Abstract
The effective use of human-derived cells that are difficult to freeze, such as parenchymal cells and differentiated cells from stem cells, is crucial. A stable supply of damage-sensitive cells, such as differentiated neuronal cells, neurons, and glial cells can contribute considerably to cell therapy. We developed a serum-free freezing solution that is effective for the cryopreservation of differentiated neuronal cells. The quality of the differentiated and undifferentiated SK-N-SH cells was determined based on cell viability, live-cell recovery rate, and morphology of cultured cells, to assess the efficacy of the freezing solutions. The viability and recovery rate of the differentiated SK-N-SH neuronal cells were reduced by approximately 1.5-folds compared to that of the undifferentiated SK-N-SH cells. The viability and recovery rate of the differentiated SK-N-SH cells were remarkably different between the freezing solutions containing 10% DMSO and that containing 10% glycerol. Cryoprotectants such as fetal bovine serum (FBS), antifreeze proteins (sericin), and sugars (maltose), are essential for protecting against freeze damage in differentiated neuronal cells and parenchymal cells. Serum-free alternatives (sericin and maltose) could increase safety during cell transplantation and regenerative medicine. Considering these, we propose an effective freezing solution for the cryopreservation of neuronal cells.
Keywords: Cryopreservation, Neuronal cells, Differentiation, Sericin, Serum-free, Regenerative therapy
Abbreviations: BSA, bovine serum albumin; 5-CFDA-AM, 5-carboxyfluorescein diacetate; DAPI, 4′,6-Diamidino-2-phenylindole, dihydrochloride; DMSO, dimethyl sulfoxide; D-MEM, Dulbecco's Modified Eagle's Medium with high glucose, l-glutamine, phenol red, and sodium pyruvate; D-PBS-free, Dulbecco's phosphate-buffered saline without Ca2+ and Mg2+ supplementation; EDTA, ethylene-diamine-tetraacetic acid; ESC, embryonic stem cells; FBS, fetal bovine serum; iPSC, induced pluripotent stem cells; NeuN, a neuronal marker; SDS, sodium dodecyl sulfate
Graphical abstract
Highlights
-
•
The timing of freezing during cell differentiation.
-
•
More effective serum-free freezing solution for differentiated neuronal cells.
-
•
Improving the quality of damage-sensitive cells, such as differentiated neuronal cells.
1. Introduction
Cell cryopreservation enables long-term stable storage, with a lower risk of deterioration and contamination than refrigeration [1]. Cells and tissues are used for basic research in medicine, biology, toxicity testing, safety testing in drug discovery, and cellular and regenerative therapy. This research requires the efficient utilization of human-derived cells that are difficult to freeze, such as parenchymal cells and cells differentiated from stem cells, such as hepatocytes, neurons, and cardiomyocytes [[2], [3], [4], [5]]. However, organs and tissues isolated from living organisms decay and deteriorate rapidly, which is a major obstacle in organ transplantation. Despite the many advances in liver transplantation, the methods of organ preservation have remained largely unchanged for almost three decades [6]. In addition to static refrigeration-based preservation methods, perfusion of organs at room temperature is performed to maintain the physiological environment; however, the organ preservation time is limited [7]. Therefore, cells isolated from organs and tissues are stored at extremely low temperatures, such as ≤−80 °C, using liquid nitrogen to suppress cell metabolism. This ensures the quality and integrity of the cells, and a stable supply can be achieved by improving the cryopreservation technology. A stable supply of damage-sensitive cells, such as neurons that are difficult to freeze, can significantly contribute to cell therapy. Cryopreservation of neuronal progenitor cells from induced pluripotent stem cells (iPSCs) has been reported [8,9]. However, we did not target the proliferative neuronal progenitor cells but the more differentiated neuron-like cells. In this study, SK-N-SH cells were used as the more differentiated neuron-like cells in a damage-sensitive cell model.
Cell damage during cryopreservation could be attributed to the intracellular and extracellular damage from ice crystals and intracellular dehydration [[10], [11], [12]]. Cryoprotectants are used to prevent or mitigate cell-frost damage. There are two types of cryoprotectants: cell membrane permeable protectants, such as dimethyl sulfoxide (DMSO) and glycerol [13,14], and cell membrane non-permeable protectants, such as fetal bovine serum (FBS) and trehalose [[15], [16], [17]]. The most commonly used cryoprotectants are DMSO and FBS, and their combination is the most widely used standard protocol [18]. However, DMSO is highly toxic to cells and it induces differentiation, growth inhibition, and cell dysfunction [[19], [20], [21], [22]]. FBS is associated with safety concerns due to infectious diseases such as bovine spongiform encephalopathy and those caused by other viruses [23,24], and quality issues due to the non-uniformity of batches. Therefore, it is necessary to develop a less toxic and safe freezing solution using a combination of DMSO and other protective agents.
Several studies have explored various freezing methods and cryopreservation solutions for stem cells [[25], [26], [27], [28], [29]]. A chemically defined solution without DMSO using vitrification was effective for the cryopreservation of human embryonic stem cells (ESCs) [27]. The use of a chemically defined solution using slow freezing for the cryopreservation of pluripotent stem cells and tissue stem cells have been widely reported [30,31]. Cell therapy with cellular and tissue-based products, such as Kymriah (Tisagenlecleucel) and TEMCELL HS Inj. (mesenchymal stem cells), and DMSO has been adopted in clinical practice. Sugars, such as trehalose and raffinose, are promising alternative to FBS, for protecting cells from damage during cryopreservation [2,3,32]. Miyamoto et al. investigated freezing solutions containing various oligosaccharides in parenchymal cells [2], pluripotent cells [28], and multipotent stem cells [29]. Trehalose, maltose, and various oligosaccharides are effective for cryopreservation of rat and human hepatic parenchymal cells (primary hepatocytes) [2]. Sericin, a natural macromolecular protein derived from silkworm, has been used to fabricate surgical sutures [33] and has recently attracted attention as a cryoprotectant [3,29,34]. It is effective for the cryopreservation of cell lines [34], primary hepatocytes [3], and adipose tissue-derived stem cells [29].
Herein, a freezing solution that is effective for differentiated cells during the freezing and thawing processes was evaluated. In this study, differentiated SK-N-SH neuronal cells were used as a damage-sensitive cell model [35,36]. Cell membrane-permeable cryoprotectants (DMSO and glycerol) and non-permeable cryoprotectants (FBS, sericin, and maltose) were added to the freezing solution. The quality of the differentiated and undifferentiated SK-N-SH cells was assessed based on cell viability, live cell recovery rate, and morphology of cultured cells, to evaluate the efficacy of the freezing solution.
2. Materials and methods
2.1. Materials
Dulbecco's modified Eagle's medium with high glucose, l-glutamine, phenol red, and sodium pyruvate (D-MEM), DMSO, glycerol, maltose, pure sericin, and all-trans-retinoic acid were purchased from Fujifilm Wako Chemicals Co. Ltd. (Osaka, Japan). Sericin hydrolysate (Seiren Co. Ltd.) with an average molecular mass of 30 kDa was used. Penicillin-streptomycin mixed solution, 2.5 g/L trypsin-1 mmol/L EDTA solution, paraformaldehyde, and tritonX-100 were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Dulbecco's phosphate-buffered saline without Ca2+ and Mg2+ supplementation (D-PBS-free), neurobasal medium, Gibco B-27 supplement, trypan blue stain (0.4%), bovine serum albumin (BSA), and 5-CFDA-AM (5-carboxyfluorescein diacetate) were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). FBS (HyClone, SH30910.03) was obtained from Funakoshi Co., Ltd. (Tokyo, Japan). All other materials and chemicals not specified above were of the highest available grade.
2.2. Cells
SK-N-SH cells (human neuroblastoma cells) and HEK293 cells (human embryonic kidney) were purchased from the Riken BRC Cell Bank (Tsukuba, Japan).
2.3. Cell culture
The maintenance medium was prepared using D-MEM as the basic composition and supplemented with 10% FBS and 1% penicillin-streptomycin to the final concentrations. SK-N-SH cells (1 × 106) and HEK293 cells were seeded in a 100-mm culture dish (Corning Co. Ltd. NY, USA) in the maintenance medium. The seeded cells were attached and spread on the dish and cultured at 37 °C under a humidified 5% CO2 atmosphere to approximately 80%–90% confluence. The cells were detached from the dish using trypsin treatment, seeded (1 × 105–1 × 106 cells), and cultured in a new dish. Cell viability was >95%, as determined using the trypan blue dye exclusion test. The final trypan blue concentration was 0.2%.
2.4. Neuronal differentiation of SK-N-SH cells
Retinoic acid was added to SK-N-SH cells to induce their differentiation into neuronal cells. SK-N-SH cells (1 × 106) were seeded onto a 100-mm culture dish in differentiation medium #1 (maintenance medium supplemented with 3 μM all-trans-retinoic acid solution). Then, the seeded cells were attached and spread onto a 100-mm culture dish and cultured at 37 °C under a humidified 5% CO2 atmosphere for one week. The morphology of the cultured cells was observed using a phase-contrast microscope (Olympus Co., Tokyo, Japan).
The neuronal cells (differentiated SK-N-SH cells) were continuously cultured at 37 °C in a humidified 5% CO2 atmosphere for five weeks by changing the neuronal differentiation media. Differentiation media #2 (maintenance medium supplemented with 10 μM all-trans-retinoic acid) and differentiation media #3 (neurobasal medium supplemented with B-27 supplements and 10 μM all-trans-retinoic acid) were used to induce mature neuronal cells (differentiated SK-N-SH cells). The morphology of the cultured cells was observed using a phase-contrast microscope (BZ-X710; Keyence Co., Osaka, Japan).
2.5. Immunofluorescence staining of undifferentiated and differentiated SK-N-SH cells
Neuronal differentiation of SK-N-SH cells was determined using the neuronal marker, NeuN. Cultured cells were washed three times with D-PBS-free and then fixed with 4% paraformaldehyde in D-PBS-free (pH 7.4) for 10 min. The samples were washed twice with D-PBS-free and then treated with 0.2% TritonX-100 in D-PBS-free for 10 min at 25 °C. Treated samples were washed twice in D-PBS-free for 5 min and incubated with 0.5% BSA in D-PBS-free for 30 min at 25 °C. After removing the 0.5% BSA in D-PBS-free, the treated samples were incubated with 1000-fold diluted primary antibody (Anti-NeuN antibody, ab177487; Abcam plc, Cambridge, UK) in 0.5% BSA in D-PBS-free, overnight at 4 °C. The treated samples were washed twice with 0.5% BSA in PBS for 5 min and then incubated with 4000-fold diluted secondary antibody (Goat Anti-Rabbit IgG H&L, ab150077; Abcam plc) and 1 μg/mL DAPI (4′,6-Diamidino-2-phenylindole, dihydrochloride; Sigma–Aldrich Co. LLC, St. Louis, MO, USA) in 0.5% BSA in D-PBS-free for 1 h at room temperature in the dark. Treated samples were washed twice with 0.5% BSA in D-PBS-free for 5 min, and the expression of NeuN protein in SK-N-SH cells was observed using a fluorescence microscope (Olympus Co., Tokyo, Japan).
2.6. Immunoblotting of undifferentiated and differentiated SK-N-SH cells
Cultured undifferentiated and differentiated SK-N-SH cells on 100 mm culture dishes were lysed using RIPA lysis buffer. The lysed supernatants were denatured by adding Laemmli's sodium dodecyl sulfate (SDS) sample buffer (2% [w/v] SDS, 62.5 mM Tris-HCl [pH 6.8], 0.005% bromophenol blue, and 7% glycerol) and heated at 95 °C for 10 min. The samples (5 μg/20 μL) were subjected to SDS polyacrylamide gel electrophoresis on 12% acrylamide gels prior to immunoblotting. Immune complexes of anti-NeuN polyclonal Abs (0.1 μg/mL) were determined using enzyme-linked color development with horseradish peroxidase-conjugated secondary Abs (0.1 μg/mL; 1:10,000, Sigma–Aldrich).
2.7. Preparation of freezing solution and cell freezing procedures
Six freezing solutions were prepared using D-MEM, which was supplemented with 10% FBS, 1% sericin, 1 M maltose, 10% DMSO, and 10% glycerol to the respective final concentrations (Table 1).
Table 1.
The composition of freezing solution.
| Solution | Composition |
|---|---|
| 1 | 1% (w/v) sericin, 0.1 M maltose, 10% (w/v) glycerol |
| 2 | 1% (w/v) sericin, 0.1 M maltose, 10% (v/v) DMSO |
| 3 | 10% (v/v) FBS, 10% (w/v) glycerol |
| 4 | 10% (v/v) FBS, 10% (v/v) DMSO |
| 5 | 10% (v/v) DMSO |
| 6 | 10% (v/v) FBS |
Six freezing solutions were prepared using D-MEM, as the basic composition, supplemented with 10% FBS, 1% sericin, 0.1 M maltose, 10% DMSO, and 10% glycerol to the final concentrations.
The cell-freezing procedure is described in Fig. S1. The cultured cells were treated with trypsin, and 1 × 106 cells were transferred to 15-mL tubes and centrifuged at 200×g for 5 min at 25 °C. After removing the culture supernatant, each cell pellet was suspended in 1 mL of freezing solution (solutions 1–6) and transferred to a 2-mL cryotube (Sumitomo Bakelite Co. Ltd., Tokyo, Japan). The cell pellet was suspended in 1 mL of maintenance medium, and the number of live cells and total cells before freezing was measured using a TC20™ Automated Cell Counter (Bio-Rad Laboratories, Hercules, CA, USA). Cryotubes were placed in a BICELL freezing treatment container (Nihon Freezer Co. Ltd., Tokyo, Japan) and frozen at −80 °C for 24 h. After 24 h, each frozen sample was transferred to liquid nitrogen and stored for 1–2 weeks.
2.8. Thawing procedures
Each stored sample was removed from liquid nitrogen and quickly thawed in a warm water bath at 37 °C, and 9 mL of maintenance medium was added to the thawed cell suspensions, followed by centrifugation at 200×g for 5 min at 25 °C. After removing the culture supernatant, each cell pellet was suspended in 1 mL of maintenance medium. The number of live cells and total cells after thawing were measured using a TC20 Automated Cell Counter. Cell viability and the live cell recovery rate before freezing and after thawing were calculated using equations A and B, respectively.
|
Cell viability lrb% = (number of live cells after thawing)/(total number of cells after thawing) × 100 |
(A) |
| Control | |
|
Cell viability before freezing lrb% = (number of live cells before freezing)/(total number of cells before freezing) × 100 |
|
|
Live cell recovery rate lrb% = (number of live cells after thawing) /(number of live cells immediately before freezing) × 100 |
(B) |
| The live cell recovery rate before freezing was set to 100 lrb%. | |
After thawing, live cells were seeded at a density of 1 × 105 cells in new dishes and cultured at 37 °C in a humidified 5% CO2 atmosphere. The morphology of the cultured cells was observed using a phase-contrast microscope.
2.9. Assessment of cell membrane damage using CFDA-AM assays
SK-N-SH cells (1 × 106) were seeded in a 100-mm culture dish in maintenance medium. The seeded cells were attached and spread on a dish and cultured overnight at 37 °C under a humidified 5% CO2 atmosphere. The cells were then incubated with maintenance medium supplemented with 4 μM CFDA-AM solution for 30 min at 37 °C under a humidified 5% CO2 atmosphere. Cell freezing and thawing were performed, as described above. The number of live cells before freezing and after thawing were evaluated under a fluorescence microscope, using cells at a density of 1 × 105 on a plate.
The number of total and live cells was determined using phase-contrast micrographs and fluorescence micrographs. The percentage of cell membrane damage was determined by measuring the fluorescence signal and cell size in SK-N-SH cells (equation C). Cell membrane damage was classified into three types based on the fluorescence signal difference. Cells stained green with high- and low-fluorescence signals were alive. On the other hand, the absence of fluorescence signal indicated dead cells due to considerable damage to cell membranes. Additionally, the size of the fluorescent cells was used to determine the extent of cell membrane damage. The cells in the phase-contrast micrographs were compared to those in the fluorescent images in the same field of view. Highly-fluorescent cells with equal sizes in both the micrographs were included in the high-fluorescence signal groups. The low-fluorescence cells of smaller sizes were classified into the low-fluorescence signal groups. The average cell diameter in phase-contrast micrographs was 15 μm, and images below 10 μm were not counted using NIH image analysis software.
|
% cell membrane damage lrb% = (number of cells with fluorescence signals)/(total number of cells) × 100 |
(C) |
Cell viability was determined by measuring the fluorescence signal in the SK-N-SH cells (equation D). Positive cells with high and low fluorescence signals were used as the live cells. No fluorescence was observed in dead cells.
| Cell viability lrb% = (number of positive cells)/(total number of cells after thawing) × 100 | (D) |
2.10. Statistical analyses
Data are presented as mean ± standard error. Each experiment was repeated in triplicate (n ≥ 3). Statistical significance was determined using the Welch's t-test. Statistical significance was set at P < 0.05.
3. Results
3.1. Experimental design for neuronal differentiation of SK-N-SH cells
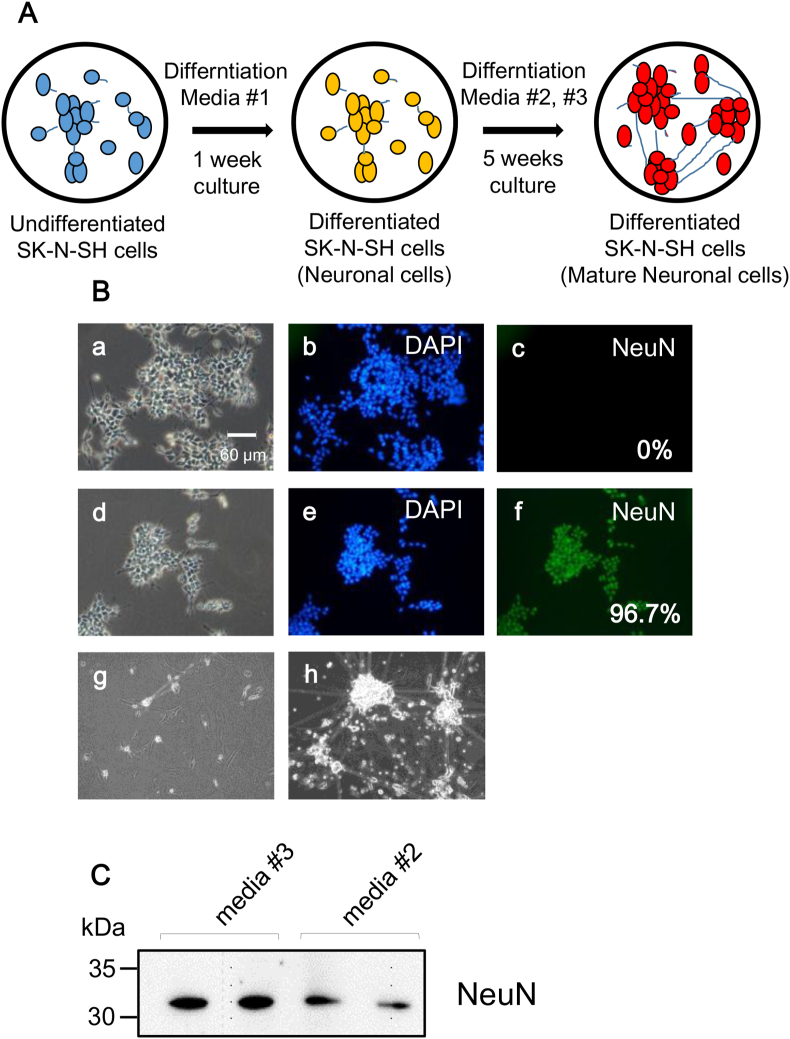
The differentiation of SK-N-SH cells into neuronal cells is shown in Fig. 1A. The undifferentiated SK-N-SH cells were differentiated into neuronal cells using the differentiation medium #1. The neuronal differentiation of SK-N-SH cells is shown in fluorescence micrographs (Fig. 1B). The expression of the neuronal marker NeuN in differentiated SK-N-SH cells was confirmed (Fig. 1B; d, e, and f). Undifferentiated SK-N-SH cells did not express the neuronal marker (Fig. 1B; a, b, and c). The percentages of differentiated SK-N-SH cells (NeuN-positive cells) before and after neural differentiation were 0% and 96.7%, respectively. The maturation of the differentiated SK-N-SH cells were induced using differentiation media #2 and #3 (Fig. 1A). The morphology of the mature neuronal cells demonstrated extensive and elongated neuritic projections (Fig. 1B, g, and h). The extent of maturation of the neuronal cells was more in differentiation medium #3 than that in differentiation medium #2, as indicated by the extensive network of neurites. NeuN protein expression was confirmed in the mature neuronal cells using immunoblotting (Fig. 1C). The expression of NeuN protein was higher in cells treated with differentiation media #3 than that in cells treated with differentiation media #2. Undifferentiated SK-N-SH cells did not express the neuronal marker (Fig. S2). Cell preparation and freezing of neuronal cells are challenging, because the cell viability and recovery rate of frozen mature neuronal cells is less than 10%. Therefore, we used neuronal cells differentiated using differentiation medium #1 in this study.
Fig. 1.
(A) Scheme of neural differentiation of SK-N-SH cells. Undifferentiated SK-N-SH cells were cultured and differentiated into neuronal cells for 1 week in differentiation media #1. Differentiated SK-N-SH cells were continuously cultured for 5 weeks by changing different neuronal differentiation media #2 and #3. Neuronal differentiation media: #1, maintenance medium supplemented with 3 μM all-trans-retinoic acid; #2, maintenance medium supplemented with 10 μM all-trans-retinoic acid; #3, neurobasal medium supplemented with B-27 supplements and 10 μM all-trans-retinoic acid. (B) The phase-contrast photomicrographs of undifferentiated (a) and differentiated SK-N-SH cells (d, g, and h). Undifferentiated SK-N-SH cells were used as control (a, b, and c). Neuronal differentiation of SK-N-SH cells was determined using a neuronal marker (NeuN; c and f) and DAPI (b, and e). Each photomicrographs of differentiated cells with differentiation media is differentiation media #1 (d, e, f), #2 (g), and #3 (h), respectively. Scale bar: 60 μm. (C) Immunoblotting with anti-NeuN polyclonal Abs in mature differentiated SK-N-SH cells. Each band was detected in differentiated cells with differentiation media #2 and #3, respectively.
3.2. Preparation of freezing solution and evaluation of cell freezing using HEK293 cells
After freezing and thawing using six freezing solutions, the cell viability and live cell recovery rate of HEK293 cells were compared (Fig. 2A and B). The six freezing solutions were prepared, as shown in Table 1. Solution 4 (containing 10% FBS and 10% DMSO) was used as the standard freezing solution. Cell viability and live cell recovery rate before freezing were 94.3 ± 1.6% and 100%, respectively (Fig. 2A and B). After thawing, the viability and recovery rate of HEK293 cells in the freezing solutions containing 10% DMSO (solutions 2, 4, and 5) were >86% and 75%, respectively (Fig. 2A and B). The viability and recovery rate of HEK293 cells in the freezing solutions containing 10% glycerol (solutions 1 and 3) were <53% and 40%, respectively (Fig. 2A and B). The viability and recovery rate of HEK293 cells were significantly different between the freezing solutions containing 10% DMSO and those containing 10% glycerol. In the solution containing 10% FBS (Solution 6), the viability and recovery rate of HEK293 cells were 1.4% and 0.6%, respectively (Fig. 2A and B).
Fig. 2.
The cell viability (A), the live cell recovery rate (B), and the phase-contrast photomicrographs (C) of cryopreserved HEK 293 cells with different freezing solutions (1)–(6). The composition of six freezing solutions was prepared using D-MEM. Solution 1. 1% sericin, 0.1 M maltose, and 10% glycerol; Solution 2. 1% sericin, 0.1 M maltose, and 10% DMSO; Solution 3. 10% FBS, and 10% glycerol; Solution 4. 10% FBS, and 10% DMSO; Solution 5. 10% DMSO; Solution 6. 10% FBS. The data settings were the cell viability (closed column) and the live cell recovery rate (open column). Scale bar: 60 μm. Data are presented as mean ± standard error. Each experiment was repeated in triplicate (n ≥ 3).
After freezing and thawing, HEK293 live cells were seeded at a density of 1 × 105 cells/well and cultured for 24 h at 37 °C under a humidified 5% CO2 atmosphere. Cell morphology was observed using phase-contrast microscopy (Fig. 2C). The morphology of cells stored in the freezing solution containing 10% DMSO (Fig. 2C, Solutions 2, 4, and 5) was similar to that of the cells stored in the freezing solution containing 10% glycerol (Fig. 2C, Solutions 1 and 3). In case of cells stored using the solution containing 10% FBS (solution 6), the cells were not attached to the dish.
3.3. Evaluation of cell freezing using undifferentiated SK-N-SH cells
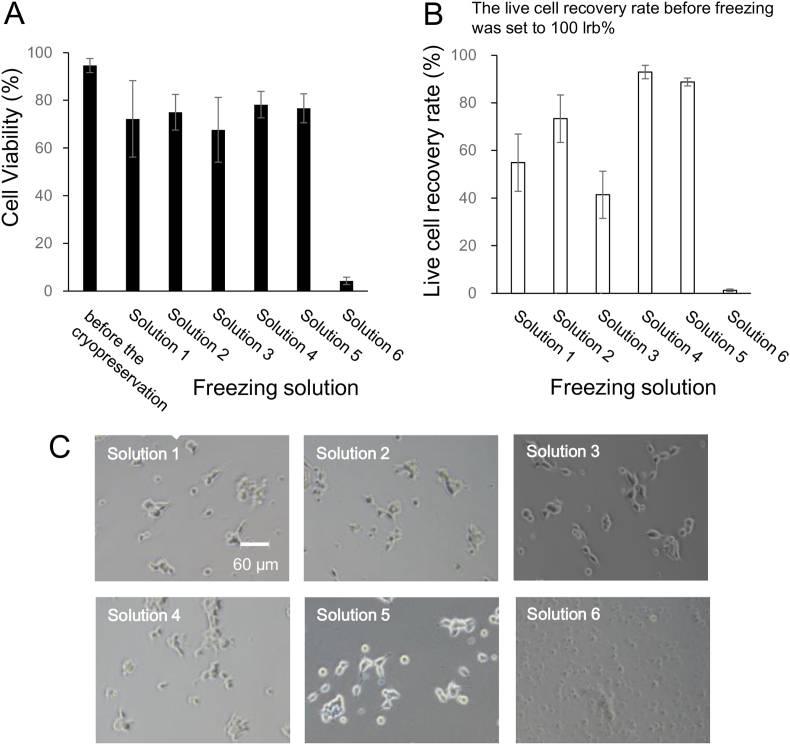
Based on the results obtained from HEK293 cells, the state of the undifferentiated and differentiated SK-N-SH cells after freezing and thawing was evaluated using six freezing solutions. The cell viability and live cell recovery rates before freezing were 94.6% and 100%, respectively (Fig. 3A and B). After thawing, the viability of SK-N-SH cells in the freezing solutions containing 10% DMSO (solutions 2, 4, and 5) and 10% glycerol (solutions 1 and 3) was 75%–78% and 67%–72%, respectively (Fig. 3A). The recovery rate of SK-N-SH cells in the freezing solution containing 10% DMSO (solutions 2, 4, and 5) was >73% (Fig. 3B), and that in the freezing solution containing 10% glycerol (solutions 1 and 3) was <54% (Fig. 3B).
Fig. 3.
The cell viability (A), the live cell recovery rate (B), and the phase-contrast photomicrographs (C) of cryopreserved undifferentiated SK-N-SH cells with different freezing solutions (1)–(6). The composition of six freezing solutions was prepared using D-MEM. Solution 1. 1% sericin, 0.1 M maltose, and 10% glycerol; Solution 2. 1% sericin, 0.1 M maltose, and 10% DMSO; Solution 3. 10% FBS, and 10% glycerol; Solution 4. 10% FBS, and 10% DMSO; Solution 5. 10% DMSO; Solution 6. 10% FBS. The data settings were the cell viability (closed column) and the live cell recovery rate (open column). Scale bar: 60 μm. Data are presented as mean ± standard error. Each experiment was repeated in triplicate (n ≥ 3).
The viability and recovery rates of SK-N-SH cells were significantly different between the freezing solutions containing 10% FBS, 10% DMSO, and the freezing solution containing 10% FBS and 10% glycerol. In the 10% FBS solution (Solution 6), the viability and recovery rate of SK-N-SH cells were 4.3% and 1.3%, respectively (Fig. 3A and B).
After freezing and thawing, SK-N-SH live cells were seeded at a density of 1 × 105 cells/well and cultured for 24 h at 37 °C under a humidified 5% CO2 atmosphere. The morphology of the cultured cells was observed using a phase-contrast microscope (Fig. 3C). There was no difference in the morphology of SK-N-SH cells stored in the various freezing solutions (solutions 1, 2, 3, 4, and 5). However, in case of cells stored in the solution with 10% FBS solution (solution 6), the cells were not attached after 24 h of incubation.
3.4. Evaluation of cell membrane damage using undifferentiated SK-N-SH cells
To investigate cell membrane damage during the freezing and thawing processes, the cell status after thawing was evaluated using the 5-CFDA-AM assay. 5-CFDA-AM is a cell-permeant esterase substrate; cell membrane integrity is required for the intracellular retention of the fluorescent product. The extracellular release of the fluorescent product is an index for evaluating cell membrane damage.
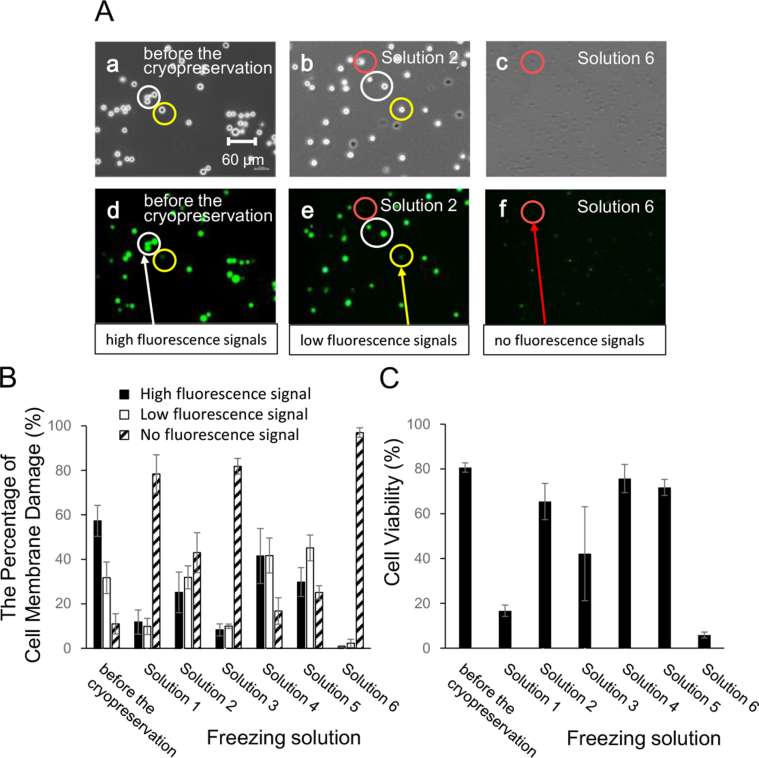
Fig. 4A shows representative fluorescence micrographs (undifferentiated SK-N-SH cells before the cryopreservation; solution 2: 1% sericin, 0.1 M maltose, and 10% DMSO; solution 6: 10% FBS) of 5-CFDA-AM-stained cells after thawing. In the 5-CFDA-AM-stained cells before freezing, high-intensity fluorescence signals were observed (white circle: high-fluorescence signals), and no dead cells were observed in the phase-contrast micrographs (Fig. 4A; undifferentiated SK-N-SH cells before cryopreservation: a, and d). In the 5-CFDA-AM-stained cells after thawing, there was a change in the fluorescence signals, indicating cell membrane damage (yellow circle: low-fluorescence signals). Dead cells (red circle: no fluorescence signals) were observed in phase-contrast and fluorescence micrographs (Fig. 4A; solution 2: b, and e). In the 5-CFDA-AM-stained cells stored in solution 6, after thawing, there were no viable cells or fluorescence signals, and the shape and size of the cells did not match the fluorescence signals. Dead cells (red circle: no fluorescence signals) were observed in phase-contrast and fluorescence micrographs (Fig. 4A; solution 6: c, and f).
Fig. 4.
Cell membrane damage using undifferentiated SK-N-SH cells. (A) The phase-contrast photomicrographs (a, b, and c) of cryopreserved undifferentiated SK-N-SH cells with the different freezing solutions. The fluorescence signal of 5-CFDA-AM-stained cells was observed by fluorescence microscopy (d, e, and f). Each photomicrograph is undifferentiated SK-N-SH cells before the cryopreservation (a, and d), solution 2 (b, and e), solution 6 (c, and f), respectively. Undifferentiated SK-N-SH cells before the cryopreservation were used as control. The fluorescence signal settings were as follows: high (white circle), low (yellow circle), and no fluorescence signals (red circle). Scale bar: 60 μm. The percentage of cell membrane damage (B) and the cell viability (C) of cryopreserved undifferentiated SK-N-SH cells with different freezing solutions (1)–(6). The composition of six freezing solutions were prepared using D-MEM. Solution 1. 1% sericin, 0.1 M maltose, and 10% glycerol; Solution 2. 1% sericin, 0.1 M maltose, and 10% DMSO; Solution 3. 10% FBS, and 10% glycerol; Solution 4. 10% FBS, and 10% DMSO; Solution 5. 10% DMSO; Solution 6. 10% FBS. The cell membrane damage settings (B) were as follows: high (closed column), low (open column), and no fluorescence signals (hatched column). The cell viability settings (C) were as follows: high and low fluorescence signals (viable cells), and no fluorescence signals (dead cells). Data are presented as mean ± standard error. Each experiment was repeated in triplicate (n ≥ 3).
The percentage of cell membrane damage was determined by measuring the fluorescence signal in the SK-N-SH cells (Fig. 4B). The percentage of high- and low-fluorescence signals before freezing were 57.3% and 31.7%, respectively (Fig. 4B). After thawing of the cells, the percentages of high-fluorescence signals in the cells stored with freezing solution containing 10% DMSO (solutions 2, 4, and 5) and 10% glycerol (Solution 1, and 3) were 25%–42% and 8%–12%, respectively (Fig. 4B). In cells stored with solution 6 containing 10% FBS, the percentage of the fluorescence signal was 0.8%.
Cell viability before and after freezing was determined using the fluorescence signals (Fig. 4C). Live and dead cells were identified as cells with fluorescence signals (high and low intensity) and no fluorescence, respectively. Cell viability before freezing was 80.6 ± 2.1% (Fig. 4C). After thawing, the viability of the cells in the freezing solution containing 10% DMSO (solutions 2, 4, and 5) and 10% glycerol (solutions 1 and 3) was 65%–76% and 16%–43%, respectively (Fig. 4C). The viability was significantly different between cells stored in the freezing solutions containing 10% DMSO (solutions 2, 4, and 5) and that stored in the freezing solutions containing 10% glycerol (solutions 1 and 3). In solution 6 containing 10% FBS, the cell viability was 6% (Fig. 4C).
3.5. Evaluation of cell freezing using differentiated SK-N-SH cells
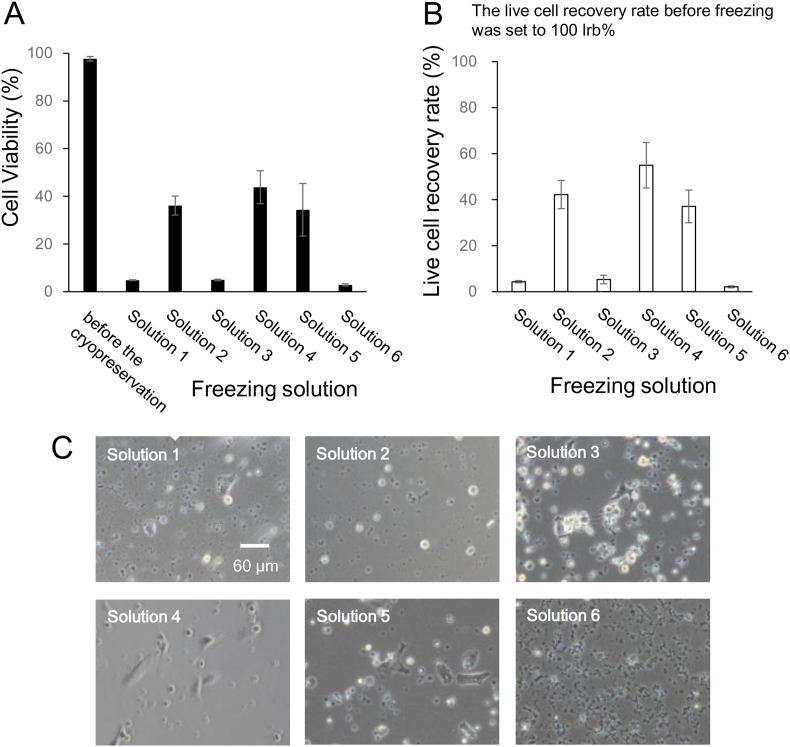
To investigate the effect of SK-N-SH cell differentiation on cryopreservation, undifferentiated SK-N-SH cells (Fig. 1B; Solutions 1, 2, and 3) were differentiated (neuronal cells, Fig. 1B; Solutions 4, 5, and 6) using all-trans-retinoic acid. Cell viability and the living cell recovery rate before freezing were 97.7 ± 0.9% and 100%, respectively (Fig. 5A and B). After thawing, the viability and the recovery rate of differentiated SK-N-SH cells stored in the freezing solutions containing 10% DMSO (solutions 2, 4, and 5) were 34%–44% and 37%–55%, respectively (Fig. 5A and B). The viability and recovery rate of differentiated SK-N-SH cells stored in the freezing solutions containing 10% glycerol (solutions 1 and 3) were <5% and 6%, respectively (Fig. 5A and B). The viability and recovery rate were significantly different between the cells stored in freezing solutions containing 10% DMSO (solutions 2, 4, and 5) and that stored in solutions containing 10% glycerol (solutions 1 and 3). In cells stored in solution 6, which contained 10% FBS, the viability and recovery rate were 2.9% and 2.1%, respectively (Fig. 5A and B).
Fig. 5.
The cell viability (A), the live cell recovery rate (B), and the phase-contrast photomicrographs (C) of cryopreserved differentiated SK-N-SH cells with different freezing solutions (1)–(6). The composition of six freezing solutions was prepared using D-MEM. Solution 1. 1% sericin, 0.1 M maltose, and 10% glycerol; Solution 2. 1% sericin, 0.1 M maltose, and 10% DMSO; Solution 3. 10% FBS, and 10% glycerol; Solution 4. 10% FBS, and 10% DMSO; Solution 5. 10% DMSO; Solution 6. 10% FBS. The data settings were the cell viability (closed column) and the live cell recovery rate (open column). Scale bar: 60 μm. Data are presented as mean ± standard error. Each experiment was repeated in triplicate (n ≥ 3).
After freezing and thawing, the differentiated SK-N-SH live cells were seeded at a density of 1 × 105 cells/well and cultured for 24 h at 37 °C under a humidified 5% CO2 atmosphere. The morphology of the cultured cells was observed using a phase-contrast microscope (Fig. 5C). There was no difference in the morphology of differentiated SK-N-SH cells stored in the various freezing solutions (solutions 1, 2, 3, 4, and 5). In case of cells stored in solution 6, containing 10% FBS, the differentiated SK-N-SH cells were not attached to the plastic dish after 24 h of incubation.
4. Discussion
This study proposes a more effective freezing solution for the freezing and thawing of cells. Specifically, in damage-sensitive cells, such as differentiated cells (neurons, and cardiomyocytes) and parenchymal cells [[2], [3], [4], [5]], it is possible to (1) improve cell quality, (2) ensure cell safety, and (3) supply large quantities of quality-controlled cells. In this study, we used undifferentiated and differentiated SK-N-SH neuronal cells as models for damage-sensitive cells. We efficiently supplied undifferentiated and differentiated neuronal cells using serum-free freezing solutions.
4.1. Differentiation of undifferentiated SK-N-SH cells into neuronal cells and timing of cell freezing
Tissue stem cells, embryonic stem (ES) cells [37,38], and induced pluripotent stem (iPS) cells [39,40] can be differentiated into target cells to enable effective cell therapy [41]. However, differentiation into target cells is time-consuming and expensive. Optimizing a cell freezing and thawing process for the differentiated cells would enable the supply of target cells at short notice. We induced the maturation of SK-N-SH cells in two steps: the first step, using differentiation media #1, we confirmed the differentiation of SK-N-SH cells using the neuronal marker, NeuN [35]; in the second step, when using differentiation media #2 and #3, the morphology of the differentiated neuronal cells showed an extensive network of neurites and they expressed NeuN (Fig. 1). The cell viability and recovery rates after cell freezing and thawing decreased as neuronal differentiation and maturation progressed. In particular, it is difficult to maintain the number and quality of cells because the recovery rate of frozen mature differentiated cells is low (less than 10%) (data not shown). Therefore, in this study, the differentiated neuronal cells were frozen after the first step of neural differentiation.
4.2. Effect of different freezing solutions on undifferentiated and differentiated SK-N-SH cells
Standard HEK293 cells were used for evaluating the freezing solutions. After thawing, the viability and recovery rate of HEK293 cells in the freezing solutions containing 10% DMSO (solutions 2, 4, and 5) were high, and the cell morphology was similar to that before freezing. There was no significant difference between the freezing solutions containing 10% DMSO (solutions 2 and 5) and the standard freezing solution containing 10% DMSO and 10% FBS (Solution 4). Compared with that in the freezing solution containing 10% DMSO and 10% glycerol, the viability and the recovery rate of cells stored in 10% DMSO after thawing were 1.6- and 1.8-times higher (Fig. 2A and B), respectively; there were no differences in cell morphology after thawing (Fig. 2C). These results indicated that DMSO penetrated the cells more efficiently than glycerol.
The freezing solutions were evaluated using undifferentiated SK-N-SH cells. There was no significant difference between the viability of undifferentiated SK-N-SH cells stored in freezing solutions containing 10% DMSO and in that with 10% glycerol. However, there was a significant difference between the recovery rate of undifferentiated SK-N-SH cells stored in freezing solutions containing 10% DMSO and in that with 10% glycerol. The live cell recovery rates of the freezing solutions containing 10% DMSO were higher than that of solutions containing 10% glycerol (>73% vs. <54%, respectively).
Undifferentiated SK-N-SH cells were used to evaluate cell membrane damage during the freezing and thawing processes. Cell membrane damage was classified into three types based on the fluorescence signals and cell sizes (Fig. 4A). The percentage of cell membrane damage was determined using fluorescence signals in the undifferentiated SK-N-SH cells (Fig. 4B). High and no fluorescence signals indicated live and dead cells, respectively. A low-fluorescence signal because of the release of the fluorescent products, indicated cell membrane damage, which ultimately leads to cell death by apoptosis. Compared to solution 4 (containing 10% DMSO and 10% FBS), solution 5 (containing 10% DMSO) increased the percentage of cell membrane damage. Therefore, extracellular protectants such as FBS and sericin [3,29,34] are effective in protecting cell membranes during freezing and thawing. Subsequently, the cell viability after freezing and thawing was calculated using 5-CFDA-AM assays [42]. High- and low-fluorescence signals were counted as live cells (Fig. 5A). There was no significant difference between the assessed cell viability using the trypan blue exclusion test (Fig. 3A) and the 5-CFDA-AM assay (Fig. 4C).
The freezing solutions were evaluated using differentiated SK-N-SH cells. The viability and recovery rate of differentiated SK-N-SH cells was reduced by approximately 1.5-times compared to that of undifferentiated SK-N-SH cells. The viability and recovery rate of the differentiated SK-N-SH cells were significantly different between the freezing solutions containing 10% DMSO and the freezing solutions containing 10% glycerol (Fig. 5A and B). The morphology of the differentiated SK-N-SH cells after thawing was similar to that before freezing. There was no difference in the morphology of the differentiated SK-N-SH cells stored in different freezing solutions (solutions 1, 2, 3, 4, and 5).
We examined the survival rate, membrane damage rate, and recovery rate of undifferentiated and differentiated SK-N-SH cells after freeze-thawing using different freezing solutions. DMSO has antioxidant properties; it reduces oxidative stress [43] and inhibits the formation of ice crystals during cell freezing. Disaccharides such as trehalose, sucrose, and maltose stabilize cell membranes and proteins as water substitutes [44,45]. It is necessary to develop a serum-free (or animal component-free) freezing solution to eliminate mycoplasma, bovine spongiform encephalopathy, and other viruses for ensuring safe cell medical treatment [23,24,46,47]. Therefore, we used a less toxic freezing solution with DMSO, sericin, and maltose combinations. Cryoprotection using this freezing solution was effective in undifferentiated SK-N-SH cells, but not in differentiated SK-N-SH cells. The survival and recovery rates of the DMSO-based freezing solutions were higher than that of the glycerol-based freezing solutions. This study used slow freezing with a DMSO-containing freezing solution for undifferentiated and differentiated SK-N-SH cells. Slow freezing is very convenient for cryopreserving a large number of cells simultaneously. However, ice crystal formation occurs throughout the cells during the freezing process if the cells are not sufficiently dehydrated. It is necessary to investigate vitrification and slow freezing with chemically defined freezing solutions (such as ethylene glycol, polyethylene glycol) without DMSO [27,31]. Damage-sensitive cells could be recovered by devising a suitable freezing strategy and optimizing the composition of the freezing solution.
This study proposes an effective freezing solution using slow freezing for cryopreservation. More differentiated neuron-like cells and parenchymal cells are damage-sensitive cells compared to stem cells (iPSCs, ESCs, and tissue stem cells). Therefore, cryoprotectants such as FBS, antifreeze proteins (sericin), and sugars (maltose) are important in protecting against freeze damage in these damage-sensitive cells. Serum-free alternatives are necessary to increase the safety during cell transplantation and regenerative medicine. Neuronal cells obtained by neuronal differentiation are expected to be used for screening compounds in drug development. We efficiently supplied undifferentiated and differentiated neuronal cells with serum-free freezing solutions supplemented with sericin, maltose, and 10% DMSO using slow freezing. In the future, we will examine the effective cryopreservation of differentiated neurons and glial cells from human stem cells using slow freezing and other freezing methods.
5. Conclusions
This study determined the appropriate timing during cell differentiation for freezing and proposed a more effective freezing solution for more differentiated neuronal cells. It is possible to improve cell quality and ensure the safety of damage-sensitive cells, such as differentiated neuron-like cells. We efficiently supplied undifferentiated and differentiated neuronal cells with serum-free freezing solutions supplemented with sericin, maltose, and 10% DMSO using slow freezing.
Author contributions
Conceptualization, K.Y., Y.N., and Y.M.; methodology, K.Y., Y.N., N.T., W.K., K.M., K.N., T.Y., and Y.M.; investigation, K.Y., Y.N., N.T., W.K., K.M., K.N., T.Y., and Y.M.; resources, K.Y. and Y.M.; data curation, K.Y., Y.N., N.T., W.K., and Y.M.; writing—original draft preparation, K.Y. and Y.M.; writing—review and editing, K.Y., W.K., K.M., and Y.M.; visualization, K.Y., Y.N., W.K., and Y.M.; supervision, K.Y. and Y.M.; project administration, Y.M.; funding acquisition, K.Y. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by JSPS KAKENHI Grant Numbers JP18H03556, JP15H03039, JP16K11121, and JP20K09608.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We greatly appreciate the support of the students at the Tokyo University of Science for supporting our experiments. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2021.12.007.
Contributor Information
Kenji Yamatoya, Email: k.yamatoya.fh@juntendo.ac.jp.
Yuya Nagai, Email: 6417636@gmail.com.
Naozumi Teramoto, Email: teramoto.naozumi@it-chiba.ac.jp.
Woojin Kang, Email: kwjbear@gmail.com.
Kenji Miyado, Email: miyado-k@ncchd.go.jp.
Kazuya Nakata, Email: nakata@go.tuat.ac.jp.
Tohru Yagi, Email: yagi.t.ab@m.titech.ac.jp.
Yoshitaka Miyamoto, Email: miyamoto-ys@ncchd.go.jp, myoshi1230@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Miyamoto Y., Ikeuchi M., Noguchi H., Hayashi S. Long-term cryopreservation of human and other mammalian cells at -80 °C for 8 years. Cell Med. 2018;10 doi: 10.1177/2155179017733148. 2155179017733148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyamoto Y., Suzuki S., Nomura K., Enosawa S. Improvement of hepatocyte viability after cryopreservation by supple-mentation of long-chain oligosaccharide in the freezing medium in rats and humans. Cell Transplant. 2006;15(10):911–919. doi: 10.3727/000000006783981404. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto Y., Teramoto N., Hayashi S., Enosawa S. An improvement in the attaching capability of cryopreserved human hepatocytes by a proteinaceous high molecule, sericin, in the serum-free solution. Cell Transplant. 2010;19(6):701–706. doi: 10.3727/096368910X508799. [DOI] [PubMed] [Google Scholar]
- 4.Ladewig J., Koch P., Endl E., Meiners B., Opitz T., Couillard-Despres S., et al. Lineage selection of functional and cryopreservable human embryonic stem cell-derived neurons. Stem Cell. 2008;26(7):1705–1712. doi: 10.1634/stemcells.2008-0007. [DOI] [PubMed] [Google Scholar]
- 5.Xu C., Police S., Hassanipour M., Li Y., Chen Y., Priest C., et al. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med. 2011;6(1):53–66. doi: 10.2217/rme.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todo S., Nery J., Yanaga K., Podesta L., Gordon R.D., Starzl T.E. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261(5):711–714. [PMC free article] [PubMed] [Google Scholar]
- 7.Nasralla D., Coussios C.C., Mergental H., Akhtar M.Z., Butler A.J., Ceresa C.D.L., et al. Consortium for Organ Preservation in Europe. A randomized trial of nor-mothermic preservation in liver transplantation. Nature. 2018;557(7703):50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 8.Sugai K., Sumida M., Shofuda T., Yamaguchi R., Tamura T., Kohzuki T., et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen Ther. 2021;18:321–333. doi: 10.1016/j.reth.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigor'eva E.V., Malankhanova T.B., Surumbayeva A., Pavlova S.V., Minina J.M., Kizilova E.A., et al. Generation of GABAergic striatal neurons by a novel iPSC differentiation protocol enabling scalability and cryopreservation of progenitor cells. Cytotechnology. 2020;72(5):649–663. doi: 10.1007/s10616-020-00406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazur P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J Gen Physiol. 1963;47(2):347–369. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazur P., Leibo S.P., Chu E.H. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71(2):345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson J.O., Cravalho E.G., Borel Rinkes I.H., Tompkins R.G., Yarmush M.L., Toner M. Nucleation and growth of ice crystals inside cultured hepatocytes during freezing in the presence of dimethyl sulfoxide. Biophys J. 1993;65(6):2524–2536. doi: 10.1016/S0006-3495(93)81319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polge C., Smith A.U., Parkes A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 14.Lovelock J.E., Bishop M.W. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183(4672):1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- 15.Anchordoguy T., Crowe J.H., Griffin F.J., Clark W.H., Jr. Cryopreservation of sperm from the marine shrimp Sicyonia ingentis. Cryobiology. 1988;25(3):238–243. doi: 10.1016/0011-2240(88)90031-4. [DOI] [PubMed] [Google Scholar]
- 16.George M.A., Johnson M.H. Cytoskeletal organization and zona sensitivity to digestion by chymotrypsin of frozen-thawed mouse oocytes. Hum Reprod. 1993;8(4):612–620. doi: 10.1093/oxfordjournals.humrep.a138106. [DOI] [PubMed] [Google Scholar]
- 17.Crowe J.H., Crowe L.M. Preservation of mammalian cells-learning nature's tricks. Nat Biotechnol. 2000;18(2):145–146. doi: 10.1038/72580. [DOI] [PubMed] [Google Scholar]
- 18.Christopher B.M. In: Cryopreservation and freeze-drying protocols. 2nd ed. John G.D., Glyn N.S., editors. Humana Press; Totowa, New Jersey, United States: 2007. Cryopreservation of animal and human cell lines; pp. 227–236. [Google Scholar]
- 19.Orkin S.H., Harosi F.I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler S., Pellizzer C., Paparella M., Hartung T., Bremer S. The effects of solvents on embryonic stem cell differentiation. Toxicol In Vitro. 2006;20(3):265–271. doi: 10.1016/j.tiv.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 21.Yu H.N., Lee Y.R., Noh E.M., Lee K.S., Song E.K., Han M.K., et al. Tumor necrosis factor-alpha enhances DMSO-induced differentiation of HL-60 cells through the activation of ERK/MAPK pathway. Int J Hematol. 2008;87(2):189–194. doi: 10.1007/s12185-008-0037-z. [DOI] [PubMed] [Google Scholar]
- 22.Hanslick J.L., Lau K., Noguchi K.K., Olney J.W., Zorumski C.F., Mennerick S., et al. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol Dis. 2009;34(1):1–10. doi: 10.1016/j.nbd.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leunda A., Van Vaerenbergh B., Baldo A., Roels S., Herman P. Laboratory activities involving transmissible spongiform encephalopathy causing agents: risk assessment and biosafety recommendations in Belgium. Prion. 2013;7(5):420–433. doi: 10.4161/pri.26533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merten O.W. Virus contaminations of cell cultures - a biotechnological view. Cytotechnology. 2002;39(2):91–116. doi: 10.1023/A:1022969101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujioka T., Yasuchika K., Nakamura Y., Nakatsuji N., Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2004;48(10):1149–1154. doi: 10.1387/ijdb.041852tf. [DOI] [PubMed] [Google Scholar]
- 26.Ohnuki M., Takahashi K., Yamanaka S. Generation and characterization of human induced pluripotent stem cells. Curr Protoc Stem Cell Biol. 2009;9:4A.2.1–4A.2.25. doi: 10.1002/9780470151808.sc04a02s9. [DOI] [PubMed] [Google Scholar]
- 27.Nishigaki T., Teramura Y., Suemori H., Iwata H. Cryopreservation of primate embryonic stem cells with chemically-defined solution without Me2SO. Cryobiology. 2010;60(2):159–164. doi: 10.1016/j.cryobiol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto Y., Noguchi H., Yukawa H., Oishi K., Matsushita K., Iwata H., et al. Cryopreservation of induced pluripotent stem cells. Cell Med. 2012;3(1–3):89–95. doi: 10.3727/215517912X639405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto Y., Oishi K., Yukawa H., Noguchi H., Sasaki M., Iwata H., et al. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant. 2012;21(2–3):617–622. doi: 10.3727/096368911X605556. [DOI] [PubMed] [Google Scholar]
- 30.Seremak J., Eroglu A. Chemically defined and xeno-free cryopreservation of human-induced pluripotent stem cells. Methods Mol Biol. 2021;2180:569–579. doi: 10.1007/978-1-0716-0783-1_29. [DOI] [PubMed] [Google Scholar]
- 31.Gilfanova R., Callegari A., Childs A., Yang G., Luarca M., Gutierrez A.G., et al. A bioinspired and chemically defined alternative to dimethyl sulfoxide for the cryopreservation of human hematopoietic stem cells. Bone Marrow Transplant. 2021;56(11):2644–2650. doi: 10.1038/s41409-021-01368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y., Foote R.H., Brockett C.C. Effect of sucrose, trehalose, hypotaurine, taurine, and blood serum on survival of frozen bull sperm. Cryobiology. 1993;30(4):423–431. doi: 10.1006/cryo.1993.1042. [DOI] [PubMed] [Google Scholar]
- 33.Shitole M., Dugam S., Tade R., Nangare S. Pharmaceutical applications of silk sericin. Ann Pharm Fr. 2020;78(6):469–486. doi: 10.1016/j.pharma.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki M., Kato Y., Yamada H., Terada S. Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol Appl Biochem. 2005;42:183–188. doi: 10.1042/BA20050019. [DOI] [PubMed] [Google Scholar]
- 35.Pizzi M., Boroni F., Bianchetti A., Moraitis C., Sarnico I., Benarese M., et al. Expression of functional NR1/NR2B-type NMDA receptors in neuronally differentiated SK-N-SH human cell line. Eur J Neurosci. 2002;16(12):2342–2350. doi: 10.1046/j.1460-9568.2002.02403.x. [DOI] [PubMed] [Google Scholar]
- 36.Harasym E., McAndrew N., Gomez G. Sub-micromolar concentrations of retinoic acid induce morphological and functional neuronal phenotypes in SK-N-SH neuroblastoma cells. In Vitro Cell Dev Biol Anim. 2017;53(9):798–809. doi: 10.1007/s11626-017-0190-x. [DOI] [PubMed] [Google Scholar]
- 37.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 38.Reubinoff B.E., Pera M.F., Fong C.Y., Trounson A., Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by de-fined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 42.Schirmer K., Chan A.G., Greenberg B.M., Dixon D.G., Bols N.C. Methodology for demonstrating and measuring the photocytotoxicity of fluoranthene to fish cells in culture. Toxicol In Vitro. 1997;11(1–2):107–119. doi: 10.1016/s0887-2333(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 43.Iida C., Fujii K., Koga E., Washino Y., Ichi I., Kojo S. Inhibitory effect of dimethyl sulfoxide (DMSO) on necrosis and oxidative stress caused by D-galactosamine in the rat liver. J Nutr Sci Vitaminol. 2007;53(2):160–165. doi: 10.3177/jnsv.53.160. [DOI] [PubMed] [Google Scholar]
- 44.Womersley C., Uster P.S., Rudolph A.S., Crowe J.H. Inhibition of dehydration-induced fusion between liposomal membranes by carbohydrates as measured by fluorescence energy transfer. Cryobiology. 1986;23(3):245–255. doi: 10.1016/0011-2240(86)90050-7. [DOI] [PubMed] [Google Scholar]
- 45.Eroglu A., Russo M.J., Bieganski R., Fowler A., Cheley S., Bayley H., et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol. 2000;18(2):163–167. doi: 10.1038/72608. [DOI] [PubMed] [Google Scholar]
- 46.Bielanski A., Algire J., Randall G.C., Surujballi O. Risk of transmission of Mycobacterium avium ssp. paratuberculosis by embryo transfer of in vivo and in vitro fertilized bovine embryos. Theriogenology. 2006;66(2):260–266. doi: 10.1016/j.theriogenology.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Bielanski A., Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24(10):2457–2467. doi: 10.1093/humrep/dep117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.