Abstract
Over the past two decades, opioid abuse has risen especially among women. In both sexes hippocampal neural circuits involved in associative memory formation and encoding of motivational incentives are critically important in the transition from initial drug use to drug abuse/dependence. The opioid circuit particularly the mossy fiber pathway, are crucial for associative memory processes important for addiction. Our anatomical studies, especially those utilizing electron microscopic immunocytochemistry, have provided unique insight into sex differences in the distribution of opioid peptides and receptors in specific hippocampal circuits and how these distributions are altered following stress and oxycodone-associative learning processes. Here we review the hippocampal opioid system in rodents with respect to ovarian hormones effects and baseline sex differences then sex differences following acute and chronic stress. Next, we review sex differences in the hippocampal opioid system in unstressed and chronically stressed rats following oxycodone conditioned place preference. We show that opioid peptides and receptors are distributed within hippocampal circuits in females with elevated estrogen states in a manner that would enhance sensitivity to endogenous and exogenous opioids. Moreover, chronic stress primes the opioid system in females in a manner that would promote opioid-associative learning processes. In contrast, chronic stress has limited effects on the opioid system in males and reduces its capacity to support opioid-mediated learning processes. Interestingly, acute stress appears to prime males for opioid associative learning. On a broader scale the findings highlighted in this review have important implications in understanding sex differences in opioid drug use and abuse.
Keywords: enkephalin, mu opioid receptor, delta opioid receptor, kappa opioid receptor, electron microscopy, RNA expression
Introduction
i.1. Background.
Opioid abuse, predominately of the prescription medication oxycodone (Oxy), has risen drastically particularly over the past two decades (Centers for Disease Control, 2015; Kibaly et al., 2021). Of notable concern is the dramatic increase in the rate of drug overuse among women which can lead to opioid dependence (VanHouten et al., 2019; Strang et al., 2020). Prior studies have shown that women have altered sensitivity to morphine across the menstrual cycle suggesting the potential involvement of ovarian hormones in drug addiction processes (Ribeiro-Dasilva et al., 2011). Similar opioid sensitivity is observed across the estrous cycle of a rodent model of heroin self-administration (Lacy et al., 2016).
In both the sexes, transition from drug use to drug abuse rely on associative memory and motivational incentives (Koob and Volkow, 2010) which critically involve hippocampal circuits connected either directly or indirectly to the mesolimbic reward system (Vorel et al., 2001; Luo et al., 2011) (Fig. 1). These circuits are primary targets of abused drugs, including mu opioid receptor (MOR) agonists such as Oxy (Koob and Volkow, 2016). Within the rodent hippocampus, opioid signaling in the CA3 subregion (Fig. 2A) plays a critical role in both spatial memory and contextual associative learning (Meilandt et al., 2004; Kesner and Warthen, 2010). Moreover, a form of opioid-mediated long-term potentiation (LTP) has been demonstrated in mossy fiber - CA3 pyramidal cell synapses (MF-CA3 synapse) in female rats at high estrogen states, but not low estrogen states or in male rats (Harte-Hargrove et al., 2015), further supporting the concept that hormonal states can alter opioid-associative learning processes.
Fig. 1. Hippocampal participation in addictive disease circuitry.
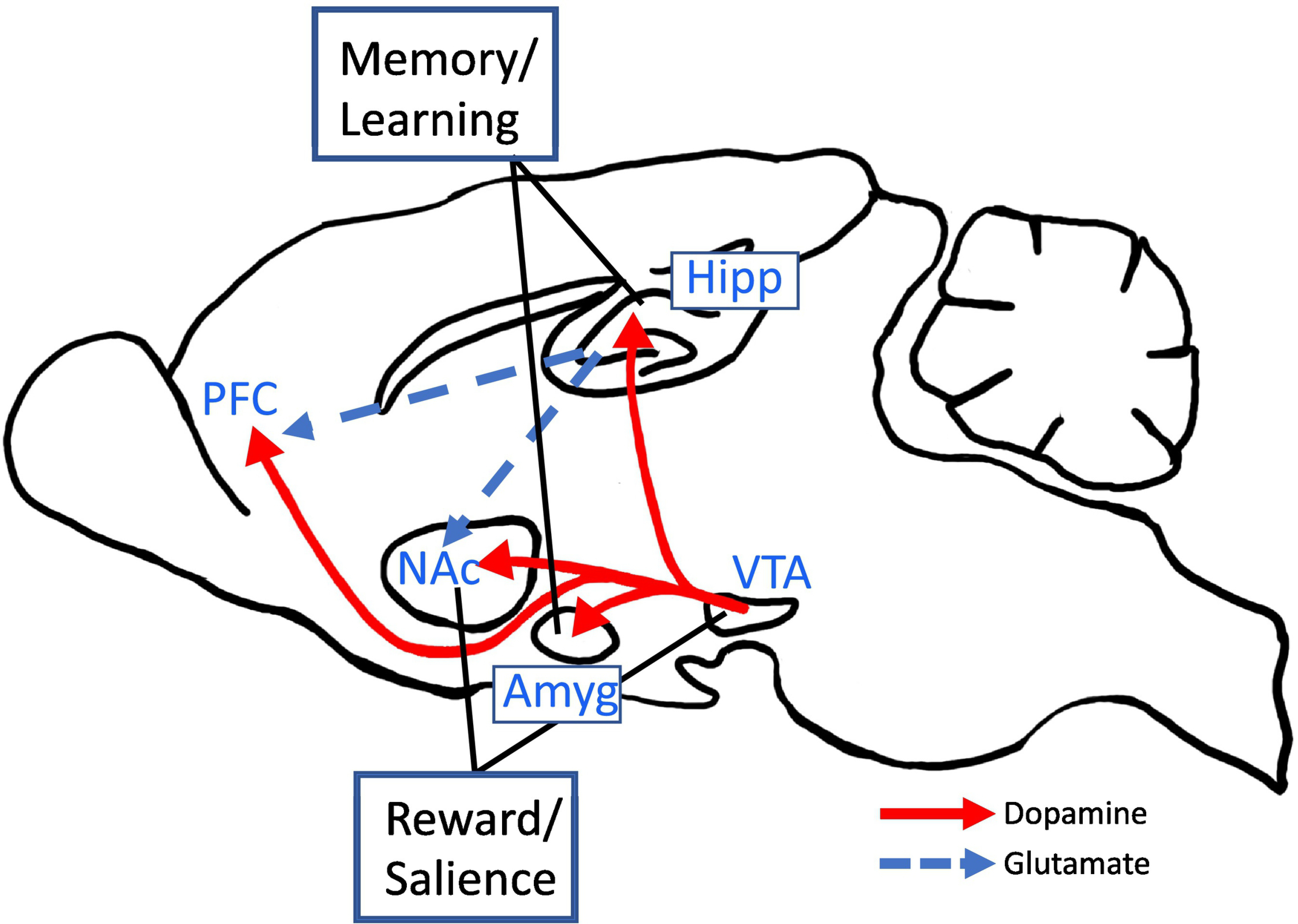
The hippocampus plays a significant role in the learning aspect of addictive diseases. Associative learning enhances the classical dopamine reward system which is important for transitioning from initial drug use to abuse/dependence. Hipp - hippocampus; PFC - prefrontal cortex; NAc - nucleus accumbens; VTA - ventral tegmental area; Amyg - amygdala.
Fig. 2. Schematic denoting regions of the hippocampus used for light and electron microscopy analysis.
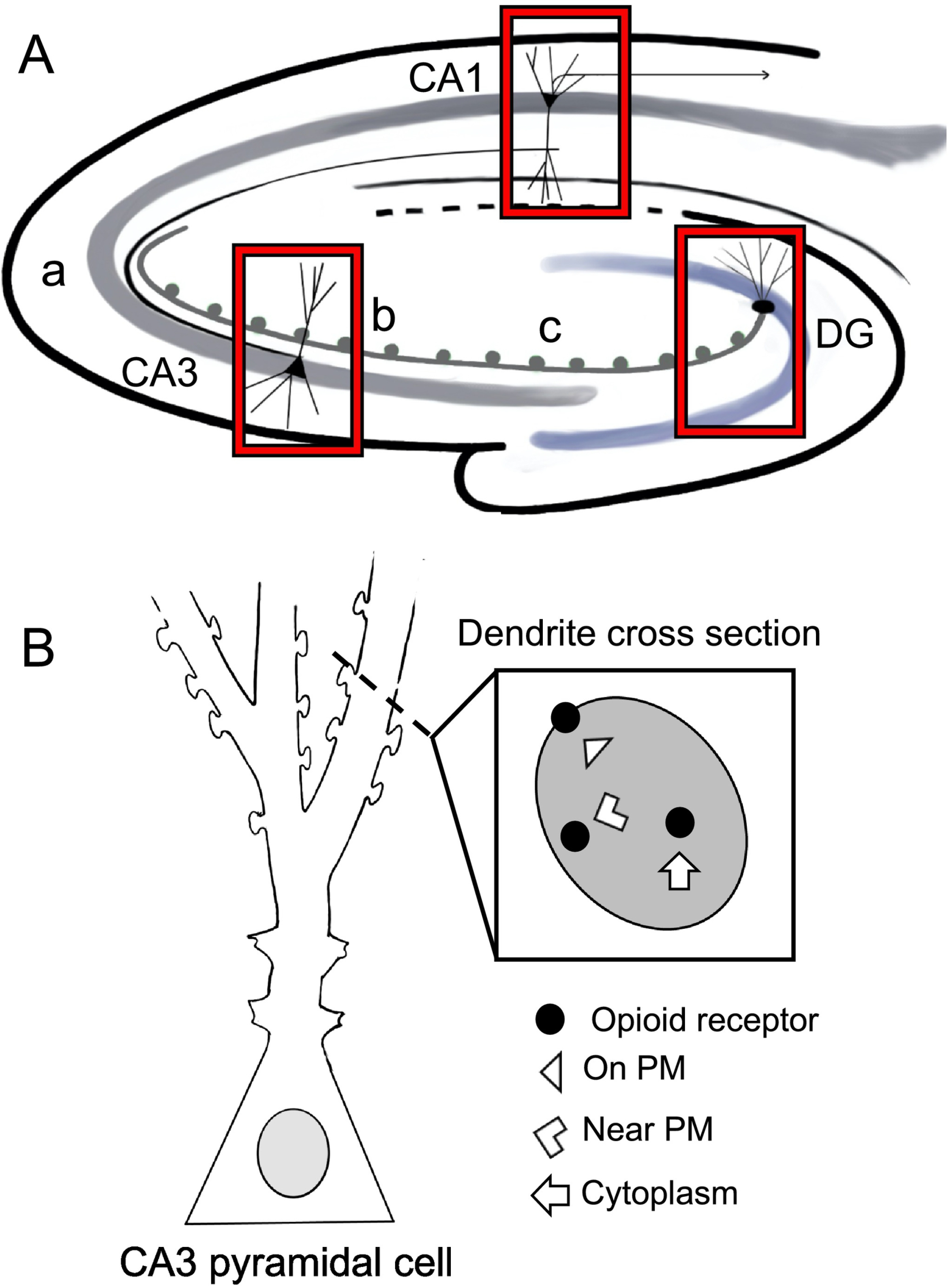
A. Schematic of a coronal section through the hippocampus showing the CA1, CA3 (a, b and c subregions) and dentate gyrus (DG) regions sampled for light and electron microscopy. B. Schematic of a dendritic cross section showing the subcellular compartmentalization of opioid receptors used for electron microscopy analysis.
Our early light and electron microscopic (EM) studies have provided detailed descriptions of hippocampal opioid circuits in rodents (Drake et al., 2007; McEwen and Milner, 2017). Additionally, our studies over the last several years have shown significant sex differences in the hippocampal opioid system that provide important insights in the role of this system in opioid-associative learning processes. Following an overview of the hippocampal opioid system, we review the hippocampal opioid system with respect to: 1) ovarian hormones effects; 2) baseline sex differences; 3) sex differences following acute stress; 4) sex differences following chronic stress; 5) sex differences in other systems related to opioids; 6) Oxy conditioned place preference (CPP); and 7) chronic stress and Oxy CPP.
i.2. Methodological approaches for analysis of hippocampal opioid system.
Three main anatomical approaches have been key in revealing sex differences in the rodent hippocampal opioid system in response to hormonal fluctuations and stress as well as following Oxy-CPP. These methods will be briefly reviewed.
1). Quantitative light microscopic immunocytochemistry densitometry:
Quantitative light microscopic densitometry can be used to determine the relative differences in the levels of immunoreactivity in terminal fields (e.g., mossy fibers) or dendritic lamina (e.g., CA1 or CA3 pyramidal cell dendrites) (Auchus and Pickel, 1992; Pierce et al., 1999; Williams et al., 2011a; Randesi et al., 2018). Our prior studies have shown that levels of the opioid peptides Leu-Enkephalin (LEnk) and dynorphin (DYN) in the mossy fiber pathway as determined by optical densitometric analysis correlates with the number of dense-core vesicles for these peptides (Pierce et al., 1999; Pierce et al., 2014).
2). Dual labeling EM immunocytochemistry:
Quantitative dual labeling immuno-EM provides a unique perspective on the proportion of receptors available for ligand binding in particular cell compartments (Milner, 2011). It is the only method available to date that can analyze changes in the redistribution of receptors in select neuronal compartments (i.e., dendrites and spines) following experimental manipulations. Figure 2B shows a conceptual overview by which a protein (e.g., MOR) is identified using silver-intensified gold particles (SIG) with immunoEM (Milner, 2011). The presence of plasmalemma receptors (on PM) identified by SIGs corresponds to sites of receptor binding (Boudin et al., 1998). Receptors identified by SIGs near the plasmalemma (i.e, near PM) may be inserted into or removed from the plasmalemma from a pool of receptors. SIG labeling in the cytoplasm represents receptors that may be stored, in transit to/from the cell body or other cellular compartments, as well as in the process of being degraded or recycled (Pierce et al., 2009; Fernandez-Monreal et al., 2012). The functionality of using SIG in receptor trafficking has been supported by the internalization of epitope-tagged MORs in nucleus accumbens following morphine administration (Haberstock-Debic et al., 2003).
3). In situ hybridization:
In situ hybridization allows for the visualization of mRNA expression in specific cell types and localization cells in different subregions (Johnson et al., 2021). Using ACD RNAscopetm and HALO software, mRNA expression levels are determined by calculating the numbers of probes per cell.
1. Review of the rodent hippocampal opioid system
The anatomy and function of the hippocampal opioid system will be briefly reviewed. For a more detailed review of the anatomy and physiology of the hippocampal opioid system see our prior review (Drake et al., 2007).
1.1. Opioid peptides:
LEnk and DYNs are prominent in mossy fiber terminal projections of granule cells in the dentate gyrus (DG) (Gall et al., 1981; Commons and Milner, 1995; Torres-Reveron et al., 2009b) (Fig. 3). These mossy fibers innervate hilar mossy cells in the DG and CA3 pyramidal cells in the stratum lucidum (Drake et al., 2007). LEnks also are in: 1) the lateral perforant path (LPP) and 2) the temporal-ammonic tract (TAT), excitatory pathways that arise from the entorhinal cortex, and 3) scattered GABA interneurons primarily in CA1 and CA3 (Gall et al., 1981; Johnson et al., 2021). Albeit at much lower levels than LEnk and DYN, endomorphins are found in occasional fibers scattered throughout hippocampus (Pierce and Wessendorf, 2000) and beta endorphin is found in progenitor cells in the dentate gyrus (Persson et al., 2003).
Fig. 3. Schematic of opioid peptide pathways within the hippocampus.
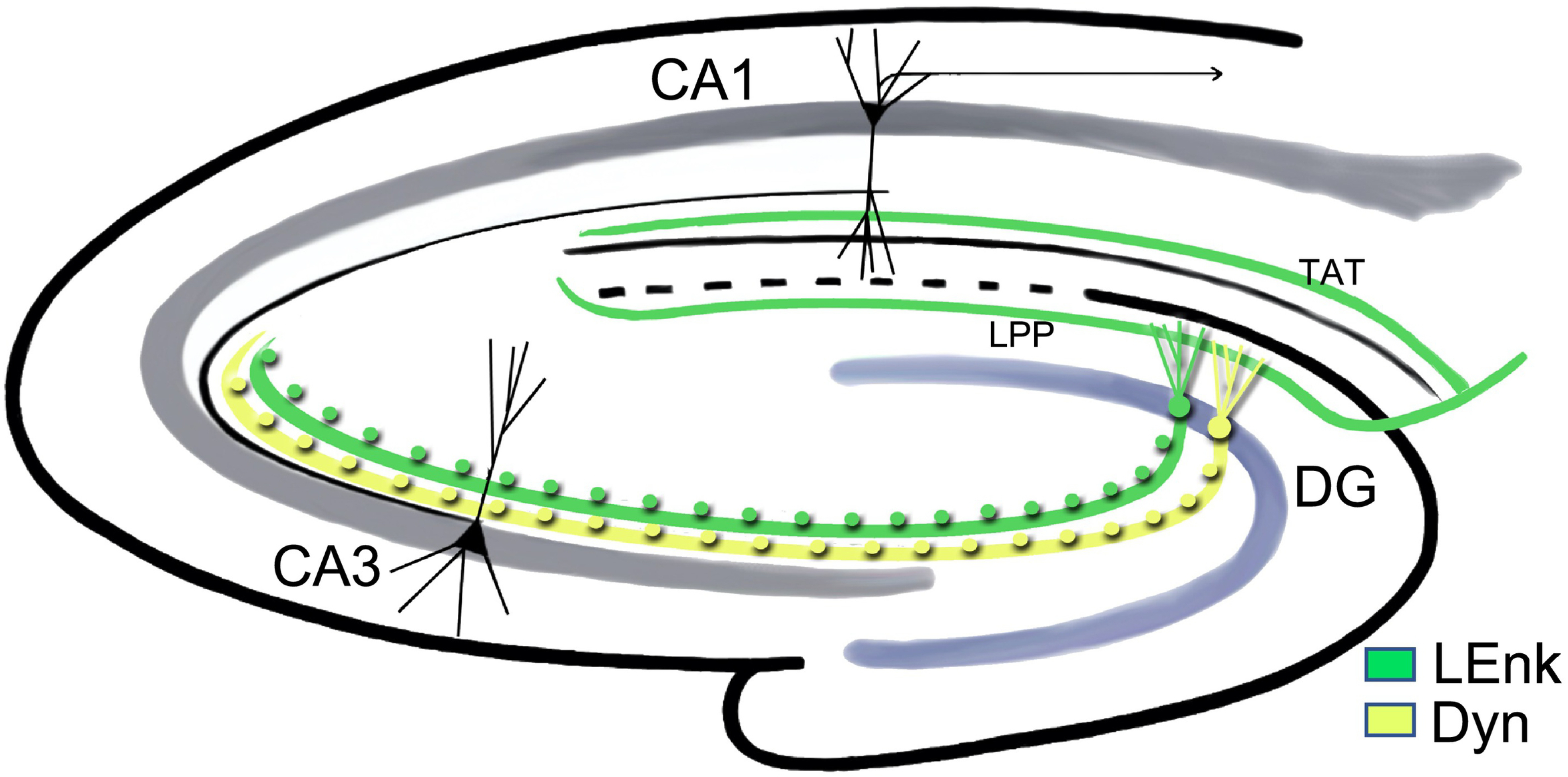
Leu-enkephalin (LEnk) and dynorphins (DYN) are found in the mossy fiber pathway originating from granule cells in the DG. The mossy fibers transverse through the CA3 (green and yellow fibers) in stratum lucidum (SLu). LEnks, originating from the entorhinal cortex, are found in lateral perforant pathway (LPP) and the temporal-ammonic tract (TAT) pathway. Scattered interneurons containing LEnk (not shown) are found throughout the hippocampus.
There are some notable species differences in the opioid system between rats and mice. Compared to the rat, LEnk levels in the mossy fiber pathway are noticeably less in the mouse (Gall et al., 1990; Van Kempen et al., 2013). The opioid peptide containing mossy fiber pathway in the mouse contains high levels of cholecystokinin (CCK), the rat does not (Gall et al., 1990; Chandy et al., 1995).
In contrast to small clear vesicles that contain glutamate or GABA, opioid peptides are released from dense-core vesicles (DCVs) after high-frequency (>50 Hz) stimulation (Pierce et al., 1999; McLaughlin et al., 2003) and activate extra synaptic receptors via “volume transmission” (Agnati et al., 1986; Drake et al., 2002). However, LEnks can also be released at low frequencies (2 Hz) (Wong and Moss, 1992; Han, 2003; Liang et al., 2010). Moreover, enkephalins and dynorphins are released in response to seizures and stress (Pierce et al., 1999; McLaughlin et al., 2003).
1.2. Opioid receptors:
Enkephalins and endomorphins are endogenous ligands for mu and delta opioid receptors (MORs and DORs, respectively) (Sandin et al., 2000; Persson et al., 2003; Drake et al., 2007). In the rat hippocampus, MORs (MOR1A) are prominent in parvalbumin (PARV)-labeled GABAergic basket cells that innervate DG granule cell and CA3 pyramidal cell somata and proximal dendrites (Drake et al., 2007) (Fig. 4). MORs are also in mossy fiber terminals and axons (MOR1D) (Abbadie et al., 2000) and cholinergic and GABAergic septal afferents (Leranth and Frotscher, 1989; Kaplan et al., 2004). DORs are prominent in GABA interneurons that project to the distal dendrites of granule and pyramidal cells and contain somatostatin (SOM), neuropeptide Y (NPY) and corticotrophin releasing factor (CRF) (Drake et al., 2007; Williams and Milner, 2011) and inhibit the distal dendrites of granule and pyramidal cells (Piguet and North, 1993; Drake et al., 2007). DORs, and MORs to a lesser extent, are in granule and pyramidal cell dendrites (Piguet and North, 1993; Williams et al., 2011a; Rubin et al., 2020; Johnson et al., 2021). Activation of MORs and DORs largely leads to disinhibition of interneurons, leading to activation of interneuron targets, but can directly inhibit granule and pyramidal cells (Drake et al., 2007). Activation of LEnk containing mossy fibers and the lateral perforant pathway promotes long-term potentiation (LTP) in CA3 pyramidal cells via MORs and DORs (Derrick et al., 1992; Do et al., 2002; Harte-Hargrove et al., 2015). Opioid signaling in the CA3 region has been shown to play a critical role in spatial memory and in contextual associative learning (Meilandt et al., 2004; Kesner and Warthen, 2010).
Fig. 4. Schematic of a granule cell and associated interneuron interactions in the dentate gyrus.
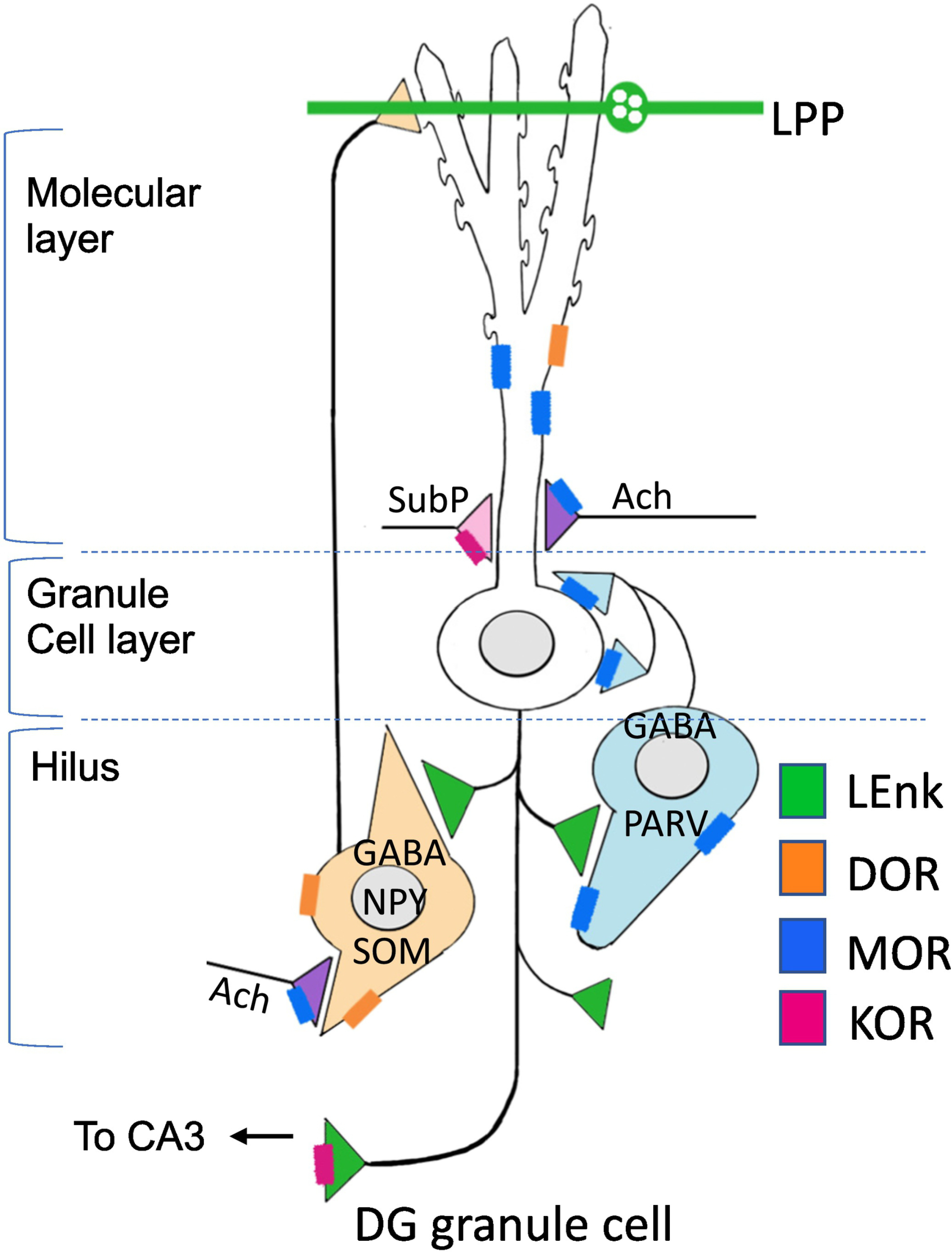
Mu opioid receptors (MORs; blue) are found on inhibitory GABAergic PARV-interneurons that project to the somata and proximal dendrites of granule cells. Activation of MORs lead to a disinhibition and subsequent activation of the granule cells. Delta opioid receptors (DORs; orange) are found on neuropeptide Y/somatostatin (NPY/SOM) interneurons that contain primarily project to the distal dendrites of granule cells. Kappa opioid receptors (KORs; pink) are primarily found in substance P (subP; pink) afferents that arise from the hypothalamus. LEnk (green) is found in granule cells and in the LPP.
DYNs are the endogenous ligands for kappa opioid receptors (KORs) and also have a high affinity for MOR1D (Drake et al., 2007; Pasternak and Pan, 2013). In the rat hippocampus, KORs are on a few scattered interneurons in the hilus and in the CA1–3 some of which contain NPY and SOM (Halasy et al., 2000; Rácz and Halasy, 2002; Johnson et al., 2021). In the guinea pig, KORs are in substance P afferents to granule cells (Drake et al., 1996; Drake et al., 1997). Endogenous DYNs block the induction of LTP and reduce excitatory neurotransmission in granule cell-perforant path synapses (Wagner et al., 1993). LTP is also inhibited by the activation of the KORs in the dentate gyrus (Morris and Johnston, 1995). KORs also are associated with inhibition of spatial learning as well as context induced fear (Sandin et al., 1998).
1.3. Opioid related peptides.
Nociceptin/orphanin FQ (N/OFQ) is a more recently identified fourth member of the opioid peptide family. This endogenous ligand binds to its own receptor, ORL1 or NOP receptor, which are distinct from conventional opioid receptors (Moulédous, 2019). Furthermore, activation of NOP receptors has a modulatory role on MORs (Toll et al., 2016). Numerous N/OFQ-containing interneurons are found in the DG as well as in the CA1, CA2, and CA3 (Moulédous, 2019). NOP binding is primarily found in lamina containing pyramidal and granule cell dendrites in the hippocampus (Moulédous, 2019). The N/OFQ system inhibits transmission and synaptic plasticity (Moulédous, 2019).
2. Effects of the hormonal milieu on the hippocampal opioid system in females
2.1. Estrous cycle:
As the majority of studies examining sex differences in the hippocampal opioid system have been done in naturally cycling female rodents, we will briefly review estrous cyclic phases used in these studies. Estrous phases used are: 1) Proestrus which gives rise to a peak in the levels of 17-beta estradiol (E2), luteinizing hormone (LHRH), and follicle stimulating hormone (FSH); It precedes ovulation and is analogous to the human follicular phase (Turner and Bagnara, 1971; McLean et al., 2012). 2) Estrus which lasts 1–2 days, with ovulation occurring within the first 24 hours (Goldman et al., 2007). Estrogen levels begin to decline and progestin levels are elevated (McLean et al., 2012). 3) Diestrus [combination of diestrus I (metestrus) and diestrus II] has the lowest levels of estrogens and progestins.
2.2. Ovarian hormone receptors and opioid system.
The distribution of estrogen receptors (ERs) and the effects of estrogen on hippocampus function have been extensively reviewed (Waters et al., 2009; Spencer-Segal et al., 2012; McEwen and Milner, 2017). Thus, we will discuss these findings primarily as they relate to the rodent hippocampal opioid system. ERα is found in CA1 and CA3 pyramidal cells and DG granule cells, especially in dendritic spines, as well as interneurons and glia (Milner et al., 2001; Romeo et al., 2005; Mitterling et al., 2010). ERα-containing interneurons often colocalize CCK and NPY (Hart et al., 2007; Ledoux et al., 2009), and occasionally found in parvalbumin-containing interneurons (Weiland et al., 1997). In CA1, ERα is associated with clusters of vesicles in perisomatic inhibitory boutons; these vesicles mobilize towards synapses following estrogen treatment (Hart et al., 2007). ERα is found in numerous axon terminals (Milner et al., 2001; Mitterling et al., 2010), some of which contain acetylcholine (Towart et al., 2003), but ERα is not detected in LEnk and DYN-containing mossy fiber terminals and axons (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009b). In contrast, ERβ is colocalized in some LEnk- and DYN mossy fibers terminals and smaller terminals in the hilus of the DG (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009b). Within the mossy fiber terminals, ERβ has a subcellular association with the plasmalemma and small synaptic vesicles (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009b). Moreover, like ERα, ERβ is found in CA1 and CA3 pyramidal cells and DG granule cells as well as interneurons in the hippocampus (Milner et al., 2005; Mitterling et al., 2010). In adult rodents, the progesterone receptor (PR) is colocalized in CA3 in both LEnk and DYN mossy fiber axons (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009b; Mitterling et al., 2010). However, in developing rats, PR is transiently expressed in Cajal-Retzius cells contained in the outer molecular layer of the DG (Newell et al., 2018), a region which overlaps with LEnk inputs arising from the entorhinal cortex (Drake et al., 2007).
2.3. Ovarian hormones and opioid peptides.
In cycling rats, LEnk levels in stratum lucidum of CA3 and the hilus of the dentate gyrus are highest when estrogen levels are elevated (proestrus/estrus) (Fig. 5) (Torres-Reveron et al., 2008; Pierce et al., 2014). Similarly, 24 hours after estradiol (E2) replacement in ovariectomized (OVX) rats, LEnk levels in the DG hilus and CA3c increase (Torres-Reveron et al., 2008). Further, LEnk levels increase in the mossy fiber pathway with age but not with either E2 alone or E2 plus progesterone replacement in the mossy fiber pathway (Williams et al., 2011c).
Fig. 5. Summary of sex and estrous cycle differences in opioid markers in the rat hippocampal mossy fiber pathway.
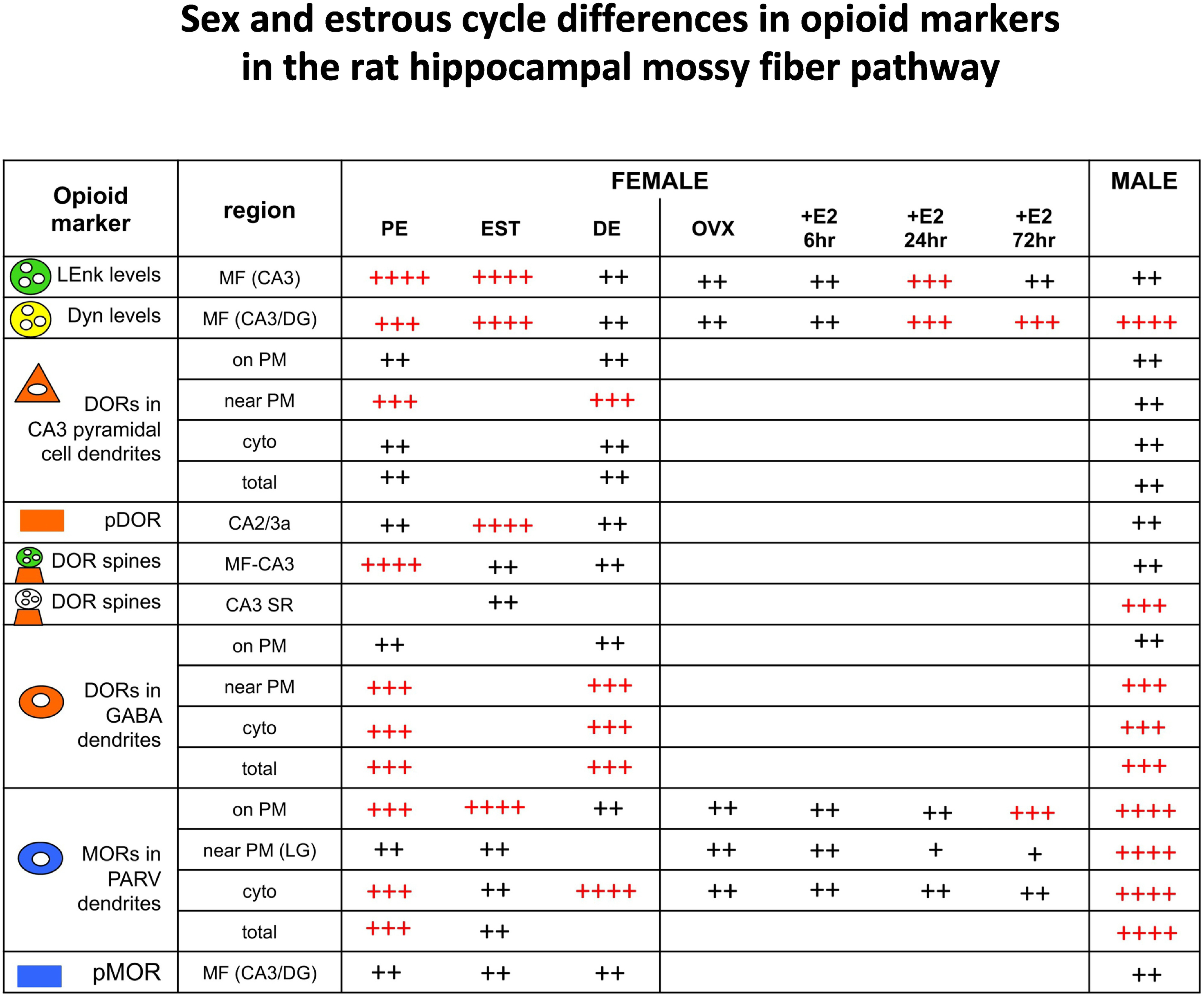
Comparisons of opioid markers in the DG and CA3 between females at different estrous states and males. Red ++++ and +++ indicate elevated levels; Black ++ and + indicate lower levels. Results for LEnk from Torres-Reveron et al (2008) and Pierce et al (2014); for DYN from Torres-Reveron et al (2009) and Gregorie et al (unpublished); for DORs from Mazid et al (2016); Harte-Hargrove et al (2015); Ryan et al (2018); for pDOR from Burstein et al (2013); for MORs from Milner et al (2013); and for pMOR from Gonzales et al. (2011).
In rats, DYN levels in the DG and CA3 lamina are the highest in the estrous phase compared to proestrus or diestrus (Torres-Reveron et al., 2009b) (Fig. 5). In OVX rats, 24 hours following either E2 or medroxyprogesterone replacement, DYN levels are increased in both DG and CA3 (Torres-Reveron et al., 2009b). Elevated DG DYN levels are also observed 72 h following E2 replacement in OVX rats (Torres-Reveron et al., 2009b). Decreased DYN in the mossy fiber pathway occurs with age as well as with E2 + progesterone replacement in young adult OVX rats (Williams et al., 2011c).
Unlike rats, mice do not have fluctuations in mossy fiber LEnk or DYN levels across the estrous cycle (Van Kempen et al., 2013). Further, no change in mossy fiber pathway LEnk levels was observed in OVX mice lacking either ERα or ERβ with and without E2 replacement (Van Kempen et al., 2013). However, OVX mice lacking ERα, but not ERβ, mossy fiber DYN levels increased 48 hours following E2 replacement (Van Kempen et al., 2013) (Table 1). This suggests that the absence of ERα unmasks a potential regulation of DYN expression by ERβ in response to E2.
Table 1.
Comparison of hippocampal opioid system in mouse vs rat.
| Mossy fiber pathway | Sex differences | Cycle differences observed | Other |
|---|---|---|---|
|
| |||
| LEnk | Mouse ≪ rat | Mouse – no | CCK colocalization |
| Rat - yes | Mouse ≫ rat | ||
| Dyn | Mouse ≪ rat | Mouse – no | Increase in OVX mouse lacking ERα + given E2 replacement |
| Rat - yes | |||
| MOR in PARV dendrites in DG hilus | Mouse – no | ||
| Rat -yes | |||
2.4. Ovarian hormones and opioid receptor distributions.
MORs:
In the hippocampus, administration of E2 alone or in combination with progesterone to OVX rats decreases the density of MORs as measured by autoradiography (Slamberová et al., 2003). E2 administration to OVX rats increases MOR binding in hippocampal homogenates (Piva et al., 1995), but does not alter MOR mRNA expression in the hippocampus (Quiñones-Jenab et al., 1997).
Similar to males, females MOR-immunoreactivity (-ir) is in numerous PARV-labeled perikarya, dendrites, and terminals in the dentate hilar region (Torres-Reveron et al., 2009a). The number of PARV-labeled cells is not affected by estrous cycle phase or estrogen levels (Torres-Reveron et al., 2009a). However, variation in ovarian steroid levels alters the subcellular distribution of MORs within PARV-labeled dendrites but not in terminals (Torres-Reveron et al., 2009a) (Fig. 5). In normal cycling rats, the density of MORs on the plasma membrane of small PARV-labeled dendrites is higher in proestrus rats than in diestrus rats (Torres-Reveron et al., 2009a). Moreover, subsequent studies show that estrus females have more MORs on the plasma membrane of PARV dendrites compared to proestrus females (Milner et al., 2013). Likewise, in OVX rats MORs have a higher density on the plasma membrane of small PARV-labeled dendrites 72 hours after E2 exposure (Torres-Reveron et al., 2009a). Thus, elevated estrogen levels increase plasmalemmal MORs on PARV-dendrites, indicating that more MORs are available for ligand binding. In contrast to rats, there were no differences in the subcellular distribution of MORs in PARV containing dendrites in the DG of female mice from different estrous cycles (unpublished) (Table. 1).
Similar to other G-protein coupled receptors, ligand binding typically initiates the phosphorylation of MORs and DORs which is important for receptor uncoupling, internalization and trafficking (Law et al., 2000; Deng et al., 2001; Pradhan et al., 2009; Doll et al., 2011; Dang and Christie, 2012). This phosphorylation can occur in the presence of other opioid receptor agonists via a few identified signaling mechanism resulting in the reduction of functional receptors and consequently leading to desensitization (Williams et al., 2013). There are no estrous cycle differences in the levels of pMOR-labeling in the mossy fiber pathway (Gonzales et al., 2011). This suggests that endogenous MOR signaling may not be affected by changes in hormonal milieu across the estrous cycle.
DORs:
At the light microscopic level, DOR levels in the stratum radiatum of CA3 are significantly elevated in diestrus rats compared to proestrus rats (Mazid et al., 2016). At the EM level, the subcellular distribution of DORs within CA3 pyramidal cell dendrites is similar in proestrus and diestrus rats (Mazid et al., 2016) (Fig. 5). However, the number of DOR-containing CA3 dendritic spines contacted by mossy fibers in proestrus females is greater than diestrus females (Harte-Hargrove et al., 2015; Mazid et al., 2016). The subcellular distribution of DORs in GABAergic hilar interneuron dendrites is also similar in proestrus and diestrus females (Mazid et al., 2016).
Unlike CA3, the subcellular distribution of DORs within CA1 pyramidal cells is affected by estrous cycle stage (Fig. 6). Estrus rats have elevated DORs on and near the plasma membrane of CA1 dendrites compared to proestrus rats (Williams et al., 2011a; Rubin et al., 2020). Conversely, proestrus and diestrus rats compared to estrus rats have higher levels of cytoplasmic DORs in CA1 dendrites (Williams et al., 2011a; Rubin et al., 2020). However, there is no difference in the numbers of DOR-containing spines on CA1 dendrites between the estrous cycle stages. These findings suggest that high estrogen levels in the absence of high progesterone levels, decrease the availability of DORs for ligand binding the CA1 dendrites.
Fig. 6. Summary of sex and estrous cycle differences in opioid markers in the rat hippocampal CA1.
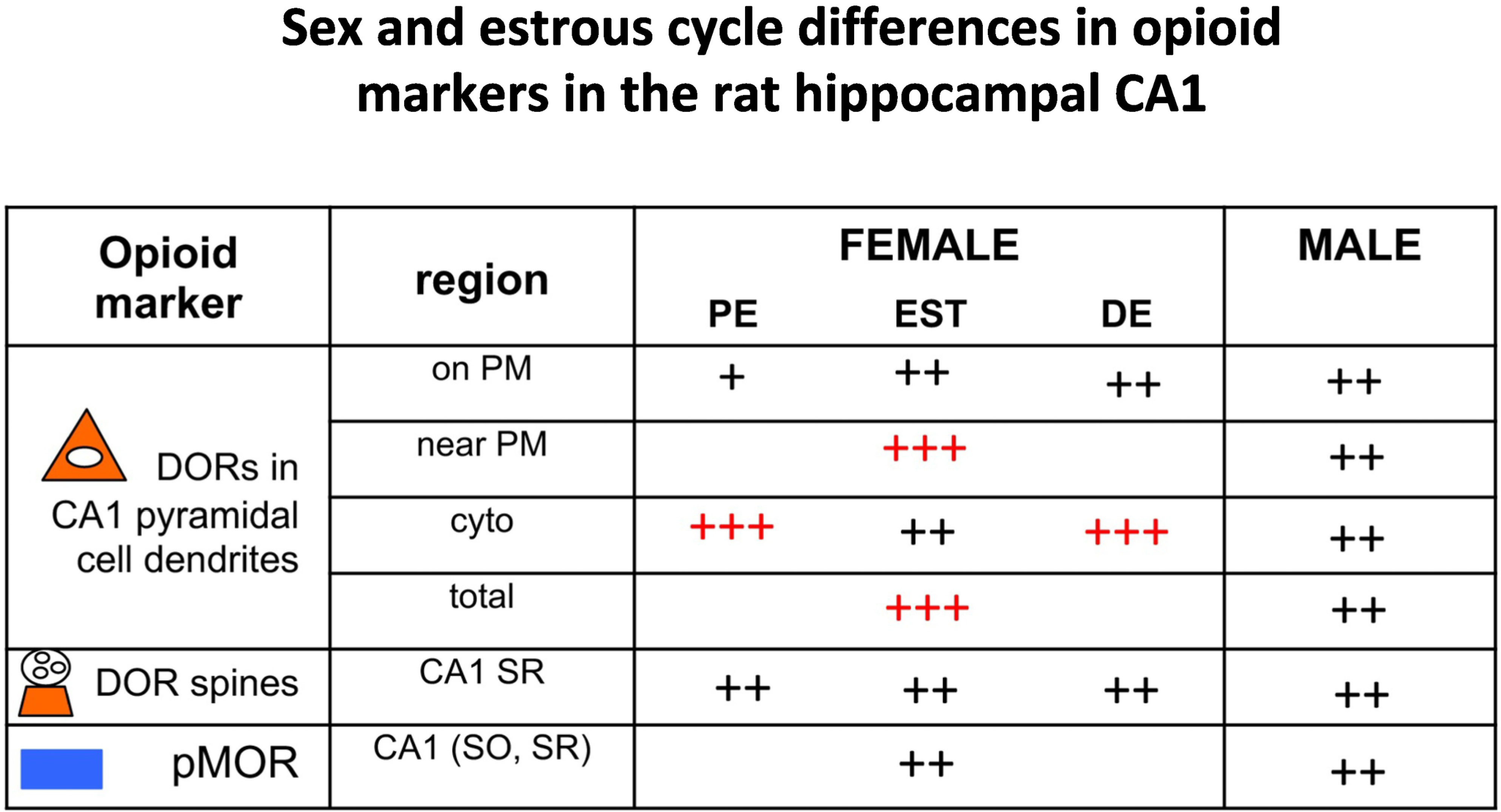
Comparisons of opioid markers in the CA1between females at different estrous states and males. Red ++++ and +++ indicate elevated levels; Black ++ and + indicate lower levels. Results for DORs from Williams et al (2011); Rubin et al (2020); and for pMOR from Bellamy et al (2019).
Rats in estrus have a higher level of pDORs in CA2/3a compared to the proestrus and diestrus females as well as males (Burstein et al., 2013). This is in line with estrus rats having increased LEnk and DOR expression and suggests potentially increased DOR signaling when high estrogen is concomitant with reduced progesterone.
2.5. Functional considerations:
Proestrus females, but not diestrus females and males, show a strong MOR regulation of mossy fiber transmission and a DOR-dependent form of mossy fiber-CA3 LTP (Harte-Hargrove et al., 2015). This novel form of opioid-dependent LTP in proestrus females is concomitant with elevations of mossy fiber LEnk levels and DORs in CA3 pyramidal cell spines contacted by mossy fibers (MF-CA3 synapses) (Pierce et al., 2014; Mazid et al., 2016) as well as MORs on the plasma membrane of GABAergic interneurons (Torres-Reveron et al., 2009a; Milner et al., 2013). Together, these results suggest in elevated estrogen states: 1) greater amounts of LEnks are produced and could be released from granule cells after stimulation [e.g., stress (McLaughlin et al., 2003)] and 2) MORs/DORs are positioned on different neuronal subpopulations in a manner that can promote excitation (i.e., disinhibition) (Drake et al., 2007).
3. Sex differences in the hippocampal opioid system: unstressed (control) rats
3.1. Sex differences and opioid peptides.
Proestrus/estrus (elevated estrogen) female rats have twice the LEnk levels in the mossy fiber pathway than diestrus female and male rats (Torres-Reveron et al., 2008; Pierce et al., 2014) (Fig. 5). As the mossy fiber pathway transverses through the CA3, the CA3 can be divided into 3 subregions: CA3c is the most proximal region to the DG, whereas CA3b is in the middle and CA3a is the most distal (Sun et al., 2017). Both the CA3a and CA3b regions encode for spatial information in short-term memory (Cherubini and Miles, 2015). The CA3b is also responsible for greater associative learning as it has the strongest excitation and reduced inhibition compared to the other 2 regions (Sun et al., 2017). Although LEnk and DYN levels in mossy fibers are more limited in mice compared to rats (see section 1), female mice have higher LEnk and DYN levels in specific subregions of CA3 compared to male mice (Van Kempen et al., 2013). In particular, LEnk is elevated in CA3b whereas DYN is higher in CA3a in the mossy fiber pathway of female compared to male mice (Van Kempen et al., 2013).
There are few sex differences in opioid peptide mRNA expression in the rat hippocampus (Johnson et al., 2021). In the granule cell layer of the DG, females have more cells with low expression of PENK (proenkephalin; precursor protein for Enk) compared to males. There are no differences in the expression of PDYN (pro-Dynoprhin; precursor protein for DYN) in either the dorsal or ventral blade of the DG granule cell layer between estrus females and males. Similarly, there are no sex difference in the expression of LEnk in interneurons in any hippocampal region (Johnson et al., 2021).
3.2. Sex differences and opioid receptors.
MORs:
At the EM level, the numbers of MOR-containing PARV cells in the hilus of the dentate gyrus are similar in females (estrus) and males (Ryan et al., 2018). However, estrus females have lower overall density of MORs near the plasma membrane and cytoplasm as well as total in PARV labelled dendrites compared to males (Ryan et al., 2018). The subcellular distribution of MORs within PARV-labeled dendrites in the hilus of the DG is similar in PE females and males (Milner et al., 2013).
The levels of pMORs in the DG or CA3 are not affected by sex or estrous cycle stage (Gonzales et al., 2011) (Fig. 5). Similarly, in the CA1, pMOR levels are not different between estrus females and male rats (Bellamy et al., 2019) (Fig. 6).
OPRM1 (Opioid receptor, mu1) is found in interneurons in CA1, CA2/3a, CA3b, and DG (sub-granular zone and granule cell layer). The number of OPRM1 expressing interneurons is similar in estrus females compared to males (Johnson et al., 2021).
DORs:
By light microscopy, there are no sex differences in the levels of DORs in SR of the CA3 region (Mazid et al., 2016). However, by EM, proestrus females compared to males had more DORs near the plasma membrane of CA3 pyramidal dendrites (Mazid et al., 2016) as well as thrice as much DORs in MF-CA3 synapses (Harte-Hargrove et al., 2015). Proestrus females have more DOR-labeled cells in the hilus of the DG compared to males (Williams et al., 2011a). Additionally, there are no sex differences in the subcellular distribution of DORs in GABAergic dendrites in the hilus of the DG as analyzed by EM (Mazid et al., 2016; Ryan et al., 2018).
In contrast to CA3, DOR levels in the CA1, as assessed by light microscopy, are lower in PE females compared to males (Williams et al., 2011a). Moreover, at the EM level, PE females compared to males have fewer plasmalemmal DORs and elevated cytoplasmic DORs in distal dendrites of CA1 pyramidal cells (Williams et al., 2011a; Rubin et al., 2020), indicating DOR trafficking away from available binding sites (Fig. 6).
There are no sex differences in the expression of OPRD1 (Opioid receptor, delta) in interneurons in CA1, CA2/3a, CA3b, and central hilus of the DG (Johnson et al., 2021). However, when the CA1 region was divided into lamina, colocalization of OPRD1/OPRM1 was found to be higher in unstressed (US) males compared to US females (Johnson et al., 2021).
KORs:
There are no sex differences in the number of OPRK1 (Opioid receptor, kappa) expressing cells in the hippocampus (Johnson et al., 2021).
3.3. Functional considerations:
Similar functional differences have been observed in the opioid system of proestrus females compared to males as were observed between females in high estrogen states compared females with low estrogen states (as described above in section 2.4, estrous cycle differences). In particular, proestrus females compared to males have greater amounts of LEnk available for release after stimulation of granule cells. Moreover, the redistribution and expression of MORs/DORs in pyramidal cells and interneurons would enhance excitability and plasticity processes to a greater extent in females with elevated estrogen states than in males. Thus, in addition to an estrous cycle effect, there is a sex difference in the hippocampal opioid system.
4. Sex differences in hippocampal response to acute immobilization stress (AIS)
Stress is a common facet of life across species and generally involves a heightened arousal state induced by an aversive situation or stimulus. The hippocampus is not only critically involved in memory formation, but also plays a role in terminating the stress response through glucocorticoid-mediated negative feedback of the hypothalamic-pituitary-adrenal axis (Herman and Cullinan, 1997; McEwen, 2007). With one of the highest concentrations of receptors for corticosteroids in the mammalian brain, the hippocampus is highly sensitive to stress (Reul and de Kloet, 1985; Conrad et al., 1999). The response to acute stress involves adaptive mechanisms that enable escape or triumph over the aversive situation; however, these mechanisms can have adverse consequences particularly in the hippocampus. Here we review our recent findings on sex differences in hippocampal response to acute immobilization stress (AIS).
4.1. Sex differences and opioid peptides following AIS.
After AIS (30 min), LEnk levels in the mossy fiber pathway in CA3 decrease in proestrus females to a level similar to that of males (Pierce et al., 2014) (Fig. 7).
Fig. 7. Summary of sex differences in opioid markers in the rat hippocampal mossy fiber pathway following stress.
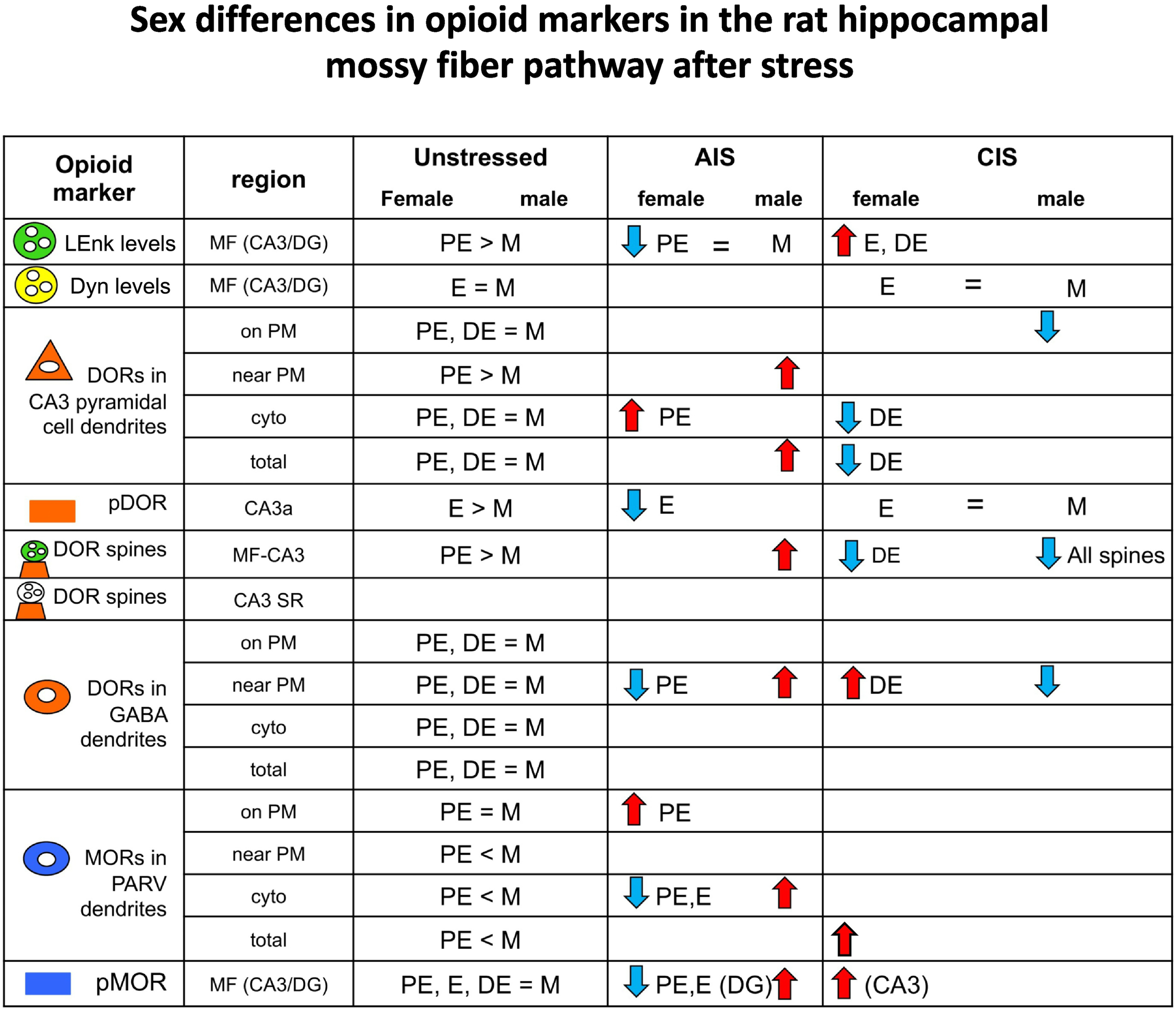
Comparisons of opioid markers in the DG and CA3 between females from different estrous states and males following acute immobilization stress (AIS) and chronic immobilization stress (CIS). Results for LEnk from Pierce et al (2014); results for DYN from Gregorie et al. (unpublished; CIS); results for pMOR from Gonzales et al., 2011(AIS); CIS - Bellamy et al 2019 (CIS); for pDOR from Burstein et al., 2013 (AIS); Bellamy et al., 2019 (CIS). for DORs from Mazid et al (2016); for MORs from Milner et al (2013). >, <, = signs indicate baseline differences. Arrows indicate the direction of change from baseline following AIS or CIS.
4.2. Sex differences and opioid receptors following AIS.
MORs:
Following AIS, females show an increase in plasmalemmal MORs in proestrus but not estrus rats and a reduction of cytoplasmic MORs in small PARV-labeled dendrites in both proestrus and estrus rats (Milner et al., 2013). (Fig. 7). These results suggest that activation of MORs following AIS in only high estrogen state females would lead to a greater inhibition of the PARV-labeled interneurons dendrites (Drake et al., 2007). In contrast, AIS in males induces an increase in cytoplasmic MORs in PARV dendrites (Milner et al., 2013).
Following AIS, proestrus and estrus females had reduced pMOR levels in the DG and no change in pMOR levels in CA3 compared to corresponding controls (Gonzales et al., 2011) (Fig. 7). Conversely, pMOR levels in the CA3 and DG subregions of the mossy fiber pathway significantly increase in male rats following AIS (Gonzales et al., 2011).
DORs:
Following AIS, proestrus female rats show increases in cytoplasmic DORs in CA3 pyramidal cell dendrites and decreases in near plasma membrane DORs in hilar GABAergic dendrites (Mazid et al., 2016) (Fig. 7). In contrast, AIS in males increases near plasmalemmal and total DORs in CA3 pyramidal cell dendrites as well as in hilar GABAergic dendrites (Mazid et al., 2016). Further, AIS increases the percent of DOR-labelled spines contacted by mossy fibers in CA2/3 in males but not females (Mazid et al., 2016). Following AIS, no significant differences in pDOR levels in CA2/3 are seen across estrous cycle phase or sex (Burstein et al., 2013).
4.3. Functional considerations.
In general, AIS has differential effects on the opioid system in males and females, depending on the estrogen state. In females, the decreases in mossy fiber LEnk levels as well DOR trafficking within CA3 pyramidal cells and GABA interneurons seen after AIS resemble that seen in diestrus females and thus would reduce excitation and plasticity processes (see section 2). In males, AIS would be expected to enhance opioid-mediated excitation and plasticity processes (Fig. 8).
Fig. 8. Following AIS opioid receptors are redistributed in a manner that would promote opioid-associated learning in male rats.
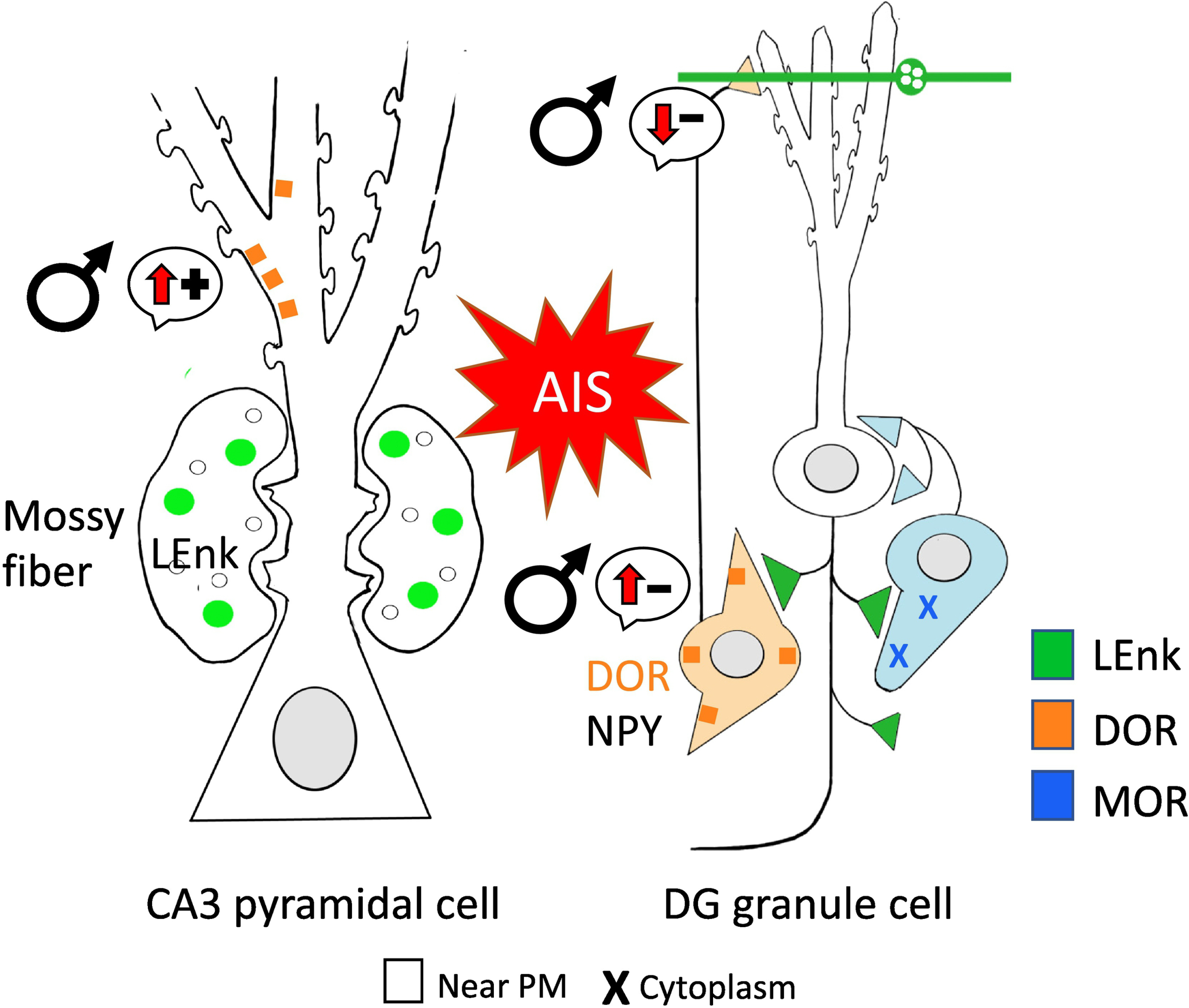
Schematic of CA3 pyramidal cell shows DORs (orange) are significantly higher near the plasmalemma following AIS in males suggesting increased opioid-ligand mediated synaptic plasticity. Schematic of DG granule cell shows DORs are also significantly higher near the plasmalemma of GABAergic NPY-interneuron. This leads to enhanced disinhibition and subsequent opioid-mediated excitation. MORs (blue) also are elevated in the cytoplasm of basket cells, indicating increased reserve pools. (−): indicates disinhibition; (+): indicates activation. Arrows indicate direction of change.
5. Sex differences in hippocampal response to chronic immobilization stress (CIS)
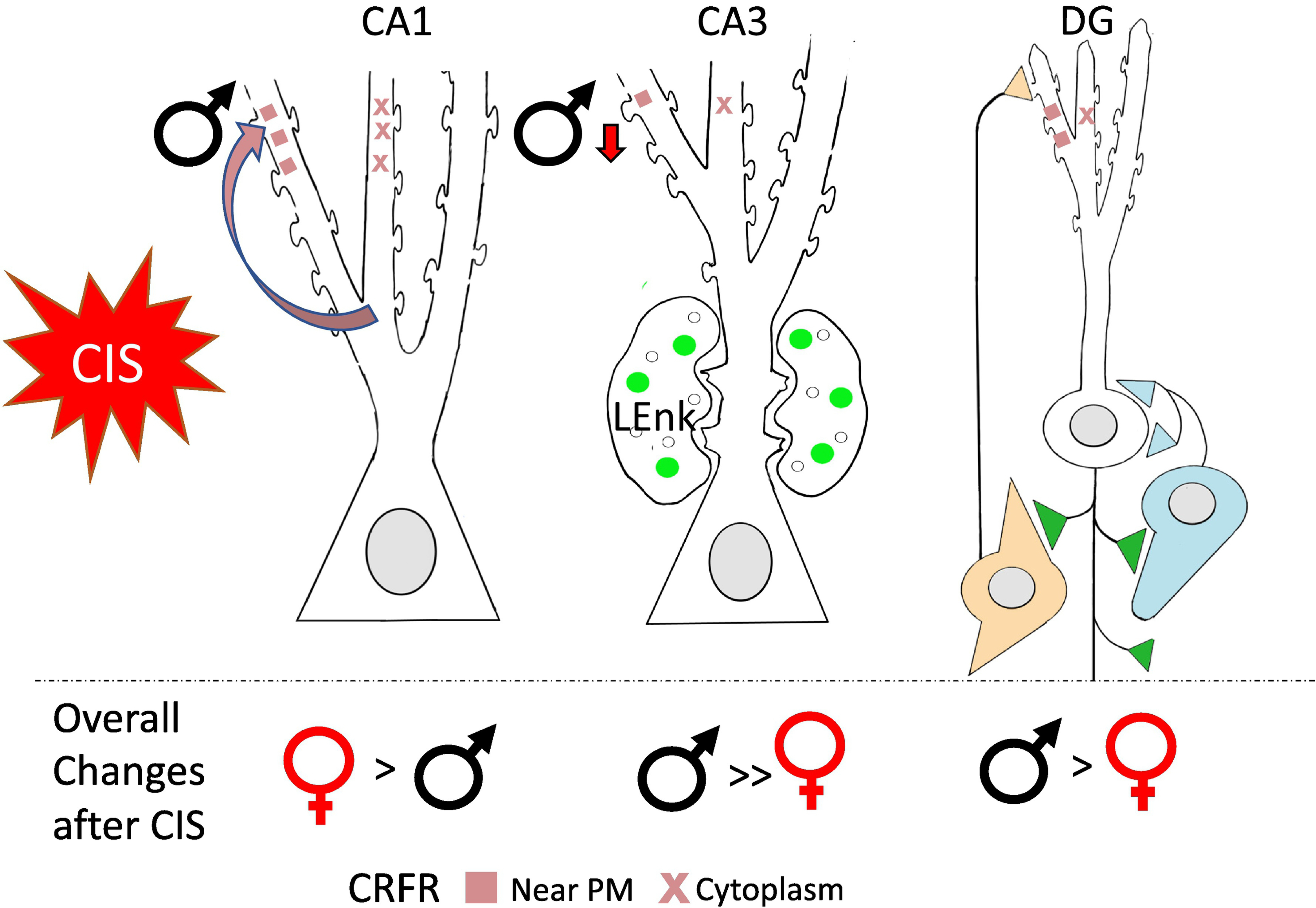
Exposure to prolonged or recurrent stress has sexually dimorphic effects on hippocampal-related learning and circuitry. Chronic stress in males impairs cognitive performance, reduces LTP, and results in atrophy and debranching in CA3 pyramidal cells apical dendrites (McEwen, 1999; McEwen and Milner, 2007). Conversely, in females chronic stress enhances some aspects of cognitive performance and does not result in CA3 pyramidal cell atrophy (Conrad et al.; Luine et al., 2007). Further, chronic stress has been shown to differentially impact the hippocampal opioid system as reviewed below.
5.1. Effects of CIS on hippocampal structure.
The effect of chronic stress on hippocampal structure and function has been extensively reviewed in prior publications (McEwen, 2007; McEwen et al., 2015; McEwen and Milner, 2017; McEwen and Akil, 2020) Of particular relevance to the hippocampal opioid system chronic stress in males, but not females, has been shown to result in dendritic retraction of CA3 pyramidal cells and PARV interneuron loss (Watanabe et al., 1992; Galea et al., 1997; Vyas et al., 2002; McEwen and Milner, 2017) (Fig. 9).
Fig. 9. Following CIS opioid receptors are redistributed in a manner may promote opioid-associated learning in female rats and cause structural changes in males.
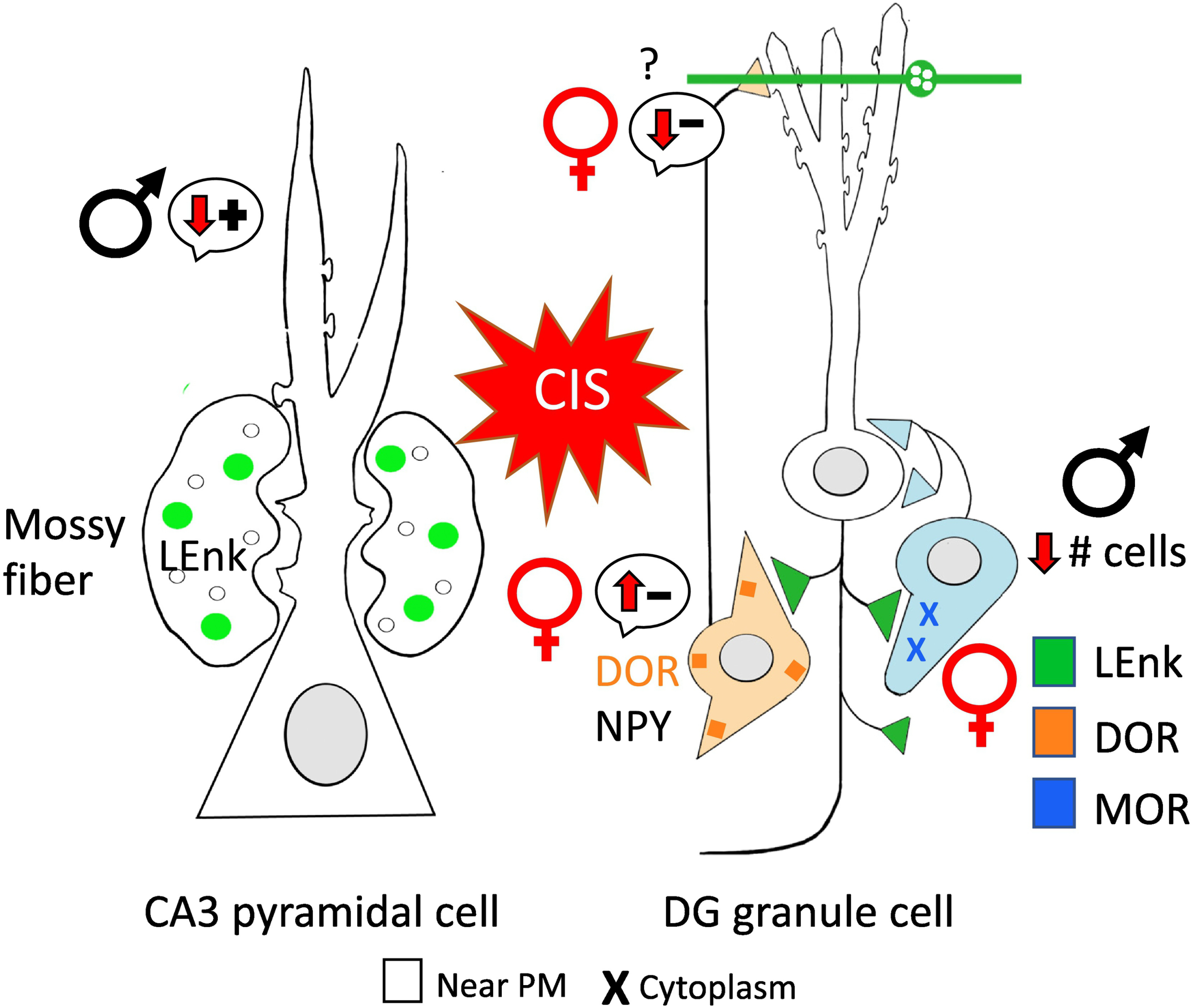
Schematic of CA3 pyramidal cell shows males have significant adverse dendritic retractions following CIS subsequently reducing the possibility of opioid mediated synaptic plasticity, Furthermore, males have decreased plasmalemma DORs in CA3 pyramidal cell dendrites thus, further reducing the opioid-mediated plasticity. In the DG, males also have reduced numbers of basket cells (know to contain MORs) following CIS. Conversely, the schematic of the DG granule cell shows that DORs increase near the plasmalemma of GABAergic NPY-interneurons in females, enhancing disinhibition leading to opioid-mediated excitation. Moreover, females have elevated total levels of MORs in basket cells. (−): indicates disinhibition; (+): indicates activation. Arrows indicate direction of change.
5.2. Sex differences and opioid peptides following CIS.
CIS increases LEnk levels in the mossy fiber pathway in estrus and diestrus rats to levels seen in proestrus rats but does not alter LEnk levels male rats (Pierce et al., 2014) (Fig. 7). Further, CIS (estrus) females compared to unstressed (US) females and US/CIS males have elevated expression of PENK in granule cells (Johnson et al., 2021). Moreover, CIS elevated PENK expression in interneurons located in CA1, CA2, and CA3 in both females and males compared to US counterparts (Johnson et al., 2021).
Following CIS, DYN levels in stratum lucidum of CA3b are higher in male rats compared to females (Gregiore et al unpublished). In contrast to LEnk, CIS males compared to US males and US/CIS females have elevated PDYN expression in the granule cells located in the dorsal blade of the DG (Johnson et al., 2021).
5.3. Sex differences and opioid receptors following CIS.
MORs:
The number of PARV-labeled neurons in the DG hilus, which colocalize MORs (Drake et al., 2007), are decreased following CIS in males, but not females (Czeh et al., 2005; Hu et al., 2010; Milner et al., 2013). At the EM level, CIS reduces the size of dendrites and terminals dually labeled for MOR and PARV in the DG in females, but not males (Milner et al., 2013). Further, CIS in females, but not males, results in a higher density of cytoplasmic MORs in PARV dendrites in the DG (Milner et al., 2013). Additionally, pMOR levels in all hippocampal regions are unchanged in both females and males after CIS (Bellamy et al., 2019).
CIS (estrus) females compared to CIS males have greater overall expression of OPRM1 in the CA3 region (Randesi et al., 2018). However, in situ hybridization showed that CIS males compared to CIS (estrus) females have elevated expression of OPRM1 in granule cells (Johnson et al., 2021).
DORs:
By light microscopy, CIS increased the number of DOR labeled interneurons in the hilus of the dentate gyrus in females but not males (Mazid et al., 2016)(Fig. 7). Additionally, CIS decreases the number of NPY containing neurons, which colocalize DORs (Drake et al., 2007) in males but not females (Mazid et al., 2016). Following CIS, EM studies show that cytoplasmic and total DORs decrease in the CA3 pyramidal cell dendrites in females (Mazid et al., 2016). In contrast, CIS in males results in a decrease in plasmalemmal DORs in CA3 pyramidal cell dendrites (Mazid et al., 2016). However, in the DG the proportion of DORs near the plasmalemma of GABAergic dendrites is elevated in females but decreased in males following CIS (Mazid et al., 2016) (Fig. 9). In CA1, CIS increased cytoplasmic and total DORs in pyramidal cell dendrites in females (Rubin et al., 2020). Moreover, CIS increases near plasmalemma DORs in pyramidal cell dendrites in males so that they were equal to that seen in females (Rubin et al., 2020). Furthermore, pDOR levels in CA2/3a are unchanged in both females and males after CIS (Bellamy et al., 2019).
Similar to increases in DOR-immunolabeled cells (see above), CIS females compared to CIS males have greater overall expression of OPRD1 in the CA2/3 region (Randesi et al., 2018) (Fig. 10). In situ hybridization shows that CIS elevates OPRD1 in CA1,CA2 and CA3 pyramidal cells and DG granule cells in both females and males (Johnson et al., 2021). Additionally, CIS increases OPRD1 probe copies in most cell types throughout the hippocampus in both females and males (Johnson et al., 2021).
Fig. 10. Summary of sex and estrous cycle differences in opioid markers in the rat hippocampal CA1 after CIS.
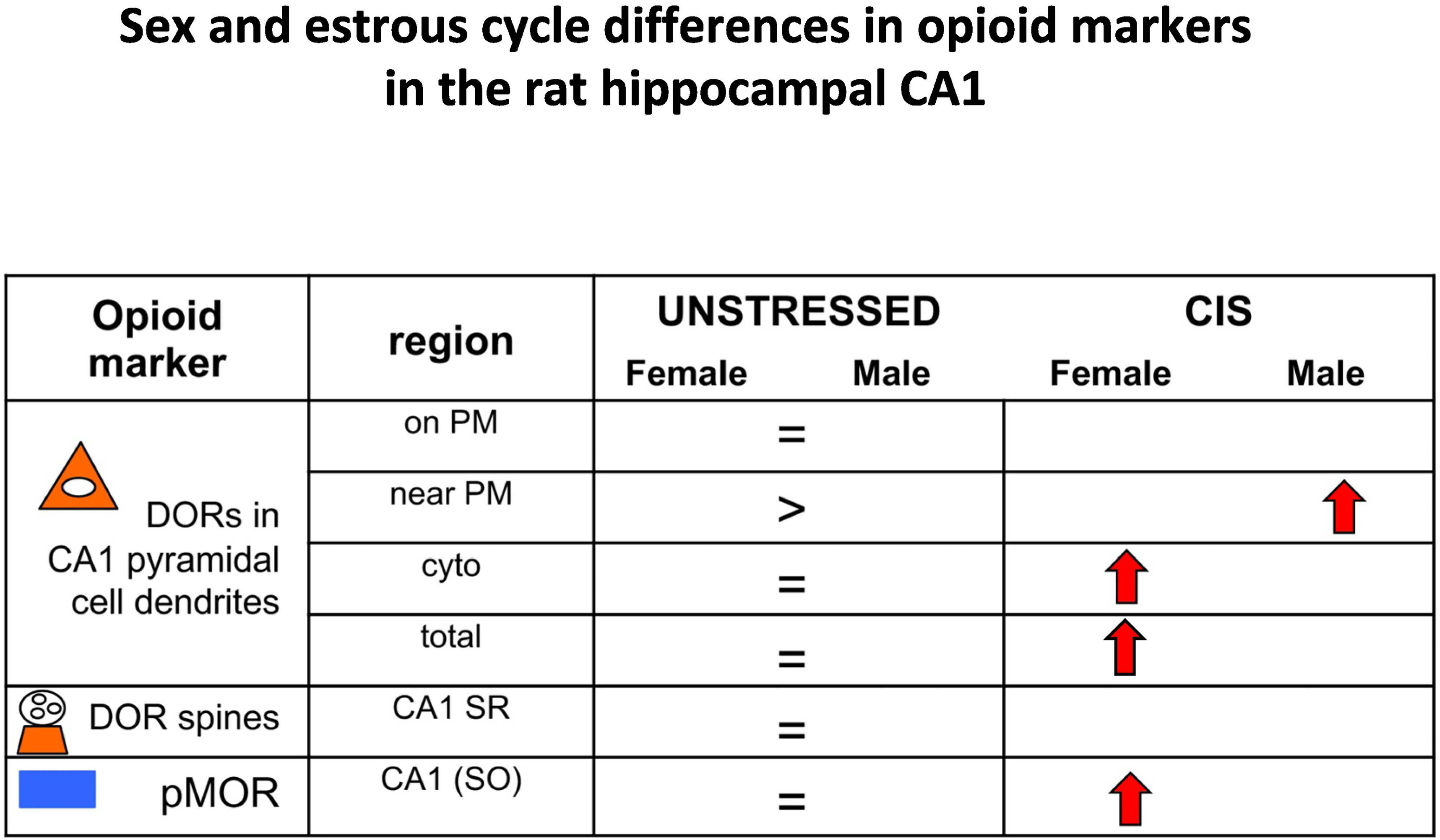
Comparisons of opioid markers between females and males following chronic immobilization stress (CIS). Results for DORs from Williams et al (2011) and Rubin et al (2020); for pMOR from Bellamy et al. (2019). >,= indicate baseline differences. Arrows indicate direction of change following CIS.
KORs:
CIS does not affect the numbers of KOR-immunolabeled cells in either sex (unpublished). However, CIS elevates the expression of OPRK1 in hilar interneurons in both males and females as well as OPRK1 in CA2/3a interneurons in males (Johnson et al., 2021).
5.4. Functional considerations:
Our studies revealed that CIS (30 min per day for 10 days) “primes” the opioid system in all females in a manner that would promote excitation and learning processes following subsequent exposure to an opiate ligand; conversely, CIS essentially “shuts off” the opioid system in males (Milner et al., 2013; Pierce et al., 2014; Mazid et al., 2016). The opioid system in all females after CIS resembles that of females in elevated estrogen states: 1) LEnk is ~2X higher in mossy fibers; 2) DORs are increased in MF-CA3 synapses; 3) MORs are increased in PARV interneuron dendrites; 4) in CIS females, DORs mobilize to the near-plasmalemma dendrites of hilar NPY/SOM interneurons known to project to granule cell dendrites where they converge with entorhinal afferents (Milner and Bacon, 1989; Milner and Veznedaroglu, 1992). This finding suggests that a second mechanism for enhancing hippocampal excitation and perhaps learning processes comes into play in females after CIS. Specifically, since DORs inhibit NPY release, activation of DORs on NPY-containing interneurons could promote lateral perforant pathway LTP (Sperk et al., 2007). Furthermore, CIS in the absence of behavior increases cytoplasmic DORs in females and near plasmalemmal DORs in males in CA1 pyramidal cell dendrites (Rubin et al., 2020). However, CIS with behavioral enrichment increases DORs in dendritic spines and thus helps females “prime” for sensitivity to DOR agonists (Rubin et al., 2020).
To conclude, CIS impairs spatial memory in males but not in females. Further, in females, CIS enhances and primes the opioid system to be more sensitive to agonists.
6. Other systems that differ between the sexes and change following CIS in parallel with the opioid system
6.1. Corticotropin releasing factor (CRF) system.
CRF peptide:
The opioid system substantially overlaps with the CRF system. DOR is colocalized with CRF in interneurons throughout the hippocampus, many of which also contain SOM (Williams and Milner, 2011) suggesting that these cells regulate perforant path input to granule cells (Freund and Buzsáki, 1996). Although DOR/CRF interneurons occur with equal frequency in proestrus females and males, proestrus females have greater numbers of CRF terminals that lack DORs (Williams and Milner, 2011). Taken together, these results indicate that DORs are positioned to regulate CRF peptide release but their ability to exert such regulation may be compromised in females with elevated estrogen states. Following CIS, overall Crf gene expression in the DG/CA1 region is elevated in males but unchanged in females (Randesi et al., 2018). As sex-dependent changes in CRF receptor expression accompany this elevation in Crf (see below), CIS may differentially affect the balance of excitation and inhibition in females and males.
CRF receptor:
DORs can modulate CRF receptor (CRFR) signaling through cyclic AMP-phosphokinase A pathways (Williams et al., 2011b). By EM, DORs and CRFRs are colocalized in CA1 pyramidal cell dendrites (Williams et al., 2011b). In CA1, proestrus females compared to males have an increased number of DOR/CRFR pyramidal cell dendrites and an elevated plasma membrane associated CRFR in these dual labeled dendrites (Williams et al., 2011b). In CA3, diestrus females and males have similar subcellular distributions of CRFR in pyramidal cells (McAlinn et al., 2018), many of which contain DORs (McEwen and Milner, 2017), except for increased plasmalemmal CRFR1 in males. In the DG, CRFR1 is found in interneurons with a topographic pattern resembling those containing NPY/SOM; males had higher total and cytoplasmic levels of CRFR in hilar interneurons compared to DE females (McAlinn et al., 2018). Following CIS, near plasmalemmal, cytoplasmic, and total CRFRs in CA1 dendrites are elevated in males while DE females had reduced total CRFRs following CIS (McAlinn et al., 2018). Additionally, overall Crhr1 gene expression in the CA3 region is down-regulated in males but not females following CIS (Randesi et al., 2018). Concomitant with these changes, plasmalemmal associated DORs decrease in CA3 dendrites in males, but not females following CIS (Mazid et al., 2016). As DORs are thought to be neuroprotective (Hayashi et al., 2002; Charron et al., 2008; Feng et al., 2009), this redistribution of CRFR1 and DORs away from the plasmalemma in CA3 pyramidal cells in males could make them more susceptible to damage from CRF following CIS (Maecker et al., 1997) (Fig. 11).
Fig. 11. CIS further alters plasmalemmal associated corticotrophin releasing factor (CRF) receptor levels in rat hippocampal neurons in a manner that differentially alters CRF sensitivity in males and females.
Baseline levels of plasmalemmal CRFR are higher in CA1 pyramidal cells in females and in CA3 pyramidal cells and hilar interneuron in males (not shown). Following CIS, plasmalemmal associated CRFRs are elevated in CA1 pyramidal cells and reduced in CA3 pyramidal cells in males, suggesting greater CRF sensitivity in males than in females in stress environments. Results from McAlinn et al. 2018.
6.2. Plasticity and other related signaling genes.
In addition to changes in opioid and CRF gene expression, CIS alters the expression of other relevant genes in the hippocampus (Randesi et al., 2018). Following CIS, Avpr1a (another stress gene), Arc, Cdh2, Ntrk2 (plasticity genes), Akt1, and Arrb1 (kinase/signaling genes) were all down-regulated in the hippocampus of male but not female (estrus) rats (Randesi et al., 2018). The sex-dependent changes in gene expression following CIS may contribute to the attenuation of Oxy-CPP in males exposed to CIS (see section 8).
7. Sex differences in opioid system and related signaling pathways following Oxy CPP
Conditioned place preference (CPP) is a behavioral model used to measure the association between a rewarding stimulus and a contextual environment (Mucha et al., 1982; Prus et al., 2009; McKendrick and Graziane, 2020) (for a comprehensive review see Tzschentke, 1998; Tzschentke, 2007). As associative learning critically involves the hippocampus and is important for the transition from initial drug use to abuse/dependence (Koob and Volkow, 2010), this behavior model was used to further study the anatomical changes in the hippocampus due to reward-related effects of opioids (McKendrick and Graziane, 2020). In brief, CPP consists of three phases; habituation, conditioning and post-conditioning. In context to Pavlovian learning, the animal learns an to associate the unconditioned response of euphoria or anhedonia of a drug to a neutral stimulus (i.e., environmental cues). Following one or more conditioning sessions, the animal is then tested for preference or avoidance of the previously drug-paired environment without the drug. This allows for the quantifiable expression of the overall preference (CPP) or aversion (CPA) to the drug-induced conditioning stimulus. If an animal acquires CPP, then this can be interpreted as being mediated by the rewarding effects of a drug (McKendrick and Graziane, 2020). As our study focuses on associative learning, an important phase of opioid use disorder (Koob and Volkow, 2010; 2016), we selected an Oxy dose of 3 mg/kg (I.P.) because it has shown to induce 90–100% CPP in female rats (Olmstead and Burns, 2005) and in both sexes in our earlier studies (Ryan et al 2018; Randesi et al., 2019).
7.1. Sex differences in the rat hippocampal opioid system after Oxy-CPP.
In agreement with prior studies examining morphine-CPP in male rats (Billa et al., 2010), both male and female rats acquire Oxy-CPP (Ryan et al., 2018). However, females spend about twice as much time in the Oxy-paired chamber (Ryan et al., 2018). Following Oxy-CPP, LEnk-mossy fiber levels are elevated in CA3b of Oxy-males and sprouted in CA3a of Oxy-females (Ryan et al., 2018) (Fig. 12), suggesting that opioid sensitivity is enhanced albeit via different mechanisms. In particular, pools of LEnk available for release in mossy fibers would be increased in males whereas mechanisms for increase synaptic plasticity in CA3 pyramidal dendrites would be increases in females following Oxy CPP (Scharfman and MacLusky, 2014). Immuno-EM revealed that DORs redistributed to MF-CA3 synapses in Oxy-males (Ryan et al., 2018); a similar redistribution in females has been previously shown to be important for opioid-mediated LTP (Harte-Hargrove et al., 2015) suggesting a potential mechanism for Oxy CPP to enhance LTP processes in males. In the DG hilus of Oxy-females, plasmalemmal and total MORs increased in PARV dendrites and near-plasmalemmal DORs increased in GABAergic dendrites known to contain NPY (Ryan et al., 2018). These findings are consistent with prior studies showing that morphine-CPP increases total MORs in synaptic homogenates in male rats (Billa et al., 2010). The redistribution of MORs and DORs within interneurons in Oxy-females would potentially enhance disinhibition of granule cells via two different circuits. Specifically, elevations in plasmalemmal associated DORs on GABAergic dendrites known to contain NPY could promoting LTP in the lateral perforant pathway (Sperk et al., 2007); and elevations in plasmalemmal associated MORs in PARV dendrites would disinhibit granule cell soma (Drake et al., 2007).
Fig. 12. Redistribution of opioid receptors following oxycodone CPP may facilitate opioid associated learning particularly in females.
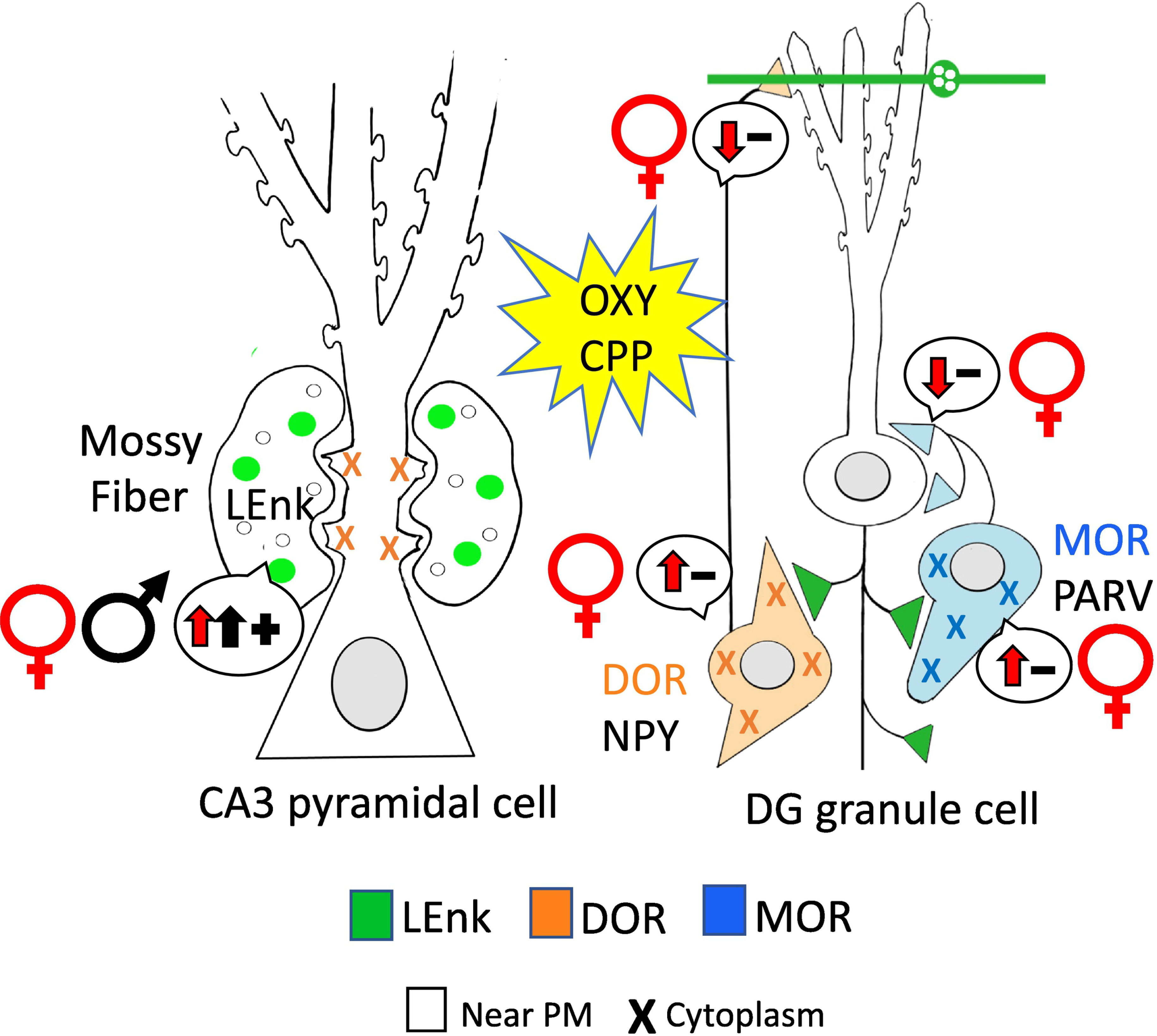
Following Oxy-CPP, DORs in mossy fiber-CA3 synapses increase males to levels of that seen in proestrus (elevated estrogen levels) females suggesting both sexes would have mechanisms in place that would increased in opioid-mediated long-term potentiation. In the DG granule cells MORs and DORs redistribute in females in a manner that would increased disinhibition and subsequent activation of granule cells. (−): indicates disinhibition; (+): indicates activation. Arrows indicate direction of change. Results from Ryan et al 2018.
Following Oxy-CPP, pMOR levels in CA1 and CA3a,b are elevated in females whereas pDOR levels in CA2/3a are higher in males (Bellamy et al., 2019). As phosphorylation is important for opioid receptor internationalization and trafficking (Law et al., 2000; Deng et al., 2001; Doll et al., 2011), these findings suggest that Oxy CPP differentially activates opioid receptors in females and males in select hippocampal circuits. Importantly, our recent studies have shown that Oxy injections not paired with CPP have little effect on the distribution of DORs and MORs as well as the phosphorylation of MORs and DORs in hippocampal neurons in either female or male rats (Ashirova et al., 2021). Together, these results indicate that Oxy-CPP induces sex-dependent redistributions of opioid receptors in hippocampal circuits in a manner facilitating opioid-associative learning processes (Fig. 8).
7.2. Other systems that change with Oxy CPP.
Sex differences in the opioid system following Oxy-CPP are paralleled by changes in plasticity, stress and kinase markers in hippocampal neurons (Randesi et al., 2019) (Fig. 13). Oxy CPP females exhibit: a) increases in activity regulated cytoskeletal-associated protein (ARC)-ir in CA3 pyramidal cells; b) decreases in Npy gene expression in the medial hippocampus but higher numbers of NPY-labeled hilar interneurons compared to males; c) increases in Crhr2 expression in CA2/3; d) increases in AKT serine/threonine kinase 1 (Akt1) expression in medial hippocampus; and e) decreases in phosphorylated mitogen activated protein kinase (pMAPK)-ir in CA1 and DG. However, Oxy CPP males had: a) increases in brain derived-neurotrophic factor (Bdnf) expression, which is known to be produced in granule cells; b) elevated Mapk1 expression and pMAPK-ir in the DG hilus which harbors newly generated granule cells; and c) increases in CRF receptor-ir in CA3 pyramidal cell soma. As previously discussed (Randesi et al., 2019), these findings suggest additional mechanisms by which Oxy CPP could interact with the hippocampal opioid system to differentially enhance plasticity and learning processes in females and males.
Fig. 13: Following oxycodone CPP, hippocampal plasticity, stress and associated kinase mRNA and protein levels have sex-specific alterations in rats.
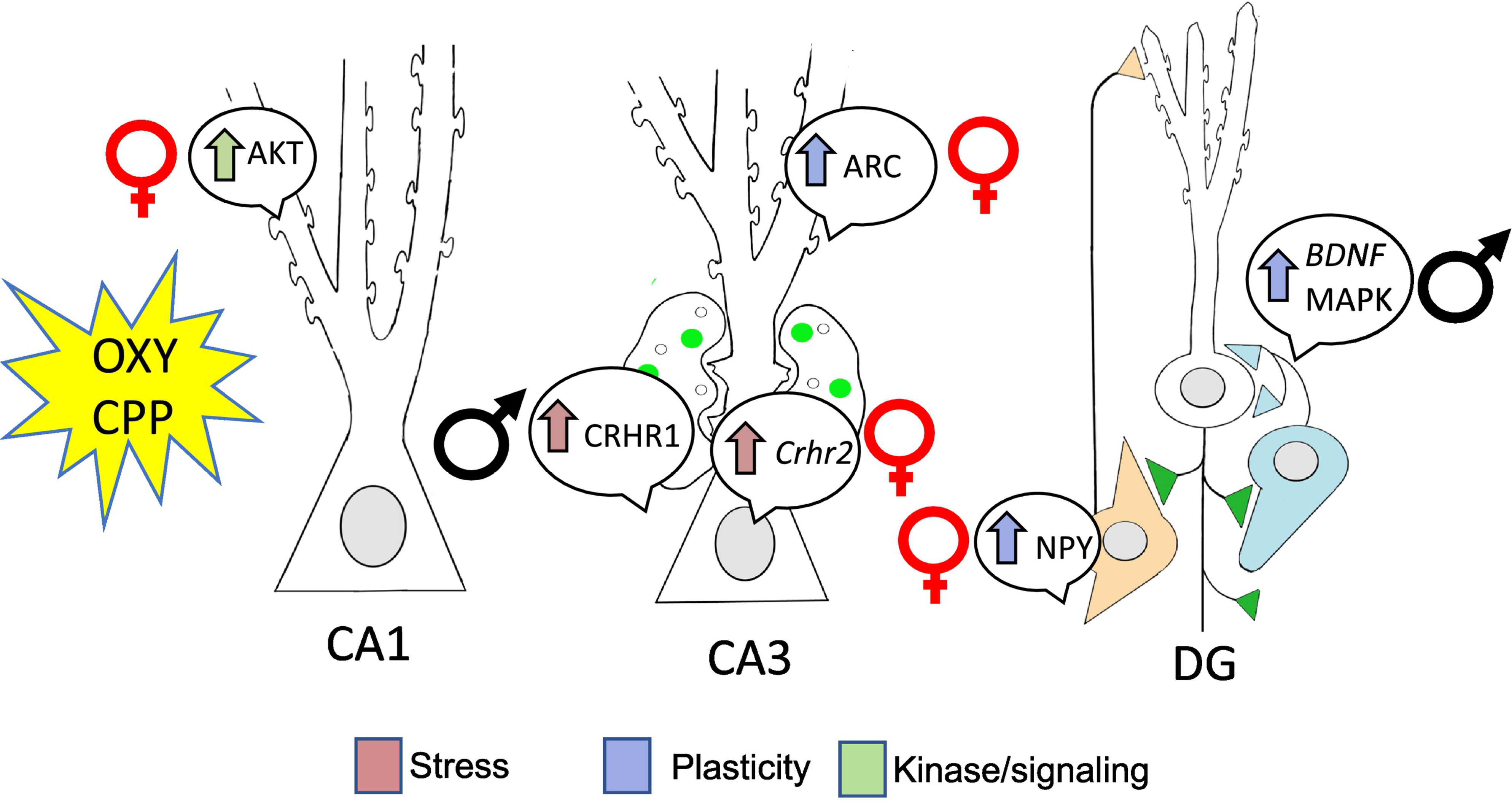
Oxy-CPP resulted in alterations in plasticity, stress and associated kinase markers in both males and females and are congruent with the sex-specific changes seen in the opioid system. The changes observed may suggest why CIS males did not acquire OXY-CPP. Arrows indicated direction of change. Placement mRNA and proteins bubbles indicate the regional changes. Results from Randesi and Contoreggi et al 2019.
8. Sex differences in hippocampal opioid system following CIS and Oxy CPP
8.1. Sex differences in the LEnk and opioid receptor trafficking following CIS and Oxy CPP.
As discussed in sections 5 and 6, CIS differentially influences the opioid system as well plasticity and stress markers in hippocampal neurons in female and male rats in a manner that would “prime” females for opioid mediated learning processes but negatively impact learning processes in males. In particular, our studies indicate that CIS in female rats results in a redistribution of MORs and DORs within hippocampal pyramidal cell and GABAergic interneurons in a manner that is similar to that seen in elevated estrogen states in unstressed rats.
Following CIS, females, but not males, acquire Oxy-CPP (Reich et al., 2019). The distribution of opioid receptors in hippocampal circuits of CIS Oxy females are positioned for enhancing excitability and plasticity processes. Like CIS Sal-females, CIS Oxy-females have elevated DOR densities in MF-CA3 synapses know to promote opioid mediated LTP (Harte-Hargrove et al., 2015). Not only do CIS Oxy-females have more DOR-labeled hilar interneurons than CIS Sal-females but CIS Sal- and Oxy-females compared to both groups of CIS males had elevated total levels of DORs and MORs in GABAergic interneuron dendrites. These findings suggest an increased capacity for greater synthesis and/or storage of these receptors in circuits important for opioid-mediated disinhibition (Drake et al., 2007). In contrast to CIS Oxy-females, low levels of DORs in MF-CA3 synapses and hilar GABAergic interneurons in CIS Oxy-males which may contribute to their inability to acquire Oxy CPP (Fig. 14). This idea is supported by previous findings that DOR antagonist pretreatment blocks acquisition of morphine-CPP in male rats and correlates with increased levels of DOR dimers in hippocampal post-synaptic densities (Billa et al., 2010). However, CIS Oxy-males compared to CIS Sal-males have more plasmalemmal MORs on large PARV-containing interneuron dendrites suggesting a limited ability for increased granule cell disinhibition Drake et al., 2007).
Fig. 14: Opioid receptors redistribute following CIS in a manner that would promote oxycodone associative learning in female rats but not males.
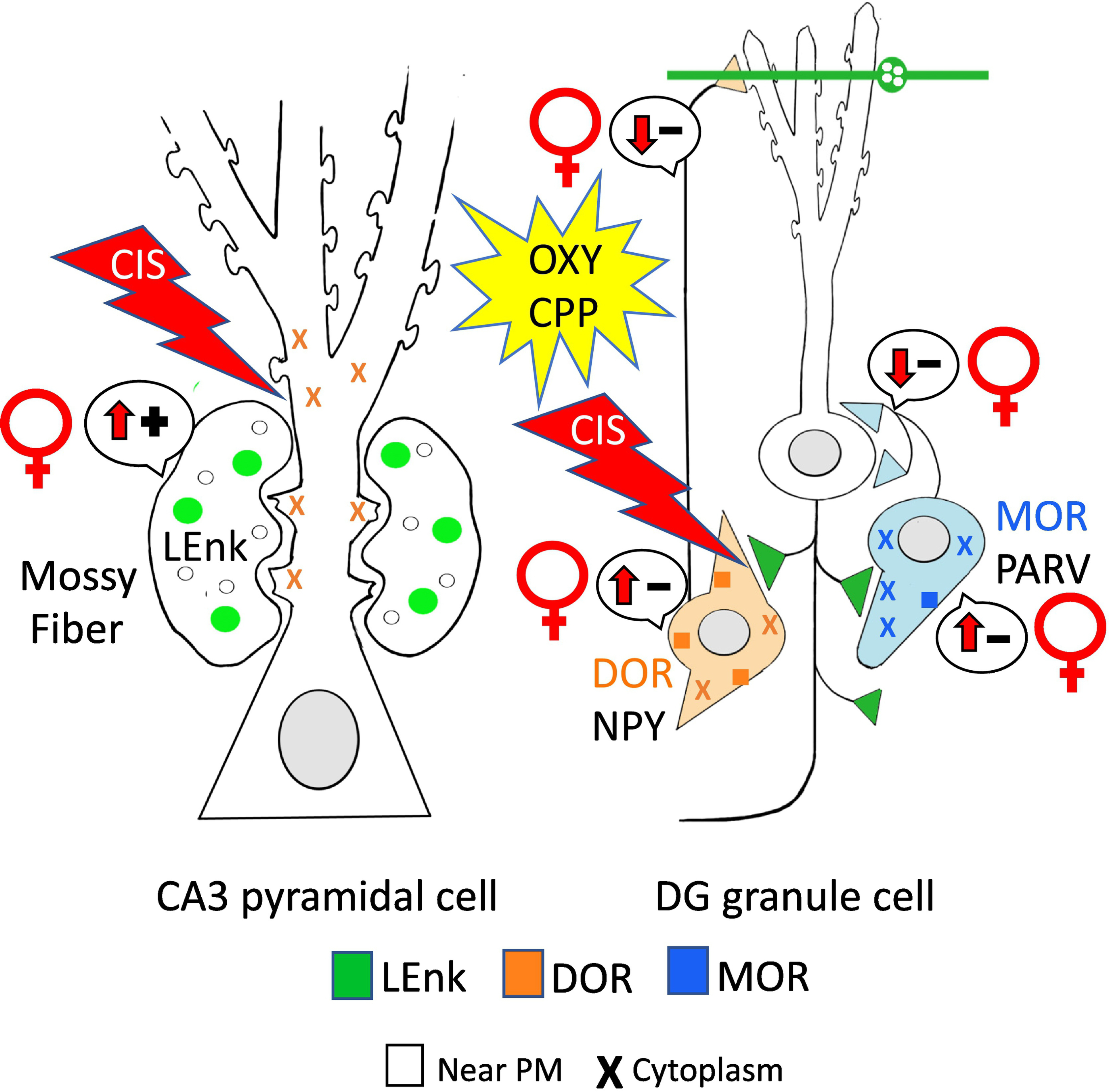
In CA3 pyramidal cells, total and cytoplasmic DORs were elevated in CIS Sal females. Following Oxy CPP, DORs had a similar distribution in CIS females. Moreover, the number of DORs mossy fiber CA3 synapses in CIS Sal females was similar to that observed in females in elevated estrogen states suggesting that mechanisms that promote DOR-mediated LTP are in place following CIS. In DG, CIS Oxy-females had greater numbers of MORs and DORs in interneurons suggesting mechanisms for enhanced disinhibition are still in place. In contrast, CIS results in few distribution changes in Sal- and Oxy males, which may contribute to the reduced ability of males to acquire Oxy-CPP. (−): indicates disinhibition; (+): indicates activation. Arrows indicate direction of change. Results from Reich et al 2019.
8.2. Sex differences in phosphorylated MORs and DORs in CIS rats following Oxy CPP.
As discussed in section 5, CIS alone has little effect on pMOR and pDOR levels in the hippocampus in both the sexes. In CIS females, Oxy CPP increases pDOR levels in pyramidal cells and their dendrites in CA2/3a (Bellamy et al., 2019). However, in CIS males, which did not acquire Oxy CPP, there were no changes in either pMOR or pDOR levels in the hippocampus (Bellamy et al., 2019). Consistent with the results from naïve rats, these findings indicate that only rats that acquire Oxy-CPP have activated opioid receptors in the hippocampus.
9. Sex differences in the rat hippocampal opioid system after acute THC.
Several lines of evidence indicate interactions between the opioid and cannabinoid systems. Anatomically, the opioid and cannabinoid systems extensively overlap in the hippocampus. Cannabinoid receptors type 1 (CB1) are densely expressed on GABAergic interneuron terminals (Nyiri et al., 2005) and many CB1 terminals arise from CCK-containing interneurons known to synapse on PARV-containing interneurons and affect their network properties (Marsicano and Lutz, 1999; Tsou et al., 1999; Karson et al., 2009). CB1s also are in terminals that synapse on DOR-containing pyramidal cells and regulate cannabinoid-dependent suppression of excitatory transmission (Kawamura et al., 2006). In female CB1 knockout mice, mossy fiber LEnk levels and the number of DOR containing hilar NPY neurons are reduced (Rogers et al., 2016). Type 2 cannabinoid receptors (CB2) are highly expressed in pyramidal cell bodies and dendrites (Brusco et al., 2008b; Brusco et al., 2008a). Behaviorally, pre-exposure to CB1 agonists strengthens morphine CPP in adult male rats (Manzanedo et al., 2004) and both CB1 agonist and Δ9Tetrahydrocannabinoid (THC), the primary psychoactive constituent of cannabis (Mechoulam and Parker, 2013), can increase heroin self-administration (Solinas et al., 2005).
Sex-dependent alteration in the hippocampal opioid system occur following a single dose of THC (Windisch et al., 2021). Acute THC altered the opioid system in proestrus/estrus females such that it resembled vehicle-injected diestrus females and males. Namely, mossy fiber LEnk levels in CA2/3a were decreased, pDOR levels in CA2/3a pyramidal cells were increased, pMOR levels were increased in CA3b laminae except SLu, and both cytoplasmic and total DORs in large CA3 pyramidal cell dendrites were decreased with a concomitate increase in near plasmalemma DORs in small CA3 dendrites. Similar to CIS, acute THC eliminated the estrogen effects on mossy fiber LEnk levels. This together with the lack of change in DOR-labeled SLu spines, suggests that females may have a reduced capacity for opioid-mediated LTP in MF-CA3 synapses (Harte-Hargrove et al., 2015) following acute exposure to cannabinoid agonists such as THC (Fig 15).
Fig. 15: Redistribution of opioid receptors following acute THC may differentially affect opioid-associated plasticity in female and male rats.
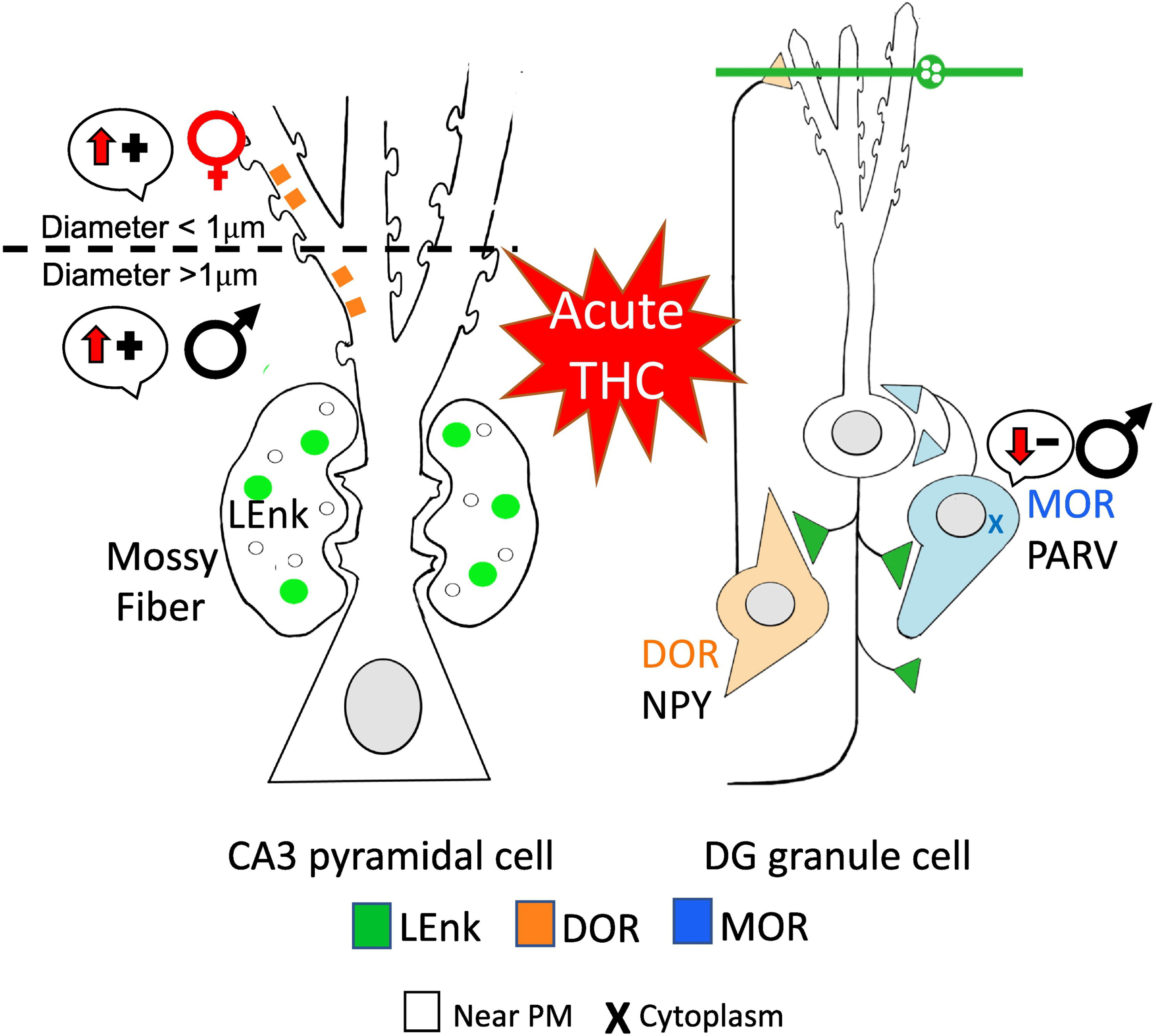
Following acute THC, DORs are trafficked to different regions of CA3 dendrites in females and males suggesting sex-specific mechanisms for increased opioid-mediated synaptic plasticity. In GABAergic PARV interneurons, MORs are internalized in males which would leading to decreased disinhibition, thus suggesting that acute THC would reduce opioid-mediated learning processes in males. (−): indicates disinhibition; (+): indicates activation. Arrows indicate direction of change. Results from Windisch and Mazid et al 2021
In males, acute THC had no effect on the levels of LEnk, pDOR, or pMOR within the CA3 region but did result in the internalization of MORs in PARV-containing hilar interneuron dendrites which would decrease disinhibition of granule cells. Further, acute THC in males resulted in decreased DORs in MF-CA3 synapses and increased near plasmalemma DORs in large CA3 pyramidal cell dendrites (Fig 15). These proximal dendrites are postsynaptic to “autoassociative” recurrent collaterals of CA3 thought to be important for key features of episodic memory (Scharfman and MacLusky, 2017). Therefore, increased DORs in these proximal dendrites may promote these processes following acute THC exposure. Overall, acute THC resulted in sex-specific changes within the rat hippocampal opioid system which could differentially promote synaptic plasticity and/or opioid-associated learning processes in both females and males.
10. Sex Differences in the Effects of Opioids Across Development.
Few studies have examined developmental differences in hippocampal changes induced by drugs of abuse. For opioids, learning and memory deficits as well as reduced hippocampal BDNF levels have been observed following prenatal morphine exposure in rats (Ahmadalipour et al., 2015). Further, females appear more sensitive to the deleterious effects of prenatal opioid exposure as memory deficits and changes in hippocampal gene expression occurred following a shorter duration of prenatal morphine exposure than that required for similar effects to occur in their male counterparts (Nasiraei-Moghadam et al., 2013). Interestingly, the prenatal morphine induced memory deficits in males, but not females, were reversed during their subsequent postnatal development. However, no studies to date have examined morphological changes in the hippocampus induced by prenatal opioid exposure.
Adolescence is a critical period of development characterized by robust behavioral, morphological, hormonal, and neurochemical changes including in brain regions implicated in drug reward and reinforcement [see review see (Windisch and Kreek, 2020)]. Adolescent opioid exposure has been shown to result in significant and prolonged effects including increased opioid reward, reduced analgesic efficacy, and exacerbated somatic withdrawal severity during subsequent opioid use in adulthood (Schwarz and Bilbo, 2013; Zhang et al., 2016; Salmanzadeh et al., 2017; Ghasemi et al., 2019). This suggests that adolescent opioid exposure results in significant and long-lasting changes in the brain. Additionally, adolescent opioid exposure has profound effects on sexual maturation in both sexes and circulating androgens in males (Cicero et al., 1989; Cicero et al., 1991; Yilmaz et al., 1999; Byrnes, 2005; Hofford et al., 2011). Adolescent oxycodone self-administration in mice results in significant alterations in the expression synaptic plasticity genes in the hippocampus (Zhang et al., 2015); however, similar to prenatal exposure, no studies to date have examined morphological changes in the hippocampus induced by opioids during adolescence. The initial and long-term consequences of exposure to opioids on the hippocampus across the lifespan remains a critical unanswered question.
Summary and conclusion.
As reviewed, our studies have revealed significant sex differences in the hippocampal opioid system especially after chronic stress which have important implications for Oxy associated learning processes. In both sexes, Oxy-CPP redistributes opioid receptors in hippocampal circuits in a manner facilitating opioid-associative learning processes. However, the number of circuits is greater in females. Moreover, CIS primes the hippocampal opioid system for Oxy-CPP in female but not male rats for opioid associative learning processes. These sex-specific changes in opioid-receptor trafficking are accompanied by a down-regulation of opioid, stress, plasticity, and kinase/signaling genes in the hippocampus of CIS males. Such changes likely contribute to the attenuation of Oxy-CPP in males following CIS. Therefore, these findings suggest why these changes seen in male rats following CIS may contribute to their diminished capacity to acquire Oxy-CPP.
Further, the changes in the female opioid system following CIS not only primes them for Oxy-CPP but also makes them more sensitive to opioids in general following chronic stress. On a broader scale, these sex-differences in the hippocampal opioid system may contribute the greater susceptibility of females to opioid addiction and relapse, especially after chronic stress (Becker et al., 2017). In particular, following chronic stress, opioid peptides and receptors are positioned within at least three hippocampal circuits in females, but not males, in a manner that would facilitate opioid associated learning processes.
As hormonal levels may exert a functional effect on the hippocampal opioid system, this may be advantageous in providing a sex-specific therapy for females. For instance, monitoring a menstrual cycle phase may be useful in disrupting the association of a drug to an environmental cue. Indeed, others (American Addiction Centers, 2020) have suggested that hormone levels may be important to consider for reducing cue induced craving in women.
Opioid abuse has drastically risen over the past two decades particularly in the rate of drug overuse among women (Centers for Disease Control, 2015). In both sexes, hippocampal neural circuits involved in associative memory formation and encoding of motivational incentives are critically involved in transition from drug use to drug abuse (Koob and Volkow, 2010). Further, there is a growing appreciation for the integral role stress plays in the transition from drug use/abuse to dependence (Koob and Schulkin, 2019; Ruisoto and Contador, 2019). The hippocampus is highly sensitivity to both acute and chronic stress (Kim and Diamond, 2002; McEwen, 2007). As previously covered, there are clear basal differences in the hippocampal opioid system between sexes that are differentially altered by subsequent stress or drug exposure. Additional research is needed to clarify the impact of age/development on the hippocampal opioid system and the subsequent impact drug dependence risk. Overall, the findings highlighted in this review forms a vital part of the bigger more pressing issue of drug abuse and addiction.
HIGHLIGHTS.
High estrogens in females increase hippocampal opioid system to promote excitation
Opioid expression and distribution favors enhanced plasticity in females
Acute stress enhances opioid-mediated plasticity in males but not females
Chronic stress reduces opioid-mediated plasticity in males but primed in females
Sex-dependent redistribution during opioid place conditioning facilitates learning
Acknowledgements:
Supported by the National Institutes of Health grant DA08259
Abbreviations
- AIS
Acute immobilization stress
- Akt1
AKT serine/threonine kinase 1
- ARC
activity regulated cytoskeletal-associated protein
- Bdnf
brain derived-neurotrophic factor
- CIS
Chronic immobilization stress
- CPP
Conditioned Place Preference
- CRF
corticotrophin releasing factor
- Crhr2
corticotropin releasing factor receptor 2
- DCV
dense-core vesicle
- DG
dentate gyrus
- DOR
Delta Opioid Receptor
- DYN
Dynorphin
- E2
Estradiol
- EM
electron microscopy
- EM-1
Endomorphin 1
- EM-2
Endomorphin 2
- ER
Estrogen receptor
- FSH
follicle stimulating hormone
- Ir
immunoreactivity
- LEnk
Leu-enkephalin
- LPP
lateral perforant path
- LTP
long-term potentiation
- LHRH
luteinizing hormone
- MAPK
mitogen activated protein kinase
- MF
Mossy Fiber
- MOR
Mu-opioid receptor
- Npy
neuropeptide Y
- OVX
ovariectomized
- OXY
Oxycodone
- PARV
Parvalbumin
- PM
Plasma Membrane
- PR
Progesterone receptor
- SIG
silver-intensified gold particles
- SOM
somatostatin
- TAT
temporal-ammonic tract
- US
Unstressed
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Pan Y, Drake CT, Pasternak GW (2000) Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100:141–153. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F (1986) A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand 128:201–207. [DOI] [PubMed] [Google Scholar]
- Ahmadalipour A, Sadeghzadeh J, Vafaei AA, Bandegi AR, Mohammadkhani R, Rashidy-Pour A (2015) Effects of environmental enrichment on behavioral deficits and alterations in hippocampal BDNF induced by prenatal exposure to morphine in juvenile rats. Neuroscience 305:372–383. [DOI] [PubMed] [Google Scholar]
- American Addiction Centers (2020). “How Hormones Affect Addiction.” Retrieved October 18, 2021, from https://rehabs.com/pro-talk/how-hormones-affect-addiction/.
- Ashirova E, Contoreggi NH, Johnson MA, Al-Khayat FJ, Calcano GA, Rubin BR, O’Cinneide EM, Zhang Y, Zhou Y, Gregoire L, McEwen BS, Kreek MJ, Milner TA (2021) Oxycodone injections not paired with conditioned place preference have little effect on the hippocampal opioid system in female and male rats. Synapse 75:e22182. [DOI] [PubMed] [Google Scholar]
- Auchus AP, Pickel VM (1992) Quantitative light microscopic demonstration of increased pallidal and striatal met5-enkephalin-like immunoreactivity in rats following chronic treatment with haloperidol but not with clozapine: implications for the pathogenesis of neuroleptic-induced movement disorders. Exp Neurol 117:17–27. [DOI] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017) Sex differences, gender and addiction. Journal of neuroscience research 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy JR, Rubin BR, Zverovich A, Zhou Y, Contoreggi NH, Gray JD, McEwen BS, Kreek MJ, Milner TA (2019) Sex and chronic stress differentially alter phosphorylated mu and delta opioid receptor levels in the rat hippocampus following oxycodone conditioned place preference. Neuroscience letters:134514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Xia Y, Moron JA (2010) Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: study of mu-opioid and delta-opioid receptor expression at the synapse. Eur J Neurosci 32:625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Pickel VM, Beaudet A (1998) Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. The Journal of neuroscience : the official journal of the Society for Neuroscience 18:8473–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES (2008a) Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci 1139:450–457. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, Onaivi ES (2008b) Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse 62:944–949. [DOI] [PubMed] [Google Scholar]
- Burstein SR, Williams TJ, Lane DA, Knudsen MG, Pickel VM, McEwen BS, Waters EM, Milner TA (2013) The influences of reproductive status and acute stress on the levels of phosphorylated delta opioid receptor immunoreactivity in rat hippocampus. Brain research 1518:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM (2005) Chronic morphine exposure during puberty decreases postpartum prolactin secretion in adult female rats. Pharmacol Biochem Behav 80:445–451. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (2015). “Drug overdose deaths hit record numbers in 2014.” Retrieved July 15, 2021, from https://www.cdc.gov/media/releases/2015/p1218-drug-overdose.html.
- Chandy J, Pierce JP, Milner TA (1995) Rat hippocampal mossy fibers contain cholecystokinin-like immunoreactivity. Anat Rec 243:519–523. [DOI] [PubMed] [Google Scholar]
- Charron C, Messier C, Plamondon H (2008) Neuroprotection and functional recovery conferred by administration of kappa- and delta1-opioid agonists in a rat model of global ischemia. Physiology & Behavior 93:502–511. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Miles R (2015) The CA3 region of the hippocampus: how is it? What is it for? How does it do it? Front Cell Neurosci 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Adams ML, Giordano A, Miller BT, O’Connor L, Nock B (1991) Influence of morphine exposure during adolescence on the sexual maturation of male rats and the development of their offspring. the journal of pharmacology and experimental therapeutics 256:1086–1093. [PubMed] [Google Scholar]
- Cicero TJ, O’Connor L, Nock B, Adams ML, Miller BT, Bell RD, Meyer ER (1989) Age-related differences in the sensitivity to opiate-induced perturbations in reproductive endocrinology in the developing and adult male rat. J Pharmacol Exp Ther 248:256–261. [PubMed] [Google Scholar]
- Commons KG, Milner TA (1995) Ultrastructural heterogeneity of enkephalin-containing terminals in the rat hippocampal formation. The Journal of comparative neurology 358:324–342. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS (1999) Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem 72:39–46. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A (2003) Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiology of Learning and Memory 79:32–40. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E (2005) Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology 30:67–79. [DOI] [PubMed] [Google Scholar]
- Dang VC, Christie MJ (2012) Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165:1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HB, Yu Y, Wang H, Guang W, Wang JB (2001) Agonist-induced mu opioid receptor phosphorylation and functional desensitization in rat thalamus. Brain research 898:204–214. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Rodriguez SB, Lieberman DN, Martinez JL Jr. (1992) Mu opioid receptors are associated with the induction of hippocampal mossy fiber long-term potentiation. J Pharmacol Exp Ther 263:725–733. [PubMed] [Google Scholar]
- Do VH, Martinez CO, Martinez JL Jr., Derrick BE (2002) Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J Neurophysiol 87:669–678. [DOI] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Poll F, Koch T, Hollt V, Schulz S (2011) Agonist-selective patterns of micro-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. British journal of pharmacology 164:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA (1997) Kappa opioid receptor-like immunoreactivity is present in substance P-containing subcortical afferents in guinea pig dentate gyrus. Hippocampus 7:36–47. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA (2007) Opioid systems in the dentate gyrus. Progress in brain research 163:245–263. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chang PC, Harris JA, Milner TA (2002) Neurons with mu opioid receptors interact indirectly with enkephalin-containing neurons in the rat dentate gyrus. Experimental neurology 176:254–261. [DOI] [PubMed] [Google Scholar]
- Drake CT, Patterson TA, Simmons ML, Chavkin C, Milner TA (1996) Kappa opioid receptor-like immunoreactivity in guinea pig brain: ultrastructural localization in presynaptic terminals in hippocampal formation. J Comp Neurol 370:377–395. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chao D, He X, Yang Y, Kang X, HL L, Xia Y (2009) A novel insight into neuroprotection against hypoxic/ischemic stress. Sheng li xue bao : [Acta physiologica Sinica] 61:585–592. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Monreal M, Brown TC, Royo M, Esteban JA (2012) The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:13200–13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS (1997) Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81:689–697. [DOI] [PubMed] [Google Scholar]
- Gall C, Brecha N, Karten HJ, Chang KJ (1981) Localization of enkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J Comp Neurol 198:335–350. [DOI] [PubMed] [Google Scholar]
- Gall C, Lauterborn J, Isackson P, White J (1990) Seizures, neuropeptide regulation, and mRNA expression in the hippocampus. Prog Brain Res 83:371–390. [DOI] [PubMed] [Google Scholar]
- Ghasemi E, Pachenari N, Semnanian S, Azizi H (2019) Adolescent morphine exposure increases nociceptive behaviors in rat model of formalin test. Dev Psychobiol 61:254–260. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL (2007) The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97. [DOI] [PubMed] [Google Scholar]
- Gonzales KL, Chapleau JD, Pierce JP, Kelter DT, Williams TJ, Torres-Reveron A, McEwen BS, Waters EM, Milner TA (2011) The influences of reproductive status and acute stress on the levels of phosphorylated mu opioid receptor immunoreactivity in rat hippocampus. Frontiers in endocrinology 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL (2003) Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasy K, Rácz B, Maderspach K (2000) Kappa opioid receptors are expressed by interneurons in the CA1 area of the rat hippocampus: a correlated light and electron microscopic immunocytochemical study. J Chem Neuroanat 19:233–241. [DOI] [PubMed] [Google Scholar]
- Han JS (2003) Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends in neurosciences 26:17–22. [DOI] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS (2007) Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci 27:2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Varga-Wesson A, Duffy AM, Milner TA, Scharfman HE (2015) Opioid receptor-dependent sex differences in synaptic plasticity in the hippocampal mossy fiber pathway of the adult rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 35:1723–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Tsao LI, Su TP (2002) Antiapoptotic and cytotoxic properties of delta opioid peptide [D-Ala(2),D-Leu(5)]enkephalin in PC12 cells. Synapse (New York, NY) 43:86–94. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends in Neurosciences 20:78–84. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Wellman PJ, Eitan S (2011) Social influences on plasma testosterone levels in morphine withdrawn adolescent mice and their drug-naive cage-mates. Psychoneuroendocrinology 36:728–736. [DOI] [PubMed] [Google Scholar]
- Hu W, Zhang M, Czéh B, Flügge G, Zhang W (2010) Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 35:1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Contoreggi NH, Kogan JF, Bryson M, Rubin BR, Gray JD, Kreek MJ, McEwen BS, Milner TA (2021) Chronic stress differentially alters mRNA expression of opioid peptides and receptors in the dorsal hippocampus of female and male rats. J Comp Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan TJ, Skyers PR, Tabori NE, Drake CT, Milner TA (2004) Ultrastructural evidence for mu-opioid modulation of cholinergic pathways in rat dentate gyrus. Brain research 1019:28–38. [DOI] [PubMed] [Google Scholar]
- Karson MA, Tang A, Milner TA, Alger BE (2009) Synaptic cross-talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci 29:4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M (2006) The CB1 cannabinoid receptor Is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26:2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK (2010) Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus 20:550–557. [DOI] [PubMed] [Google Scholar]
- Kibaly C, Alderete JA, Liu SH, Nasef HS, Law PY, Evans CJ, Cahill CM (2021) Oxycodone in the Opioid Epidemic: High ‘Liking’, ‘Wanting’, and Abuse Liability. Cell Mol Neurobiol 41:899–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM (2002) The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 3:453–462. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Schulkin J (2019) Addiction and stress: An allostatic view. Neurosci Biobehav Rev 106:245–262. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA (2016) The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology 233:3201–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Kouhen OM, Solberg J, Wang W, Erickson LJ, Loh HH (2000) Deltorphin II-induced rapid desensitization of delta-opioid receptor requires both phosphorylation and internalization of the receptor. The Journal of Biological Chemistry 275:32057–32065. [DOI] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS (2009) Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci 29:1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Frotscher M (1989) Organization of the septal region in the rat brain: cholinergic-GABAergic interconnections and the termination of hippocampo-septal fibers. The Journal of comparative neurology 289:304–314. [DOI] [PubMed] [Google Scholar]
- Liang J, Ping XJ, Li YJ, Ma YY, Wu LZ, Han JS, Cui CL (2010) Morphine-induced conditioned place preference in rats is inhibited by electroacupuncture at 2 Hz: role of enkephalin in the nucleus accumbens. Neuropharmacology 58:233–240. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ (2007) Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 19:743–751. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G (2011) Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science (New York, NY) 333:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R (1997) Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain research 744:166–170. [DOI] [PubMed] [Google Scholar]
- Manzanedo C, Aguilar MA, Rodriguez-Arias M, Navarro M, Minarro J (2004) Cannabinoid agonist-induced sensitisation to morphine place preference in mice. Neuroreport 15:1373–1377. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11:4213–4225. [DOI] [PubMed] [Google Scholar]
- Mazid S, Hall BS, Odell SC, Stafford K, Dyer AD, Van Kempen TA, Selegean J, McEwen BS, Waters EM, Milner TA (2016) Sex differences in subcellular distribution of delta opioid receptors in the rat hippocampus in response to acute and chronic stress. Neurobiology of Stress 5:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlinn HR, Reich B, Contoreggi NH, Kamakura RP, Dyer AG, McEwen BS, Waters EM, Milner TA (2018) Sex differences in the subcellular distribution of corticotropin-releasing factor receptor 1 in the rat hippocampus following chronic immobilization stress. Neuroscience 383:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1999) Stress and hippocampal plasticity. Annu Rev Neurosci 22:105–122. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA (2007) Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev 55:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Milner TA (2017) Understanding the broad influence of sex hormones and sex differences in the brain. Journal of neuroscience research 95:24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Akil H (2020) Revisiting the Stress Concept: Implications for Affective Disorders. J Neurosci 40:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray JD, Nasca C (2015) 60 YEARS OF NEUROENDOCRINOLOGY: Redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol 226:T67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick G, Graziane NM (2020) Drug-Induced Conditioned Place Preference and Its Practical Use in Substance Use Disorder Research. Front Behav Neurosci 14:582147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C (2003) Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:5674–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp:e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL Jr. (2004) Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Bacon CE (1989) Ultrastructural localization of somatostatin-like immunoreactivity in the rat dentate gyrus. The Journal of comparative neurology 290:544–560. [DOI] [PubMed] [Google Scholar]
- Milner TA, Veznedaroglu E (1992) Ultrastructural localization of neuropeptide Y-like immunoreactivity in the rat hippocampal formation. Hippocampus 2:107–125. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE (2001) Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol 429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE (2005) Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol 491:81–95. [DOI] [PubMed] [Google Scholar]
- Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, Williams TJ, Schierberl KC, Torres-Reveron A, Gonzales KL, McEwen BS, Waters EM (2013) Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse (New York, NY) 67:757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP (2011) Degenerating processes identified by electron microscopic immunocytochemical methods. Neurodegeneration, Methods and Protocols:23–59. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA (2010) Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol 518:2729–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ, Johnston HM (1995) A role for hippocampal opioids in long-term functional plasticity. Trends Neurosci 18:350–355. [DOI] [PubMed] [Google Scholar]
- Moulédous L (2019) The Nociceptin/Orphanin FQ System and the Regulation of Memory. Handb Exp Pharmacol 254:259–278. [DOI] [PubMed] [Google Scholar]
- Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P (1982) Drug reinforcemnt studied by the use of place conditioning in rat. Brain Res. 243(1):91–105. [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S, Sherafat MA, Safari MS, Moradi F, Ahmadiani A, Dargahi L (2013) Reversal of prenatal morphine exposure-induced memory deficit in male but not female rats. J Mol Neurosci 50:58–69. [DOI] [PubMed] [Google Scholar]
- Newell AJ, Lalitsasivimol D, Willing J, Gonzales K, Waters EM, Milner TA, McEwen BS, Wagner CK (2018) Progesterone receptor expression in cajal-retzius cells of the developing rat dentate gyrus: Potential role in hippocampus-dependent memory. J Comp Neurol 526:2285–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF (2005) CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience 136:811–822. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Burns LH (2005) Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology (Berl) 181:576–581. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX (2013) Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65:1257–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS (2003) Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci 17:1159–1172. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA (1999) Morphometry of a peptidergic transmitter system: Dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus 9:255–276. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kelter DT, McEwen BS, Waters EM, Milner TA (2014) Hippocampal mossy fiber leu-enkephalin immunoreactivity in female rats is significantly altered following both acute and chronic stress. J Chem Neuroanat 55:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Kievits J, Graustein B, Speth RC, Iadecola C, Milner TA (2009) Sex differences in the subcellular distribution of AT(1) receptors and nadph oxidase subunits in the dendrites of C1 neurons in the rat rostral ventrolateral medulla. Neuroscience 163:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce TL, Wessendorf MW (2000) Immunocytochemical mapping of endomorphin-2-immunoreactivity in rat brain. J Chem Neuroanat 18:181–207. [DOI] [PubMed] [Google Scholar]
- Piguet P, North RA (1993) Opioid actions at mu and delta receptors in the rat dentate gyrus in vitro. J Pharmacol Exp Ther 266:1139–1149. [PubMed] [Google Scholar]
- Piva F, Limonta P, Dondi D, Pimpinelli F, Martini L, Maggi R (1995) Effects of steroids on the brain opioid system. J Steroid Biochem Mol Biol 53:343–348. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL (2009) In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One 4:e5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosecrans JA (2009) Conditioned place preference. In: Methods of behavioral analysis in neuroscience, 2nd ed., pp 59–76. Boca Raton, FL, US: CRC Press/Routledge/Taylor & Francis Group. [PubMed] [Google Scholar]
- Quiñones-Jenab V, Jenab S, Ogawa S, Inturrisi C, Pfaff DW (1997) Estrogen regulation of mu-opioid receptor mRNA in the forebrain of female rats. Brain Res Mol Brain Res 47:134–138. [DOI] [PubMed] [Google Scholar]
- Rácz B, Halasy K (2002) Kappa opioid receptor is expressed by somatostatin- and neuropeptide Y-containing interneurons in the rat hippocampus. Brain Res 931:50–55. [DOI] [PubMed] [Google Scholar]
- Randesi M, Zhou Y, Mazid S, Odell SC, Gray JD, Correa da Rosa J, McEwen BS, Milner TA, Kreek MJ (2018) Sex differences after chronic stress in the expression of opioid- and neuroplasticity-related genes in the rat hippocampus. Neurobiology of Stress 8:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randesi M, Contoreggi NH, Zhou Y, Reich B, Bellamy JR, Yu F, Gray JD, McEwen BS, Milner TA, Kreek MJ (2019) Sex Differences in Neuroplasticity- and Stress-Related Gene Expression and Protein Levels in the Rat Hippocampus Following Oxycodone Conditioned Place Preference. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich B, Zhou Y, Goldstein E, Srivats SS, Contoreggi NH, Kogan JF, McEwen BS, Kreek MJ, Milner TA, Gray JD (2019) Chronic immobilization stress primes the hippocampal opioid system for oxycodone-associated learning in female but not male rats. Synapse (New York, NY):e22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505–2511. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Dasilva MC, Shinal RM, Glover T, Williams RS, Staud R, Riley JL 3rd, Fillingim RB (2011) Evaluation of menstrual cycle effects on morphine and pentazocine analgesia. Pain 152:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SA, Kempen TA, Pickel VM, Milner TA (2016) Enkephalin levels and the number of neuropeptide Y-containing interneurons in the hippocampus are decreased in female cannabinoid-receptor 1 knock-out mice. Neurosci Lett 620:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS (2005) Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology 81:391–399. [DOI] [PubMed] [Google Scholar]
- Rubin BR, Johnson MA, Berman JM, Goldstein E, Pertsovskaya V, Zhou Y, Contoreggi NH, Dyer AG, Gray JD, Waters EM, McEwen BS, Kreek MJ, Milner TA (2020) Sex and chronic stress alter delta opioid receptor distribution within rat hippocampal CA1 pyramidal cells following behavioral challenges. Neurobiology of Stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisoto P, Contador I (2019) The role of stress in drug addiction. An integrative review. Physiol Behav 202:62–68. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Zhou Y, Contoreggi NH, Bshesh FK, Gray JD, Kogan JF, Ben KT, McEwen BS, Jeanne Kreek M, Milner TA (2018) Sex Differences in the Rat Hippocampal Opioid System After Oxycodone Conditioned Place Preference. Neuroscience 393:236–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmanzadeh H, Azizi H, Semnanian S (2017) Adolescent chronic escalating morphine administration induces long lasting changes in tolerance and dependence to morphine in rats. Physiol Behav 174:191–196. [DOI] [PubMed] [Google Scholar]
- Sandin J, Ogren SO, Terenius L (2000) Endomorphin-2 but not Leu-enkephalin modulates spatial learning when microinjected in the CA3 region of the rat hippocampus. Neuroreport 11:3659–3662. [DOI] [PubMed] [Google Scholar]
- Sandin J, Nylander I, Georgieva J, Schött PA, Ogren SO, Terenius L (1998) Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience 85:375–382. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ (2014) Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiology of disease 72 Pt B:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ (2017) Sex differences in hippocampal area CA3 pyramidal cells. J Neurosci Res 95:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD (2013) Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J Neurosci 33:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberová R, Rimanóczy A, Bar N, Schindler CJ, Vathy I (2003) Density of mu-opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus 13:461–471. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR (2005) Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology 30:2046–2057. [DOI] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S (2012) Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 202:131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, Colmers WF (2007) Neuropeptide Y in the dentate gyrus. In: Progress in Brain Research (Scharfman HE, ed), pp 285–297: Elsevier. [DOI] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL (2020) Opioid use disorder. Nat Rev Dis Primers 6:3. [DOI] [PubMed] [Google Scholar]
- Sun Q, Sotayo A, Cazzulino AS, Snyder AM, Denny CA, Siegelbaum SA (2017) Proximodistal Heterogeneity of Hippocampal CA3 Pyramidal Neuron Intrinsic Properties, Connectivity, and Reactivation during Memory Recall. Neuron 95:656–672.e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT (2016) Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol Rev 68:419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Drake CT, McEwen BS, Milner TA (2008) Ovarian steroids modulate leu-enkephalin levels and target leu-enkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain research 1232:70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, Drake CT, Milner TA (2009a) Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Experimental neurology 219:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, Drake CT, McEwen BS, Milner TA (2009b) Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience 159:204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA (2003) Subcellular relationships between cholinergic terminals and estrogen receptor-alpha in the dorsal hippocampus. J Comp Neurol 463:390–401. [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM (1999) Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience 93:969–975. [DOI] [PubMed] [Google Scholar]
- Turner CD, Bagnara JT (1971) General Endocrinology. Philadelphia: W.B. Saunders. [Google Scholar]
- Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology 56:613–672. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462. [DOI] [PubMed] [Google Scholar]
- Van Kempen TA, Kahlid S, Gonzalez AD, Spencer-Segal JL, Tsuda MC, Ogawa S, McEwen BS, Waters EM, Milner TA (2013) Sex and estrogen receptor expression influence opioid peptide levels in the mouse hippocampal mossy fiber pathway. Neurosci Lett 552:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanHouten JP, Rudd RA, Ballesteros MF, Mack KA (2019) Drug Overdose Deaths Among Women Aged 30–64 Years - United States, 1999–2017. MMWR Morbidity and mortality weekly report 68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001) Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science (New York, NY) 292:1175–1178. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002) Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. The Journal of Neuroscience 22:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C (1993) Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature 363:451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS (1992) Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research 588:341–345. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA (2009) Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res 1290:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland NG, Orikasa C, Hayashi S, McEwen BS (1997) Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol 388:603–612. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Milner TA (2011) Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience 179:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Torres-Reveron A, Chapleau JD, Milner TA (2011a) Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiology of learning and memory 95:206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Akama KT, Knudsen MG, McEwen BS, Milner TA (2011b) Ovarian hormones influence corticotropin releasing factor receptor colocalization with delta opioid receptors in CA1 pyramidal cell dendrites. Experimental neurology 230:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Mitterling KL, Thompson LI, Torres-Reveron A, Waters EM, McEwen BS, Gore AC, Milner TA (2011c) Age- and hormone-regulation of opioid peptides and synaptic proteins in the rat dorsal hippocampal formation. Brain Res 1379:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch KA, Kreek MJ (2020) Review of addiction risk potential associated with adolescent opioid use. Pharmacol Biochem Behav 198:173022. [DOI] [PubMed] [Google Scholar]
- Windisch KA, Mazid S, Johnson MA, Ashirova E, Zhou Y, Gergoire L, Warwick S, McEwen BS, Kreek MJ, Milner TA (2021) Acute Delta 9-tetrahydrocannabinol administration differentially alters the hippocampal opioid system in adult female and male rats. Synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL (1992) Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 12:3217–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz B, Konar V, Kutlu S, Sandal S, Canpolat S, Gezen MR, Kelestimur H (1999) Influence of chronic morphine exposure on serum LH, FSH, testosterone levels, and body and testicular weights in the developing male rat. Arch Androl 43:189–196. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Windisch K, Altschuler J, Rahm S, Butelman ER, Kreek MJ (2016) Adolescent oxycodone self administration alters subsequent oxycodone-induced conditioned place preference and anti-nociceptive effect in C57BL/6J mice in adulthood. Neuropharmacology 111:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brownstein AJ, Buonora M, Niikura K, Ho A, Correa da Rosa J, Kreek MJ, Ott J (2015) Self administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience 285:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


