Abstract
Parkinson’s disease is a neurodegenerative disease characterized by the formation of neuronal inclusions of α-synuclein in patient brains. As the disease progresses, toxic α-synuclein aggregates transmit throughout the nervous system. No effective disease-modifying therapy has been established, and preventing α-synuclein aggregation is thought to be one of the most promising approaches to ameliorate the disease. In this study, we performed a two-step screening using the thioflavin T assay and a cell-based assay to identify α-synuclein aggregation inhibitors. The first screening, thioflavin T assay, allowed the identification of 30 molecules, among a total of 1262 FDA-approved small compounds, which showed inhibitory effects on α-synuclein fibrilization. In the second screening, a cell-based aggregation assay, seven out of these 30 candidates were found to prevent α-synuclein aggregation without causing substantial toxicity. Of the seven final candidates, tannic acid was the most promising compound. The robustness of our screening method was validated by a primary neuronal cell model and a Caenorhabditis elegans model, which demonstrated the effect of tannic acid against α-synuclein aggregation. In conclusion, our two-step screening system is a powerful method for the identification of α-synuclein aggregation inhibitors, and tannic acid is a promising candidate as a disease-modifying drug for Parkinson’s disease.
Subject terms: High-throughput screening, Parkinson's disease
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease (AD), and the number of patients is increasing with the aging of the global population1. PD is clinically characterized by progressive motor and non-motor symptoms. Although the currently available dopamine replacement therapies can ameliorate some of the symptoms, no curative therapy is available at present, and symptoms become disabling as the disease progresses. Therefore, disease-modifying therapies that slow or halt disease progression are urgently required.
The pathological hallmarks of PD include the appearance of neuronal inclusions called Lewy bodies, which are mainly composed of aberrant α-synuclein (αSyn) aggregates, and progressive neuronal loss mainly involving dopaminergic neurons in the substantia nigra2. Cumulative evidence has indicated that pathological αSyn and αSyn aggregates play a central role in the pathogenesis of PD, and its cell-to-cell propagation is associated with disease progression3. Therefore, the suppression of αSyn aggregation and/or the depolymerization of αSyn aggregates have been considered to be promising therapies for PD. Potential drugs that target αSyn aggregation act via the following strategies: suppression of αSyn expression4,5, modulation of the protein degradation machinery6, inhibition of the aggregation of αSyn7,8, and depolymerization of αSyn fibrils9. Among them, inhibition of the aggregation of αSyn is the most straightforward strategy that targets the pathological process underlying PD10,11. High throughput screening (HTS) of 1000 pharmacophores with the second-harmonic generation method identified BIOD303 as a modulator of the conformation of monomeric αSyn which reduced αSyn aggregation11. Recently, HTS by the thioflavin T (ThT) assay identified SynuClean-D12 and ZPD-213 as αSyn aggregation inhibitors from 14,400 compounds of Maybridge HitFinder Collection14. Although these reports have shown that HTS for αSyn aggregation inhibitors is a feasible strategy to identify drug candidates for PD, the cell-free screening methods employed in these studies could have not properly evaluated the influence of the cellular milieu on the action of the tested compounds. Moreover, ThT assay only monitors the fibrillation process of αSyn monomer, whereas the desired candidate compounds for patients is to inhibit the propagation of existing fibrils. To overcome these limitations, we propose a two-step screening method consisting of the conventional HTS ThT assay followed by a cell-based assay that allows the evaluation of seeding capability of the fibrils.
In this study, we evaluated 1262 FDA-approved compounds to enable future drug repositioning of the candidates. We took advantage of the high-throughput nature of the ThT assay, together with a cell-based system, which can evaluate both the efficacy and safety of the candidates. We identified seven compounds, and the robustness of our strategy was validated by the analysis of tannic acid (TA), which was the most effective molecule identified in our screening.
Results
ThT fluorescence assay for the screening of αSyn fibrillation inhibitors
Pujols et al. reported that the ThT fluorescence assay was the robust method to evaluate the kinetics of fibrillation of amyloid proteins including αSyn, and was useful for screening of αSyn fibrillation inhibitors14. In this study, we used this method as our first step of screening. Amyloid formation of αSyn was observed as a sigmoidal increase in ThT fluorescence (Fig. S1A), as previously described14. We then corroborated that the presence of ThT did not affect the kinetics of fibril formation (Fig. S1B) or the fibril morphology, visualized by transmission electron microscopy (TEM) (Fig. S1C). Also, the increase in ThT fluorescence was suppressed by the addition of Congo Red, which is known to have an inhibitory effect against αSyn fibrillation (Fig. S1D). For the quantitative evaluation of fibrillation, we determined the lag time until the ThT fluorescence intensity exceeded 1000 A.U., and the maximum ThT intensity. The lag time was counted as 2505 min if the fluorescence intensity did not exceed 1000 A.U., which was the time we finished the experiments. Congo Red significantly delayed the lag time (Fig. S1E) and inhibited the formation of amyloid fibrils (Fig. S1F).
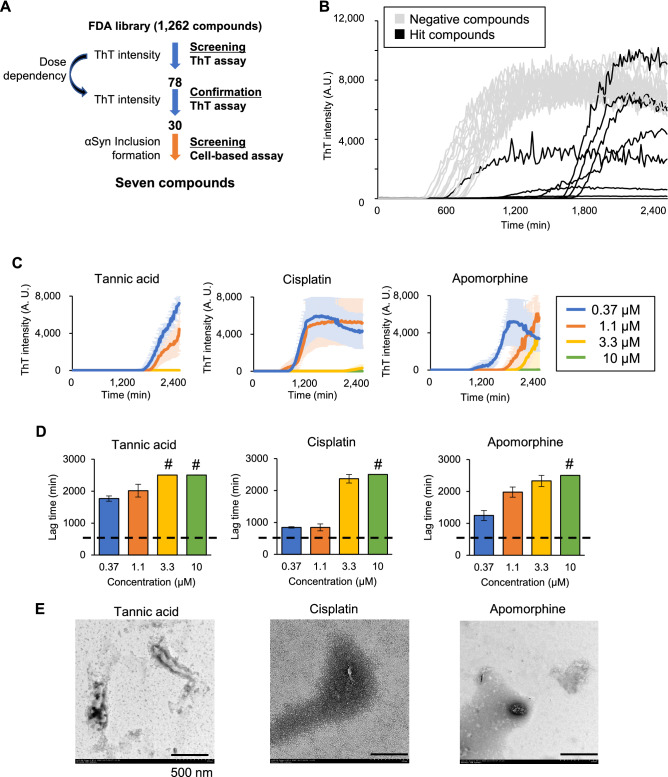
We screened 1262 FDA-approved compounds by the ThT assay as a first step of the screening for αSyn aggregation inhibitors, then, after confirming the dose-dependency of the positive compounds, we analyzed their effects using the cell-based assay (Fig. 1A). A single concentration (10 μM) for all the compound was tested in duplicate in the ThT assay. We established the lag time and the value of maximum ThT intensity within a same plate as the criteria to evaluate the efficacy of the tested compounds to suppress fibrillation. A compound was considered a hit when any of both parameters was found within the top ten percentile in the duplicate assay. Representative ThT kinetics of the wells with positive and negative compounds are shown in Fig. 1B. The 78 compounds that were positive in the first screening at the concentration of 10 μM were analyzed in the next screening using four different compound concentrations (10, 3.3, 1, and 0.37 μM), tested in triplicate. We ranked the compounds according to their lag time at 10 μM. If the lag time values at 10 μM were not different between compounds, we used the lag time at 3.3 μM, or lower concentrations until a difference was observed. The top 30 hits are listed in Table 1. Representative ThT kinetics and the lag times of the top 3 compounds at different concentrations are shown in Fig. 1C,D. The inhibition of fibril formation was confirmed by TEM (Fig. 1E).
Figure 1.
ThT fluorescence assay used for the screening of αSyn fibrillation inhibitors. (A) Schematic illustration of the two-step screening. (B) ThT kinetics of the wells with representative positive hits (black lines) and negative compounds (gray lines). (C) ThT kinetics of the top 3 hit compounds at different concentrations. Data are shown as the mean ± SEM of three independent wells (n = 3). Concentrations of the drugs are as indicated in the right box. (D) Representative lag times of the top 3 hit compounds at different concentrations. Dashed line represents the average lag times in the absence of any compound. Sharp marks (#) indicate that the reaction was stopped since it reached to the maximum duration (2505 min). Data are shown as the mean ± SEM of three independent wells (n = 3). (E) TEM visualization of fibrillation reaction products treated with the indicated compounds. Bar scale: 500 nm.
Table 1.
Top 30 hit compounds identified in the ThT screening assay for αSyn fibrillation inhibitors.
| Compound | Mean lag time (min) n = 3 | ||||
|---|---|---|---|---|---|
| 0.37 μM | 1.1 μM | 3.3 μM | 10 μM | ||
| 1 | Tannic acid | 1770 | 2015 | 2505 | 2505 |
| 2 | Cisplatin | 845 | 845 | 2370 | 2505 |
| 3 | Apomorphine hydrochloride | 1245 | 1980 | 2330 | 2505 |
| 4 | Norepinephrine | 1125 | 1530 | 2040 | 2505 |
| 5 | Diflunisal | 1730 | 1850 | 1965 | 2505 |
| 6 | Rabeprazole sodium | 985 | 1665 | 2015 | 2500 |
| 7 | Althiazide | 1120 | 1555 | 1780 | 2200 |
| 8 | Dopamine hydrochloride | 1065 | 1295 | 1620 | 2085 |
| 9 | Hymechrome | 1800 | 1705 | 1820 | 2070 |
| 10 | Pantoprazole | 950 | 1215 | 1785 | 2070 |
| 11 | Tamoxifen citrate | 1255 | 1305 | 1515 | 2070 |
| 12 | Donepezil hydrochloride | 1345 | 1340 | 1560 | 2030 |
| 13 | Hyoscyamine | 1405 | 1450 | 2195 | 2010 |
| 14 | Glucosamine hydrochloride | 1190 | 1760 | 2095 | 1925 |
| 15 | Methyldopa | 1440 | 1415 | 1240 | 1925 |
| 16 | Chlorophyllide Cu complex Na salt | 820 | 930 | 1000 | 1920 |
| 17 | Isotretinoin | 1495 | 1485 | 2050 | 1885 |
| 18 | Pirenperone | 1355 | 1450 | 1865 | 1845 |
| 19 | Entacapone | 1385 | 1695 | 1830 | 1800 |
| 20 | Nisoldipine | 1285 | 1450 | 1490 | 1795 |
| 21 | Chloroxine | 1345 | 1445 | 1875 | 1785 |
| 22 | Paroxetine hydrochloride | 1330 | 1385 | 1620 | 1705 |
| 23 | Risedronate sodium | 1260 | 1520 | 1640 | 1700 |
| 24 | Nateglinide | 955 | 1370 | 1360 | 1630 |
| 25 | Diethylcarbamazine citrate | 1060 | 1350 | 1470 | 1625 |
| 26 | Deferoxamine mesylate | 1260 | 1335 | 1255 | 1580 |
| 27 | Fenoldopam mesylate | 1120 | 1180 | 1390 | 1400 |
| 28 | Oxidopamine hydrochloride | 1045 | 1055 | 1125 | 1365 |
| 29 | Oxyquinoline sulfate | 575 | 725 | 815 | 1060 |
| 30 | Neomycin sulfate | 670 | 815 | 810 | 980 |
Cell-based assay for the screening of αSyn aggregation inhibitors
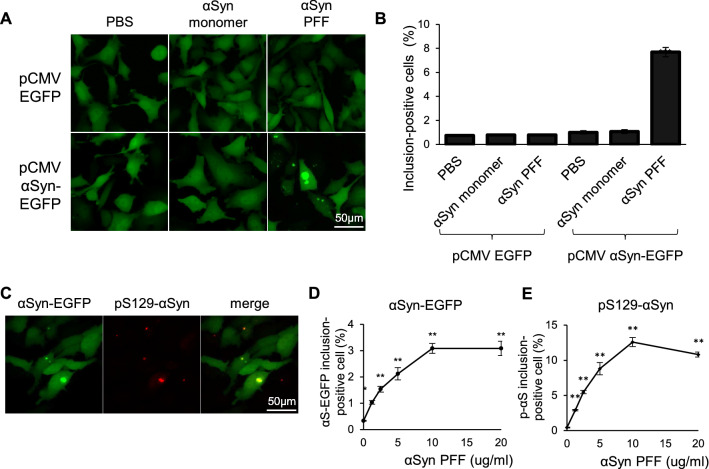
Exogenous αSyn preformed fibril (PFF) can seed the formation of Lewy body-like intracellular inclusions in cultured cells15. Based on this report, we developed a 96-well plate cell-based assay coupled with an automatic quantification system to further analyze the effects of the hit compounds identified by the ThT screening. To create cellular models of αSyn aggregation, we transiently overexpressed αSyn conjugated with enhanced green fluorescent protein (αSyn-EGFP) in HeLa cells, and treated the cells with PFF of αSyn. We confirmed the formation of large intracellular inclusions only when cells were treated with both αSyn-EGFP overexpression and αSyn PFF (Fig. 2A). The percentage of cells containing obvious EGFP inclusion bodies was significantly higher in the αSyn-EGFP overexpressing cells treated with αSyn PFF than in the other groups (Fig. 2B). The number of nuclei (Fig. S2A) and the percentage of EGFP-positive cells (Fig. S2B) were not significantly altered by PFF treatment. To investigate the biological relevance of the EGFP inclusions, we stained for αSyn phosphorylated on Ser129 (pS129 αS), which is considered to be the pathological form of αSyn. We found that αSyn-EGFP inclusions colocalized with pS129 αS (Fig. 2C). The percentage of cells containing EGFP inclusions and pS129 αS increased in a concentration-dependent manner up to 10 μg/mL of αSyn PFF (Fig. 2D,E). Taken together, our cell-based assay evaluating αSyn-EGFP inclusions was confirmed to detect changes in the amount of aggregation of pathological αSyn, and it is hence useful for drug screening.
Figure 2.
Establishment of a cell-based assay to evaluate αSyn aggregation. (A) Representative images of αSyn-EGFP aggregation in HeLa cells. HeLa cells were transfected with the indicated plasmids, and then treated with PBS, αSyn monomers, or PFF. (B) Quantification of the percentage of cells containing obvious αSyn-EGFP inclusion bodies in (A). Data are shown as the mean ± SEM of twelve independent wells (n = 12; **P < 0.01; two-way ANOVA with the Tukey test). (C) Representative immunocytochemistry images of PFF-treated HeLa cells. Cells were transfected with pCMV αSyn-EGFP, and then treated with αSyn PFF. Phosphorylated αSyn (pS129 αS) was stained using a specific antibody. (D,E) Quantification of the percentage of cells containing obvious αSyn-EGFP (D) and p129-αSyn (E) inclusion bodies. Data are shown as the mean ± SEM of four independent wells (n = 4; *P < 0.05; **P < 0.01; one-way ANOVA with the Dunnett test compared with the no αSyn PFF control).
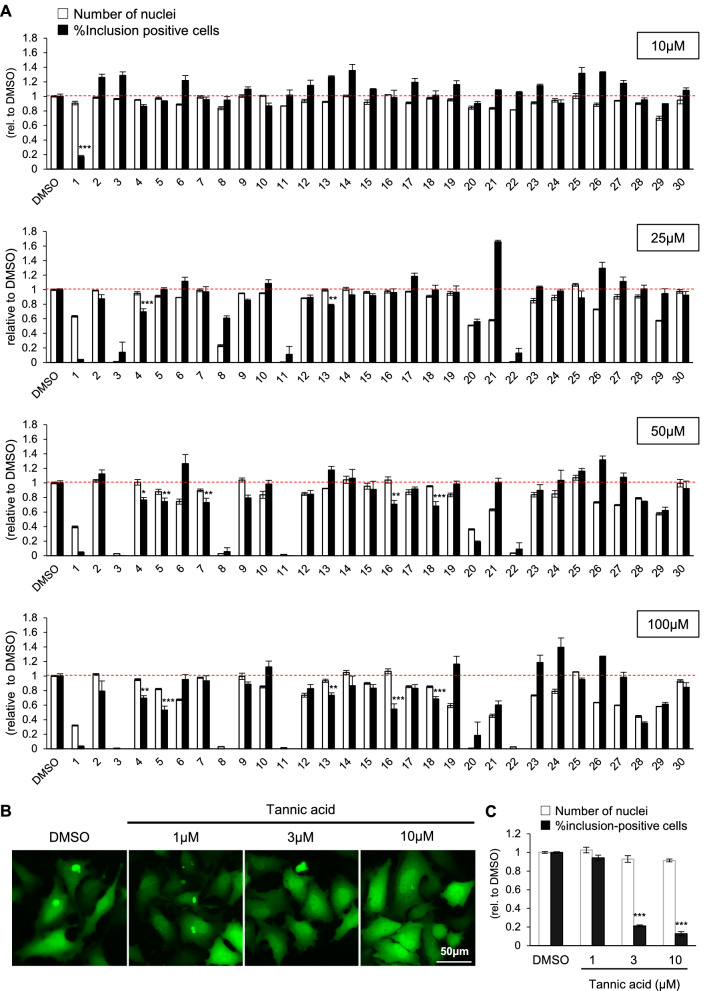
As in previous reports, we used the αSyn aggregation inhibitor rifampicin as a positive control in our assay16. Our cell-based assay demonstrated that rifampicin reduces the percentage of cells containing EGFP inclusions in a dose-dependent manner (Fig. S3, black bars). On the other hand, the dose-dependent decrease in the number of nuclei suggested that rifampicin is cytotoxic (Fig. S3A, white bars). Considering that the optimal concentration may be different among drugs, we tested four concentrations (10, 25, 50, and 100 μM) for each compound (Fig. 3A). A compound was defined as a hit by its efficacy (a significant reduction in αSyn-EGFP inclusions), in combination with its safety (< 20% cell loss). As a result, the following seven drugs were finally selected as hit compounds: TA, norepinephrine, diflunisal, althiazide, hyoscyamine, chlorophyllide Cu complex Na salt, and pirenperone (Table 2). We confirmed that at an optimal concentration of these compounds, there was no significant cell toxicities (Fig. S3B). Among them, TA showed the most prominent reduction in the number of αSyn-EGFP inclusions. TA also inhibited αSyn inclusions in a dose-dependent manner (Fig. 3B,C). Additionally, in order to exclude the possibility that TA disassembles mature fibrils in the extracellular space, we incubated PFF with TA for 24 h and then evaluated the morphology by TEM, confirming that the fibrils were stable in the presence of TA (Fig. S3C).
Figure 3.
Cell-based screening of αSyn aggregation inhibitors. (A) Quantification of the number of nuclei (white bars) and the percentage of cells containing obvious αSyn-EGFP inclusion bodies (black bars), standardized by those of cells treated with DMSO. Concentration of the drugs are indicated at the right. The numbers under the graph correspond to the numbers in Table 1. Data are shown as the mean ± SEM of three independent wells (n = 3; *P < 0.05; one-way ANOVA with the Dunnett test compared with the DMSO control). (B) Representative images of tannic acid-treated HeLa cells. Cells were transfected with pCMV αSyn-EGFP, followed by treatment with αSyn PFF and the indicated concentrations of tannic acid. (C) Quantification of the number of nuclei (white bars) and the percentage of cells containing obvious αSyn-EGFP inclusion bodies (black bars) in B standardized by those of cells treated with DMSO. Data are shown as the mean ± SEM of three independent wells (n = 3; *P < 0.001; one-way ANOVA with the Dunnett test compared with the no tannic acid control).
Table 2.
Seven final αSyn aggregation inhibitor candidates identified by the two-step screening.
| Compound | %Inhibition | %Viability | Conc. (μM) | |
|---|---|---|---|---|
| 1 | Tannic acid | 83 | 91 | 10 |
| 4 | Norepinephrine | 30 | 95 | 100 |
| 5 | Diflunisal | 47 | 82 | 100 |
| 7 | Althiazide | 27 | 90 | 50 |
| 13 | Hyoscyamine | 26 | 93 | 100 |
| 16 | Chlorophyllide Cu complex Na salt | 46 | 106 | 100 |
| 18 | Pirenperone | 32 | 85 | 100 |
TA inhibits aSyn aggregation in primary cultured neuronal cells and a C. elegans PD model
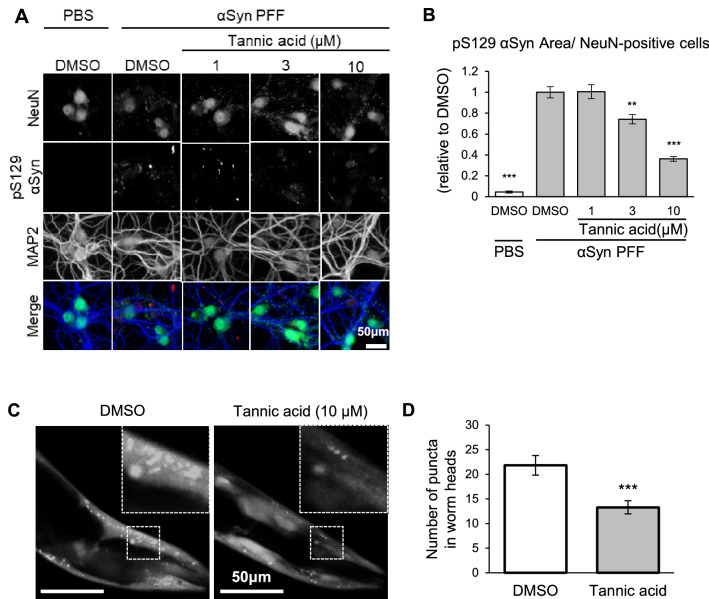
Next, to investigate the effects of TA on neuronal cells, which are the cells mainly affected by αSyn in Parkinson’s disease, we used primary cultured neurons from embryonic mouse cortex. The endogenous α-synuclein in mouse neurons is subject to aggregation and phosphorylation when exogeneous α-synuclein PFF is administered17. Treatment with αSyn PFF and TA started on days in vitro (DIV) 7 and the cells were fixed after 7 days (DIV 14). The level of pS129 αS per neuron significantly decreased with TA treatment in a dose-dependent manner, indicating that TA may prevent the PFF-induced aggregation of endogenous αSyn in mouse neurons (Fig. 4A,B). Then, to investigate the effects of TA in vivo, we used the NL5901 strain of C. elegans, which has been established as a model for PD. In NL5901 worms, expressing αSyn-YFP in muscle cells, αSyn forms aggregates with aging18. Age-synchronized 3-day-old young adult worms were transferred to a plate containing 10 μM TA, and the number of aggregates in 6-day-old wormswere evaluated. The number of aggregates was suppressed by about 30% upon treatment with TA (Fig. 4C,D).
Figure 4.
Tannic acid inhibits the formation of αSyn aggregates in mouse primary cultured neurons and PD model C. elegans. (A) Representative immunocytochemistry images of primary cultured neuronal cells treated with αSyn PFF and tannic acid. Primary neurons were treated with the indicated combinations at DIV 7, and were stained at DIV 14. (B) Quantification of pS129 αSyn in PFF- and tannic acid-treated neurons. Relative pS129 αSyn area per number of NeuN-positive neuronal cells was standardized by that of cells treated with αSyn PFF and DMSO. Data are shown as the mean ± SEM of three independent wells (n = 3; **P < 0.01; ***P < 0.001; one-way ANOVA with the Dunnett compared with the αSyn PFF without tannic acid control) (C) Representative images of the heads of PD model C. elegans treated with DMSO or tannic acid. (D) Quantification of the number of obvious puncta in C. elegans treated with tannic acid or the DMSO control. Data are shown as the mean ± SEM of twenty-eight worms (n = 28; ***P < 0.001; Student t-test).
Discussion
In this study, we used a two-step screening method to find candidate αSyn aggregation inhibitors. Taking advantage of the high-throughput ability of our method, we first evaluated 1262 FDA-approved compounds by the ThT assay and selected 30 hit compounds. To evaluate the efficacy and safety of these compounds in the cell-based assay, we developed a 96 well-based automatic quantification system to detect αSyn-EGFP inclusions. This system facilitated the analysis of several distinct concentrations of the compounds at one time, resulting in an increased chance to identify hit compounds. Seven out of 30 candidates (23.3%) were identified as potential αSyn aggregation inhibitors by this second step. A high hit-ratio of the second screening indicated the usefulness of the first ThT assay. Of note, the other 23 compounds were unable to inhibit αSyn aggregation in the cellular milieu owing to a weaker efficacy than expected (15/23, 65%) and/or cell toxicity (15/23, 65%). The discrepancy in compound efficacy between the ThT assay and the cell-based model may be mainly attributed to the complex environment within the cellular milieu. Compared with the simplicity of the in vitro ThT assay, the dynamics of αSyn in the living cell will be affected by various other molecules and protein quality control systems in vivo. The membrane permeability, intracellular modification, and metabolism of the compounds may also affect the results. Therefore, substances that exert their effects in the cell-based assay can be considered as promising candidates. Taken together, our two-step screening system may be a feasible strategy to identify drug candidates for inhibitors of pathological αSyn aggregation.
Our screening of 1262 FDA-approved compounds identified TA as the most effective candidate to inhibit αSyn aggregation. TA showed a reduction of 80% or more in αSyn inclusion-positive cells without any significant cytotoxicity. TA is a polyphenol compound, and previous studies have demonstrated that some polyphenols, including TA, suppress the aggregation of αSyn19–21. Therefore, the identification of TA from our screening contributes to validate the robustness of our screening system.
In this study, the efficacy of TA as an αSyn aggregation inhibitor was further validated in neuronal cells and a C. elegans model of PD. To the best of our knowledge, this is the first report showing the effects of TA on the inhibition of αSyn aggregation in a neuronal model and an animal model. As TA also suppresses Aβ aggregation, which is a hallmark of AD, this compound might have the potential of broad application to many neurodegenerative diseases. Interestingly, it has been reported that the oral administration of TA to an AD mouse model reduced Aβ deposits in the brain, although TA itself is not expected to cross the blood–brain barrier (BBB). This suggests that some metabolites of TA might remain active and pass across the BBB. Moreover, we also expect that the inhibition of αSyn aggregation in peripheral organs might exert beneficial effects on PD pathology. Cumulative reports have suggested the progression of PD pathology through the transmission of αSyn aggregates from the gut and olfactory bulb to the brain. From this point of view, the suppression of αSyn aggregation in the peripheral organs, such as the gut by TA may ameliorate brain pathology. Further detailed investigation of the effects of TA on PD and other neurodegenerative disorders is required in the future.
Other than TA and other polyphenol compounds, we found diflunisal, an FDA-approved drug for transthyretin (TTR) familial amyloid polyneuropathy22. Diflunisal inhibits TTR aggregation by stabilizing the tetrameric conformation of TTR23,24. Interestingly, αSyn is more stable and resistant to aggregation in its tetrameric form25. Further studies will be required to confirm the action of diflunisal on αSyn aggregation.
The limitations of our study include the following points: first, the two assays performed were based on artificial conditions, while the hit compounds will require a further validation under more physiological conditions. In this regard, we have already investigated the effects of TA in neuronal cells without performing transfection, and in nematodes without adding PFF. Second, we did not test a sufficient range of concentrations for all the compounds in the ThT assay, just a single concentration of 10 µM, which could result in false-negative results. In addition, the cell-based assay may also have produced false negative results since some compounds were not effective but still not toxic at the maximum concentration of 100 μM.
In summary, our two-step screening of 1262 FDA-approved compounds identified seven candidate drugs as αSyn aggregation inhibitors. In particular, TA appeared to be the most promising of the candidates, and further studies towards its clinical application are expected in the future.
Materials and methods
Plasmids
The plasmid containing the human αSyn was originally created in a previous study by amplification from cDNA of human brain (Cap site cDNA dT: Nippon gene) by PCR26. The MultiSite Gateway® donor vectors (Invitrogen, MA, USA) were used to clone human wild type (WT) αSyn to generate the entry clones pENTR-L1-ACC-αSyn and -R5. Entry clones were then recombined with pcDNA™-DEST40 (Invitrogen) and pENTR-L5-GGS6-EGFP-L227 (a gift from Professor H. Kuroyanagi) to create the final expression clone αSyn-GGS6-EGFP.
Preparation of αSyn
Human WT αSyn was purified from Escherichia coli (E. coli) as described previously28. Briefly, a plasmid containing WT human αSyn was expressed in E. coli BL21 (DE3) (Novagen, Merck, San Diego, CA, USA). The cells were suspended in purification buffer, disrupted by sonication, and centrifuged. Streptomycin sulfate (final 2.5% [w/w]) was added to the supernatant and centrifuged. The supernatant was then heated at 90 °C in a water bath and centrifuged. The supernatant was precipitated by solid ammonium sulfate to 70% saturation, centrifuged, dialyzed overnight, and applied onto a Resource-Q column (GE Healthcare, Little Chalfont, UK) with 50 mM Tris–HCl buffer (pH 7.5) containing 0.1 mM dithiothreitol and 0.1 mM phenylmethylsulfonyl fluoride as the running buffer, and eluted with a linear gradient of 0 to 1 M NaCl. αSyn-enriched fractions (as determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis/Coomassie blue staining) were pooled and further purified by size exclusion chromatography using a Superdex 200 10/300 GL column (GE Healthcare) equilibrated with 50 mM Tris–HCl (pH 7.5) and 150 mM NaCl. The purified fractions were combined and dialyzed against deionized water at 4 °C overnight. Sample solutions were flash-frozen in liquid nitrogen and lyophilized.
In vitro fibrillation of αSyn
The in vitro fibrillation assay was performed as reported previously14, with some modifications. Briefly, lyophilized αSyn was dissolved in a buffer containing 250 mM NaCl, 50 mM Tris–HCl (pH 7.4), 10 μM ThT, filtered through a 0.22 µm membrane to remove αSyn aggregates, and adjusted to a final concentration of 500 μg/mL. For the evaluation of ThT concentration dependency (Fig. S1B), the concentration of ThT ranged between 1 and 20 µM. The fibrillation reaction was carried out in a 96-well sealed plate (Costar Assay Plate, Corning, USA). Each well contained 100 μL of reaction mixture with or without compounds at 10 µM. The reaction 96-well microplate was subjected to cyclic agitation with a 3 min orbital shaking period at 2000 rpm, followed by a 12 min quiescent period, at 37 °C. Amyloid formation was monitored by ThT intensity fluorescence (excitation at 450 nm and emission at 485 nm) every 15 min. Fluorescence measurement and shaking procedures were performed using a MTP-900 microplate reader (Corona Electric Co., Tokyo, Japan). All the samples were measured in duplicate, and the formation of fibrillar aggregates was characterized by the lag time, defined as the time required to reach a fluorescence value of 1000 A.U., and the maximum ThT intensity, the highest intensity value in the measuring period, as the parameters in αSyn fibrillation.
TEM analysis
Fibrils were adsorbed onto 400-mesh grids and negatively stained with 1% phosphotungstic acid, and their structures were observed using an H-7650 TEM (Hitachi High Technologies Corporation, Tokyo, Japan).
Cell culture
HeLa cells were purchased from KAC (KAC, Kyoto, Japan). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, MO, USA, D5796) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a 95% air, 5% CO2 humidified incubator. Cells were routinely subcultured when confluent. The maximum passage of the cell line was 20 times.
Creation of a cell-culture model of αSyn aggregation
HeLa cells were transfected with the αSyn-EGFP plasmid using FuGENE HD Transfection Reagent (Promega, WI, USA) following the manufacturer’s instructions, to overexpress αSyn. After 24 h, the culture medium was replaced with culture medium containing αSyn PFF with or without the drug compounds. After 24 h of incubation, cells were fixed with 4% paraformaldehyde (Wako, Japan) for 30 min at room temperature, and then the cells were immunostained for pS129 αS and microscopically analyzed.
Immunostaining
Fixed HeLa cells were washed in phosphate-buffered saline (PBS), then incubated in PBS with 10% Block Ace (Yukizirushi, Tokyo, Japan) for 1 h and subsequently with primary anti-phosphorylated αSyn (pS129 αS) (1:1000; Abcam, Cambridge, UK) for 4 °C overnight. After several washes in PBS, the cells were incubated with Cy3-conjugated anti-rabbit antibody (Jackson ImmunoResearch, PA, USA) for 1 h at room temperature. After several washes in PBS, the cells were counterstained with Hoechst 34580 (Invitrogen).
Image acquisition and analysis
Images of the treated cells were obtained using IN Cell Analyzer (GE Healthcare). Thirty-six images of a culture well were taken at 20× magnification. Automated quantification of the number of nuclei, cells expressing EGFP, and cells expressing αSyn-EGFP, or with pS129 αS inclusions were performed using the software IN Cell Developer Toolbox (GE Healthcare).
Cell toxicity assay
HeLa WT cells were incubated with the optimal concentration of compounds as listed in Table 2 for 24 h and the number of nuclei were counted as described above.
Primary neuronal culture model of αSyn aggregation and TA treatment
Pregnant C57BL/6J mice, (RRID:IMSR_JAX:000664) were obtained from Charles River Laboratories Japan (Yokohama, Japan). Primary cortical neurons were prepared from E15.5 pups and cultured in MACS Neuro Medium (Miltenyi Biotec, Bergisch Gladbach, Germany) supplemented with MACS NeuroBrew-21 (Miltenyi Biotec), 0.5 mM l-glutamine, penicillin, and streptomycin (all from Invitrogen) on tissue culture plates coated with poly-l-ornithine (Fujifilm, Tokyo, Japan) at a density of 5 × 104 cells/cm2. At 3 days in vitro (DIV), a half volume of fresh media containing 2′-deoxy-5-fluorouridine (final concentration, 3.3 µM) (Tokyo Chemical Industry, Tokyo, Japan) was added to inhibit glial proliferation. At 7 DIV, half of the media was changed, and αSyn PFF (final concentration, 10 µg/mL) with or without TA was added. αSyn PFF were incubated for 7 days and then analyzed by immunocytochemistry.
C. elegans experiments
Standard methods were used to culture C. elegans on nematode growth medium (NGM) agar seeded with OP50 E. coli, as previously described29,30. The worms were maintained at 20 °C unless otherwise indicated.
N2 WT (Bristol) and NL590118 were obtained from the Caenorhabditis elegans Genetic Center.
Quantification of αSyn aggregates formed in C. elegans
The quantification of aggregates was performed as previously described12,18 with brief modifications. Briefly, NL5901 (Punc-54:α-SYN:YFP) worms were age-synchronized and left overnight to hatch. Synchronized nematodes were cultured and grown on NGM plates seeded with E. coli OP50 until they reached the young adult stage (day 3). Afterwards, animals were transferred onto 5-Fluoro-2′-deoxyuridine-containing plates with or without TA (10 μM). αSyn aggregates were counted for each animal 3 days after treatment. For each independent experiment, 28 worms for each treatment were examined under a Zeiss LSM 700 microscope. Aggregates were defined as discrete bright structures with boundaries distinguishable from the surrounding fluorescence. Measurements of the aggregates were performed visually on all the aggregates observed in the head region of the worms. The experiments were performed blinded. The experimenter who performed the quantification was unaware of the worm’s group.
Statistical analysis
All data were analyzed using JMP software. For the statistical analysis of 2 groups, the paired or unpaired t-test was used as described in the figure legends. In all experiments, data were expressed as the mean ± SD and a p value of < 0.05 was considered to indicate a statistically significant difference between 2 groups. One-way analysis of the variance (ANOVA) with the Dunnett post-hoc test for more than three groups.
Statement
All animal experiments were conducted with approval from the Animal Care and Use Committee and the Committee for Safe Handling of Living Modified Organisms of the Graduate School of Medicine, Osaka University. This study was carried out in compliance with the ARRIVE guidelines. All methods were carried out in accordance with relevant guidelines and regulations.
Supplementary Information
Acknowledgements
This work was supported by the Core Research for Evolutional Science and Technology (Grant No. JPMJCR17H6 and JPMJCR18H4); JSPS KAKENHI (Grant No. JP18H02741 and JP17H05700); Japan Agency for Medical Research and Development (Grant Nos. JP18hm0102037, JP19dm0207070, and JP18km0405206); Japan Society for the Promotion of Science (Core-to-Core Program A (Advanced Research Networks)); Project Medical Evolution Expedited Tackle (MEET), Osaka University Graduate School of Medicine and Mitsubishi Tanabe Pharma Corporation. We also thank Corona Electric Company for kindly providing the microplate reader, Hidehito Kuroyanagi (Tokyo Medical and Dental University) for kindly providing the pENTR-L5-GGS6-EGFP-L2 plasmid, and Prof. Yasushi Kawata (Tottori University) for kindly gifting the plasmid of human αSyn, and Center for Medical Research and Education, Graduate School of Medicine, Osaka University, for technical assistance. The authors declare no conflicts of interest in association with this study.
Abbreviations
- αSyn
α-Synuclein
- DMSO
Dimethyl sulfoxide
- FDA
Food and drug administration
- PD
Parkinson’s disease
- PFF
Preformed fibril
- RRID
Research Resource Identifier
- TA
Tannic acid
Author contributions
M.H., Y.K., C.A., K.K., C.C., J.D., K.N., K.Y., K.N., K.B., S.N., and K.I. performed the experiments. T.T., Y.G., Y.N., H.M., and K.I. designed the experiments. M.H., Y.K., C.A., T.T., K.Y., K.N., K.B., S.N., Y.G., Y.N., H.M., and K.I. discussed the results and wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hideki Mochizuki, Email: hmochizuki@neurol.med.osaka-u.ac.jp.
Kensuke Ikenaka, Email: ikenaka@neurol.med.osaka-u.ac.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-04131-9.
References
- 1.Ray Dorsey E, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki K, et al. Parkinson’s disease is a type of amyloidosis featuring accumulation of amyloid fibrils of α-synuclein. Proc. Natl. Acad. Sci. USA. 2019;116:17963–17969. doi: 10.1073/pnas.1906124116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefanis L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012;2:1–23. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamori M, Junn E, Mochizuki H, Mouradian MM. Nucleic acid-based therapeutics for Parkinson’s disease. Neurotherapeutics. 2019;16:287–298. doi: 10.1007/s13311-019-00714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittal S, et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science. 2017;357:891–898. doi: 10.1126/science.aaf3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Perera G, Bhadbhade M, Halliday GM, Dzamko N. Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells. J. Biol. Chem. 2019;294:14241–14256. doi: 10.1074/jbc.RA119.008733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatenhorst L, et al. Fasudil attenuates aggregation of α-synuclein in models of Parkinson’s disease. Acta Neuropathol. Commun. 2016;4:39. doi: 10.1186/s40478-016-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perni M, et al. A natural product inhibits the initiation of α-synuclein aggregation & suppresses its toxicity. Proc. Natl. Acad. Sci. USA. 2017;114:E1009–E1017. doi: 10.1073/pnas.1610586114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehay B, et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015;14:855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner J, et al. Anle138b: A novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 2013;125:795–813. doi: 10.1007/s00401-013-1114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moree B, et al. Small molecules detected by second-harmonic generation modulate the conformation of monomeric α-synuclein and reduce its aggregation in cells. J. Biol. Chem. 2015;290:27582–27593. doi: 10.1074/jbc.M114.636027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujols J, et al. Small molecule inhibits α-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2018;115:10481–10486. doi: 10.1073/pnas.1804198115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peña-Díaz S, et al. ZPD-2, a small compound that inhibits α-synuclein amyloid aggregation and its seeded polymerization. Front. Mol. Neurosci. 2019;12:1–12. doi: 10.3389/fnmol.2019.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pujols J, et al. High-throughput screening methodology to identify alpha-synuclein aggregation inhibitors. Int. J. Mol. Sci. 2017;18:478. doi: 10.3390/ijms18030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luk KC, et al. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umeda T, et al. Rifampicin is a candidate preventive medicine against amyloid-β and tau oligomers. Brain. 2016;139:1568–1586. doi: 10.1093/brain/aww042. [DOI] [PubMed] [Google Scholar]
- 17.Volpicelli-Daley LA, Luk KC, Lee VMY. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014;9:2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ham TJ, et al. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet. 2008;4:e1000027. doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for α-synuclein fibrils in vitro. J. Neurochem. 2006;97:105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 20.Ardah MT, et al. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014;6:1–17. doi: 10.3389/fnagi.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi R, et al. Phenolic compounds prevent the oligomerization of α-synuclein and reduce synaptic toxicity. J. Neurochem. 2015;134:943–955. doi: 10.1111/jnc.13180. [DOI] [PubMed] [Google Scholar]
- 22.Berk JL, et al. Repurposing diflunisal for familial amyloid polyneuropathy: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2013;310:2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller SR, Sekijima Y, Kelly JW. Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab. Investig. 2004;84:545–552. doi: 10.1038/labinvest.3700059. [DOI] [PubMed] [Google Scholar]
- 24.Sekijima Y, Maria AD, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 25.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–111. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi H, Kusaka E, Hongo K, Mizobata T, Kawata Y. Amyloid fibril formation of α-synuclein is accelerated by preformed amyloid seeds of other proteins: Implications for the mechanism of transmissible conformational diseases. J. Biol. Chem. 2005;280:38609–38616. doi: 10.1074/jbc.M508623200. [DOI] [PubMed] [Google Scholar]
- 27.Kuroyanagi H, Watanabe Y, Suzuki Y, Hagiwara M. Position-dependent and neuron-specific splicing regulation by the CELF family RNA-binding protein UNC-75 in Caenorhabditis elegans. Nucleic Acids Res. 2013;41:4015–4025. doi: 10.1093/nar/gkt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakuda K, et al. Ultrasonication-based rapid amplification of α-synuclein aggregates in cerebrospinal fluid. Sci. Rep. 2019;9:6001. doi: 10.1038/s41598-019-42399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikenaka K, et al. dnc-1/dynactin 1 knockdown disrupts transport of autophagosomes and induces motor neuron degeneration. PLoS ONE. 2013;8:e54511. doi: 10.1371/journal.pone.0054511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikenaka K, et al. A behavior-based drug screening system using a Caenorhabditis elegans model of motor neuron disease. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-46642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.