Abstract
Background:
Febrile seizures are the most common type of seizures in children. While in most children the outcome is favorable, children with febrile status epilepticus may exhibit modest cognitive impairment. Whether children with other forms of complex febrile seizure, such as repetitive febrile seizures within the same illness are at risk for cognitive deficits is not known. In this study we used a well-established model of experimental febrile seizures in rat pups to compare the effects of febrile status epilepticus and recurrent febrile seizures on subsequent spatial cognition and anxiety.
Methods:
Male and female rat pups were subjected to hyperthermic seizures at postnatal day 10 and were divided into groups of rats with continuous seizures for ≥40 min or recurrent febrile seizures. They were then tested as adults in the active avoidance and spatial accuracy tests to assess spatial learning and memory and the elevated plus maze to measure anxiety.
Results:
Febrile status epilepticus rats demonstrated impaired spatial cognition in active avoidance and spatial accuracy and exhibited reduced anxiety-like behavior in the elevated plus maze. Rats with recurrent febrile seizures did not differ significantly from the controls on any measures. There were also significant sex-related differences with FSE females performing far better than FSE males in active avoidance but demonstrating a navigational learning impairment relative to CTL females in spatial accuracy. However, once learned, FSE females performed the spatial accuracy task as well as CTL females.
Conclusion:
There is a duration-dependent effect of febrile seizures on subsequent cognitive and behavioral outcomes. Febrile status epilepticus resulted in spatial cognitive deficits and reduced anxiety-related behaviors whereas rats with recurrent febrile seizures did not differ from controls. Sex had a remarkable effect on spatial cognitive outcome where FSE males fared worse than FSE females. The results demonstrate that sex should be considered as a biological variable in studies evaluating the effects of seizures on the developing brain.
Keywords: Active avoidance test, spatial accuracy test, elevated plus maze, learning, memory, spatial cognition
Introduction
During early development, neural circuits and networks may require precise electrical signaling combined with sensory inputs to develop normally [1-3]. The development and refinement of cell morphology and signal coordination within and between circuits that underlie cognition, including learning and memory, are dependent upon the efficacy of circuit temporal organization [4-6]. Seizures are an intense disruptor of normal neural electrochemical signaling and function and thus have the potential of causing permanent cognitive impairment [5, 7].
Febrile seizures (FS) are the most common epileptic seizures in childhood, affecting 2-5% of children between six months and six years of age in the United States and Western Europe, with a peak incidence in the second year of life [8, 9]. The majority of FS in children are short in duration and are associated with favorable cognitive outcomes [10, 11]. Likewise, in animal models, short FS result in no cognitive impairment [12]. However, in cases of complex FS defined as having a focal onset with a duration of >10 mins, multiple seizures within a single illness [13] or febrile status epilepticus (FSE)[14] where FS last 30 mins or more, children are at increased risk for detrimental outcomes [10, 15-19]. Febrile seizures evolve into FSE in 5-9% of children, making it the most frequent cause of status epilepticus in children [13, 20].
Most children with FS, including FSE, do well long-term [21]; however, the impact of potentially incurring lifelong cognitive impairment increases with a high population incidence rate of FSE, thus making this disorder a critically important phenomenon to characterize. To probe both the causal relationship and underlying mechanisms of FSE and memory problems, an experimental FSE (FSE) model has been heavily adopted and validated [22-28]. Although it is now well established that prolonged FSE results in cognitive impairment and developmental delay in humans [15, 19] and spatial learning and memory impairments in the rodent model [22-28], questions remain regarding whether the duration of febrile seizure or recurrent FS (RFS), another form of complex FS, are also risk factors for cognitive impairment in the animal model.
In this study we carried out multiple cognitive assessments in male and female FSE, RFS and normothermic controls (CTL). We hypothesized that shorter duration RFS would not have as great an effect on spatial cognition and anxiety measures as FSE. We assayed spatial cognition in each group with the aversive active avoidance task on a rotating arena, and the operant appetitive reward spatial accuracy test. As anxiety may play a confounding role in spatial cognition [29], especially in conditions using aversive conditioning or the open field, understanding how anxiety may confound seizure related cognitive comorbidities in early life is important. If FSE animals exhibit heightened anxiety compared with controls, an aversive stimulus may lead to elevated stress levels and negatively impact cognition.
We replicated previous findings demonstrating FSE male cognitive impairment on the active avoidance task [4, 5] while also showing that FSE females and RFS males [30] or females were not impaired on the active avoidance task. However, FSE females demonstrated a learning impairment relative to CTL females on the spatial accuracy task, although once meeting criterion FSE females performed as well as CTL females. Anxiety measures did not explain poor cognitive outcomes in FSE males but the lack of anxiety in FSE females may correlate with good outcomes in the active avoidance task.
Methods
Experimental Overview
Male and female rat pups underwent experimental FS or served as non-seizure controls at P10. Starting at P45, rats were assessed for spatial learning and memory deficits in two cognitive assays: 1)The active avoidance test, which pairs an aversive stimulus with a region of space; and 2) The spatial accuracy test, which pairs an appetitive operant reward with a circumscribed region of space. We began with the active avoidance test and ended with the spatial accuracy test. Between these cognitive tasks, anxiety-like behavior was measured in the elevated plus maze.
Animals
All procedures were approved by the University of Vermont’s Institutional Animal Care and Use Committee (IACUC), and in accordance with the National Institutes of Health’s guidelines. Sprague-Dawley (Charles River, Montreal) pups were subjects in this study. All rat pups were weaned at postnatal day (P) 21 and were housed in groups until P35. By P40 all rats were individually housed and were maintained on a 12-hour light/dark cycle at all stages. Both male and female rats were used in this study; febrile animals were compared with normothermic littermate controls. A total of 32 rat pups (14 ♂ and 18 ♀) were studied.
Induction of RFS and FSE
Febrile seizures were induced as described previously [5, 26, 27, 30, 31]. Before hyperthermia, measures were taken to avoid burns to sensitive areas like the paws, ears and tails using a glycerin-based hydrating ointment. After protection was applied, rats were placed two at a time inside a glass 3-liter flask and subjected to a continuous stream of hot air. The beginning of the seizure was determined by the onset of behaviors such as freezing followed by chewing automatisms. This was considered as seizure onset and the core temperature was noted at this time. Hyperthermia was maintained for 60 min and behavioral manifestations of seizures was noted. During hyperthermia, core temperatures were checked every 2 mins to ensure body temperature remained between 39.5 and 41.5°C. Weight-matched littermate controls (CTL) were maternally deprived for the same period that seizure animals underwent treatment to control for stress. At the end of hyperthermia, animals were removed from the hyperthermia chamber and placed on a cool surface, bringing core temperatures back to basal levels.
Active avoidance task
Animals underwent testing in the active place avoidance task (Biosignal; Brooklyn, New York) at P50-P60. The active avoidance rotating platform task [32, 33] is a measure of spatial cognitive function that is hippocampus [34, 35] and amygdala [36] dependent. In this task, animals must attend to their ever-changing position in the room frame lest they be rotated into a pre-determined zone where they receive a mild electrical shock [5, 26, 27, 37-39].
One day prior to the active avoidance task rats were anesthetized and a stainless-steel pin was implanted subcutaneously between the shoulders. The swivel was attached to a cable with an LED at the end allowing for automated tracking and shock delivery. The arena consists of an 82 cm diameter steel disc lit from both above and below. The arena is centered in a room where it is approximately 50 cm from black curtains on the S and E sides and 50 cm from white walls on the N and W sides. The N and W walls have an 11 cm gray power-strip that forms a continuous line 50 cm above the floor of the arena. Two rectangular spatial cues (30 cm X 43 cm) depicting a red star (centered at W position) and a black circle (centered at N position), both on a white background, were placed 18 cm above the arena floor. An additional rectangular polarizing cue (53 cm X 84 cm) made of white paper with five 2.5 cm wide diagonal black stripes centered at the N position, 5 cm above the gray power strip.
On the first day of training, the animal was connected to the shock cable and introduced to the rotating arena for a 10 min habituation without shock. Number of entrances in the inactivated shock zone were calculated to determine if the rats explored the shock zone region equally. On all subsequent sessions rats received a 0.4 mA shock in an unmarked 876 cm2 wedge-shaped sector covering a 60° arc in the NE sector of the arena. The shock zone was stable in the room frame while the arena rotated. The entrance latency of the shock was 1 ms, the shock duration was 0.5 sec, and the inter-shock latency was 2 sec. Rats were trained in eight 10 min sessions per day for two days (16 sessions total). Performance measures were recorded and analyzed using custom software (Biosignal; Brooklyn, New York).
Raised plus maze
A raised plus maze was used to measure anxiety in control (CTL), RFS and FSE rats starting at P104. The maze consisted of four total arms, each 51 cm long, two of which were surrounded by opaque walls 42 cm high and across from each other and on the end, so the rat is surrounded on three sides. The other two arms of the maze remained open, so that the animal could survey its surroundings. The maze stands 74 cm above the ground. All rats entered the maze facing the northward enclosed arm. Each subject underwent two 5 min trials where behavior was recorded on video and analyzed. The amount of time each rat spent in either the open or closed arms or the middle of the maze was quantified by reviewing the video recordings. The percentage of time spent in the three conditions (open arm, closed arm or middle) was used to compare anxiety levels between groups [40].
Spatial accuracy task
Starting at P124, an appetitive operant reward task was used to assay spatial learning and memory by conditioning preference for a specific circumscribed goal zone [41-43] that ranged in diameter down to 10% of the 183 cm diameter arena. The task has two modes for continuous spatial behavior, one for goal-directed navigation, when the animal is seeking the goal zone and the other for random searching for a food reward after the pellet has been dropped into a random arena location following a successful pause within the goal zone. The visible and hidden versions of the spatial accuracy task were modeled after the cue and place navigation tasks in the Morris water maze [44]. Hippocampal lesions cause a profound and enduring place navigational impairment that cannot be attributed to motor, motivational or reinforcement deficits. In contrast to the water maze task, the spatial accuracy task allows for continuous measurement of multiple goal navigation epochs [42].
Rats were food restricted and maintained at 85% percent body weight during spatial accuracy training. Rat location was tracked and recorded via a firewire camera (30 Hz sampling rate) placed over the 183 cm diameter arena and analyzed with Biosignal software (Tracker, Bio-signal Group Corp, Brooklyn, USA) and custom software (MATLAB v R2019A, MathWorks, Natick, MA). Training began with a 2 cm diameter white bottle cap used as a visible goal. The bottle cap was placed 63 cm from the arena wall, near the arena center in the southeast quadrant. A polarizing cue card (color code gray 9.5; Color-Aid Corp., Hudson Falls, NY) was placed along the north sector of the arena wall, covering approximately 45° of arc. Animals were advanced to successive visible goal phases after reaching a criterion of 20 rewards. In phases 1-4 of training, 500 ms dwell-time in a target zone successively ranging in diameter from 122 cm (Phase 1), 64 cm (Phase 2), 32 cm (Phase 3) and 18 cm (Phase 4) elicited a +5V TTL pulse via a peripheral component interconnect. This pulse triggered release of a food pellet reward (Bioserv, New Jersey; 20 mg dustless precision pellets) from a custom overhead feeder, which fell to a random arena location. A refractory reward period of 5 sec was set to encourage the animal to leave the target area and forage for the fallen pellet before returning to the goal zone. In this manner, measures of continuous navigation to and from the goal zone were possible over the course of each 30 min training session. Finally, in Phase 5 of training, the diameter of the goal zone was maintained at 18 cm, but the visible goal cue was removed. In these hidden goal training sessions, the animal’s spatial behavior provided a proxy measure of self-localization relative to the estimated goal zone location as determined by stationary room and arena cues. Previous studies with goal zones at similar distances from polarizing cue cards found that behavioral choices correlated with place field location [45]. In the hidden goal sessions, the floor was cleaned with soap and water and the arena floor rotated ~ 45 degrees to minimize the influence of odor cues that might indicate the goal zone location.
Spatial accuracy behavior was quantified by the: 1) Number of trials to criterion; 2) Number of rewards; 3) Number of entrances; 4) Ratio of rewards to entrances; 5) Mean Speed; 6) Proportion of time in each arena quadrant; and 7) Proportion of time in goal.
In a previous spatial accuracy study in a 76 cm diameter arena, criterion at each phase was set as 30 rewards in a 30 min session (1reward/min) [42]. As the current iteration of the task uses an environment more than twice this diameter, we lowered the reward criterion to 20 rewards. This gave rats more time to forage for pellets and return to the goal zone while maintaining rigorous performance standards. Goal dwell time of at least 2% was also a key performance measure as this indicated statistical significance with respect to spatial sampling in the arena and more than twice the minimum amount of time needed to trigger 20 rewards. Proportion of time in goal was an important complementary measure to reward number as it represents the quality of self-localization relative to the goal zone, particularly during the hidden goal task in phase 5.
Sample size/power calculations and statistics
The primary outcome goal was to address the hypothesis that there would be significant differences in total shocks in the CTL, RFS and FSE during the active avoidance task. Based on a recent study using active avoidance we estimated that FSE rats would have approximately 20±2 shocks/trial versus 10±2 shocks in the CTL, we calculate a sample size of 7 rats in the CTL, RFS and FSE groups would detect a 50% (90% power, 5% α) difference in number of shocks over the 16 trials. The study of sex differences by group was not powered a priori.
Statistics
Active avoidance and elevated plus maze data were analyzed using GraphPad prism. Where noted, statistical significance was determined using either a student’s t-test, one-way analysis of variance (ANOVA), or two-way ANOVA using multiple comparisons and a confidence interval of 95%. P values less than 0.05 were considered significant.
For spatial accuracy data GEE (General Estimating Equations; SPSS; Armonk, NY), a class of regression marginal model, was used to analyze multivariable relationships between clustered behavioral data for individual animals [46]. Models were adjusted according to the distribution of each analyzed variable, i.e., gamma with log link model was used for non-normally distributed data while poisson loglinear model was used for count data. Main effects of group x training phase interactions are reported as well as the group effects for each performance variable of interest and group x condition (visible or hidden goal) interaction.
Results
Overview
Hyperthermia induction was maintained for 60 mins in 15 rats. All animals subjected to hyperthermia exhibited seizures and none died. During the 60 min period of hyperthermia, rats were scored on their total number of seizures and seizure duration. Six rats experienced 5-8 (mean = 6.8±1.2 seizures) separate, repetitive seizures and were designated as the Recurrent Febrile Seizure (RFS) group. The remaining 9/15 hyperthermic rats experienced no less than 15-20 seizures (16.8±0.7 seizures), which transitioned from brief and separate to prolonged and continuous, were designated as the experimental Febrile Status Epilepticus (eFSE) group. The average time spent in febrile status was 31.5±1.36 min. Hyperthermia or maternal separation protocols resulted in the following group designations: CTL = 17(8 ♂; 9 ♀); RFS = 6 (3 ♂; 3 ♀); FSE = 9 (4 ♂; 5 ♀).
The experiment started with active avoidance as our primary motivation was to replicate previous findings in this task [4, 5] and to test for the influence of sex and seizure duration with respect to cognitive outcome. Active avoidance was then followed by the elevated plus maze and the spatial accuracy task. As we found group and sex differences in the active avoidance experiment, we wished to know whether poor performance by FSE males in comparison to females correlated with putative differences in anxiety levels on the elevated plus maze. Although FSE animals exhibited less anxiety-like behavior, this result was primarily driven by FSE females. We then wished to know whether sex and group differences in spatial cognitive performance during active avoidance would carry over to an appetitive spatial task that emphasized navigational accuracy and self-localization relative to a fixed goal zone. As a prior study had shown that rats could be trained to alternate from active avoidance to food reward during a foraging task [6], we anticipated that we could train the animals on the spatial accuracy task following active avoidance. While it was possible that prior fear conditioning during active avoidance training could negatively affect spatial accuracy training, we found that all male rats failed to participate and did not meet criterion on the earlier and less challenging phases of the task, primarily by not moving away from the walls or freezing in place. Only females participated in spatial accuracy training and carried through to the final phase of the experiment. While both CTL and FSE females performed well on the active avoidance task, the FSE females exhibited a spatial accuracy learning deficit when the visible goal zone marker was removed. Results from each experiment are described in detail below.
Active Avoidance
During habituation there were no differences in number of crossings between the groups in the non-activated shock zone (AverageCTL = 38.35±2.32; AverageRFS = 39.50±4.55; AverageFSE = 41.11±2.54; F (2, 29) = 0.2556; p=0.7762). This data indicates that there were no differences in activity level and mobility between the groups prior to active avoidance training.
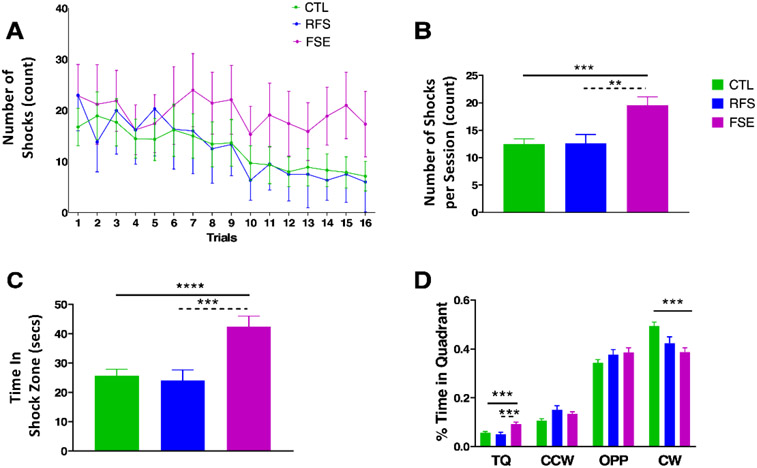
There were group differences in number of shocks during active avoidance (Fig. 1A) with a mixed-effects analysis showing a significant group effect where FSE were impaired in comparison to the CTL and RFS groups. There was an interaction between shocks and trials (F (16, 493) = 21.52; p<0.0001) with a significant difference between groups (F(1.765, 435.0) = 22.35; p<0.0001). Tukey’s multiple comparisons test showed significant differences between CTL and FSE (p=0.0007) and RFS and FSE (p=0.0007), but no differences between CTL and RFS. There was a significant group effect for mean number of shocks per trial (F(2,510)= 9.321; p=0.0001). There was a significant difference between CTL and FSE (p=0.0001) and RFS and FSE (p=0.0049) but no differences between CTL and RFS (Fig. 1B),
Figure 1:
Active place avoidance results. A) Comparison of number of shocks across 16 trials administered over two days in the CTL, RFS and FSE groups. There was a significant difference in groups (p<0.0001) with significant differences between CTL and FSE (p=0.0007) and RFS and FSE (p=0.0007); B) Mean difference in shocks/session. There were significantly more shocks in the FSE group compared to the CTL and RFS groups. No differences were seen in the CTL and RFS groups; C) Mean time in shock quadrant. The FSE group spent significantly more time in the shock zone than the CTL and RFS groups. No differences were seen in the CTL and RFS groups; D) Percent time in the target (shock) quadrant (TQ), counterclockwise (CCW) to the TQ, opposite (OPP) to the TQ and clockwise (CW) to the TQ. Rats in the EFS spent more time in the TQ than the CTL and RFS groups. ** = p<0.01; *** =p<0.001; ****=p<0.0001.
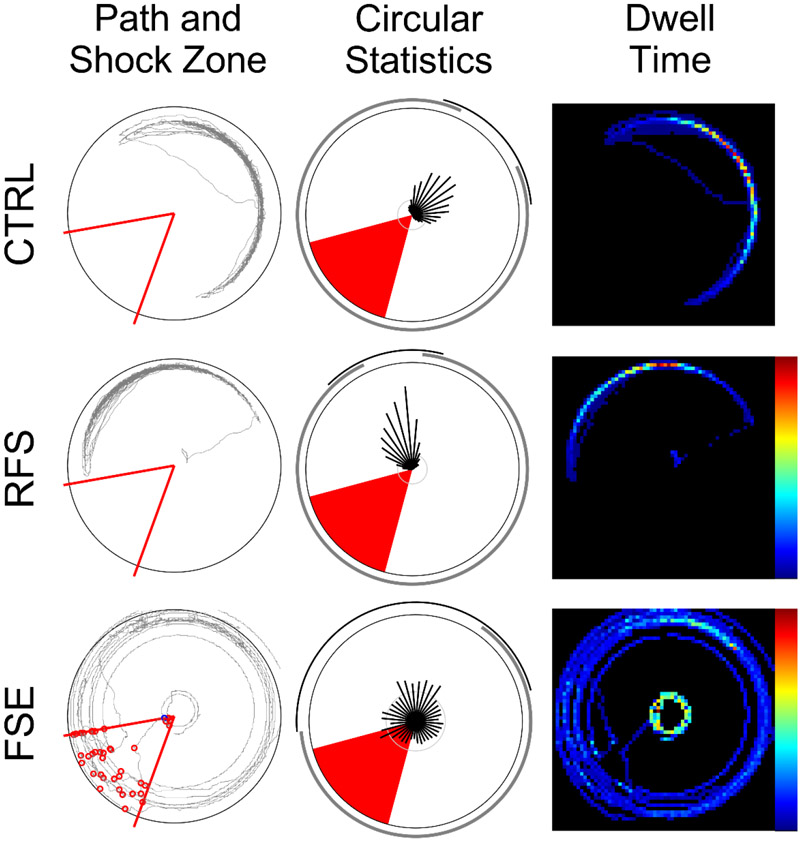
FSE rats spent significantly more time in the shock zone than the CTL and RFS rats (F(2,510)=9.622; p<0.0001) with significant differences between CTL and FSE (p<0.0003) and FSE and RFS (p=0.0008; Fig. 1C). Both control and RFS rats spent less time in the target quadrant than the FSE rats (F(2,510)=5.34) and the CTL more time in the CW quadrant than the FSE and RFS rats (F(2,510)= 8.806; p=0.0002, Fig. 1D). Sample path and dwell-time maps during an active avoidance training session demonstrates that CTL and RFS rats spent less time in the shock zone than the FSE rat (Fig. 2).
Figure 2:
Individual examples of active avoidance on the rotating arena from a CTL, RFS and FSE animal (Left panel). Path taken by each rat (grey line) over the surface of the arena. Middle panel: The red sector indicates the location of the shock zone, and the red circles indicate epochs where the animal received mild electric shock. Polar analysis of spatial sampling where the vector, angle and length of the black lines emanating from the plot center indicate the proportion of time spent at each angle of the arena (0-360° in 10° bins). The outside arc (thin black line) for each plot indicates the distribution of the longest vector lengths that account for at least 50% of the session duration. Right panel. Dwell time map (64 pixel resolution) where black pixels indicate non-sampled regions and the blue to red color continuum indicates the least to most sampled locations on the arena surface. Over the course of the 16 training trials, FSE animals performed worse on the active avoidance task than RFS or CTL.
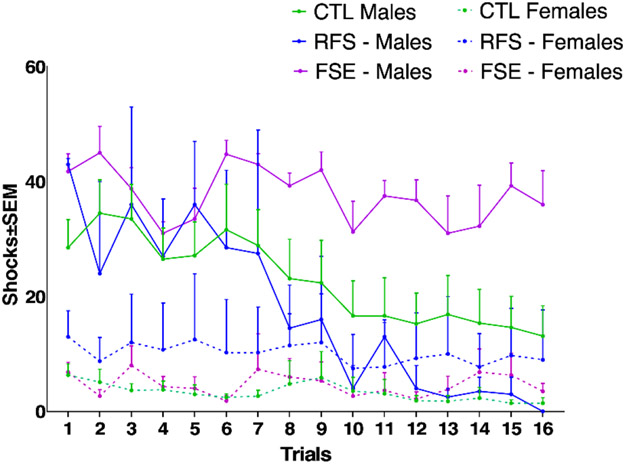
The effect of sex was also evaluated in the three groups. In all three groups (CTL, RFS and FSE), females performed better than males (Fig. 3). There was an interaction between number of shocks and trial (F (4, 499) = 7.456; p<0.0001) with a significant group effect (F (3.140, 313.3) = 101.5; p<0.0001). While RFS males and females did not differ significantly (p = 0.1594), significant differences were found between CTL males and females and FSE males and females (p <0.0001). FSE males exhibited a clear spatial learning and memory deficit in the active avoidance task, maintaining an average of over 30 shocks per session on both training days. Longer duration febrile status in males therefore leads to cognitive comorbidities while shorter duration febrile seizures do not. Seizures of any length had no effect on active avoidance in females. This result is similar to a recent study that found recurrent flurothyl induced seizures as pups had a significant effect on active avoidance outcomes in adult males but not adult females [47]. Sex is therefore a significant determinant of cognitive outcome post early life seizure.
Figure 3:
Comparison of mean number of shocks per session in the CTL, RFS and FSE groups separated by sex. There was an interaction between shocks and trials (p<0.0001) with a significant difference in groups (p<0.0001). While the difference between the RFS male and RFS females did not differ significantly, the differences between CTL males and females and FSE males and females were highly significant (p <0.0001).
Raised plus maze
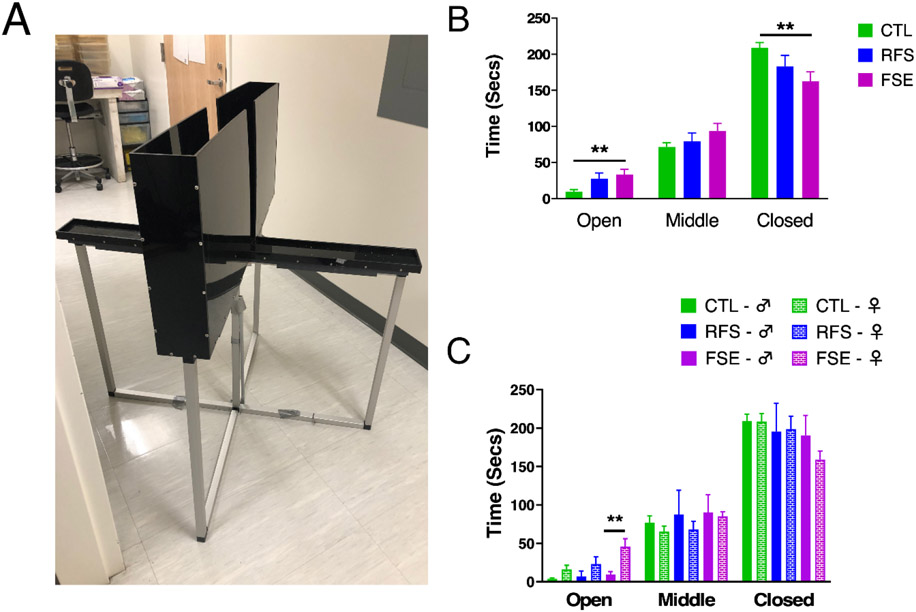
All the rats explored the maze (Fig. 4A). No differences were noted in the three groups between the first and second training session. For statistical analysis Days 1 and 2 were combined. In the combined data there were group differences in the amount of time spent in the open arm (F(2,57)=6.369; p=0.0032) and closed arm (F(2,57)= 5.626; p=0.0059). CTL animals spent less time in the open arm than FSE rats (p=0.0045) and more time in the closed arm (p=0.005). As the raised plus maze serves as a measure of anxiety in a rodent model [48], these results indicate the CTL group had a significantly higher level of anxiety than the FSE rats. Rats with RFS were intermediate between the CTL and FSE and did not differ statistically from either group (Fig. 4A).
Figure 4:
Elevated plus maze; A) Picture of elevated plus maze; B) CTL animals spent less time in the open arm than FSE rats and more time in the closed arm; C) Elevated plus maze performance in animals separated by sex. FSE females spent significantly more time in the open arm than males. ** = p<0.01
Sex differences were evaluated with male and female rats compared in ach group and a 2-way ANOVA revealed a difference for time in the open arm (F (5, 41) = 7.359; p< 0.0001). Tukey's multiple comparisons test showed that FSE females spent significantly more time in the open arm than the FSE males (p = 0.0015, Fig. 4B).
To determine if there was a relationship between active avoidance performance and raised plus maze, mean number of shocks per trial were correlated with time in the open arms, middle and closed arms. No significant differences were noted when the animals were analyzed in total or when the CTL, RFS and EFS groups when analyzed individually. While anxiety-level appeared to have no relationship to spatial cognitive deficits, the finding that FSE females spend more time in the open arm suggests that a lack of anxiety might be a factor that promotes better post FSE cognitive outcomes in females rather than males.
Spatial Accuracy Test
A high attrition rate was incurred in early training sessions as many rats were thigmotaxic and exhibited a reluctance to enter the middle of the arena. Only 3/17 of the CTL, 1/6 of the RFS and 4/9 of the FSE rats completed the task. All rats that completed spatial accuracy were female, resulting in a significant sex difference in attrition (Fisher’s exact test, p=0.0013). However, chi-square analysis found no significant seizure duration group differences in attrition (X2 = 2.5272, p = 0.283). We interpret this as an effect of training the animals in the aversive active avoidance task prior to the appetitive spatial accuracy task, as the delivery of the shock in the active avoidance task and the delivery of the pellets in the spatial accuracy task sounded similar. This may have resulted in hesitancy to explore the arena, particularly by males. As no differences were found between CTL and RFS in the first 2 tasks, we placed the one RFS rat who learned the task in the CTL group.
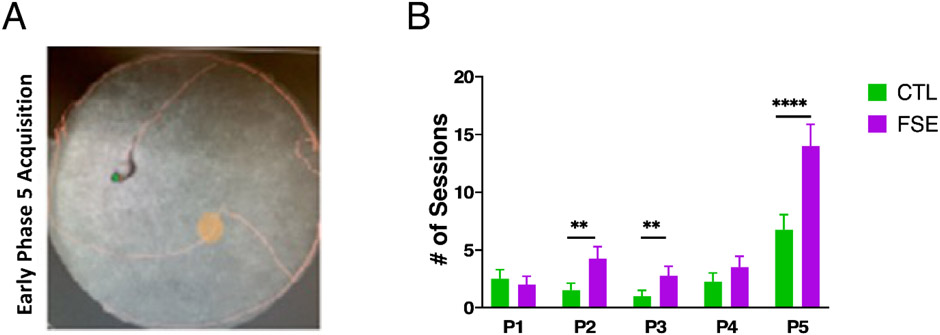
As shown in Fig. 5, the number of sessions to meet criteria in the CTL and FSE females were similar in the hippocampal-independent visible goal version of the task (P1-P4). With respect to the hippocampal-dependent hidden goal task (P5), both groups took more sessions to reach criterion than earlier phases. GEE found a significant group x training phase interaction regarding the number of training trials to criterion of either 20 rewards or spending 2% of the session within the goal zone (Wald Value = 102.63, p < 0.0001). While some differences are apparent in early training phases, the most robust group differences were found in the hidden goal sessions in phase 5 where FSE animals required nearly twice as many training trials (Fig 5, Supp. Table 1). Fig. 5A and Fig. 6A provide examples of performance during early phase 5 training trials where the major challenge for rats in both groups is to ‘dial in’ their pauses within the boundary of the hidden goal zone and self-localize relative to stationary room and arena cues.
Figure 5:
Spatial Accuracy performance; A) Photo representing the rat’s path around the arena (orange line) and a path through the hidden goal zone (red circle) during an early training trial in phase 5; B) Mean number of training trials to criterion of either 20 rewards or 2% of session time within the goal zone. While there are marginal group differences in phase 2 and 3 of spatial accuracy training, during phase 5 the FSE females require twice as many sessions to reach criterion than the CTL females.
Figure 6:
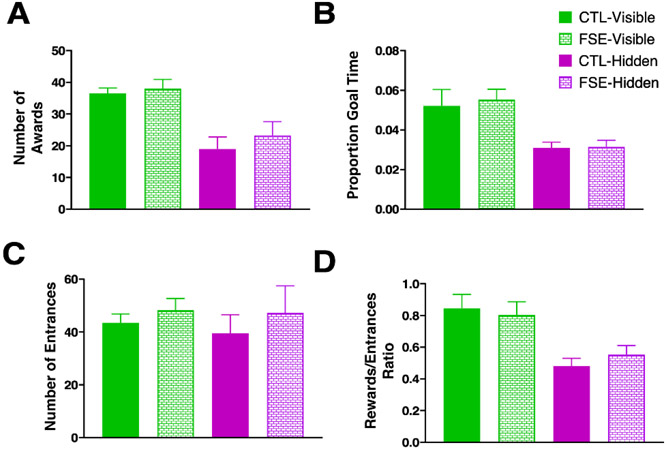
Spatial accuracy results during the best performance in the FSE and CTL rats during the visible and hidden portion of the tasks; A) Number of awards; B) Percent time in the goal arm; C) Number of entrances; D) Rewards/entrances ratio; Once the task was learned, there were no group performance differences between either the visible or hidden goal versions of the spatial accuracy task.
In comparing the peak performance of both groups, as determined by highest rewards and proportion goal time, no significant group effects were found for either the visible or hidden goal conditions (Fig. 7A-D) for number of rewards (Visible: Wald value = 0.200, p = 0.654; Hidden: Wald value = 0.546, p = 0.460), proportion time in goal ( Visible: Wald value = 0.101, p = 0.751; Hidden: Wald value = 0.015, p = 0.903), number of entrances (Visible: Wald value = 0.751, p = 0.386; Hidden: Wald value = 0.406, p = 0.524) and reward to entrance ratio (Visible: Wald value = 0.413, p = 0.521; Hidden: Wald value = 0.753, p = 0.386).
Figure 7:
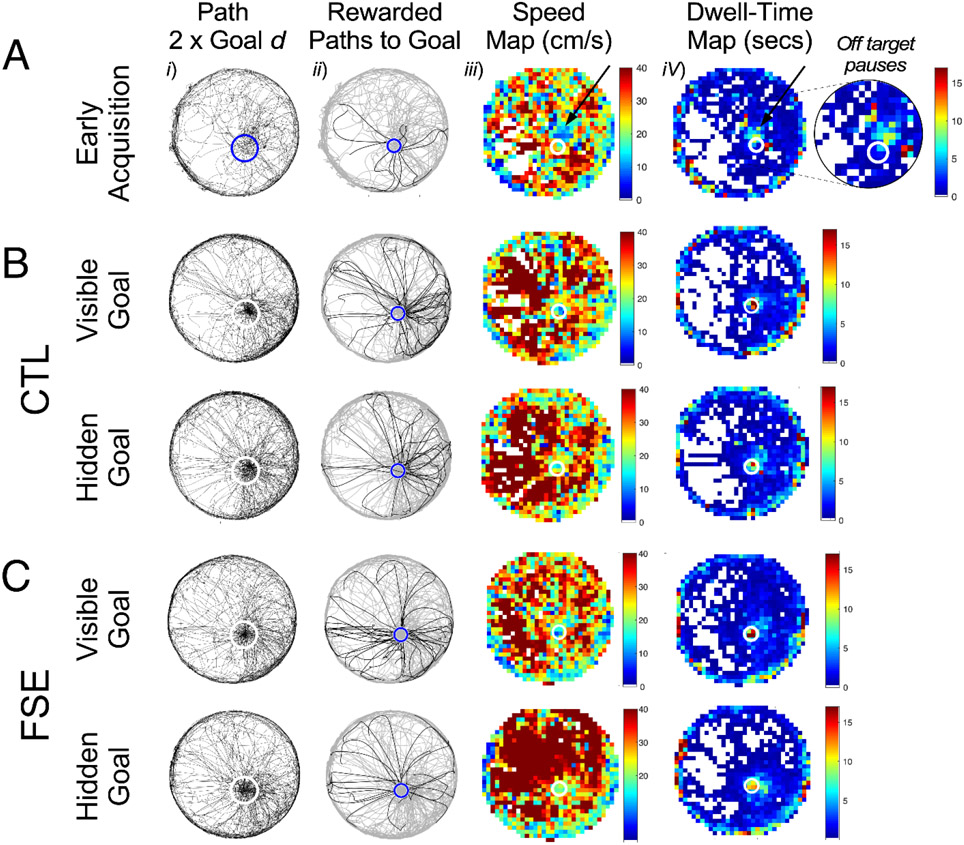
Spatial accuracy acquisition and peak performance in CTL and FSE. A. Measurements of spatial accuracy performance during an early session of phase 5 training and off-target goal pauses. Left to right columns: Animal path (black lines) relative to two times the diameter of the goal zone (blue or white circle); Animal path (grey lines) and rewarded paths (black lines) to goal (blue circle); Speed and dwell-time measurements in 32x 32 pixel arrays demonstrating self-localization relative to estimated hidden goal position. Black arrows and zoomed sector indicate off-target pauses relative to the goal zone (white circles). As in A, examples of peak spatial accuracy performance in a CTL (B) and FSE rat (C). Although FSE rats require more training to accurately locate the hidden goal, once learned they perform as well as CTL.
GEE also found a significant group x condition effect for the number of rewards (Wald value = 25.81, p < 0.0001), the reward to entrance ratio (Wald value = 21.22, p < 0.0001) and proportion time in goal zone (Wald value = 74.95, p < 0.0001). Both groups received fewer rewards, had a lower reward to entrance ratio and spent less time in the hidden goal zone version of the accuracy task than the visible goal zone version (Supp. Table 2-4). Taken together, the data indicate that despite differences in task acquisition, both groups ultimately exhibited similar levels of spatial accuracy and that both groups found the hidden goal zone version of the task more difficult than the visible goal version.
Importantly, no significant group effect was found for mean speed in the visible goal condition (AverageCTL = 33.02 ± 4.2 cm/s and AverageFSE = 38.00 ± 2.9 cm/s; Wald value = 0.780, p = 0.349) or the hidden goal condition (AverageCTL = 19.00 ± 3.8 cm/s and AverageFSE = 22.93 ±.4.5 cm/s; Wald value = 0.451, p = 0.502). This result suggests that FSE had no effect on motor properties in relation to task performance.
We conclude that task demands during spatial accuracy revealed spatial learning differences in female FSE rats that were not detected during active avoidance. However, with additional training, the performance of FSE females was equal to CTL females.
Discussion
Experimental FSE male rat pups have long-lasting spatial cognitive deficits in the active avoidance while FSE females exhibit navigational learning deficits in the spatial accuracy task as well as reduced anxiety in the elevated plus maze. FSE spatial cognitive deficits confirms prior results in this model [26, 27, 31]. However, rats exposed to the same degree of hyperthermia but who developed a series of discrete seizures (RFS), rather than FSE, did not differ significantly from the controls in any of the measures. These findings suggest there is a seizure frequency or duration threshold for cognitive deficit. This notion is supported by findings of Chang et al. [12] where a single experimental FS on P10 or FS induced daily from P10 to P12 (three repetitive FS in total) did not result in water maze impairment whereas rats with three FS daily, from P10 to P12, did exhibit water maze deficits. While seizures in our study occurred within one hyperthermic session, this group would most closely align with the repetitive FS described by these authors. While the RFS does not overtly affect spatial cognitive performance on the active avoidance task, this does not mean the rats were unaffected by RFS. A recent study showed that RFS can significantly alter temporal dynamics of both oscillations and cellular action potentials in the hippocampal circuit [30]. Prior work from our laboratory has also shown that significant changes in the temporal coordination of FSE CA1 neurons relative to local theta may still be found even if the FSE animals were able learn the active avoidance task [6]. It is therefore possible that RFS can modify CA1 neuronal input/output dynamics, yet not be sufficient to impair spatial behavior [30].
Another significant finding in this study was the marked effect sex had on spatial cognition. In particular, FSE rats demonstrated significant sex differences on both days of training. FSE males demonstrated no signs of learning the task by the end of training and FSE females performed well from the beginning of training. These findings support a recent observation from another early-life seizure model in our laboratory where male and female rat pups were given 50 flurothyl-induced seizures over 10 days starting at P15 [47]. When tested as adults, male rats with early-life seizures exhibited marked impairments in active avoidance, whereas female rats were unaffected. Similarly, Akman and colleagues [49] found that three episodes of kainic acid-induced status epilepticus on P4-6 caused more transient learning delays in the Barnes maze on P16-19 in males but not females. In rodents, sexual dimorphism is documented in epilepsy after early life stress [50, 51] and in the therapeutic response after neonatal ischemic injury [52]. McNally et al. [53] used a neonatal murine hypoxia-ischemia model to test whether the severity of hippocampal and cortical injury predicts seizure susceptibility 8 days after hypoxia-ischemia. Hippocampal and cortical injury directly correlated with seizure susceptibility in male, but not female pups. Thus, there are sex-specific consequences of neonatal hypoxia-ischemia on seizure susceptibility in a murine neonatal model.
Physiological sex differences over the course of development could account for differences in seizure vulnerability and cognitive outcome in prepubescent pups. Female sex hormones such as estradiol, even at the early developmental stage of P10, may offer some protection against early-life neurological insults [54-56]. There are also established sex differences early in postnatal development relative to depolarizing GABAergic signaling [57]. There is a sex-dependent developmental shift of GABA conductance and chloride gradient, leading to differences in the timing of key developmental stages [58].
Previous work has also shown sexual dimorphism in relation to fear conditioning where males show stronger freezing responses than females, correlating with increased dentate gyrus plasticity in males [59, 60]. This fear conditioning dimorphism was also found to be testosterone independent [60]. Hyper-arborization of dentate granule cells post FSE [5] could further affect plasticity in the perforant pathway and lead to more freezing in FSE males rather than avoidance. Ultimately, that FSE females fared better than FSE males in the 2 spatial cognitive tasks reconfirms the importance of exploring sex as a variable for cognitive outcome following early-life seizures [61]. Further supporting the finding that sex differences play a major role in cognitive impairment following FSE are the findings from the spatial accuracy test. Despite starting the task with 14 male rats, none were able to complete training. While this result was likely influenced by prior conditioned fear training on the active avoidance task in the open arena, the experiment did find a difference between FSE and CTL females where FSE females took twice as many trials to learn the task. However, once criterion was met, their performance was no different than CTL. This indicates that the place accuracy task is more difficult to learn than active avoidance and, with this increased difficulty, FSE female rats demonstrated slower learning dynamics than the CTL group. This finding further supports the idea that, in the assessment of spatial cognition performance, deficits are dependent on task demands [62]. A possible explanation for FSE learning difficulty in the spatial accuracy task are larger place fields that have been previously associated with the FSE model [27], which could result in less accurate cognitive maps with which to guide goal navigation [63, 64].
In the elevated plus maze rats with FSE had less anxiety-like behaviors than the CTL rats. Interestingly this reduction in anxiety-like behavior was more apparent in the female rats. The amygdala-hippocampal circuit is a critical network underlying fear learning and anxiety [65-68]. Amygdala injury can result in increased [66, 69] as well as decreased anxiety-like behaviors [70-72]. Previous studies of MRI obtained shortly after experimental FSE showed changes of T2 reaction times in the hippocampus and basolateral amygdala that were predictive of subsequent spatial cognition [27]. Our findings suggest that amygdala injury from the FSE could account for the decreased anxiety-like behavior in FSE females, although the mechanism of such injury is not clear. It is also possible that a degree of decreased anxiety in FSE females could mediate improved outcomes of FSE females on the active avoidance task in comparison to FSE males [29].
In summary, this study demonstrates that longer duration FSE, but not short duration RFS result in spatial cognitive impairment and reduced anxiety-like behavior. However, the degree of deficit was strongly determined by sex as FSE male rats exhibited more robust spatial deficits in the active avoidance task while FSE female rats only exhibited a navigational learning impairment in the more complex spatial accuracy task. These findings emphasize the need for future research that examines sex as a biological variable with respect to the adverse effects of seizures on the physiology of the developing brain and corresponding cognitive outcomes.
Supplementary Material
Highlights.
Febrile status epilepticus in rat pups cause cognitive and behavioral deficits
Recurrent febrile seizures do not result in cognitive or behavioral deficits
Outcome following seizures is strongly related to sex
Female rats have substantially better outcomes following febrile seizures than males
Acknowledgements:
The authors thank Reese Green for her assistance in collecting data for the spatial accuracy task. The authors thank Madeline Shultes and Francisco Velasquez for assistance. We also thank Dr. John Kubie for input regarding development and implementation of the spatial accuracy task. This work was supported by NIH Grants NS108765; NS108296.
Footnotes
Conflicts of Interest:
Authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Dominguez S, Ma L, Yu H, Pouchelon G, Mayer C, Spyropoulos GD, Cea C, Buzsaki G, Fishell G, Khodagholy D, Gelinas JN. A transient postnatal quiescent period precedes emergence of mature cortical dynamics. Elife 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wiesel TN, Hubel DH. Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J Neurophysiol 1963;26: 978–93. [DOI] [PubMed] [Google Scholar]
- [3].Kloc ML, Velasquez F, Niedecker RW, Barry JM, Holmes GL. Disruption of hippocampal rhythms via optogenetic stimulation during the critical period for memory development impairs spatial cognition. Brain Stimul 2020;13: 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barry JM, Mahoney JM, Holmes GL. Coordination of hippocampal theta and gamma oscillations relative to spatial active avoidance reflects cognitive outcome after febrile status epilepticus. Behav Neurosci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patterson KP, Barry JM, Curran MM, Singh-Taylor A, Brennan G, Rismanchi N, Page M, Noam Y, Holmes GL, Baram TZ. Enduring Memory Impairments Provoked by Developmental Febrile Seizures Are Mediated by Functional and Structural Effects of Neuronal Restrictive Silencing Factor. J Neurosci 2017;37: 3799–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barry JM, Sakkaki S, Barriere SJ, Patterson KP, Lenck-Santini PP, Scott RC, Baram TZ, Holmes GL. Temporal Coordination of Hippocampal Neurons Reflects Cognitive Outcome Post-febrile Status Epilepticus. EBioMedicine 2016;7: 175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baram TZ, Donato F, Holmes GL. Construction and disruption of spatial memory networks during development. Learn Mem 2019;26: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Verity CM, Butler NR, Goldring J. Febrile convulsions in a national cohort followed up from birth. I. Prevalence and recurrence in the first five years of life. Brit. Med. J 1985;290: 1307–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia 1994;35 Suppl 2: S1–S6. [DOI] [PubMed] [Google Scholar]
- [10].Verity CM, Greenwood R, Golding J. Long-term intellectual and behavioral outcomes of children with febrile convulsions. N. Engl. J. Med 1998;338: 1723–1728. [DOI] [PubMed] [Google Scholar]
- [11].Chang YC, Guo NW, Huang CC, Wang ST, Tsai JJ. Neurocognitive attention and behavior outcome of school-age children with a history of febrile convulsions: a population study. Epilepsia 2000;41: 412–420. [DOI] [PubMed] [Google Scholar]
- [12].Chang YC, Huang AM, Kuo YM, Wang ST, Chang YY, Huang CC. Febrile seizures impair memory and cAMP response-element binding protein activation. Ann. Neurol 2003;54: 701–705. [DOI] [PubMed] [Google Scholar]
- [13].Berg AT, Shinnar S. Complex febrile seizures. Epilepsia 1996;37: 126–133. [DOI] [PubMed] [Google Scholar]
- [14].Hesdorffer DC, Shinnar S, Lewis DV, Moshe SL, Nordli DR Jr., Pellock JM, MacFall J, Shinnar RC, Masur D, Frank LM, Epstein LG, Litherland C, Seinfeld S, Bello JA, Chan S, Bagiella E, Sun S. Design and phenomenology of the FEBSTAT study. Epilepsia 2012;53: 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weiss EF, Masur D, Shinnar S, Hesdorffer DC, Hinton VJ, Bonner M, Rinaldi J, Van de Water V, Culbert J, Shinnar RC, Seinfeld S, Gallentine W, Nordli DR Jr., Frank LM, Epstein L, Moshe SL, Sun S. Cognitive functioning one month and one year following febrile status epilepticus. Epilepsy Behav 2016;64: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scott RC. Consequences of febrile seizures in childhood. Curr. Opin. Pediatr 2014;26: 662–667. [DOI] [PubMed] [Google Scholar]
- [17].Shinnar S, Berg AT, Moshé SL, Petix M, Maytal J, Kang H, Goldensohn ES, Hauser WA. Risk of seizure recurrence following a first unprovoked seizure in childhood: a prospective study. Pediatrics 1990;85: 1076–85. [PubMed] [Google Scholar]
- [18].Martinos MM, Yoong M, Patil S, Chin RF, Neville BG, Scott RC, de Haan M. Recognition memory is impaired in children after prolonged febrile seizures. Brain : a journal of neurology 2012;135: 3153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hesdorffer DC, Benn EK, Bagiella E, Nordli D, Pellock J, Hinton V, Shinnar S, Team FS. Distribution of febrile seizure duration and associations with development. Ann Neurol 2011;70: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shinnar S, Hesdorffer DC, Nordli DR Jr., Pellock JM, O'Dell C, Lewis DV, Frank LM, Moshé SL, Epstein LG, Marmarou A, Bagiella E. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology 2008;71: 170–6. [DOI] [PubMed] [Google Scholar]
- [21].Martinos MM, Pujar S, O'Reilly H, de Haan M, Neville BGR, Scott RC, Chin RFM. Intelligence and memory outcomes within 10years of childhood convulsive status epilepticus. Epilepsy Behav 2019;95: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dube C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann. Neurol 2000;47: 336–344. [PMC free article] [PubMed] [Google Scholar]
- [23].Dubé CM, Baram TZ. Complex febrile seizures - an experimental model in immature rodents. In: Pitkänen A, Schwartzkroin PA, Moshé S, editors. Models of Seizures and Epilepsy. Burlington, Massachusetts: Elsevier; 2006, p. 333–340. [Google Scholar]
- [24].Dube CM, McClelland S, Choy MK, Brewster AL, Noam Y, Baram TZ. Fever, febrile seizures and epileptogenesis. 2012. [PubMed] [Google Scholar]
- [25].McClelland S, Flynn C, Dube C, Richichi C, Zha Q, Ghestem A, Esclapez M, Bernard C, Baram TZ. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann. Neurol 2011;70: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barry JM, Sakkaki S, Barriere SJ, Patterson KP, Lenck-Santini PP, Scott RC, Baram TZ, Holmes GL. Temporal Coordination of Hippocampal Neurons Reflects Cognitive Outcome Post-febrile Status Epilepticus. EBioMedicine 2016; 7: 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Barry JM, Choy M, Dube C, Robbins A, Obenaus A, Lenck-Santini PP, Scott RC, Baram TZ, Holmes GL. T2 relaxation time post febrile status epilepticus predicts cognitive outcome. Exp. Neurol 2015;269: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Barry JM, Mahoney JM, Holmes GL. Coordination of hippocampal theta and gamma oscillations relative to spatial active avoidance reflects cognitive outcome after febrile status epilepticus. Behav Neurosci 2020;134: 562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem 2010;17: 522–30. [DOI] [PubMed] [Google Scholar]
- [30].Kloc ML, Daglian J, Holmes GL, Baram TZ, Barry JM. Recurrent febrile seizures alter intra-hippocampal temporal coordination but do not cause spatial learning impairments. Epilepsia 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dube CM, Zhou JL, Hamamura M, Zhao Q, Ring A, Abrahams J, McIntyre K, Nalcioglu O, Shatskih T, Baram TZ, Holmes GL. Cognitive dysfunction after experimental febrile seizures. Exp. Neurol 2009;215: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cimadevilla JM, Fenton AA, Bures J. New spatial cognition tests for mice: passive place avoidance on stable and active place avoidance on rotating arenas. Brain Res Bull 2001;54: 559–63. [DOI] [PubMed] [Google Scholar]
- [33].Cimadevilla JM, Kaminsky Y, Fenton A, Bures J. Passive and active place avoidance as a tool of spatial memory research in rats. J Neurosci Methods 2000;102: 155–64. [DOI] [PubMed] [Google Scholar]
- [34].Cimadevilla JM, Fenton AA, Bures J. Transient sex differences in the between-sessions but not in the within-session memory underlying an active place avoidance task in weanling rats. Behav Neurosci 2001;115: 695–703. [DOI] [PubMed] [Google Scholar]
- [35].Cimadevilla JM, Fenton AA, Bures J. Functional inactivation of dorsal hippocampus impairs active place avoidance in rats. Neurosci Lett 2000;285: 53–6. [DOI] [PubMed] [Google Scholar]
- [36].Vafaei AA, Jezek K, Bures J, Fenton AA, Rashidy-Pour A. Post-training reversible inactivation of the rat's basolateral amygdala interferes with hippocampus-dependent place avoidance memory in a time-dependent manner. Neurobiol Learn Mem 2007;88: 87–93. [DOI] [PubMed] [Google Scholar]
- [37].Baglietto MG, Battaglia FM, Nobili L, Tortorelli S, De NE, Calevo MG, Veneselli E, De NM. Neuropsychological disorders related to interictal epileptic discharges during sleep in benign epilepsy of childhood with centrotemporal or Rolandic spikes. Dev. Med. Child Neurol 2001;43: 407–412. [DOI] [PubMed] [Google Scholar]
- [38].Popp SS, Lei B, Kelemen E, Fenton AA, Cottrell JE, Kass IS. Intravenous antiarrhythmic doses of lidocaine increase the survival rate of CA1 neurons and improve cognitive outcome after transient global cerebral ischemia in rats. Neuroscience 2011;192: 537–549. [DOI] [PubMed] [Google Scholar]
- [39].Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science 2006; 313: 1141–1144. [DOI] [PubMed] [Google Scholar]
- [40].Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 2006;143: 387–93. [DOI] [PubMed] [Google Scholar]
- [41].Kubie JL, Fenton A, Novikov N, Touretzky D, Muller RU. Changes in goal selection induced by cue conflicts are in register with predictions from changes in place cell field locations. Behav Neurosci 2007;121: 751–63. [DOI] [PubMed] [Google Scholar]
- [42].Mouchati PR, Kloc ML, Holmes GL, White SL, Barry JM. Optogenetic "low-theta" pacing of the septohippocampal circuit is sufficient for spatial goal finding and is influenced by behavioral state and cognitive demand. Hippocampus 2020;30: 1167–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bures J, Fenton AA, Kaminsky Y, Rossier J, Sacchetti B, Zinyuk L. Dissociation of exteroceptive and idiothetic orientation cues: effect on hippocampal place cells and place navigation. Philos. Trans. R. Soc. Lond B Biol. Sci 1997;352: 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 1982;297: 681–683. [DOI] [PubMed] [Google Scholar]
- [45].Lenck-Santini PP, Save E, Poucet B. Evidence for a relationship between place-cell spatial firing and spatial memory performance. Hippocampus 2001;11: 377–390. [DOI] [PubMed] [Google Scholar]
- [46].Ziegler A, Kastner C, Blettner M. The generalised estimating equations: an annotated bibliography. Biometical Journal 1998;40: 115–139. [Google Scholar]
- [47].Niedecker RW, Kloc ML, Holmes GL, Barry JM. Effects of early life seizures on coordination of hippocampal-prefrontal networks: Influence of sex and dynamic brain states. Epilepsia 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].La-Vu M, Tobias BC, Schuette PJ, Adhikari A. To Approach or Avoid: An Introductory Overview of the Study of Anxiety Using Rodent Assays. Front Behav Neurosci 2020;14: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Akman O, Moshé SL, Galanopoulou AS. Early life status epilepticus and stress have distinct and sex-specific effects on learning, subsequent seizure outcomes, including anticonvulsant response to phenobarbital. CNS Neurosci Ther 2015; 21: 181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jarvis S, Glinianaia SV, Arnaud C, Fauconnier J, Johnson A, McManus V, Topp M, Uvebrant P, Cans C, Krägeloh-Mann I. Case gender and severity in cerebral palsy varies with intrauterine growth. Arch Dis Child 2005;90: 474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Desgent S, Duss S, Sanon NT, Lema P, Lévesque M, Hébert D, Rébillard RM, Bibeau K, Brochu M, Carmant L. Early-life stress is associated with gender-based vulnerability to epileptogenesis in rat pups. PLoS One 2012;7: e42622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Carter BM, Sullivan BJ, Landers JR, Kadam SD. Dose-dependent reversal of KCC2 hypofunction and phenobarbital-resistant neonatal seizures by ANA12. Sci Rep 2018;8: 11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].McNally MA, Chavez-Valdez R, Felling RJ, Flock DL, Northington FJ, Stafstrom CE. Seizure Susceptibility Correlates with Brain Injury in Male Mice Treated with Hypothermia after Neonatal Hypoxia-Ischemia. Dev Neurosci 2019: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McCarthy MM. Estradiol and the developing brain. Physiol Rev 2008;88: 91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Veliskova J, De Jesus G, Kaur R, Velisek L. Females, their estrogens, and seizures. Epilepsia 2010;51: 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Veliskova J, DeSantis KA. Sex and hormonal influences on seizures and epilepsy. Hormones and Behavior 2013;63: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ben-Ari Y Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci 2002;3: 728–739. [DOI] [PubMed] [Google Scholar]
- [58].Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci 2008; 28: 1557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 1994;661: 25–34. [DOI] [PubMed] [Google Scholar]
- [60].Anagnostaras SG, Maren S, DeCola JP, Lane NI, Gale GD, Schlinger BA, Fanselow MS. Testicular hormones do not regulate sexually dimorphic Pavlovian fear conditioning or perforant-path long-term potentiation in adult male rats. Behav Brain Res 1998;92: 1–9. [DOI] [PubMed] [Google Scholar]
- [61].Christian CA, Reddy DS, Maguire J, Forcelli PA. Sex Differences in the Epilepsies and Associated Comorbidities: Implications for Use and Development of Pharmacotherapies. Pharmacol Rev 2020;72: 767–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Barry JM, Tian C, Spinella A, Page M, Holmes GL. Spatial cognition following early-life seizures in rats: Performance deficits are dependent on task demands. Epilepsy Behav 2016;60: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford,New York: Clarendon Press ; Oxford University Press; 1978. [Google Scholar]
- [64].Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 1982;297: 681–3. [DOI] [PubMed] [Google Scholar]
- [65].LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci 2000;23: 155–84. [DOI] [PubMed] [Google Scholar]
- [66].Patel D, Kas MJ, Chattarji S, Buwalda B. Rodent models of social stress and neuronal plasticity: Relevance to depressive-like disorders. Behav Brain Res 2019;369: 111900. [DOI] [PubMed] [Google Scholar]
- [67].Narayanan RT, Seidenbecher T, Kluge C, Bergado J, Stork O, Pape HC. Dissociated theta phase synchronization in amygdalo- hippocampal circuits during various stages of fear memory. Eur J Neurosci 2007;25: 1823–31. [DOI] [PubMed] [Google Scholar]
- [68].Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 2016;321: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron 2014;82: 966–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Popovitz J, Mysore SP, Adwanikar H. Long-Term Effects of Traumatic Brain Injury on Anxiety-Like Behaviors in Mice: Behavioral and Neural Correlates. Front Behav Neurosci 2019;13: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Boldt L, Koska I, Maarten van Dijk R, Talbot SR, Miljanovic N, Palme R, Bleich A, Potschka H. Toward evidence-based severity assessment in mouse models with repeated seizures: I. Electrical kindling. Epilepsy Behav 2021;115: 107689. [DOI] [PubMed] [Google Scholar]
- [72].Smolensky IV, Zubareva OE, Kalemenev SV, Lavrentyeva VV, Dyomina AV, Karepanov AA, Zaitsev AV. Impairments in cognitive functions and emotional and social behaviors in a rat lithium-pilocarpine model of temporal lobe epilepsy. Behav Brain Res 2019;372: 112044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.