Abstract
Member IV of the Ediacaran Doushantuo Formation records the recovery from the most negative carbon isotope excursion in Earth history. However, the main biogeochemical controls that ultimately drove this recovery have yet to be elucidated. Here, we report new carbon and nitrogen isotope and concentration data from the Nanhua Basin (South China), where δ13C values of carbonates (δ13Ccarb) rise from − 7‰ to −1‰ and δ15N values decrease from +5.4‰ to +2.3‰. These trends are proposed to arise from a new equilibrium in the C and N cycles where primary production overcomes secondary production as the main source of organic matter in sediments. The enhanced primary production is supported by the coexisting Raman spectral data, which reveal a systematic difference in kerogen structure between depositional environments. Our new observations point to the variable dominance of distinct microbial communities in the late Ediacaran ecosystems, and suggest that blooms of oxygenic phototrophs modulated the recovery from the most negative δ13Ccarb excursion in Earth history.
Subject terms: Carbon cycle, Geochemistry, Precambrian geology
Variable dominance of distinct microbial communities during the late Ediacaran, recorded in C and N cycles perturbations and in Raman structural heterogeneities of organic matter, modulated the recovery from the most negative δ13Ccarb excursion in Earth’s history.
Introduction
The Ediacaran Shuram excursion (c. 570 – c. 551 Ma) records a significant C cycle perturbation in Earth history with unusually light carbonate carbon isotope values (δ13Ccarb) down to −12‰ and a characteristic lack of covariation between carbonate and sedimentary organic carbon isotope trends1,2. This isotope excursion was first described from the Shuram Formation in Oman3. Afterwards, correlative studies from Australia, California, and South China1,4,5 showed this excursion was a global phenomenon. Although intensive studies have been conducted, the mechanisms driving the origin and termination of the Shuram excursion remain debated (see reviews in refs. 6–8).
There are various hypotheses proposed to explain the origin of the Shuram excursion, and these can be summarised in two principal groups. The first group proposes that the excursion results from secondary, post-depositional alteration during burial diagenesis, along with meteoric water or authigenic carbonate precipitation9–11. The second group, however, suggests that the excursion is a primary depositional carbon isotope signature related to the oxidation of massive 13C−depleted dissolved organic matter (DOC)12 or other types of organic carbon pools13,14 during a globally synchronous ocean oxygenation event3,5. In the first case, the diagenetic overprint of the isotopic signal would have required sediments to be chemically preconditioned so that local processes produce the same signal globally. The second case would imply the existence of a massive DOC pool in which partial or complete oxidation would have required abundant oxidants. Both hypotheses are controversial with equivocal solutions. However, they may not necessarily be mutually exclusive if some C-recycling processes started in the water column and continued during diagenesis.
While it is possible that the Shuram excursion was a consequence of diagenesis, increasing evidence support that the excursion can also represent the oxidation of the marine organic carbon pool. The abundance of cyanobacterial-like microfossils in the black shales of the underlying Member II15, combined with 13C−enriched carbonate16 and widespread phosphorite deposits in the Doushantuo Fm.15,17,18 suggest the existence of elevated concentrations of organic matter from primary producers in the environment. In addition, if the DOC oxidation drove the excursion, it is expected that there would be a close relationship between the oxygenation of the water column and the Shuram excursion. In support of this, shallow oxic conditions are described from different environments5,19–21. In these studies, Fe speciation and S isotopes are used to conclude that organic-rich shales of Member IV were deposited in euxinic environments. However, these studies also report high δ238U, δ98Mo, and Mo concentrations that suggest extensive ocean oxygenation during the deposition of the Member IV. Lastly, recent quantitative models propose a sufficient and realistic oxidant budget for heterogeneous or partial oxidation of the DOC occurring only on shelf areas of the global Ediacaran oceans22, or the total DOC exhaustion when surplus oxidant is generated through bacterial reduction of sulfate weathered from evaporite deposits8.
The recovery from the Shuram excursion to pre-excursion δ13Ccarb values has been previously associated with the complete oxidation of a DOC pool12, decreasing input of weathered detrital organic carbon13, decreasing expelled hydrocarbons from sedimentary organic matter14, or reduced local DOC availability via persistent consumption of reduced carbon at the shelfs7. In the South China Nanhua Basin, the recovery from the Shuram excursion is recorded in Member IV of the Doushantuo Formation (ca. 560 − 551 Ma). This member consists of organic−rich shales of variable thickness (1 − 30 m) deposited during a sea-level highstand with total organic carbon (TOC) contents up to 15%23.
Here, we use new bulk C and N isotope and Raman data from Member IV shales in six sections that span shallow to deep environments in the Nanhua Basin to understand the biogeochemistry in the basin and its relationship to the Shuram excursion, specifically with the recovery to pre-excursion δ13Ccarb values. Future studies should focus on sedimentological and mineralogical observation to further explore the diagenetic phenomena associated with the oxidation of organic matter during this critical Neoproterozoic period24.
Results
Chemostratigraphic profiles
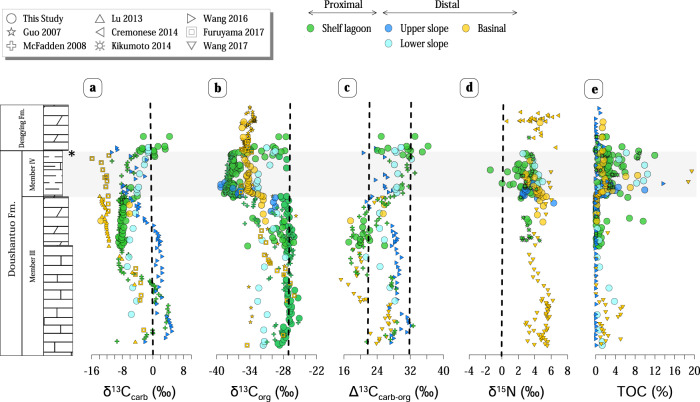
C and N concentration and isotope data from six shelf−to−basin depositional environments of the Nanhua Basin are illustrated in Fig. 1, summarised in Table 1 and detailed in Table S1. Table 1 shows stratigraphic end-members of Member IV (described in Supplementary Information), representing the chemo-stratigraphic trends for the evolution of the measured C and N geochemical parameters. Proximal sections include Xiangerwan, Zhimaping, and Qinglinkou, which are located in the shelf lagoon paleo-environment25. Distal sections include Taoying (upper slope), Xiajiaomeng (lower slope), and Fengtan (basin)25 (see Fig. S1a–c). The proposed stratigraphic correlation of Member IV of these sections is based on new and published data from the studied and nearby sections that constrain the lateral variability and isotopic expression of the Shuram excursion in South China5,26–28. More details on the geological context of the studied sections can be found in Supplementary Information. In addition, potential postdepositional alteration of isotope signatures was evaluated based on δ13Corg-TOC, TOC-TN, TN-δ15N, and Mn/Sr-δ13Ccarb plots and resulted in no significant alteration detected in the studied samples (see Supplementary Information).
Fig. 1. New chemostratigraphic columns of carbon and nitrogen isotope compositions in Doushantuo Member IV.
a Carbonate carbon isotopes. b Organic carbon isotopes. c Carbonate and organic carbon isotopes difference. d Sedimentary nitrogen isotopes. e Total organic carbon content. Data are classified by depositional environments and, where applicable, shown in comparison with published data5,26,28–30,68. *U − Pb age of 551.1 ± 0.7 Ma69. Vertical dashed lines from left to right show Peedee belemnite, isotopic fractionation imparted to biomass by primary producers typically around −27‰, photosynthetic range 22–32‰ based on compilations of Δ13Ccarb-org throughout the Phanerozoic70, and lastly δ15N for atmospheric N2.
Table 1.
Summary of geochemical results.
| δ13Ccarb (‰) | δ13Corg (‰) | δ15N (‰) | TOC (%) | n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Environment | Section | Base | Top | Base | Top | Base | Top | Base | Top | |
| Shelf Lagoon | Xiangerwan | −4.1 | −0.6 | −37.7 | −36.9 | +4.4 | +3.6 | 2.7 | 4.6 | 7 |
| Zhimaping | −7.1 | −0.2 | −38.6 | −34.6 | +3.7 | +2.9 | 5.0 | 1.3 | 51 | |
| Qinglinkou | −5.8 | −3.6 | −37.0 | −27.7 | +3.3 | +2.3 | 0.3 | 9.6 | 15 | |
| Slope | Taoying | −4.3* | −11.3* | −35.7 | −38.5 | +2.5 | +3.7 | 1.1 | 1.7 | 10 |
| Xiajiaomeng | −3.7 | −1.2 | −32.8 | −29.8 | +4.3 | +2.6 | 8.3 | 6.2 | 6 | |
| Basin | Fengtan | −11.5** | −15.9** | −32.0 | −34.4 | +5.4 | +3.4 | 0.3 | 0.9 | 21 |
Data show end-member values of Member IV (base and top). In sections where data at the base and top of Member IV were not available, data from the Member III-Member IV and Member IV-Dengying Fm. boundaries were used and are expressed in bold. (*) represents data from an analogous upper slope environment section (Siduping30). (**) represents data from the Fengtan section28. n= number of samples.
The carbon isotope composition of carbonates and organic matter (δ13Ccarb and δ13Corg) from the proximal sections preserve the most similar signal to the Shuram excursion in Oman3. In this environment, values of δ13Ccarb from the uppermost part of Member III to the Member IV-Dengying Fm. boundary exhibit a trend that gradually shifts from −7.1‰ to −0.2‰ (Fig. 1a). Similarly, values of δ13Corg of the same interval present a shift from ∼−28‰ to −38.6‰ and recover again to ∼−28‰ at the Member IV-Dengying Fm. boundary (Fig. 1b). These characteristic trends are consistent with previously reported data from nearby outcrop samples2,5,26,29. On the other hand, the distal sections present values of δ13Corg from −32.8‰ to −38.5‰ and δ13Ccarb values from −4.3‰30 to −15.9‰28 upward section. In contrast to the recovery trend in the proximal sections, these isotope trends for distal sections vary toward more 13C-depletions at the top of Member IV. The exception to this is found in the Xiajiaomeng section, located in the distal lower slope environment and presents the same δ13C patterns as the proximal sections with increasing δ13Corg from −32.8‰ to −29.8‰ and δ13Ccarb values from −3.7‰ to −1.2‰ upward section.
Previous late Ediacaran δ15N data from the studied area are scarce and have been reported from only two sections in proximal environments26,31, and one in distal environments30, and have a range in values between +5.4‰ and +2.3‰ (n = 83) (Fig. 1d) towards the top of the Doushantuo Fm. Results from this study are thus consistent with previous data. However, they present a new and complete shelf−to−basin N profile, exclusively spanning Member IV. Towards the top of Member IV, most data show similar decreasing trends except the Taoying section, which instead presents increasing δ15N values from +2.5‰ to +3.7‰. δ15N variations from the base to the top of Member IV in the distal environments range from +5.4‰ to +2.6‰. In the proximal area, however, Zhimaping shows more significant δ15N variations with a characteristic trend formed by two different cycles upward section separated by sharp boundaries with values between +3.7‰ to 0‰ and from +3.6‰ to –1.4‰ respectively.
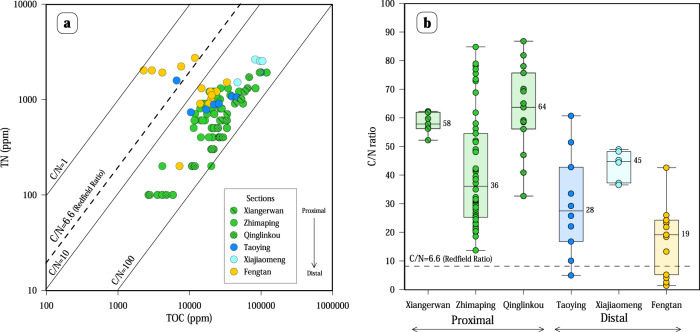
Similarly, reported Precambrian C/N ratio data are limited, and most of these data have a high C/N ratio32. Measured C/N ratios of Member IV black shales also markedly exceed the Redfield ratio of 6.633, as shown in Fig. 2, with 94% of values between 10 and 90 (n = 104). Sections from the proximal area collectively show the highest C/N ratios, close to 90. Comparably, results from distal sections (Taoying and Fengtan) show the lowest C/N ratios, significantly below the Redfield Ratio, with values as low as 1.3. The statistical C/N distribution between environments also reflects higher mean values in the proximal sections (n = 73) compare to the distal sections (n = 31), which show the lowest mean values in the basin.
Fig. 2. Measured C and N abundances in the different depositional environments.
a C/N ratio presented by stratigraphic sections. The dashed line represents the Redfield ratio (C/N = 106/16). b Statistical distribution of C/N ratios from shallow to deep environments showing the full range of ratios (ticked lines), the mean (lines with a number), and one standard deviation distribution (box).
Raman spectral characteristics of organic matter
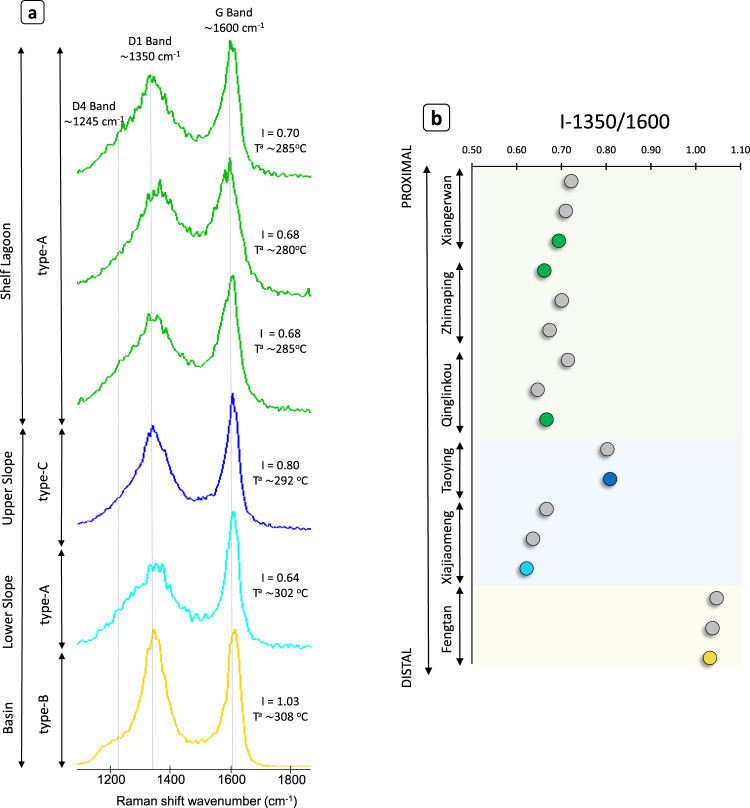
Raman spectra of organic matter in the studied samples show the typical features of disordered organic matter, with two broad bands at ∼1350 cm−1 (or D1 band) and ∼1600 cm−1 (G band). These characteristic D1 and G bands, together with their intensity ratio, defined as I-1350/1600, allow the identification of different predominant types of kerogen structures shown in Fig. 3. For example, kerogen structure type-A predominates in proximal and lower slope sections, and it is characterized by a wide D1 band, a resolvable shoulder of the D4 band at ∼1245 cm−1, and a more intense and narrower G band with a I-1350/I-1600 between 0.64 and 0.70. Kerogen structure type-B dominates in the basinal environment (Fengtan) and is characterized by narrower D1 and G bands with similar intensities and the I-1350/I-1600 1.03 (Fig. 3). In addition, the upper slope area shows a mixed type-C kerogen structure characterized by a more intense G band, similar to type-A, and narrower D1 and G bands, similar to type-B and with an intermediate I-1350/I-1600 value of 0.8 (Fig. 3). Importantly, these spectra of organic matter are used to estimate peak metamorphic temperatures (Supplementary Information; Table S2) with results between 280–289 oC in the proximal sections and between 300–309 oC in the distal sections, thereby confirming the authigenecity of organic matter.
Fig. 3. Raman spectra of organic matter in Doushantuo Member IV.
a Raman spectra are organized by depositional environments and show the disordered but variable structure of the different kerogen types proposed (A, B and C). The two broad bands at ∼1350 cm−1 and ∼1600 cm−1 are used to calculate peak metamorphic temperatures and yield an intensity ratio defined as I-1350/1600 (I in this figure). D4 band is a secondary band that shows a well-developed shoulder in the basin sample. b Compilation of I-1350/1600 results from seventeen samples of the studied sections showing the distribution of the calculated intensity ratio along the basin. The colored circles represent samples shown in a.
Nutrient availability in Late Ediacaran oceans
Phosphorous and nitrogen are two limiting nutrients that play a significant role in regulating the primary production of organic matter, so understanding their availability in the Ediacaran oceans is essential to evaluate the impact of organic matter production on the Shuram excursion recovery. The occurrence of widespread phosphorite deposits in Member IV34,35 and blooms of oxygenic photosynthesis during the Ediacaran15, and notably similar at both ends of the Proterozoic36, point to the high availability of dissolved phosphorous that led to eutrophication in the water column with high primary productivity in surface waters and high organic carbon fluxes into sediments.
Nitrogen is the other major control for marine primary production. In general, the balance in the N input-output processes will determine the δ15N composition of oceanic N, where N2-fixation and denitrification are the major processes37. N enters the ocean mainly through the biological fixation of atmospheric N2, while denitrification is the main pathway of N loss from the ocean38. Both processes present characteristic isotopic fractionations (ε = δ15Nproduct – δ15Nreactant): N-fixation ∼0 to −4‰38, and denitrification between +15‰ and +30‰39. Our results reveal homogeneous spatial δ15N variations with values between +5.4‰ to +2‰, comparable with modern shelf sediments. In modern oceans, nitrate is partially reduced by microbial denitrification into N2 or N2O with significant isotopic fractionation, resulting in 15N-rich nitrate, which is then assimilated into biomass38. In other words, denitrification or assimilation processes exceed the supply of nitrate into the modern ocean. Thus, within the modern δ15N values range, the shift from the base to the top of Member IV reflects a dynamic environmental scenario from initial denitrification or nitrate assimilation dominance towards a new ecological state evaluated below.
Two principal mechanisms can account for the decrease of δ15N: increasing nitrate availability and/or enhanced N-fixation. In the first case, although this scenario is compatible with gradual oxidation of the Ediacaran oceans3,5, prolonged anoxic conditions in the deep ocean during the Ediacaran25,40 likely contributed to extensive denitrification that removed nitrate from seawater. Thus, nitrate unlikely exceeded denitrification, which does not support a model of increased nitrate availability. Alternatively, high P conditions, likely promoted by combined continental weathering41 and P remineralization42, would have led to active biological N2-fixation to overcome the N loss due to denitrification. This is because dissolved N is tightly correlated with dissolved P in open ocean water, with an N/P ratio of 16/133, and to maintain the ratio relatively constant, instantaneous changes in the nitrate reservoir through denitrification would have led to increased N2-fixation43 to return the nitrate reservoir to its initial size, closer to the Redfield ratio. In addition, high phosphorus availability in the proximal area, supported by the occurrence of widespread phosphorite deposits, could also explain the lowest δ15N values reached in the Zhimaping section, as low as –1.4‰, as areas with high phosphorus content would have experienced more intense N2-fixation to maintain the N/P ratio constant. Hence, the general decrease of δ15N values in the Member IV is best explained as a new equilibrium in the N cycle, whereby N2-fixation becomes dominant over denitrification as the predominant biological process modulating sedimentary δ15N values.
Extensive primary production drove the recovery of the Shuram excursion
One of the principal characteristics of the Shuram excursion is the lack of covariation between carbonate and sedimentary organic carbon δ13C trends. Covariation between coeval δ13Ccarb and δ13Corg isotope records has been used to assess changes in the ancient dissolved inorganic carbon (DIC) pool. This is because, under the conventional understanding of the carbon cycle, δ13C of organic and carbonate carbon is derived from the same marine DIC reservoir and covary accordingly12. Therefore, coupled δ13Ccarb and δ13Corg signatures reflect primary δ13C signatures of seawater while decoupled δ13Ccarb and δ13Corg signatures are interpreted as evidence for diagenetic alteration in sediments9–11 or oxidation of organic matter in the water column3,5,12.
Paired data sets of δ13Ccarb and δ13Corg in this study are mostly decoupled during the Shuram excursion and exhibit a coupled trend only in the uppermost part of Member IV. This is consistent with previous data reported in South China5,26 and shows the same pattern as the Shuram excursion in Oman3. Although it cannot be entirely ruled out that the excursion was a consequence of diagenesis, in the context of this study, the negative δ13Ccarb excursions are interpreted as the result of the remineralization of a massive 13C−depleted DOC pool12. The turning point to coupled and more positive trends would reflect a transition towards a new environmental state proposed to be dominated by oxygenic photosynthetic primary producers under extensive bloom conditions.
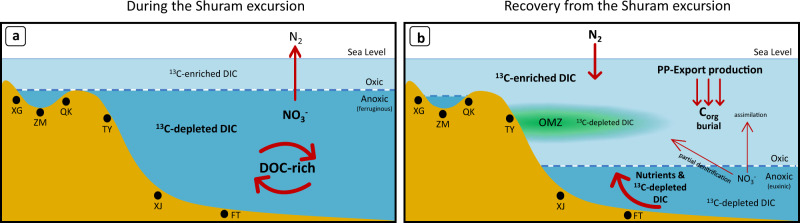
The new proposed environmental state is illustrated in Fig. 4, which represents a late Ediacaran C and N cycle evolution during the Shuram excursion and the recovery to pre-excursion values. Before the recovery, Fig. 4a, the environmental scenario was characterized by denitrification as the principal N metabolic pathway and heterotrophic remineralization of the existing DOC that led to 13C−depleted rich DIC pool and, thus, low δ13Ccarb. However, during the recovery (Fig. 4b), extensive primary productivity in shallow waters led to increased export production and extensive organic carbon burial. Consequently, the 13C−enriched DIC budget was considerably increased and eventually counteracted the existing 13C−depleted DIC, probably aided by decreasing DOC oxidation suggested previously3,7, and drove positive shifts in both δ13Ccarb and δ13Corg, thus the recovery from the Shuram excursion. In this latter scenario, N2-fixation became the primary N metabolism in the N biogeochemical cycle that, together with phosphorous availability mentioned above, led to extensive primary production in the surface.
Fig. 4. Schematic diagram showing the biogeochemical evolution of the late Ediacaran ocean.
a During the Shuram excursion, denitrification was the dominant N biogeochemical process. Recycling and remineralization of DOC by heterotrophic organisms led to a 13C−depleted rich DIC pool. Anoxic waters were predominantly ferruginous23,34. b During the recovery from the Shuram excursion N2-fixation became the main N biogeochemical process. Extensive PP in shallow waters favored the increase of organic carbon fluxes towards deep waters and the 13C−enriched DIC budget, which counteracted the 13C−depleted DIC pool and led to positive shifts in δ13Ccarb and δ13Corg. Anoxic waters were predominantly euxinic23. XG Xiangerwan, ZM Zhimaping, QK Qinglinkou, TY Taoying, XJ Xiajiaomeng, FT Fengtan. DOC dissolved organic carbon, DIC dissolved inorganic carbon, PP Primary production, OMZ Oxygen Minimum Zone. Text in bold represents the dominant processes/fractions.
The dominance of primary over secondary production has also been proposed for the contemporaneous Shuram Formation in Oman, where preserved organic biomarkers reveal multiple sources of syngenetic organic matter with more significant contributions from bacterial than algal biomass during the recovery of the Shuram excursion44. In addition, the δ15N shift toward lower values, and thus dominance of N2-fixation metabolism, would have promoted the ecological advantage to N2-fixing cyanobacteria relative to eukaryotic algae, which lack the capacity for N2-fixation and instead preferentially assimilate nitrate45.
The variable dominance of distinct microbial communities can be tested from the C/N data. Laboratory experiments reveal that during early and maximum blooms, dominated by primary producers such as cyanobacteria, C/N ratios tend to increase above the Redfield ratio to a maximum because rates of total N uptake exceed those of regeneration46. However, during postbloom periods, dominated by consumer interactions, C/N ratio tends to decrease close to the Redfield ratio because of the degradation of low molecular weight organic carbon47. The C/N ratios show a heterogeneous distribution between the studied sections (Fig. 2), and are consistent with the dominance of primary or secondary producers in the different environments. For example, values near the Redfield ratio in the distal environments (C/N between 1.3 and 26) suggest the dominance of secondary production. However, values higher than the Redfield ratio in proximal environments (C/N between 20 and 85) are consistent with those reported from this period in South China26,30 and elsewhere31 and represent periods of blooms of primary production.
Source mixing between primary and secondary organic matter
The new photosynthetic C produced near the surface may have largely coexisted with older and recalcitrant DOC principally stored in deep waters but ultimately brought to shallow depths via upwelling12,48. The heterogenous δ13C trends in the basin reflect a source mixing between autotrophic and heterotrophic pools and describe a transient C-cycle characterized by a longer C residence time during the late Ediacaran than today’s ocean-atmosphere system, which is 105 years. Thus, it is suggested that a heterogeneous decrease of the DOC pool size after extensive remineralization into 13C−depleted carbonate would have led to the δ13C trends heterogeneity along the basin, as previously proposed7.
Three different factors may have accounted for this heterogenous DOC pool size reduction. First, the presence of anoxic-euxinic conditions in the mid-slope and deep basinal environments25,40 allowed anaerobic degradation of organic matter to dominate the production of 13C−depleted DIC buried in carbonate precipitates. Second, the chemically resistant nature of the refractory DOC in the deep ocean, which accounts for 72% of the total organic carbon in the modern oceans49, led to less efficient microbial oxidation. This is because the refractory fraction consists of high molecular weight and structurally complex compounds rich in carbon resistant to microbial oxidation and degradation50,51. Third, upwelling during transgression would have delivered 13C−depleted bicarbonate together with regenerated nutrients from the deep sea to the photic zone, which would fuel primary productivity and trigger a turnover of 13C−depleted mid-slope and basinal waters.
Extensive primary production in shelf environments would lead to a sulfidic oxygen minimum zone (OMZ) immediately below the productivity zone23,52, which would favor the preservation of organic matter. This scenario explains why the distal lower slope section (Xiajiaomeng), interpreted to be located immediately below the OMZ, presents a carbon isotope trend similar to the proximal environment, characterized by high rates of organic carbon burial. An OMZ also helps to explain why only the Taoying section, located in the upper slope, shows an increase in δ15N values likely due to anammox and heterotrophic denitrification, which led to substantial N-loss. This is because in OMZs, and other low oxygen systems, nitrogen loss by denitrification or assimilation exceeds nitrogen supply in the zone, and the remaining nitrate will become progressively enriched in 15N(53). Any of the three factors mentioned above, or a combination of them, imply a dynamic situation where DOC is continuously produced and recycled, in which case the different δ13Ccarb and δ13Corg trends represent higher primary than secondary production rates, allowing the DOC pool to wax and wane.
Structural heterogeneity of organic matter
In order to independently confirm the occurrence of different sources of biomass in the Nanhua Basin, Raman spectra of organic matter are investigated. The Raman spectra of organic matter in the studied samples reflect the presence of different types of kerogen structure (type-A, type-B, and type-C; Fig. 3). One reason that may account for different kerogen structures is the metamorphic grade that affected the organic matter. However, the peak temperature analysis (see Supplementary Information; Table S2) shows that all the evaluated samples are below or near the base of the greenschist metamorphic grade, around 280−308 oC in proximal and distal environments, respectively. In addition, there is no correlation between temperature and spectral characteristics in the studied samples (Fig. 3a). For example, spectra of organic matter in the shelf lagoon and lower slope have a similar shape and I-1350/1600 values but differ by ∼15–20 oC in their peak metamorphic temperature. Similarly, the Raman spectra of organic matter between the lower slope and basin show similar peak metamorphic temperatures between 302 and 308 oC, but remarkably different shapes and I-1350/1600 values. Therefore, these observations imply that all these rocks were exposed to the same temperature and pressure regime of regional metamorphism, and thus, the observed different kerogen structures are not associated with metamorphic temperature variations along the basin.
An alternative interpretation for kerogen variability could be the differential degradation of organic matter in the column water. In marine environments, the two principal factors that may impact organic matter degradation are temperature and redox conditions. Paleotemperatures inferred from isotope compositions (δ18O and δ30Si) in chert have been used to estimate the late Neoproterozoic seawater temperature to be around 25–28 oC54,55. This range is only slightly higher than modern ocean surface water, which is about 20 oC. Thus, the kinetics of thermal mechanisms affecting organic matter degradation (aerobic respiration, fermentation, etc.) likely worked similarly to today. Hence, redox conditions might be a major factor affecting organic matter degradation. Redox conditions in the Nanhua Basin have been studied and inferred from multiple geochemical proxies. For example, sulfur isotope composition of pyrite and iron speciation in different proximal and distal sections suggested prevailing ferruginous/euxinic deep waters during the late Ediacaran5,23,25. Likewise, high uranium and negative molybdenum isotope trends measured in the same environments were interpreted as extensive ocean oxygenation during the deposition of Member IV black shales20,21.
In the scenario that only a single type of organic matter was present and preserved, the basin-wide stratified redox conditions would have allowed a relatively homogeneous chemical degradation of biological organic matter throughout the basin. Furthermore, the likely similar sinking path in the water column and the eventual anoxic/euxinic layer at the sea bottom and sediment-water interface would have caused organic matter sequentially to lose the same functional groups and undergo aromatization during degradation irrespectively of the environment in it was deposited. Consequently, a similar Raman spectra fingerprint could be expected, regardless of its position in the basin. However, Raman results described in this study show well-differentiated spectra that require alternative explanations.
A plausible solution for different kerogen structures could be the variable composition of organic matter associated with various biomass sources, as previously argued56. In recent studies, heterogeneities in the Raman spectral I-1350/1600 parameter of organic matter in microfossils have been interpreted to be associated with the presence of organic compounds derived from different sources. This is because the structural ordering of organic matter depends on the original composition of the biological precursor57–59. This heterogeneity is remarkably evident among the studied sections with I-1350/1600 values between 0.62 and 1.05 (Fig. 3b and Table S2, SI) and with a remarkable distribution easily correlated with the different environments. In addition, specific characteristics of Raman spectra also may point to various biomass sources. For example, the D4 band is present in all samples but is exceptionally well developed in the most distal section (Fig. 3 and Fig. S4b), where it becomes a distinctive feature. The D4 band (at ∼1245 cm−1) often appears as a broad shoulder of the D1 band60. The most common interpretations of the origin of the D4 band focus on the increase of structures with sp2-sp3 hybridization and C-C or C = C bonds61. It is generally absent in purely graphitic or highly carbonized materials but present in less mature OM where the presence of C-C on aromatic rings provides robust chemical structures that may prevent degradation62.
As mentioned in the previous section, refractory DOC is formed by structurally specific compounds rich in C-C and C = C bonds resistant to microbial oxidation and degradation50,51. This observation allows interpreting that kerogen structure type-B, with distinctive D4 band, represents resistant organic matter rich in C-C and C = C bonds, thus refractory DOC that would have persisted in the water column. A widely accepted mechanism links the chemical diversity of refractory DOC to microbial diversity63,64. These studies identified associations between the production of refractory DOC with specific bacterial and archaeal groups whereby these organisms altered the molecular structure of DOM and made it resistant to further degradation, preserving fixed carbon in the ocean. It was also observed that the rate of formation of refractory DOC was dependent on the rate of microbial activity63. Therefore, it is suggested that kerogen structure type-B represents refractory DOC that dominated in the distal environment and was partly sourced by microbial activity or heterotrophic remineralization. Accordingly, kerogen structure type-A is suggested to represent photosynthetic production based on its predominance in shallow environments. In the surface ocean, the photosynthesis of organic molecules from CO2 by phytoplankton is the source of most of the ocean’s labile DOC65, which is compositionally distinct from refractory DOC. For example, proteins, carbohydrates and aliphatic materials are enriched in labile DOC relative to the signatures of the deep ocean, which is rich in resistant aromatic compounds66. Thus, the less pronounced D4 band shoulder, thus less C-C or C = C bonds, in proximal samples could represent fewer aromatic compounds and, therefore, labile organic matter.
However, this Raman spectra evaluation, considered alone, cannot be used to distinguish primary from secondary productivity alone, but along with C and N isotope data, their combination shows promising results. The described Raman characteristics of the organic matter from the basin have a distribution remarkably similar to the C isotope trends described in this study. Specifically, the shelf lagoon and lower slope sections, with kerogen structure denominated as type-A and I-1350/1600 values between 0.62-0.72, are characterized by δ13C recovery trends. In contrast, the basin section, which hosts kerogen of type-B and I-1350/1600 values around 1.05, shows a δ13C trend towards lower values. The correlation between kerogen types and δ13C trends further indicates that kerogen type-A was the most dominant type in the Nanhua Basin and represents organic matter sourced from primary production. If so, dominant kerogen type-B sourced from heterotrophic production during the Shuram excursion was overcome by kerogen type-A sourced from extensive primary production, pointing to a new environmental 13C−enriched DIC scenario that eventually promoted the recovery from the Shuram excursion.
High primary production in Member IV of the Doushantuo Formation is reflected by the occurrence of organic-rich shales and phosphorite deposits. This increased primary production was likely stimulated by phosphate availability and the consequent increased N2-fixation and CO2-fixation. The enhanced primary production is consistent with recently modelled organic carbon fluxes during the Ediacaran, which predicts a higher proportion of organic carbon burial than today67. The ensuing recovery to near-zero δ13Ccarb values was modulated by primary producers, who fix both C and N. This new biological pump would have caused increased exports of nutrients and organic carbon to sediments. The identification of different types of organic matter by Raman spectroscopy lends independent support for the presence of various sources of biomass, which is most parsimoniously interpreted as primary and secondary given identical metamorphic grades. Hence, the dominance of oxygenic photosynthesis resulted in the recovery of the Shuram excursion. Secular changes in δ13C and δ15N chemostratigraphy and Raman structural order are useful tools to understand the evolution of late Ediacaran environmental conditions and provide an explanation for the recovery from the most negative carbon isotope excursion in Earth’s history.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
F.C. acknowledges support from NERC for a DTP scholarship (NE/L002485/1). We acknowledge support from the BGS for grant IP-1829-0618 for stable isotope analyses. C.L. acknowledges support from NSFC (grants # 41825019, 42130208, 41821001). We thank Gary Tarbuck, James Davy, Robert Burton and Andi Smith for their assistance in the laboratory work. The following are also thanked for their contributions to the field trips and sample collection and processing: G. Shields, S. Poulton, M. Zhu, H. Ling, Y. Zhou, Xi Chen, Da Li, M. Chen and Z. Tian.
Author contributions
F.C. and D.P. designed the study, interpreted the data, and prepared the original manuscript. F.C and C.L. collected samples in the field. F.C. and M.L. carried out nitrogen isotopic analysis. F.C., M.L. and C.L. carried out carbon and oxygen isotopic analyses. F.C. and D.P. carried out Raman analysis. All authors discussed the results and commented on the manuscript.
Peer review information
Nature Communications thanks Xinming Chen and Michael Meyer for their contribution to the peer review of this work.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files (Supplementary Tables).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-27812-5.
References
- 1.Calver CR. Isotope stratigraphy of the Ediacarian (Neoproterozoic III) of the Adelaide Rift Complex, Australia, and the overprint of water column stratification. Precambrian Res. 2000;100:121–150. [Google Scholar]
- 2.Jiang G, Kaufman AJ, Christie-Blick N, Zhang S, Wu H. Carbon isotope variability across the Ediacaran Yangtze platform in South China: Implications for a large surface-to-deep ocean δ13C gradient. Earth Planet. Sci. Lett. 2007;261:303–320. [Google Scholar]
- 3.Fike DA, Grotzinger JP, Pratt LM, Summons RE. Oxidation of the Ediacaran Ocean. Nature. 2006;444:4–7. doi: 10.1038/nature05345. [DOI] [PubMed] [Google Scholar]
- 4.Verdel C, Wernicke BP, Bowring SA. The Shuram and subsequent Ediacaran carbon isotope excursions from southwest Laurentia, and implications for environmental stability during the metazoan radiation. Bull. Geol. Soc. Am. 2011;123:1539–1559. [Google Scholar]
- 5.McFadden KA, et al. Pulsed oxidation and biological evolution in the Ediacaran Doushantuo Formation. Proc. Natl Acad. Sci. 2008;105(9):3197–3202. doi: 10.1073/pnas.0708336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grotzinger JP, Fike DA, Fischer WW. Enigmatic origin of the largest-known carbon isotope excursion in Earth’s history. Nat. Geosci. 2011;4:285–292. [Google Scholar]
- 7.Li C, et al. Uncovering the spatial heterogeneity of Ediacaran carbon cycling. Geobiology. 2017;15:211–224. doi: 10.1111/gbi.12222. [DOI] [PubMed] [Google Scholar]
- 8.Shields GA, et al. Unique Neoproterozoic carbon isotope excursions sustained by coupled evaporite dissolution and pyrite burial. Nat. Geosci. 2019;12:823–827. [Google Scholar]
- 9.Cui H, Kaufman AJ, Xiao S, Zhou C, Liu XM. Was the Ediacaran Shuram Excursion a globally synchronized early diagenetic event? Insights from methane-derived authigenic carbonates in the uppermost Doushantuo Formation, South China. Chem. Geol. 2017;450:59–80. [Google Scholar]
- 10.Derry LA, Kaufman AJ, Jacobsen SB. Sedimentary cycling and environmental- change in the Late Proterozoic — evidence from stable and radiogenic isotopes. Geochim. Cosmochim. Acta. 1992;56(3):1317–1329. [Google Scholar]
- 11.Schrag DP. Authigenic carbonate and the history of the global carbon cycle. Science. 2013;339:1383. doi: 10.1126/science.1229578. [DOI] [PubMed] [Google Scholar]
- 12.Rothman DH, Hayes JM, Summons RE. Dynamics of the Neoproterozoic carbon cycle. Proc. Natl Acad. Sci. USA. 2003;100(14):8124–8129. doi: 10.1073/pnas.0832439100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang G, et al. The origin of decoupled carbonate and organic carbon isotope signatures in the early Cambrian (ca. 542 – 520 Ma) Yangtze platform. Earth Planet. Sci. Lett. 2012;317–318:96–110. [Google Scholar]
- 14.Lee C, Love GD, Fischer WW, Grotzinger JP, Halverson GP. Marine organic matter cycling during the Ediacaran Shuram excursion. Geology. 2015;43:1103–1106. [Google Scholar]
- 15.She Z, Strother P, Papineau D. Terminal Proterozoic cyanobacterial blooms and phosphogenesis documented by the Doushantuo granular phosphorites II: Microbial diversity and C isotopes. Precambrian Res. 2014;251:62–79. [Google Scholar]
- 16.Ader M, et al. A multilayered water column in the Ediacaran Yangtze platform? Insights from carbonate and organic matter paired δ13C. Earth Planet. Sci. Lett. 2009;288:213–227. [Google Scholar]
- 17.Cui H, et al. Phosphogenesis associated with the Shuram Excursion: Petrographic and geochemical observations from the Ediacaran Doushantuo Formation of South China. Sediment. Geol. 2016;341:134–146. [Google Scholar]
- 18.Gao Y, Zhang X, Zhang G, Chen K, Shen Y. Ediacaran negative C-isotopic excursions associated with phosphogenic events: Evidence from South China. Precambrian Res. 2018;307:218–228. [Google Scholar]
- 19.Scott C, et al. Tracing the stepwise oxygenation of the Proterozoic ocean. Nature. 2008;452:456–459. doi: 10.1038/nature06811. [DOI] [PubMed] [Google Scholar]
- 20.Sahoo SK, et al. Oceanic oxygenation events in the anoxic Ediacaran ocean. Geobiology. 2016;14:457–468. doi: 10.1111/gbi.12182. [DOI] [PubMed] [Google Scholar]
- 21.Ostrander CM, et al. Multiple negative molybdenum isotope excursions in the Doushantuo Formation (South China) fingerprint complex redox-related processes in the Ediacaran Nanhua Basin. Geochim. Cosmochim. Acta. 2019;261:191–209. [Google Scholar]
- 22.Shi W, Li C, Algeo TJ. Quantitative model evaluation of organic carbon oxidation hypotheses for the Ediacaran Shuram carbon isotopic excursion. Sci. China Earth Sci. 2017;60:2118–2127. [Google Scholar]
- 23.Li C, et al. A Stratified Redox Model for the Ediacaran Ocean. Science. 2010;328:80–83. doi: 10.1126/science.1182369. [DOI] [PubMed] [Google Scholar]
- 24.Papineau D, Yin J, Devine KG, Liu D, She Z. Chemically Oscillating Reactions during the Diagenetic Formation of Ediacaran Siliceous and Carbonate Botryoids. Minerals. 2021;11:1060. [Google Scholar]
- 25.Jiang G, Shi X, Zhang S, Wang Y, Xiao S. Stratigraphy and paleogeography of the Ediacaran Doushantuo Formation (ca. 635–551Ma) in South China. Gondwana Res. 2011;19:831–849. [Google Scholar]
- 26.Kikumoto R, et al. Nitrogen isotope chemostratigraphy of the Ediacaran and Early Cambrian platform sequence at Three Gorges, South China. Gondwana Res. 2014;25:1057–1069. [Google Scholar]
- 27.Lu M, et al. The DOUNCE event at the top of the Ediacaran Doushantuo Formation, South China: Broad stratigraphic occurrence and non-diagenetic origin. Precambrian Res. 2013;225:86–109. [Google Scholar]
- 28.Furuyama S, et al. Chemostratigraphy of the Ediacaran basinal setting on the Yangtze platform, South China: Oceanographic and diagenetic aspects of the carbon isotopic depth gradient. Isl. Arc. 2017;26:1–14. [Google Scholar]
- 29.Wang X, Jiang G, Shi X, Xiao S. Paired carbonate and organic carbon isotope variations of the Ediacaran Doushantuo Formation from an upper slope section at Siduping, South China. Precambrian Res. 2016;273:53–66. [Google Scholar]
- 30.Wang W, et al. Integrated carbon, sulfur, and nitrogen isotope chemostratigraphy of the Ediacaran Lantian Formation in South China: Spatial gradient, ocean redox oscillation, and fossil distribution. Geobiology. 2017;15:552–571. doi: 10.1111/gbi.12226. [DOI] [PubMed] [Google Scholar]
- 31.Ader M, et al. Ocean redox structure across the Late Neoproterozoic Oxygenation Event: A nitrogen isotope perspective. Earth Planet. Sci. Lett. 2014;396:1–13. [Google Scholar]
- 32.Beaumont V, Robert F. Nitrogen isotope ratios of kerogens in Precambrian cherts: A record of the evolution of atmosphere chemistry? Precambrian Res. 1999;96:63–82. [Google Scholar]
- 33.Redfield AC, Ketchum BH, Richards FA. The influence of organisms on the composition of sea-water. Sea. 1963;2:26–77. [Google Scholar]
- 34.Xiao S, et al. Integrated chemostratigraphy of the Doushantuo Formation at the northern Xiaofenghe section (Yangtze Gorges, South China) and its implication for Ediacaran stratigraphic correlation and ocean redox models. Precambrian Res. 2012;192:125–141. [Google Scholar]
- 35.Cui H, et al. Redox architecture of an Ediacaran ocean margin: Integrated chemostratigraphic (δ13C-δ34S-87Sr/86Sr-Ce/Ce*) correlation of the Doushantuo Formation, South China. Chem. Geol. 2015;405:48–62. [Google Scholar]
- 36.Papineau D. Global biogeochemical changes at both ends of the proterozoic: insights from phosphorites. Astrobiology. 2010;10:165–181. doi: 10.1089/ast.2009.0360. [DOI] [PubMed] [Google Scholar]
- 37.Ader M, et al. Interpretation of the nitrogen isotopic composition of Precambrian sedimentary rocks: Assumptions and perspectives. Chem. Geol. 2016;429:93–110. [Google Scholar]
- 38.Sigman, D. M., Karsh, K. L. & Casciotti, K. L. Nitrogen isotopes in the ocean. Encycl. Ocean Sci. 320–322 (2009).
- 39.Mariotti A, et al. Experimental determination of nitrogen kinetic isotope fractionation: Some principles; illustration for the denitrification and nitrification processes. Plant Soil. 1981;62:413–430. [Google Scholar]
- 40.Wang J, Chen D, Yan D, Wei H, Xiang L. Evolution from an anoxic to oxic deep ocean during the Ediacaran-Cambrian transition and implications for bioradiation. Chem. Geol. 2012;306–307:129–138. [Google Scholar]
- 41.Lan Z, et al. An integrated chemostratigraphic (δ13C-δ18O-87Sr/86Sr-δ15N) study of the Doushantuo Formation in western Hubei Province, South China. Precambrian Res. 2019;320:232–252. [Google Scholar]
- 42.Laakso TA, Sperling EA, Johnston DT, Knoll AH. Ediacaran reorganization of the marine phosphorus cycle. Proc. Natl Acad. Sci. 2020;117:1–7. doi: 10.1073/pnas.1916738117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenton TM, Watson AJ. Regulation of nitrate, phosphate, and oxygen in the Ocean. Regulation. 2000;14:225–248. [Google Scholar]
- 44.Lee C, et al. Carbon isotopes and lipid biomarkers from organic-rich facies of the Shuram Formation, Sultanate of Oman. Geobiology. 2013;11:406–419. doi: 10.1111/gbi.12045. [DOI] [PubMed] [Google Scholar]
- 45.Tyrrell T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature. 1999;400:525–531. [Google Scholar]
- 46.Bronk DA, Gilbert PM, Malone TC, Banahan S, Sahlsten E. Inorganic and organic nitrogen cycling in Chesapeake Bay: Autotrophic versus heterotrophic processes and relationships to carbon flux. Aquat. Microb. Ecol. 1998;15:177–189. [Google Scholar]
- 47.Kepkay PE, Jellett JF, Niven SEH. Respiration and the carbon-to-nitrogen ratio of a phytoplankton bloom. Mar. Ecol. Prog. Ser. 1997;150:249–261. [Google Scholar]
- 48.Wang H, et al. Depositional environment and micropore characteristics of the Ediacaran Doushantuo Formation black shale in Western Hubei. China Arab. J. Geosci. 2016;9:1–13. [Google Scholar]
- 49.Hansell DA, Carlson CA, Repeta DJ, Schlitzer R. Dissolved organic matter in the ocean a controversy stimulates new insights. Oceanography. 2009;22:202–211. [Google Scholar]
- 50.Clayton C. Carbon isotope fractionation during natural gas generation from kerogen. Mar. Pet. Geol. 1991;8:232–240. [Google Scholar]
- 51.Barber RT. Dissolved Organic Carbon from Deep Waters resists Microbial Oxidation. Nature. 1968;220:274–275. doi: 10.1038/220274a0. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Shi X, Jiang G. Pyrite morphology and redox fluctuations recorded in the Ediacaran Doushantuo Formation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012;333–334:218–227. [Google Scholar]
- 53.Mulder A, van de Graaf AA, Robertson LA, Kuenen JG. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 1995;16:177–183. [Google Scholar]
- 54.Marin-Carbonne J, Robert F, Chaussidon M. The silicon and oxygen isotope compositions of Precambrian cherts: A record of oceanic paleo-temperatures? Precambrian Res. 2014;247:223–234. [Google Scholar]
- 55.Robert F, Chaussidon M. A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature. 2006;443:969–972. doi: 10.1038/nature05239. [DOI] [PubMed] [Google Scholar]
- 56.Dodd MS, et al. Widespread occurrences of variably crystalline 13 C-depleted graphitic carbon in banded iron formations. Earth Planet. Sci. Lett. 2019;512:163–174. [Google Scholar]
- 57.Foucher F, Ammar MR, Westall F. Revealing the biotic origin of silicified Precambrian carbonaceous microstructures using Raman spectroscopic mapping, a potential method for the detection of microfossils on Mars. J. Raman Spectrosc. 2015;46:873–879. [Google Scholar]
- 58.Qu Y, Engdahl A, Zhu S, Vajda V, McLoughlin N. Ultrastructural Heterogeneity of Carbonaceous Material in Ancient Cherts: Investigating Biosignature Origin and Preservation. Astrobiology. 2015;15:825–842. doi: 10.1089/ast.2015.1298. [DOI] [PubMed] [Google Scholar]
- 59.Qu Y, et al. Carbonaceous biosignatures of diverse chemotrophic microbial communities from chert nodules of the Ediacaran Doushantuo Formation. Precambrian Res. 2017;290:184–196. [Google Scholar]
- 60.Lahfid A, et al. Evolution of the Raman spectrum of carbonaceous material in low-grade metasediments of the Glarus Alps (Switzerland) Terra Nov. 2010;22:354–360. [Google Scholar]
- 61.Li X, Hayashi J. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel. 2006;85:1700–1707. [Google Scholar]
- 62.Ferralis N, Matys ED, Knoll AH, Hallmann C, Summons RE. Rapid, direct and non-destructive assessment of fossil organic matter via microRaman spectroscopy. Carbon N. Y. 2016;108:440–449. [Google Scholar]
- 63.Ogawa H, Amagai Y, Koike I, Kaiser K, Benner R. Production of refractory dissolved organic matter by bacteria. Science. 2001;292:917–920. doi: 10.1126/science.1057627. [DOI] [PubMed] [Google Scholar]
- 64.Osterholz H, et al. Deciphering associations between dissolved organic molecules and bacterial communities in a pelagic marine system. ISME J. 2016;10:1717–1730. doi: 10.1038/ismej.2015.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moran MA, et al. Deciphering ocean carbon in a changing world. Proc. Natl Acad. Sci. USA. 2016;113:3143–3151. doi: 10.1073/pnas.1514645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jørgensen L, et al. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011;126:139–148. [Google Scholar]
- 67.Canfield DE, Knoll AH, Poulton SW, Narbonne GM, Dunning GR. Carbon isotopes in clastic rocks and the Neoproterozoic carbon cycle. Am. J. Sci. 2020;320:97–124. [Google Scholar]
- 68.Cremonese L, Shields-Zhou GA, Struck U, Ling HF, Och LM. Nitrogen and organic carbon isotope stratigraphy of the Yangtze Platform during the Ediacaran-Cambrian transition in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014;398:165–186. [Google Scholar]
- 69.Condon D, et al. U–Pb ages from the Neoproterozoic Doushantuo Formation, China. Science. 2005;308:95–98. doi: 10.1126/science.1107765. [DOI] [PubMed] [Google Scholar]
- 70.Hayes JM, Strauss H, Kaufman AJ. The abundance of 13C in marine organic matter and isotopic fractionation in the global biogeochemical cycle of carbon during the past 800 Ma. Chem. Geol. 1999;161:103–125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files (Supplementary Tables).