Abstract
Background:
Animal and epidemiological studies suggest that prenatal exposure to polycyclic aromatic hydrocarbons (PAHs) may negatively impact toddler neurodevelopment.
Methods:
We investigated this association in 835 mother-child pairs from CANDLE, a diverse pregnancy cohort in the mid-South region of the U.S. PAH metabolite concentrations were measured in mid-pregnancy maternal urine. Cognitive and Language composite scores at ages 2 and 3 years were derived from the Bayley Scales of Infant and Toddler Development, 3rd edition (Bayley-3). Behavior Problem and Competence scores at age 2 were derived from the Brief Infant and Toddler Social Emotional Assessment (BITSEA). We used multivariate linear or Poisson regression to estimate associations with continuous scores and relative risks (RR) of neurodevelopment delay or behavior problems per 2-fold increase in PAH, adjusted for maternal health, nutrition, and socioeconomic status. Secondary analyses investigated associations with PAH mixture using Weighted Quantile Sum Regression (WQS) with a permutation test extension.
Results:
1- hydroxypyrene was associated with elevated relative risk for Neurodevelopmental Delay at age 2 (RR = 1.20, 95% CI: 1.03,1.39). Contrary to hypotheses, 1-hydroxynaphthalene was associated with lower risk for Behavior Problems at age 2 (RR = 0.90, 95% CI: 0.83,0.98), and combined 1- and 9-hydroxyphenanthrene was associated with 0.52-point higher (95% CI: 0.11,0.93) Cognitive score at age 3. For PAH mixtures, a quintile increase in hydroxy-PAH mixture was associated with lower Language score at age 2 (βwqs = −1.59; 95% CI: −2.84,−0.34; ppermutation=0.07) and higher Cognitive score at age 3 (βwqs = 0.96; 95% CI: 0.11,1.82; ppermutation=0.05). All other estimates were consistent with null associations.
Conclusion:
In this large southern U.S. population we observed some support for adverse associations between PAHs and neurodevelopment.
Keywords: Polycyclic aromatic hydrocarbons, pediatric neurodevelopment, prenatal
Graphical Abstract
1. INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs)1, a group of chemicals formed during the incomplete combustion of oil, coal, gas, and other substances (Agency for Toxic Substances and Disease Registry (ATSDR), 1995), are linked to cytotoxic, genotoxic, endocrine disrupting, and carcinogenic effects in both animal and human studies (Schroeder, 2011; Deanna D Wormley et al., 2004). PAHs are predominantly metabolized into hydroxy-PAHs (OH-PAHs)(Onyemauwa et al., 2009), which exhibit enhanced genotoxicity over parent PAHs(Wei et al., 2010). Prenatal exposure to PAHs is associated with a number of adverse health outcomes in the fetus, infants and children, including preterm birth, intrauterine growth restriction and reduced birthweight, airway inflammation and asthma, and neurodevelopmental deficits (Edwards et al., 2010; Jedrychowski et al., 2012, 2015, 2014; F. Perera et al., 2005; F. P. Perera et al., 2005). Research in animals suggests several potential pathophysiologic mechanisms for perturbations in healthy brain development. These include direct neurotoxic and genotoxic effects (Saunders et al., 2003, 2002), neuronal death (Dutta et al., 2010), and the disruption of pathways regulating neuroplasticity and neurodifferentiation (Slotkin and Seidler, 2009), any of which may have long lasting, if not permanent, effects on the brain. Animal studies have also observed impaired cortical function and behavior changes in rodents exposed either prenatally or directly to PAHs (McCallister et al., 2008; Zhang et al., 2016).
International and U.S.-based studies have examined the impact of prenatal PAH exposure on neurodevelopment in infancy, early childhood, and into the school years, with mixed findings across age and cohort (Mortamais et al., 2017; F. Perera et al., 2005; Perera et al., 2012, 2007, 2006; Peterson et al., 2015; Tang et al., 2008; Vishnevetsky et al., 2015). In the lifestage most relevant to this study, i.e. the toddler years, evidence from 4 studies based in New York City and China show inconsistent associations between prenatal PAH exposure and neurodevelopment, with two out of three studies finding neurodevelopmental deficits at 12 and 24 months (Lin et al., 2021; Perera et al., 2006; Tang et al., 2008), and at 36 months in one of two studies (Perera et al., 2007, 2006). Sample sizes were small (<300 participants) across all studies and adjustment for confounding was minimal in both of the Chinese cohorts that observed neurodevelopmental deficits associated with PAH exposure, which may partially explain the inconsistency in findings (Lin et al., 2021; Tang et al., 2008). The limited geographical scope, small sample sizes, differing neurodevelopmental assessments in existing studies and inadequate confounding adjustment represented in the current evidence precludes confidence in the findings and clear extrapolation to other populations in the U.S.
This study builds on understanding of the potential adverse neurodevelopmental toxicity of PAH exposure in pregnancy in several ways. First, we examine associations between individual PAH metabolites and early-life neurodevelopment in a diverse and well-characterized large prospective cohort of mother-child pairs from the urban southern U.S. with rich adjustment for sociodemographic and maternal health confounders. In addition to examining effects of individual PAH metabolites individually, we employ novel methods to examine the association of exposure to PAH mixtures on neurodevelopment. We are also, to our knowledge, the first to assess the potential buffering role of nutritional factors. A mother’s prenatal nutrition, including factors known to influence a child’s neurodevelopment, such as folate (Castillo-Lancellotti et al., 2013; Julvez et al., 2009; Roth et al., 2011) and vitamin D (Melough et al., 2021; Tylavsky et al., 2015), may modify the neurotoxic effects of in utero PAH exposure. We previously observed effect modification in prenatal PM10 exposure and child IQ by maternal plasma folate levels (Loftus et al., 2019) in this cohort and hypothesized that nutritional factors may similarly modify PAH and neurodevelopmental associations. Therefore, we tested the hypothesis that adverse associations between PAH and neurodevelopment would be stronger among children whose mothers had lower prenatal plasma levels of either vitamin D or folate.
2. METHODS
2.1. Study Population
This study includes mother and child pairs participating in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study, a prospective pregnancy cohort in Shelby County, Tennessee (Sontag-Padilla et al., 2015) which was designed to identify early-life determinants of neurocognitive development. The study design, recruitment experience, and data collection have been described elsewhere (LeWinn et al., 2020). Briefly, between 2006 and 2011, CANDLE recruited pregnant women aged 16–40 years old with a low-risk pregnancy (e.g. without chronic hypertension requiring therapy, insulin-dependent diabetes, renal disease) in the second trimester of a singleton pregnancy with the intent to deliver at one of five health care settings in Shelby County. Of the 5228 women screened, 3320 were determined to be eligible and of these, 1503 enrolled in CANDLE (Sontag-Padilla et al., 2015).
Data collection for the CANDLE cohort included two prenatal clinic visits, multiple postnatal study visits attended by children and mothers, and two home visits in early childhood. All participants provided informed consent. Research activities were approved by the University of Tennessee Health Sciences Center Institutional Review Board (IRB). The current analyses were conducted as part of the PATHWAYS Consortium, a cohort award of the Environmental Influences on Child Health Outcomes (ECHO) initiative, and were approved by the IRB of the University of Washington, Seattle.
The analytic sample included CANDLE mother-child dyads with a mid-pregnancy urinary PAH measure (median gestational age=22.9 weeks, range 15.3 to 29.6 weeks) and at least one neurodevelopmental outcome at the child’s age 2- or 3-year visit (n=965)., We excluded (n=11) children born prior to 32 weeks gestation due to the known much higher risk for neurodevelopmental delay in children born very pre-term (Blencowe et al., 2013; Soleimani et al., 2014). Because one of the primary aims of this study was to assess associations of PAH exposure and neurodevelopment independent of the effects of prenatal maternal smoking, we also excluded women who reported any smoking during pregnancy or with urinary cotinine levels > 200 ng/mL at the second or third trimester study visits (n=119), considered a common cutoff to define smokers (Schick et al., 2017), yielding a final analytic sample of 835 children. Figure 1 illustrates cohort retention from enrollment to the time of outcome assessments and sizes of the analytic samples.
Figure 1. Flowchart for inclusion in the analytic samples in CANDLE participants.
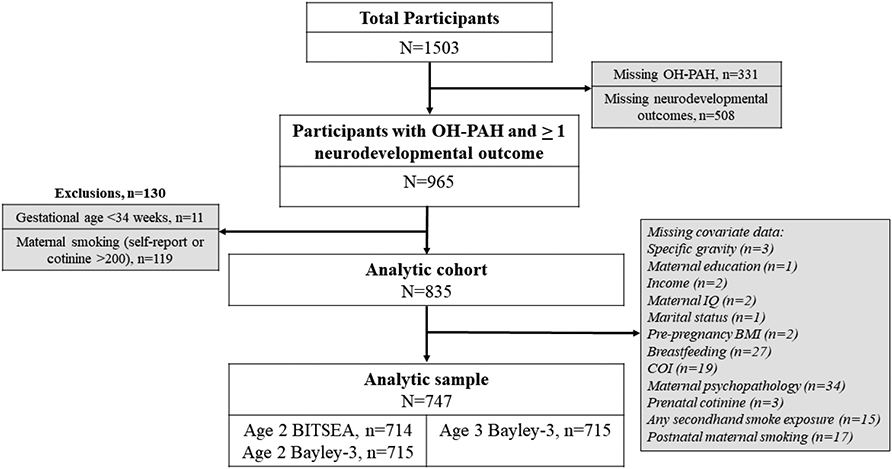
Abbreviations: BITSEA= Brief Infant and Toddler Social Emotional Assessment, BMI=Body mass index, OH-PAH = Hydroxy-Polycyclic Aromatic Hydrocarbon
2.2. PAH Assessment
Maternal prenatal PAH exposure was determined using urinary monohydroxylated OH-PAH metabolite concentrations from mid-pregnancy. Extraction of OH-PAHs from urine was performed by liquid-liquid extraction followed by LC-MS/MS analysis, as described elsewhere (Guo et al., 2013). Briefly, urine samples (500 μL) were fortified with 10 ng each of an isotopically labeled internal standard mixture, and mixed with 1 mL of 0.5 M ammonium acetate buffer containing 200 units/mL of β-glucuronidase/sulfatase enzyme (MP Biomedicals, LLC, Solon, OH, USA). The samples were gently mixed and incubated overnight at 37°C, and then diluted with 2 mL of HPLC-grade water and extracted with 7 mL of 80% pentane: 20% toluene (v:v), by shaking on a reciprocating shaker for one hour, centrifuged at 3600 x g for 20 minutes, the supernatant was transferred into a new glass tube for instrumental analysis. The chromatographic separation of OH-PAHs was accomplished using a Waters Acquity I-Class UPLC system (Waters; Milford, MA, USA) connected with an Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm, Waters; Milford, MA, USA). Identification and quantification of PAH metabolites was performed on an ABSCIEX 5500 triple quadrupole mass spectrometer (Applied Biosystems; Foster City, CA, USA). Quality assurance protocols include, analysis of two Standard Reference Materials (SRM 3672, SRM 3673) containing certified values for several OH-PAHs PAHs metabolites (1- and 2-hydroxynaphthalene; 1-, 2-, 3-, and 9-hydroxyphenanthrene; 2-, 3-, and 9-hydroxyfluorene, and 1-hydroxypyrene), method blanks, matrix (urine) blanks, and matrix (urine) spiked samples. Recoveries of analytes in SRMs ranged from 79 to 109%. Synthetic urine purchased from Cerilliant (Round Rock, Texas, USA) was used for the matrix blank and matrix spiked samples and the recoveries of target analytes spiked into synthetic urine matrix was >80% HPLC-grade water was used for sample/procedural blanks. A quadratic 13-point standard calibration curve (0.02 ng/mL – 200 ng/mL) with a 1/x weighting (and a regression coefficient value >0.99 for each analyte) was used for quantification. Periodic injections of PAH metabolite calibration standards are included throughout the sample run to ensure instrument stability in responses. Periodic instrumental blanks are also injected throughout the sample run to ensure no carryover or contamination. The laboratory participated in several external quality assurance schemes to validate OH-PAHs assay successfully(Kannan et al., 2021). The limits of detection (LOD) ranged from 0.02 to 0.12 ng/mL. Samples below the limit of detection (LOD) were calculated as: LOD/√2. For this analysis we established that each OH-PAH metabolite was detected in greater than 80% of the study population, which yielded sufficient samples for the following OH-PAHs: 1- and 2-hydroxynaphthalene; 1-, 2-, 3-, and 9-hydroxyphenanthrene; 2-, 3-, and 9-hydroxyfluorene, and 1-hydroxypyrene. Measurements were unable to distinguish the hydroxyfluorenes or 1- and 9-hydroxyphenanthrene, and so these measurements were quantified together and combined into 2/3/9-hydroxyfluorene and 1/9-hydroxyphenanthrene, respectively. While raw OH-PAH measures (in ng/mL) were used in the main analysis, we calculated and adjusted OH-PAHs for urine specific gravity in sensitivity analyses using the following formula:
Where P is the measured urinary PAH concentration, SG is the specific gravity for each participant, and SGmedian is the median SG(Levine and Fahy, 1945).
2.3. Neurodevelopmental and Behavioral Outcomes
Child behavioral assessments were performed using the Brief Infant-Toddler Social and Emotional Assessment (BITSEA) (Briggs-Gowan et al., 2004). The BITSEA (Briggs-Gowan et al., 2004) measures caregiver-report of social-emotional and behavioral function and was administered at the age 2 visit. The BITSEA yields continuous Problem and Competence scores that are summed across the 42-item questionnaire. Using cut-points, we also identified children meeting the threshold for Behavior Problems, defined as BITSEA Problem scores ≥75th percentile for age (Briggs-Gowan et al., 2013, 2004). Competence scores were not examined dichotomously in this analysis due to the low proportion (<10%) of children in this study with BITSEA Competence scores ≥ 75th percentile for age.
Child neurodevelopment assessments were performed using the Bayley Scales of Infant Development, Third Edition (Bayley-3) (Bayley, 2006), which is used to identify infants and young children at risk for neurodevelopmental delay (Bayley, 2006). Trained developmental psychologists administered the Bayley-3 at the age 2 and age 3 visit. Scores are standardized to age and sex to yield Cognitive and Language composite scores with a normative mean of 100 (standard deviation=15; SD). We also identified children at risk of Neurodevelopmental Delay, defined as Bayley Cognitive or Language composite scores <85 (Bayley, 2006).
The primary outcomes for this study were the continuous BITSEA Problem and Competence and Bayley-3 Cognitive and Language scores at each age. Binary outcomes indicating whether the child’s results exceeded thresholds for Behavior Problems and Neurodevelopmental Delay were considered secondary outcomes. For the Bayley-3 Cognitive, Language, and BITSEA Competence scores, higher scores represent better neurodevelopmental or behavioral function, whereas higher scores on the BITSEA Problem scale score indicate poorer behavioral development.
2.4. Covariates
Maternal characteristics were collected at prenatal and postnatal study visits, including pre-pregnancy BMI, age at delivery, race and ethnicity, educational attainment, marital status, insurance coverage, parity, gestational age and birthweight of the child, and child breastfeeding. Maternal IQ was directly assessed using the Wechsler Abbreviated Scale of Intelligence (WASI) short form (Axelrod, 2002). Maternal psychopathology was assessed using the Brief Symptom Inventory (BSI) (Derogatis, 1993).Maternal parenting knowledge was ascertained using the Knowledge of Infant Development Inventory (KIDI) (MacPhee, 1981). Child opportunity at the neighborhood level was measured using the total score of the Child Opportunity Index and was based on maternal address at enrollment (Acevedo-Garcia et al., 2014). A postnatal home visit included direct observation of the care-taking environment using the Home Observation Measurement of the Environment (HOME) inventory (Frankenburg and Coons, 1986). Post-natal tobacco exposure from the child’s mother or from other family members in the home was self-reported at age 4. Maternal cotinine (in ng/mL), used to measure prenatal secondhand tobacco exposure, was assessed using urine samples collected in the second and third trimester and the average of the two samples was used for analysis.
2.5. Prenatal Folate and Vitamin D Assessment in Maternal Plasma
Maternal blood samples were collected in the 2nd and 3rd trimesters. Plasma was separated by centrifuging at 3000 pm for 10 minutes and stored at −70°C until analysis. Folate concentrations were assessed using the 96-well plate adaptation of the Lactobacillus case microbiological assay, with a minimum detection limit of 3 ng/mL (Roy et al., 2018) and the mean of the two measures was used for analysis. Vitamin 25(OH)D levels were available from the 2nd trimester sample, quantified by commercial enzymatic immunoassay (IDS, Boldon, Tyne and Wear, UK) (Tylavsky et al., 2015). Minimum detection range of this assay was 2 ng/mL, with interassay variability <6% and precision within 1 SD of mean, using NIST SRM972 as standard. All measurements were performed within 3 months of sample collection. Serum folate and vitamin D were dichotomized at “possible” deficiency, defined as ≤13.4 ng/mL for folate and <20 ng/mL for vitamin D (Holick, 2007; Organization, 2015) for effect modification analyses.
2.5. Statistical Analysis
We used descriptive statistics to characterize the study sample and the distributions of maternal OH-PAH exposure in the analytic sample. Multivariate linear regression with robust standard errors was used to estimate associations of individual OH-PAHs and the primary continuous neurodevelopmental outcome scores with corresponding 95% confidence intervals (95% CI). Separate models were run for each OH-PAH and each neurodevelopmental outcome. Individual OH-PAHs were natural-log transformed prior to analysis and modeled continuously for all analyses. Multivariate Poisson regression was used to estimate the relative risk (RR) and corresponding 95% CIs for the associations of each OH-PAH and dichotomous outcomes of neurodevelopmental delay and social-emotional/behavior problems. For all models, OH-PAH concentrations were log transformed (not specific gravity-adjusted) and analyzed in separate regression models that included specific gravity as a covariate. To ease in interpretability, estimates were multiplied by ln(2) to calculate estimates per 2-fold increase in ln-OH-PAH.
We took a staged approach to confounder adjustment and inclusion of precision variables. All covariates were identified a priori as potential confounders between OH-PAH and neurodevelopmental outcomes or well-established predictors of pediatric neurodevelopment (Eriksen et al., 2013; Kendler et al., 2015; Lawlor et al., 2006; Lewinn et al., 2020; Ritchie and Tucker-Drob, 2018; Turkheimer et al., 2003). The minimally adjusted model includes child age at assessment (continuous year, to two decimal places), child sex (binary), and specific gravity (modeled as a 4-degree of freedom natural spline). The fully adjusted model, considered the main analyses, includes terms in the minimally adjusted model as well as maternal education (categorical), adjusted income by household count (continuous) (Burniaux et al., 1998), maternal race (binary, Black vs. not), insurance status (binary, Medicaid or no insurance vs. Private/Mixed Private with Medicaid or Medicare/Other, maternal IQ (continuous), maternal age at child’s birth (continuous), marital status (married or living with partner vs. not, child birth order (binary, first born vs. not), recruitment site (binary; Memphis/safety-net hospital vs. other), pre-pregnancy BMI (continuous), breastfeeding status (categorical; None, <6 months., ≥ 6 months), Child Opportunity Index (COI total score, modeled as splines with 4 parameters), prenatal maternal mental health using the Global Severity Index from the Brief Symptom Inventory (GSI total score, continuous), prenatal maternal cotinine level (continuous), postnatal secondhand smoke exposure (self-reported; any vs. none; measured at child’s visit at age 4), and postnatal maternal smoking (self-reported; any vs. none; measured at age 4).
2.5.1. Secondary Analyses
We used Weighted Quantile Sum regression (WQS) (Brunst et al., 2017; Carrico et al., 2015; Czarnota et al., 2015a, 2015b) to characterize the association between OH-PAH mixtures and neurodevelopmental outcomes. OH-PAHs were normalized by converting to quintiles, and WQS scores comprised of weighted sums of individual OH-PAHs were estimated. Weights were selected using bootstrap resampling methods (1000 bootstrap runs for each analysis) to optimize the association between the WQS score and outcomes in multivariate linear regression models adjusted for the covariates in the fully adjusted models. We estimated mixture effects in the positive or negative direction separately. We applied a permutation test extension to WQS regression, an approach we recently developed to allow accurate estimation of p-values in full-sample WQS analyses (Loftus et al., 2021). We estimated a permutation test p-value (ppermutation) for every full sample WQS analysis that resulted in a 95% CI that did not include the null. We applied three levels of evidence against the null: 1) full-sample 95% CIs overlap the null (weakest evidence); 2) full-sample 95% CIs do not overlap the null but ppermutation>0.05 (moderate evidence); and 3) full-sample 95% CIs do not overlap the null and ppermutation<0.05 (strongest evidence). Evidence for differences in all other analyses was evaluated using a significance threshold of p<0.05.
We examined potential effect modification by prenatal folate or vitamin D deficiency using linear regression with robust SEs and included all covariates in the primary fully adjusted models. Models included interaction terms between either continuous ln-transformed OH-PAHs and dichotomous folate or vitamin D deficiency status, and p-values for interaction were estimated using the Wald test.
All statistical analyses were conducted at the UW Pathways Data Center and analyzed using STATA IC 16.1 and R Studio (RStudio Team, 2020; StataCorp, 2020). The R package “gWQS” version 2.0 package was used for the WQS analyses (Renzetti et al., 2020).
2.5.2. Sensitivity Analyses
We performed four sets of sensitivity analyses for all analyses of OH-PAHs and continuous neurodevelopmental outcomes. The purpose of these sensitivity analyses were to evaluate the robustness of the estimates of the primary analyses to different modeling approaches: (1) method of adjusting OH-PAHs for urinary dilution; (2) inclusion of additional covariates for the purposes of adjustment for confounding and improvement of precision that were excluded from the fully adjusted model due to data missingness; (3) inclusion of covariates hypothesized to be confounding factors that may also mediate associations between OH-PAH exposure and neurodevelopmental outcomes and (4) to examine patterns of associations (e.g. non-linearity, outliers) in evident findings. First, the main analyses were repeated to use specific gravity-corrected OH-PAH as the predictor variable in place of using raw OH-PAH with specific gravity as a covariate in the model. Second, we repeated all main analyses with additional adjustment for the Knowledge of Infant Development inventory (KIDI) Total score (maternal-report, assessed at maternal visit 2, continuous, missing n=33), and Home Observation Measurement of the Environment Inventory (HOME) total score (objectively assessed by rater at home visit 2, measured continuously, missing in n=334). Third, we repeated the main analyses after additional adjustment for birthweight (in grams, continuous) and gestational age (in weeks, continuous), which were excluded from the fully adjusted model because they were hypothesized to be both confounders as well as potential mediators between prenatal PAH exposure and neurodevelopment. Finally, in OH-PAH and neurodevelopmental associations from the main analyses where estimates excluded the 95% CI, we used generalized additive models (GAMs) to explore whether the exposure-response relationships deviated from linearity(Wood, 2011). Models were run with all covariates in the fully adjusted model and we inspected exposure-response curves and associated 95% CI for evidence of deviation from linearity.
We were primarily interested in examining associations of PAH exposure and neurodevelopment in non-smoking women due to the well-known deleterious effects of tobacco smoke, a known source of PAH exposure, on the developing brain. We nonetheless recognized the interest the effects of PAH in populations of women who smoke, particularly because existing cohorts of PAHs and neurodevelopment in the U.S. have excluded smoking women from their studies. Therefore, as a final sensitivity analysis we repeated the primary analyses including prenatal smokers and adjusting for pre- and post-natal smoking (any vs. none and adjustment for continuous prenatal cotinine).
3. RESULTS
The demographic, maternal, and child characteristics of the analytic cohort at study baseline and at subsequent maternal and child home visits from birth to early life are shown in Table 1. Sixty-three percent of mothers were Black, 31% White, 1% Asian, and 5% of mixed or other race. Approximately half had Medicaid or no health insurance at enrollment. Sixty percent of children were first-born, and 29% of mothers reported that their child was breastfed for at least 6 months. Twenty four percent of children had postnatal secondhand smoke exposure from a family member living in the home and 8% of mothers reported postnatal smoking (Table 1).
Table 1.
Characteristics of mothers and children in CANDLE with prenatal OH-PAH and early life neurodevelopmental outcomes
| Total | Alla N = 835 |
Seen at visit 2a N = 793 |
Seen at visit 3a N = 798 |
|---|---|---|---|
| Child age at visit 2, mean ± SD | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| Child age at visit 3, mean ± SD | 3.1 ± 0.1 | 3.1 ± 0.1 | 3.1 ± 0.1 |
| Child sex, n (%) | |||
| Male | 405 (48.5%) | 387 (48.8%) | 391 (49.0%) |
| Female | 430 (51.5%) | 406 (51.2%) | 407 (51.0%) |
| Gestational age, mean ± SD | 38.9 ± 1.5 | 38.9 ± 1.4 | 38.9 ± 1.5 |
| Birthweight, mean ± SD | 3275.3 ± 506.2 | 3290.2 ± 500.0 | 3276.5 ± 501.4 |
| Maternal age at birth, mean ± SD | 27.1 ± 5.6 | 27.2 ± 5.6 | 27.2 ± 5.6 |
| Maternal race, n (%) | |||
| Black | 524 (62.8%) | 489 (61.7%) | 500 (62.7%) |
| White | 262 (31.4%) | 258 (32.5%) | 252 (31.6%) |
| Asian | 9 (1.1%) | 9 (1.1%) | 9 (1.1%) |
| Other or Multiple | 40 (4.8%) | 37 (4.7%) | 37 (4.6%) |
| Maternal ethnicity | |||
| Not Hispanic or Latino | 816 (98.4%) | 774 (98.3%) | 781 (98.6%) |
| Hispanic/Latino | 13 (1.6%) | 13 (1.7%) | 11 (1.4%) |
| Child is first born | |||
| No | 501 (60.0%) | 476 (60.0%) | 482 (60.4%) |
| Yes | 334 (40.0%) | 317 (40.0%) | 316 (39.6%) |
| Maternal Education | |||
| < High School | 67 (8.0%) | 57 (7.2%) | 63 (7.9%) |
| High School | 383 (45.9%) | 361 (45.6%) | 361 (45.3%) |
| College or Technical school | 271 (32.5%) | 263 (33.2%) | 262 (32.9%) |
| Graduate or Professional degree | 113 (13.5%) | 111 (14.0%) | 111 (13.9%) |
| Maternal IQ (WASI total score), mean ± SD | 96.6 ± 16.3 | 97.0 ± 16.2 | 96.7 ± 16.2 |
| Medicaid only | |||
| Medicaid or none | 428 (51.3%) | 395 (49.8%) | 405 (50.8%) |
| Private or other | 407 (48.7%) | 398 (50.2%) | 393 (49.2%) |
| Marital Status | |||
| Married | 488 (58.5%) | 473 (59.7%) | 474 (59.5%) |
| No | 346 (41.5%) | 319 (40.3%) | 323 (40.5%) |
| Pre-pregnancy maternal BMI, mean ± SD | 28.1 ± 7.9 | 28.2 ± 7.9 | 28.1 ± 7.9 |
| Child was breastfed | |||
| None | 257 (31.8%) | 238 (30.9%) | 243 (31.4%) |
| <6 months | 319 (39.5%) | 308 (39.9%) | 304 (39.3%) |
| ≥6 months | 232 (28.7%) | 225 (29.2%) | 227 (29.3%) |
| Child Opportunity Index score, mean ± SD | 0.04 ± 0.43 | 0.04 ± 0.43 | 0.04 ± 0.43 |
| Maternal GSI total score (from BSI), mean ± SD | 50.2 ± 9.2 | 50.2 ± 9.1 | 50.1 ± 9.1 |
| KIDI total score, mean ± SD | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| HOME total score, mean ± SD | 39.2 ± 4.4 | 39.2 ± 4.4 | 39.1 ± 4.4 |
| Any secondhand smoke exposure | |||
| No | 622 (75.9%) | 595 (76.3%) | 601 (76.5%) |
| Yes | 198 (24.1%) | 185 (23.7%) | 185 (23.5%) |
| Postnatal maternal smoking | |||
| No | 757 (92.5%) | 724 (93.1%) | 727 (92.7%) |
| Yes | 61 (7.5%) | 54 (6.9%) | 57 (7.3%) |
Missingness: Gestational age (n=4), birthweight (n=5), maternal ethnicity (n=6), maternal education (n=1), maternal IQ (n=2), marital status (n=1), breastfeeding (n=18), Child Opportunity Index score (n=19), maternal GSI score (n=24), KIDI score (n=33), HOME score (n=334), secondhand smoke exposure (n=15), postnatal maternal smoking (n=17)
Abbreviations: BITSEA= Brief Infant and Toddler Social Emotional Assessment, BMI=Body mass index, BSI = Brief Symptom Index, GSI = Global Severity Index, HOME = Home Observation Measurement of the Environment, KIDI = Knowledge of Infant Development Inventory, SD = Standard deviation
Tables 2 and 3 display the distribution of neurodevelopmental scores at each visit and urinary OH-PAH concentrations (ng/mL), respectively. OH-PAH concentrations (in geometric mean) ranged from 0.08 to 4.14 ng/mL and were lowest for 2- and 3-hydroxyphenanthrene and highest for 2-hydroxynaphthalene (Table 3). Descriptive examination of the distribution of OH-PAHs across selected characteristics showed that PAH concentrations were consistently higher in mothers who were black, had less than a high school education, were on Medicaid, and who lived in homes with secondhand smoke exposure (Supplemental Table 1).
Table 2.
Distribution of neurodevelopment scores in CANDLE children
| Study visit and outcome | N | Mean ± SD |
|---|---|---|
| Child visit 2 | ||
| BITSEA Problem | 719 | 9.71 ± 6.41 |
| BITSEA Competence | 719 | 18.14 ± 2.58 |
| Bayley-3 Cognitive | 720 | 98.76 ± 13.48 |
| Bayley-3 Language | 720 | 99.51 ± 15.42 |
| Child visit 3 | ||
| Bayley-3 Cognitive | 722 | 97.32 ± 10.69 |
| Bayley-3 Language | 722 | 101.90 ± 12.31 |
Abbreviations: BITSEA= Brief Infant and Toddler Social Emotional Assessment, SD = Standard deviation
Table 3.
Distribution of urinary prenatal OH-PAH metabolite concentrations (ng/mL) in the CANDLE cohort
| Metabolite | % Detected | Geometric Meana |
50th % | 95th % | Range |
|---|---|---|---|---|---|
| 1-hydroxynaphthalene | 100.0 | 1.01 | 0.86 | 11.05 | 0.04, 331.00 |
| 2-hydroxynaphthalene | 99.8 | 4.14 | 4.39 | 21.70 | 0.02, 228.00 |
| 2-hydroxyphenanthrene | 85.4 | 0.08 | 0.08 | 0.34 | 0.02, 6.61 |
| 3-hydroxyphenanthrene | 85.2 | 0.08 | 0.08 | 0.29 | 0.02, 4.34 |
| 1,9-hydroxyphenanthrene | 83.6 | 0.24 | 0.30 | 1.32 | 0.02, 18.39 |
| 2/3/9-hydroxyfluorene | 96.2 | 0.79 | 0.84 | 3.42 | 0.06, 47.1 |
| 1-hydroxypyrene | 88.0 | 0.12 | 0.13 | 0.62 | 0.02, 4.91 |
All distributions represent volumetric concentrations of raw OH-PAHs and based on detectible values
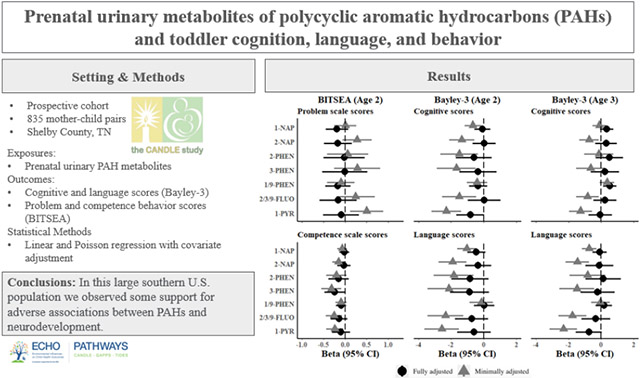
3.1. Associations with individual metabolites
3.1.1. BITSEA age 2
At age 2, we observed negative associations between BITSEA Competence scores and OH-PAH concentrations across most metabolites, all of which were attenuated in the fully adjusted models (Figure 2). There was little evidence for associations between any OH-PAH and Problem scores and no evidence in fully adjusted models for any metabolites (Figure 2). In fully adjusted models, a 2-fold increase in 3-hydroxyphenanthrene was associated with a 0.24-point decrease in Competence score (95% CI: −0.48 to 0.00) (Figure 2). Null associations were observed for other OH-PAHs and Competence scores (Figure 2).
Figure 2. Estimated associations of OH-PAH metabolites and neurodevelopmental scores at age 2 and 3 in the CANDLE cohort.
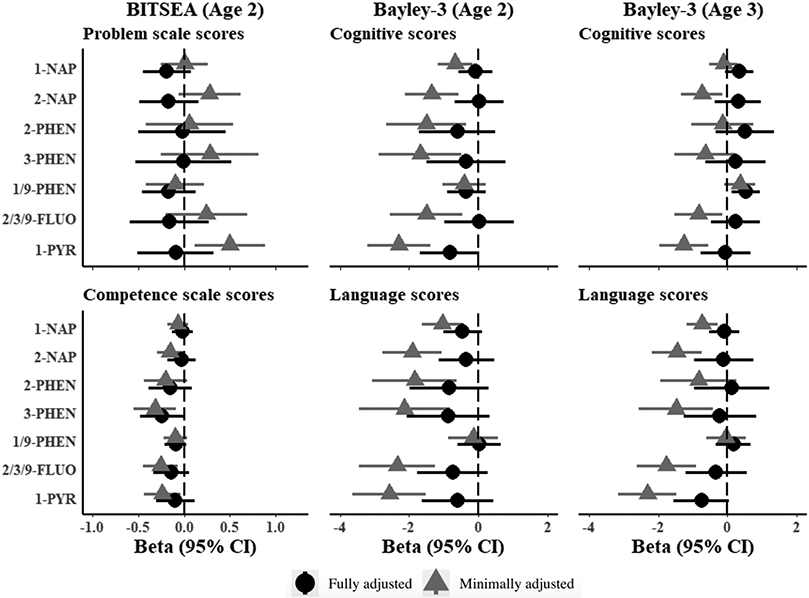
Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions. Minimally adjusted models include child sex, age at assessment, and specific gravity and restricted to participants with complete covariate data in the fully adjusted model. Fully adjusted models include child sex, age at assessment, specific gravity, race, study site, maternal age, maternal education, maternal IQ, income adjusted for household size, marital status, parity, insurance, pre-pregnancy BMI, breastfeeding, maternal psychopathology, COI, prenatal cotinine, postnatal secondhand tobacco smoke exposure, postnatal maternal smoking. All estimates represent effect per 2-fold increase in log OH-PAH. Higher scores represent denote positive outcomes for all neurodevelopmental outcomes with the exception of BITSEA Problem scores, where higher scores denote worse outcomes. Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR= 1-hydroxypyrene, 95% CI = 95% Confidence Interval, BITSEA= Brief Infant and Toddler Social Emotional Assessment
3.1.2. Bayley-3 age 2
At age 2, deficits in Bayley-3 Cognitive scores were observed in minimally adjusted models across all but one OH-PAH, which were all attenuated after full adjustment (Figure 2). A 2-fold increase in 1-hydroxypyrene was associated with a 0.82-point lower Cognitive score, although confidence intervals in fully adjusted models included the null (95% CI −1.68 to 0.04). No other metabolites were associated with Bayley-3 Cognitive scores in fully adjusted models (Figure 2). For Bayley-3 Language scores at age 2, deficits were observed in most minimally adjusted models, but these were also uniformly attenuated after full adjustment (Figure 2).
3.1.3. Bayley-3 age 3.
At age 3 Bayley-3 assessments, negative associations were observed for several, but not all, metabolites (Figure 2) on Language and Cognitive scores in minimally adjusted models. After full adjustment, a 2-fold increase in 1/9-hydroxyphenanthrene was associated with 0.52 point higher Cognitive score (95% CI: 0.11 to 0.93) (Figure 2). Estimates reached borderline significance for 1-hydroxypyrene and Language scores, although confidence intervals included the null (β per 2-fold increase = −0.75, 95% CI: −1.56 to 0.05). All other estimates were consistent with null associations (Figure 2).
3.1.4. Behavior Problems and Neurodevelopmental Delay
Using clinically relevant cut-offs, 19% of children met the threshold for Behavior Problems on the age 2 BITSEA assessment, and 16% and 6% met the threshold for Neurodevelopmental Delay on the Bayley-3 at age 2, and 3, respectively (Table 4). Contrary to our hypothesis, increasing 1-hydroxynaphthalene was associated with a lower risk for BITSEA Behavior problems at age 2 in fully adjusted models (RR = 0.90, 95% CI: 0.83 to 0.98). In contrast, increasing 1-hydroxypyrene was associated with an elevated relative risk for Neurodevelopmental Delay at age 2 (RR for 2-fold increase = 1.20, 95% CI: 1.03 to 1.39). No other OH-PAHs were associated with clinical cutoffs for Neurodevelopmental Delay or Behavior Problems at the age 2 and 3 assessments (Table 4).
Table 4.
Associations of prenatal urinary OH-PAH metabolites and risk of behavior problems or neurodevelopmental delay by metabolite and model
| Age 2 Behavior Problems N=138/719 (19%) |
Neurodevelopmental Delay N=119/720 (16%) |
Age 3 Neurodevelopmental Delay N=42/722 (6%) |
||||
|---|---|---|---|---|---|---|
| OH-PAH Metabolite | Minimala RR (95% CI) |
Fullb RR(95% CI) |
Minimala RR (95% CI) |
Fullb RR (95% CI) |
Minimala RR (95% CI) |
Fullb RR (95% CI) |
| 1-hydroxynaphthalene | 0.95 (0.86, 1.04) | 0.90 (0.83, 0.98) | 1.09 (1.00, 1.18) | 1.06 (0.98, 1.15) | 0.92 (0.79, 1.06) | 0.90 (0.78, 1.05) |
| 2-hydroxynaphthalene | 1.07 (0.96, 1.20) | 0.92 (0.82, 1.03) | 1.16 (1.03, 1.31) | 1.07 (0.94, 1.20) | 1.03 (0.83, 1.28) | 0.96 (0.76, 1.21) |
| 2-hydroxyphenanthrene | 1.00 (0.85, 1.18) | 1.00 (0.84, 1.19) | 1.09 (0.93, 1.28) | 1.05 (0.89, 1.25) | 0.87 (0.58, 1.32) | 0.79 (0.50, 1.25) |
| 3-hydroxyphenanthrene | 1.06 (0.90, 1.26) | 0.99 (0.82, 1.18) | 1.17 (0.99, 1.38) | 1.13 (0.95, 1.34) | 0.88 (0.57, 1.35) | 0.82 (0.53, 1.29) |
| 1/9-hydroxyphenanthrene | 1.00 (0.91, 1.10) | 0.99 (0.91, 1.09) | 1.04 (0.92, 1.17) | 1.04 (0.93, 1.16) | 1.00 (0.81, 1.23) | 0.98 (0.79, 1.20) |
| 2/3/9-hydroxyfluorene | 1.07 (0.93, 1.23) | 0.98 (0.84, 1.14) | 1.14 (0.97, 1.34) | 1.02 (0.85, 1.22) | 1.11 (0.79, 1.55) | 0.99 (0.69, 1.40) |
| 1-hydroxypyrene | 1.20 (1.06, 1.37) | 1.03 (0.88, 1.20) | 1.30 (1.12, 1.51) | 1.20 (1.03, 1.39) | 1.11 (0.81, 1.53) | 0.99 (0.71, 1.38) |
All estimates are for Poisson regressions adjusted for child sex, age at assessment, and specific gravity and restricted to participants with complete covariate data in the fully adjusted model.
All estimates are for Poisson regressions adjusted for child sex, age at assessment, specific gravity, race, study site, maternal age, maternal education, maternal IQ, income adjusted for household size, marital status, parity, insurance, pre-pregnancy BMI, breastfeeding, maternal psychopathology, COI, prenatal cotinine, postnatal secondhand tobacco smoke exposure, postnatal maternal smoking.
Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene, 95% CI = 95% Confidence Interval, BITSEA= Brief Infant and Toddler Social Emotional Assessment, OH-PAH = Hydroxy-Polycyclic Aromatic Hydrocarbon, RR = Relative Risk
3.2. Associations with PAH mixtures (WQS Analyses)
Results from analyses of urinary OH-PAHs as mixtures using WQS regression are provided in Table 5 and Supplemental Table 2. We did not find evidence to support associations of urinary PAH mixtures with BITSEA Problem scores and Competence scores at age 2 in either the positive or negative direction. We observed moderate strength in evidence for associations of the OH-PAH mixture with Bayley-3 Language scores at age 2, and Cognitive scores at age 3. A 1-quintile increase in OH-PAH mixture with high index weights for 2/3/9-hydroxyfluorene and 1-hydroxynaphthalene was associated with a lower age 2 Bayley-3 Language score (βwqs = −1.59; full sample 95% CI: −2.84 to −0.34; ppermutation=0.07; Table 5 and Supplemental Table 2). A 1-quintile increase in OH-PAH mixture with high index weights for 1/9-hydroxyphenanthrene, 2-hydroxynaphthalene, and 2-hydroxyphenanthrene was associated with higher Bayley-3 Cognitive scores at age 3 (βwqs = 0.96; full sample 95% CI: 0.11 to 1.82; 1.82; ppermutation=0.05) (Table 5 and Supplemental Table 2). There was no evidence for associations between OH-PAH mixtures and Bayley-3 Language scores in either direction at age 3.
Table 5.
Estimated effects of OH-PAH matrix and child neurodevelopment from Weighted Quantile Sum regression in the CANDLE cohort
| Outcome and Model | Beta | WQS index 95% CI |
P valuea |
|---|---|---|---|
| Age 2 | |||
| BITSEA Problem | |||
| Positive | −0.28 | (−0.82, 0.27) | |
| Negative | −0.49 | (−1.08, 0.10) | |
| BITSEA Competence | |||
| Positive | −0.14 | (−0.39, 0.12) | |
| Negative | −0.22 | (−0.47, 0.04) | |
| Bayley-3 Cognitive | |||
| Positive | −0.44 | (−1.55, 0.66) | |
| Negative | −0.92 | (−1.98, 0.13) | |
| Bayley-3 Language | |||
| Positive | −0.61 | (−1.65, 0.44) | |
| Negative | −1.59 | (−2.84, −0.34) | 0.07 |
| Age 3 | |||
| Bayley-3 Cognitive | |||
| Positive | 0.96 | (0.11, 1.82) | 0.05 |
| Negative | 0.33 | (−0.53, 1.20) | |
| Bayley-3 Language | |||
| Positive | 0.16 | (−0.80, 1.13) | |
| Negative | −0.76 | (−1.81, 0.28) | |
P values were derived from permutation tests
Abbreviations: 95% CI = 95% Confidence Interval, BITSEA= Brief Infant and Toddler Social Emotional Assessment, WQS = Weighted Quantile Sum Regression
3.3. Effect Modification by Maternal Folate and Vitamin D
Seventeen percent of mothers were deficient or possibly deficient in folate, and 44% had vitamin D deficiency or possible deficiency.
There was no evidence for interactions of OH-PAHs and BITSEA outcomes for either folate or vitamin D deficiency (Figure 3). At age 2, there was evidence for interaction in associations of 1-hydroxynaphthalene and age 2 Bayley-3 Language scores for folate deficiency, however, the direction was contrary to what was expected. In mothers without folate deficiency, we observed lower Bayley-3 Language scores with higher 1-hydroxynaphthalene levels, whereas there was no evidence for associations for folate deficient mothers (β in non-deficient mothers = −0.71; 95% CI: −0.10, −1.32; β in deficient mothers = 0.77; 95% CI: 1.70, −0.15; Wald p=0.006) (Figure 3). Effects of 1-hydroxynapthalene were modest in the overall cohort and 95% confidence intervals included the null (β = −0.45, 95% CI: −1.01, 0.10) (Figure 3). There was no evidence for differences by folate deficiency on age 3 Bayley-3 outcomes (Figure 3). For vitamin D deficiency, we observed some evidence for differences for 1-hydroxynaphthalene and Bayley-3 Cognitive scores at ages 2 and 3 (Figure 4). At age 2, increasing 1-hydroxynaphthalene was associated with lower Cognitive scores in mothers without vitamin D deficiency and higher scores in vitamin D deficient mothers, but confidence intervals for both estimates included the null (β in non-deficient mothers = −0.45, 95% CI: 0.14 to −1.04; β in deficient mothers = 0.45 95% CI: 1.19 to −0.28; Wald p=0.05) (Figure 4). Similar results were observed at age 3, (Figure 4).
Figure 3. Estimated associations of OH-PAH metabolites and neurodevelopmental scores at age 2 and 3 in the CANDLE cohort by maternal folate deficiency.
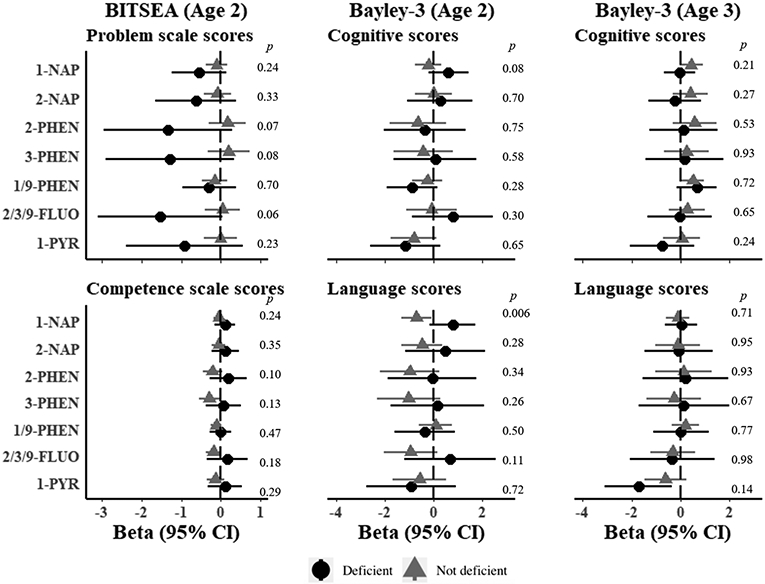
Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions with interaction terms between possible folate deficiency (folate ≤ 13.4 ng/mL) and individual OH-PAHs. All estimates represent effect per 2-fold increase in log OH-PAH. P-values for interaction calculated from Wald tests. Higher scores represent denote positive outcomes for all neurodevelopmental outcomes with the exception of BITSEA Problem scores, where higher scores denote worse outcomes. Estimates are adjusted for child sex, age at assessment, specific gravity, race, study site, maternal age, maternal education, maternal IQ, income adjusted for household size, marital status, parity, insurance, pre-pregnancy BMI, breastfeeding, maternal psychopathology, COI, prenatal cotinine, postnatal secondhand tobacco smoke exposure, postnatal maternal smoking. Abbreviations: OH-PAH = Hydroxy-Polycyclic Aromatic Hydrocarbon, 1-NAP= 1-hydroxynaphthalene; 2-NAP=2-hydroxynaphthalene; 2-PHEN=2-hydroxyphenanthrene; 3-PHEN=3-hydroxyphenanthrene; 1/9-PHEN=1/9-hydroxyphenanthrene; 2/3/9-FLUO=2/3/9-hydroxyfluorene; 1-PYR=1-hydroxypyrene; 95% CI = 95% Confidence Interval , BITSEA= Brief Infant and Toddler Social Emotional Assessment
Figure 4. Estimated associations of OH-PAH metabolites and neurodevelopmental scores at age 2 and 3 in the CANDLE cohort by maternal vitamin D deficiency.
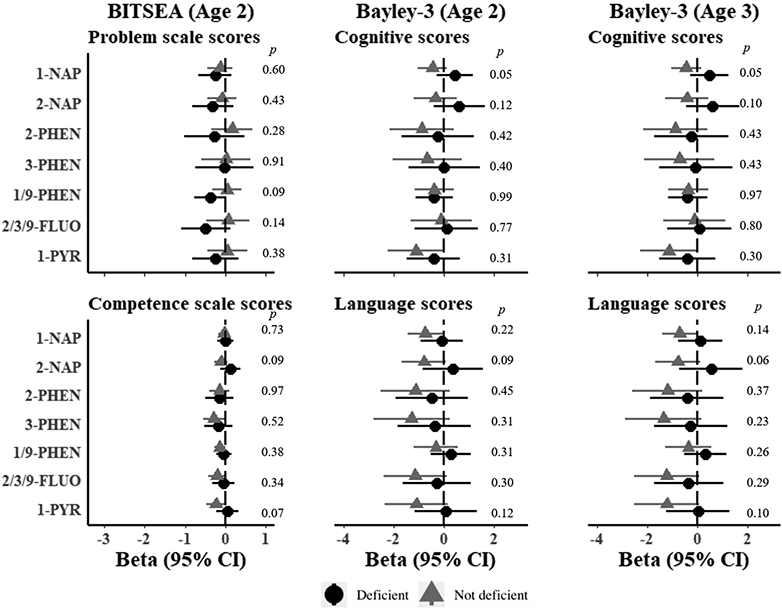
Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions with interaction terms between possible vitamin D deficiency (< 20 ng/mL) and individual OH-PAHs. All estimates represent effect per 2-fold increase in log OH-PAH. P-values for interaction calculated from Wald tests. Higher scores represent denote positive outcomes for all neurodevelopmental outcomes with the exception of BITSEA Problem scores, where higher scores denote worse outcomes. Estimates are adjusted for child sex, age at assessment, specific gravity, race, study site, maternal age, maternal education, maternal IQ, income adjusted for household size, marital status, parity, insurance, pre-pregnancy BMI, breastfeeding, maternal psychopathology, COI, prenatal cotinine, postnatal secondhand tobacco smoke exposure, postnatal maternal smoking. Abbreviations: OH-PAH = Hydroxy-Polycyclic Aromatic Hydrocarbon, 1-NAP= 1-hydroxynaphthalene; 2-NAP=2-hydroxynaphthalene; 2-PHEN=2-hydroxyphenanthrene; 3-PHEN=3-hydroxyphenanthrene; 1/9-PHEN=1/9-hydroxyphenanthrene; 2/3/9-FLUO=2/3/9-hydroxyfluorene; 1-PYR=1-hydroxypyrene; 95% CI = 95% Confidence Interval; BITSEA= Brief Infant and Toddler Social Emotional Assessment
3.4. Sensitivity Analyses
Results of sensitivity analyses to utilize alternative methods for the modeling of OH-PAH dilution (i.e. for specific gravity adjustment), for additional adjustment for confounding or precision variables, for consideration of mediating variables, and for consideration of non-linear OH-PAH and neurodevelopmental associations and are shown in Supplemental Tables 3 through 8 and Supplemental Figure 1. Application of alternative methods for specific gravity adjustment resulted in a modest shift in estimates away from the null, but most findings were not materially changed (Supplemental Tables 3 and 4). New notable adverse effects were observed for 1-hydroxypyrene and Cognitive scores at age 2 (β = −0.87; 95% CI: −1.72 to −0.02) (Supplemental Tables 3 and 4). Other estimates at age 2, including adverse effects for 2-hydroxyphenanthrene and BITSEA Competence scores and positive associations between 1/9-hydroxyphenanthrene and age 3 Cognitive scores, were consistent with the primary analyses. Adjustment for birthweight and gestational age resulted in a similar shift in estimates away from the null (Supplemental Tables 5 and 6). Maternal 1-hydroxypyrene was associated with a 0.93-point lower Cognitive score at age 2 (95% CI: −1.79, −0.06) (Supplemental Table 5). Other results were consistent with the primary analyses. Analyses which were restricted to 449 participants with KIDI and HOME scores and included additional adjustment for those scores are shown in Supplemental Tables 7 and 8. Compared to fully adjusted estimates in the full analytic sample, fully adjusted (but not additional adjustment for KIDI and HOME score) estimates in the restricted sample were shifted away from the null for most metabolites, but confidence intervals were also widened. Additional adjustment for KIDI and HOME scores among the restricted sample had little effect on the estimates (Supplemental Tables 7 and 8). At age 2, 1-hydroxynapthlathene was associated with lower Language scores in both fully adjusted models and with additional adjustment for KIDI and HOME scores, a finding we had not observed in the primary analysis with the full analytic sample (Supplemental Table 7). Results of GAM analyses to examine non-linearity in associations for evident findings in the main analyses are shown in Supplemental Figure 1. Plots of the partial residuals showed evidence of outliers in the association of 3-hydroxyphenanthrene and age 2 BITESA Competence, and adverse associations were attenuated to the null after removing the top and bottom 2.5% of 3-hydroxyphenanthrene observations (data not shown). All other associations were consistent with linearity (Supplemental Figure 1).
Results of analyses repeated to include 119 maternal smokers that were excluded a priori from the main analytic sample and adjusted for pre- and post-natal smoking are shown in Supplemental Tables 9 and 10. Null associations were observed across most metabolites. At age 2, 2/3/9-hydroxyfluorene was associated with a 0.96 lower Bayley-3 Language score (95% CI −1.86 to −0.05), which we had not observed in prior analyses.
4. DISCUSSION
To the best of our knowledge, this is the largest study to analyze prenatal PAH exposure and neurodevelopment and the first study to utilize novel methods to study mixtures of PAH metabolites and neurodevelopment. We examined associations with validated measures of age 2 behavior encompassing Problems and Competency (BITSEA) and of age 2 and 3 year Cognition and Language (Bayley-3). Most associations were null and were mixed in direction across metabolites and outcomes. Of the individual OH-PAHs, we observed that 1-hydroxypyrene was associated with a higher risk for meeting clinical cutoff scores for neurodevelopmental delay at age 2. Other associations were in the opposite direction. Higher levels of 1/9-hydroxyphenanthrene were associated with higher Cognitive scores at age 3 and 1-hydroxynaphthalene was associated with a lower risk of the clinical cutoff for Behavior Problems at age 2, a finding we had not observed when behavior problems from the BITSEA were analyzed as a continuous score. Descriptively, we observed that OH-PAH metabolite concentrations were higher in mothers with a high school education or less, or those with Medicaid insurance without health insurance.
Our WQS analyses questioned whether mixtures of OH-PAHs may be important for neurodevelopmental outcomes, even for metabolites where no effects were seen when analyzed individually. We observed negative associations between age 2 Bayley Language scores and an OH-PAH mixtures with high index weights for 2/3/9-hydroxyfluorene and 1-hydroxynaphthalene, although these were not significant after application of the permutation tests, and positive associations between age 3 Bayley Cognitive scores and an OH-PAH mixture with high index weights for 1/9-hydroxyphenanthrene, 2-hydroxynaphthalene, and 2-hydroxyphenanthrene, which were robust to the permutation test. The mechanisms underlying potential adverse effects of particular PAH combinations remain to be understood. Existing toxicological studies have focused on the effects of individual PAHs or sums over parent compounds or the total PAHs experienced via a specific pathway (e.g. air concentrations). Yet it is likely the harmful effects of PAHs in human populations occur from the experience of exposure to complex mixtures with unique pathophysiological mechanisms reflecting the components (Agency for Toxic Substances and Disease Registry (ATSDR), 1995).
We also provide novel data on potential interaction of nutritional factors (vitamin D and folate sufficiency) with maternal prenatal OH-PAHs,. We did not observe support for our hypotheses that maternal deficiency in folate or vitamin D would be associated with more pronounced adverse effects of maternal OH-PAHs on child neurodevelopment A previous CANDLE study reported a positive and dose-dependent association of maternal 25(OH)D levels with cognitive and language scores at age 2 years (Tylavsky et al., 2015) and IQ at age 4 (Melough et al., 2020), motivating our interest in examining a potential protective modifying effect of vitamin D (and folate) status. In this study overall, effect estimates based on these factors were generally not in the hypothesized direction, i.e., they did not suggest sufficient vitamin D or folate in pregnancy protected against adverse PAH effects. For example, for 1-hydroxynapthalene and age 2 Language, a positive effect was observed in the group with mothers who met the clinical cutoff for folate deficiency, whereas the estimate was negative for mothers who met definition of sufficient levels of folate in pregnancy. A somewhat similar unexpected interaction effect for this metabolite (1-hydroxynaphthalene) was observed in relation to vitamin D status and cognition. Toddlers of mothers who met the clinical cut off for deficiency of vitamin D in pregnancy had on average higher cognitive scores at age 2 and 3 with increasing exposure to this metabolite. Contrarily, the effect estimate was negative in the toddlers of mothers with sufficient vitamin D in pregnancy. Our findings related to 1-hydroxynaphthalene are difficult to explain from a biological mechanism perspective and may reflect type 1 error.
The mechanisms through which PAHs are believed to cause harm to the developing brain are not well understood. Proposed mechanisms include damages to DNA resulting in activation of apoptotic pathways(Nicol et al., 1995), endocrine disruption (Takeda et al., 2004), oxidative stress(Saunders et al., 2006), and disruption to pathways regulating fetal development of the central nervous system. Toxicological studies point to impairment to long-term potentiation in the hippocampus(D. D. Wormley et al., 2004; Deanna D Wormley et al., 2004), a critical component of learning and memory, and dysregulation of N-methyl-D-aspartate receptor mRNA expression, essential to multiple processes of neuronal developmental include neuronal differentiation, synapse formation, and synaptic plasticity(Brown et al., 2007; McCallister et al., 2008; D. D. Wormley et al., 2004; Deanna D Wormley et al., 2004; Zhang et al., 2016).
Direct comparison of the magnitude of our observed associations to other published work is challenged by the fact that most studies to date have analyzed PAH exposures dichotomously (e.g. high vs. low exposure), do not employ urinary metabolite levels for exposure assessment, and in most cases use a summed measure of prenatal PAH exposure, not individual metabolites. Findings are mixed across studies and within cohort over time. In the United States, the Columbia Center for Children’s Environmental Health (CCCEH) cohort in New York City, comprised of African-American and Dominican mothers, prenatal PAH exposure based on maternal personal sampling of airborne PAH and neurodevelopmental outcomes assessed as 12, 24, and 36 months. No differences were found in neurodevelopmental outcomes at age 12 and 24 months, but at 36 months children in the highest quartile of PAH exposure experienced a nearly 6 point drop in Bayley-2 Mental Development Index scores at 36 months and a nearly 3-fold odds of being developmentally delayed compared to children’s whose mothers below the 4th quartile (Perera et al., 2006). These effect sizes were greater than those observed for any individual OH-PAH metabolite in our own study that utilized the newer Bayley-3 assessment, which separates the previous Mental Development Index into the cognitive and language domains. Later analyses at age 5 showed that children with prenatal air PAH exposures above the median scored 4.3 to 4.7 points lower on IQ and verbal scores compared to those below the median (Perera et al., 2009). A second NYC cohort, comprised of a diverse sample of approximately 100 mother-child pairs located in the vicinity of the World Trade Center disaster, observed no direct associations between continuous prenatal cord blood PAH levels and Bayley-2 neurodevelopmental scores at age 3, although the authors did observe interaction by environmental tobacco smoke exposure. (Perera et al., 2007). Cross-sectional evidence in the U.S. comes from children aged 6-15 years participating in the National Health and Nutrition Examination Survey (NHANES) (Abid et al., 2014). Children with urine OH-PAH levels of 2/3-hydroxyfluorene above the median had a 2-fold greater odds of receiving special education services compared to children with concentrations below the median but results were null when OH-PAHs were modeled continuously. No associations were found for any other OH-PAHs, parent compounds, or for other outcomes, including parent-reported ADHD and learning disability (Abid et al., 2014).
Internationally, three cohorts in China have examined prenatal PAH exposures in urine or cord blood and toddler-age neurodevelopment using the Gesell Developmental Schedules (GDS), with mixed findings across studies(Cao et al., 2020; Lin et al., 2021; Tang et al., 2008). The first study was conducted in 158 mother-child pairs residing in Taiyuan City, a region with heavy pollution from coal burning and chemical and metallurgical industries (Cao et al., 2020). Inverse trends were observed for several individual urinary OH-PAH metabolites as well as sum OH-PAHs for GDS motor score, but there were no associations for other domains of the GDS, including adaptive behavior, language, and social scores. In the second study, conducted in Tongliang where the major source of PAH exposure was a seasonally operated coal-fired power plant, the authors observed large but imprecise adverse effect sizes at age 2 when comparing woman above vs. below the median umbilical cord blood leukocyte benzo(a)pyrene (BaP)-DNA adduct levels and motor, language, and average GDS scores (Tang et al., 2008). Later findings showed no associations between cord leukocyte BaP-DNA adducts and age 5 year IQ, although they observed interactions between BaP-DNA adducts and secondhand tobacco smoke exposure on IQ (Perera et al., 2012). The second study conducted in Qingdao examined associations of BaP-DNA adducts collected in umbilical cord blood leukocytes as well as postnatal urine OH-PAHs collected in the children at 12 months of age (Lin et al., 2021). The authors observed negative associations prenatally for BaP-DNA adducts and fine and gross motor domains and social behavior quotient scores and for postnatal associations of 1/9-hydroxyphenanthrene and fine motor skills. Covariate adjustment in the Tongliang and Qingdao Chinese studies was minimal however and lacked the demographic and prenatal characteristics included in other studies of prenatal PAH exposure and neurodevelopment, including this study (e.g. maternal IQ, home or neighborhood characteristics), and results of these studies may reflect confounding bias. In our own study we observed significant attenuation of estimates between minimally and fully adjusted models, supporting the need for careful consideration of confounding. Other international cohorts in Europe have assessed PAH exposures and neurodevelopment at older ages than examined in this study, with evidence for neurodevelopmental or behavioral deficits in some (Edwards et al., 2010; Genkinger et al., 2015; Jedrychowski et al., 2015), but not all, studies (Jorcano et al., 2019; Mortamais et al., 2017).
Differing sources and magnitudes of PAH exposures may contribute to the mixed findings across studies. We observed approximately similar distributions in urinary metabolite concentrations in CANDLE mothers to those in other PAH exposure studies of pregnant and non-pregnant women conducted in the U.S. and Canada, as well as in the cross-sectional assessments of postnatal urine OH-PAHs in toddlers (Cathey et al., 2018; Lin et al., 2021; Nethery et al., 2012; Wheeler et al., 2014). Some epidemiological studies of PAHs and neurodevelopment, such as the one in Tongliang, China, were situated in an area with a known source of high PAH exposure (i.e. coal-fired power plant), while other cohorts, such as those in New York City, and Krakow, Poland, were recruited in urban or inner-city settings (Edwards et al., 2010; Tang et al., 2008).
Our study suggests that both individual OH-PAHs, particularly 1-hydroxypyrene, as well as mixtures of hydroxyfluorene, hydroxynaphthalene, and hydroxyphenanthrene, may adversely influence neurodevelopment in very early childhood. Research on sources of individual OH-PAHs suggests that 1-hydroxypyrene levels are consistently associated with smoking, occupational exposures, living in areas of higher ambient air pollution, proximity to major roadways (i.e. higher traffic exposure), and the consumption of charbroiled and barbequed meat (Ciarrocca et al., 2014; Kang et al., 1995; Nethery et al., 2012; Roggi et al., 1997; Rostron et al., 2020; Strickland et al., 1996; Yuan et al., 2015). Naphthalene, fluorene, and phenanthrene share sources in common with 1-hydroxypyrene, including tobacco smoke and industrial sources (i.e. oil and coal tar refineries) (Ding et al., 2005; Jia and Batterman, 2010). Additional common sources of naphthalene include off-gassing of mothballs, pesticides, and fumigants (Jia and Batterman, 2010).
Interpretation of our results should consider several study limitations. OH-PAH exposures were based a single urine collection during pregnancy and we lacked multiple measurements to capture variability in concentrations over pregnancy although evidence also shows that PAHs can accumulate in fatty tissue compartments such as fat and brain(Pastor-Belda et al., 2019) and urinary OH-PAHs may reflect chronic exposures. Urine is known to approximate short-term exposures to PAH, with a half-life of 6-35 hours (Jongeneelen et al., 1990), and does not represent a measure of cumulative PAH burden over pregnancy. One study in Puerto Rico where OH-PAH urine measurements were performed at two points in pregnancy showed weak to moderate intraclass correlation coefficients, ranging from 0.1 to 0.4 (Cathey et al., 2018). A second study in Canada observed no consistent trends in OH-PAH concentrations across trimesters of pregnancy (Nethery et al., 2012). Furthermore, PAH exposure occurs as a complex mixture of unsubstituted PAHs and PAHs with hydroxy, oxo, nitro, and organic functional groups(Andersson and Achten, 2015; Gbeddy et al., 2020) and the monohydroxylated PAH metabolites may not fully capture this complex exposure nor fully characterize the toxicity of other potentially more toxic metabolites, such as tetrols (Luo et al., 2019). Similar limitations apply to assessments of prenatal nutritional and smoking status. We defined all study hypotheses and analyses a priori and did not adjust analyses for multiple comparisons; as a result we cannot exclude the possibility for chance findings. Vitamin D deficiency was based on a single measure in pregnancy and folate deficiency based on two prenatal measures, and the conflicting results we observed for these modifying factors may reflect bias from misclassification. Likewise we used up to two measures of urinary cotinine to define prenatal smoking status and to adjust for prenatal secondhand smoke exposure and lacked a biomarker for longer-term or cumulative smoking. While this variability is consistent with other prenatal exposures commonly assessed with a single urine specimen, such as phthalates (Johns et al., 2015), it nevertheless suggests that multiple assessments may yield more accurate estimates of PAH exposure and nutritional and smoking status throughout pregnancy. Our use of a single measure may introduce nondifferential exposure misclassification and bias our findings toward the null.
There were however, several important strengths of this study. This includes the diverse and well-characterized prospective cohort from the U.S. South, a traditionally understudied region, with excellent retention of the families over time and rich information about socio-demographics, prenatal health and nutrition, factors in the home associated with neurodevelopmental outcomes, and measurement of actual urinary biomarkers of exposures. The attenuation of findings in our final models with adjustment for these important cofactors underscores the importance of adequate confounder control in environmental neurodevelopmental epidemiology and strengthens confidence in our null findings after full adjustment. This is the largest study to examine associations of PAH and toddler age neurodevelopment. While the use of urine to measure PAH exposure does have the limitations described above, it has the benefit of capturing all routes of exposure in a mother, both hair and diet. This is also the first study to apply methods to assess OH-PAH mixtures and to have improved upon existing work in WQS with the application of the permutation test.
Future work in this area of inquiry in the ECHO PATHWAYS consortium will examine whether these findings persist in children at older ages and include children with harmonized data in additional cohorts. As children age, assessments can provide additional, sometimes more sensitive measures of cognition and behavior, particularly for subscales of IQ and for multiple domains of behavior problems and syndromes (e.g. attention, ADHD). Due to the short window of time between the age 2 and 3 year assessment we did not seek to perform a repeated measures analysis of developmental trajectory, but future studies with additional endpoints at future ages could investigate longitudinal associations.
Conclusions
Importantly, in this well characterized, large, socioeconomically and racially diverse U.S. cohort, we observed that prenatal PAH exposure, measured both as individual urinary OH-PAHs as well as complex mixtures, had mixed associations with neurodevelopmental outcomes in toddler-age children, with evidence for higher risk of neurodevelopmental delay but also evidence of positive effects for some metabolites and outcomes. We found no evidence for effect modification based on maternal vitamin D or folate status. These results complement and build upon a growing literature to further discern potential neurodevelopmental harms associated with prenatal PAH exposure and young children. While the magnitude of deficits observed for some of the tested associations is small (e.g., 20% more likely to meet clinical cutoff for age 2 year risk for neurodevelopment delay for doubling of maternal PAH), these confer significant burden and concern for affected families and communities. Approximately one in six children in the U.S. have a developmental disability, and the prevalence of developmental disabilities, ADHD, autism spectrum disorder, and intellectual disabilities have steadily increased over time (Zablotsky et al., 2019). Results from this and prior studies suggest that populations already susceptible to health disparities, such as low socioeconomic status, have higher PAH exposures and may be particularly vulnerable. Future studies are needed to hone the science of PAH exposure and child neurodevelopment, including assessment of critical windows of exposure, additional inquiry into mixture related effects, evaluation of the trajectory of outcomes from early to later childhood, and assessment of potential protective effect modifiers.
Supplementary Material
Highlights:
This study examined associations of prenatal PAH and child neurodevelopment
The study population was a large and diverse longitudinal birth cohort
We employed novel methods to examine PAH mixtures and child neurodevelopment
This study is the first to examine differences in PAH effects by prenatal nutrition
We observed some support for adverse associations between PAHs and neurodevelopment
Acknowledgements:
ECHO PATHWAYS is funded by NIH (1UG3OD023271-01, 4UH3OD023271-03). This research was also supported by the University of Washington EDGE Center of the National Institutes of Health under award number: P30ES007033. The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute and NIH (R01 HL109977). We are grateful for the participation of families enrolled in the CANDLE study, as well as the dedication of CANDLE research staff and investigators.
Footnotes
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT author statement
Erin R. Wallace: Conceptualization, Methodology, Formal analysis, Writing – Original Draft, Visualization
Yu Ni: Methodology, Formal analysis, Visualization, Writing – Review & Editing
Christine T. Loftus: Methodology, Writing – Review & Editing
Alexis Sullivan: Methodology, Writing – Review & Editing
Erin Masterson: Methodology, Writing – Review & Editing
Adam A. Szpiro: Methodology, Writing – Review & Editing
Drew B. Day: Formal analysis, Writing – Review & Editing
Morgan Robinson: Investigation, Writing – Review & Editing
Kurunthachalam Kannan: Investigation, Writing – Review & Editing
Fran A. Tylavasky: Writing – Review & Editing
Sheela Sathyanarayana: Methodology, Writing – Review & Editing
Nicole R. Bush: Methodology, Writing – Review & Editing
Kaja Z. LeWinn: Methodology, Writing – Review & Editing
Catherine J. Karr: Conceptualization, Methodology, Writing – Review & Editing, Supervision, Funding acquisition
1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene, 95% CI = 95% Confidence Interval, BITSEA= Brief Infant and Toddler Social Emotional Assessment, BMI=Body mass index, BSI = Brief Symptom Index, GSI = Global Severity Index, HOME = Home Observation Measurement of the Environment, KIDI = Knowledge of Infant Development Inventory, OH-PAH = Hydroxy-Polycyclic Aromatic Hydrocarbon, RR = Relative Risk, SD = Standard deviation, WQS = Weighted Quantile Sum Regression
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abid Z, Roy A, Herbstman JB, Ettinger AS, 2014. Urinary polycyclic aromatic hydrocarbon metabolites and attention/deficit hyperactivity disorder, learning disability, and special education in U.S. children aged 6 to 15. Journal of environmental and public health 2014, 628508. 10.1155/2014/628508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Garcia D, McArdle N, Hardy EF, Crisan UI, Romano B, Norris D, Baek M, Reece J, 2014. The child opportunity index: improving collaboration between community development and public health. Health affairs (Project Hope) 33, 1948–1957. 10.1377/hlthaff.2014.0679 [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 1995. Toxicological profile for polycyclic aromatic hydrocarbons, U.S. Department of Health and Human Services. [PubMed]
- Andersson JT, Achten C, 2015. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycyclic aromatic compounds 35, 330–354. 10.1080/10406638.2014.991042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod BN, 2002. Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment 9, 17–23. 10.1177/1073191102009001003 [DOI] [PubMed] [Google Scholar]
- Bayley N, 2006. Bayley scales of infant and toddler development. PsychCorp, Pearson. [Google Scholar]
- Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, Chou D, Say L, Modi N, Katz J, Vos T, Marlow N, Lawn JE, 2013. Preterm birth–associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatric Research 2013 74:1 74, 17–34. 10.1038/pr.2013.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, Cicchetti DV, 2004. The Brief Infant-Toddler Social and Emotional Assessment: screening for social-emotional problems and delays in competence. Journal of pediatric psychology 29, 143–155. 10.1093/jpepsy/jsh017 [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, McCarthy K, Augustyn M, Caronna E, Clark R, 2013. Clinical validity of a brief measure of early childhood social-emotional/behavioral problems. Journal of pediatric psychology 38, 577–587. 10.1093/jpepsy/jst014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson C. v., Ramesh A, Sheng L, McCallister MM, Jiang GCT, Aschner M, Hood DB, 2007. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. NeuroToxicology 28, 965–978. 10.1016/J.NEURO.2007.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Sanchez Guerra M, Gennings C, Hacker M, Jara C, Bosquet Enlow M, Wright RO, Baccarelli A, Wright RJ, 2017. Maternal Lifetime Stress and Prenatal Psychological Functioning and Decreased Placental Mitochondrial DNA Copy Number in the PRISM Study. American journal of epidemiology 186, 1227–1236. 10.1093/aje/kwx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burniaux J-M, Dang T-T, Fore D, Förster M, d'Ercole MM, Oxley H, 1998. Income Distribution and Poverty in Selected OECD Countries. 10.1787/730801800603 [DOI] [Google Scholar]
- Cao X, Li J, Cheng L, Deng Y, Li Y, Yan Z, Duan L, Yang J, Niu Q, Perera F, Nie J, Tang D, 2020. The associations between prenatal exposure to polycyclic aromatic hydrocarbon metabolites, umbilical cord blood mitochondrial DNA copy number, and children’s neurobehavioral development. Environmental Pollution 265. 10.1016/J.ENVPOL.2020.114594 [DOI] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P, 2015. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of agricultural, biological, and environmental statistics 20, 100–120. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Lancellotti C, Tur JA, Uauy R, 2013. Impact of folic acid fortification of flour on neural tube defects: a systematic review. Public health nutrition 16, 901–911. 10.1017/S1368980012003576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, McElrath TF, Cantonwine DE, Pace G, Alshawabkeh A, Cordero JF, Meeker JD, 2018. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environmental pollution (Barking, Essex : 1987) 232, 556–562. 10.1016/j.envpol.2017.09.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrocca M, Rosati MV, Tomei F, Capozzella A, Andreozzi G, Tomei G, Bacaloni A, Casale T, Andrè JC, Fioravanti M, Cuartas MF, Caciari T, 2014. Is urinary 1-hydroxypyrene a valid biomarker for exposure to air pollution in outdoor workers? A meta-analysis. Journal of Exposure Science & Environmental Epidemiology 24, 17–26. 10.1038/jes.2012.111 [DOI] [PubMed] [Google Scholar]
- Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK, Hartge P, Ward MH, Wheeler DC, 2015a. Analysis of Environmental Chemical Mixtures and Non-Hodgkin Lymphoma Risk in the NCI-SEER NHL Study. Environmental health perspectives 123, 965–970. 10.1289/ehp.1408630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnota J, Gennings C, Wheeler DC, 2015b. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer informatics 14, 159–171. 10.4137/CIN.S17295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, 1993. BSI brief symptom inventory. Administration, scoring, and procedures manual. [Google Scholar]
- Ding YS, Trommel JS, Yan XJ, Ashley D, Watson CH, 2005. Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from domestic cigarettes. Environmental science & technology 39, 471–478. 10.1021/es048690k [DOI] [PubMed] [Google Scholar]
- Dutta K, Ghosh D, Nazmi A, Kumawat KL, Basu A, 2010. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PloS one 5, e9984. 10.1371/journal.pone.0009984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, Flak E, Li Z, Wang S, Rauh V, Perera F, 2010. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environmental health perspectives 118, 1326–1331. 10.1289/ehp.0901070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen HLF, Kesmodel US, Underbjerg M, Kilburn TR, Bertrand J, Mortensen EL, 2013. Predictors of intelligence at the age of 5: Family, pregnancy and birth characteristics, postnatal influences, and postnatal growth. PLoS ONE 8. 10.1371/JOURNAL.PONE.0079200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenburg WK, Coons CE, 1986. Home Screening Questionnaire: its validity in assessing home environment. The Journal of pediatrics 108, 624–626. 10.1016/s0022-3476(86)80853-8 [DOI] [PubMed] [Google Scholar]
- Gbeddy G, Egodawatta P, Goonetilleke A, Ayoko G, Chen L, 2020. Application of quantitative structure-activity relationship (QSAR) model in comprehensive human health risk assessment of PAHs, and alkyl-, nitro-, carbonyl-, and hydroxyl-PAHs laden in urban road dust. Journal of hazardous materials 383, 121154. 10.1016/j.jhazmat.2019.121154 [DOI] [PubMed] [Google Scholar]
- Genkinger JM, Stigter L, Jedrychowski W, Huang T-J, Wang S, Roen EL, Majewska R, Kieltyka A, Mroz E, Perera FP, 2015. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure, antioxidant levels and behavioral development of children ages 6-9. Environmental research 140, 136–144. 10.1016/j.envres.2015.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Senthilkumar K, Alomirah H, Moon H-B, Minh TB, Mohd MA, Nakata H, Kannan K, 2013. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environmental science & technology 47, 2932–2938. 10.1021/es3052262 [DOI] [PubMed] [Google Scholar]
- Holick MF, 2007. Vitamin D deficiency. New England Journal of Medicine 357, 266–281. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera FP, Tang D, Stigter L, Mroz E, Flak E, Spengler J, Budzyn-Mrozek D, Kaim I, Jacek R, 2012. Impact of barbecued meat consumed in pregnancy on birth outcomes accounting for personal prenatal exposure to airborne polycyclic aromatic hydrocarbons: Birth cohort study in Poland. Nutrition (Burbank, Los Angeles County, Calif.) 28, 372–377. 10.1016/j.nut.2011.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R, Sowa A, 2015. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environmental science and pollution research international 22, 3631–3639. 10.1007/s11356-014-3627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Majewska R, Camman D, Spengler JD, Mroz E, Stigter L, Flak E, Jacek R, 2014. Separate and joint effects of tranplacental and postnatal inhalatory exposure to polycyclic aromatic hydrocarbons: prospective birth cohort study on wheezing events. Pediatric pulmonology 49, 162–172. 10.1002/ppul.22923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Batterman S, 2010. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. International journal of environmental research and public health 7, 2903–2939. 10.3390/ijerph7072903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A, Meeker JD, 2015. Exposure assessment issues in epidemiology studies of phthalates. Environment international 85, 27–39. 10.1016/j.envint.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneelen FJ, van Leeuwen FE, Oosterink S, Anzion RB, van der Loop F, Bos RP, van Veen HG, 1990. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. British journal of industrial medicine 47, 454–461. 10.1136/oem.47.7.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcano A, Lubczyńska MJ, Pierotti L, Altug H, Ballester F, Cesaroni G, el Marroun H, Fernández-Somoano A, Freire C, Hanke W, Hoek G, Ibarluzea J, Iñiguez C, Jansen PW, Lepeule J, Markevych I, Polańska K, Porta D, Schikowski T, Slama R, Standl M, Tardon A, Vrijkotte TGM, von Berg A, Tiemeier H, Sunyer J, Guxens M, 2019. Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environment international 131, 104927. 10.1016/j.envint.2019.104927 [DOI] [PubMed] [Google Scholar]
- Julvez J, Fortuny J, Mendez M, Torrent M, Ribas-Fitó N, Sunyer J, 2009. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatric and perinatal epidemiology 23, 199–206. [DOI] [PubMed] [Google Scholar]
- Kang DH, Rothman N, Poirier MC, Greenberg A, Hsu CH, Schwartz BS, Baser ME, Groopman JD, Weston A, Strickland PT, 1995. Interindividual differences in the concentration of 1-hydroxypyrene-glucuronide in urine and polycyclic aromatic hydrocarbon-DNA adducts in peripheral white blood cells after charbroiled beef consumption. Carcinogenesis 16, 1079–1085. 10.1093/carcin/16.5.1079 [DOI] [PubMed] [Google Scholar]
- Kannan K, Stathis A, Mazzella MJ, Andra SS, Barr DB, Hecht SS, Merrill LS, Galusha AL, Parsons PJ, 2021. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. International Journal of Hygiene and Environmental Health 234. 10.1016/J.IJHEH.2021.113741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Turkheimer Eric, Ohlsson H, Sundquist J, Sundquist K, 2015. Family environment and the malleability of cognitive ability a Swedish national home-reared and adopted-away cosibling control study. Proceedings of the National Academy of Sciences of the United States of America 112, 4612–4617. 10.1073/PNAS.1417106112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Najman JM, Batty GD, O’Callaghan MJ, Williams GM, Bor W, 2006. Early life predictors of childhood intelligence: Findings from the Mater-University study of pregnancy and its outcomes. Paediatric and Perinatal Epidemiology 20, 148–162. 10.1111/J.1365-3016.2006.00704.X [DOI] [PubMed] [Google Scholar]
- Levine L, Fahy J, 1945. Evaluation of urinary lead concentrations. I. The significance of the specific gravity. J Ind Hyg Toxicol 27, 217–223. [Google Scholar]
- LeWinn KZ, Bush NR, Batra A, Tylavsky F, Rehkopf D, 2020. Identification of Modifiable Social and Behavioral Factors Associated With Childhood Cognitive Performance. JAMA pediatrics 174, 1063–1072. 10.1001/jamapediatrics.2020.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinn KZ, Bush NR, Batra A, Tylavsky F, Rehkopf D, 2020. Identification of Modifiable Social and Behavioral Factors Associated with Childhood Cognitive Performance. JAMA Pediatrics 174, 1063–1072. 10.1001/JAMAPEDIATRICS.2020.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Sun Z, Liu Y, Zhang Qian, Zhang Qi, Wang B, Gao R, Yu W, 2021. Influence of postnatal polycyclic aromatic hydrocarbon exposure on the neurodevelopment of toddlers at the age of 12 months. NeuroToxicology 82, 45–49. 10.1016/j.neuro.2020.10.013 [DOI] [PubMed] [Google Scholar]
- Loftus CT, Bush NR, Day DB, Ni Y, Tylavsky FA, Karr CJ, Kannan K, Barrett ES, Szpiro AA, Sathyanarayana S, LeWinn KZ, 2021. Exposure to prenatal phthalate mixtures and neurodevelopment in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. Environment international 150, 106409. 10.1016/j.envint.2021.106409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus CT, Hazlehurst MF, Szpiro AA, Ni Y, Tylavsky FA, Bush NR, Sathyanarayana S, Carroll KN, Karr CJ, LeWinn KZ, 2019. Prenatal air pollution and childhood IQ: Preliminary evidence of effect modification by folate. Environmental Research 176. 10.1016/J.ENVRES.2019.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Stepanov I, Hecht SS, 2019. Chemical biomarkers of exposure and early damage from potentially carcinogenic airborne pollutants. Ann. Cancer Epidemiol 3. [Google Scholar]
- MacPhee D, 1981. Knowledge of Infant Development Inventory. [Google Scholar]
- McCallister MM, Maguire M, Ramesh A, Aimin Q, Liu S, Khoshbouei H, Aschner M, Ebner FF, Hood DB, 2008. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology 29, 846–854. 10.1016/j.neuro.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melough MM, Murphy LE, Graff JC, Derefinko KJ, LeWinn KZ, Bush NR, Enquobahrie DA, Loftus CT, Kocak M, Sathyanarayana S, Tylavsky FA, 2021. Maternal Plasma 25-Hydroxyvitamin D during Gestation Is Positively Associated with Neurocognitive Development in Offspring at Age 4-6 Years. The Journal of nutrition 151, 132–139. 10.1093/jn/nxaa309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, van Drooge BL, Macià D, Martínez-Vilavella G, Reynes C, Sabatier R, Rivas I, Grimalt J, Forns J, Alvarez-Pedrerol M, Querol X, Sunyer J, 2017. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environment international 105, 12–19. 10.1016/j.envint.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Nethery E, Wheeler AJ, Fisher M, Sjödin A, Li Z, Romanoff LC, Foster W, Arbuckle TE, 2012. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: A pilot study among pregnant women. Journal of Exposure Science and Environmental Epidemiology. 10.1038/jes.2011.32 [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Harrison ML, Laposa RR, Gimelshtein IL, Wells PG, 1995. A teratologic suppressor role for p53 in benzo(a)pyrene–treated transgenic p53-deficient mice. Nature Genetics 10, 181–187. 10.1038/NG0695-181 [DOI] [PubMed] [Google Scholar]
- Onyemauwa F, Rappaport SM, Sobus JR, Gajdosová D, Wu R, Waidyanatha S, 2009. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 877, 1117–1125. 10.1016/j.jchromb.2009.02.067 [DOI] [PubMed] [Google Scholar]
- Organization, W.H., 2015. Serum and red blood cell folate concentrations for assessing folate status in populations. World Health Organization. [Google Scholar]
- Pastor-Belda M, Campillo N, Arroyo-Manzanares N, Torres C, Pérez-Cárceles MD, Hernández-Córdoba M, Viñas P, 2019. Bioaccumulation of Polycyclic Aromatic Hydrocarbons for Forensic Assessment Using Gas Chromatography-Mass Spectrometry. Chemical Research in Toxicology 32, 1680–1688. 10.1021/ACS.CHEMRESTOX.9B00213 [DOI] [PubMed] [Google Scholar]
- Perera F, Li TY, Lin C, Tang D, 2012. Effects of prenatal polycyclic aromatic hydrocarbon exposure and environmental tobacco smoke on child IQ in a Chinese cohort. Environmental research 114, 40–46. 10.1016/j.envres.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Perera F, Tang D, Whyatt R, Lederman SA, Jedrychowski W, 2005. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 14, 709–714. 10.1158/1055-9965.EPI-04-0457 [DOI] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, Rauh V, 2009. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 124, e195–202. 10.1542/peds.2008-3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, Diaz D, Dietrich J, Reyes A, Kinney PL, 2005. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology 26, 573–587. 10.1016/j.neuro.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai W-Y, Tang D, Diaz D, Hoepner L, Barr D, Tu Y-H, Camann D, Kinney P, 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental health perspectives 114, 1287–1292. 10.1289/ehp.9084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Rauh V, Tu YH, Tsai WY, Becker M, Stein JL, King J, Del Priore G, Lederman SA, 2007. Relationship between polycyclic aromatic hydrocarbon-DNA adducts, environmental tobacco smoke, and child development in the World Trade Center cohort. Environmental health perspectives 115, 1497–1502. 10.1289/ehp.10144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, Semanek D, Perera F, 2015. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA psychiatry 72, 531–540. 10.1001/jamapsychiatry.2015.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, Curtin P, Just AC, Bello G, Gennings C, 2020. gWQS: Generalized Weighted Quantile Sum Regression. [Google Scholar]
- Ritchie SJ, Tucker-Drob EM, 2018. How Much Does Education Improve Intelligence? A Meta-Analysis. Psychological Science 29, 1358–1369. 10.1177/0956797618774253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggi C, Minoia C, Sciarra GF, Apostoli P, Maccarini L, Magnaghi S, Cenni A, Fonte A, Nidasio GF, Micoli G, 1997. Urinary 1-hydroxypyrene as a marker of exposure to pyrene: an epidemiological survey on a general population group. The Science of the total environment 199, 247–254. 10.1016/s0048-9697(97)05458-2 [DOI] [PubMed] [Google Scholar]
- Rostron BL, Corey CG, Chang JT, van Bemmel DM, Miller ME, Chang CM, 2020. Changes in Cigarettes per Day and Biomarkers of Exposure Among US Adult Smokers in the Population Assessment of Tobacco and Health Study Waves 1 and 2 (2013-2015). Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 22, 1780–1787. 10.1093/ntr/ntaa038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C, Magnus P, Schjølberg S, Stoltenberg C, Surùn P, McKeague IW, Smith GD, Reichborn-Kjennerud T, Susser E, 2011. Folic acid supplements in pregnancy and severe language delay in children. Jama 306, 1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kocak M, Hartman TJ, Vereen S, Adgent M, Piyathilake C, Tylavsky FA, Carroll KN, 2018. Association of prenatal folate status with early childhood wheeze and atopic dermatitis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 29, 144–150. 10.1111/pai.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team, 2020. RStudio: Integrated Development Environment for R. [Google Scholar]
- Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S, 2006. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: Role of oxidative stress. Journal of Applied Toxicology 26, 427–438. 10.1002/JAT.1157 [DOI] [PubMed] [Google Scholar]
- Saunders CR, Ramesh A, Shockley DC, 2002. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicology letters 129, 33–45. 10.1016/s0378-4274(01)00467-2 [DOI] [PubMed] [Google Scholar]
- Saunders CR, Shockley DC, Knuckles ME, 2003. Fluoranthene-induced neurobehavioral toxicity in F-344 rats. International journal of toxicology 22, 263–276. 10.1080/10915810305114 [DOI] [PubMed] [Google Scholar]
- Schick SF, Blount BC, Jacob PR, Saliba NA, Bernert JT, El Hellani A, Jatlow P, Pappas RS, Wang L, Foulds J, Ghosh A, Hecht SS, Gomez JC, Martin JR, Mesaros C, Srivastava S, St Helen G, Tarran R, Lorkiewicz PK, Blair IA, Kimmel HL, Doerschuk CM, Benowitz NL, Bhatnagar A, 2017. Biomarkers of exposure to new and emerging tobacco delivery products. American journal of physiology. Lung cellular and molecular physiology 313, L425–L452. 10.1152/ajplung.00343.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H, 2011. Developmental Brain and Behavior Toxicity of Air Pollutants: A Focus on the Effects of Polycyclic Aromatic Hydrocarbons (PAHs). Critical Reviews in Environmental Science and Technology 41, 2026–2047. 10.1080/10643389.2010.495644 [DOI] [Google Scholar]
- Slotkin TA, Seidler FJ, 2009. Benzo[a]pyrene impairs neurodifferentiation in PC12 cells. Brain research bulletin 80, 17–21. 10.1016/j.brainresbull.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani F, Zaheri F, Abdi F, 2014. Long-term neurodevelopmental outcomes after preterm birth. Iranian Red Crescent medical journal 16, e17965–e17965. 10.5812/ircmj.17965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, Tylavsky F, 2015. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description. RAND Corporation, Santa Monica, CA. 10.7249/RR1336 [DOI] [Google Scholar]
- StataCorp, 2020. Stata Statistical Software: Release 16.
- Strickland P, Kang D, Sithisarankul P, 1996. Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environmental health perspectives 104 Suppl, 927–932. 10.1289/ehp.96104s5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Tsukue N, Yoshida S, 2004. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environmental sciences : an international journal of environmental physiology and toxicology 11, 33–45. [PubMed] [Google Scholar]
- Tang D, Li T, Liu JJ, Zhou Z, Yuan T, Chen Y, Rauh VA, Xie J, Perera F, 2008. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environmental health perspectives 116, 674–679. 10.1289/ehp.10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II, 2003. Socioeconomic status modifies heritability of IQ in young children. Psychological Science 14, 623–628. 10.1046/J.0956-7976.2003.PSCI_1475.X [DOI] [PubMed] [Google Scholar]
- Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Völgyi E, Diaz-Thomas AM, Ferry RJJ, 2015. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients 7, 9918–9930. 10.3390/nu7125499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnevetsky J, Tang D, Chang H-W, Roen EL, Wang Y, Rauh V, Wang S, Miller RL, Herbstman J, Perera FP, 2015. Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicology and teratology 49, 74–80. 10.1016/j.ntt.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Lin Y, Zhang A-Q, Guo L-H, Cao J, 2010. Evaluation of the noncovalent binding interactions between polycyclic aromatic hydrocarbon metabolites and human p53 cDNA. The Science of the total environment 408, 6285–6290. 10.1016/j.scitotenv.2010.09.024 [DOI] [PubMed] [Google Scholar]
- Wheeler AJ, Dobbin NA, Héroux M-E, Fisher M, Sun L, Khoury CF, Hauser R, Walker M, Ramsay T, Bienvenu J-F, LeBlanc A, Daigle E, Gaudreau E, Belanger P, Feeley M, Ayotte P, Arbuckle TE, 2014. Urinary and breast milk biomarkers to assess exposure to naphthalene in pregnant women: an investigation of personal and indoor air sources. Environmental health : a global access science source 13, 30. 10.1186/1476-069X-13-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN, 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Statist. Soc. B 73, 3–36. [Google Scholar]
- Wormley DD, Chirwa S, Nayyar T, Wu J, Johnson S, Brown LA, Harris E, Hood DB, 2004. Inhaled benzo(a)pyrene impairs long-term potentiation in the F1 generation rat dentate gyrus. Cellular and molecular biology (Noisy-le-Grand, France) 50, 715–721. [PubMed] [Google Scholar]
- Wormley Deanna D, Ramesh A, Hood DB, 2004. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicology and applied pharmacology 197, 49–65. 10.1016/j.taap.2004.01.016 [DOI] [PubMed] [Google Scholar]
- Yuan T-H, Shie R-H, Chin Y-Y, Chan C-C, 2015. Assessment of the levels of urinary 1-hydroxypyrene and air polycyclic aromatic hydrocarbon in PM2.5 for adult exposure to the petrochemical complex emissions. Environmental Research 136, 219–226. 10.1016/j.envres.2014.10.007 [DOI] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, Maenner MJ, Schieve LA, Danielson ML, Bitsko RH, Blumberg SJ, Kogan MD, Boyle CA, 2019. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009-2017. Pediatrics 144. 10.1542/peds.2019-0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Tian F, Zheng J, Li S, Qiang M 2016. Chronic Administration of Benzo(a)pyrene Induces Memory Impairment and Anxiety-Like Behavior and Increases of NR2B DNA Methylation. PloS one 11, e0149574. 10.1371/journal.pone.0149574 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


