Abstract
Over the past two decades, evidence has emerged for the existence of a distinct population of endogenous progenitor cells in adult articular cartilage, predominantly referred to as articular cartilage-derived progenitor cells (ACPCs). This progenitor population can be isolated from articular cartilage of a broad range of species, including human, equine, and bovine cartilage. In vitro, ACPCs possess mesenchymal stromal cell (MSC)-like characteristics, such as colony forming potential, extensive proliferation, and multilineage potential. Contrary to bone marrow-derived MSCs, ACPCs exhibit no signs of hypertrophic differentiation and therefore hold potential for cartilage repair. As no unique cell marker or marker set has been established to specifically identify ACPCs, isolation and characterization protocols vary greatly. This systematic review summarizes the state-of-the-art research on this promising cell type for use in cartilage repair therapies. It provides an overview of the available literature on endogenous progenitor cells in adult articular cartilage and specifically compares identification of these cell populations in healthy and osteoarthritic (OA) cartilage, isolation procedures, in vitro characterization, and advantages over other cell types used for cartilage repair. The methods for the systematic review were prospectively registered in PROSPERO (CRD42020184775).
Subject terms: Translational research, Regeneration, Adult stem cells, Stem-cell research
Introduction
Hyaline cartilage facilitates smooth movement of articular joints and transmission of mechanical forces. The mechanical strength of cartilage tissue is provided by the combination of highly organized collagen arcades and negatively charged proteoglycans that draw water into the tissue1. Persisting damage to this structural organization leads to a change in the distribution of forces and loss in mechanical strength2. Cartilage injury can be post-traumatic, where defects are generally isolated, or it can occur during the progression of osteoarthritis (OA) where defects can emerge simultaneously. Both focal defects and OA impair quality of life leading to pain, reduced mobility, and disability3,4. As the healthy articular cartilage is an avascular tissue, its endogenous healing capacity is limited.
Adult chondrocytes, the cells residing in articular cartilage, are used to treat cartilage defects in autologous chondrocyte implantation5. Due to the low cell density in cartilage, chondrocytes are culture-expanded to obtain a sufficient number of cells for treatment. Expansion of chondrocytes is limited in population doublings6, as they tend to acquire a fibroblastic appearance and lose their chondrogenic phenotype7,8, before becoming senescent. Alternatively, the use of mesenchymal stromal cells (MSCs) for cartilage repair has been evaluated extensively in clinical studies9. Despite their capacity to generate cartilaginous tissue, MSCs have a tendency for differentiation into hypertrophic chondrocytes and subsequent endochondral ossification10. In contrast, MSCs are suggested to have chondro-inductive effects when combined with autologous chondrons for the treatment of focal cartilage defects11.
A distinct population of endogenous progenitor cells that resides in articular cartilage, named articular cartilage-derived progenitor cells (ACPCs), has been described in the last two decades12–15. The key in vitro characteristics of ACPCs include stem cell-like properties such as clonal expansion, extensive proliferation, and differentiation potential into multiple mesenchymal lineages, including the chondrogenic lineage. ACPCs were first identified in bovine cartilage16, and later also in different species, including equine7,13 and human cartilage17,18. Interestingly, ACPCs were shown not to upregulate type X collagen gene expression in vitro, a marker for hypertrophic differentiation during redifferentiation, contrary to MSCs7,13. The use of an endogenous cartilage progenitor cell population for treatment of cartilage defects and tissue engineering purposes therefore seems favorable over the use of other cell types14,19,20. Yet, isolation protocols and specific characterization for these cells differ greatly amongst researchers. In addition, a wide range of terms is being used to name the cells, like chondrogenic progenitor cells, cartilage stem cells, mesenchymal progenitor cells, or cartilage-derived stem/progenitor cells. For clarity, this review refers to ACPCs to address all endogenous progenitor populations identified in adult hyaline cartilage and characterized in vitro.
The purpose of this review is to systematically evaluate the available literature on ACPCs derived from healthy and diseased adult articular cartilage. We summarize the state-of-the-art research and discuss its potential for clinical use in cartilage repair therapies.
Results
The literature search yielded 1017 studies in EMBASE and 662 studies in PubMed. After duplicate removal, 1064 studies were identified. After title and abstract screening, the full text of 180 studies was screened. A total of 84 studies were then found eligible based on the inclusion and exclusion criteria (Fig. 1).
Fig. 1. Flow diagram of the literature search.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) workflow showing systematic selection process for studies.
Markers to identify ACPCs in vivo
The presence of ACPCs was first described by Dowthwaite et al. in 200416. Enhanced expression of fibronectin and one of its key receptors, integrin-α5β1, was found in the superficial zone of bovine articular cartilage. Isolation of this fraction resulted in a population with high clonogenicity. As a unique marker or marker set is lacking, MSC or chondrocyte markers are mostly used for identification (Table 1). Classical MSC markers CD10521–23, CD16624, CD14625, VCAM26, or combinations including these markers27,28 have been used by others. In essence, this results in the identification of an MSC-like population in articular cartilage. Additional markers have been described to identify ACPCs in the tissue more specifically. Proteins involved in the Notch signaling pathway, like Notch-1, Notch-2, Delta, and Jagged26,29, or integrin-α5β121, proteoglycan 4 (PRG4, or lubricin)30, and laminin31 are used. Alternative approaches to identify ACPCs in cartilage tissue have focussed on visual distinction by an elongated cell morphology of ACPCs in cartilage tissue samples31,32, cell clustering of ACPCs33, proliferation marker Ki-6733,34, and migration of ACPCs upon stimulation of the cartilage35.
Table 1.
Identifying an ACPC population in articular cartilage.
| Species | Anatomical location of cartilage | Disease model/state | Method of progenitor identification in tissue | Outcomes | |
|---|---|---|---|---|---|
| Tao et al.22 | Murine | Knee | Unknown | CD105+ in the superficial layer | CD105+ cells in the superficial layer increased after induced OA and FN treatment. CD105+/CD166+ cells increased consistently |
| Tong et al.34 | Rat | Knee | Unknown | Ki-67/BrdU labeling | Prevalence of ACPCs increases in OA. The highest frequency in the superficial layer. Inhibition of NF-κB pathway increased ACPCs in OA progression and lowered OARSI scores |
| Zhang et al.21 | Rat | Hip | Unknown | CD105+/integrin-α5β1+ co-expression | CD105+/integrin-α5β1+ cells are activated by partial-thickness cartilage defects |
| Cai et al.45 | Rat | Knee | ACLT-induced OA | CD44E+/CD90+ co-expression | Recovery of CD44E+/CD90+ cells in cartilage after ACLT and treatment with HA and magnoflorine |
| Walsh et al.23 | Porcine | Knee | Unknown | Mechanical loading of immature, adolescent, and mature cartilage followed by surface marker expression, gene expression, and histology | Increased expression of CD105 and CD29 in immature cartilage; decreased expression of ACAN, Col-X and SOX9 in immature cartilage, increased expression of Col-I, Col-II in immature cartilage |
| Dowthwaite et al.16 | Bovine | Articular cartilage (surface, middle, and deep zone) | Unknown | Expression of integrin-α5, integrin-β1, fibronectin, and Notch-1 | All markers are mainly expressed in the superficial zone |
| Jang et al.35 | Bovine | Stifle (tibial plateau) | Unknown | Calcein-AM/Ethidium homodimer staining of cells migrated into fibrin in partial- and full-thickness defects, treated with low-intensity pulsed ultrasound | More cells migrated in low-intensity pulsed treated defects. FAK activation increased in treated samples |
| Seol et al.32 | Bovine and human | Stifle (bovine) and talus (human) | Healthy | Morphology | Increased number of elongated cells in impacted cartilage explants of both species |
| Ustunel et al.29 | Human | Knee (intercondylar notch) | Healthy | Expression of Notch-1, Notch-2, Notch-3, Notch-4, Delta, Jagged-1, and Jagged-2 | Notch-1 and Delta were abundantly expressed in the superficial zone |
| Grogan et al.26 | Human | Knee | Healthy and OA | Expression of Notch-1, VCAM, and Stro-1 | All markers show expression throughout all cartilage layers; expression in the superficial zone is increased |
| Pretzel et al.24 | Human | Knee | Healthy and OA | Expression of CD166 | High percentage (22%) of CD166+ cells. The highest prevalence in the superficial and middle zone |
| Su et al.25 | Human | Knee (femoral condyles) | OA | Expression of CD146 | CD146+ cells observed in OA cartilage and are smaller in size than CD146− cells |
| Hoshiyama et al.33 | Human | Knee (femoral condyles) | OA | Cell clustering; expression of Stro-1, FGF-2, Ki-67 | More cell clustering and higher expression of all markers in cells adjacent to cartilage damage |
| Schminke et al.31 | Human | Knee (lateral femoral condyles) | Healthy and OA | Morphology; expression of laminin-α1 and laminin-α5 in the pericellular matrix. | More laminins expressed in the pericellular matrix of cells with an elongated morphology |
| De Luca et al.30 | Human | Hip (femoral head and neck) | Healthy and OA | Expression of PRG-4 | Expression of PRG-4 shifts from the superficial layer (healthy cartilage) to deeper zones (OA cartilage) |
| Wang et al.28 | Human | Knee (tibial plateau) | OA | CD271+ and CD105+ cell distribution in WORMS grade 1–2 versus 3–4 cartilage | Enhanced expression of CD105 and CD271 in the superficial zone of grade 3–4 cartilage |
ACLT anterior cruciate ligament transection, FAK focal adhesion kinase, FN fibronectin, HA hyaluronic acid, OA osteoarthritis, PRG-4 proteoglycan 4, VCAM vascular cell adhesion molecule.
Methods for isolation of ACPCs from cartilage
A protocol for selective isolation of ACPC by differential adhesion to fibronectin (DAF) was established16,17,29, taking advantage of the enriched expression of the fibronectin receptor16 and the finding that isolation based on integrins resulted in selection for stem cells rather than transit-amplifying cells36. In two-thirds of the studies using DAF, this protocol is followed by isolation of colonies, that are subsequently formed by the cells that adhere (generally) in 20 min13,14,17,18,22,29,37–43. Six out of nineteen studies did not perform colony isolation and the complete pool of cells that adhered to fibronectin was isolated34,44–48.
Alternatively, ACPCs are sorted from the total cell population either via immunomagnetic separation or fluorescence-activated cell sorting (FACS). ACPCs were isolated by FACS based on co-expression of CD105 and CD16615, a marker combination that defines a subset of bone marrow-derived MSCs49 and was proposed to select for ACPCs. Another marker set used for cell sorting that resulted in an ACPC population is CD9+/CD90+/CD166+50.
Finally, cells migrating out of cartilage explants, whether or not the cartilage is stimulated in any way, hold progenitor characteristics such as multilineage differentiation potential and colony forming efficiency (CFE)19,28,32,51–53. These migratory cells were distinctly different from chondrocytes and osteoblasts54. To stimulate migration of cells, explants were stimulated by nerve growth factor (NGF)52, platelet lysate19, or migrating cells were isolated after partial digestion of the tissue by collagenase28. Cell migration could also be triggered by induction of injury32,53. Cells with progenitor characteristics migrated towards the site of cartilage injury and a role in the repair of adult cartilage upon damage was suggested by the authors32.
Nine studies did not report on any distinct method to isolate a population from the total cell population7,30,55–61. Five others performed an isolation step after one or two passages in culture24,25,62–64. It can therefore be questioned whether these are investigating a population that is different from what is generally referred to as chondrocytes, as most of the studies were also lacking a chondrocyte control group.
In vitro characterization of ACPCs after isolation
Isolated ACPCs are characterized based on their proliferative potential, CFE, differentiation potential, and expression of markers that are also used for their isolation (Table 2). ACPCs could be maintained in culture for up to 30–60 population doublings17,18,37–39 and early-passage cells were able to form colonies in culture7,16,18,19,28,32,39,46,52,65,66. Moreover, human ACPCs were found to maintain telomere length and telomerase activity up to at least 20 population doublings18,37. However, ACPCs derived from OA cartilage contained a subpopulation of cells that have reduced proliferative potential and undergo early senescence when cultured in vitro18.
Table 2.
Isolation and characterization of ACPCs.
| Study | Species | Anatomical location of cartilage | Disease state | Isolation procedure of cells | Cell characterization | Compared to (cell type) | |
|---|---|---|---|---|---|---|---|
| Differential adhesion to fibronectin | Marker expression | ||||||
| Tao et al.22 | Murine | Knee | Unknown | DAF followed by colony isolation | Proliferation; migration; chondrogenic differentiation | CD34, CD45, CD105, CD166 | Chondrocytes |
| Tong et al.34 | Rat | Hip and knee | Unknown | DAF | Chondrogenic, osteogenic, and adipogenic differentiation | CD90, CD44, CD45, CD31, CD34 | Chondrocytes; BM-MSCs |
| He et al.44 | Rat | Knee | Unknown | DAF | Osteogenic and adipogenic differentiation | CD90, CD73, CD105, CD34, HLA-DR (after one passage) | — |
| Cai et al.45 | Rat | Knee | OA (ACLT-induced) | DAF | Chondrogenic differentiation | CD44E/CD90 coexpression | — |
| Li et al.46 | Rabbit | Knee (surface zone cartilage) | Healthy | DAF | CFE; chondrogenic, osteogenic, and adipogenic differentiation in alginate beads | — | Chondrocytes; IFP-stem cells |
| Dowthwaite et al.16 | Bovine | Articular cartilage (surface, middle, and deep zone) | Unknown | DAF | Adhesion to FN; CFE | α5 and β1 integrin (immunolocalization) | — |
| Khan et al.37 | Bovine | Juvenile metacarpophalangeal joint | Healthy | DAF followed by colony isolation | Population doublings; telomerase activity; telomere length; gene expression; chondrogenic differentiation | Sox-9; Notch-1; PCNA (all immunolocalization) | Full-depth and superficial zone chondrocytes |
| Marcus et al.38 | Bovine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | Population doublings | — | — |
| McCarthy et al.14 | Equine | Metacarpal joint | Unknown | DAF followed by colony isolation | Chondrogenic, osteogenic, and adipogenic differentiation | Notch-1; Stro-1; CD90; CD166 (all immunolocalization) | BM-MSCs |
| Levato et al.13 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | Chondrogenic, osteogenic, and adipogenic differentiation | CD13; CD29; CD31; CD44; CD45; CD49d; CD73; CD90; CD105; CD106; CD146; CD166 (all gene expression) | BM-MSCs |
| Ustunel et al.29 | Human | Knee (intercondylar notch) | Healthy (ACLT repair) | DAF followed by colony isolation | — | Notch-1; Notch-2; Notch-3; Notch-4; Delta; Jagged-1; Jagged-2 (all immunolocalization in colonies) | Chondrocytes |
| Williams et al.17 | Human | Knee | Healthy | DAF followed by colony isolation | Population doublings; chondrogenic, osteogenic, and adipogenic differentiation; karyotyping; telomere length analysis; cell engraftment in ovo | Notch-1; CD90; Stro-1; Jagged-1; Delta-1 (all immunolocalization) | Full-depth chondrocytes |
| Nelson et al.18 | Human | Knee (tibial plateau) | OA | DAF followed by colony isolation | CFE on FN; growth kinetics; chondrogenic, osteogenic, and adipogenic differentiation; | STRO-1 | — |
| Fellows et al.39 | Human | Knee (tibial plateau) | Healthy and OA | DAF followed by colony isolation | CFE on FN; growth kinetics; chondrogenic, osteogenic, and adipogenic differentiation; telomere length analysis | — | — |
| Shafiee et al.40 | Human | Articular cartilage (not specified) | Unknown | DAF followed by colony isolation | Cell cycle analysis; karyotyping; proliferation; chondrogenic differentiation | CD166; CD133; CD106; CD105; CD90; CD73; CD 45; CD34; HLA-DR | Nasal septum-progenitors; BM-MSCs; AD-MSCs |
| Vinod et al.41 | Human | Knee (superficial layer) | Healthy | DAF followed by colony isolation | Chondrogenic, osteogenic, and adipogenic differentiation | CD105; CD73; CD90; CD34; CD45; CD29; CD49e; CD151; CD166 | Chondrocytes |
| Zhang et al.47 | Murine and human | Knee | Healthy and OA | DAF | Chondrogenic, osteogenic, and adipogenic differentiation; proliferation; high-throughput RNA sequencing | PRDM16; NCAM-1; N-cadherin; CPNE-1; CTGF (protein expression). CD29; CD44; CD90; CD45; CD34 | — |
| Kachroo et al.48 | Human | Knee | OA | DAF | Gene expression | — | Non-DAF cells; fresh cartilage cells |
| Vinod et al.42 | Human | Knee | Healthy and OA | DAF followed by colony isolation | Population doublings; chondrogenic, osteogenic, and adipogenic differentiation; gene expression | CD105, CD73, CD90, CD34, CD45, CD14, CD54, CD44, CD9, CD106, CD29, CD151, CD49e, CD166; CD146 | Chondrocytes |
| Vinod et al.43 | Human | Knee | Healthy and OA | DAF followed by colony isolation | Expression of immunogenic markers HLA-A2; HLA-B7; HLA-DR; CD80; CD86; CD14 | — | Chondrocytes |
| Cell sorting | |||||||
| Karlsson et al.65 | Bovine | Knee (femoral condyle) | Healthy | FACS for Notch-1 or cell size | CFE (in agarose); chondrogenic, osteogenic, and adipogenic differentiation | — | Notch-1− cells and larger/small cells |
| Alsalameh et al.15 | Human | Knee (femoral condyle and tibial plateau) | Healthy and OA | Immunomagnetic cell separation for CD105+/CD166+ | Chondrogenic, osteogenic, and adipogenic differentiation | CD105; CD166 (immunolocalization) | BM-MSCs |
| Fickert et al.50 | Human | Knee | OA | FACS for CD9+/CD90+/CD166+ | Chondrogenic, osteogenic, adipogenic differentiation | — | — |
| Pretzel et al.24 | Human | Knee | OA | Immunomagnetic cell separation for CD166 (after one passage) | Chondrogenic, osteogenic, adipogenic differentiation | CD105; CD166 (immunolocalization) | — |
| Peng et al.62 | Human | Hip (femoral head) | Healthy and OA | Immunomagnetic cell separation for CD105+/CD166+ (after one passage) | Chondrogenic differentiation | — | — |
| Su et al.25 | Human | Knee (femoral condyles) | OA | FACS for CD146 (after one passage) | Chondrogenic, osteogenic, and adipogenic differentiation; gene expression; CFE (after three passages) | CD29; CD31; CD45; CD133; CD44; CD34; CD73; CD90; CD146; CD105; CD166; HLA-ABC; HLA-DR | Unsorted chondrocytes; AD-MSCs |
| Unguryte et al.106 | Human | Knee | OA | FACS for ALDH activity | Gene expression | CD29; CD49a; CD49c; CD105; CD349; Notch1; CD54; CD55; CD56; CD63; CD47; CD140b; CD146; CD166 | ALDH− and ALDH-diminished-expressing cells |
| Xia et al.63 | Human | Knee (femoral condyles) | OA | FACS for CD105+/CD166+ (after two passages) | Cell proliferation; gene and miRNA expression; chondrogenic, osteogenic, and adipogenic differentiation | CD29; CD44; CD73; CD90; CD105; CD166; CD19; CD34; CD45; HLA-DR | — |
| Kachroo et al.66 | Human | Knee | OA | FACS for CD49e+ | CFE | CD49e; CD29 | Fresh chondrocytes; CD49e− cells |
| Migration from tissue | |||||||
| Joos et al.51 | Human | Knee | OA | Outgrowth from cartilage tissue | Chondrogenic, osteogenic, and adipogenic differentiation; cell migration; chemotaxis | CD9; CD54; CD146; CD14; CD73; CD166; CD29; CD88; CD184; CD34; CD90; MSCA-1; CD44; CD105; Stro-1 (and quadruplicate combinations of these markers) | BM-MSCs |
| Jiang et al.52 | Human | Knee (femoral condyles) | OA | Cell migration through a membrane stimulated by NGF | CFE; chondrogenic, osteogenic, and adipogenic differentiation | CD90; CD73; CD105; CD166; CD44; CD29; CD34; CD45 | — |
| Carluccio et al.19 | Human | Hip | OA | Outgrowth from cartilage tissue using platelet lysate | Growth kinetics; CFE; chondrogenic, osteogenic, and adipogenic differentiation; migration; chemotaxis; secretory profile; gene expression | Cyclin D1; α-tubulin (protein expression); CD44; CD166; HLA-ABC; HLA-DR; CD90; CD105; CD73; CD146; CD106; CD45; CD34; CD29 | Chondrocytes |
| Enzymatic | |||||||
| Seol et al.32 | Bovine | Stifle (tibial plateau) | Healthy | Trypsin treatment after injury | Migration; chemotaxis; chondrogenic, osteogenic, and adipogenic differentiation; RNA microarray; CFE | — | BM-MSCs; chondrocytes |
| Zhou et al.53 | Bovine | Stifle (tibial plateau) | Healthy | Trypsin treatment after injury | Gene expression; chondrogenic differentiation | — | Chondrocytes; synoviocytes; synovial fluid cells |
| Wang et al.28 | Human | Knee (tibial plateau) | OA | Collagenase treatment followed by outgrowth | Growth kinetics; CFE; chondrogenic, osteogenic, and adipogenic differentiation | CD29; CD31; CD44; CD45; CD73; CD90; CD105; CD166; CD271 | — |
| Other isolation procedures | |||||||
| Hattori et al.107 | Bovine | Stifle | Healthy | Hoechst 33342- | Chondrogenic differentiation | — | Hoechst 33342+ population |
| Yu et al.72 | Bovine | Stifle (femoral condyle) | Healthy | Colony formation of single live cells | Chondrogenic, osteogenic, and adipogenic differentiation; gene expression; migration | — | Chondrocytes |
| Thornemo et al.64 | Human | Knee | Healthy | Cluster growth in agarose (after one passage) | Chondrogenic, osteogenic, and adipogenic differentiation | — | Periosteal cells; BM-MSCs; fibroblasts |
| Grogan et al.26 | Human | Knee | Healthy and OA | Hoechst 33342- | Chondrogenic, osteogenic, and adipogenic differentiation | — | Hoechst 33342+ population |
| No isolation procedure described | |||||||
| Barbero et al.7 | Human | Knee (femoral condyle) | Healthy | — | CFE; proliferation rate; chondrogenic, osteogenic, and adipogenic differentiation | — | — |
| Tallheden et al.55 | Human | Knee | Healthy | — | Chondrogenic, osteogenic, and adipogenic differentiation | — | BM-MSCs |
| Bernstein et al.56 | Human | Knee | OA | — | Chondrogenic, osteogenic, and adipogenic differentiation | CD9; CD166; CD90; CD54; CD44; CD45; CD105; CD73; CD54 (quadruple combinations) | — |
| Salamon et al.61 | Human | Knee | OA | — | Growth kinetics; adipogenic and osteogenic differentiation | CD29; CD44; CD105; CD166 | AD-MSCs |
| Mantripragada et al.58 | Human | Knee (femoral condyle) | OA | — | CFE; chondrogenic differentiation | — | — |
| Mantripragada et al.57 | Human | Knee (femoral condyle) | OA | — | CFE; chondrogenic differentiation | — | — |
| De Luca et al.30 | Human | Hip (femoral head and neck) | OA | — | CFE; chondrogenic, osteogenic, and adipogenic differentiation; immunomodulatory properties | CD14; CD34; CD44; CD45; CD71; CD105; CD166; CD90; CD73; CD151 | BM-MSCs; AD-MSCs |
| Mantripragada et al.59 | Human | Knee (femoral condyle) | OA | — | CFE; chondrogenic differentiation | — | BM-MSCs; IFP-cells; synovium-derived cells; periosteal cells |
| Mantripragada et al.60 | Human | Knee (femoral condyle) | OA | — | CFE; chondrogenic differentiation | — | IFP-cells; synovium-derived cells; periosteal cells |
ACLT anterior cruciate ligament transection, AD adipose tissue-derived, ALDH aldehyde dehydrogenase, CFE colony-forming efficiency, DAF differential adhesion to fibronectin, FN fibronectin, IFP infrapatellar fat pad, NGF nerve growth factor, OA osteoarthritis.
ACPCs could be differentiated into the chondrogenic, osteogenic, and adipogenic lineage, a feature that MSCs also possess67. There is one report of reduced osteogenic differentiation potential of ACPCs42, while 20% of the studies looking into multilineage potential found indications for reduced osteogenesis12,13,19,20,50,68.
Surface marker expression of ACPCs was in general similar to MSCs, with ACPCs being positive for CD90, CD105, CD73, and CD166, while negative for hematopoietic markers, highlighting the challenge to distinguish the two cell types13,19,28,30,40–42,51,52,56,63. Of note, about half of the studies mentioned here examine immunophenotype of cells in culture13,19,22,28,30,34,40,42,44,47,51,56,61,66,69, while cells tend to change their phenotype during in vitro expansion70,71. Moreover, investigating marker expression by gene expression or flow cytometry on the bulk populations makes it problematic to define whether these markers are co-expressed or not.
In vitro comparison of ACPCs to other cell types with regard to surface marker expression
Cell surface marker expression and in vitro performance of ACPCs were directly compared to MSCs from various sources, like bone marrow13–15,30,32,34,40,51,55,59,64, adipose tissue25,30,40, and infrapatellar fat pad46,59,60. Other cell types compared are chondrocytes17,19,22,25,29,32,34,37,41,42,46,48,53,65,66,72 and other intra-articular cells, like synoviocytes53,59,60, synovial fluid cells53, and periosteal cells59,60,64 (Table 2).
Table 3.
Application and translation of ACPCs.
| Study | Species | Anatomical location of cartilage | Disease state | Isolation procedure of cells | Other cell types compared | Application(s) | Outcomes |
|---|---|---|---|---|---|---|---|
| In vitro studies | |||||||
| He et al.44 | Rat | Knee | Unknown | DAF | — | Effect of LLP on cytotoxicity, chondrogenesis, proliferation, migration, chemotaxis, gene, and protein expression | No difference in cytotoxicity, proliferation; migration, chemotaxis, and chondrogenesis were increased by LLP; Sox9, Col-II, and Acan gene expression increased with LLP |
| Melero-Martin et al.74 | Bovine | Juvenile metatarsophalangeal joint (superficial zone) | Healthy | DAF | — | Effect of cryopreservation on proliferation, viability, and chondrogenesis. Comparison between media and FBS, TGF-β1, and FGF concentrations | Cell density increased 53-fold with optimized FBS concentration up to 40% and feeding rate above 10 μL/cm2/h. Cell density increased 33-fold when media was supplemented with 1 ng/mL TGF-β1 and 40% FBS. Chondrogenic differentiation potential was maintained |
| Melero-Martin et al.76 | Bovine | Juvenile metatarsophalangeal joint (superficial zone) | Healthy | DAF | — | Effect of seeding density, passage number, and feeding strategy on cell density | Optimal growth kinetics at 104 cells/cm2 seeding density and 73 h passage length. However, looking at costs of expansion, a longer culture time was preferred |
| Melero-Martin et al.75 | Bovine | Juvenile metatarsophalangeal joint (superficial zone) | Healthy | DAF | — | Growth kinetics 2D versus 3D microcarriers and differentiation potential afterward | Expansion slower than in 2D, but upscaling possible and chondrogenic differentiation potential maintained; bead-to-bead migration possible (subcultivation without harvesting) |
| Seol et al.32 | Bovine | Stifle (tibial plateau) | Healthy | Enzymatic: Trypsin treatment after injury | BM-MSCs; chondrocytes | Migration of GFP-labeled grafted ACPCs into an impacted area on osteochondral explant | The number of labeled cells in the impact site increased drastically from 2 to 12 days (no quantification) |
| Jang et al.35 | Bovine | Stifle (tibial plateau) | Unknown | Enzymatic: Trypsin treatment after injury | — | Cell migration under influence of low-intensity pulsed ultrasound | Low-intensity pulsed ultrasound stimulated migration of isolated ACPCs into scratch |
| Zhou et al.81 | Bovine | Stifle (tibial plateau) | Healthy | Enzymatic: Trypsin treatment after injury | Chondrocytes; synoviocytes | Phagocytic capacity | ACPCs internalized more cell-debris than chondrocytes; similar to synoviocytes and (murine cell line) macrophages; ACPCs overexpressed markers associated with phagocytosis and internalized more FN fragments than chondrocytes |
| Morgan et al.85 | Bovine | Immature metacarpophalangeal joint | Healthy | DAF followed by colony isolation | — | Determination of optimal potent chondrogenic factors | BMP9 increased aggrecan and Col-II gene expression, low Col-X expression, more anisotropic collagen fibril deposition |
| Koelling et al.87 | Human | Knee | OA | Outgrowth from cartilage tissue | — | Effect of sex hormones on the regenerative potential | Sex hormones influence the regenerative potential of progenitor cells |
| Joos et al.51 | Human | Knee | OA | Outgrowth from cartilage tissue | — | Cell migration under the influence of IL-1Β and TNF-α | Cell migration was inhibited by both IL-1Β and TNF-α |
| Peng et al.62 | Human | Hip (femoral head) | Healthy and OA | Immunomagnetic cell separation for CD105+/CD166+ (after one passage) | — | Effect of Wnt-signaling on chondrogenic differentiation | Inhibition of Wnt/β-catenin promoted proliferation and differentiation |
| Jiang et al.52 | Human | Knee (femoral condyles) | OA | Cell migration through Transwell stimulated by NGF | — | Influence of NGF on chondrogenesis | Chondrogenesis was not stimulated by NGF |
| Schminke et al.31 | Human | Knee (lateral femoral condyles) | OA | Outgrowth from cartilage tissue | — | Effect of laminin or nidogen-2 on gene expression; Nidogen-2 siRNA applied | SOX9 and ACAN increased by nidogen-2. COL2A1 increased and COL1A1 decreased by laminin. ACPCs expressed more Nidogen-2 compared to both chondrocyte types. siRNA knockdown of nidogen-2 caused increased RUNX2 and decreased SOX9 protein expression |
| Anderson et al.68 | Human | Knee (femoral condyles) | Healthy | DAF followed by colony isolation | — | Response to normoxia and hypoxia in pellets | Variation in intrinsic chondrogenicity between clones. ACPCs demonstrate a consistently low COLX gene and protein expression in physoxia |
| Nguyen et al.108 | Human | Hip and knee | OA | — | — | Expansion with FBS versus PL | PL induces re-entry of the cell cycle, stimulates proliferation; PL-expanded cells better at producing cartilage; PL induces cell outgrowth from cartilage pieces |
| Anderson et al.109 | Human | Knee (femoral condyles) | Healthy | DAF followed by colony isolation | — | Tissue self-assembly on membranes | Oriented cartilaginous tissue self-assembly by ACPCs on FN membranes. Higher GAG and collagen when compared to chondrocytes; surface lubricin was lower in ACPCs |
| Riegger et al.27 | Human | Knee (femoral condyles) | OA | Outgrowth from cartilage tissue | — | Treatment of cells with explant supernatants (impacted or treated with compounds); chondrogenic capacities; gene expression for pro- and anti-inflammatory factors | Enhanced proliferation, migration, and expression of immunomodulatory mediators. Chondrogenic capacity was impaired |
| Vinod et al.110 | Human | Knee (superficial layer) | Healthy | DAF followed by colony isolation | — | Micron-sized superparamagnetic iron oxide (M-SPIO) particle uptake and function thereafter | Viability, cell-markers, and chondrogenesis reduced with increasing concentration M-SPIO; osteogenic and adipogenic differentiation were unchanged |
| Vinod et al.41 | Human | Knee (superficial layer) | Healthy | DAF followed by colony isolation | Chondrocytes | Cocultures of ACPCs and chondrocytes in different ratios | No difference in surface marker expression, gene expression, or growth kinetics |
| Vinod et al.111 | Human | Knee (superficial layer) | OA | DAF followed by colony isolation | — | Trilineage differentiation and viability of ACPCs in PRP clots | Maintained differentiation potential and viability in PRP clots |
| Kachroo et al.77 | Human | Knee | OA | DAF followed by colony isolation | — | Expansion of ACPCs with 10% FBS versus 10% hPL | hPL-expanded ACPCs had more population doublings, higher expression of CD146, and increased gene expression of COL2A1, ACAN, COL1A1, COL10A1 |
| Mantripragada et al.60 | Human | Knee (femoral condyle) | OA | — | Growth of ACPCs in high glucose (25 mM) and low glucose (5 mM) | CFE was inhibited by glucose | |
| Vinod et al.69 | Human | Knee | OA | DAF or differential adhesion to laminin followed by colony isolation | — | Comparison of fibronectin versus laminin adhesion assay for ACPC isolation | Higher population doublings in laminin-selected ACPCs; No difference in expression of CD105, CD73, CD90, CD34, CD45, HLA-DR, CD146, CD166, CD49e, and CD29; increased expression of COL2A1 in laminin-selected ACPCs; Increased osteogenic and adipogenic differentiation |
| Wang et al.28 | Human | Knee (tibial plateau) | OA | Collagenase treatment followed by outgrowth | — | Differentiation; gene expression; migration (upon treatment with OA SF); comparison of grade 1–2 and 3–4 ACPCs | Grade 3–4 ACPCs showed enhanced migratory, osteogenic, and adipogenic potential; decreased chondrogenic potential |
| Vinod et al.112 | Human | Knee | Healthy | DAF followed by colony isolation | — | Chondrogenesis under influence of a pulsed electromagnetic field | No difference between TGF-β2-treated ACPC pellets and pellets treated with a pulsed electromagnetic field |
| Tissue engineering studies | |||||||
| Li et al.46 | Rabbit | Knee (surface zone cartilage) | Healthy | DAF | Chondrocytes; IFP-stem cells | Effect of intermittent hydrostatic pressure on ACPCs in alginate beads | Increase in migration, proliferation, GAG production, Col-II production, chondrogenic gene expression under influence of intermittent hydrostatic pressure |
| Schmidt et al.20 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | BM-MSCs | 3D culture in agarose in normoxic versus hypoxic conditions. Monocultures of ACPCs and MSCs, and zonal construct of ACPC/MSC | Higher production of glycosaminoglycans by ACPCs in normoxia and hypoxia. Weaker type I collagen staining in ACPC constructs, low ALP expression |
| Neumann et al.78 | Human | Knee (tibial plateau) | Healthy | DAF followed by colony isolation | — | BMP-2 overexpression through adenovirus; Scaffold culture loaded versus unloaded | Loading induced chondrogenesis; chondrogenesis reduced by BMP2 overexpression |
| Shafiee et al.40 | Human | Articular cartilage (not specified) | Unknown | DAF followed by colony isolation | Nasal septum progenitors (NSPs); BM-MSCs; AD-MSCs | Chondrogenesis and proliferation on nanofibrous scaffolds (PCL/PLLA). | Expression of SOX9 and ACAN higher in NSPs compared to ACPCs; COL1 and COL2 lower in ACPCs compared to NSP and AD-MSC |
| Biofabrication studies | |||||||
| Levato et al.13 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | Chondrocytes; BM-MSCs | Cartilage formation in (layered) casted GelMA hydrogel constructs; cartilage formation in layered bioprinted cartilage construct (MSCs in middle/deep layer, ACPCs in superficial layer) | ACPCs produced a higher amount and better-quality neo-cartilage matrix compared to chondrocytes, but not MSCs; Interplay of ACPCs with chondrocytes and MSCs supported neo-cartilage synthesis in layered co-cultures |
| Lim et al.94 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | — | Chondrogenic differentiation in DLP-printed bio-resin constructs | DLP-printed bio-resin supported chondrogenic differentiation of ACPCs |
| Mouser et al.89 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | — | Encapsulation in GelMA/gellan/HAMA hydrogels and 3D (zonal) bioprinting | Successful chondrogenic differentiation in hydrogel |
| Bernal et al.95 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | — | Fibrocartilage formation in volumetric bioprinted meniscus-shaped constructs | GAG, type I and II collagen production; increased compressive modulus after chondrogenic culture |
| Diloksumpan et al.90 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | — | Encapsulation in GelMA in a biofabricated osteochondral plug | ACPCs produce cartilage matrix and differentiation of ACPCs was not hampered by the presence of a bone scaffold |
| Mancini et al.91 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | BM-MSCs | Encapsulation in hyaluronic acid/poly(glycidol) hybrid hydrogel in a layered biofabricated osteochondral plug in an equine model | No difference in histological scoring. Repair tissue was stiffer in ACPC/MSC zonal constructs compared to constructs containing MSCs only |
| Peiffer et al.92 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | — | Encapsulation of ACPCs in hydrogel reinforced with a melt electrowritten scaffold printed on curvature | Cartilage-like tissue formation throughout the construct with high shape fidelity |
| Piluso et al.93 | Equine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | BM-MSCs; DPSCs | Cytocompatibility of riboflavin and sodium persulfate; cytocompatibility in silk fibroin hydrogel | Riboflavin did not affect viability, sodium persulfate decreased viability after three hours in high concentration. ACPCs in hydrogel maintained viability over 28 days of culture |
| In vitro and in vivo studies | |||||||
| Tao et al.22 | Murine | Knee | Unknown | DAF followed by colony isolation | Chondrocytes | Effect of FN on proliferation, migration, and chondrogenesis. Effect of FN in early in vivo OA model | Increased proliferation, migration, and Col-II and Aggrecan expression by FN. Inhibited by integrin-α5β1 inhibitor. FN promoted cartilage repair in vivo and increased CD105+ and CD166+ cells |
| Wang et al.113 | Murine | Joint (not further specified) | Unknown | DAF followed by colony isolation | — | EVs from MRL/MpJ super-healer mice-ACPCs were used for intra-articular injection in an OA model and for chondrocyte migration and proliferation | Super-healer mice ACPC-EVs could ameliorate OA severity in vivo and improve chondrocyte function in vitro |
| Tong et al.34 | Rat | Hip and knee | Unknown | DAF | Chondrocytes; BM-MSCs | Chondrogenesis under influence of IL-1Β and NF-κB pathway inhibitor | NF-κB pathway inhibitor was successful in rescuing ACPC chondrogenesis |
| Cai et al.45 | Rat | Knee | OA (ACLT-induced) | DAF | — | Chondrogenesis and migration under influence of magnoflorine | Chondrogenesis and migration were stimulated by magnoflorine |
| Liu et al.86 | Rat | Knee | Unknown | — | — | Effect of kartogenin on ACPCs | Kartogenin promoted proliferation; increased percentage of G2-M stage cells, increased gene expression of IL-6 and Gp130; phosphorylation of Stat3 enhanced. In vivo destabilization of the medial meniscus: increased cartilage thickness after kartogenin injection; upregulation of Stat3 phosphorylation; enhanced distribution of CD44+/CD105+ cells |
| Williams et al.17 | Caprine | Knee | Healthy | DAF followed by colony isolation | Full-depth chondrocytes | Caprine in vivo cartilage defect filling with cell-seeded type I/III collagen membrane | Good integration with surrounding cartilage. No difference between full-depth chondrocytes and ACPCs |
| Tallheden et al.55 | Human | Knee | Healthy | — | BM-MSCs | In vivo osteochondrogenic assay in SCID mice | Cartilage matrix formation in the chondrocyte group compared to bone matrix formation in the MSC group |
| Carluccio et al.19 | Human | Hip | OA | Outgrowth from cartilage tissue using platelet lysate | Chondrocytes | In vivo ectopic chondrogenesis and osteogenesis (pellet and biomaterials) | ACPCs (PL expanded) provided a better option than chondrocytes for stable cartilage regeneration |
| In vivo studies | |||||||
| Marcus et al.38 | Bovine | Metacarpophalangeal joint | Healthy | DAF followed by colony isolation | — | Intramuscular injection in SCID mice | ACPCs were able to survive but failed to produce cartilage matrix (while chondrocytes did) |
| Frisbie et al.96 | Equine | Trochlear ridge of the femur (superficial zone) | Healthy | DAF followed by colony isolation | — | In vivo chondral defect filling in autologous fibrin, comparison of autologous and allogeneic cells | Autologous cells provide a benefit in outcomes in terms of pain, synovial effusion, range of motion, radiographs, and histology. No apparent benefit of allogeneic cells |
| In human studies | |||||||
| Jiang et al.12 | Human | Knee (femoral condyle) | OA | — | — | MACT procedure using ACPCs | Significant clinical improvement based on IKDC and Lysholm scores; full coverage of defect site after one year; hyaline-like cartilage architecture |
ACLT anterior cruciate ligament transection, AD adipose tissue-derived, ALP alkaline phosphatase, CFE colony-forming efficiency, DAF differential adhesion to fibronectin, DLP digital light processing, EV extracellular vesicle, FBS fetal bovine serum, FN fibronectin, GAG glycosaminoglycan, IFP infrapatellar fat pad, IKDC International Knee Documentation Committee, LLP Link protein N-terminal peptide, MACT matrix-assisted autologous chondrocyte transplantation, NGF nerve growth factor, NSP nasal septum progenitor, OA osteoarthritis, PCL polycaprolactone, PL platelet lysate, PLLA polycaprolactone/polylactic acid, PRP platelet-rich plasma, SCID severe combined immunodeficient mice.
A clear distinction between MSCs and ACPCs based on the expression of markers was only reported once, when equine ACPCs were compared to bone marrow-derived MSCs, an increase in gene expression for CD44 was found13. One-third of the studies directly compare ACPCs to chondrocytes, as these also reside in adult hyaline cartilage, and distinction of these cell types is crucial for isolation and application. The proliferation of ACPCs was faster than chondrocytes in one study19, but slower in a different report42. In addition, ACPCs were found to form more colonies compared to chondrocytes32. A distinction was made between chondrocytes and ACPCs based on high expression of CD9017,25,34, CD4434, CD10546, CD16646, Notch-117, and HLA-ABC25 in ACPCs while culture-expanded chondrocytes showed little to no expression of these markers. Co-expression of CD44 and CD90 was found to distinguish between rat chondrocytes and ACPCs34,45. When ACPCs were sorted from the total pool of chondrocytes by CD49e-expression, a difference was found in the expression of CD29 in chondrocytes (50%) versus ACPCs (100%)66. When ACPCs were treated with platelet lysate, an increased expression of CD166 and decreased expression of CD106 compared to chondrocytes was found19.
Differences between species
Identification of similarities and differences in ACPCs between species is challenging, due to the diversity of isolation procedures and variety of study objectives. Colony formation was identified in several human studies, as well as in the first report on bovine ACPCs. CFE in bovine cartilage cells was reported to be 0.6%16, while all other literature on non-human cells lacked this analysis. In human cells, consistency is found to some extent. CFE in healthy cartilage cells on fibronectin-coated dishes was 1.47%39, while this was almost double (2.8%) in OA cells in the same study. Others reported on CFEs of <0.1%18 and 0.66%66 of OA cells on fibronectin-coated dishes. When OA cells were seeded on uncoated culture plastic, a CFE of <0.01% was found57–59. The percentage of colony forming cells increased when cells were culture expanded. Passage one OA cells (isolation method not specified) had 18% CFE30 and the same passage cells that migrated from OA tissue in response to platelet lysate had 7.8% CFE19. Cells that migrated from OA tissue with NGF and were expanded for four passages had increased their CFE to 38.6%52. When CD105+/CD166+-sorted cells were quantified, CFEs of 3.5% (healthy) and 8% (OA) were found in one study15 and 15% (healthy) and 17% (OA) were found in another24. Of note, the latter used cells that were culture expanded for one passage. Overall, when comparing human ACPC studies, it seems that OA tissue contains more colony forming cells than healthy cartilage. Also, CFE increases after culture expansion, possibly as a consequence of culture-related changes in immunophenotype71.
Differences between ACPCs from healthy and osteoarthritic cartilage
ACPCs have been identified in hyaline cartilage from different pathological states. Identification and characterization can contribute to our understanding of their role in homeostasis and disease, as well as their accessibility for clinical use.
In healthy articular cartilage, ACPCs most likely reside in the superficial zone, as Notch-1-expressing cells are found here16 and possess progenitor cell characteristics17,26,29. In addition, enhanced expression of fibronectin and one of its receptors, integrin-α5 and -β1, was found in the superficial zone16. As a direct consequence, most of the cells isolated via DAF originated from the superficial zone. The same group also showed that the CFE of surface zone cells is higher compared to deep zone cells16.
Upon damage of cartilage, ACPCs seem to migrate towards the site of injury73. Cells that migrated into the site of injury were found to possess progenitor-like characteristics32. An increase of CD271-expression was seen in ACPCs from increased OA severity28. Classical MSC markers CD10521,22, VCAM26, or combinations including these markers27,28 were all enhanced in OA cartilage or upon trauma. A shift of expression of PRG430 from the superficial layer to deeper zones was seen in OA, whereas CD271- and CD105-positive cells shifted towards the superficial zone in OA28. In OA cartilage, cell clusters were observed which express ACPC-associated markers like Notch-1, Stro-127, VCAM, FGF-2, and Ki-6726,33. These cells proliferated faster and produced more cartilaginous nodules in vitro compared to cells isolated from macroscopically healthy cartilage33. Contradictory, others found that ACPCs derived from healthy cartilage proliferated faster than OA-derived ACPCs63. Lastly, a high number of CD105/CD166-positive15 and CD146-positive cells25 was found in OA cartilage and these cells had multilineage potential. OA-derived cells also formed more colonies compared to cells from normal human cartilage39 and this increased with OA severity28.
In vitro culture of ACPCs
Three studies made an attempt to optimize growth kinetics examining factors like seeding density, culture systems, and serum concentrations74–76. The authors reported on optimal expansion conditions when the medium was supplemented with fetal bovine serum (FBS) and transforming growth factor (TGF)-β1 at 40% and 1 ng/mL, respectively74. However, more recent studies have not used FBS concentrations that were as high as 40%. A passage length of 5 days was optimal for cell yield and the authors reported on reduced costs of expansion by 60%76. Furthermore, a method for expansion on microcarriers eliminated the need for a harvesting step and was thus suggested to prevent dedifferentiation75. A direct comparison of fibronectin versus laminin, another important cell adhesion molecule, for differential adhesion of ACPCs resulted in higher population doubling, increased gene expression of type II collagen, and increased osteogenic and adipogenic differentiation potential of laminin-selected ACPCs69. Likewise, expansion with platelet lysate compared to FBS showed more population doublings and increased expression of chondrogenic genes aggrecan and type II collagen, but at the same time expression of type X collagen was also increased77. Others found increased gene expression of aggrecan, type II collagen, and Sox9, as well as proteoglycan and type II collagen production of ACPCs by application of intermittent hydrostatic pressure46 or mechanical stimulation in a bioreactor system78, and inhibition of CFE by high glucose levels during growth culture60. Moreover, normoxic versus hypoxic conditions revealed greater production of glycosaminoglycans, low alkaline phosphatase expression, and weaker type I collagen staining in both conditions compared to MSCs20. In line, consistently low levels of type X collagen were expressed by ACPCs when normoxia and hypoxia were compared68. A reduction of oxygen tension during culture is also known to delay chondrocyte aging and improve their chondrogenic potential79,80.
In brief, the optimization of culture conditions for ACPCs has been investigated extensively. There are no uniform protocols for expansion and optimal differentiation for cartilage formation. Consensus on these matters would aid in comparing outcomes of studies in the future.
Upon ex vivo injury of bovine cartilage, migratory cells with progenitor characteristics were found32,35. Additional research showed that the phagocytic capacity of these ACPCs was higher compared to chondrocytes and comparable to synoviocytes and macrophages, suggesting a macrophage-like role for ACPCs in cartilage injury81. After treating ACPCs with supernatant from injured explants, proliferation, migration, and expression of immunomodulatory mediators were enhanced, while chondrogenic capacity was impaired27.
Stimulation of chondrogenesis in ACPCs was successful by inhibition of the nuclear factor-κB pathway, the major signaling pathway involved in OA82. Inhibition of this pathway was achieved by an inhibiting peptide34 and magnoflorine45, both resulting in increased chondrogenesis. Interleukin-1Β and tumor necrosis factor-α, inflammatory factors involved in OA, were reported to inhibit migration of ACPCs51. Similarly, β-Catenin and NGF are elevated in OA83,84. Inhibition of the Wnt/β-Catenin pathway promoted proliferation and differentiation62, while NGF failed to stimulate chondrogenesis in ACPCs52. The specific role of these compounds in OA remains to be investigated.
Alternatively, chondrogenesis could be triggered by the direct activation of chondrogenic pathways. Combined mechanical stimulation and shear stress-induced chondrogenesis through an increase in endogenously produced TGF-β1, while overexpression of BMP2 reduced chondrogenesis78. Also, BMP9 was a potent stimulator of chondrogenesis85. Direct treatment of ACPCs with extracellular matrix components Link protein N-terminal peptide44 or nidogen-231 increased expression of chondrogenic genes. The proliferation of ACPCs was promoted by kartogenin86, a small molecule that induces chondrogenic differentiation of MSCs. Finally, sex hormones estrogen and testosterone influenced human ACPC performance87.
To summarize, initial results indicate that ACPCs respond to injury and chondrogenesis can be induced in vitro, which could make the cells interesting as therapeutic targets. These findings could be used to provoke neo-cartilage formation or inhibit inflammation in OA.
Application and translation of progenitors
The potential of ACPCs for tissue engineering, biofabrication, and clinical application has been investigated widely (Table 3). Biofabrication allows for the production of constructs consisting of (bio)materials, bioactive cues, and/or cells, with a detailed predefined architecture88. The extensive proliferative potential of ACPCs combined with their chondrogenic capacity make these cells good candidates to use in tissue engineering and biofabrication approaches to repair or regenerate articular cartilage.
Under the influence of intermittent hydrostatic pressure, the performance of rabbit ACPCs embedded in alginate was enhanced significantly. These cultures were pretreated for 1 week with a TGF-β3-containing medium but did not receive any exogenous growth factors thereafter. After two and four weeks, glycosaminoglycan, collagen, and DNA content were significantly higher than groups not treated with intermittent hydrostatic pressure46. Two studies investigating equine ACPC performance in hydrogels both reported good outcomes. When the cells were embedded in gelatin methacrolyl (gelMA) hydrogel cultured in a chondrogenic medium, mainly a difference was found in the expression of zonal markers compared to bone marrow-derived MSCs. Expression of PRG4 was increased in ACPC-loaded gels, while type X collagen expression was decreased compared to MSCs13. Furthermore, when equine ACPCs were embedded in gelMA/gellan and gelMA/gellan/HAMA hydrogels and cultured in a chondrogenic medium, these produced more glycosaminoglycans and type II collagen than chondrocytes, whereas the performance of MSCs in the same gels was comparable to ACPCs89. Similar to hydrogels, printed scaffolds have also been successfully seeded with ACPCs. Human ACPCs seeded on fibrin-polyurethane composite scaffolds were responsive to mechanical stimulation. The cells produced more glycosaminoglycans and aggrecan gene expression was increased without the addition of exogenous growth factors78. Furthermore, human ACPCs could also be seeded onto polycaprolactone/polylactic electrospun nanofibrous scaffolds where the cells attached and spread over the fibers. Further research has to shed light on the chondrogenic performance of the cells in this specific setting40.
Besides tissue engineering, ACPCs were successfully used in several biofabrication techniques. It was shown that equine ACPCs have the potential to be bioprinted and while exact mechanisms remain to be elucidated, an interplay between MSCs, ACPCs, and chondrocytes was found to be important for neo-cartilage synthesis13. The same cells were also successfully used for encapsulation in various hydrogels90–93 in combination with biofabrication techniques like extrusion-based bioprinting13,89, digital light processing94, and volumetric bioprinting95, while maintaining cell viability. While these are only the first indications to use ACPCs with various techniques, additional research is necessary to assess chondrogenic performance of the cells in these settings. Nevertheless, initial results are promising to move forward with this cell population.
Several attempts were made to take the next steps in the application of ACPCs for in vivo cartilage formation and repair. These are important to translate in vitro findings and define the potential of ACPCs for the clinic.
When DAF-selected ACPCs were applied in a caprine model for cartilage defect filling using a cell-seeded type I/III collagen membrane (Chondro-Gide®), ACPC-seeded scaffolds showed good lateral integration with the surrounding tissue and type II collagen-positive repair tissue. However, no difference was found between chondrocyte- or ACPC-treated defects17. In the same study, engraftment into the growth plate of developing chick hind limbs of isolated and culture-expanded ACPCs was shown. Contradictory, DAF-selected bovine ACPCs that were injected intramuscularly in immune-deficient mice failed to produce cartilage matrix38. In an equine model, DAF-selected ACPCs were applied in a layered biofabricated osteochondral plug and showed good integration with the native cartilage, but the repair tissue contained mainly type I collagen91. When autologous and allogeneic ACPCs were directly compared in an equine cartilage defect model, an advantage of autologous over allogeneic cells was seen in histological outcomes96.
When human ACPCs were used in immune-deficient mice, the cells were successful in the production of cartilage matrix, whereas MSCs produced mainly bone55. The cells in this study were not isolated using any distinct method for ACPC isolation but were 2D expanded in low density with low glucose. Furthermore, migratory human ACPCs expanded using platelet lysate outperformed chondrocytes in an in vivo ectopic chondrogenesis assay in athymic mice19.
Finally, an attempt was made to proceed to human application, by using ACPCs to replace chondrocytes for matrix-assisted autologous chondrocyte transplantation, similar to the caprine study mentioned earlier. The pilot study with 15 patients12 reported on repair tissue rich in type II collagen and proteoglycans and without types I and X collagen. Furthermore, IKDC and Lysholm questionnaire scores improved significantly. However, there was no direct comparison between ACPCs and expanded chondrocytes in this study.
While the discussed studies provide initial evidence of in vivo chondrogenic potential of these cells, further investigation is essential to ascertain promise cartilage repair and clinical translatability.
Discussion
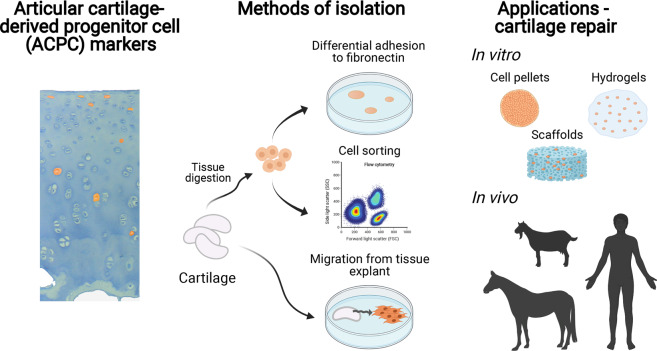
With this review, we aimed to systemically evaluate the available literature on adult ACPCs and their use for cartilage tissue engineering and repair therapies. We are the first to provide a thorough overview of research from the last two decades that demonstrates the presence of a progenitor cell population residing in adult hyaline cartilage (Fig. 2). Although a great effort was made to study the identity and applications of ACPCs, many uncertainties remain. As a result of differences in isolation protocols, characterization, and culture expansion, most cell populations discussed in the literature are likely to be heterogeneous populations and difficult to compare between laboratories. This stresses the need for this systematic review to expose certain inconsistencies and arrive at a shared definition of ACPCs.
Fig. 2. Isolation, characterization, and application of articular cartilage-derived progenitor cells.
Schematic overview of the identification of articular cartilage-derived progenitor cells (ACPCs) in cartilage, isolation methods, and applications of ACPCs. Created with BioRender.com.
The reviewed literature employs a wide variety of procedures for the isolation and characterization of ACPCs. Broadly speaking, three main methods for ACPC isolation are described. The method using DAF, used in 42% of the investigated studies, is based on the enriched expression of integrin-α5β1, as first described by Dowthwaite et al.16. The other two main methods are based on the expression of (a combination of) cell surface markers (19%)15,50 or migratory capacity (6%)51. Most populations isolated through these methods employed multilineage potential, responded to acute injury or mobilized during OA, and were able to produce hyaline cartilage extracellular matrix in vitro or in vivo.
The heterogeneity in isolation and characterization creates discrepancies between donors and laboratories. Direct comparisons of ACPC populations isolated through different procedures are lacking and would aid to improve our understanding of the populations. The identification of a unique cell marker would facilitate extensive and coordinated research into the cell type. This could pave the way towards clinical application or cell targeting to promote cartilage regeneration in OA. Recently, Gdf5-expressing cells in developing joints were identified to contribute to joint cell lineages97. Co-expression of Lgr5 and Col22a1 was identified as an important lineage marker towards juvenile articular chondrocytes in the developing mouse joint98. Additionally, single-cell RNA sequencing has revealed several novel markers that are potentially specific for ACPCs in human OA cartilage99.
The available literature suggests that ACPCs resemble MSCs67 in vitro based on surface marker expression and multilineage potential. The comparison to MSCs is often made due to the fact that MSCs (derived from various tissues) are a useful cell type for clinical application and are currently applied9. As the general view on the origin and role of MSCs is changing100, characterization of ACPCs based on MSC features might not be the way to go and other routes should be investigated. More recent work has shed light on the cellular basis of bone and cartilage formation by identifying skeletal stem cells in mice and humans101,102, a cell type that might be closer related to (the origin of) ACPCs in adult hyaline cartilage. Although the comparison to clinically used chondrocytes is relevant, research into similarities between ACPCs and skeletal stem cells or more downstream progenitor cells is lacking and finding resembling features would contribute to knowledge about the origin and identity of ACPCs.
Establishing the role of ACPCs in cartilage development and homeostasis, as well as their response upon injury or in OA would provide additional insights into their physiological function in mature cartilage. Regeneration in the early stages of OA could be stimulated or progression of the disease halted. Several studies discussed here suggested that ACPCs have a possible role in immunomodulation, based on their capacity to migrate upon injury32,35, excretion of inflammatory mediators27 and phagocytic capacities81. Others found a higher prevalence of these cells upon cartilage damage and OA21,22,26–28,30,32–35. Of note, these are all in vitro indications for which in vivo validation is essential. During OA, cell density and clustering in cartilage increases103, for which ACPCs might partly be responsible. On the other hand, there is contradicting evidence that Prg4-expressing cells from the synovium migrate into sites of acute cartilage injury and contribute to cartilage repair97. In order to expand the application of ACPCs to OA besides repair of chondral defects alone, immunomodulatory properties should be demonstrated in vivo as is known for MSCs104.
The described ACPC populations generally surpassed other cell types in proliferative potential and producing cartilage extracellular matrix in vitro19,20,25,42,46. In addition, most studies implanting animal-derived ACPCs in vivo confirmed their chondrogenic potential17,38,91, and even two studies using human cells reported on successful neo-cartilage formation19,55. As isolation methods do not seem to be associated with in vivo outcomes of cell performance and tissue formation, the challenge remains to compare findings between studies. Furthermore, differences between species and pathological states could influence cell performance. Donor age might play an important role, although none of the studies investigated this specifically. Nevertheless, the cells’ potency of prolonged in vitro expansion17,19,37 combined with limited tendency towards hypertrophic differentiation13,14,19,68 and their ability to form neo-cartilage can make ACPCs an appropriate cell type for repair of focal chondral defects.
Despite the great deal of research that has been done on ACPCs, certain actions need to take place in order to close the gaps and reach consensus between researchers and laboratories. As noted before, isolation based on a unique marker is crucial to ascertain similarity in cell population between laboratories. Comparison of culture media and additives for ACPCs in a recent systematic review105 highlights the importance of consistency to align research. As ACPCs currently have no discrete set of cell surface markers that can be used to isolate the cells from tissue, the question remains whether ACPCs are a distinct cell type or it refer to a heterogeneous mix of many cell types. The establishment of a cell marker and consistency in isolation and culture protocols ascertain comparability between populations. Another limitation might be the availability of tissue for cell isolation. Cartilage from OA patients is generally more accessible than healthy cartilage, as it is redundant after knee replacement surgery. A direct comparison of ACPC populations of healthy and OA cartilage would shed light on differences in performance. In the same view, investigation of allogeneic use of ACPCs is valuable, as this would greatly improve the potential of application by availability, reduction of costs, and preselection of chondrogenic cells.
The current systematic review is limited by the restriction to cell populations that are isolated from adult hyaline cartilage. The comparison and relation to cell types in the developing joint are lacking and would contribute to our further understanding of the origin of the populations discussed here and their role in joint development and homeostasis. However, the current review and discussed literature are predominantly directed at clinical translation as opposed to etiology or the role of a cartilage progenitor cell in development.
Arriving at a shared definition of a homogenous cell population that can be isolated and characterized in a comparable manner is crucial. This work could be used as a basis for research groups and clinicians to harmonize study protocols and characterization. First, studies should report on the origin of the cell in terms of species, anatomical location of the hyaline cartilage, and disease state. Second, the method of isolation should be described in detail and preferably identical to one of the established protocols. Finally, the phenotype of the isolated populations should be examined directly following isolation and culture media (and additives) as well as expansion time and/or passage number should be reported and synchronized.
To conclude, the available literature indicates that a cell population with progenitor-like characteristics resides in adult hyaline cartilage, which has extensive chondrogenic and proliferative potential. These features highlight the suitability of ACPCs as a cell source for focal chondral repair. In addition, it is crucial to investigate the role of ACPCs in development and disease, in order to determine their potential to slow down or reverse OA. If the current challenges can be overcome and consensus can be reached on this population, ACPCs hold great potential as a cell type for tissue engineering and for the repair of cartilage damage in both focal cartilage injury and OA.
Methods
Literature search
A systematic search of the literature was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines on adult endogenous ACPCs. The review protocol was prospectively registered with PROSPERO (registration number CRD42020184775). The electronic databases of EMBASE and PubMed were searched using the following search terms: (cartilage AND (articular OR hyaline OR knee OR hip OR ankle)) AND (progenitor OR progenitor cell OR multipotent cell OR chondroprogenitor OR multipotent cell OR cartilage-derived OR articular cartilage-derived OR (stem cell OR MSC OR mesenchymal stem cell OR mesenchymal stromal cell AND (cartilage-derived OR cartilage resident))). A final search was performed on 17 February 2021. Two authors (M.R. and J.V.K.) independently screened all selected studies for eligibility, first by title and abstract followed by full-text screening. After duplicate removal, inconsistencies between the researchers were discussed in a consensus meeting.
Inclusion and exclusion criteria
Inclusion criteria that were used during the title, abstract, and full-text screening for eligible studies included: adult endogenous cartilage stem/progenitor cells; knee, hip, or ankle cartilage; in vitro and/or in vivo and/or in man studies; English language; reviews, case reports, conference papers, studies of which the full texts were not retrievable, studies investigating cell line of chondroprogenitors, cells other than endogenous cartilage-derived progenitors, and lineage-tracing studies were excluded. Extracted data from the selected studies included species, anatomical location of cartilage, isolation procedure, cell characterization, and application of the cells. The quality of a study was considered inferior if methods or results are poorly reported. Study limitations/inconsistencies are discussed at the end of a paragraph in the results.
Acknowledgements
This work is supported by the partners of Regenerative Medicine Crossing Borders (RegMed XB), a public–private partnership that uses regenerative medicine strategies to cure common chronic diseases. This collaboration project is financed by the Dutch Ministry of Economic Affairs by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public–private partnerships. The research was also supported by the Dutch Arthritis Foundation (LLP-12 and LLP-22). J.V.K. acknowledges ZonMw (The Netherlands Organization for Health Research and Development) and the strategic theme “Regenerative Medicine & Stem Cells” of the University Medical Center Utrecht. R.L. acknowledges funding from the European Research Council (Grant Agreement No. 949806) and the Horizon 2020 Research and Innovation Program under Grant Agreement No. 814444 (MEFISTO).
Author contributions
M.R. and J.V.K. performed the literature searches and reviewed the bibliography; M.R. wrote the manuscript; M.R., J.V.K., R.L., J.M., and L.A.V. reviewed and edited the manuscript; all authors approved the manuscript for submission.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing interests
The authors declare no competing interests. This research was performed at University Medical Center Utrecht. L.A.V. is currently employed by CO.DON AG.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klein TJ, et al. Strategies for zonal cartilage repair using hydrogels. Macromol. Biosci. 2009;9:1049–1058. doi: 10.1002/mabi.200900176. [DOI] [PubMed] [Google Scholar]
- 2.Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am. J. Sports Med. 2004;32:1451–1458. doi: 10.1177/0363546504263234. [DOI] [PubMed] [Google Scholar]
- 3.Heir S, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am. J. Sports Med. 2010;38:231–237. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 4.Cross M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Evans C, Georgescu H. Observations on the senescence of cells derived from articular cartilage. Mech. Ageing Dev. 1983;22:179–191. doi: 10.1016/0047-6374(83)90111-2. [DOI] [PubMed] [Google Scholar]
- 7.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 8.von der Mark K, Gauss V, von der Mark H, Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 9.Vonk LA, De Windt TS, Slaper-Cortenbach ICM, Saris DBF. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: a concise review. Stem Cell Res. Ther. 2015;6:1–11. doi: 10.1186/s13287-015-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller MB, et al. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-β isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010;192:158–166. doi: 10.1159/000313399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Windt TS, et al. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients. Stem Cells. 2017;35:1984–1993. doi: 10.1002/stem.2657. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, et al. Human cartilage-derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration. Stem Cells Transl. Med. 2016;5:733–744. doi: 10.5966/sctm.2015-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levato R, et al. The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017;61:41–53. doi: 10.1016/j.actbio.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy HE, Bara JJ, Brakspear K, Singhrao SK, Archer CW. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet. J. 2012;192:345–351. doi: 10.1016/j.tvjl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 16.Dowthwaite GP, et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 17.Williams R, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson L, McCarthy HE, Fairclough J, Williams R, Archer CW. Evidence of a viable pool of stem cells within human osteoarthritic cartilage. Cartilage. 2014;5:203–214. doi: 10.1177/1947603514544953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carluccio S, et al. Progenitor cells activated by platelet lysate in human articular cartilage as a tool for future cartilage engineering and reparative strategies. Cells. 2020;9:1052. doi: 10.3390/cells9041052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt S, et al. Differential production of cartilage ECM in 3D agarose constructs by equine articular cartilage progenitor cells and mesenchymal stromal cells. Int. J. Mol. Sci. 2020;21:7071. doi: 10.3390/ijms21197071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K, et al. Chondrogenic cells respond to partial-thickness defects of articular cartilage in adult rats: an in vivo study. J. Mol. Histol. 2016;47:249–258. doi: 10.1007/s10735-016-9668-1. [DOI] [PubMed] [Google Scholar]
- 22.Tao T, et al. Fibronectin enhances cartilage repair by activating progenitor cells through integrin alpha5beta1 receptor. Tissue Eng. Part A. 2018;24:1112–1124. doi: 10.1089/ten.TEA.2017.0322. [DOI] [PubMed] [Google Scholar]
- 23.Walsh SK, Schneider SE, Amundson LA, Neu CP, Henak CR. Maturity-dependent cartilage cell plasticity and sensitivity to external perturbation. J. Mech. Behav. Biomed. Mater. 2020;106:103732. doi: 10.1016/j.jmbbm.2020.103732. [DOI] [PubMed] [Google Scholar]
- 24.Pretzel D, et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res. Ther. 2011;13:R64. doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su X, et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J. Orthop. Res. 2015;33:84–91. doi: 10.1002/jor.22731. [DOI] [PubMed] [Google Scholar]
- 26.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res. Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riegger J, Palm H, Brenner R. The functional role of chondrogenic stem/progenitor cells: novel evidence for immunomodulatory properties and regenerative potential after cartilage injury. Eur. Cells Mater. 2018;36:110–127. doi: 10.22203/eCM.v036a09. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y-X, et al. Biological potential alterations of migratory chondrogenic progenitor cells during knee osteoarthritic progression. Arthritis Res. Ther. 2020;22:62. doi: 10.1186/s13075-020-2144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ustunel I, et al. The immunohistochemical localization of notch receptors and ligands in human articular cartilage, chondroprogenitor culture and ultrastructural characteristics of these progenitor cells. Acta Histochem. 2008;110:397–407. doi: 10.1016/j.acthis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 30.De Luca P, et al. Human diseased articular cartilage contains a mesenchymal stem cell-like population of chondroprogenitors with strong immunomodulatory responses. J. Clin. Med. 2019;8:423. doi: 10.3390/jcm8040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schminke B, Frese J, Bode C, Goldring MB, Miosge N. Laminins and nidogens in the pericellular matrix of chondrocytes: their role in osteoarthritis and chondrogenic differentiation. Am. J. Pathol. 2016;186:410–418. doi: 10.1016/j.ajpath.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Seol D, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64:3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoshiyama Y, et al. Chondrocyte clusters adjacent to sites of cartilage degeneration have characteristics of progenitor cells. J. Orthop. Res. 2015;33:548–555. doi: 10.1002/jor.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong W, et al. In vivo identification and induction of articular cartilage stem cells by inhibiting NF-κB signaling in osteoarthritis. Stem Cells. 2015;33:3125–3137. doi: 10.1002/stem.2124. [DOI] [PubMed] [Google Scholar]
- 35.Jang KW, et al. Low-Intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound Med. Biol. 2014;40:1177–1186. doi: 10.1016/j.ultrasmedbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 37.Khan IM, Bishop JC, Gilbert S, Archer CW. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthr. Cartil. 2009;17:518–528. doi: 10.1016/j.joca.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Marcus P, De Bari C, Dell’Accio F, Archer CW. Articular chondroprogenitor cells maintain chondrogenic potential but fail to form a functional matrix when implanted into muscles of SCID mice. Cartilage. 2014;5:231–240. doi: 10.1177/1947603514541274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fellows CR, et al. Characterisation of a divergent progenitor cell sub-populations in human osteoarthritic cartilage: the role of telomere erosion and replicative senescence. Sci. Rep. 2017;7:41421. doi: 10.1038/srep41421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafiee A, Kabiri M, Langroudi L, Soleimani M, Ai J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J. Biomed. Mater. Res. Part A. 2016;104:600–610. doi: 10.1002/jbm.a.35603. [DOI] [PubMed] [Google Scholar]
- 41.Vinod E, Kachroo U, Ozbey O, Sathishkumar S, Boopalan P. Comparison of human articular chondrocyte and chondroprogenitor cocultures and monocultures: to assess chondrogenic potential and markers of hypertrophy. Tissue Cell. 2019;57:42–48. doi: 10.1016/j.tice.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Vinod E, Kachroo U, Rebekah G, Yadav BK, Ramasamy B. Characterization of human articular chondrocytes and chondroprogenitors derived from non-diseased and osteoarthritic knee joints to assess superiority for cell-based therapy. Acta Histochem. 2020;122:151588. doi: 10.1016/j.acthis.2020.151588. [DOI] [PubMed] [Google Scholar]
- 43.Vinod E, Ramasamy B, Kachroo U. Comparison of immunogenic markers of human chondrocytes and chondroprogenitors derived from non-diseased and osteoarthritic articular cartilage. J. Orthop. Trauma Rehabil. 2020;27:63–67. [Google Scholar]
- 44.He R, et al. Link protein N-terminal peptide as a potential stimulating factor for stem cell-based cartilage regeneration. Stem Cells Int. 2018;2018:1–11. doi: 10.1155/2018/3217895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai, Z. et al. Prevent action of magnoflorine with hyaluronic acid gel from cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. Biomed. Pharmacother. 126, 109733 (2020). [DOI] [PubMed]
- 46.Li Y, Zhou J, Yang X, Jiang Y, Gui J. Intermittent hydrostatic pressure maintains and enhances the chondrogenic differentiation of cartilage progenitor cells cultivated in alginate beads. Dev. Growth Differ. 2016;58:180–193. doi: 10.1111/dgd.12261. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S, et al. Core regulatory RNA molecules identified in articular cartilage stem/progenitor cells during osteoarthritis progression. Epigenomics. 2019;11:669–684. doi: 10.2217/epi-2018-0212. [DOI] [PubMed] [Google Scholar]
- 48.Kachroo U, Vinod E. Comparative analysis of gene expression between articular cartilage-derived cells to assess suitability of fibronectin adhesion assay to enrich chondroprogenitors. Knee. 2020;27:755–759. doi: 10.1016/j.knee.2020.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J. Cell. Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 50.Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res. Ther. 2004;6:422–432. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joos H, Wildner A, Hogrefe C, Reichel H, Brenner RE. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res. Ther. 2013;15:R119. doi: 10.1186/ar4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Y, et al. Cartilage stem/progenitor cells are activated in osteoarthritis via interleukin-1β/nerve growth factor signaling. Arthritis Res. Ther. 2015;17:327. doi: 10.1186/s13075-015-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou C, Zheng H, Seol D, Yu Y, Martin JA. Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J. Orthop. Res. 2014;32:981–988. doi: 10.1002/jor.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koelling S, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Tallheden T, et al. Phenotypic plasticity of human articular chondrocytes. J. Bone Jt. Surg. Am. 2003;85:93–99. doi: 10.2106/00004623-200300002-00012. [DOI] [PubMed] [Google Scholar]
- 56.Bernstein P, Sperling I, Corbeil D, Hempel U, Fickert S. Progenitor cells from cartilage-no osteoarthritis-grade-specific differences in stem cell marker expression. Biotechnol. Prog. 2013;29:206–212. doi: 10.1002/btpr.1668. [DOI] [PubMed] [Google Scholar]
- 57.Mantripragada VP, et al. Progenitor cells from different zones of human cartilage and their correlation with histopathological osteoarthritis progression. J. Orthop. Res. 2018;36:1728–1738. doi: 10.1002/jor.23829. [DOI] [PubMed] [Google Scholar]
- 58.Mantripragada VP, et al. Primary cells isolated from human knee cartilage reveal decreased prevalence of progenitor cells but comparable biological potential during osteoarthritic disease progression. J. Bone Jt. Surg. Am. 2018;100:1771–1780. doi: 10.2106/JBJS.18.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantripragada VP, et al. Native-osteoarthritic joint resident stem and progenitor cells for cartilage cell-based therapies: a quantitative comparison with respect to concentration and biological performance. Am. J. Sports Med. 2019;47:3521–3530. doi: 10.1177/0363546519880905. [DOI] [PubMed] [Google Scholar]
- 60.Mantripragada, V. P. et al. Influence of glucose concentration on colony-forming efficiency and biological performance of primary human tissue–derived progenitor cells. Cartilage10.1177/1947603520906605 (2020). [DOI] [PMC free article] [PubMed]
- 61.Salamon A, et al. Articular cartilage-derived cells hold a strong osteogenic differentiation potential in comparison to mesenchymal stem cells in vitro. Exp. Cell Res. 2013;319:2856–2865. doi: 10.1016/j.yexcr.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Peng X, et al. Wnt/β-catenin signaling regulates the proliferation and differentiation of mesenchymal progenitor cells through the p53 pathway. PLoS ONE. 2014;9:e97283. doi: 10.1371/journal.pone.0097283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia Z, et al. Altered function in cartilage derived mesenchymal stem cell leads to OA-related cartilage erosion. Am. J. Transl. Res. 2016;8:433–446. [PMC free article] [PubMed] [Google Scholar]
- 64.Thornemo M, et al. Clonal populations of chondrocytes with progenitor properties identified within human articular cartilage. Cells Tissues Organs. 2005;180:141–150. doi: 10.1159/000088242. [DOI] [PubMed] [Google Scholar]
- 65.Karlsson C, Stenhamre H, Sandstedt J, Lindahl A. Neither Notch1 expression nor cellular size correlate with mesenchymal stem cell properties of adult articular chondrocytes. Cells Tissues Organs. 2008;187:275–285. doi: 10.1159/000113409. [DOI] [PubMed] [Google Scholar]
- 66.Kachroo U, Ramasamy B, Vinod E. Evaluation of CD49e as a distinguishing marker for human articular cartilage derived chondroprogenitors. Knee. 2020;27:833–837. doi: 10.1016/j.knee.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 68.Anderson DE, Markway BD, Bond D, McCarthy HE, Johnstone B. Responses to altered oxygen tension are distinct between human stem cells of high and low chondrogenic capacity. Stem Cell Res. Ther. 2016;7:154. doi: 10.1186/s13287-016-0419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vinod, E. et al. Comparison of the efficiency of laminin versus fibronectin as a differential adhesion assay for isolation of human articular cartilage derived chondroprogenitors. Connect. Tissue Res. 10.1080/03008207.2020.1761344 (2020). [DOI] [PubMed]
- 70.Mayne R, Vail MS, Mayne PM, Miller EJ. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc. Natl Acad. Sci. USA. 1976;73:1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diaz-Romero J, et al. Immunophenotypic analysis of human articular chondrocytes: Changes in surface markers associated with cell expansion in monolayer culture. J. Cell. Physiol. 2005;202:731–742. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 72.Yu Y, Zheng H, Buckwalter JA, Martin JA. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthr. Cartil. 2014;22:1318–1326. doi: 10.1016/j.joca.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015;11:206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melero Martin JM, Smith M, Al-Rubeai M. Cryopreservation and in vitro expansion of chondroprogenitor cells isolated from the superficial zone of articular cartilage. Biotechnol. Prog. 2005;21:168–177. doi: 10.1021/bp049821o. [DOI] [PubMed] [Google Scholar]
- 75.Melero-Martin JM, Dowling M-A, Smith M, Al-Rubeai M. Expansion of chondroprogenitor cells on macroporous microcarriers as an alternative to conventional monolayer systems. Biomaterials. 2006;27:2970–2979. doi: 10.1016/j.biomaterials.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 76.Melero-Martin JM, Dowling M-A, Smith M, Al-Rubeai M. Optimal in-vitro expansion of chondroprogenitor cells in monolayer culture. Biotechnol. Bioeng. 2006;93:519–533. doi: 10.1002/bit.20735. [DOI] [PubMed] [Google Scholar]
- 77.Kachroo, U. et al. Comparison of human platelet lysate versus fetal bovine serum for expansion of human articular cartilage–derived chondroprogenitors. Cartilage10.1177/1947603520918635 (2020). [DOI] [PMC free article] [PubMed]
- 78.Neumann AJ, et al. Human articular cartilage progenitor cells are responsive to mechanical stimulation and adenoviral-mediated overexpression of bone-morphogenetic protein 2. PLoS ONE. 2015;10:e0136229. doi: 10.1371/journal.pone.0136229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nevo Z, Beit-Or A, Eilam Y. Slowing down aging of cultured chick chondrocytes by maintenance under lowered O2 tension. Mechanis. 1988;45:157–165. doi: 10.1016/0047-6374(88)90105-4. [DOI] [PubMed] [Google Scholar]
- 80.Foldager CB, et al. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop. 2011;82:234–240. doi: 10.3109/17453674.2011.566135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou C, Zheng H, Buckwalter JA, Martin JA. Enhanced phagocytic capacity endows chondrogenic progenitor cells with a novel scavenger function within injured cartilage. Osteoarthr. Cartil. 2016;24:1648–1655. doi: 10.1016/j.joca.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roman-Blas JA, Jimenez SA. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Zhu M, et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J. Bone Miner. Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iannone F, et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology. 2002;41:1413–1418. doi: 10.1093/rheumatology/41.12.1413. [DOI] [PubMed] [Google Scholar]
- 85.Morgan, B. J. et al. Bone morphogenetic protein-9 is a potent chondrogenic and morphogenic factor for articular cartilage chondroprogenitors. Stem Cells Dev. 29, 882–894 (2020). [DOI] [PMC free article] [PubMed]
- 86.Liu T, et al. Kartogenin mediates cartilage regeneration by stimulating the IL-6/Stat3-dependent proliferation of cartilage stem/progenitor cells. Biochem. Biophys. Res. Commun. 2020;532:385–392. doi: 10.1016/j.bbrc.2020.08.059. [DOI] [PubMed] [Google Scholar]
- 87.Koelling S, Miosge N. Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthritis Rheum. 2010;62:1077–1087. doi: 10.1002/art.27311. [DOI] [PubMed] [Google Scholar]
- 88.Mouser VHM, et al. Three-dimensional bioprinting and its potential in the field of articular cartilage regeneration. Cartilage. 2017;8:327–340. doi: 10.1177/1947603516665445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mouser VHM, et al. Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs. Connect. Tissue Res. 2018;61:137–151. doi: 10.1080/03008207.2018.1553960. [DOI] [PubMed] [Google Scholar]
- 90.Diloksumpan P, et al. Combining multi-scale 3D printing technologies to engineer reinforced hydrogel-ceramic interfaces. Biofabrication. 2020;12:025014. doi: 10.1088/1758-5090/ab69d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mancini IAD, et al. A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: in vivo performance in a long-term equine model. Biofabrication. 2020;12:035028. doi: 10.1088/1758-5090/ab94ce. [DOI] [PubMed] [Google Scholar]
- 92.Peiffer QC, et al. Melt electrowriting onto anatomically relevant biodegradable substrates: resurfacing a diarthrodial joint. Mater. Des. 2020;195:109025. doi: 10.1016/j.matdes.2020.109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piluso S, et al. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J. Mater. Chem. B. 2020;8:9566–9575. doi: 10.1039/d0tb01731k. [DOI] [PubMed] [Google Scholar]
- 94.Lim, K. S. et al. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs. Biofabrication10, 034101 (2018). [DOI] [PubMed]
- 95.Bernal, P. N. et al. Volumetric bioprinting of complex living-tissue constructs within seconds. Adv. Mater. 31, 1904209 (2019). [DOI] [PubMed]
- 96.Frisbie DD, McCarthy HE, Archer CW, Barrett MF, McIlwraith CW. Evaluation of articular cartilage progenitor cells for the repair of articular defects in an equine model. J. Bone Jt. Surg. Am. 2015;97:484–493. doi: 10.2106/JBJS.N.00404. [DOI] [PubMed] [Google Scholar]
- 97.Decker RS, et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 2017;426:56–68. doi: 10.1016/j.ydbio.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng C, et al. Lgr5 and Col22a1 mark progenitor cells in the lineage toward juvenile articular chondrocytes. Stem Cell Rep. 2019;13:713–729. doi: 10.1016/j.stemcr.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ji Q, et al. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann. Rheum. Dis. 2019;78:100–110. doi: 10.1136/annrheumdis-2017-212863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caplan A. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 101.Chan CKF, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chan CKF, et al. Identification of the human skeletal stem cell. Cell. 2018;175:43.e21–56.e21. doi: 10.1016/j.cell.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surg. Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 104.Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vinod, E., Parameswaran, R., Ramasamy, B. & Kachroo, U. Pondering the potential of hyaline cartilage–derived chondroprogenitors for tissue regeneration: a systematic review. Cartilage10.1177/1947603520951631 (2020). [DOI] [PMC free article] [PubMed]
- 106.Unguryte A, et al. Human articular chondrocytes with higher aldehyde dehydrogenase activity have stronger expression of COL2A1 and SOX9. Osteoarthr. Cartil. 2016;24:873–882. doi: 10.1016/j.joca.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem. Biophys. Res. Commun. 2007;358:99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen VT, Cancedda R, Descalzi F. Platelet lysate activates quiescent cell proliferation and reprogramming in human articular cartilage: Involvement of hypoxia inducible factor 1. J. Tissue Eng. Regen. Med. 2018;12:e1691–e1703. doi: 10.1002/term.2595. [DOI] [PubMed] [Google Scholar]
- 109.Anderson DE, Markway BD, Weekes KJ, McCarthy HE, Johnstone B. Physioxia promotes the articular chondrocyte-like phenotype in human chondroprogenitor-derived self-organized tissue. Tissue Eng. Part A. 2018;24:264–274. doi: 10.1089/ten.tea.2016.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vinod E, et al. Comparison of incremental concentrations of micron-sized superparamagnetic iron oxide for labelling articular cartilage derived chondroprogenitors. Acta Histochem. 2019;121:791–797. doi: 10.1016/j.acthis.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 111.Vinod E, Vinod Francis D, Manickam Amirtham S, Sathishkumar S, Boopalan PRJVC. Allogeneic platelet rich plasma serves as a scaffold for articular cartilage derived chondroprogenitors. Tissue Cell. 2019;56:107–113. doi: 10.1016/j.tice.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Vinod E, Kachroo U, Rebekah G, Thomas S, Ramasamy B. In vitro chondrogenic differentiation of human articular cartilage derived chondroprogenitors using pulsed electromagnetic field. J. Clin. Orthop. Trauma. 2021;14:22–28. doi: 10.1016/j.jcot.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang R, et al. Intra-articular delivery of extracellular vesicles secreted by chondrogenic progenitor cells from MRL/MpJ superhealer mice enhances articular cartilage repair in a mouse injury model. Stem Cell Res. Ther. 2020;11:93. doi: 10.1186/s13287-020-01594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.