Abstract
Melia azedarach L. is a valuable source of antioxidants and secondary metabolites. This study is a first extensive report about the effect of different serialization protocols and plant growth regulators (PGRs) on explant disinfection efficiency, callus induction and secondary metabolites production and accumulation in callus cultures of M. azedarach L. In this regard, the effect of plant growth regulators on callus induction and secondary metabolites production were examined. In addition, different sterilization agents were evaluated for disinfection of chinaberry leaf explants. The results showed that the lowest percentage of explant contamination and browning with the highest percentage of callus induction and callus growth obtained with explants pretreated with benomyl (2 g/L) for 2 h and sterilized with 7% H2O2 for 10 min and NaOCl 2% (without pH adjustment) for 12 min. Although adjusting the pH of NaOCl to pH = 7 and 10 significantly reduced the microbial contamination and increased the percentage of contamination-free cultures of M. azedarach L., adversely influenced the explant viability and callus induction and growth. The highest percentage of callus induction obtained on the MS medium containing 3 mg/L NAA/2,4-D and 1 or 3 mg/L Kin/BAP, and the highest callus yield (1804.833 mg/explant) belonged to the MS medium supplemented with 5 mg/L 2,4-D and 5 mg/L Kin. The callus cultures grown on the MS medium supplemented with 3 mg/L NAA and 1 mg/L Kin produced the highest amount of Quercetin (2.06 mg/g fresh weight), Rutin (5.56 mg/g fresh weight) and Kaempferol (1.84 mg/g fresh weight).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-022-01343-8.
Keywords: Disinfection, Hydrogen peroxide, Kaempferol, Melia azedarach L., Quercetin, Rutin
Introduction
Melia azedarach L. (known as Chinaberry) is one of the most valuable pharmaceutical plants. It has therapeutic properties such as antihyperglycemic, anticarcinogenic, anti-inflammatory, antiviral, insecticidal and antioxidant effects. A large numbers of secondary metabolites such as alkaloids, phenolic compounds, and vitamins have been recognized in M. azedarach L. (Yalla et al. 2021; Coria et al. 2008). The success of a plant tissue culture relies on many factors, such as culture medium composition, particularly plant growth regulators (PGRs), age and other physiological aspects of donor plant (Zare et al. 2009; Asghari Zakaria et al. 2011).
In vitro fungal and bacterial contaminations are also one of the most important limiting factors, particularly in woody plants. In order to overcome fungal and bacterial contaminations in the disinfection stage, different surface sterilizing agents including sodium and calcium hypochlorite, ethanol, mercuric chloride, silver nitrate, antibiotics, and fungicide were used in laboratories worldwide. Nevertheless, the efficiency of these treatments is low in woody plants, or some of them are very toxic to plant tissues. Furthermore, the efficiency of sterilization methods depends on different parameters, such as the physiological state of the mother plant, the size, age and type of the explant, the type and concentration of sterilization agent, and exposure duration (Teixeira da Silva et al. 2015). So, it is difficult to present a standard disinfection protocol for all plant species and explants. Therefore, the sterilization procedure should be optimized for any species to gain the maximum sterilized viable explants. Assareh and Sardabi (2005) reported that among calcium and sodium hypochlorite and mercuric chloride agents used for surface sterilization of Ziziphus spina-christi explants, Ca(OCl)2 (5% for 20 min) was the most efficient in explants sterilization. In contrast, in Ghaffoor et al. (2003) studies, NaOCl was found to be efficient for removing the contaminations in Solanum tuberosum L. The reports on tissue culture of woody plants indicate that surface disinfection was not efficient to produce clean cultures, and always is accompanied with a high level of bacterial and fungal contamination (Pence 2005).
Biotechnological techniques, especially plant cell and tissue culture, play an essential role in exploring alternative approaches for the production of desirable therapeutic compounds from plants (Farjaminezhad et al. 2013) and environmental adaptation (Farjaminezhad and Garoosi 2021). The callus induction and establishment of suspension cultures are two basic techniques, which have been used for production of wide range of phytochemicals, including therapeutic and antioxidant compounds (Colgecen et al. 2011). Flavonoids are a large group of phenolic compounds, which are generally found in nearly all plant species. Flavonoids are known as potential metal chelators and antioxidants. Several therapeutic and disease inhibition properties of flavonoids have been reported by researchers, which make them interesting nutraceuticals in human nutrition and healthcare (Prochazkova et al. 2011). There are reports about the production of flavonoids using different biotechnological methods, including callus and cell suspension cultures (Haida et al. 2019). So, the present study was aimed to the optimize the sterilization method for M. azedarach L. leaf explants using different sterilization agents such as H2O2 and NaOCl, and pH adjustment of NaOCl. Moreover, the effects of different factors on callus induction from leaf explants of M. azedarach L. and production of secondary metabolites including rutin, quercetin and kaempferol in callus cultures was investigated.
Materials and methods
Explant preparation and sterilization
In this study, young leaves of Chinaberry (M. azedarach L.) were prepared from the Qazvin province [36°19′ N 50°00′ E and 1287 m above sea level (m a.s.l.)], Iran. The young leaves were pretreated with a soft commercial detergent and washed for 30 min. The leaf explants were sterilized using different protocols as described below.
Experiment 1: the effects of different concentrations of H2O2 and NaOCl, and pH adjustment of NaOCl on the sterilization and growth of explants
The leaf explants were dipped in benomyl (C14H18N4O3) (a systemic fungicide) solution (3 g/L) for 2 h, and then treated with 5% or 7% (v/v) H2O2 for 10 or 15 min, then soaked in 70% (v/v) ethanol for 20 s, and finally soaked in 2% (w/v) NaOCl with different pH (pH = 7 or 10 or without pH adjustment) containing 2 drops of Tween-20 for 12 min (Additional file 1: Table S1). After that, the leaves were rinsed with sterile distilled water (SDW) for three times and then sliced into small fragments (0.5 cm squares), and cultured on MS (Murashige and Skoog 1962) medium supplemented with different concentrations of auxins [α-naphthalene acetic acid (NAA) or 2,4-Dichlorophenoxyacetic acid (2,4-D) at 3 and 5 mg/L concentrations] and cytokinins [6-benzylaminopurine (BAP) or Kinetin (Kin) at 1, 3 and 5 mg/L concentrations].
Common disinfection method including sterilization with 70% (v/v) ethanol for 20 s + 2% (w/v) NaOCl without pH adjustment for 12 min considered as control (Additional file 1: Table S1).
Experiment 2: the effect of benomyl inclusion in culture medium on the contaminations and growth of the explants
The leaf explants were surface sterilized using 5% (v/v) H2O2 for 10 min, 70% (v/v) ethanol for 20 s and 2% (w/v) NaOCl with different pH [pH = 10 or without pH adjustment (pH ≥ 12)] plus Tween-20 for 12 min (Additional file 1: Table S2). Finally, the leaves were rinsed with distilled water for three times and then sliced into small fragments (0.5 cm squares) and cultured on the MS medium supplemented with 1 mg/L NAA or 2,4-D + 1 mg/L BAP or Kin and benomyl fungicide (100 or 500 mg/L).
Common disinfection method [including 70% (v/v) ethanol for 20 s + 2% (w/v) NaOCl without pH adjustment for 12 min] was considered as control (Additional file 1: Table S2).
Medium preparation and cultures incubation condition
MS medium were supplemented with 3% sucrose and solidified using 0.8% plant agar. The pH of the medium was adjusted to 5.7–5.8 before autoclaving at 121 °C for 20 min. Cultures were maintained in a growth chamber at 24 ± 1 °C and 16-h photoperiod (cool Wight florescent light; 55 μmol/m2s) and sub-cultured at monthly intervals. The percentage of callus induction, bacterial and fungal contaminations, viable and browning explants was recorded 4 weeks after culture. The callus fresh weight (mg/single explant) was measured 3 months after culture.
Secondary metabolites analysis
According to the results of the experiments 1 and 2, the leaf explants were surface sterilized using A2 sterilization method, and cultured on the MS medium containing different combinations of plant growth regulators (PGRs) for callus induction and growth. The cultures were maintained in above maintained conditions and sub-cultured at 4 weeks interval. The secondary metabolites of the calli derived from leaf explants were extracted using Wagner (1979) method. Briefly, 0.1 g of fresh callus pulverized with liquid nitrogen, and suspended in 5 mL acidified methanol (methanol 99:1 acetic acid). The samples were incubated at room temperature for 72 h and centrifuged at 4000 rpm for 10 min. The supernatant was collected and used for determination of total phenolic, flavonoids and anthocyanin content.
Total phenolic content (TPC) were measured using colorimetric Folin-Ciocalteu spectrophotometric method described by Al-Farsi et al. (2005) and quantified using the standard curve of gallic acid (Sigma) (0, 0.001, 0.0015, 0.002 and 0.003 g/L of methanol) (y = 14.333x − 0.00003 and R2 = 0.9933). Briefly, 3 mL of diluted Folin-Ciocalteu (1:10 with deionized water) were added to the 400 μL extract and incubated for 5 min in a water bath at 22 °C. Then, 3 mL sodium bicarbonate solution (7%) was added and the samples were incubated at 22 °C for 90 min. The absorbance of the samples were recorded at 725 nm using a spectrophotometer (SmartSpec Plus spectrophotometer, Bio-Rad, Hercules, CA, USA). Total phenolic content of the callus cells was expressed as mg of gallic acid equivalents (GAE) per 1 g fresh weight (FW). TPC calculated using equation 1 (Shubhangi et al. 2017):
| 1 |
where T is total phenolic content (mg GAE/g FW), C is the concentration of total phenolic content (TPC) (mg/mL), V is the final volume of the extract (mL), and M is the fresh weight of callus sample (g).
The total flavonoid content (TFC) in the callus cultures was determined using an aluminum chloride (AlCl3) colorimetric method reported by Anjum et al. (2017). Briefly, 0.25 mL of 10% AlCl3 and 0.25 mL of 1 M potassium acetate (CH3COOK) were added to 1 mL of methanolic extract. Then, the absorbance of the mixture was measured immediately at 498 nm against the control sample containing acidified methanol instead of the callus extract. The standard curve was prepared using different concentrations (0, 0.4, 0.8, 1.6, and 2.4 g/L) of quercetin in methanol and used for quantification of TFC in the callus samples (y = 0.0573x + 0.0025, R2 = 0.9888). The TFC of the callus expressed as mg of quercetin equivalents/1 g FW using the equation 1, where C is the concentration of flavonoid (mg/mL), V is the final volume of extract (mL), and M is the weight of the sample (g FW).
In order to quantification of anthocyanin content, the absorbance of the extracts measured at 550 nm. Then, the concentration of anthocyanin was calculated using the formula A = ɛbc and molar extinction coefficient (ɛ550 = 33,000 × 106 M−1 cm−1), and expressed as µM/g FW. Where A is the absorbance of the extracts, b is the width of the cuvette (cm), and c is the concentration of anthocyanin (M).
HPLC analysis of rutin, quercetin and kaempferol
The separation, determination, and quantification of rutin, quercetin and kaempferol performed using high-performance liquid chromatography (HPLC) analysis. The secondary metabolites of the calli were extracted using Zu et al. (2006) method. Briefly, 0.5 g of the fresh callus pulverized in liquid nitrogen, and 1.5 mL ethanol (90% v/v) were added and vortexed for 5–10 min. Then, the samples were sonicated in a bath sonicator (Bandelin electronic®, Germany) for 15 min at 35 °C and incubated at room temperature for 3 h. The vortex and sonication were repeated twice. Finally, the samples were centrifuged (Sigma 1-14 K, Germany) at 10,000 rpm for 15 min. The supernatant was collected and filtered using a 0.22 μm filter. The filtrate was dried at 45 °C, till final volume was up to 100 μL and stored at − 20 °C until analysis. The standard curve was prepared using different concentrations of rutin (Sigma-Aldrich, USA) (5, 50, 100 ppm); quercetin (Sigma-Aldrich, Q4951, CAS: 117-39-5) and kaempferol (Sigma-Aldrich, 60010, CAS: 520-18-3) (5, 50, 100 and 500 ppm) and used for quantification in the callus samples.
HPLC analyses were performed on a Sykam HPLC system (SYKAM 1130, Sykam GmbH, Germany). HPLC separations carried out on a HIQ SIL C18V reversed-phase column (ø 4.6 × 250 mm) packed with 5 μm diameter particles, the mobile phase was methanol–acetonitrile–water (40:15:45, v/v/v). Rutin, quercetin and kaempferol were detected by diode-array detector DAD following HPLC separation at 257 nm for rutin and 368 nm for quercetin and kaempferol. Flow rate and injection volume was 1.0 mL/min and 20 μL, respectively. All chromatographic operations were carried out at room temperature.
Statistical analysis
Disinfection experiments conducted in a factorial (disinfection and PGRs as factors) arrangement based on a completely randomized design with six replicates with 12 explants per replication. Secondary metabolites (TPC, TFC, AC, rutin, quercetin and kaempferol) measurements carried out in a completely randomized design (CRD) with three replications. Experimental data subjected to analysis of variance (ANOVA) (p < 0.05) and mean comparison using Duncan’s Multiple Range Test (DMRT) (at p < 0.05) using IBM SPSS Ver.21 statistical software (IBM Corporation and Others, Armonk, NY, USA). The results expressed as mean ± SE. The graphs produced using Microsoft Office Excel 2010.
Results
Disinfection
Experiment 1: the effects of different concentrations of H2O2 and NaOCl, and pH adjustment of NaOCl on the contamination and growth of explants
Some of the cultures were contamination–free and the explants exhibited rapid swelling and cell proliferation especially from the edge of cuttings, which was led to the callus formation within 2–3 weeks (Additional file 1: Fig. S1). Embryogenic calli had green spots that showed signs of differentiation and morphogenesis after culture on the medium containing high proportion of cytokinin/auxin (Additional file 1: Fig. S2). The results indicated that the percentage of bacterial and fungal contamination, clean explants, explants viability and browning and callus induction and growth were significantly influenced by disinfection methods. Furthermore, there were significant differences between PGRs treatments in terms of explants viability and browning (%), and callus induction and growth (Additional file 1: Table S3). The highest bacterial (15.97%) and fungal (29.49%) contaminations occurred in the control sterilization method. The highest percentage of clean explants was achieved in the A3–A7 disinfection methods, while the highest percentage of viable explants was obtained in the control, A1, A2 and A5 disinfection methods (Table 1). Furthermore, the lowest percentage of explant browning and the highest percentage of callus induction and growth achieved with the A2 disinfection method (Table 1). As shown in Table 1, in the methods including NaOCl pH adjustment (A3, A5 and A7) the minimum percentage of explants with fungal and bacterial contamination was occurred, but the percentage of explants with callus induction and growth response was also lower. For instance, the application of 2% NaOCl with pH = 7 for 12 min (method A3) significantly decreased the explant viability and increased the browning explants as compared to 2% NaOCl without pH adjustment (method A2). Furthermore, the methods A2 and A3 in which employed benomyl pretreatment and H2O2 had an inhibitory effect on the bacterial contamination compared to the method A1 in which only explants pretreated with benomyl solution (3 mg/L) for 2 h (Table 1). Furthermore, the highest percentage of callus induction was obtained in MS medium supplemented with 3 mg/L NAA + 1 mg/L Kin or BAP and 3 mg/L NAA or 2,4-D + 3 mg/L BAP (Table 2). While, the highest callus yield (fresh weight) was obtained in the MS medium containing 5 mg/L 2,4-D + 5 mg/L Kin and 5 mg/L NAA + 5 mg/L BAP (Table 2).
Table 1.
The effect of different disinfection methods on the percentage of microbial contamination and in vitro response of M. azedarach L. leaf explants
| Methods of disinfection | Bacterial contamination (%) | Fungal contamination (%) | Clean explant (%) | Viability (%) | Browning (%) | Callus induction (%) | weight of callus (mg/explant) |
|---|---|---|---|---|---|---|---|
| Control | 15.97 ± 3.10d | 29.49 ± 4.08c | 54.5 ± 4.8d | 1.00 ± 0.00a | 0.00 ± 0.00a | 26.78 ± 5.58b | 873.733 ± 34.418ab |
| A1 | 8.74 ± 1.56c | 1.08 ± 0.45a | 90.2 ± 1.6bc | 1.00 ± 0.00a | 0.00 ± 0.00a | 5.91 ± 1.59c | 484.568 ± 26.896bc |
| A2 | 5.57 ± 1.21bc | 7.45 ± 1.47b | 87.0 ± 2.4c | 99.65 ± 0.35a | 0.35 ± 0.35a | 38.56 ± 5.49a | 1191.294 ± 37.148a |
| A3 | 3.39 ± 0.94ab | 0.00 ± 0.00a | 96.6 ± 0.9ab | 86.45 ± 3.29b | 14.20 ± 3.25b | 1.52 ± 0.91c | 158.921 ± 96.003c |
| A4 | 0.40 ± 0.40a | 0.00 ± 0.00a | 99.6 ± 0.4a | 79.25 ± 3.58c | 21.14 ± 3.67c | 9.41 ± 2.37c | 554.056 ± 89.829bc |
| A5 | 0.86 ± 0.60a | 0.69 ± 0.48a | 98.5 ± 0.8a | 99.31 ± 0.48a | 0.79 ± 0.55a | 10.77 ± 2.82c | 282.111 ± 100.367c |
| A6 | 1.06 ± 0.60a | 1.32 ± 0.63a | 97.6 ± 0.8a | 77.87 ± 3.94c | 22.72 ± 4.01c | 3.91 ± 1.43c | 130.382 ± 60.386c |
| A7 | 0.37 ± 0.37a | 0.00 ± 0.00a | 99.6 ± 0.4a | 54.48 ± 4.64d | 45.52 ± 4.64d | 9.44 ± 3.76c | 108.147 ± 47.128c |
In each column, different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
Table 2.
The effect of the different types and concentrations of PGRs on the explant viability, browning, and callus induction from leaf explants of M. azedarach L.
| Concentration of PGRs (mg/L) | Viability (%) | Browning (%) | Callus induction (%) | Callus weight (mg/explant) |
|---|---|---|---|---|
| 3 NAA + 1 Kin | 91.33 ± 4.00ab | 8.67 ± 4.00cd | 18.17 ± 3.60a | 455.737 ± 17.445b−e |
| 3 NAA + 1 BAP | 83.70 ± 5.98bc | 16.30 ± 0.50abc | 19.00 ± 3.79a | 814.529 ± 252.15b−e |
| 3 2,4-D + 1 Kin | 88.30 ± 5.03ab | 11.89 ± 3.18bcd | 15.32 ± 3.89ab | 66.444 ± 39.585de |
| 3 2,4-D + 1 BAP | 90.82 ± 3.50ab | 9.17 ± 3.53cd | 16.00 ± 3.83ab | 103.150 ± 41.373de |
| 3 NAA + 3 Kin | 78.80 ± 6.07c | 21.19 ± 4.07a | 14.49 ± 3.92abc | 987.000 ± 28.824bc |
| 3 NAA + 3 BAP | 77.66 ± 6.18c | 22.33 ± 4.18a | 18.35 ± 3.13a | 851.095 ± 30.287bcd |
| 3 2,4-D + 3 Kin | 76.80 ± 5.23c | 23.19 ± 3.43a | 10.14 ± 3.46abc | 78.800 ± 48.109de |
| 3 2,4-D + 3 BAP | 83.21 ± 5.62bc | 18.06 ± 3.01ab | 20.15 ± 3.14a | 488.714 ± 19.267b−e |
| 5 NAA + 1 Kin | 91.97 ± 4.18ab | 9.03 ± 4.18cd | 11.85 ± 3.69abc | 154.706 ± 83.611de |
| 5 NAA + 1 BAP | 89.30 ± 4.07ab | 10.70 ± 4.07bcd | 3.67 ± 3.67bc | 53.647 ± 53.647de |
| 5 2,4-D + 1 Kin | 91.66 ± 3.76ab | 8.33 ± 3.76cd | 0.98 ± 0.98c | 2.765 ± 2.765e |
| 5 2,4-D + 1 BAP | 91.24 ± 4.04ab | 9.93 ± 4.48bcd | 10.29 ± 3.31abc | 112.353 ± 53.227de |
| 5 NAA + 5 Kin | 96.98 ± 1.65a | 3.32 ± 1.58d | 15.47 ± 3.68ab | 222.167 ± 92.369cde |
| 5 NAA + 5 BAP | 96.14 ± 1.84a | 5.25 ± 1.91d | 14.09 ± 3.41abc | 1073.722 ± 63.623b |
| 5 2,4-D + 5 Kin | 91.20 ± 5.05ab | 8.79 ± 3.05cd | 15.98 ± 3.46ab | 1804.833 ± 85.304a |
| 5 2,4-D + 5 BAP | 92.90 ± 3.14a | 7.09 ± 3.14d | 11.73 ± 2.90abc | 342.063 ± 179.899b−e |
In each column, different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
Experiment 2: the effect of benomyl inclusion in the culture medium on the contamination and growth of explants
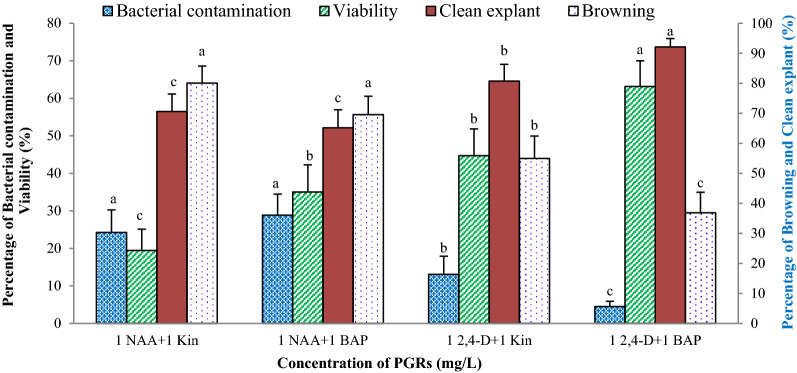
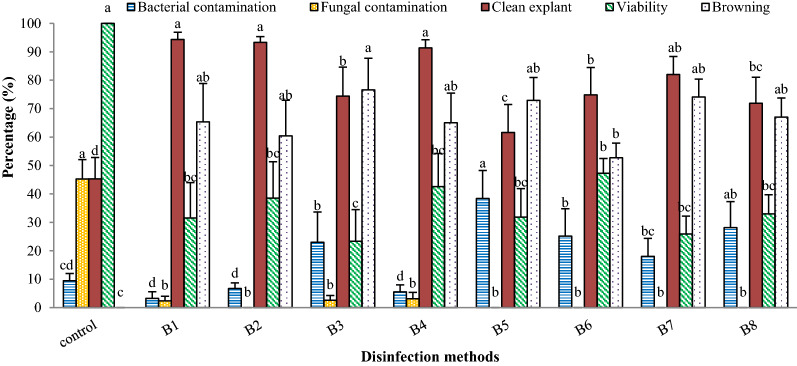
In this experiment, the effect of disinfection methods and the inclusion of benomyl in the culture medium on the explant contamination and viability were assessed. The percentage of bacterial contamination, and viable and browning explants were significantly influenced by the disinfection method, PGRs combination, and their interaction. The percentage of explant browning in the medium containing NAA was higher than that of the medium supplemented with 2,4-D, while the explant viability in the media containing 2,4-D was higher than those containing NAA. Furthermore, explant viability influenced by cytokinin type, so in both 2,4-D and NAA containing media, BAP improved the explant viability as compared to kinetin (Fig. 1). As a result, the maximum and minimum explant viability was observed in the medium supplemented with 1 mg/L 2,4-D + 1 mg/L BAP and 1 mg/L NAA + 1 mg/L Kin, respectively. Inclusion of benomyl (100 and 500 mg/L) in the culture medium composition significantly reduced the percentage of fungal contamination in M. azedarach L. leaf explant cultures. So, the percentage of viable explants and fungal contamination in the control method were higher than the others. Disinfection methods B1 and B5 showed the lowest and highest bacterial contamination (%), respectively (Fig. 2). In this experiment, the callus induction only obtained from the explants disinfected with the method B4 and cultured on the MS medium supplemented with 1 mg/L NAA + 1 mg/L BAP and 1 mg/L 2,4-D + 1 mg/L Kin or BAP.
Fig. 1.
The effect of plant growth regulators on in vitro responses of M. azedarach L. leaf explants in the culture media containing benomyl (Experiment 2). Bars with different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
Fig. 2.
The effect of different disinfection methods and inclusion of benomyl in culture medium on the bacterial contamination and in vitro response of M. azedarach L. leaf explants. Bars with different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
Total phenolic content (TPC), total flavonoid content (TFC) and Anthocyanin (AC)
Spectrophotometric assay of the secondary metabolites content in the M. azedarach L. calli obtained from different PGRs combinations (Additional file 1: Fig. S3) indicated that the amount of TPC, TFC, and AC were significantly influenced by the PGRs (Additional file 1: Table S4). According to the results, TPC in the methanolic extract was varied from 0.001 to 0.109 mg GAE/g FW in 5 mg/L NAA + 5 mg/L Kin and 3 mg/L 2,4-D + 3 mg/L BAP treatments, respectively. The highest amount of TFC was obtained in 3 mg/L 2,4-D + 3 mg/L BAP (193.051 mg QE/g FW) treatment, while the lowest value was observed in 5 mg/L NAA + 1 mg/L Kin and 5 mg/L 2,4-D + 5 mg/L Kin (58.583 and 64.638 mg QE/g FW, respectively) treatments. The highest and lowest amount of anthocyanin (AC), were obtained from the calli grown on the MS medium supplemented with 3 mg/L 2,4-D + 1 mg/L BAP or 3 mg/L Kin and 3 mg/L NAA + 3 mg/L BAP, respectively (Table 3).
Table 3.
The effect of plant growth regulators on total phenolic (TPC), Total flavonoid (TFC), and anthocyanin (AC) content in the callus cultures of M. azedarach L.
| Concentration of PGRs (mg/L) | TPC (mg GAE/g FW) | TFC (mg QE/g FW) | AC (μm/g FW) |
|---|---|---|---|
| 1 NAA + 1 KIN | 0.010 ± 0.005fg | 82.805 ± 4.545b−g | 1.650 ± 0.170bcd |
| 1 NAA + 1 BAP | 0.021 ± 0.006d−h | 80.402 ± 11.385b−g | 1.508 ± 0.302bcd |
| 1 2,4-D + 1 KIN | 0.027 ± 0.009d−g | 67.522 ± 4.774efg | 1.647 ± 0.042bcd |
| 1 2,4-D + 1 BAP | 0.030 ± 0.006d−g | 68.868 ± 5.132d−g | 1.111 ± 0.208cd |
| 3 NAA + 1 KIN | 0.026 ± 0.007d−g | 66.465 ± 10.599fg | 1.084 ± 0.116cd |
| 3 NAA + 1 BAP | 0.051 ± 0.002cd | 102.509 ± 13.155b−e | 1.623 ± 0.563bcd |
| 3 NAA + 3 KIN | 0.066 ± 0.011bc | 101.644 ± 17.790b−g | 1.946 ± 0.481abc |
| 3 NAA + 3 BAP | 0.022 ± 0.010d−g | 101.836 ± 14.609b−e | 0.152 ± 0.077e |
| 3 2,4-D + 1 KIN | 0.094 ± 0.007ab | 107.507 ± 9.145bc | 2.458 ± 0.281ab |
| 3 2,4-D + 1 BAP | 0.043 ± 0.002c−f | 100.202 ± 2.888b−f | 2.707 ± 0.386a |
| 3 2,4-D + 3 KIN | 0.090 ± 0.001ab | 109.237 ± 6.528b | 2.667 ± 0.545a |
| 3 2,4-D + 3 BAP | 0.109 ± 0.007a | 193.051 ± 16.732a | 2.455 ± 0.293ab |
| 5 NAA + 1 KIN | 0.013 ± 0.007efg | 58.583 ± 13.115g | 1.525 ± 0.417bcd |
| 5 NAA + 1 BAP | 0.012 ± 0.006fg | 68.291 ± 9.497d−g | 1.6061 ± 0.102bcd |
| 5 NAA + 5 KIN | 0.001 ± 0.014g | 73.385 ± 13.732c−g | 1.448 ± 0.288cd |
| 5 NAA + 5 BAP | 0.017 ± 0.012efg | 69.829 ± 10.656d−g | 1.216 ± 0.218cd |
| 5 2,4-D + 1 KIN | 0.046 ± 0.009cde | 100.875 ± 2.217b−f | 1.865 ± 0.084abc |
| 5 2,4-D + 1 BAP | 0.041 ± 0.016c−f | 103.278 ± 0.254bcd | 1.872 ± 0.148abc |
| 5 2,4-D + 5 KIN | 0.022 ± 0.019d−g | 64.639 ± 11.731g | 0.673 ± 0.139de |
| 5 2,4-D + 5 BAP | 0.017 ± 0.008efg | 70.406 ± 2.141d−g | 0.741 ± 0.255de |
In each column, different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
As shown in Table 3, increasing the concentration of 2,4-D from 1 to 3 mg/L significantly increased the amount of TPC, TFC, and AC contents. After that, increasing the 2,4-D concentration from 3 to 5 mg/L significantly decreased the production and accumulation of these secondary metabolites in the callus cultures of M. azedarach L. (Table 3). However, these increase and decreases were varied depending on the type and concentration of cytokinin. For instance, increasing the concentration of 2,4-D from 3 to 5 mg/L in the medium containing 5 mg/L Kin or BAP significantly decreased the AC content, but this reduction was not statistically significant in the medium containing 1 mg/L Kin or BAP (Table 3). On the other hand, increasing the 2,4-D concentration from 3 to 5 mg/L significantly reduced the TPC and AC contents of the callus cells in both media containing 1 and 5 mg/L of Kin or BAP. Furthermore, the amount of TFC in the callus cells grown on the medium containing 5 mg/L 2,4-D + 5 mg/L Kin or BAP was significantly lower than that of containing 5 mg/L 2,4-D + 1 mg/L Kin or BAP. While, there were no significant differences between 1, 3, and 5 mg/L concentrations of Kin or BAP in the media containing 5 mg/L NAA (Table 3).
Rutin, quercetin and kaempferol content
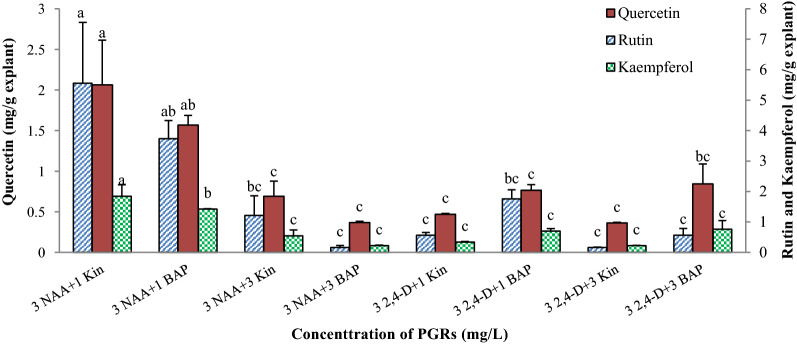
The amount of rutin, quercetin and kaempferol in callus cultures were analyzed using HPLC (Additional file 1: Fig. S4a, S4b), and the results indicated that the production and accumulation of these secondary metabolites significantly influenced by both type and concentration of plant growth regulators (Additional file 1: Table S5). So that, the highest amount of rutin, quercetin and kaempferol were obtained from the medium supplemented with 3 mg/L NAA + 1 mg/L Kin and 3 mg/L NAA + 1 mg/L BAP treatments (Fig. 3).
Fig. 3.
The effect of the plant growth regulators type and concentrations on the amount of rutin, quercetin and kaempferol in the callus cultures of M. azedarach L. Bars with different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
Discussion
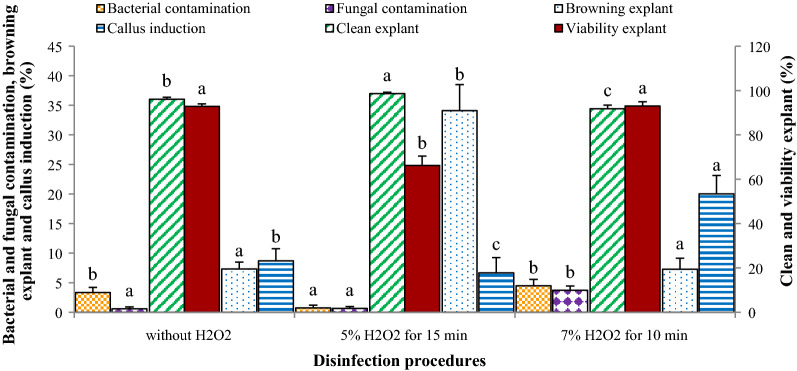
Tissue culture techniques play a vital role in plant biotechnology, especially in medicinal plants. Therefore, establishment of successful cultures and overcome to in vitro contamination requires an efficient protocol for explant disinfection. On the other hand, the preparation and establishment of clean in vitro cultures, especially in woody plants and plant materials prepared from natural habitats is a critical stage in successful plant cell and tissue culture. Disinfection procedures may differ for different plants, depending on their morpho-physiological characteristics (Srivastava et al. 2010). H2O2 has been reported in a few reports as a potent oxidizing and sterilization agent for the surface disinfection of different plant materials (Mihaljević et al. 2013; Hesami et al. 2019). In our study, utilization of H2O2 (A2, A3, A6, and A7 methods) increased the percentage of browning explants (from 7.312 to 20.698%) and decreased the explants viability and bacterial contamination (from 92.854 to 79.614% and 3.336 to 2.599%, respectively) in comparison to the methods without this agent (A1, A4, and A5 methods) (Table 1; Fig. 4). Increasing the concentration of H2O2 from 5% (methods A6 and A7) to 7% (method A2 and A3) significantly decreased the percentage of browning explants (from 34.121 to 7.275%) and subsequently increased the percentage of explants viability and callus induction (Table 1; Fig. 4). Curvetto et al. (2006) reported that clean bulblet explants in Lilium improved with increasing the H2O2 concentration. Accordingly, in Bakhsh et al. (2016) study, the highest contamination-free of gossypium hirsutum L. explants were achieved by utilization of H2O2. In our study, M. azedarach L. leaf explants viability and callus induction in methods including 7% H2O2 for 10 min were 1.5-times and 3-times, respectively, higher than those of 5% H2O2 for 15 min. On the other hand, a higher concentration of H2O2 with lower exposure duration has no adverse effects on explant viability and callus induction from M. azedarach L. leaf explants. Our results are in agreement with Hesami et al. (2019) and Mihaljević et al. (2013) observations in surface sterilization of chrysanthemum and sour cherry, respectively.
Fig. 4.
The effect of H2O2 inclusion and concentration in disinfection protocol on the M. azedarach L. leaf explants contamination and viability. Bars with different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
In the present study utilization of a two-step disinfection procedure improved the disinfection efficiency and in vitro response of M. azedarach L. leaf explants. So that, the clean explants achieved from two agents surface disinfection [pretreatment (step one) with 0.5% NaOCl + 3 mg/L benomyl and treatment (step 2) with 2% NaOCl for 12 min (methods A4 and A5)] was higher than those obtained from one step surface disinfection, in which only 2% NaOCl treatment was applied for 12 min (control treatment) (Table 1). Similarly, in Kozak and Stelmaszczuk (2013) study, 60% and 80% clean cultures of Allium karataviense explants were obtained, when 4 and 2% NaOCl applied for 30 min followed by 1% NaOCl for 15 min, respectively. Furthermore, Sivanesan et al. (2014) reported 100% clean cultures of Crocus vernus L. corms by utilization of 2% NaOCl for 10 min and 0.01% HgCl2. Similarly, Siavash Moghaddam et al. (2011) achieved 40% clean explant with utilization of 1 g/L benomyl and 10–15% clorox®, and this value enhanced up to 60% with the addition of plant preservative mixture (PPM) to the culture medium. Furthermore, in the present study, although adjustment of NaOCl pH to pH = 7 and 10 significantly reduced the microbial contamination and increased the percentage of clean explants in M. azedarach L. in vitro cultures, but adversely influenced the explant viability and callus induction (Table 1; Fig. 5). It has been shown that with reducing the pH of NaOCl solution, the chlorine as –OCl increases, which has been considered as a strong oxidizing agent and most effective on disinfecting functions (Fukuzaki 2006). It seems that these elevated levels of –OCl in NaOCl solution with pH = 7 and 10 exert cytotoxic effects on M. azedarach L. leaf explants; and led to the cell death and reduced in vitro responses of the explants.
Fig. 5.
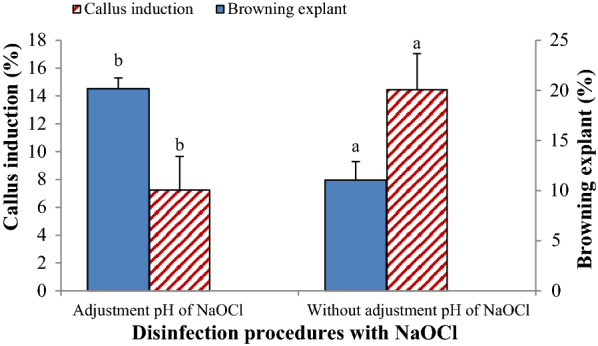
The effect of NaOCl pH adjustment on M. azedarach L. leaf explant disinfection and in vitro culture responses in the media containing benomyl. Bars with different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
Fungicides and bactericides can be useful to proper disinfection and have been used in previous investigations (Sharan et al. 2010). In the aqueous solutions, benomyl breaks down into two compounds, methyle-2-benzimidazolecarbamate and butyl isocyanate, which are toxic to fungi (Pence 2005). The results of the present study showed that, inclusion of benomyl in the culture medium significantly reduced the fungal contamination as compared the control treatment. So, about 80% clean explant was obtained by addition of 100 or 500 mg/L benomyl in the culture medium. Our findings are in agreement with Haldeman et al. (1987) observation, who reported 100% clean cultures of Camellia sinensis shoot tip explants in MS medium supplemented with 1 g/L benomyl. Furthermore, Siavash Moghaddam et al. (2011) achieved up to 50% clean explant in Centella asiatica L. by inclusion 100 mg/L benomyl in the culture medium composition.
Plants show different in vitro growth response to different types and concentrations of plant growth regulators, especially the combination of auxins and cytokinins (Guo et al. 2009; Firoozi et al. 2019). In the present study, all used PGRs treatment induced callogenesis from M. azedarach L. leaf explants, but with different percentage and callus growth rate. The moderate concentration of NAA or 2,4-D (3 mg/L) combined with 1 or 3 mg/L Kin/BAP resulted in the highest callus induction and growth. So that, increasing the concentration of NAA or 2,4-D up to 5 mg/L was led to reduced callus induction and growth and the lowest percentage of callus induction obtained in the higher ratios of Aux/Cyt (5 mg/L 2,4-D + 1 mg/L Kin and 5 mg/L NAA + 1 mg/L BAP). These results are in agreement with Firoozi et al. (2019) report in saffron. Nevertheless, Castellar and Iborra (1997) reported the highest callus induction from saffron in vitro cultures on the MS medium supplemented with 10 mg/L NAA and 5 mg/L BAP.
Among the phytochemicals, flavonoids and phenolic compounds are well-known compounds which have antioxidant and anticancer properties may be due to their strong free radical scavenging activity (Tungmunnithum et al. 2018). There has been a growing interest in the improving production of these valuable phytochemicals in the plant cells through in vitro cell culture techniques. Manipulation and optimization of cell culture conditions and medium compositions such as PGRs provide a practical approach for manipulation and improving the cell growth and secondary metabolite production. PGRs act as one of the critical factors influencing the growth and development of plant cells in both whole plant and in vitro cultures (Farjaminezhad et al. 2013; Firoozi et al. 2019). Our results indicated that in vitro production of bioactive secondary metabolites in M. azedarach L. callus cultures was significantly influenced by the type and concentrations of auxins and cytokinins. So, the medium supplemented with moderate concentration (3 mg/L) of 2,4-D in combination with 1 or 3 mg/L BAP/Kin increased the amount of TPC, TFC, and AC in the callus cultures. So that, the amount of TFC and AC obtained from the calli grown on the medium supplemented with a moderate concentration of 2,4-D was 1.5–2 times higher than those attained from the medium supplemented with other concentrations of 2,4-D or NAA. Regardless of the type of auxins, increasing the auxin concentrations from 1 to 3 were enhanced the amount of TPC, TFC, and AC. In contrast, increasing the auxin concentration from 3 to 5 significantly reduced the TPC, TFC and AC production and accumulation in the M. azedarach L. callus cultures (Table 3; Fig. 6A). These results revealed that both type and concentration of auxin play a significant role in the production and accumulation of secondary metabolites in the M. azedarach L. callus cultures. Similarly, Javed et al. (2017) reported that the highest amount of TPC and TFC in Stevia rebaudiana in vitro cultures produced in 2 mg/L NAA + 0.5 mg/L BAP treatment. Where, the lowest TPC was obtained from 2 mg/L 2,4-D + 1 mg/L BAP, and 0.5 mg/L 2,4-D + 0.5 mg/L Kin. Also, the amount of TPC, TFC and AC were increased by increasing the cytokinin concentration from 1 to 3. In contrast, the amount of these metabolites declined by increasing the cytokinin concentration from 3 to 5 mg/L (Fig. 6B). Contrariwise, among the treatments assessed in the present study, the calli grown on MS medium containing 3 mg/L NAA + 1 mg/L Kin produced the highest amount of rutin, quercetin and kaempferol (5.556, 2.063 and 1.843 mg/g FW, respectively), which respectively was about 34, 5.68 and 8.23 times higher than those obtained from 3 mg/L 2,4-D + 3 mg/L Kin. Furthermore, the calli grown on MS medium supplemented with 3 mg/L NAA + 1 mg/L BAP produced 3.733, 1.568 and 1.423 mg/g FW of rutin, quercetin and kaempferol, respectively. On the other hand, the production of these secondary metabolites inhibited through 2,4-D. The highest and lowest suppression was occurred in the MS medium containing 3 mg/L 2,4-D + 3 mg/L Kin and 3 mg/L 2,4-D + 1 mg/L BAP, respectively. Nair et al. (1992) suggested that the suppression effects of 2,4-D to secondary metabolite production may be attributed to the herbicidal properties of 2,4-D. Furthermore, the results of the present study revealed that with increasing the concentration of cytokinin from 1 to 3 mg/L, the amount of rutin (4.5-times), quercetin (4-times) and kaempferol (3.4-times) in callus cultures were decreased significantly (p ≤ 5%). These decreases were varied depending on the auxin type, so that, the amount of decline of rutin, quercetin and kaempferol in the treatments containing NAA were significantly higher than those of the containing 2,4-D. On the other hands, the calli grown on the medium containing Kin produced the higher amount of rutin, quercetin and kaempferol as compared to those on the medium containing BAP, especially at 1 mg/L level. Farjaminezhad and Garoosi (2021) reported the higher azadirachtin production in Azadirachta indica cell suspension culture in the medium containing 2 mg/L Kin. In Lian et al. (2002) study on Panax ging, saponin productivity increased when the medium supplemented with a low concentration of cytokinins, but the growth of the cells was not affected. Cytokinins have also been reported to be effective in the induction of artemisinin production in Artemisia missouriensis in vitro cultures (Zia et al. 2007). Zia et al. (2007) reported that inclusion of 8.88 µM BAP produced 3.05 µg/g artemisinin in A. absinthium callus cultures, which was higher than that of the media containing NAA or Kin.
Fig. 6.
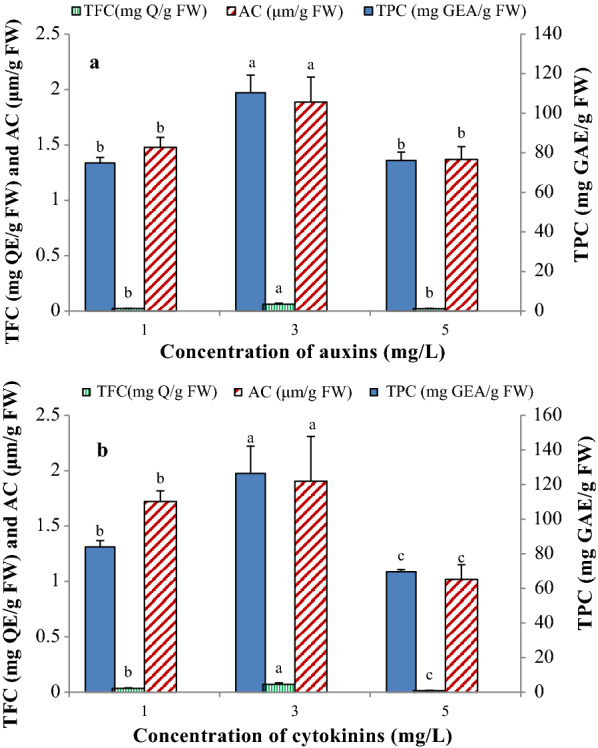
The effect of auxins (A) and cytokinins (B) concentration on TPC, TFC and AC content in the callus cultures of M. azedarach L. Bars with different letters indicate a significant difference (P ≤ 5%) according to Duncan’s Multiple-Range Tests (DMRTs)
In the present study, we established an efficient protocol for disinfection and in vitro culture of M. azedarach L. Utilization of two different disinfection agents (first with H2O2 fallowed by NaOCl) improved the sterilization efficiency and in vitro response in M. azedarach L. leaf explants. Here we demonstrated the production and accumulation of secondary metabolites including rutin, quercetin and kaempferol in callus cultures of M. azedarach L. Furthermore, the amount of these secondary metabolites influenced by both type and concentration of plant growth regulators. These findings provide a newfound knowledge and the practical approach for biotechnological studies in this medicinal plant and production of valuable secondary metabolites.
Supplementary Information
Additional file 1: Figure S1. In vitro culture of M. azedarach L.; A1: leaf explants, B1: viable and browning leaf explants after disinfection and in vitro culture, C1: swollen leaf explants and initiation of callus induction on MS medium containing 1 mg/L NAA + 1 mg/L Kin, D1 and E1: callus induced on MS medium containing 1 mg/L NAA + 1 mg/L BAP and 3 mg/L NAA + 1 mg/L BAP (is embryogenic callus), respectively, A2 and B2: callus initiation and growth on MS + 1 mg/L 2,4-D + 1 mg/L Kin, C2, D2 and E2: callus growth on MS medium containing 1 mg/L 2,4-D + 1 mg/L Kin, 1 mg/L NAA + 1 mg/L Kin and 3 mg/L 2,4-D + 3 mg/L BAP, respectively. Figure S2. In vitro culture of M. azedarach L.; A1: callus induction on MS medium containing 3 mg/L NAA + 1 mg/L Kin containing green cells in callus and B1: 3 mg/L NAA + 1 mg/L BAP containing green and globular shape spots in callus (are embryogenic callus), C1: embryogenic callus that have taken root, A2: globular and torpedo shape embryos, B2: globular embryos under a stereoscope and C2: torpedo shape embryos under a stereoscope. Figure S3. In vitro culture of M. azedarach L.; Callus growth on MS + A1: 1 mg/L NAA + 1 mg/L Kin, B1: 1 mg/L NAA + 1 mg/L BAP, C1: 1 mg/L 2,4-D + 1 mg/L Kin, D1: 1 mg/L 2,4-D + 1 mg/L BAP, A2: 3 mg/L NAA + 1 mg/L Kin, B2: 3 mg/L NAA + 1 mg/L BAP, C2: 3 mg/L NAA + 3 mg/L Kin, D2: 3 mg/L NAA + 3 mg/L BAP, A3: 3 mg/L 2,4-D + 1 mg/L Kin, B3: 3 mg/L 2,4-D + 1 mg/L BAP, C3: 3 mg/L 2,4-D + 3 mg/L Kin, D3: 3 mg/L 2,4-D + 3 mg/L BAP, A4: 5 mg/L NAA + 1 mg/L Kin, B4: 5 mg/L NAA + 1 mg/L BAP, C4: 5 mg/L NAA + 5 mg/L Kin, D4: 5 mg/L NAA + 5 mg/L BAP, A5: 5 mg/L 2,4-D + 1 mg/L Kin, B5: 5 mg/L 2,4-D + 1 mg/L BAP, C5: 5 mg/L 2,4-D + 5 mg/L Kin, D5: 5 mg/L 2,4-D + 5 mg/L BAP, respectively. Figure S4. HPLC chromatogram of rutin, quercetin and kaempferol in the standard mixture (A) and M. azedarach L. calli grown on the MS medium containing 3 mg/L NAA + 3 mg/L Kin (B). Table S1. Different disinfection methods used in experiment 1 for sterilization of M. azedarach L. leaf explants. Table S2. Different disinfection methods and inclusion of benomyl in culture medium used in experiment 2 for sterilization of M. azedarach L. leaf explants. Table S3. The effect of different disinfection methods on disinfection indices of leaf explants in M. azedarach L. (Experiment 1). Table S4. Effect of PGRs treatment on total flavonoid, total phenol and anthocyanin contents in M. azedarach L. calli. Table S5. Effect of PGRs treatment on rutin, quercetin and kaempferol in M. azedarach L. by HPLC analysis.
Acknowledgements
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. NZ and RA supervised the research. Material preparation, data collection and analysis were performed by FA, NZ and PS, FA wrote the first draft of the manuscript and all authors reviewed and commented on the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the University of Mohaghegh Ardabili under a Grant Number (51-1796, 1395.08.03).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
The authors give consent for the publication of the manuscript to the AMB express.
Competing interests
The authors declare that they have no knowledge of competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Farsi M, Alsalvar C, Morris A, Baron M, Shadih F. Comparison of antioxidant activity, anthocyanins, carotenoids and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem. 2005;53:7592–7599. doi: 10.1021/jf050579q. [DOI] [PubMed] [Google Scholar]
- Anjum S, Abbasi BH, Hano C. Trends in accumulation of pharmacologically important antioxidant-secondary metabolites in callus cultures of Linum usitatissimum L. Plant Cell Tissue Organ Cult. 2017;129:73–227. doi: 10.1007/s11240-016-1158-3. [DOI] [Google Scholar]
- Asghari Zakaria R, Haghighat Hour M, Zare N. Callus production and regeneration of the medicinal plant Papaverorientale. Afr J Biotechnol. 2011;10:11152–11156. doi: 10.5897/AJB11.204. [DOI] [Google Scholar]
- Assareh MH, Sardabi H. Macropropagation and micropropagation of Ziziphus spina-christi. Pesqui Agropecu Bras. 2005;40:459–465. doi: 10.1590/S0100-204X2005000500006. [DOI] [Google Scholar]
- Bakhsh A, Anayol E, Sancak C, Ozcan S. An efficient and cost effective sterilizing method with least microbial contamination and maximum germination ratio for in vitro cotton (Gossypium hirsutum L.) culture. J Anim Plant Sci. 2016;26:868–873. [Google Scholar]
- Castellar MR, Iborra JL. Callus induction from explants of Crocus sativus L. J Plant Biochem Biot. 1997;6:97–100. doi: 10.22364/eeb.14.25. [DOI] [Google Scholar]
- Colgecen H, Koca U, Toker G. Influence of different sterilization methods on callus initiation and production of pigmented callus in Arnebia densiflora Ledeb. Turk J Biol. 2011;35:513–520. doi: 10.3906/biy-0911-161. [DOI] [Google Scholar]
- Coria C, Almiron W, Valladares G, Carpinella C, Ludueña F, Defago M, Palacios S. Larvicide and oviposition deterrent effects of fruit and leaf extracts from Melia azedarach L. on Aedes aegypti (L.) (Diptera: Culicidae) Bioresour Technol. 2008;99:3066–3070. doi: 10.1016/j.biortech.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Curvetto N, Marinangeli P, Mockel G. Hydrogen peroxide in micropropagation of Lilium. A comparison with a traditional methodology. Biocell. 2006;30:497–500. doi: 10.32604/biocell.2006.30.497. [DOI] [PubMed] [Google Scholar]
- Farjaminezhad R, Garoosi G. Improvement and prediction of secondary metabolites production under yeast extract elicitation of Azadirachta indica cell suspension culture using response surface methodology. AMB Expr. 2021;11:43. doi: 10.1186/s13568-021-01203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjaminezhad R, Zare N, Asghari-Zakaria R, Farjaminezhad M. Establishment and optimization of cell growth in suspension culture of Papaver bracteatum: a biotechnology approach for thebaine production. Turk J Biol. 2013;37:689–697. doi: 10.3906/biy-1304-54. [DOI] [Google Scholar]
- Firoozi B, Zare N, Sofalian O, Sheikhzade-Mosadegh P. In vitro indirect somatic embryogenesis and secondary metabolites production in the saffron: emphasis on ultrasound and plant growth regulators. Tarim Biliml Derg. 2019;25:1–10. doi: 10.15832/ankutbd.538973. [DOI] [Google Scholar]
- Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11:147–157. doi: 10.4265/bio.11.147. [DOI] [PubMed] [Google Scholar]
- Ghaffoor A, Bahar Shah G, Waseem K. In vitro response of potato (Solanum tuberosum L.) to various growth regulators. Biotechnol. 2003;2:191–197. doi: 10.3923/biotech.2003.191.197. [DOI] [Google Scholar]
- Guo X, Fu X, Zang D, Ma Y. Effect of auxin treatments, cutting’s collection date and initial characteristics on Paeonia ‘Yang Fei Chu Yu’ cutting propagation. Sci Hortic. 2009;119:177–181. doi: 10.1016/j.scienta.2008.07.022. [DOI] [Google Scholar]
- Haida Z, Syahida A, Ariff SM, Mahmood Maziah M, Hakiman M. Factors affecting cell biomass and flavonoid production of Ficus deltoidea var. kunstleri in Cell suspension culture system. Sci Rep. 2019;9:9533. doi: 10.1038/s41598-019-46042-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldeman JH, Thomas RL, McKamy DL. Use of benomyl and rifampicin for in vitro shoot tip culture of Camellia sinensis and C. japonica. Hortic Sci. 1987;22:306–307. [Google Scholar]
- Hesami M, Naderi R, Tohidfar M. Modeling and optimizing in vitro sterilization of chrysanthemum via MultilayerPerceptron-Non-dominated Sorting Genetic Algorithm-II (MLP-NSGAII) Front plant sci. 2019;10:282. doi: 10.3389/fpls.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed R, Yucesan B, Zia M, Gurel E. Differential effects of plant growth regulators on physiology, steviol glycosides content, and antioxidant capacity in micropropagated tissues of Stevia rebaudiana. Biologia. 2017;72(10):1156–1165. doi: 10.1515/biolog-2017-0133. [DOI] [Google Scholar]
- Kozak D, Stelmaszczuk M. Comparison of Allium aflatunense B. Fedtsch. ‘Purple Sensation’ and Allium karataviense Regel. ‘Ivory Queen’ regenerative capabilities in tissue culture. Acta Sci Pol Hortoru. 2013;12:197–213. [Google Scholar]
- Lian ML, Chakrabarty D, Paek KY. Effect of plant growth regulators and medium composition on cell growth and saponin production during cell-suspension culture of mountain ginseng (Panax ginseng C.A. mayer) J Plant Biol. 2002;45:201–206. doi: 10.1007/BF03030360. [DOI] [Google Scholar]
- Mihaljević I, Dugalić K, Tomaš V, Viljevac M, Pranjić A, Cmelik Z, Puškar B, Jurković Z. In vitro sterilizationprocedures for micropropagation of 'Oblačinska' sour cherry. J Agric Sci. 2013;58:117–126. doi: 10.2298/JAS1302117M. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assay with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nair AJ, Sudhakaran PR, Madhusudanan JR, Ramakrishna SV. Berberine synthesis by callus and cell suspension cultures of Coscinium fenestratum. Plant Cell Tissue Organ Cult. 1992;29:7–10. doi: 10.1007/BF00036139. [DOI] [Google Scholar]
- Pence VC. In vitro collecting (IVG). 1. The effect of collecting method and antimicrobial agents on contamination in temperate and tropical collections. In Vitro Cell Dev. 2005;41:324–332. doi: 10.1079/IVP2004629. [DOI] [Google Scholar]
- Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Sharan M, Dhumne IL, Sharon M. Micropropagation of Chlorophytum borivilliens through direct organogenesis. Adv Appl Sci Res. 2010;1:41–46. [Google Scholar]
- Shubhangi K, Kirti S, Sofiya M, Suchita G. Quantitative estimation of total phenolics and flavonoids in Soymidafebrifuga leaves. Am J Phytomed Clin Ther. 2017;5:20. [Google Scholar]
- Siavash Moghaddam S, Hawa Binti J, Maheran Abdul A, Rusli I. Optimization of an efficient semi-solid culture protocol for sterilization and plant regeneration of Centella asiatica (L.) as a medicinal herb. Molecules. 2011;16:8981–8991. doi: 10.3390/molecules16118981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan I, Jana S, Jeong RR. In vitro shoot regeneration and microcorm development in Crocusvernus (L.) Hill. Pak J Bot. 2014;46:693–697. [Google Scholar]
- Srivastava P, Kasoju N, Bora U, Chaturvedi R. Accumulation of betulinic, oleanolic, and ursolic acids in in vitro cell cultures of Lantana camara L. and their significant cytotoxic effects on HeLa cell lines. Biotechnol Bioproc E. 2010;15:1038–1046. doi: 10.1007/s12257-010-0054-7. [DOI] [Google Scholar]
- Teixeira da Silva JA, Winarto B, Dobránszki J, Zeng S. Disinfection procedures for in vitro propagation of Anthurium. Folia Hortic. 2015;27:3–14. doi: 10.1515/fhort-2015-0009. [DOI] [Google Scholar]
- Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ. Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol. 1979;64:88–93. doi: 10.1104/pp.64.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalla R, Satyendra M, Nidhi M. Medicinal uses and biological activity of the secondary metabolites of Meliaazedarach Linn.: an overview. Plant Cell Biotechnol Mol Biol. 2021;22(33–34):106–123. [Google Scholar]
- Zare N, Valizadeh M, Tohidfar M, Mohammadi SA, Malboobi MA, Habashi AA. Selection of regenerative genotypes from Iranian alfalfa cultivars. J Food Agric Environ. 2009;7:567–572. [Google Scholar]
- Zia M, Mannan A, Chaudhary MF. Effect of growth regulators and amino acids on Artemisinin production in the callus of Artemisia absinthium. Pak J Bot. 2007;39:799–805. [Google Scholar]
- Zu Y, Chunying L, Yujie F, Chunjian Z. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharmaceut Biomed. 2006;41:714–719. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. In vitro culture of M. azedarach L.; A1: leaf explants, B1: viable and browning leaf explants after disinfection and in vitro culture, C1: swollen leaf explants and initiation of callus induction on MS medium containing 1 mg/L NAA + 1 mg/L Kin, D1 and E1: callus induced on MS medium containing 1 mg/L NAA + 1 mg/L BAP and 3 mg/L NAA + 1 mg/L BAP (is embryogenic callus), respectively, A2 and B2: callus initiation and growth on MS + 1 mg/L 2,4-D + 1 mg/L Kin, C2, D2 and E2: callus growth on MS medium containing 1 mg/L 2,4-D + 1 mg/L Kin, 1 mg/L NAA + 1 mg/L Kin and 3 mg/L 2,4-D + 3 mg/L BAP, respectively. Figure S2. In vitro culture of M. azedarach L.; A1: callus induction on MS medium containing 3 mg/L NAA + 1 mg/L Kin containing green cells in callus and B1: 3 mg/L NAA + 1 mg/L BAP containing green and globular shape spots in callus (are embryogenic callus), C1: embryogenic callus that have taken root, A2: globular and torpedo shape embryos, B2: globular embryos under a stereoscope and C2: torpedo shape embryos under a stereoscope. Figure S3. In vitro culture of M. azedarach L.; Callus growth on MS + A1: 1 mg/L NAA + 1 mg/L Kin, B1: 1 mg/L NAA + 1 mg/L BAP, C1: 1 mg/L 2,4-D + 1 mg/L Kin, D1: 1 mg/L 2,4-D + 1 mg/L BAP, A2: 3 mg/L NAA + 1 mg/L Kin, B2: 3 mg/L NAA + 1 mg/L BAP, C2: 3 mg/L NAA + 3 mg/L Kin, D2: 3 mg/L NAA + 3 mg/L BAP, A3: 3 mg/L 2,4-D + 1 mg/L Kin, B3: 3 mg/L 2,4-D + 1 mg/L BAP, C3: 3 mg/L 2,4-D + 3 mg/L Kin, D3: 3 mg/L 2,4-D + 3 mg/L BAP, A4: 5 mg/L NAA + 1 mg/L Kin, B4: 5 mg/L NAA + 1 mg/L BAP, C4: 5 mg/L NAA + 5 mg/L Kin, D4: 5 mg/L NAA + 5 mg/L BAP, A5: 5 mg/L 2,4-D + 1 mg/L Kin, B5: 5 mg/L 2,4-D + 1 mg/L BAP, C5: 5 mg/L 2,4-D + 5 mg/L Kin, D5: 5 mg/L 2,4-D + 5 mg/L BAP, respectively. Figure S4. HPLC chromatogram of rutin, quercetin and kaempferol in the standard mixture (A) and M. azedarach L. calli grown on the MS medium containing 3 mg/L NAA + 3 mg/L Kin (B). Table S1. Different disinfection methods used in experiment 1 for sterilization of M. azedarach L. leaf explants. Table S2. Different disinfection methods and inclusion of benomyl in culture medium used in experiment 2 for sterilization of M. azedarach L. leaf explants. Table S3. The effect of different disinfection methods on disinfection indices of leaf explants in M. azedarach L. (Experiment 1). Table S4. Effect of PGRs treatment on total flavonoid, total phenol and anthocyanin contents in M. azedarach L. calli. Table S5. Effect of PGRs treatment on rutin, quercetin and kaempferol in M. azedarach L. by HPLC analysis.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.