Abstract
Traditional therapies need high systematic dosages that not only destroys cancerous cells but also healthy cells. To overcome this problem recent advancement in nanotechnology specifically in nanomaterials has been extensively done for various biological applications, such as targeted drug delivery. Nanotechnology, as a frontier science, has the potential to break down all the obstacles to be more effective and secure drug delivery system. It is possible to develop nanopolymer based drug carrier that can target drugs with extreme accuracy. Polymers can advance drug delivery technologies by allowing controlled release of therapeutic drugs in stable amounts over long duration of time. For controlled drug delivery, biodegradable synthetic polymers have various benefits over non-biodegradable polymers. Biodegradable polymer either are less toxic or non-toxic. Polylactic Acid (PLA) is one of the most remarkable amphipathic polymers which make it one of the most suitable materials for polymeric micelles. Amphiphilic nanomaterial, such as Polyethylene Glycol (PEG), is one of the most promising carrier for tumor targeting. PLA–PEG as a copolymer has been generally utilized as drug delivery system for the various types of cancer. Chemotherapeutic drugs are stacked into PLA–PEG copolymer and as a result their duration time delays, hence medications arrive at specific tumor site.
Keywords: Cancer therapeutics, Nanotechnology, Targeted drug delivery, Polymer, Carrier
Introduction
Cancer or neoplasm is the uncontrolled growth and rapid development of abnormal cells. The significant growth of cell and cell death resistance, caused by the alteration in genes, is recognized by the impassion of abnormalities and mutagenic phases. Cancer may be either benign or malignant, where benign tumor remains limited to original tissues, malignant tumor may spread to other tissues and organs. It is one of the most life threatening health problems to the human life throughout the world. In 2012, globally, approximately 14 million new tumor cases and more than 8 million cancer related deaths were reported (Wang et al. 2018). In 2020 as reported by Globocan around 18 million cancer cases and 9.5 million cancer related death were observed (Asia et al. 2020). In general, lung, prostate, stomach and liver cancer are most common in men, while breast colorectal, lung, cervical and thyroid cancer are found in woman (Bray et al. 2018; de Oliveira et al. 2021). Surgery, chemotherapy, radiation, bone marrow transplant, immunotherapy, hormonal therapies are major therapies available. Among these the traditional Chemotherapy is most widely used (Wang et al. 2018). In chemotherapy small toxic chemotherapeutic molecules bind with DNA fragments to cause cell death in cancer tissues. Anti-metabolites such as methotrexate and 5-fluorouracil directly interrupt with the growth of cancer cells (Leuschner and Kumar 2005).
In 1960s, surgery and radiotherapy were the main hallmarks of solid tumour treatments (Arruebo et al. 2011). Surgeries operations are invasive that necessitate specialised equipment, trained manpower, and high costs operations. These surgeries are ineffective to cure metastatic cancers, and even in localised tumours. They also fail to remove cancer within surgical margins, causing the patient to undergo a re-operation. Most patients handle radiotherapy well but some patients can suffer side effects, such as nausea, tissue injury, irritation, swelling and discomfort (de Oliveira et al. 2021). In androgen (hormonal) ablation, reduction of the tumor cells along with partial recovery is seen in prostate cancer patients. Later the tumor again arises in a poorly differentiated androgen-independent form within 2 years, during which there is no treatment available to prolong the patient's lifespan. Surgical removal of the tumor cells is invasive and has adverse effects that vary depending on the tumor type. When a primary tumor is removed, it also leads to a growth in dormant cancer cells metastases (Leuschner and Kumar 2005). Currently available chemotherapeutic drugs such as paclitaxel, docetaxel inhibits cell division by acting on microtubules. These drugs are systemically very active and efficient. High systematic dosages are needed to achieve high therapeutic efficiency, but heavy doses do not necessarily mean to cure it. The rate of drug release is followed by an initial burst that can cause depletion of the active drug within a short life span (Ghasemi et al. 2018). They sometimes trigger a varieties of toxicity, such as cardio toxicity, nephrotoxicity, hepatotoxicity and hematotoxicity (Wadhawan et al. 2019), bone marrow cell death (de Oliveira et al. 2021). It does not kill single cancerous cells or at specific tumor site. These medications target rapid proliferating cells, thereby cannot distinguish between malignant and non-cancerous cells (Wadhawan et al. 2019). Hair loss (Wadhawan et al. 2019), anemia (low red blood cells counts), nausea, alopecia, fatigue (Leuschner and Kumar 2005), mood changes, infertility, development of new cancer (de Oliveira et al. 2021) are the instantaneous side effects. Still there are some side effects of these traditional therapeutics, such as high levels of stress among patients (Wadhawan et al. 2019). Multi-drug resistance has another downside of chemotherapy.
In Biomedical research sector some new therapies have been introduced, such as photodynamic therapy, photothermal gene therapy and immune therapy. Although having these therapeutic agents exhibit great results, it also have various disadvantages, such as low targeting ability, poor pharmacokinetics and non-specified bio-distribution. Moreover, the major concerns shown by these therapeutic agents are their poor solubility and hydrophobicity. It is necessary to overcome these drawbacks as it will help to boost the anti-tumor efficiency (Wang et al. 2018; de Oliveira et al. 2021).
Nanotechnology as a targeted drug delivery
In current scenario there is of need for new cancer treatments and drug delivery mechanisms that particularly affects the tumor cells (Parveen and Sahoo 2008). Recent advancements in nanotechnology specifically in nanomaterials are extensively used for many biological applications, such as in drug delivery and tissue engineering. Nanotechnology, as a frontier science, has the potential to break down all the obstacles and to be more effective and secure drug delivery system (DDS) (Parveen and Sahoo 2008). Paul Herlich, immunologist proposed that it may be possible to develop a drug carrier which can target drugs with extreme accuracy with the help of bionanotechnology (Lai et al. 2014). Nanotechnology ultimately resolves the disadvantages of active ingredients, by linking the drug with the carrier particles (micelles, nanocapsules, and nanospheres).
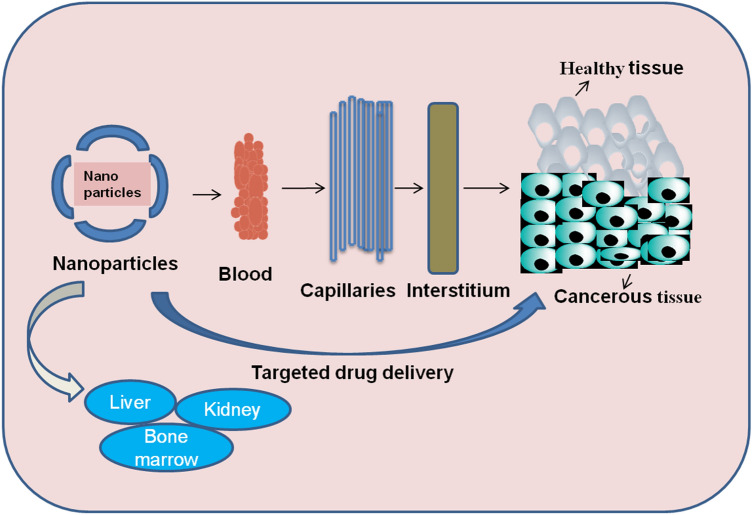
Numerous nanocarriers which comprise micelles, liposomes and nanocapsules, have been considered in anticancer trials. They possess the ability to combine both pharmacodynamics and pharmacokinetics properties, thereby enhancing their therapeutic index. Nanosized delivery systems can move far deeper into tissue by fine capillaries (Fig. 1), breaching the fenestration present in the epithelial lining (e.g., liver). They are usually uptaken more effectively by cells than micrometer-sized delivery systems (Cegnar et al. 2005). Nanomedicine provides materials ranging in size from 1 to 100 nm that are used as drug nanocarriers with unique properties, such as size, solubility, high specificity. Nanoparticles have higher permeability and retention effect in which nanoparticles conjugated drugs can accumulate more in tumor tissue (Avramović et al. 2020).
Fig. 1.
Route of Nanoparticle particularly at cancerous tissue
An Ideal carrier must have high performance of drug loading and should have license from the Food and Drug Administration (FDA) to be used in medicinal materials, anti-cancer medicines and other drug carriers (Sim et al. 2018; Alyafeeu et al. 2018). Moreover, it must be biocompatible (Pereira et al. 2016), biodegradable (Chang et al. 2019) and non-toxic (Jelonek et al. 2015). After encapsulation of drug, carrier protects and delivers drug selectively at tumor site in a controlled manner (Afsharzadeh et al. 2020). They also have unique property of high degradation and capable of being biologically degraded into appropriate molecules that can be metabolized by normal metabolic pathways and removed from the body (Sim et al. 2018). Most importantly it must protect the drug from premature degradation (Hruby and Ulbrich 2005). Carrier must possess some other characteristics, such as high specificity, efficient drug release, extended drug circulatory system time, passive tumor tissue targeting (Kim et al. 2014; Colone et al. 2020).
Heavy dosages delivered into the targeted site can speed up the treatment with high efficacy against tumor (Dariva et al. 2020). The key advantage of using nanocarriers is the distribution of drug across blood with particular targeting ligand, resulting in greater drug metabolism specifically at the cancerous tissue. Moreover, they do not kill healthy cells (Fig. 1). Nowadays, nanoparticles can be used in several different ways, such as dermal delivery, as antimicrobial and anticancer agents, carries peptide and protein medications, such as insulin, as well as carriers of anti-inflammatory and respiratory drugs.
Some factors affecting nanoparticles fabrication in vivo are Particle size, Surface properties, Stability of drugs, while encapsulation, Immunogenicity, Biocompatible coatings (Leuschner and Kumar 2005).
In general, two approaches to NP formulation are Top-down and Bottom-up.
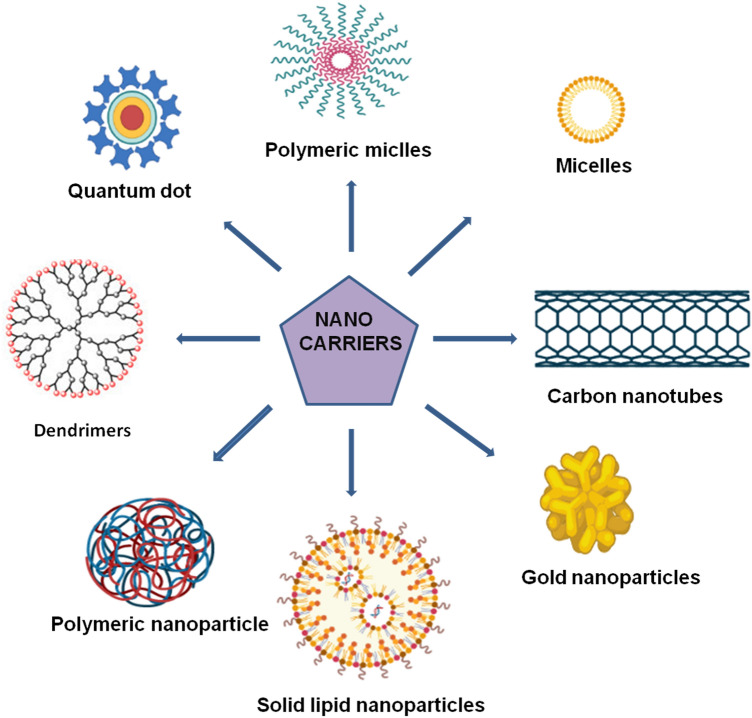
Bottom-up technique is self-assembly forms a larger particle through tiny building blocks. Liposomes, micelles, polymeric nanoparticles, and dendrimers are only a few examples of bottom-up approach. Nanoparticles come in a variety of shapes and sizes, and they have the ability to change a variety of aspects of cancer. Other nanoparticle that are used in cancer drug delivery are polysaccharide nanoparticles, silica nanoparticles, and gold nanoparticles (Fig. 2). However, use of these nanocarriers became restricted because of few limitations.
Fig. 2.
Different types of Nanocarriers used in drug delivery
Among all metal-based nanoparticles, gold nanoparticles have shown a great potential as drug delivery carriers and they can be easy functionalized with different types of molecules. The major drawback associated with gold nanoparticles are their non-biodegradable nature, thus their surface modification can change toxicity, biodistribution, and pharmacokinetics properties during the transportation of the drug (Kong et al. 2017; Ajnai et al. 2014). Silica nanoparticles are now extensively used as nanocarriers for the delivery of various drugs having different physiochemical, pharmacokinetic and pharmacodynamic properties. The major disadvantage of porous silica nanoparticles is attributed to the surface density of silanol groups interacting with the surface of the phospholipids of the red blood cell membranes resulting in hemolysis. In addition, metabolic changes induced by porous silica nanoparticles promote melanoma (Bharti et al. 2015). Polysaccharides can be used as matrices for encapsulation, immobilization, and controlled release for many active compounds. However, their high molecular weight and low solubility limits their potential in drug delivery (Barclay et al. 2019).
Polymer in cancer drug delivery
Polymers advance the drug delivery system in controlled manner of therapeutic drugs, i.e., hydrophilic and hydrophobic drugs in stable long duration of time. Pharmacokinetic and pharmacodynamic properties are modified by conjugating the therapeutic drug to the polymer via a number of mechanisms including enhanced plasma half-life, resistance from proteolytic enzymes, decreased immunogenicity, increased solubility of low MW products and capacity for selective distribution (Liechty et al. 2010; Li et al. 2013). As a consequence polymers offer a potential strategy for more successful and convenient treatment of cancer to patient.
The two primary types of polymers used in drug delivery are Biodegradable and Non-Biodegradable polymers. Non-Biodegradable polymers cause toxicity problems, mostly due to accumulation in the body. The basic mechanism of drug release from non-degradable matrices is diffusion, which is based on polymer permeability and drug characteristics (Cegnar et al. 2005). To resolve these problems, researchers began developing biodegradable polymers in the early 1970s. They made significant progress (clinical application) particularly in the areas of cancer in last few decades (Whittlesey and Shea 2004).
Benefits of biodegradable polymers over non-biodegradable polymers
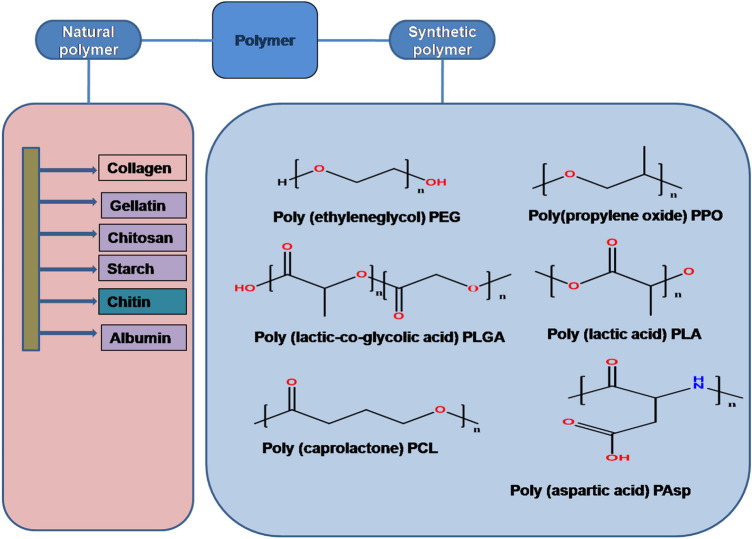
Biodegradable polymers may be either synthetic or natural (Fig. 3). Polymers degrade into enzymatically or non-enzymatically in vivo and byproducts produced are excreated by different metabolic routes. Several synthetic and natural biodegradable polymers have been studied for drug targeting and extended drug release but only few of them are biocompatible and biodegradable that are currently in use (Jain et al. 1998). They either are low toxic or non-toxic. They are suitable for various types of applications due to their mechanical properties (Vahdati 2019). Synthetic polymers release therapeutic drugs continuously for long duration of time, on the other hand natural polymers are confined to organic solvents and release drug in shorter period of time (Parveen and Sahoo 2008).
Fig. 3.
Classification of different types of polymer
Synthetic polymers
They have been explored since the 1940s, so that they can be used as nanomedicines for different fields. Before the discovery of various synthetic polymers, dextrans, dextrin and other oligosaccharides were utilized as coatings for different nanoparticles for the treatment of diseases. Helmut Ringsdorf proposed the idea for using polymer with drug conjugated in 1975 which comprises of three segments, a solubilizer or the hydrophilic section to guarantee the water solvency, a medication which generally bound to the polymeric spine through a linker, and a focusing on moiety to give transport of a specific natural objective.
Synthetic polymers, provide more homogeneous composition and hence having a higher purity. Polyesters such as Polylactide glycolic acid (PLGA), Poly ester amide (PEA), PGA, PEG, PLA, etc. are some of the examples of synthetic polymers (Lai et al. 2014). The Advantages and disvantages of these synthetic polymers are shown in Table 1.
Table 1.
Advantages and disadvantages of different nanopolymers
| S. No. | Polymer | Advantages | Disadvantages | References |
|---|---|---|---|---|
| 1 | PLGA | Biocompatible, non-toxic polymer and has sustained release properties | Hydrophobicity | (Taghipour-Sabzevar et al. 2019) |
| 2 | PCL | Biodegradable, suitable for long term drug delivery system | Takes longer time to degrade in human body | (Aramudan and Senthil 2016) |
| 3 | PLA | Non-toxic, biodegradable, | Poor hydrophobicity | (Liu et al. 2020) |
| 4 | PEG | Hydrophobic, high drug loading capacity | Sometimes triggers immune response | (Taghipour-Sabzevar et al. 2019) |
| 5 | PPE | Faster degradation rate, best for nucleic acid delivery | Causes difficulty in synthesis | (Kamaly et al. 2016) |
| 6 | PEA | Biodegradable, hydrophobic due to presence of hydrophobic amino acids | Alteration of amide and ester bond leads to slow degradation | (Kamaly et al. 2016) |
| 7 | PPO | Biodegradable, biocompatible | Large scale production is difficult | (Kamaly et al. 2016) |
| 8 | PGA | High crystallinity | Poor solubility | (Rezvantalab et al. 2018) |
Polymeric nanoparticles selectively formulate, secure and distribute various drugs delivered in a regulated and tunable manner particularly at tumor site. Thus provides an enormous approach in the treatment of cancer (Afsharzadeh et al. 2020). The degradability of synthetic polymers is controlled by their chemical composition, which plays a crucial role in scaffold design. Engineers are able to manipulate the synthetic polymers with a wide range of characteristics. Subsequently, polymers can be multifunctional and show ease of tailoring (Liu et al. 2020).
PLA and PEG
Various Polyesters such as PEG, PLGA, PLA, PCL, etc. have been extracted from the renewal resources and have been approved by FDA for clinical uses (Massadeh et al. 2016) (Table 2). PEG is biodegradabe, biocompatible, high drug loading ability-; has extended circulation half-life, flexibility and simple functionalization with complex ligands (Ghasemi et al. 2018). Being an amphiphilic nanomaterial PEG is one of the most promising carriers for tumour targeting. It is the most popular hydrophilic agent owing to its various other advantages, such as low polydispersity, no charge, and linearity. It is commonly used protective coating medium for drug delivery liposomes and nanomicelles. Moreover, circulation time of the PEG conjugated drug increases. There are neglible or very less chances of degradation (Bunker 2012).
Table 2.
List of Food and Drug (FDA) approved PLA, PEG, PEG–PLA copolymer
| Type of carrier | Polymer | Drug/ nucleic acid | Particle size (nm) | Target | References |
|---|---|---|---|---|---|
| Nanoparticle | PLA–PEG | Nisin | 200 | Cytotoxicity against cancer cell line (AGS, KYSE-30, K562) and induces apoptosis | (Elmowafy et al. 2019) |
| Micelles | PEG–PLA | Paclitaxel (genexol-PM) | 20–50 | Metastatic breast cancer | (Meng et al. 2021) |
| Micelles | PEG–PLA | Gemcitabine + Paclitaxel | 20–50 | Lung cancer | (Tyler et al. 2016) |
| Micelles | PEG–PLA | Triolimus (pac litaxel, 17-AAG, rapamycin) | 5–100 | Breast cancer | (Tyler et al. 2016) |
| Micelles | PEG–PLA | Docetaxel | 1–100 | Solid tumor (such as lung, cervical, gastroesophageal cancer) and metastatic cancer | (Tyler et al. 2016) |
| Nanoparticles in microparticle | PEG–PLA | pDNA | <200 | Breast cancer cell (ZR-75–1) | (Elmowafy et al. 2019) |
| Nanoparticle | PLA | Curcumin | 60–70 | Liver fibrosis | (Elmowafy et al. 2019) |
| Nanoparticle | mPEG–PLA | Nimodipine | 75–85 | Target to the cerebrospinal fluid increased compared to nimodipine alone | (Zhang et al. 2006) |
| Nanoparticle | PEG | SN38 | – | Antitumor activity (invitro and invivo) | (Mishra et al. 2016) |
| Nanoparticle | PEG | Doxorubicin | 80–90 | Breast and Ovarian cancer | (Mishra et al. 2016) |
PLA is a prominent biomedical material that is substituting traditional petrochemical-based polymers in pharmaceutical industry(Liu et al. 2020). Nowadays, it is one of the most remarkable amphipathic polymers and most suitable materials for polymeric micelles. Three kinds of stereoisomers present in PLA are poly (l-lactide) (PLLA), poly (d-lactide) (PDLA), poly (d,l-lactide) (PDLLA) (Farah et al. 2016). Some unique properties of the PLLA and PDLA are that they can frame stereocomplexes. More percentage of PLLA enhances the mechanical flexibility of scaffold (Abd Alsaheb et al. 2015).
PLA has been authorized by the FDA in drug conjugated carriers, due to its appropriate biocompatibility, renewability, better safety, long lasting immunity, and high mechanical properties. Low molecular weight of PLA is selected, because time taken for degradation is less (Li et al. 2017). In contrast, polylactic acid and its degradation elements such as H2O and CO2 are ideal for medical application due to their non-carcinogenic and non-toxic effects on the human body (Abd Alsaheb et al. 2015). Addition of surfactants or altering the molecular weight, size, temperature, and moisture of PLA may readily change its chemical and physical characteristics particularly its biocompatibility and biodegradability. This facilitates the creation of a desired drug delivery system in a variety of formulations (Li et al. 2017).
Copolymer of PLA–PEG
Block copolymers, peptide polymeric derivatives, dendrimers, branched polymers are among the highly complicated polymer structures generated by specialized polymer chemistry and engineering (Cegnar et al. 2005). Block copolymers can be engineered for efficient drug delivery by changing their chemical characteristic. In drug-conjugated micelles the covalent bond between both polymer and chemotherapeutic agent or drug must be cleaved. However, drugs are sited in entrapped micelles mostly by interactions throughout shell or core of the microspheres. The rate of discharging the drug is attained through diffusion (Danafar et al. 2016).
Polymeric micelles based on block copolymers have been of considerable concern for cancer therapeutics (Sant et al. 2005). Copolymer increases the solubility of hydrophobic drugs which help to administer them intravenously in an easier way (Torchilin 2004). The prime purpose of copolymerization is to provide low aqueous solubility by making core as hydrophobic component and shell as hydrophilic part for drug agents. The hydrophilicity of shell offers other benefits, i.e., better circulation and blood stability (Hami et al. 2014). Furthermore, it increases tumor aggregation and polymeric micelles may be functional with targeting ligands. Amphiphilic copolymers also have the potential to improve the hydrophobic drugs water solubility by 5000 times (Wang et al. 2018). PLA–PEG copolymer has gained much attention among the amphiphilic copolymers for cancer drug delivery (Zhao et al. 2015).
Characteristics and properties of PEG–PLA copolymer
PLA–PEG micelles have shown many advantages over standard drug carriers that are used widely. Chemotherapeutic drugs are stacked into PLA–PEG copolymer after that their duration time delays, hence medications arrive at specific tumor site. PLA–PEG block copolymer is non-accumulative and non-poisonous in vivo. Degraded byproducts are introduced in TCA cycle and are later excreted by the kidney. Copolymerization improves drug loading, minimize the burst effect, extend drug residence time in vivo. It also prevent macrophages from engulfing (Xiao et al. 2010). Belonging to the class of hydrophilic polymers (PEG), PEG–PLA provide great stabilization to nanoparticles against opsonisation (Elmowafy et al. 2019).
Polymer composition largely influences the polymer degradation and the rate of drug release. Thus, with increase in hydrophilic PEG segment, the diffusion occurs between drug and water. As a result both drug release and copolymer hydrolytic erosion are increased. Water solubility of the PLA–PEG can be enhanced by the use of hydrophilic PEG. In PEG–PLA copolymer PEG can reduce the threat of protein degradation and improve the blood circulation (Zhu et al. 1990). Modifications in crystallization, hydrophilicity and degradation rate of PLA–PEG copolymer show tremendous possibilities to be utilized in drug delivery. Unbending nature of PEG and the PLA fragment gives secrecy properties due to which nanoparticles can escape from the immune system of the individual. The hydrophobic core can be obscured by the PEGylated shell, leaving the carriers unrecognized by the reticulo-endothelial system, contributing to extended blood circulation (Afsharzadeh et al. 2020).
The ideal temperature for synthesis of PEG–PLA copolymer is between 180 and 190 °C otherwise the products turned dark brown or reaction do not occurs. Moreover, the mechanical properties of PLA–PEG copolymer depends on the composition of polymers (Zhu et al. 1990). Other physicochemical and biological properties, including nontoxicity and decreased absorption by the reticulo-endothelial system after intravenous injection, PEG–PLA micelles have been widely employed as drug delivery vehicles for cancer therapy(Wang et al. 2018).
Factors influencing the release of PLA–PEG block copolymer nanoparticles drug
The first factor that influences its release is particle size of copolymerization nanoparticle: The PEG/PLA ratio can be used to adjust the particle size of block copolymer (PEG–PLA) nanoparticles. Different sizes of copolymer will cause the nanomatrix to degrade or diffuse at different rates, resulting in variation in drug release (Xiao et al. 2010). Second factor is the method of preparing of block copolymer nanoparticles: different formulation methods can significantly affect polymer crystal structure, drug delivery, and carrier material sustainability, nanoparticle internal compactness, size, and surface structure. All of these factors interfere with the rate of drug release (Xiao et al. 2010). Moreover, drug loading in PEG–PLA is another significant factor that influences drug release (Xiao et al. 2010).
Method of preparation of PLA–PEG copolymer
Various methods are used to prepare PEG–PLA block copolymers in different forms such as nanospheres, nanocapsules, nanomicelles, and polymersomes along with different compositions. All these methods of synthesis of PLA–PEG copolymers are feasible and convenient; making it easily accessible for the treatment of cancer. The above mentioned methods are briefly explained as follows:-
Nanomicelles
The preparation of PEG–PLA copolymer nanomicelles depends upon the hydrophilicity of copolymer as these copolymer are easily capable of forming the micelles by self-assembly action in the water. There are two methods for preparation, i.e., direct dissolution method and film rehydration method. Direct dissolution method is commonly used. After dissolving directly in water, the transparent micellar solution can be formed instantaneously above its critical micelle concentration (Zhang et al. 2009). Second method is film rehydration in which drugs and copolymers are being dissolved in the volatile solvent forming a membrane. The micelles are formed by the addition of water or buffer solution (Zhan et al. 2010). Organic solvents are used in such cases, where copolymer do not dissolve with water. Then the PEG–PLA copolymers is dissolved in the organic solvent. Dialysis or evaporation is used to remove organic solvents (Li et al. 2009). Then the prepared mixture is dialyzed. Dynamic light scattering helps to measure the size as well as shape of the PEG–PLA copolymers (Kataoka et al. 2001).
Polymersomes
Ultrasonic dispersion, injection and film rehydration are the method used to prepare copolymer polymersomes. In case of the film rehydration method, the volatile organic solvent is used to dissolve the copolymers (Li et al. 2007). The solvent injection method is used to load amphotericin B polymersomes (Jain and Kumar 2010).
Nanospheres
Emulsification solvent evaporation and emulsification solvent diffusion are the methods used in a preparation for nanosphere copolymer. Nanosphere is a two-step process, the first step includes emulsification, i.e., first, the drug along with the copolymer is dissolved in organic solvent, such as acetone, dimethylformamide or dimethylacetamide. Then emulsion is formed by the addition in the water phase and stirring. Second, dialysis and evaporation helps to remove organic solvent (Hammady et al. 2009).
Nanocapsules
The widest method for the preparation of nanocapsules is the interfacial polymerization method in which the block copolymer and drugs are being dissolved slowly into a water-miscible organic solvent into an aqueous solution by stirring (Pereira et al. 2008).
Among all the methods which are discussed above for the preparation of various PEG–PLA block copolymer, the method of direct dissolution and interfacial polymerization used for nanomicelles and nanocapsules come out to be the most feasible methods. Owing to their wide reach, easy preparation and affordability they can be easily accessed for preparation of copolymers.
Synthesis of PLA–PEG copolymer
The copolymers used must be well-defined to manage the characteristics of a micellar device. Effective polymerization reaction would be determined by the quality of the polymer to be synthesised. Method for polymerization are categorized under Anionic ring and Open ring polymerization (Sant et al. 2005).
Open ring polymerization
The synthesis of different PEG–PLA copolymer of different block lengths is carried out: 2000:3000 (copolymer 1), 2000:6000 (copolymer 2), 5000:7000 (copolymer 3), 5000:9000 (copolymer 4) and 5000:15,000 (copolymer 5) which is by ring opening polymerization (Sadeghi et al. 2015).
Commercially available asymmetric PEGs or its end-group derivatives such as a-Methoxy-N-hydroxyl-PEG are used to initiate the polymerization for preparing mPEG–PLA through ring-opening polymerization (Xiao et al. 2010). Polyesters of d,l-lactide is desirable hence to provide safety, effectiveness and solubility (Sant et al. 2005). Stannous octoate [Sn(Oct)2] (Zhang et al. 2009) has higher catalytic ability, frequently act as catalysts.
Anionic ring opening polymerization
The synthesised block copolymer mPEG–PLA is made using an alternative anionic ring-opening polymerization with butyl lithium, sodium alkoxide and potassium alkoxide (Otsuka et al. 2000; Stefani et al. 2006). It is done via anionic ring-opening polymerization through lactic acid and ethylene oxide. The initiator used is 3,3diethoxy-potassium propanol (Otsuka et al. 2000).
The preparation of mPEG–PLA and its modification should be done in ring opening polymerization. As it has numerous advantages over anionic polymerization having higher efficacy, easy to prepare, and less time taking.
Different types of drugs conjugated with PLA–PEG and their mechanism
Paclitaxel
Among the various anticancer drugs the Paclitaxel is a prominent that also acts as a microtubule stabilizing agent. It is one the most vital anticancer drugs which is widely used in the treatment of various cancers, such as breast cancer, lung cancer and ovarian cancer (Singla et al. 2002). Paclitaxel drug efficacy is hindered by its natural rapid clearance, high toxicity and low water solubility. In addition, this drug has nonselective bio distribution that ends up with poor accumulation at the tumour site, resulting in inefficient suppression of tumor and consequential side effects. To improve the pharmacokinetics, paclitaxel (Xin 2012) is fabricated that resulted in the expansion of novel polymeric DDS including nanospheres, microsphere formulations, liposomes, macromolecular conjugates and micelles (Guo et al. 2011; Zhong et al. 2016; Yu et al. 2010).
The anticancer activity of paclitaxel conjugated with PEG–PLA was found to be effective towards H7402 cells. It inhibits cell replication by promoting the microtubules assembly particularly from tubulin heterodimers but prevents their depolymerisation. Then cells are arrested in the G2/M phase in cell cycle and eventually undergo programmed cell death (Apoptosis) (Kampan et al. 2015). In another study paclitaxel loadings in the PLA–PEG stabilized with graphene oxide nanocomposite was found to be adequate for sustainable release, regulated by the molecular weight of the PLA–PEG copolymer and its percentage in the composite. The PLA–PEG composites stabilized with graphene containing paclitaxel shown antitumor efficacy in vitro against cancer cells (A549 lung cancer cells) (Angelopoulou et al. 2015).
Galbanic acid
Galbanic acid (GBA) is a vital sesquiterpene coumarin derivative, having various curative advantages, possessing anticancer properties (Afsharzadeh et al. 2019). The primary disadvantage of the GBA is its low solubility ultimately affecting its clinical applications, so designing a nanostructure which can carry GBA and permit the drug to be administered in an aqueous medium and increases its therapeutic efficiency. Biotechnologist encapsulated the GBA in lipid nanomaterials formulations improving its aqueous solubility media and observed an extensive standing apoptotic effects (Eskandani et al. 2015).
GBA with PLA–PEG uses single emulsion-solvent evaluation method to deliver GBA into cancer cells (Afsharzadeh et al. 2019). As a powerful anticancer drug galbanic acid triggers Apoptosis of lung cancer (H460 cells) involving caspase activation and Mcl-1 suppression (Oh et al. 2015). PEG–PLA a self-constructed nanoparticles (PEG–PLA–NPs) conjugated with galbanic acid suppresses the prostate specific membrane antigen of prostate cancer cell via Apoptotic pathway. The result obtained reflects the galbanic acid encapsulation into the PLA–PEG can also be used certainly for colorectal cancer treatment (Afsharzadeh et al. 2020) (Table 3).
Table 3.
PLA–PEG conjugated with different drugs
| Carrier | Drugs | Loading mode | Targetting site | References |
|---|---|---|---|---|
| PLA–PEG | Docetaxel and Galbanic acid | Encapsulation | Prostate cancer | (Afsharzadeh et al. 2020) |
| PLA–PEG | Tat conjugated Paclitaxel | Entrapment | A549 lung Cell line | (Koutsiouki et al. 2017) |
| PLA–PEG | Nubcp9 peptide | Encapsulation | Mcf-7 breast cancer | (Wadhawan et al. 2019) |
| PLA–PEG | Doxorubicin and ce9-np | Encapsulation | Mcf-cancer cells | (Dariva et al. 2020) |
| PLA–PEG | Curcumin | Encapsulation | Anticancerous properties | (Thong et al. 2014) |
| PLA–PEG | Doxorubicin | Encapsulation | Ovarian cell line | (Hami et al. 2014) |
NuBCP-9 peptide
Encapsulation of NuBCP-9 is done in PEG-modified polylactic acid diblock copolymer (NuBCP-9/PLA–PEG) using a process double emulsion solvent evaporation. The PEG chain length increases up to 4 KDa (Tomar et al. 2013). The final emulsifier concentration for PLA–PEG NPs is 0.4 %. The outcomes of delivery of anticancerous peptides (NuBCP-9) with PLA–PEG copolymer for targeting intracellular proteins were adequate (Kumar et al. 2014). PLA–PEG conjugated NuBCP-9 is a potential anti-cancer peptide that trigger cancer cells by programmed cell death (Apoptosis), exposing the BCL-2 BH3 domain and inhibiting the BCL-xL defense mechanism. NuBCP-9 encapsulation is more effectual than NuBCP-9-R8 for inducing apoptosis. No side effects of weight loss or toxic effects shown after administration of the NuBCP-9/NPs (Kumar et al. 2014).
In NuBCP-9, PEG chain length is important for loading in and out from polymeric Nanoparticles. It is shown that increasing the length of the PEG chain continuously enhance the NuBCP-9 loading. Furthermore, with increasing the length of the PEG chain, the release time of drug is increased. It also enhances its flexibility, hydrophilicity, and hydration (Essa et al. 2010).
Curcumin
Curcumin (cur) is a polyphenol compound having low molecular weight and extracted from Curcuma plant (Zhao et al. 2015). Various pharmacological activities are shown by the Curcumin including anti-proliferative, anti-inflammatory (Wu et al. 2011), anti-parasitic, anti-cancerous (Srivastava et al. 2011). However, some major barriers cause an obstacle are its poor water solubility, oxidation and heat, quick metabolism at physiological pH with poor oral absorption (Basile et al. 2009). Copolymers have proved to be an enticing delivery carrier for poorly soluble drugs.
Arginine–glycine–aspartic acid (RGD) based nano-formulations have shown to be effective in targeting tumor therapy. UV–Vis spectrophotometer is used to determine the concentration of cur in the micelles. The PEG–PLA-cur micelles greatly increases drug absorption in B16 and HUVEC cell lines via receptor-mediated endocytosis. Cur-micells inhibited tumour development more effectively in B16 tumor-bearing cells and shows great potential with the RGD targeted drug delivery (Zhao et al. 2015). As the PLA/PEG ratio is increased, the encapsulation efficiency and solubility of curcumin improved significantly. When the PLA/PEG ratio is lowered curcumin's effectiveness remained 50%. It is around 90% effectiveness when the PLA/PEG ratio is increased to 3:1 from 1:3. However, the rate of curcumin release declines (Thong et al. 2014).
Conclusion and its future aspects
The development of various innovative and effective drug delivery mechanisms has resulted from the synchronous improvement of polymer technology and pharmaceutical science. These approaches overcome the constraints of traditional dosages, resulting in better medication therapy and therapeutic response. Polymers nowadays are used in drug delivery as they have various advantages over traditional drug delivery system. The drug release can be controlled in a better way with the help of PLA–PEG via conjugating with various drugs that have shown a significant variation in drug release as per our requirement. This review contributes the creation of innovative drug delivery methods as well as the performance carriers. As a result these drug delivery system reduces various adverse effects of therapeutic drugs.
Given their inherent benefits, several reasons are there for optimistic future of copolymer (PLA–PEG) in drug delivery. As a result designing polymeric NPs with cell-specific surface receptors is a viable technique that might lead to major advancements of intelligent drug delivery systems with applications in vaccine administration, drug targeting etc. However, now Studies are ongoing on PLA–PEG, there is long way to go in terms of making more secure, steady technologies and promoting large scale preparation for diagnostic and therapeutic need. PEG–PLA copolymers predicted to provide further advanced features and functions for treatment with an immense potential applications in the pharmaceutical sector.
Low investment and high manufacturing costs of designing the polymers limits the usage of polymeric nanotechnology. As a result, it appears that providing this therapy to ordinary people is virtually unsustainable in the current situation in developing countries. More research and government help are needed to deliver these powerful medicines to the masses in developing countries.
Acknowledgements
Authors acknowledge the support of the University Grants Commission (UGC), Basic Scientific Research, Government of India (sanction No. F.30-301/2016 [BSR] dt.16.02.2017).
Author contributions
RM: Manuscript writing, figures and tables, abstract and keywords, references, editing. TT: Manuscript writing, figure and tables, abstract and keywords, editing. MC: Conceptualization, overall guidance, final editing.
Accession numbers
Nil.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Rohit Mundel, Tanya Thakur contributed equally.
References
- Abd Alsaheb RA, Aladdin A, Othman NZ, Abd Malek R, Leng OM, Aziz R, El Enshasy HA. Recent applications of polylactic acid in pharmaceutical and medical industries. J Chem Pharm Res. 2015;712:51–63. [Google Scholar]
- Afsharzadeh M, Abnous K, Yazdian-Robati R, Ataranzadeh A, Ramezani M, Hashemi M. Formulation and evaluation of anticancer and antiangiogenesis efficiency of PLA–PEG nanoparticles loaded with galbanic acid in C26 colon carcinoma, in vitro and in vivo. J Cell Physiol. 2019;234(5):6099–6107. doi: 10.1002/jcp.27346. [DOI] [PubMed] [Google Scholar]
- Afsharzadeh M, Hashemi M, Babaei M, Abnous K, Ramezani M. PEG–PLA nanoparticles decorated with small-molecule PSMA ligand for targeted delivery of galbanic acid and docetaxel to prostate cancer cells. J Cell Physiol. 2020;235(5):4618–4630. doi: 10.1002/jcp.29339. [DOI] [PubMed] [Google Scholar]
- Ajnai G, Chiu A, Kan T, Cheng CC, Tsai TH, Chang J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J Exp Clin Med (taiwan) 2014;6(6):172–178. doi: 10.1016/j.jecm.2014.10.015. [DOI] [Google Scholar]
- Alyafeeu YA, Alaamery M, Bawazeer S, Almutairi MS, Alghamdi B, Alomran N, Sheereen A, Daghestani M, Massadeh S. Preparation of anastrozole loaded PEG–PLA nanoparticles: evaluation of apoptotic response of breast cancer cell lines. Int J Nanomed. 2018;13:199–208. doi: 10.2147/IJN.S151139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelopoulou A, Voulgari E, Diamanti EK, Gournis D, Avgoustaki K. Graphene oxide stabilized by PLA–PEG copolymers for the controlled delivery of paclitaxel. Eur J Pharm Biopharm. 2015;93:18–26. doi: 10.1016/j.ejpb.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Aramudan S, Senthil KL (2016) Etoposide delivery : mPEG–PCL based copolymeric micelles assessed by various in-vitro anti-cancer activity. Int J Res Pharm Sci 7(2):122–131. www.ijrps.pharmascope.org
- Arruebo M, Vilaboa N, Sáez-gutierrez B, et al. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3:3279–3330. doi: 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asia S, Asia S, Hdi H. All Cancers. Glob Cancer Obs. 2020;419:199–200. [Google Scholar]
- Avramović N, Mandić B, Savić-Radojević A, Simić T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics. 2020;12(4):298. doi: 10.3390/pharmaceutics12040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay TG, et al. Review of polysaccharide particle-based functional drug delivery. Carbohydr Polym. 2019;221:94–112. doi: 10.1016/j.carbpol.2019.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives : molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78(10):1305–1315. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Bharti C, Gulati N, Nagaich U, Pal A. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124. doi: 10.4103/2230-973x.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker A. Poly(ethylene glycol) in drug delivery, why does it work, and can we do better? All atom molecular dynamics simulation provides some answers. Phys Procedia. 2012;34:24–33. doi: 10.1016/j.phpro.2012.05.004. [DOI] [Google Scholar]
- Cegnar M, Kristl J, Kos J. Nanoscale polymer carriers to deliver chemotherapeutic agents to tumours. Expert Opin Biol Ther. 2005;5(12):1557–1569. doi: 10.1517/14712598.5.12.1557. [DOI] [PubMed] [Google Scholar]
- Chang YC, Chen Y, Ning J, Hao C, Rock M, Amer M, Feng S, Falahati M, Wang LJ, Chen RK, Zhang J, Ding JL, Li L. No such thing as trash: a 3D-printable polymer composite composed of oil-extracted spent coffee grounds and polylactic acid with enhanced impact toughness. ACS Sustain Chem Eng. 2019;7(18):15304–15310. doi: 10.1021/acssuschemeng.9b02527. [DOI] [Google Scholar]
- Colone M, Calcabrini A, Stringaro A. Drug delivery systems of natural products in oncology. Molecules. 2020;25(19):1–4560. doi: 10.3390/molecules25194560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danafar H, Rostamizadeh K, Davaran S, Hamidi M. Drug-conjugated PLA–PEG–PLA copolymers : a novel approach for controlled delivery of hydrophilic drugs by micelle formation. Pharm Dev Technol. 2016;22(8):947–957. doi: 10.3109/10837450.2015.1125920. [DOI] [PubMed] [Google Scholar]
- Dariva CG, Figueiredo JPH, Ferreira C, et al. Development of red-light cleavable PEG–PLA nanoparticles as delivery systems for cancer therapy. Colloids Surf B. 2020;196:111354. doi: 10.1016/j.colsurfb.2020.111354. [DOI] [PubMed] [Google Scholar]
- de Oliveira SA, Borges R, Rosa D dos S, de Souza ACS, Seabra AB, Baino F, Marchi J. Strategies for cancer treatment based on photonic nanomedicine. Materials. 2021;14(6):1435. doi: 10.3390/ma14061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmowafy EM, Tiboni M, Soliman ME. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J Pharm Investig. 2019 doi: 10.1007/s40005-019-00439-x. [DOI] [Google Scholar]
- Eskandani M, Barar J, Ezzati J, et al. Formulation, characterization, and geno/cytotoxicity studies of galbanic acid-loaded solid lipid nanoparticles. Pharm Biol. 2015;53(10):1525–1538. doi: 10.3109/13880209.2014.991836. [DOI] [PubMed] [Google Scholar]
- Essa S, Rabanel JM, Hildgen P. Effect of polyethylene glycol (PEG) chain organization on the physicochemical properties of poly (d, l-lactide) (PLA) based nanoparticles. Eur J Pharm Biopharm. 2010;75:96–106. doi: 10.1016/j.ejpb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications. Adv Drug Deliv Rev. 2016;107:367–392. doi: 10.1016/j.addr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Ghasemi R, Abdollahi M, Emamgholi Zadeh E, et al. mPEG–PLA and PLA–PEG–PLA nanoparticles as new carriers for delivery of recombinant human Growth Hormone (rhGH) Sci Rep. 2018;8:9854. doi: 10.1038/s41598-018-28092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H. Aptamer-functionalized PEG e PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32(31):8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Hami Z, Amini M, Ghazi-Khansari M, Rezayat SM, Gilani K. Doxorubicin-conjugated PLA–PEG-folate based polymeric micelle for tumor-targeted delivery: synthesis and in vitro evaluation. DARU J Pharm Sci. 2014;22(1):30. doi: 10.1186/2008-2231-22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammady T, Rabanel JM, Dhanikula RS, Leclair G, Hildgen P. Functionalized nanospheres loaded with anti-angiogenic drugs: cellular uptake and angiosuppressive efficacy. Eur J Pharm Biopharm. 2009;72(2):418–427. doi: 10.1016/j.ejpb.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Hruby M, Ulbrich K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J Control Release. 2005;103(1):137–148. doi: 10.1016/j.jconrel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Jain JP, Kumar N. Development of amphotericin B loaded polymersomes based on (PEG)3–PLA co-polymers: factors affecting size and in vitro evaluation. Eur J Pharm Sci. 2010;40(5):456–465. doi: 10.1016/j.ejps.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Jain R, Shah NH, Malick AW, Rhodes CT. Controlled drug delivery by biodegradable poly (ester) devices: different preparative approaches. Drug Dev Ind Pharm. 1998;24(8):703–727. doi: 10.3109/03639049809082719. [DOI] [PubMed] [Google Scholar]
- Jelonek K, Li S, Wu X, et al. Self-assembled filomicelles prepared from polylactide/poly (ethylene glycol) block copolymers for anticancer drug delivery. Int J Pharm. 2015;485(1–2):357–364. doi: 10.1016/j.ijpharm.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampan NC, Madondo MT, Mcnally OM, Quinn M, Plebanski M. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed Res Int. 2015;2015:413076. doi: 10.1155/2015/413076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- Kim HY, Ryu JH, Chu CW, et al. Paclitaxel-incorporated nanoparticles using block copolymers composed of poly(ethylene glycol)/poly(3-hydroxyoctanoate) Nanoscale Res Lett. 2014;9:525. doi: 10.1186/1556-276X-9-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong FY, Zhang JW, Li RF, Wang ZX, Wang WJ, Wang W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017 doi: 10.3390/molecules22091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsiouki K, Angelopoulou A, Ioannou E, et al. TAT peptide-conjugated magnetic PLA–PEG nanocapsules for the targeted delivery of paclitaxel: in vitro and cell studies. AAPS PharmSciTech. 2017;18(3):769–781. doi: 10.1208/s12249-016-0560-9. [DOI] [PubMed] [Google Scholar]
- Kumar M, Gupta D, Singh G, Sharma S, et al. Novel polymeric nanoparticles for intracellular delivery of peptide cargos: antitumor efficacy of the BCL-2 conversion peptide NuBCP-9. Can Res. 2014;74(12):3271–3281. doi: 10.1158/0008-5472.CAN-13-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai P, Daear W, Löbenberg R, Prenner EJ. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly (d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf B. 2014;118:154–163. doi: 10.1016/j.colsurfb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Leuschner C, Kumar C. Nanoparticles for cancer drug delivery. Nanofabrication towards Biomed Appl Tech Tools, Appl Impact. 2005 doi: 10.1002/3527603476.ch11. [DOI] [Google Scholar]
- Li D, Guo G, Deng X, et al. PLA/PEG–PPG–PEG/dexamethasone implant prepared by hot-melt extrusion for controlled release of immunosuppressive drug to implantable medical devices, part 2: in vivo evaluation. Drug Deliv. 2013;20(3–4):134–142. doi: 10.3109/10717544.2013.801049. [DOI] [PubMed] [Google Scholar]
- Li J, Ding J, Liu T, Liu JF, Yan L, Chen X (2017) Poly(lactic acid) controlled drug delivery. In: Di Lorenzo M, Androsch R (eds) Industrial applications of poly(lactic acid). Advances in polymer science, vol 282. Springer, Cham. Doi: 10.1007/12_2017_11
- Li S, Byrne B, Welsh JE, Palmer AF. Self-assembled poly(butadiene)-b-poly(ethylene oxide) polymersomes as paclitaxel carriers. Biotechnol Prog. 2007;23(1):278–285. doi: 10.1021/bp060208+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qi XR, Maitani Y, Nagai T. PEG–PLA diblock copolymer micelle-like nanoparticles as all-trans-retinoic acid carrier: Invitro and invivo characterizations. Nanotechnology. 2009 doi: 10.1088/0957-4484/20/5/055106. [DOI] [PubMed] [Google Scholar]
- Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annu Rev Chem Biomol Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Qin S, He M, et al. Current applications of poly (lactic acid) composites in tissue engineering and drug delivery. Compos B. 2020;199:108238. doi: 10.1016/j.compositesb.2020.108238. [DOI] [Google Scholar]
- Massadeh S, Alaamery M, Al-Qatanani S, Alarifi S, Bawazeer S, Alyafee Y. Synthesis of protein-coated biocompatible methotrexate-loaded PLA–PEG–PLA nanoparticles for breast cancer treatment. Nano Rev Exp. 2016;7(1):31996. doi: 10.3402/nano.v7.31996. [DOI] [Google Scholar]
- Meng X, Zhang Z, Tong J, Sun H, Fawcett JP, Gu J. The biological fate of the polymer nanocarrier material monomethoxy poly(ethylene glycol)-block-poly(d, l-lactic acid) in rat. Acta Pharm Sin B. 2021;11(4):1003–1009. doi: 10.1016/j.apsb.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Nayak B, Dey RK. PEGylation in anti-cancer therapy: an overview. Asian J Pharm Sci. 2016;11(3):337–348. doi: 10.1016/j.ajps.2015.08.011. [DOI] [Google Scholar]
- Oh B, Shin EA, Jung JH, Jung D, Kim B. Apoptotic effect of galbanic acid via activation of caspases and inhibition of Mcl-1 in H460 non-small lung carcinoma cells. Phytother Res. 2015;29(6):844–849. doi: 10.1002/ptr.5320. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Nagasaki Y, Kataoka K. Surface characterization of functionalized polylactide through the coating with heterobifunctional poly(ethylene glycol)/polylactide block copolymers. Biomacromol. 2000;1:39–48. doi: 10.1021/bm990005s. [DOI] [PubMed] [Google Scholar]
- Parveen S, Sahoo SK. Polymeric nanoparticles for cancer therapy. J Drug Target. 2008;16(2):108–123. doi: 10.1080/10611860701794353. [DOI] [PubMed] [Google Scholar]
- Pereira ED, Cerruti R, Fernandes E, et al. Influence of PLGA and PLGA–PEG on the dissolution profile of oxaliplatin. Polimeros. 2016;26(2):137–143. doi: 10.1590/0104-1428.2323. [DOI] [Google Scholar]
- Pereira MA, Mosqueira VCF, Vilela JMC, Andrade MS, Ramaldes GA, Cardoso VN. PLA–PEG nanocapsules radiolabeled with 99mTechnetium-HMPAO: release properties and physicochemical characterization by atomic force microscopy and photon correlation spectroscopy. Eur J Pharm Sci. 2008;33(1):42–51. doi: 10.1016/j.ejps.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Rezvantalab S, Drude NI, Moraveji MK, et al. PLGA-based nanoparticles in cancer treatment. Front Pharmacol. 2018;9:1260. doi: 10.3389/fphar.2018.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi F, Hadizadeh F, Sazmand SH, et al. Synthesis and self-assembly of biodegradable polyethylene glycol-poly (lactic acid) diblock copolymers as polymersomes for preparation of sustained release system of doxorubicin. Int J Pharm Investig. 2015;5(3):134–141. doi: 10.4103/2230-973x.160846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant VP, Gaucher G, Dufresne MH, Kang N, Maysinger D, Leroux J. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release. 2005;109(1–3):169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Sim T, Kim JE, Hoang NH, et al. Development of a docetaxel micellar formulation using poly(ethylene glycol)–polylactide–poly(ethylene glycol) (PEG–PLA–PEG) with successful reconstitution for tumor targeted drug delivery. Drug Deliv. 2018;25(1):1362–1371. doi: 10.1080/10717544.2018.1477865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(1–2):179–192. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol. 2011;11(3):331–341. doi: 10.1016/j.intimp.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Stefani M, Coudane J, Vert M. In vitro ageing and degradation of PEG–PLA diblock copolymer-based nanoparticles. Polym Degrad Stab. 2006;91(11):2554–2559. doi: 10.1016/j.polymdegradstab.2006.05.009. [DOI] [Google Scholar]
- Taghipour-Sabzevar V, Sharifi T, Moghaddam MM. Polymeric nanoparticles as carrier for targeted and controlled delivery of anticancer agents. Ther Deliv. 2019;10(8):527–550. doi: 10.4155/tde-2019-0044. [DOI] [PubMed] [Google Scholar]
- Thong PQ, et al. Impact of PLA/PEG ratios on curcumin solubility and encapsulation efficiency, size and release behavior of curcumin loaded poly(lactide)-poly(ethylenglycol) polymeric micelles. Int J Drug Deliv. 2014;6(3):279–285. [Google Scholar]
- Tomar L, Tyagi C, Kumar M, et al. In vivo evaluation of a conjugated poly (lactide-ethylene glycol) nanoparticle depot formulation for prolonged insulin delivery in the diabetic rabbit model. Int J Nanomed. 2013;8:505–520. doi: 10.2147/IJN.S38011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol Life Sci. 2004;61(19–20):2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PubMed] [Google Scholar]
- Tyler B, Gullotti D, Mangraviti A, Utsuki T, Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Deliv Rev. 2016;107:163–175. doi: 10.1016/j.addr.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Vahdati SFG (2019) Investigations on the development of biodegradable nanoparticles for anti-cancer drug. Biosci Biotechnol Res Commun 12(1):36–45, 34–41. 10.21786/bbrc/12.1/5
- Wadhawan A, Chatterjee M, Singh G. Present scenario of bioconjugates in cancer therapy: a review. Int J Mol Sci. 2019;20(21):5243. doi: 10.3390/ijms20215243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li S, Han Y, Guan J, Chung S, Wang C, Li D. Poly(ethylene glycol)-polylactide micelles for cancer therapy. Front Pharmacol. 2018;9:202. doi: 10.3389/fphar.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp Neurol. 2004;190(1):1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Wu W, Shen J, Banerjee P, Zhou S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials. 2011;32(2):598–609. doi: 10.1016/j.biomaterials.2010.08.112. [DOI] [PubMed] [Google Scholar]
- Xiao RZ, Zeng ZW, Zhou GL, et al. Recent advances in PEG–PLA block copolymer nanoparticles. Int J Nanomed. 2010;5:1057–1065. doi: 10.2147/IJN.S14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG–PCL nanoparticles. Biomaterial. 2012;33(32):8167–8176. doi: 10.1016/j.biomaterials.2012.07.046. [DOI] [PubMed] [Google Scholar]
- Yu DH, Lu Q, Xie J, Fang C, Chen HZ. Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterial. 2010;31(8):2278–2292. doi: 10.1016/j.biomaterials.2009.11.047. [DOI] [PubMed] [Google Scholar]
- Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143(1):136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xia H, Wang J, Li Y. High intensity focused ultrasound-responsive release behavior of PLA–b–PEG copolymer micelles. J Control Release. 2009;139(1):31–39. doi: 10.1016/j.jconrel.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Zhang QZ, Zha LS, Zhang Y, Jiang WM, Lu W, Shi ZQ, Jiang XG, Fu SK. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J Drug Target. 2006;14(5):281–290. doi: 10.1080/10611860600721051. [DOI] [PubMed] [Google Scholar]
- Zhao L, Yang C, Dou J, Xi Y, Lou H, Zhai G. Development of RGD-functionalized PEG–PLA micelles for delivery of curcumin. J Biomed Nanotechnol. 2015;11(3):436–446. doi: 10.1166/jbn.2015.1919. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Goltsche K, Cheng L, Xie F, Meng F, Deng C, Zhong Z, Haag R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials. 2016;84:250–261. doi: 10.1016/j.biomaterials.2016.01.049. [DOI] [PubMed] [Google Scholar]
- Zhu KJ, Xiangzhou L, Shilin Y. Preparation, characterization, and properties of polylactide (PLA)–poly(ethylene glycol) (PEG) copolymers: a potential drug carrier. J Appl Polym Sci. 1990;39(1):1–9. doi: 10.1002/app.1990.070390101. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nil.