Abstract
Introduction
Inflammatory pseudo-tumour (IPT) of the colon is a rare entity with an obscure pathophysiology and largely indeterminate aetiology.
Presentation of case
A young male patient presented with an Alvarado score of 9/10 and was admitted for appendectomy. An irregular hepatic flexure mass was discovered intraoperatively. The patient underwent an oncological right hemicolectomy with lymphadenectomy under the supposition that it was malignant and recovered with no short or long-term repercussions. Haemoxylin and eosin staining of the mass revealed features of a benign IPT.
Discussion
IPTs have clinical and radiological features that may be indistinguishable from those of malignancies, often resulting in extensive oncological resections despite recurrence and malignant transformation being negligibly rare.
Conclusion
Benign pathologies such as IPT that mimic malignancy can sometimes result in extensive investigations or radical resections, the justification of which can only be a point of contention in retrospect. The following report explores our experience with one such patient and is accompanied by a review of the literature.
Abbreviations: IPT, inflammatory pseudo-tumour; GIT, gastrointestinal tract; CT, computed tomography
Keywords: Inflammatory pseudo-tumour, Colonic mass, Hepatic flexure tumour, Colorectal surgery, General surgery
Highlights
-
•
Inflammatory pseudotumours are often mistaken for malignant lesions
-
•
This error can result in unnecessary resection being performed
-
•
We investigated whether radiography can diagnose inflammatory pseudotumours
-
•
Our findings reveal that radiography may not be effective in this context
-
•
Further studies may be required to prevent unnecessarily extensive resection
1. Introduction
This case report complies with the SCARE guidelines and has been reported in line with the SCARE checklist [1]. Inflammatory pseudo-tumours (IPTs) are rare mesenchymal masses that occur in <0.0001% of the population [2], [3], [4]. First described as IPTs in 1954 by Uniker and Iversion [4], these tumours are benign lesions associated with non-specific inflammatory cellular infiltration with spindle-cell proliferation and are often reactive, infective, or immunological in nature [5]. They have been described predominantly in the lungs to date; however, they may occur in any anatomical location, mostly in children and young adults [2], [3], [4]. Alimentary tract IPTs were first described in the stomach; however, they remain exceedingly rare, particularly in the colon, where their aetiology remains unclear [2], [3], [6]. IPTs are radiologically indistinguishable from malignant lesions, which can result in unnecessary oncological resections [6]. We report the case of a young South African man with an IPT of the colon that mimicked acute appendicitis on clinical presentation and a neoplastic lesion on macroscopic examination.
2. Presentation of case
A 29-year-old man who was previously well and had no comorbidities presented to the emergency department one night in Johannesburg, South Africa. He had a 3-day history of migratory pain extending peri-umbilically to the right lower quadrant that caused anorexia, nausea, and vomiting. There was no significant family- or social history. The patient appeared acutely unwell on examination and exhibited mild tachycardia and a body temperature of 38.4 °C. Abdominal examination revealed right lower quadrant tenderness and features of localized peritonitis. No palpable abdominal mass was visible at the time, and the results of the remaining systemic examination were unremarkable.
Chest and abdominal radiography revealed no significant findings. Significant blood results included an elevated C-reactive protein level of 227 mg/L (reference range: <10 mg/L) and leukocytosis (white cell count, 16.01 × 109/L; reference range: 3.92–10.40). The patient was determined to have acute appendicitis as his Alvarado score was 9/10. After-hours point of care sonography, computed tomography, and laparoscopic equipment were not available at the hospital due to limited resources. As such, he was admitted and underwent open emergency appendectomy on clinical grounds within an hour after presentation.
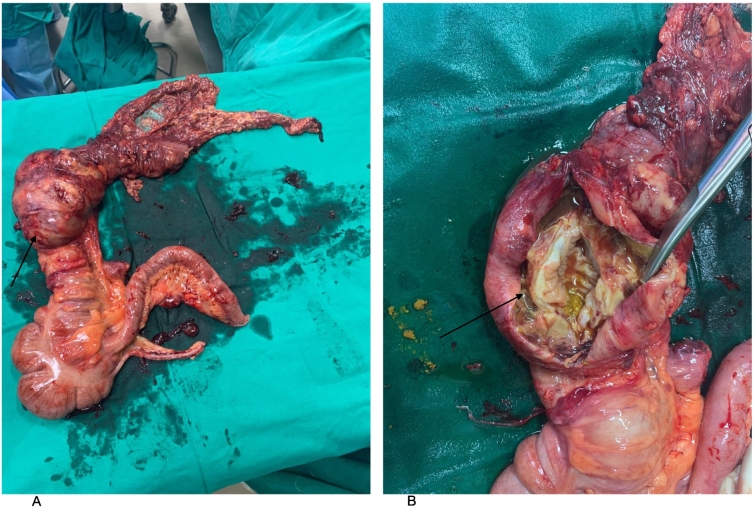
The patient was taken to operation theatre. The procedure was performed by a senior general surgery resident under the supervision of a general surgery consultant. A McBurney's incision was made intraoperatively in order to complete a limited appendectomy. No appendicular pathology was observed. A large, cystic mass, approximately 10 cm in length and 8 cm in width, was found in the hepatic flexure of the colon along with enlarged ileocolic, paracolic, and pre-caecal nodes (Fig. 1). The incision was extended to allow complete right hemicolectomy, lymphadenectomy, and end-to-end ileo-colic anastomosis. There were no peritoneal or liver deposits, and no other intra-abdominal pathologies were noted.
Fig. 1.
The right hemicolectomy specimen
A: Specimen in an anatomical position. Black arrow showing hepatic flexure mass measuring 98 × 59 × 34 mm.
B: Black arrow indicating an incision into the mass, demonstrating subserosal and cystic quality.
Gross examination of the right hemicolectomy specimen revealed a subserosal-based caecal tumour measuring 98 × 59 × 34 mm. The tumour had a central cystic cavity surrounded by a firm yellow-white mass (Fig. 2) that was located 89 mm from the distal resection margin and 179 mm from the proximal resection margin. The rest of the bowel was unremarkable.
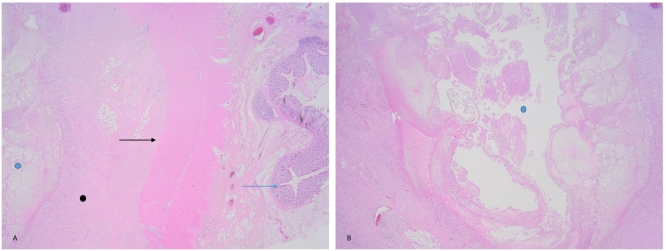
Fig. 2.
Photomicrograph of the resected specimen.
A: The blue arrow represents the colonic mucosa, and the black arrow, the muscularis propria. The tumour is represented by the black dot and is located in the adventitia [Haematoxylin and eosin (H&E) stain ×20].
B: The tumour has a central cystic cavity represented by the blue dot on A (H&E ×20). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Microscopically, a solitary tumour with suppurative and fibroblastic/myofibroblastic patterns was identified. The centre of the tumour had an abscess surrounded by granulation tissue and a proliferation of fibroblasts and myofibroblasts. The background was collagenous. Scattered inflammatory cells comprising lymphocytes, plasma cells, and eosinophils were present (Fig. 3). No definitive specific micro-organisms were identified on routine and special staining. Approximately 11 reactive lymph nodes accompanied the specimen with no malignant infiltration. A final diagnosis of IPT was made.
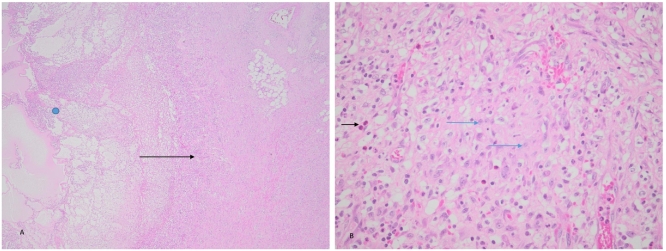
Fig. 3.
Photomicrograph of the inflammatory pseudo-tumour.
A The centre of the tumour has a central cystic cavity (blue dot) containing fibrin, neutrophils and foreign material (food). The cavity is surrounded by granulation tissue as shown by the black arrow (H&E stain ×100).
B Higher magnification of the tumour showing a fibroblastic/myofibroblastic proliferation of cells deposited in a collagenous background (blue arrow). There is a prominent admixture of lymphocytes, eosinophils (black arrow) and plasma cells (H&E stain ×400). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Postoperatively, the patient remained stable in the ward. Early feeding was initiated, and he was subsequently discharged on day 3 with normal blood test results, tolerating feeds, and reporting active bowel movement. On follow-up, his clinical course remained uneventful with no evidence of recurrence to date.
3. Discussion
IPTs of the colon are rare entities of uncertain aetiology and pathogenesis [6], [7], [8]. Evidence suggests that the pathology may be preceded by trauma, surgery, infection (viral, e.g., Epstein-Barr virus or human herpes virus 8 or bacterial, e.g., Nocardia species, Mycobacterium tuberculosis, or Klebsiella species), and autoimmune-mediated inflammation [7], [8], [9]. The heterogenous bodily sites and histological characteristics of IPTs have led to the development of various pseudonyms in literature over time [2], [3], [4], [7]. However, ‘IPT’ remains an umbrella term that encompasses a range of pathologies, which include inflammatory myofibrohistiocytic proliferation, inflammatory myofibroblastic tumours, fibroxanthoma, plasma-cell granulomas, and plasma-cell pseudo-tumours [2], [3], [4], [7], [8], [10], [11]. There is ongoing effort to define and sub-classify these tumours [7], [11]. This is particularly true for those with more aggressive phenotypes, such as inflammatory fibrosarcoma and inflammatory leiomyosarcoma, which carry a risk of recurrence and the ability to metastasize and are associated with mortality, reflecting a more malignant process [5], [10]. Similarly, the distinct morphological and molecular patterns of inflammatory myofibroblastic tumours have distinguished them as a separate body [7].
IPTs were originally described in the lungs but have been documented in extrapulmonary systems, including the genitourinary, cardiovascular, skeletal, hepatobiliary, integumentary, and gastrointestinal (GIT) systems [2], [3], [4], [7]. The stomach and omentum are the predominant sites, followed by the small intestine, colon, and oesophagus [8]. Colonic IPTs, although rare, have been documented in association with Crohn's disease and chronic diverticulitis [3], [5], [12]. A review of colonic IPTs conducted in 2015 found only 18 prior literature records of IPTs in the colon [13].
The presenting features of IPTs are usually non-specific and mostly depend on the anatomical site and mass characteristics [13], [14], [15]. As seen in our case, abdominal pain with or without a palpable abdominal mass is a common symptom of IPTs in intra-abdominal locations [6], [7], [8]. A diagnosis of acute appendicitis was initially considered in our patient due to the patient's age and presentation. Despite being a common surgical condition, a confident diagnosis may be challenging even to skilled clinicians due to their personal experience and a lack of available adjunctive investigations. Furthermore, best practice policies when determining the need for and urgency of operative intervention may vary across different institutions [14]. Laboratory investigations, imaging modalities, and expert consultation are often sought in the setting of the possibility of complications, atypical presentations or palpable mass lesions [14], [15]. In the absence of these, scoring systems may be used to aid in the diagnosis and guide pharmacological and surgical intervention [14]. An Alvarado score of 9, as seen in our patient, is considered highly indicative of an acute appendicitis, with a likelihood of 87% [16]. Notably, the score has limitations in terms of distinguishing between complicated and uncomplicated cases and may be more prone to false-negative results in patients infected with human immunodeficiency virus, a condition prevalent in the South African population [14]. In our case, the decision to take the patient to the operation theatre was made based on the observed clinical and biochemical features and the hospital policy, which informed our decision to proceed to surgery.
Radiologically, IPT lesions are also non-specific [2], [8]. On ultrasonography, they may appear hyper- or hypoechoic, well- or ill-defined, with or without septations and usually have increased doppler signals [2], [3], [4], [8]. This variability is also true for computed tomography (CT), in which they may vary in terms of attenuation, homogeneity, filling, and definition, depending on the degree of necrosis, fibrosis and infiltration [2], [3], [4], [6], [8], [9]. These features may mimic haematological or metastatic lesions, making them indistinguishable on radiological assessment alone [4], [5], [6], [9]. For this reason, we doubt whether pre-operative imaging would have facilitated a more accurate diagnosis of our patient. However, it may have prompted further investigation or supported a midline laparotomy incision in the first instance, given that the patient was clinically septic and likely to proceed to theatre regardless.
Immunohistochemical analysis of IPTs may reveal IgG4 predominance, making it indistinguishable from fibrosclerosing diseases such as IgG4-related sclerosis [7], [8], [9], [11]. They may also express p53, α-smooth- and muscle-specific actin, vimentin, and calpoinin [7], [8], [9]. Clonal anaplastic lymphoma kinase overexpression (also associated with anaplastic large cell lymphoma) is documented in 40–50% of IPTs [7], [8]. Immunohistochemical analysis has been used to identify prognostic markers to no avail due to inconsistencies in the findings [7], [8]. However, clonal translocations at the 2p23 chromosomal locus distinguished inflammatory myofibroblast tumours in over 50% of cases [7], [8].
Histologically, IPT's consist of myofibroblastic spindle cells and inflammatory cells such as lymphocytes, plasma cells, and monocytes with or without collagen [2], [7], [8], [9]. Chronic infiltrates are more common and demonstrate a granulomatous reaction [2], [7], [8]. Interleukin-1 is produced by macrophages and contributes to the surrounding tissue inflammation, constitutional symptoms, and the theoretical risk of malignant transformation [2], [3], [4], [7], [8]. Despite this, malignant transformation is rare. [2], [3], [4], [7], [8] However, recurrence has been documented in the more aggressive subtypes, namely inflammatory fibrosarcoma (37%) and inflammatory myofibroblast tumours (25%) [7], [8]. The recurrence rate of GIT IPTs in uncertain but has been documented to recur at a rate of 18–40% [7], [8]. For this reason, follow-up with clinical examination, CT, and inflammatory mediators such as erythrocyte sedimentation rate is recommended [3], [7], [8].
These lesions, whose pathology often mimics more sinister lesions, are commonly excised surgically [2], [3], [4], [8], [9]. This would also be indicated if there were complications such as perforation, bleeding, or obstruction [8], [9]. However, chemotherapeutic therapies based on agents such as thalidomide, corticosteroids and non-steroidal anti-inflammatory drugs may lead to full resolution [7], [8]. In isolated cases, radiotherapy may be used as an adjunct treatment [6], [7], [8].
4. Conclusion
A key point of contention from this case is the importance of radiological imaging in the workup of patients with abdominal pathologies. However, based on the literature, the imaging may not have informed the diagnosis. The patient may theoretically have had the mass lesion discovered and histology confirmed by colonoscopic biopsy while being treated with antibiotics if no clear indications for emergency exploration were apparent on imaging. This may have prevented radical operative resection and the subsequent emotional burden of a presumed malignancy diagnosis [2], [6].
A review of the literature revealed that IPTs are a complex entity where there can be a move toward a framework to develop a classification system for these scarce mesenchymal masses, like in this case report. Colonic IPTs are non-specific in presentation and can be associated with Crohn's disease or chronic diverticulitis or can clinically mimic acute appendicitis, as in the present case.
Provenance and peer review were not commissioned; the paper was externally peer reviewed.
Data availability statement
Raw data were generated at Tembisa hospital, Johannesburg, South Africa. Derived data supporting the findings of this study are available from the corresponding author on request.
Sources of funding
None.
Ethical approval
Obtained from the University of Pretoria Ethics Committee. Approval number 327/2021.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Research registration
N/A.
Guarantor
Dr. Sphamandla Zulu
CRediT authorship contribution statement
Dr. Sphamandla Zulu: Patient care, data collection, analysis, writing original draft, editing, getting ethics approval and patient consent.
Dr. Charné Kruger: Patient care, data collection and writing of original draft.
Dr. Nolitha Morare: Review and editing.
Dr. Sharol Ngwenya: Review and editing.
Professor Daniel Montwedi: Review, editing and approval of the final manuscript.
Declaration of competing interest
None.
Acknowledgements
Dr. B Khulu, head of general surgery at Tembisa hospital for all your assistance and guidance.
References
- 1.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. Epub 2020 Nov 9. PMID: 33181358. [DOI] [PubMed] [Google Scholar]
- 2.Narla L.D., Newman B., Spottswood S.S., Narla S., Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23(3):719–729. doi: 10.1148/rg.233025073. [DOI] [PubMed] [Google Scholar]
- 3.Ahuja J., Fasih N., Elkeilani A. Inflammatory pseudotumor of the colon. Radiol. Case Rep. 2015;6(4):515. doi: 10.2484/rcr.v6i4.515. Ahuja, J., Fasih, N., & Elkeilani, A. (2015). Inflammatory pseudotumor of the colon. Radiology case reports, 6(4), 515. doi:10.2484/rcr.v6i4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alshammari H.K., Alzamami H.F., Ashoor M., Almarzouq W.F., Kussaibi H. A rare presentation of inflammatory myofibroblastic tumor in the nasolabial fold. Case Rep. Otolaryngol. 2019;2019:3257697. doi: 10.1155/2019/3257697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehmbey G., Al-Jashaami L., Nanda R. Pseudotumor of the sigmoid colon causing bowel obstruction. ACG Case Rep. J. 2019;6(2) doi: 10.14309/crj.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gharbi Khalid, Lkousse Mohammed Amine, Atmani Jamal, Ismail Younes, Elfarouki Abdeltif, Errami Adil Ait Errami Adil Ait, Oubaha Sofia, Samlani Zohor, Krati Khadija. Myofibroblastic inflammatory bowel tumor: unusual location, case report. Int. J. Clin. Exp. Med. Sci. 2020;6(2):25–27. doi: 10.11648/j.ijcems.20200602.12. [DOI] [Google Scholar]
- 7.Gleason B.C., Hornick J.L. Inflammatory myofibroblastic tumours: where are we now? J. Clin. Pathol. 2008;61(4):428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 8.Patnana M., Sevrukov A.B., Elsayes K.M., Viswanathan C., Lubner M., Menias C.O. Inflammatory pseudotumor: the great mimicker. Am. J. Roentgenol. 2012;198(3) doi: 10.2214/AJR.11.7288. W217–W227. [DOI] [PubMed] [Google Scholar]
- 9.Zeng X., Huang H., Li J., Peng J., Zhang J. The clinical and radiological characteristics of inflammatory myofibroblastic tumor occurring at unusual sites. Biomed. Res. Int. 2018;2018 doi: 10.1155/2018/5679634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Urquijo M., Romero-Davila A., Kettenhofen S.E., Gonzalez-Ramirez R., Gil-Galindo G. An inflammatory myofibroblastic tumor of the appendix mimicking an appendicular Malignant Lesion. Clin. Pathol. 2020;13 doi: 10.1177/2632010X20905843. 2632010X20905843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallen M.E., Hornick J.L. The 2020 WHO classification: what's new in soft tissue tumor pathology? Am. J. Surg. Pathol. 2021;45(1) doi: 10.1097/PAS.0000000000001552. W217–W227. [DOI] [PubMed] [Google Scholar]
- 12.Mouelhi Leila, Abbes Leila, Houissa Fatma, Debbeche Radhouane, Mekki Hayfa, Rejeb Majd Ben, Trabelsi Sinda, Salem Mohamed, Najjar Taoufik. Inflammatory pseudotumor of the liver associated with Crohn's disease. J. Crohn's Colitis. December 2009;3(4):305–308. doi: 10.1016/j.crohns.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y., Li L.P., Wang J., Lun Z.J., Li W., Yang Z. Inflammatory pseudotumor of the colon causing intussusception: a case report and literature review. World J. Gastroenterol. 2015;21(2):704–710. doi: 10.3748/wjg.v21.i2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Saverio S., Podda M., De Simone B., Ceresoli M., Augustin G., Gori A., Boermeester M., Sartelli M., Coccolini F., Tarasconi A., De' Angelis N., Weber D.G., Tolonen M., Birindelli A., Biffl W., Moore E.E., Kelly M., Soreide K., Kashuk J., Ten Broek R., Catena F. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J. Emerg. Surg. 2020;15(1):27. doi: 10.1186/s13017-020-00306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlberg D.J., Lee S.D., Dubin J.S. Lower abdominal pain. Emerg. Med. Clin. North Am. 2016;34(2):229–249. doi: 10.1016/j.emc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Snyder M.J., Guthrie M., Cagle S. Acute appendicitis: efficient diagnosis and management. Am. Fam. Phys. 2018;98(1):25–33. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at Tembisa hospital, Johannesburg, South Africa. Derived data supporting the findings of this study are available from the corresponding author on request.