journal.pone.0139975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MCU, Nielsen TO, Moorman PG, Earp HS, Millikan RC, n.d. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study 11. [DOI] [PubMed] [Google Scholar]
- Cashman KD, 2020. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif. Tissue Int 106, 14–29. 10.1007/s00223-019-00559-4 [DOI] [PubMed] [Google Scholar]
- Cauchi JP, Camilleri L, Scerri C, 2016. Environmental and lifestyle risk factors of breast cancer in Malta—a retrospective case-control study. EPMA J 7, 20. 10.1186/s13167-016-0069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Chlebowski RT, Wactawski-Wende J, Robbins JA, Rodabough RJ, Chen Z, Johnson KC, O’Sullivan MJ, Jackson RD, Manson JE, 2013. Calcium Plus Vitamin D Supplementation and Health Outcomes Five Years After Active Intervention Ended: The Women’s Health Initiative. J. Womens Health 22, 915–929. 10.1089/jwh.2013.4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ, 2011. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC–Oxford study. Public Health Nutr 14, 340–346. 10.1017/S1368980010002454 [DOI] [PubMed] [Google Scholar]
- D’Aloisio AA, Nichols HB, Hodgson ME, Deming-Halverson SL, Sandler DP, 2017. Validity of self-reported breast cancer characteristics in a nationwide cohort of women with a family history of breast cancer. BMC Cancer 17, 692. 10.1186/s12885-017-3686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi F, Brenner RV, Evans SRT, Schumaker LM, Shabahang M, Nauta RJ, Buras RR, 1995. Modulation of vitamin D receptor and estrogen receptor by 1,25(OH)2-vitamin D3 in T-47D human breast cancer cells. J. Steroid Biochem. Mol. Biol 54, 147–153. 10.1016/0960-0760(95)00128-M [DOI] [PubMed] [Google Scholar]
- D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T, 2013. UV Radiation and the Skin. Int. J. Mol. Sci 14, 12222–12248. 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsen K, Veierød MB, Brustad M, Braaten T, Engelsen O, Lund E, 2011. Vitamin D-effective solar UV radiation, dietary vitamin D and breast cancer risk. Int. J. Cancer 128, 1425–1433. 10.1002/ijc.25463 [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE, 2011. Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses’ Health Study II. Breast Cancer Res 13, R50. 10.1186/bcr2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LS, Satagopan J, Sima CS, Orlow I, Mujumdar U, Coble J, Roy P, Yoo S, Sandler DP, Alavanja MC, 2014. Sun Exposure, Vitamin D Receptor Genetic Variants, and Risk of Breast Cancer in the Agricultural Health Study. Environ. Health Perspect 122, 165–171. 10.1289/ehp.1206274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault M-C, Clavel-Chapelon F, 2011. Joint Effects of Dietary Vitamin D and Sun Exposure on Breast Cancer Risk: Results from the French E3N Cohort. Cancer Epidemiol. Biomarkers Prev 20, 187–198. 10.1158/1055-9965.EPI-10-1039 [DOI] [PubMed] [Google Scholar]
- Estébanez N, Gómez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T, 2018. Vitamin D exposure and Risk of Breast Cancer: a meta-analysis. Sci. Rep 8, 9039. 10.1038/s41598-018-27297-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ, 2014. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 14, 342–357. 10.1038/nrc3691 [DOI] [PubMed] [Google Scholar]
- Forrest KYZ, Stuhldreher WL, 2011. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res 31, 48–54. 10.1016/j.nutres.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Fuhrman BJ, Freedman DM, Bhatti P, Doody MM, Chang S-C, Linet MS, Sigurdson AJ, 2013. Sunlight, Polymorphisms of Vitamin D-related Genes and Risk of Breast Cancer 17. [PMC free article] [PubMed] [Google Scholar]
- Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P, 2011. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer 128, 1414–1424. 10.1002/ijc.25439 [DOI] [PubMed] [Google Scholar]
- Gaudet MM, Gierach GL, Carter BD, Luo J, Milne RL, Weiderpass E, Giles GG, Tamimi RM, Eliassen AH, Rosner B, Wolk A, Adami H-O, Margolis KL, Gapstur SM, Garcia-Closas M, Brinton LA, 2018. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res. canres;0008–5472.CAN-18–0502v2. 10.1158/0008-5472.CAN-18-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginde AA, Liu MC, Camargo CA, 2009. Demographic Differences and Trends of Vitamin D Insufficiency in the US Population, 1988–2004. Arch. Intern. Med 169, 626. 10.1001/archinternmed.2008.604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham ED, Garland FC, Garlandm CF, n.d. Sunlight and Breast Cancer Incidence in the USSR. Int. J. Epidemiol 5. [DOI] [PubMed] [Google Scholar]
- Grant WB, 2002. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 94, 1867–1875. 10.1002/cncr.10427 [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Beyeler N, 2020. Cancer and climate change. Lancet Oncol 21, e519–e527. 10.1016/S1470-2045(20)30448-4 [DOI] [PubMed] [Google Scholar]
- Hiller TWR, O’Sullivan DE, Brenner DR, Peters CE, King WD, 2020. Solar Ultraviolet Radiation and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Environ. Health Perspect 128, 016002. 10.1289/EHP4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, 2007. Vitamin D Deficiency. N Engl J Med 16. [DOI] [PubMed] [Google Scholar]
- John EM, Schwartz GG, Dreon DM, Koo J, n.d. Vitamin D and Breast Cancer Risk: The NHANES I Epidemiologic Follow-up Study, 1971–1975 to 1992 9. [PubMed] [Google Scholar]
- John EM, Schwartz GG, Koo J, Wang W, Ingles SA, 2007. Sun Exposure, Vitamin D Receptor Gene Polymorphisms, and Breast Cancer Risk in a Multiethnic Population. Am. J. Epidemiol 166, 1409–1419. 10.1093/aje/kwm259 [DOI] [PubMed] [Google Scholar]
- Kantor ED, Rehm CD, Du M, White E, Giovannucci EL, 2016. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA 316, 1464. 10.1001/jama.2016.14403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan QJ, Kimler BF, Fabian CJ, 2010. The Relationship Between Vitamin D and Breast Cancer Incidence and Natural History. Curr. Oncol. Rep 12, 136–142. 10.1007/s11912-010-0081-8 [DOI] [PubMed] [Google Scholar]
- Kim Y, Je Y, 2014. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br. J. Cancer 110, 2772–2784. 10.1038/bjc.2014.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R, 2007. Vitamin D and Reduced Risk of Breast Cancer: A Population-Based Case-Control Study. Cancer Epidemiol. Biomarkers Prev 16, 422–429. 10.1158/1055-9965.EPI-06-0865 [DOI] [PubMed] [Google Scholar]
- Kroll MH, Bi C, Garber CC, Kaufman HW, Liu D, Caston-Balderrama A, Zhang K, Clarke N, Xie M, Reitz RE, Suffin SC, Holick MF, 2015. Temporal Relationship between Vitamin D Status and Parathyroid Hormone in the United States. PLOS ONE 10, e0118108. 10.1371/journal.pone.0118108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn T, Kaaks R, Becker S, Eomois P-P, Clavel-Chapelon F, Kvaskoff M, Dossus L, Tjønneland A, Olsen A, Overvad K, Chang-Claude J, Lukanova A, Buijsse B, Boeing H, Trichopoulou A, Lagiou P, Bamia C, Masala G, Krogh V, Sacerdote C, Tumino R, Mattiello A, Buckland G, Sánchez M-J, Menéndez V, Chirlaque M-D, Barricarte A, Bueno-de-Mesquita HB, van Duijnhoven FJB, van Gils CH, Bakker MF, Weiderpass E, Skeie G, Brustad M, Andersson A, Sund M, Wareham N, Khaw KT, Travis RC, Schmidt JA, Rinaldi S, Romieu I, Gallo V, Murphy N, Riboli E, Linseisen J, 2013. Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: A nested case-control study: 25(OH)vitamin D and the risk of breast cancer. Int. J. Cancer 133, 1689–1700. 10.1002/ijc.28172 [DOI] [PubMed] [Google Scholar]
- Kuper H, Yang L, Sandin S, Lof M, Adami H-O, Weiderpass E, 2009. Prospective Study of Solar Exposure, Dietary Vitamin D Intake, and Risk of Breast Cancer among Middle-aged Women. Cancer Epidemiol. Biomarkers Prev 18, 2558–2561. 10.1158/1055-9965.EPI-09-0449 [DOI] [PubMed] [Google Scholar]
- Li Y, Ma L, 2020. Exposure to solar ultraviolet radiation and breast cancer risk: A dose-response meta-analysis. Medicine (Baltimore) 99, e23105. 10.1097/MD.0000000000023105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-W, Wheeler DC, Park Y, Cahoon EK, Hollenbeck AR, Freedman DM, Abnet CC, 2012. Prospective study of ultraviolet radiation exposure and risk of cancer in the United States. Int. J. Cancer 131, E1015–E1023. 10.1002/ijc.27619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE, 2019. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med 380, 33–44. 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, Gapstur SM, Thun MJ, Calle EE, 2009. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res 11, R64. 10.1186/bcr2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr SB, Garland CF, Gorham ED, Grant WB, Garland FC, 2008. Relationship between Low Ultraviolet B Irradiance and Higher Breast Cancer Risk in 107 Countries. Breast J 14, 255–260. 10.1111/j.1524-4741.2008.00571.x [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Sandler DP, Taylor JA, Weinberg CR, 2017. Serum Vitamin D and Risk of Breast Cancer within Five Years. Environ. Health Perspect 125, 077004. 10.1289/EHP943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin B, Xu B, Ji N, Yao S, Pawlish K, Llanos AAM, Lin Y, Demissie K, Ambrosone CB, Hong C-C, Bandera EV, 2020. Intake of vitamin D and calcium, sun exposure, and risk of breast cancer subtypes among black women. Am. J. Clin. Nutr 111, 396–405. 10.1093/ajcn/nqz302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, Mosekilde L, 2009. Reduced Prediagnostic 25-Hydroxyvitamin D Levels in Women with Breast Cancer: A Nested Case-Control Study. Cancer Epidemiol. Biomarkers Prev 18, 2655–2660. 10.1158/1055-9965.EPI-09-0531 [DOI] [PubMed] [Google Scholar]
- Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, Kleeberger CA, Shore DL, DeRoo LA, Taylor JA, Weinberg CR, the Sister Study Research Team, 2017. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ. Health Perspect 125, 127003. 10.1289/EHP1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier I, Platt RW, 2008. Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol 8, 70. 10.1186/1471-2288-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2020. Cancer statistics, 2020. CA. Cancer J. Clin 70, 7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Sister study follow-up timeline and follow-up response rates. Data Release 6.0 [WWW Document], n.d. URL https://sisterstudy.niehs.nih.gov/English/images/SIS-FU-TimelineDR6-FURR-508.pdf (accessed 1.4.21).
- Song D, Deng Y, Liu K, Zhou L, Li N, Zheng Y, Hao Q, Yang S, Wu Y, Zhai Z, Li H, Dai Z, 2019. Vitamin D intake, blood vitamin D levels, and the risk of breast cancer: a dose-response meta-analysis of observational studies. Aging 11, 12708–12732. 10.18632/aging.102597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MB, Michels KB, Brody JG, Byrne C, Chen S, Jerry DJ, Malecki KMC, Martin MB, Miller RL, Neuhausen SL, Silk K, Trentham-Dietz A, 2019. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res 21, 96. 10.1186/s13058-019-1168-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VoPham T, Bertrand KA, DuPré NC, James P, Vieira VM, Tamimi RM, Laden F, Hart JE, 2019. Ultraviolet radiation exposure and breast cancer risk in the Nurses’ Health Study II: Environ. Epidemiol 3, e057. 10.1097/EE9.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VoPham T, Hart JE, Bertrand KA, Sun Z, Tamimi RM, Laden F, 2016. Spatiotemporal exposure modeling of ambient erythemal ultraviolet radiation. Environ. Health 15, 111. 10.1186/s12940-016-0197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AR, Engelsen O, 2006. Calculated Ultraviolet Exposure Levels for a Healthy Vitamin D Status. Photochem. Photobiol 82, 1697. 10.1562/2006-09-01-RA-670 [DOI] [PubMed] [Google Scholar]
- Welsh J, 2012. Cellular and molecular effects of vitamin D on carcinogenesis. Arch. Biochem. Biophys 523, 107–114. 10.1016/j.abb.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J, Wietzke JA, Zinser GM, Smyczek S, Romu S, Tribble E, Welsh JC, Byrne B, Narvaez CJ, 2002. Impact of the Vitamin D3 receptor on growth-regulatory pathways in mammary gland and breast cancer. J. Steroid Biochem. Mol. Biol 83, 85–92. 10.1016/S0960-0760(02)00277-7 [DOI] [PubMed] [Google Scholar]
- Xue X, Kim MY, Gaudet MM, Park Y, Heo M, Hollenbeck AR, Strickler HD, Gunter MJ, 2013. A Comparison of the Polytomous Logistic Regression and Joint Cox Proportional Hazards Models for Evaluating Multiple Disease Subtypes in Prospective Cohort Studies. Cancer Epidemiol. Biomarkers Prev 22, 275–285. 10.1158/1055-9965.EPI-12-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Veierod MB, Lof M, Sandin S, Adami H-O, Weiderpass E, 2011. Prospective Study of UV Exposure and Cancer Incidence Among Swedish Women. Cancer Epidemiol. Biomarkers Prev 20, 1358–1367. 10.1158/1055-9965.EPI-11-0071 [DOI] [PubMed] [Google Scholar]
- Yao S, Ambrosone CB, 2013. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J. Steroid Biochem. Mol. Biol 136, 337–341. 10.1016/j.jsbmb.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Zamoiski RD, Freedman DM, Linet MS, Kitahara CM, Liu W, Cahoon EK, 2016. Prospective study of ultraviolet radiation exposure and risk of breast cancer in the United States. Environ. Res 151, 419–427. 10.1016/j.envres.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


