Abstract
Social buffering can provide protective effects on stress responses and their subsequent negative health outcomes. Although social buffering is beneficial for the recipient, it can also have anxiogenic effects on the provider of the social buffering – a phenomena referred to as stress contagion. Social buffering and stress contagion usually occur together, but they have traditionally been studied independently, thus limiting our understanding of this dyadic social interaction. In the present study, we examined the effects of preventative social buffering and stress contagion in socially monogamous prairie voles (Microtus ochrogaster). We tested the hypothesis that this dynamic social interaction is associated with coordinated alterations in behaviors, neurochemical activation, and neuroimmune responses. To do so, adult male prairie voles were stressed via an acute immobilization restraint tube (IMO) either alone (Alone) or with their previously pair-bonded female partner (Partner) in the cage for 1 h. In contrast, females were placed in a cage containing either an empty IMO tube (Empty) or one that contained their pair-bonded male (Partner). Anxiety-like behavior was tested on the elevated plus maze (EPM) following the 60-mins test and brain sections were processed for neurochemical/neuroimmune marker labeling for all subjects. Our data indicate that females in the Partner group were in contact with and sniffed the IMO tube more, showed fewer anxiety-like behaviors, and had a higher level of oxytocin expression in the paraventricular nucleus of the hypothalamus (PVN) compared to the Empty group females. Males in the Partner group had lower levels of anxiety-like behavior during the EPM test, greater activation of corticotropin-releasing hormone expressing neurons in the PVN, lower activation of serotonin neurons in the dorsal raphe, and lower levels of microgliosis in the nucleus accumbens. Taken together, these data suggest brain region- and neurochemical-specific alterations as well as neuroinflammatory changes that may be involved in the regulation of social buffering and stress contagion behaviors.
Keywords: Restraint stress, Social buffering, Corticotrophin-releasing hormone, Serotonin, Oxytocin, Microgliosis
Highlights
-
•
Presence of female partners buffered anxiety-like behavior in male voles.
-
•
Female partners spent greater amount of time with the restrainer when it contained the male partner.
-
•
Partner presence altered 5-HT and CRH expression in the brain of stressed males.
-
•
Partner presence decreased microgliosis in the NAcc in stressed males.
1. Introduction
Social relationships are integral to health and wellbeing in humans. Recently, limited social contact due to the ongoing COVID-19 pandemic has exacerbated a “loneliness epidemic” and its subsequent detrimental health consequences on a global scale (Hwang et al., 2020; Jeste et al., 2020). While social distancing mandates have been in place, the public has been encouraged to stay socially connected, as the existence of social support can lower perceived stress and ameliorate negative health outcomes – a concept known as social buffering (Gunnar and Hostinar, 2015; Kiyokawa and Hennessy, 2018; Razai et al., 2020; Wu, 2020). It has been shown that social buffering can help minimize the amount of perceived stress, aid in coping with stressors, and can speed up stress recovery (Cohen and Wills, 1985; Eisenberger et al., 2007).
Although social buffering can be helpful for alleviating stress in an individual, it can also impact those who provide the support. Social buffering inherently depends on social interactions between two parties: the one who is stressed, and the other who provides the support. As someone is helping to ameliorate stress in an individual, that stressed individual may in turn affect the emotional status of the other – a concept referred to as stress contagion (Fraser et al., 2008; Oliveira and Faustino, 2017). This paradox of social buffering and stress contagion can be viewed as two complementary, yet counterbalancing events during a dyadic social interaction in a stress context, which is thought to serve an evolutionary purpose: to coordinate behaviors between individuals (Buchanan et al., 2012). While the phenomenon has been well documented in humans, relatively little is known about the underlying regulating mechanisms of these dynamic interactions and its interactions with stress (Eisenberger, 2013).
Various animal models have been utilized to study the underlying mechanisms of social buffering. In rats, for example, the presence of partners after a stressful experience helps to reduce typical anxiety-like and fear responses as well as circulating levels of corticosterone (CORT) in the stressed animals, and this effect is modulated by familiarity, stress status, and sensory modalities (Kiyokawa and Hennessy, 2018; Patki et al., 2014). In mice, increased contact with a conspecific has analgesic effects on pain sensitivity (D'Amato and Pavone, 1996; Langford et al., 2010), in addition to its effects on reducing anxiety-like/depressive-like behaviors (Beery and Kaufer, 2015). Several brain regions and neurochemical systems have been implicated in mediating social buffering on stress responses (Peen et al., 2021; Smith and Wang, 2012). For example, social interaction reduces stress-induced Fos (a marker of neuronal activity) expression in the paraventricular nucleus of the hypothalamus (PVN) in rats (Kiyokawa et al., 2004), and blocks potentiation of glutamate synapses on PVN corticotrophin-releasing hormone (CRH) neurons in mice recovering from foot shocks (Sterley et al., 2018). In addition, oxytocin (OT) neurons in the PVN and their projections to the nucleus accumbens (NAcc) and anterior cingulate cortex (ACC) are involved in modulating social buffering effects on stress responses (Peen et al., 2021). OT also has neuroprotective effects and can buffer immune and inflammatory responses to stress (Smith and Wang, 2012). Further, stress can also have widespread effects on the immune system (Haykin and Rolls, 2021). Microglia, referred to as the resident immune cells of the brain (Lenz and Nelson, 2018), are involved in the detection and response to stress (Frank et al., 2019). However, the relationship between microglia and social behavior are in the beginning stages of being better understood (Loth and Donaldson, 2021).
Stress contagion in rodents has often been studied in the context of observational fear learning, where a non-stressed animal (often referred to as the observer) is exposed to a stressed animal (often referred to as the demonstrator) (Hernandez-Lallement et al., 2020). In rats, for example, observing social defeat or being exposed to a stressed demonstrator can induce depressive-like behaviors, elevate circulating CORT, and alter cardiovascular tone, in a pattern that matches the defeated demonstrator rat (Carnevali et al., 2017; Finnell et al., 2017). This state-matching between demonstrators and observers can also be seen in neuronal activation in various brain regions (Knapska et al., 2006). Mice also exhibit increased freezing behaviors when observing a stressed demonstrator (Jeon et al., 2010; Pisansky et al., 2017; Sanders et al., 2013). Intriguingly, stress-induced metaplasticity at glutamate synapses on PVN CRH neurons can be transmitted to an observing partner (Sterley et al., 2018), and activation of PVN OT neurons facilitates their observational fear behavior (Pisansky et al., 2017). Inactivation of the ACC impairs an observer's fear response in both rats and mice (Carrillo et al., 2019; Jeon et al., 2010). Further, serotonin (5-HT) and its interactions with OT and dopamine (DA) in the ACC are important regulators of consolation-like behaviors and sociability in mandarin voles as well (Li et al., 2020, 2021). While these studies in rats and mice have provided an invaluable foundation for studying social buffering and stress contagion, traditional laboratory rodent models do not display the same social bonding between committed partners as seen in humans. To gain further insight into the dynamic social interactions underlying social buffering and stress contagion in close social relationships, research utilizing highly social animal models that display these strong, enduring social bonds will be necessary.
The prairie vole (Microtus ochrogaster) is a socially monogamous rodent species commonly utilized for the study of social behaviors due to their unique ability to form long-term pair bonds (Aragona and Wang, 2004; Getz et al., 1981). These bonds are so enduring that partner loss/separation can lead to increased anxiety- and depressive-like behaviors, circulating CORT levels, and altered neuroimmune functioning (Bosch et al., 2009; McNeal et al., 2014; Pohl et al., 2021; Sun et al., 2014). This robust bond formation provides an excellent opportunity for using the prairie vole model to study the neurobiology of social buffering, stress contagion, and their underlying mechanisms (Lieberwirth and Wang, 2016). It has been found that stressed female voles recovering with a male partner after an acute immobilization stressor (IMO) have decreased anxiety-like behavior and circulating CORT compared to those recovering alone (Smith and Wang, 2014). Observer prairie voles display increased consolatory behavior, namely allogrooming, towards stressed partners, thus confirming that prairie voles demonstrate social buffering behaviors (Burkett et al., 2016; Smith and Wang, 2014). In addition, brain OT, particularly in the PVN and ACC, has been implicated in mediating both social buffering effects in the stressed voles and the increased allogrooming from their observing partners, respectively (Burkett et al., 2016; Smith and Wang, 2014). While these studies were primarily focused on social buffering during recovery or after the stressor was over, data from a recent study indicate that partner presence during an acute IMO stress can also provide preventative social buffering effects in prairie voles (Donovan et al., 2018). Therefore, in the present study, we focused on the reciprocal effects between the free-moving females and their stressed, bonded male partners during an acute IMO stress to test the hypothesis that social buffering and stress contagion during a stressful event are associated with coordinated alterations in behaviors, neurochemical activation, and neuroimmune responses in a socially monogamous species. We predicted that the female's presence will reduce anxiety-like behaviors of the male partners and affect the neurochemical and neuroimmune expression in the male's brain. We also predicted, by the idea of social contagion, that females will, likewise, be affected by their stressed male partner and show alterations in their anxiety-like and affiliative behaviors as well as neurochemical activity in the brain.
2. Materials and methods
2.1. Subjects
Subjects were male and female prairie voles (M. ochrogaster) captive-bred at Florida State University. All voles were weaned on postnatal day 21 and housed in Plexiglas cages (29 × 18 × 13 cm) with a same-sex conspecific. All cages contained cedar chip bedding with food and water provided ad libitum. Subjects were kept at 20 °C under a 14:10 h light:dark cycle (lights on at 0700). At the time of pairing, subjects had reached adulthood (>90 days of age) and were sexually naïve. All females were ovariectomized and allowed at least one week to recover from surgery before pairing. Male subjects were paired with unrelated, ovariectomized female partners for two weeks - a sufficient amount of time for the development of a stable pair bond (Aragona and Wang, 2004).
2.2. Experimental design
The experimental paradigm is illustrated in Fig. 1. Pair-bonded male subjects were randomly assigned into one of two experimental groups that underwent a 60-min IMO stress either alone (Alone) or with their female partner (Partner) in the cage. For the female partners, they were either exposed to an empty restraint tube (Empty) or to their pair-bonded male partners in the IMO restraint tube (Partner) for 60 min. All procedures were approved by the Institutional Animal Care and Use Committee at Florida State University and were in accordance with the guidelines set forth by the National Institutes of Health.
Fig. 1.
Schematic illustration of the experimental timeline (A) and treatment groups (B). Male voles were pair-housed with their female partners for 14 days. Thereafter, they were assigned into one of the two experimental groups where they experienced 60-mins immobilization stress (IMO) in a restraint tube either alone (Alone) or with their female partner in the cage (Partner). Females were either with a restraint tube containing their stressed male partner (Partner) or with an empty restraint tube (Empty). Following the IMO stress, both males and their female partners recovered individually for 5 min, and then underwent a 5-min elevated plus maze (EPM) test. Thereafter, their blood and brains were collected immediately. Created with BioRender.com.
2.3. Immobilization stressor (IMO) paradigm
The IMO procedure has been validated and shown to reliably induce behavioral and physiological stress responses in previous studies in prairie voles (Donovan et al., 2018; Smith et al., 2013; Smith and Wang, 2014). Briefly, male subjects were placed in 50 mL plastic centrifuge tubes which had the top 1/3 removed to adjust for animal size that were secured with mesh and Velcro straps to completely immobilize the subject inside the tube. The tube tip was removed to allow for unrestricted respiration. The IMO restrainer was then placed in a clean Plexiglas cage (45 × 20 × 25 cm) containing fresh cedar chip bedding for 60 min, during which male subjects either remained alone (Alone) or were with their female partners (Partner) (Fig. 1B). Conversely, free-moving females were in the cage under one of the two conditions: with the IMO restrainer containing the pair-bonded male (Partner) or nothing (Empty) (Fig. 1B) for 60 min, and their behaviors were recorded. Duration of the females’ anxiety-like behaviors and restrainer-directed behaviors were subsequently quantified for 30 min using J-Watcher (http://www.jwatcher.ucla.edu/) by a trained observer blind to the treatment. The anxiety-like behaviors include self-grooming and rearing, which combined as total anxiety-like behaviors. The measured restrainer-directed behaviors include olfactory investigation (sniffing), effort (biting and pulling the restrainer), and contact (physical contact with the restrainer that is not sniffing or effort). Total restrainer-directed behavior was the sum of sniffing, effort, and contact durations.
2.4. Elevated Plus Maze (EPM) test
Immediately after the IMO test, subjects were habituated alone in a clean cage for 5 min and then tested for their anxiety-like behaviors using an established EPM test (Pan et al., 2009; Smith et al., 2013; Stowe et al., 2005). Briefly, the EPM is elevated 45 cm off the ground and has two opposing open (35 × 6.5 cm) and two opposing closed arms (35 × 5 × 15 cm) that cross in the middle. All subjects were placed in the center of the EPM facing an open arm, and their behavior was recorded for 5 min. Behaviors were quantified by a trained observer blind to treatment conditions via J-Watcher. The duration of time spent in each arm and frequency of arm entries were quantified for each subject.
2.5. Blood preparation and corticosterone radioimmunoassay
Immediately after the EPM test, subjects were deeply anesthetized, and cardiac blood was collected prior to perfusion. Blood samples (∼300 μl) were collected into microcentrifuge vials containing 20 μl EDTA and immediately placed in ice. Blood was centrifuged at 6000 rpm for 15 min at 4 °C, plasma was aspirated and transferred into new tubes, then re-centrifuged at 6000 rpm for 10 min at 4 °C. Plasma was aliquoted into new microcentrifuge vials and stored at −80 °C. Plasma samples (1:800) were analyzed (in duplicates) for CORT via radioimmunoassay (RIA) using a commercially available kit according to manufacturer instructions (Diagnostic Products Corp., Los Angeles, CA). The kit has been previously validated in prairie vole studies (Donovan et al., 2020; Smith et al., 2013; Smith and Wang, 2014).
2.6. Brain tissue preparation
All subjects were perfused approximately 70–90 min after the start of the 60-min IMO test. Subjects were deeply anesthetized (with euthasol) and intracardially perfused via 0.9% saline followed by 4% paraformaldehyde in 0.1M phosphate PB buffer (PB). Brains were extracted, post-fixed in 4% paraformaldehyde in PB for 2 h, and then stored in 30% sucrose in PB at 4 °C until sectioning. Brains were coronally sectioned at 40 μm thickness via sliding microtome. Tissue was stored in 0.1 M PB with 1% sodium azide at 4 °C until immunocytochemical processing.
2.7. Immunocytochemistry
c-Fos and ionized calcium binding adaptor molecule 1 (Iba-1, a microglia-specific marker) immunoreactive staining was performed on sets of coronal sections at 200 μm intervals using established protocol (Donovan et al., 2020; Liu et al., 2019). Sections were rinsed in 0.1 M phosphate-buffered saline (PBS) 4 times for a total of 20 min and then incubated with 1% NaBH4 in 0.1 M PBS for 10 min. After rinsing, the sections were incubated in 0.3% H2O2 in 0.1 M PBS for 20 min, in 10% normal goat serum (NGS, Sigma-Aldrich, St. Louis, MO) or normal donkey serum (NDS, Sigma-Aldrich) in 0.3% Triton X-100 in 0.1 M PBS (TPBS) for 1 h, in rabbit-c-Fos (1:3K; Cat # 2250S, Cell Signaling, Danvers, MA) or rabbit anti-Iba-1 (1:10K; Cat # 019–19741, Wako Chemicals, Richmond, VA) in 0.3% TPBS with 2% NGS at room temperature for 1 h and again at 4 °C for 2 nights. Sections were then placed at room temperature for 1 h, rinsed in 0.3% TPBS, incubated in biotinylated goat antirabbit IgG (1:300, Vector Lab, Burlingame, CA) or donkey antirabbit IgG (1:500, Jackson ImmunoResearch Lab, West Grove, PA) for 2 h at room temperature. Subsequently, the sections were rinsed in 0.3% TPBS and incubated in the ABC Elite HRP Kit (Vector Lab) in 0.1 M PBS for 90 min at room temperature. c-Fos and Iba-1 staining was revealed using 3′ diaminobenzidine (DAB, Sigma-Aldrich).
Immunoreactive double-label staining for c-Fos/OT, c-Fos/vasopressin (AVP), c-Fos/CRH, and c-Fos/5-HT were also performed on sets of coronal sections at 200 μm intervals using our previously established method (Donovan et al., 2020; Liu et al., 2019). For the double-label sections, after c-fos immunolabeling as described above, sections were rinsed in 0.1 M PBS after the DAB staining and then incubated in either 5% NGS (for OT, AVP, and CRH) or 5% normal rabbit serum (NRS, Sigma-Aldrich, for 5-HT) in 0.3% TPBS for 30 min. The tissue were then incubated in one of the following primary antibodies at 4 °C for 2 nights: rb anti-OT (1:50K; Cat # 20068, ImmunoStar, Hudson, WI), rb anti-AVP (1:8K; Cat # AB1565, Millipore, Burlington, MA), gp anti-CRH (1:3K; Cat # T-5007, Peninsula Lab, San Carlos, CA), or gt anti-5-HT (1:50K; Cat # 20079, ImmunoStar). Sections were then incubated in biotinylated goat anti-rabbit IgG (1:300, for OT and AVP), goat anti-guinea pig (1:300, Vector Lab, for CRH), or rabbit anti-goat (1:300, Vector Lab, for 5-HT). Sections were subsequently stained using SG (Cat # SK-4700, Vector Lab). After staining, all sections were mounted onto slides, dehydrated in ethanol, clarified in xylene, and cover-slipped with permount.
The number of c-Fos-ir cells was counted in the NAcc, bed nucleus of the stria terminalis (BNST), amygdala (AMYG), and lateral septum (LS). The boundary for each of the brain area was outlined bilaterally in which all c-Fos-ir cells were counted. These brain regions were chosen based on their demonstrated roles in social behaviors and/or stress responses (Lieberwirth and Wang, 2016; Smith and Wang, 2012). In addition, the number of single-labeled cells for c-Fos, OT, AVP, or CRH, as well as cells double-labeled for c-Fos/OT, c-Fos/AVP, and c-Fos/CRH in the PVN were quantified bilaterally. Similarly, cells single-labeled for c-Fos or 5-HT and cells double-labeled for c-Fos/5-HT were quantified in the dorsal raphe (DR). Iba-1-labeled cells were quantified bilaterally in a specified region of interest in the NAcc, LS, DR, and PVN. All cell counting was conducted manually using the cell counter function in FIJI (National Institutes of Health, Bethesda, MD) from 2 to 3 sections per brain area/subject for each marker. Counts for cells double-labeled for c-Fos/neurochemical marker were converted to the percentage of all cells labeled for the neurochemical marker, which indicates the overall portion of the neurochemical cells that were activated. In addition, such double-labeled cells were also converted to the percentage of all c-Fos labeled cells, which indicates the portion of all activated neurons that expressed the neurochemical phenotype. These two measurements provide complementary information indicating activation of a particular neurochemical system in a behavioral and/or physiological process (Donovan et al., 2020; Liu et al., 2019).
2.8. Data analysis
All behavioral data were quantified via J-Watcher. Group differences in behavioral, neurochemical and immunolabeling data for male subjects were analyzed by t tests. Similarly, group differences in the data from female partners were also analyzed by t tests. The male and female subjects underwent different experimental conditions, so they were analyzed separately. Reported p values were not corrected for multiple comparisons (Althouse, 2016). Effect sizes are reported as Cohen's d (d).
3. Results
3.1. Partner presence altered anxiety-like and social behaviors
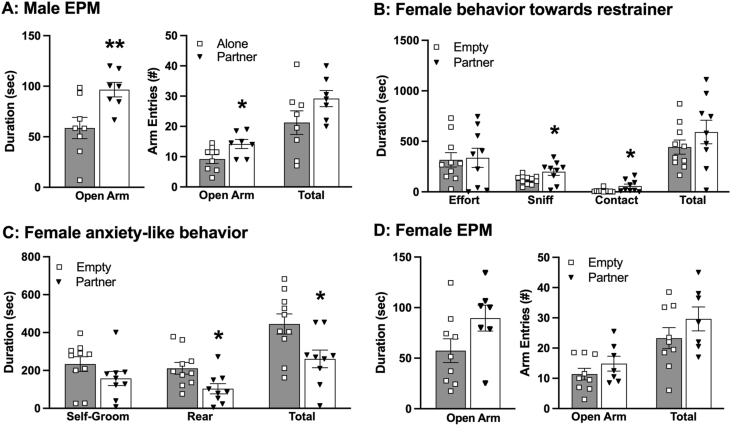
Anxiety-like behaviors of male subjects were assessed by an EPM test following the IMO test. Males that had their female partners in the cage during the IMO test spent significantly more time in the open arm of the EPM (t(13) = 2.91, p < 0.01, d = 1.53) and made more open arm entries (t(13) = 2.37, p < 0.05, d = 1.23) compared to the control males that underwent the IMO stress alone (Fig. 2A). No group differences were found in the number of total arm entries (Fig. 2A).
Fig. 2.
Behaviors of male and female voles during the immobilization (IMO) stress and the subsequent elevated plus maze (EPM) test. Males who had their partner in the cage during the IMO test (Partner) spent significantly more time in the open arm and entered the open arm more frequently during the EPM test, compared to males who experienced IMO alone (Alone) (A). During the IMO test, females spent significantly more time sniffing and in contact with the restrainer containing the stressed male partner (Partner) than with an empty restrainer (Empty). There were no significant differences in effort (biting/pulling the restrainer) or in the total time spent with the restrainer (sum of effort, sniff, and contact) (B). In addition, females in the Partner condition spent significantly less time rearing and in total anxiety-like behaviors (sum of rearing and self-grooming) than females in the Empty condition (C). Females showed no significant group differences in behaviors from the EPM test (D). Bars indicate mean ± SEM. * represents p < 0.05; ** represents p < 0.01.
During the IMO test, female partners in the cage spent greater time in contact with (t(17) = 2.27, p < 0.05, d = 1.07) and sniffing (t(17) = 2.17, p < 0.05, d = 1.01) the restrainer more when it contained the male partner compared to when it was empty (Fig. 2B). Females in the presence of their male partner in the restrainer spent less time rearing (t(17) = 2.59, p < 0.05, d = 1.20) and in total anxiety-like behaviors (rearing and self-grooming) (t(17) = 2.56, p < 0.05, d = 1.18), compared to females with the empty restrainer (Fig. 2C). No significant group differences were found in female's behaviors during the subsequent EPM test following the IMO test (Fig. 2D).
3.2. Partner presence altered neural activity in a brain region- and neurochemical-specific manner
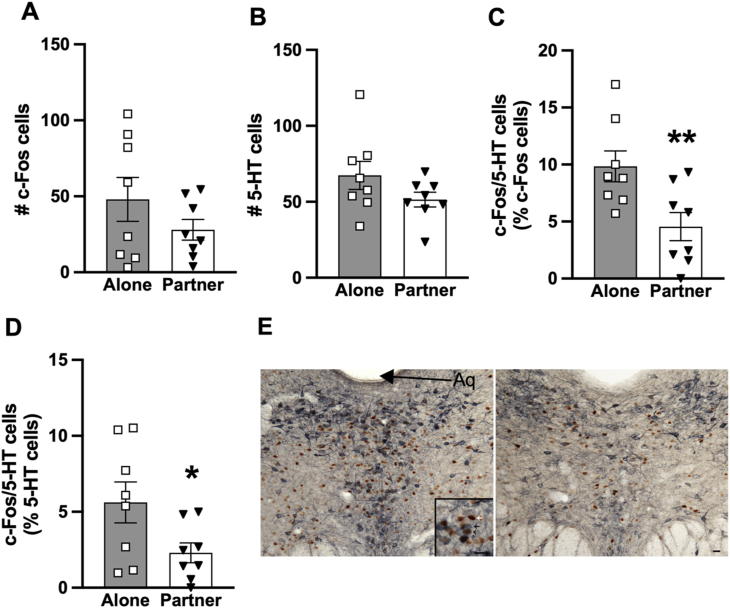
We examined neuronal activation, as indicated by c-Fos levels, in a variety of brain regions. In the DR, no group differences were found in the number of cells labeled for c-Fos or 5-HT in males (Fig. 3A and B). However, the percentage of c-Fos/5-HT double-labeled cells over total c-Fos cells (t(14) = 2.89, p < 0.01, d = 1.45; Fig. 3C) and over total 5-HT cells (t(14) = 2.22, p < 0.05, d = 1.11; Fig. 3D) were significantly lower in the males that were with their female partners during the IMO stress compared to those who underwent the IMO stress alone. No group differences were found in c-Fos and 5-HT labeling in the DR in females (data not shown).
Fig. 3.
c-Fos and 5-HT immunolabeling in the dorsal raphe (DR) of the male vole brains. No group differences were found in the total number of c-Fos-labeled cells (A) or 5-HT-labeled cells (B) in the DR. Compared to the males that received immobilization (IMO) stress alone (Alone), stressed males with their partner in the cage (Partner) had a significantly lower percentage of c-Fos expressing cells co-labeled for 5-HT (C), as well as a lower percentage of 5-HT expressing cells co-labeled for c-Fos (D). Bars indicate mean ± SEM. * represents p < 0.05; ** represents p < 0.01. Panel E shows representative images of immunostaining of c-Fos, 5-HT, and double-labeled c-Fos/5-HT cells in the DR of the Alone (left) and Partner (right) males. White arrow indicates c-Fos labeled cells; black arrow indicates 5-HT labeled cells, and black arrowhead indicates cells double-labeled for c-Fos and 5-HT. Aq, cerebral aqueduct. Scale bars = 20 μm.
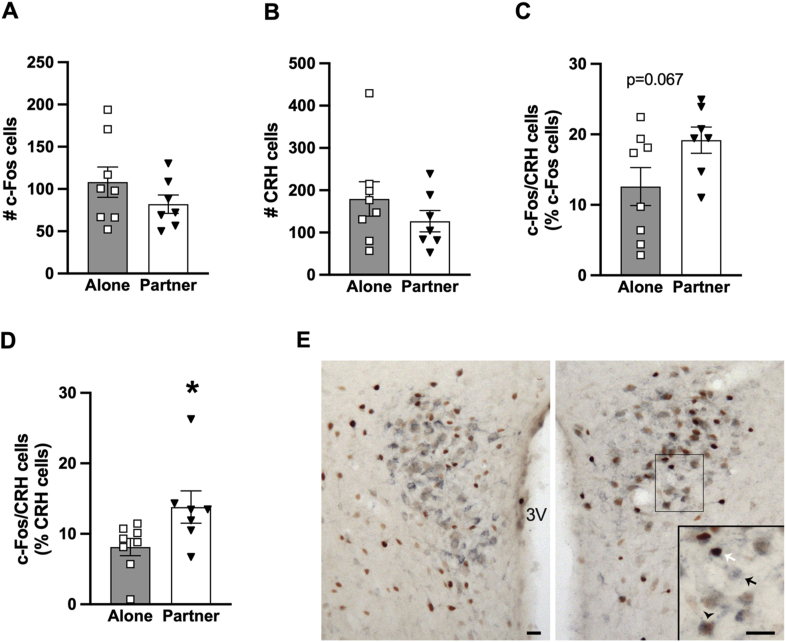
In the PVN, cells labeled for c-Fos, CRH, OT and AVP, as well as double-labeled for c-Fos with each of the neurochemical markers are summarized in Table 1. Overall, no group differences were found in the number of cells labeled for c-Fos or for CRH in both males and females (Table 1, Fig. 4A & B). In males, the percentage of c-Fos/CRH double-labeled cells over total CRH cells was significantly higher in those with their female partners during the IMO compared to ones that were stressed alone (t(13) = 2.26, p < 0.05, d = 1.15; Fig. 4D). A similar trend was found in the percentage of c-Fos/CRH over total c-Fos cells, but this difference was not statistically significant (Fig. 4C). Interestingly, females exposed to the restrainer containing male partners had more cells labeled for OT than females with the empty restrainer (t(14) = 2.85, p < 0.05, d = 1.38; Table 1). c-Fos labeling in the NAcc, BNST, AMYG, and LS are summarized in Table 2. The only significant group difference found was in the LS, in which restrained males with their female partners had lower levels of c-Fos labeling compared to the males that underwent the IMO stress alone.
Table 1.
c-Fos, AVP, OT, and CRH labeling in the PVN.
| Males |
Females |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alone (n = 8) | Partner (n = 8) | t | p | d | Empty (n = 9) | Partner (n = 9) | t | p | d | |
| c-Fos | 21.7 ± 3.9 | 39.1 ± 13.9 | 1.20 | ns | 0.60 | 38.0 ± 20.6 | 32.6 ± 16.3 | 0.20 | ns | 0.10 |
| AVP | 96.9 ± 12.7 | 68.4 ± 4.2 | 2.13 | 0.06 | 1.07 | 85.2 ± 11.6 | 87.3 ± 7.7 | 0.14 | ns | 0.07 |
| AVP/c-Fos | 0.8 ± 0.5 | 0.1 ± 0.1 | 1.36 | ns | 0.68 | 0.0 ± 0.0 | 0.1 ± 0.1 | 1.00 | ns | N/A |
| % AVP | 1.0 ± 0.6 | 0.2 ± 0.1 | 1.35 | ns | 0.67 | 0.0 ± 0.0 | 0.1 ± 0.1 | 1.00 | ns | N/A |
| % c-Fos | 2.8 ± 1.6 | 0.3 ± 0.2 | 1.52 | ns | 0.76 | 0.0 ± 0.0 | 0.1 ± 0.1 | 1.00 | ns | N/A |
| c-Fos | 18.0 ± 5.6 | 18.3 ± 5.5 | 0.04 | ns | 0.02 | 32.0 ± 17.6 | 18.9 ± 10.7 | 0.59 | ns | 0.31 |
| OT | 56.0 ± 13.2 | 59.7 ± 8.5 | 0.23 | ns | 0.11 | 68.3 ± 8.2 | 95.0 ± 4.5 | 2.85 | 0.02 | 1.38 |
| OT/c-Fos | 0.4 ± 0.2 | 0.1 ± 0.1 | 0.97 | ns | 0.48 | 0.6 ± 0.4 | 0.2 ± 0.1 | 0.86 | ns | 0.41 |
| % OT | 0.8 ± 0.5 | 0.3 ± 0.2 | 1.02 | ns | 0.51 | 0.6 ± 0.4 | 0.2 ± 0.2 | 0.93 | ns | 0.44 |
| % c-Fos | 2.2 ± 1.1 | 0.5 ± 0.4 | 1.41 | ns | 0.70 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.08 | ns | 0.04 |
| c-Fos | 108.2 ± 18.0 | 82.1 ± 10.9 | 1.20 | ns | 0.63 | 133.8 ± 40.4 | 145.7 ± 39.7 | 0.21 | ns | 0.10 |
| CRH | 179.5 ± 40.6 | 126.8 ± 25.0 | 1.07 | ns | 0.56 | 250.4 ± 46.9 | 255.1 ± 37.7 | 0.08 | ns | 0.04 |
| CRH/c-Fos | 14.4 ± 4.6 | 16.0 ± 2.7 | 0.29 | ns | 0.15 | 14.7 ± 4.0 | 17.2 ± 4.0 | 0.45 | ns | 0.21 |
| % CRH | 8.1 ± 1.2 | 13.8 ± 2.3 | 2.26 | 0.04 | 1.15 | 5.7 ± 1.2 | 7.0 ± 1.5 | 0.72 | ns | 0.34 |
| % c-Fos | 12.6 ± 2.7 | 19.2 ± 1.9 | 2.01 | 0.07 | 1.03 | 12.5 ± 2.2 | 13.9 ± 1.8 | 0.46 | ns | 0.22 |
Fig. 4.
c-Fos and corticotrophin releasing hormone (CRH) labeling in the paraventricular nucleus of the hypothalamus (PVN) of the male vole brains. No group differences were found in the total number of c-Fos-labeled cells (A) or CRH-labeled cells (B) in the PVN. Compared to the males that received immobilization (IMO) stress alone (Alone), stressed males with their partner in the cage (Partner) had a significantly higher percentage of CRH expressing cells co-labeled for c-Fos (D), and a similar trend was also found in the percentage of c-Fos expressing cells co-labeled for CRH (C). Bars indicate mean ± SEM. * represents p < 0.05. Panel E shows representative images of immunostaining of c-Fos, CRH, and double-labeled c-Fos/CRH cells in the PVN of the Alone (left) and Partner (right) males. White arrow indicates c-Fos labeled cells; black arrow indicates CRH labeled cells, and black arrowhead indicates cells double-labeled for c-Fos and CRH. 3V, third ventricle. Scale bars = 20 μm.
Table 2.
c-Fos labeled cells in selected brain areas.
| Males |
Females |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alone (n = 9) | Partner (n = 9) | t | p | d | Empty (n = 10) | Partner (n = 9) | t | p | d | |
| NAcc total | 37.9 ± 8.6 | 64.9 ± 18.9 | 1.30 | ns | 0.62 | 55.4 ± 19.6 | 50.2 ± 15.5 | 0.21 | ns | 0.10 |
| NAcc core | 17.1 ± 4.7 | 35.5 ± 12.3 | 1.40 | ns | 0.66 | 24.6 ± 8.0 | 26.1 ± 8.0 | 0.14 | ns | 0.06 |
| NAcc shell | 20.9 ± 4.6 | 29.4 ± 7.5 | 0.94 | ns | 0.46 | 30.9 ± 11.9 | 24.1 ± 7.6 (9) | 0.48 | ns | 0.23 |
| BNST total | 90.4 ± 10.5 | 101.8 ± 15.8 | 0.60 | ns | 0.28 | 103.9 ± 23.1 | 120.1 ± 24.3 | 0.48 | ns | 0.23 |
| BNSTd | 21.4 ± 4.5 | 27.4 ± 8.7 | 0.61 | ns | 0.29 | 21.6 ± 6.0 | 28.3 ± 6.6 | 0.75 | ns | 0.36 |
| BNSTv | 68.9 ± 7.2 | 74.3 ± 11.0 | 0.41 | ns | 0.19 | 82.3 ± 18.0 | 91.8 ± 19.8 | 0.35 | ns | 0.17 |
| AMYG total | 251.1 ± 41.8 | 356.0 ± 78.2 | 1.18 | ns | 0.59 | 383.1 ± 45.4 | 298.7 ± 35.6 | 1.46 | ns | 0.69 |
| MeA | 186.3 ± 35.5 | 245.0 ± 53.2 | 0.92 | ns | 0.46 | 274.3 ± 34.0 | 209.6 ± 25.6 | 1.52 | ns | 0.72 |
| ACo | 48.1 ± 11.6 | 58.5 ± 11.6 | 0.63 | ns | 0.32 | 70.8 ± 8.9 | 60.2 ± 9.0 | 0.84 | ns | 0.40 |
| CeA | 16.8 ± 3.2 | 52.5 ± 20.5 | 1.73 | ns | 0.86 | 39.9 ± 8.3 | 28.9 ± 6.7 | 1.01 | ns | 0.47 |
| LS | 74.8 ± 17.9 | 23.7 ± 5.3 | 2.74 | 0.03 | 1.36 | 81.0 ± 18.6 | 39.3 ± 12.8 | 1.80 | 0.09 | 0.84 |
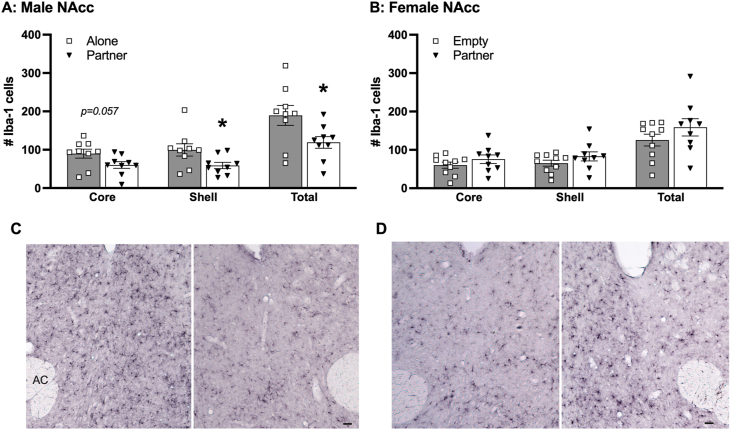
3.3. Lower levels of microgliosis in the NAcc of restrained males with female partner
Iba-1 cells were quantified in the NAcc in both males and females. Males restrained with the female partners in the cage had significantly lower levels of Iba-1 cells in the total NAcc (t(16) = 2.33, p < 0.05, d = 1.10) and in the shell (t(16) = 2.26, p < 0.05, d = 1.07), with a similar, but not statistically significant, difference in the core (Fig. 5A and C). No group differences were found in the number of Iba-1 cells in the NAcc of females (Fig. 5B and D). In addition, no group differences were found in the number of Iba-1 cells in other quantified brain areas including the DR, LS, and PVN in both males and females (Table 3).
Fig. 5.
Immunostaining of ionized calcium-binding adaptor molecule 1 (Iba-1) in the nucleus accumbens (NAcc) of the male (A & C) and female (B and D) brains. Compared to the males that received immobilization (IMO) stress alone (Alone), stressed males with their partner in the cage (Partner) had significantly lower numbers of Iba-1 labeled cells in the NAcc shell and total (core and shell), as well as a trending decrease in the NAcc core (A). No group differences were found in the number of Iba-1 labeled cells in the NAcc in females (B). Bars indicate mean ± SEM. * represents p < 0.05. Panel C shows representative images of Iba-1 staining in the NAcc of Alone (left) and Partner (right) males, whereas Panel D shows representative images of Iba-1 staining in the NAcc of Empty (left) and Partner (right) females. AC, anterior commissure. Scale bars = 50 μm.
Table 3.
Iba-1 labeled cells in selected brain areas and circulating levels of corticosterone.
| Males |
Females |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alone (n = 9) | Partner (n = 9) | t | p | d | Empty (n = 10) | Partner (n = 9) | t | p | d | |
| Iba-1 labeling | ||||||||||
| DR | 13.2 ± 1.5 | 12.9 ± 1.5 | 0.17 | ns | 0.08 | 14.3 ± 1.6 | 16.0 ± 1.4 | 0.82 | ns | 0.38 |
| LS | 10.4 ± 1.4 | 12.2 ± 1.9 | 0.78 | ns | 0.38 | 10.1 ± 1.7 | 8.7 ± 1.2 | 0.64 | ns | 0.30 |
| PVN | 64.5 ± 5.9 | 59.4 ± 2.1 | 0.78 | ns | 0.39 | 67.8 ± 7.1 | 65.3 ± 7.6 | 0.24 | ns | 0.12 |
| Corticosterone | 305.0 ± 13.8 | 349.4 ± 23.4 | 1.64 | ns | 0.78 | 500.0 ± 31.8 | 442.3 ± 20.4 | 1.49 | ns | 0.69 |
3.4. Partner presence did not alter circulating levels of CORT
Plasma samples were assessed for circulating levels of CORT. No significant group differences were found for either males or females (Table 3).
4. Discussion
Although social interactions, especially those from close ties, can buffer stress responses, little is known about the underlying mechanisms (Eisenberger, 2013; Lieberwirth and Wang, 2016). In the present study using socially monogamous prairie voles, our data showed that male's anxiety-like behavior following an acute stressor was significantly less in the presence of a female partner during the stress. The lower level of anxiety-like behavior in males was associated with changes in activation of 5-HT and CRH-expressing neurons, as well as with altered microglia expression in a brain region-specific manner. Interestingly, female partners displayed both enhanced social interactions towards restrained male partners, and lower level of anxiety-like behavior while in the presence of the stressed partner, and these changes were associated with greater OT expression in the PVN. Together, these data illustrate not only neurochemical and neuroimmune responses to stress, but also their potential roles in mediating social interactions, stress contagion, and social buffering.
4.1. Male and female behaviors, stress, and social buffering
The IMO test is a well-established paradigm that reliably induces biobehavioral stress responses in a variety of rodent species – including prairie voles (Kovács et al., 2018; Rowland and Dunn, 1995; Smith et al., 2013). In the present study, male voles in the company of their female partners during the IMO test exhibited lower levels of anxiety-like behaviors compared to control males that underwent the IMO test alone. These data are consistent with our previous findings (Donovan et al., 2018), and together with the data from other studies (Burkett et al., 2016; Donovan et al., 2018; Smith and Wang, 2014) demonstrates that partner presence during and after stressful events can attenuate stress responses in prairie voles. Such social buffering effects on stress responses have also been reported in other rodent species, including rats (Davitz and Mason, 1955) and mice (Sterley et al., 2018).
Consolatory and stress-alleviating behaviors have been documented in a variety of animal species (Meyza et al., 2017). Prairie voles display enhanced allogrooming towards their stressed partners, thereby buffering the partners' stress responses (Burkett et al., 2016; Smith and Wang, 2014). In our study, female voles spent more time interacting with an IMO restraint tube containing their male partner compared to an empty IMO tube. These data indicate that even with limited physical contact, the presence of a female partner was effective at decreasing typical anxiety-like stress responses seen after the IMO test in male voles. Apart from physical contact, alternate sensory modalities, including olfactory, auditory, and visual cues, can facilitate social connection and social buffering (Kiyokawa and Hennessy, 2018). For example, social buffering of stress responses does not require physical interactions between a dyad of male rats (Kiyokawa et al., 2013) and conspecific olfactory signals can block stress responses and associated neuronal activation in the brain of male rats (Takahashi et al., 2013). Vocalizations and visual cues are also able to reduce stress and alter affective states in isolated marmosets (Rukstalis and French, 2005) and in humans (Bartels and Zeki, 2000). In prairie voles, chemosensory cues have been shown to mediate anxiety-like behavior, social and partner preferences, paternal care, and can alter context-dependent neuronal activation and adult neurogenesis (Jean-Baptiste et al., 2008; Liu et al., 2014; Tubbiola and Wysocki, 1997; Williams et al., 1992). Our data indicate that the females' behavior towards the restrained males along with the males’ subsequently lower anxiety-like behavior are likely mediated by bi-directional partner-associated cues with limited tactile stimulation.
In our study, female voles with their stressed male partner exhibited lower levels of anxiety-like behaviors during the IMO test compared to females that faced an empty IMO restrainer. One plausible explanation could be that given the anxiogenic nature of social isolation, especially for socially monogamous prairie voles, it may be more stressful to be alone in a novel environment than to be with a familiar partner (Donovan et al., 2020; Grippo et al., 2007b; Pan et al., 2009). In the same vein, presence of a familiar partner in a novel environment, regardless of the partner's emotional state, might be anxiolytic. This is supported by our data showing that both the males and females, regardless of stress exposure, had lower levels of anxiety-like behavior when with their partner compared to their respective alone controls. Further, the transmission of stress, or stress contagion, during social interactions may be an essential precursor to the initiation of consolatory behaviors (De Waal and Preston, 2017), and, therefore, it is also possible that presence of the female partner may have attenuated the male's stress responses, and, thereby, preemptively reduced the amount of transmitted stress that the female partner received (Cohen and Wills, 1985). Unfortunately, we were unable to measure solicitation behaviors (i.e. vocalizations, pheromone release, and body movement) exhibited by the males in the restrainer as well as the receptivity of the females to these cues, and thus do not have the data to either support or repute this notion. Finally, the EPM data following the IMO paradigm showed a partner-associated, significant decrease in anxiety-like behaviors in males but only trending effects in females. These results may be due to differences in the type/magnitude of the stressors as well as sex differences in responses to anxiogenic effects of social isolation and/or anxiolytic effects of having a partner present.
4.2. Changes of neurochemical and neuroimmune markers in the male brain
Social buffering on the males’ anxiety-like behavior was also related to changes in neuronal activation in the PVN in a neurochemical-specific manner. Males that were accompanied by their female partner during the IMO stress showed greater activation of CRH-expressing neurons in the PVN, compared to males stressed alone. While increased hypothalamic–pituitary–adrenal axis (HPA) activity typically occurs under stressful conditions, including social isolation or IMO stress in prairie voles (Grippo et al., 2007a; Smith et al., 2013), it has also been implicated in the facilitation of pair bonding. This is particularly in male prairie voles, as CRH and CORT administration induces their bonding behaviors towards the female partners (DeVries et al., 2002; Devries et al., 1996). Therefore, greater CRH activation in the PVN of male voles could be stimulated by female-associated cues and may play a role in facilitating the emotional transition from anxiety to social bonding.
Our data also reveal lower activation of 5-HT neurons in the DR of males stressed in the presence of their partners compared to those stressed alone. 5-HT has oftentimes been characterized as “prosocial” due to its positive correlation with affiliative behaviors and negative association with aggression and isolation in males (Insel and Winslow, 1998; Siegel and Crockett, 2013; Young and Leyton, 2002). It should be noted, however, that 5-HT can have anxiolytic or anxiogenic effects on behaviors depending on the context (Fernandez and Gaspar, 2012; Guilherme Graeff and Zangrossi Jr., 2012). Genetic models of 5-HT depletion and drugs/lesions that inhibit 5-HT activity have anxiolytic effects in unconditioned anxiety tests such as the EPM (Fernandez and Gaspar, 2012; Lowry et al., 2005), and increased 5-HT activity can enhance anxiety-like behaviors (Lowry et al., 2005). Therefore, the lower levels of 5-HT activity in the DR in our study may indicate its role in reducing anxiety-like behavior in pair-bonded male voles – a speculation that needs to be tested in subsequent studies.
In stressed males that had their partner present, we saw greater PVN CRH neuron activation, lower DR 5-HT neuron activation, and lower levels of anxiety-like behavior. Albeit confusing, these results may be indicative of a neurochemical interaction between CRH and 5-HT to facilitate this reduction in anxiety-like behaviors. CRH-ir fibers innervate the DR, and CRH receptors can be found directly on 5-HT expressing neurons (Valentino et al., 2010). The two types of CRH receptors (CRHR1 and CRHR2) have opposing effects on 5-HT – lower amounts of CRH have a higher affinity for CRHR1 with an inhibitory effect on 5-HT release, whereas higher levels of CRH will act on CRHR2 with an excitatory effect on 5-HT release (Valentino et al., 2010). We were unable to deduce neurochemical release and binding in the current study, but the established link between these two neurochemical systems indicates a regulatory process of interest for future social buffering studies. Finally, extensive interactions between the CRH and the neuropeptide systems such as OT have been demonstrated on multiple levels including activation, release, and receptor expression (Winter and Jurek, 2019), and OT, in particular, has been shown to interact with CRH in reducing stress responses and in facilitating social interactions (Gobrogge and Wang, 2015; Windle et al., 2004). Although not tested in the present study, it would be interesting to study CRH-OT interactions in mediating social interaction, social transmission, and subsequent social buffering effects on stress responses in highly social animal models.
Examination of microgliosis in response to stress has yielded interesting results. Socially isolated female prairie voles have higher levels of Iba-1 labeling in the NAcc than co-housed controls (Donovan et al., 2020). In the present study, male voles that experienced IMO stress in the presence of their female partners showed lower levels of Iba-1 labeling in the NAcc, compared to the males that underwent the stress alone. Together, these data illustrate neuroinflammatory responses to stress as well as to the specific social relationship and social interaction. These data are consistent with the literature demonstrating that psychosocial stressors lead to increased microglial activity in the brain (Calcia et al., 2016) whereas social interaction decreases neuroinflammatory responses to stress in male mice (Gaudier-Diaz et al., 2017; Norman et al., 2010). Associative changes between microgliosis and neurochemical expression/activity have been documented. For example, increased expression of OT and OTR are associated with promoting anti-inflammatory effects, and treatment with OT can reduce microglial activation (Panaro et al., 2020; Yuan et al., 2016). In prairie voles, IMO experience leads to a lower OTR density in the NAcc, and such effects are abolished by the presence of partners (Donovan et al., 2018). Further, partner interactions can induce OT release (Burkett et al., 2016) and enhance OTR expression in the NAcc which facilitated partner preference formation in female prairie voles (Ross et al., 2009). In the present study, the lower levels of Iba-1 labeling in the NAcc in IMO males in the presence of their female partners may indicate partners’ social buffering effects by decreasing neuroinflammation via greater OT activity in the NAcc. Thus, the opposite patterns of OTR and Iba-1 expression in the NAcc in the social buffering paradigm suggest the opposing effects of OT and neuroimmune activity. Further, in a recent study in adolescent male rats, decreased microglia in the NAcc was found to be causally related to downregulation of DA D1-type receptors which regulate social play behavior, demonstrating a direct effect of microglia on the DA system in the NAcc (Kopec et al., 2018). NAcc and its neurochemicals, including OT and DA, have been implicated in social bonding behavior in prairie voles (Aragona et al., 2003; Wang and Aragona, 2004; Young et al., 2001). More interestingly, social interactions among the partners and familiar conspecifics are rewarding (Goodwin et al., 2019); NAcc OT and DA have been implicated in social reward (Berridge and Robinson, 1998; Dölen et al., 2013); and such reward component during social interactions may also play a role in social buffering (Lieberwirth and Wang, 2016). Together, these data indicate the importance of the NAcc in bidirectional interactions between microglia and neurochemicals as well as their functional roles in regulating stress responses and social interaction, reward, and buffering.
Indeed, microglia express receptors for several neurochemicals including OT, DA, CRH, and 5-HT (Herr et al., 2017; Kato et al., 2013; Loth and Donaldson, 2021) – neurochemicals that play crucial roles in social behaviors like pair bond formation in prairie voles (Aragona et al., 2003; DeVries et al., 1995, 2002). Although the research on CRH and microglial interactions are conflicting, there is evidence to suggest that CRH can induce microglial apoptosis (Ock et al., 2006), and this may be a self-regulating mechanism to control microglial presence in the brain (White et al., 1998). The interactions between 5-HT and microglia are even less studied, but in vitro studies of microglial migration demonstrate that microglial processes move towards 5-HT (Kolodziejczak et al., 2015) and 5-HT presence facilitates microglial migration towards injury (Krabbe et al., 2012). Nevertheless, the study of neuroimmune mechanisms underlying social behaviors is still in its infancy, but the continued study of the interactions of social behavior and inflammation in the context of stress is promising and requires future investigation (Muscatell, 2021). Likewise, as glia and neuron interactions are bidirectional (Eyo and Wu, 2013), the intersection of neuroimmune and neurochemical systems in the context of social and stress behavior is an essential avenue for further research.
4.3. Changes of PVN OT neurons in the female brain
Another interesting finding in the present study is the larger number of OT neurons in the PVN in females that were exposed to the stressed male partner. OT plays multifaceted roles in stress responses and social behavior (Marlin and Froemke, 2017; Nishioka et al., 1998; Smith et al., 2016; Steinman et al., 2019; Wang and Aragona, 2004; Wotjak et al., 1998). Social interaction leads to increased OT mRNA expression in the PVN in male mice (Murakami et al., 2011) and increased activation of PVN OT-containing neurons in male and female Syrian hamsters (Borland et al., 2018). In the context of stress, brain OT can have analgesic effects by reducing sensitivity to stressful stimuli (Robinson et al., 2002) as well as by suppressing the CRH system (Winter and Jurek, 2019), thereby providing a safeguarding mechanism against the effects of stress (Matsushita et al., 2019). In prairie voles, OT has been shown to modulate social salience neural networks and regulate pair bonding (Johnson et al., 2017; Liu et al., 2001; Williams et al., 1994; Young et al., 2011). Social isolation and IMO stress increase OT neurons in the PVN and facilitate OT release in the brain, and the activation of OT receptors can decrease anxiety-like behaviors (Grippo et al., 2007b; Neumann et al., 2000; Smith and Wang, 2014). OT has also been implicated in social buffering of stress responses (Lieberwirth and Wang, 2016; Peen et al., 2021). The stress response in prairie voles can be alleviated by interactions with a conspecific partner or OT administration, and such effects can be impaired by OT receptor antagonism (Burkett et al., 2016; Smith and Wang, 2014). Additionally, prairie voles exposed to a stressed partner engaged in more partner-directed social grooming behavior – an effect modulated by OT in the ACC (Burkett et al., 2016). A recent study demonstrated that the social transmission of maternal behavior, including the visual observation and acquisition of this behavior, was mediated by PVN OT (Carcea et al., 2021), supporting the pivotal role by OT in facilitating the social transmission of information and influencing social contagion.
In our study, females exhibited social approach to and interaction with the restrained male partner, leading to a social buffering effect on the male's stress responses. In addition, females displayed reductions in their own anxiety-like behaviors. Therefore, it is possible that greater amount of OT in the PVN was involved in each of those functions (i.e. social interaction, pair-bonding effects, partner-directed behaviors) directly and/or through reciprocal social contagion (Smith et al., 2016; Steinman et al., 2019). In other words, the reduction in the male's anxiety-like behavior due to the presence of the partner may, in turn, have influenced the female partner's anxiety-like behavior – a reduced anxiety state-matching between the individuals.
It should be noted that we did not see group differences in c-Fos/OT double labeling in the PVN in female voles. While this data may indicate a lack of group differences in OT neuron activation, we cannot exclude the possibility that, as Fos induction is stimulus- and neuron-type specific (Herrera and Robertson, 1996; Kawashima et al., 2014), it may not serve as an effective neuronal activation marker indicative of OT activation under our specific paradigm. Greater OT expression in the PVN has been used as an indication of increased OT production or transcription in response to the IMO experience (Grippo et al., 2007b; Liu et al., 2001, Liu et al., 2001).
Two additional sets of data are worth mentioning. First, less Fos labeling in the LS in both males and females in the partner group is interesting. The LS has been commonly seen as a node not only in mediating stress and anxiety responses (Anthony et al., 2014), but also in regulating social recognition and social interaction (Bielsky et al., 2005; Clemens et al., 2020). It has been shown that certain populations of neurons in the LS that project to the hypothalamus are important for the promotion of persistent anxiety-like behaviors (Anthony et al., 2014). The LS integrates signals from the external environment and internal state, and in symphony with neurochemicals like OT, works to elicit the appropriate behavioral response (Menon et al., 2021). The direct role of this multifaceted brain region in a social buffering interaction remains to be addressed. Second, although not tested in males, in female prairie voles, IMO stress increases circulating CORT and the partner's presence following IMO stress reverses stress-induced increases in CORT (Smith et al., 2013; Smith and Wang, 2014). As the partner's presence decreased anxiety-like behaviors in male voles, we expected lower levels of corresponding circulating CORT. The lack of such effects is surprising. We do not currently have a ready explanation for the lack of social buffering effects on circulating CORT in males. However, the trending increase in CORT in the males with the female presence is consistent with their elevated CRH activation in the PVN and supports the notion that partner associated cues and interaction activate HPA activity which facilitates social bonding in male prairie voles (DeVries et al., 2002). It should also be mentioned that the observed group differences in the neuronal/neurochemical activations and neuroimmune marker expression could be interpreted as the effects of partner-associated cues. However, the specific aspects of the social cues, the context and magnitude of the stressors, and their interactions will need to be considered and controlled for in further studies to parse out the effects of stress and social buffering.
Finally, in our initial behavioral experiment, free-moving females tended to interact more with the restrainer containing the partner, but not a conspecific stranger, and the latter seemed not as effective as the partner in alleviating the anxiety-like behaviors of the restrained stranger males (Fig. S1). These data support the finding of partner-specific social buffering in prairie voles (Burkett et al., 2016), and set up the foundation for our current study focusing on the familiar partners. However, omission of the additional controls indeed is a caveat in the present study as it limits our analysis and understanding of how social stimuli are transmitted and perceived during a stressful situation.
5. Conclusion
In summary, data from the present study shows reciprocal behavioral interactions between stressed male voles and their female partners via social signal transmission and social buffering. Such effects on social and anxiety-like behaviors were accompanied by a suite of neurochemical and neuroimmune responses. These data not only indicate the importance of social connectivity and social buffering under pair bonding conditions, but also illustrate the great utility of prairie voles as an ethologically relevant animal model for studying social buffering and stress contagion and underlying mechanistic circuitry integrating neurochemical, neuroimmune, and hormonal responses to shape these dynamic behaviors.
Authors' contributions
E.K.C. and Z.W. designed the study. E.K.C. and Y.L. performed experiments. E.K.C. and M.D. analyzed the data. E.K.C., M.D., and Z.W. wrote the manuscript. All authors discussed the results, provided critical feedback, and contributed to the final version of the manuscript.
Declaration of competing interest
All authors have no declared conflict of interest and have nothing to disclose.
Acknowledgements & Funding
This work was supported by grants from National Institutes of Health [NIMH R01-108527, NIMH R01-109450, and NIMH R21-111998] to Z.W. E.K.C. was supported by the NIH program training grant [T32 MH093311, P.K. Keel and L.A. Eckel] and the National Science Foundation Graduate Research Fellowship under Grant No. 1449440. M.D. was supported by the VA Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. The authors would like to thank Zeidi Jiminez, Isabel Fain, Caroline Henk, and Gabriela Petasne for assistance with data collection and analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2022.100427.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Althouse A.D. Adjust for multiple comparisons? It's not that simple. Ann. Thorac. Surg. 2016;101:1644–1645. doi: 10.1016/J.ATHORACSUR.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Anthony T.E., Dee N., Bernard A., Lerchner W., Heintz N., Anderson D.J. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell. 2014;156:522–536. doi: 10.1016/J.CELL.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona B.J., Liu Y., Curtis J.T., Stephan F.K., Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 2003;23:3483–3490. doi: 10.1523/jneurosci.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona B.J., Wang Z. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR J. 2004 doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Beery A.K., Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol. Stress. 2015;1:116–127. doi: 10.1016/J.YNSTR.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28:309–369. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bielsky I.F., Hu S.B., Ren X., Terwilliger E.F., Young L.J. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/J.NEURON.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Borland J.M., Aiani L.M., Norvelle A., Grantham K.N., O'Laughlin K., Terranova J.I., Frantz K.J., Albers H.E. Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacology. 2018;44:785–792. doi: 10.1038/s41386-018-0262-y. 2018, 4 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch O.J., Nair H.P., Ahern T.H., Neumann I.D., Young L.J. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T.W., Bagley S.L., Stansfield R.B., Preston S.D. The empathic, physiological resonance of stress. Soc. Neurosci. 2012;7:191–201. doi: 10.1080/17470919.2011.588723. [DOI] [PubMed] [Google Scholar]
- Burkett J.P., Andari E., Johnson Z.v., Curry D.C., de Waal F.B.M., Young L.J. Oxytocin-dependent consolation behavior in rodents. Science. 2016 doi: 10.1126/science.aac4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia M.A., Bonsall D.R., Bloomfield P.S., Selvaraj S., Barichello T., Howes O.D. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233:1637–1650. doi: 10.1007/S00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcea I., Caraballo N.L., Marlin B.J., Ooyama R., Riceberg J.S., Mendoza Navarro J.M., Opendak M., Diaz V.E., Schuster L., Alvarado Torres M.I., Lethin H., Ramos D., Minder J., Mendoza S.L., Bair-Marshall C.J., Samadjopoulos G.H., Hidema S., Falkner A., Lin D., Mar A., Wadghiri Y.Z., Nishimori K., Kikusui T., Mogi K., Sullivan R.M., Froemke R.C. Oxytocin neurons enable social transmission of maternal behaviour. Nature. 2021;596:553–557. doi: 10.1038/s41586-021-03814-7. 7873 596, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevali L., Montano N., Statello R., Coudé G., Vacondio F., Rivara S., Ferrari P.F., Sgoifo A. Social stress contagion in rats: behavioural, autonomic and neuroendocrine correlates. Psychoneuroendocrinology. 2017;82:155–163. doi: 10.1016/j.psyneuen.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Carrillo M., Han Y., Migliorati F., Liu M., Gazzola V., Keysers C. Emotional mirror neurons in the rat's anterior cingulate cortex. Curr. Biol. 2019;29:1301–1312. doi: 10.1016/j.cub.2019.03.024. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens A.M., Wang H., Brecht M. The lateral septum mediates kinship behavior in the rat. Nat. Commun. 2020;11(1 11):1–11. doi: 10.1038/s41467-020-16489-x. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Wills T.A. Psychological Bulletin; 1985. Stress, Social Support, and the Buffering Hypothesis. [DOI] [PubMed] [Google Scholar]
- D'Amato F.R., Pavone F. Reunion of separated sibling mice: neurobiological and behavioral aspects. Neurobiol. Learn. Mem. 1996;65:9–16. doi: 10.1006/nlme.1996.0002. [DOI] [PubMed] [Google Scholar]
- Davitz J.R., Mason D.J. Socially facilitated reduction of a fear response in rats. J. Comp. Physiol. Psychol. 1955;48:149–151. doi: 10.1037/h0046411. [DOI] [PubMed] [Google Scholar]
- De Waal F.B.M., Preston S.D. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 2017;18:498–509. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- DeVries A.C., DeVries M.B., Taymans S., Carter C.S. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc. Nat. Aca. Sci. United States of America. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries A.C., Devries M.B., Taymans S.E., Carter C.S. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc. Nat. Aca. Sci. United States of America. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries A.C., Guptaa T., Cardillo S., Cho M., Carter C.S. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/S0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Dölen G., Darvishzadeh A., Huang K.W., Malenka R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. 2013 501:7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M., Liu Y., Wang Z. Anxiety-like behavior and neuropeptide receptor expression in male and female prairie voles: the effects of stress and social buffering. Behav. Brain Res. 2018 doi: 10.1016/j.bbr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M., Mackey C.S., Platt G.N., Rounds J., Brown A.N., Trickey D.J., Liu Y., Jones K.M., Wang Z. Social isolation alters behavior, the gut-immune-brain axis, and neurochemical circuits in male and female prairie voles. Neurobiol. Stress. 2020;13:100278. doi: 10.1016/j.ynstr.2020.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I. An empirical review of the neural underpinnings of receiving and giving social support: implications for health. Psychosom. Med. 2013;75:545–556. doi: 10.1097/PSY.0b013e31829de2e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Taylor S.E., Gable S.L., Hilmert C.J., Lieberman M.D. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo U.B., Wu L.J. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013 doi: 10.1155/2013/456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez S.P., Gaspar P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology. 2012:144–154. doi: 10.1016/j.neuropharm.2011.08.049. Neuropharmacology. [DOI] [PubMed] [Google Scholar]
- Finnell J.E., Lombard C.M., Padi A.R., Moffitt C.M., Wilson L.B., Wood C.S., Wood S.K. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G., Fonken L.K., Watkins L.R., Maier S.F. Microglia: neuroimmune-sensors of stress. Semin. Cell Dev. Biol. 2019;94:176. doi: 10.1016/J.SEMCDB.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser O.N., Stahl D., Aureli F. Stress reduction through consolation in chimpanzees. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:8557–8562. doi: 10.1073/PNAS.0804141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudier-Diaz M.M., Zhang N., Haines A.H., Surbhi, Zhou M., DeVries A.C. Social interaction modulates the neuroinflammatory response to global cerebral ischemia in male mice. Brain Res. 2017;1673:86–94. doi: 10.1016/J.BRAINRES.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz L.L., Carter C.S., Gavish L. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav. Ecol. Sociobiol. 1981;8:189–194. doi: 10.1007/BF00299829. [DOI] [Google Scholar]
- Gobrogge K., Wang Z. Neuropeptidergic regulation of pair-bonding and stress buffering: lessons from voles. Horm. Behav. 2015;76:91–105. doi: 10.1016/J.YHBEH.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin N.L., Lopez S.A., Lee N.S., Beery A.K. Comparative role of reward in long-term peer and mate relationships in voles. Horm. Behav. 2019;111:70–77. doi: 10.1016/J.YHBEH.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Cushing B.S., Carter C.S. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom. Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Gerena D., Huang J., Kumar N., Shah M., Ughreja R., Sue Carter C. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme Graeff F., Zangrossi H., Jr. The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent. Nerv. Syst. Agents Med. Chem. 2012;10:207–217. doi: 10.2174/1871524911006030207. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R., Hostinar C.E. The social buffering of the hypothalamic–pituitary–adrenocortical axis in humans: developmental and experiential determinants. Soc. Neurosci. 2015 doi: 10.1080/17470919.2015.1070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykin H., Rolls A. The neuroimmune response during stress: a physiological perspective. Immunity. 2021;54:1933–1947. doi: 10.1016/J.IMMUNI.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lallement J., Gómez-Sotres P., Carrillo M. Towards a unified theory of emotional contagion in rodents—a meta-analysis. Neurosci. Biobehav. Rev. 2020 doi: 10.1016/j.neubiorev.2020.09.010. [DOI] [PubMed] [Google Scholar]
- Herr N., Bode C., Duerschmied D. The effects of serotonin in immune cells. Front. Cardiovas. Med. 2017 doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera D.G., Robertson H.A. Activation of c-fos in the brain. Prog. Neurobiol. 1996;50:83–107. doi: 10.1016/S0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hwang T.J., Rabheru K., Peisah C., Reichman W., Ikeda M. Loneliness and social isolation during the COVID-19 pandemic. Int. Psychogeriatr. 2020 doi: 10.1017/S1041610220000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R., Winslow J.T. Serotonin and neuropeptides in affiliative behaviors. Biol. Psychiatr. 1998 doi: 10.1016/S0006-3223(98)00094-8. [DOI] [PubMed] [Google Scholar]
- Jean-Baptiste N., Terleph T.A., Bamshad M. Changes in paternal responsiveness of prairie voles (Microtus ochrogaster) in response to olfactory cues and continuous physical contact with a female. Ethology. 2008;114:1239–1246. doi: 10.1111/J.1439-0310.2008.01577.X. [DOI] [Google Scholar]
- Jeon D., Kim S., Chetana M., Jo D., Ruley H.E., Lin S.Y., Rabah D., Kinet J.P., Shin H.S. Observational fear learning involves affective pain system and Ca v 1.2 Ca 2+ channels in ACC. Nat. Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste D.v., Lee E.E., Cacioppo S. Battling the modern behavioral epidemic of loneliness: suggestions for research and interventions. JAMA Psychiatr. 2020 doi: 10.1001/jamapsychiatry.2020.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Z.v., Walum H., Xiao Y., Riefkohl P.C., Young L.J. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm. Behav. 2017;87:16–24. doi: 10.1016/j.yhbeh.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T.A., Hayakawa K., Monji A., Kanba S. Missing and possible link between neuroendocrine factors, neuropsychiatric disorders and microglia. Front. Integr. Neurosci. 2013;7:53. doi: 10.3389/fnint.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T., Okuno H., Bito H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front. Neural Circ. 2014;8:37. doi: 10.3389/FNCIR.2014.00037/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y., Hennessy M.B. Comparative studies of social buffering: a consideration of approaches, terminology, and pitfalls. Neurosci. Biobehav. Rev. 2018 doi: 10.1016/j.neubiorev.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y., Kikusui T., Takeuchi Y., Mori Y. Partner's stress status influences social buffering effects in rats. Behav. Neurosci. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y., Kodama Y., Takeuchi Y., Mori Y. Physical interaction is not necessary for the induction of housing-type social buffering of conditioned hyperthermia in male rats. Behav. Brain Res. 2013;256:414–419. doi: 10.1016/j.bbr.2013.08.037. [DOI] [PubMed] [Google Scholar]
- Knapska E., Nikolaev E., Boguszewski P., Walasek G., Blaszczyk J., Kaczmarek L., Werka T. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc. Nat. Aca. Sci. United States of America. 2006;103:3858–3862. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczak M., Béchade C., Gervasi N., Irinopoulou T., Banas S.M., Cordier C., Rebsam A., Roumier A., Maroteaux L. Serotonin modulates developmental microglia via 5-HT2B receptors: potential implication during synaptic refinement of retinogeniculate projections. ACS Chem. Neurosci. 2015;6:1219–1230. doi: 10.1021/cn5003489. [DOI] [PubMed] [Google Scholar]
- Kopec A.M., Smith C.J., Ayre N.R., Sweat S.C., Bilbo S.D. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat. Commun. 2018;9:1–16. doi: 10.1038/s41467-018-06118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L.Á., Schiessl J.A., Nafz A.E., Csernus V., Gaszner B. Both basal and acute restraint stress-induced c-Fos expression is influenced by age in the extended amygdala and brainstem stress centers in male rats. Front. Aging Neurosci. 2018;10:248. doi: 10.3389/fnagi.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe G., Matyash V., Pannasch U., Mamer L., Boddeke H.W.G.M., Kettenmann H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav. Immun. 2012;26:419–428. doi: 10.1016/j.bbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Langford D.J., Tuttle A.H., Brown K., Deschenes S., Fischer D.B., Mutso A., Root K.C., Sotocinal S.G., Stern M.A., Mogil J.S., Sternberg W.F. Social approach to pain in laboratory mice. Soc. Neurosci. 2010;5:163–170. doi: 10.1080/17470910903216609. [DOI] [PubMed] [Google Scholar]
- Lenz K.M., Nelson L.H. Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 2018:698. doi: 10.3389/FIMMU.2018.00698. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.-F., Yuan W., He Z.-X., Ma H., Xun Y.-F., Meng L.-R., Zhu S.-J., Wang L.-M., Zhang J., Cai W.-Q., Zhang X.-N., Guo Q.-Q., Lian Z.-M., Jia R., Tai F.-D. Reduced consolation behaviors in physically stressed Mandarin voles: involvement of oxytocin, dopamine D2, and serotonin 1A receptors within the anterior cingulate cortex. Int. J. Neuropsychopharmacol. 2020;23:511–523. doi: 10.1093/IJNP/PYZ060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.-F., Zhang L.-Z., He Z.-X., Ma H., Zhang Y.-T., Xun Y.-F., Yuan W., Hou W.-J., Li Y.-T., Lv Z.-J., Jia R., Tai F.-D. Dorsal raphe nucleus to anterior cingulate cortex 5-HTergic neural circuit modulates consolation and sociability. Elife. 2021;10 doi: 10.7554/ELIFE.67638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C., Wang Z. The neurobiology of pair bond formation, bond disruption, and social buffering. Curr. Opin. Neurobiol. 2016;40:8–13. doi: 10.1016/j.conb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Curtis J.T., Fowler C.D., Spencer C., Houpt T., Wang Z.X. Differential expression of vasopressin, oxytocin and corticotrophin-releasing hormone messenger RNA in the paraventricular nucleus of the prairie vole brain following stress. J. Neuroendocrinol. 2001;13:1059–1065. doi: 10.1046/j.1365-2826.2001.00729.x. [DOI] [PubMed] [Google Scholar]
- Liu Yan, Curtis J.T., Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav. Neurosci. 2001;115:910–919. doi: 10.1037/0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y., Donovan M., Jia X., Wang Z. The ventromedial hypothalamic circuitry and male alloparental behaviour in a socially monogamous rodent species. Eur. J. Neurosci. 2019;50:3689–3701. doi: 10.1111/ejn.14550. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lieberwirth C., Jia X., Curtis J.T., Meredith M., Wang Z.X. Chemosensory cues affect amygdaloid neurogenesis and alter behaviors in the socially monogamous prairie vole. Eur. J. Neurosci. 2014;39:1632–1641. doi: 10.1111/ejn.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth M.K., Donaldson Z.R. Oxytocin, Dopamine, and opioid interactions underlying pair bonding: highlighting a potential role for microglia. Endocrinology. 2021 doi: 10.1210/endocr/bqaa223. (United States) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C.A., Johnson P.L., Hay-Schmidt A., Mikkelsen J., Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005 doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Marlin B.J., Froemke R.C. Oxytocin modulation of neural circuits for social behavior. Develop. Neurobiol. 2017 doi: 10.1002/dneu.22452. [DOI] [PubMed] [Google Scholar]
- Matsushita H., Latt H.M., Koga Y., Nishiki T., Matsui H. Oxytocin and stress: neural mechanisms, stress-related disorders, and therapeutic approaches. Neuroscience. 2019;417:1–10. doi: 10.1016/j.neuroscience.2019.07.046. [DOI] [PubMed] [Google Scholar]
- McNeal N., Scotti M.A.L., Wardwell J., Chandler D.L., Bates S.L., LaRocca M., Trahanas D.M., Grippo A.J. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci.: Basic and Clinical. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R., Süß T., Oliveira V.E. de M., Neumann I.D., Bludau A. Neurobiology of the lateral septum: regulation of social behavior. Trends Neurosci. 2021 doi: 10.1016/J.TINS.2021.10.010. [DOI] [PubMed] [Google Scholar]
- Meyza K.Z., Bartal I.B.A., Monfils M.H., Panksepp J.B., Knapska E. The roots of empathy: through the lens of rodent models. Neurosci. Biobehav. Rev. 2017;76:216–234. doi: 10.1016/J.NEUBIOREV.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami G., Hunter R.G., Fontaine C., Ribeiro A., Pfaff D. Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur. J. Neurosci. 2011;34:469–477. doi: 10.1111/J.1460-9568.2011.07761.X. [DOI] [PubMed] [Google Scholar]
- Muscatell K.A. Social psychoneuroimmunology: understanding bidirectional links between social experiences and the immune system. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2020.12.023. [DOI] [PubMed] [Google Scholar]
- Neumann I.D., Wigger A., Torner L., Holsboer F., Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J. Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Nishioka T., Anselmo-Franci J.A., Li P., Callahan M.F., Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998 doi: 10.1016/S0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Norman G.J., Karelina K., Morris J.S., Zhang N., Cochran M., Devries A.C. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosom. Med. 2010;72:519–526. doi: 10.1097/PSY.0B013E3181DE8678. [DOI] [PubMed] [Google Scholar]
- Ock J., Lee H., Kim S., Lee W.H., Choi D.K., Eun J.P., Kim S.H., In K.K., Suk K. Induction of microglial apoptosis by corticotropin-releasing hormone. J. Neurochem. 2006;98:962–972. doi: 10.1111/j.1471-4159.2006.03933.x. [DOI] [PubMed] [Google Scholar]
- Oliveira R.F., Faustino A.I. Social information use in threat perception: social buffering, contagion and facilitation of alarm responses. Commun. Integr. Biol. 2017 doi: 10.1080/19420889.2017.1325049. [DOI] [Google Scholar]
- Pan Y., Liu Y., Young K.A., Zhang Z., Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci. Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaro M.A., Benameur T., Porro C. Hypothalamic neuropeptide brain protection: focus on oxytocin. J. Clin. Med. 2020;9:1534. doi: 10.3390/jcm9051534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G., Solanki N., Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. Int. J. Neuropsychopharmacol. 2014;755:2017–2029. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peen N.F., Duque-Wilckens N., Trainor B.C. Convergent neuroendocrine mechanisms of social buffering and stress contagion. Horm. Behav. 2021 doi: 10.1016/j.yhbeh.2021.104933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisansky M.T., Hanson L.R., Gottesman I.I., Gewirtz J.C. Oxytocin enhances observational fear in mice. Nat. Commun. 2017;8:1–11. doi: 10.1038/s41467-017-02279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl T.T., Jung O., di Benedetto B., Young L.J., Bosch O.J. Microglia react to partner loss in a sex- and brain site-specific manner in prairie voles. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razai M.S., Oakeshott P., Kankam H., Galea S., Stokes-Lampard H. Mitigating the psychological effects of social isolation during the covid-19 pandemic. BMJ. 2020;369 doi: 10.1136/bmj.m1904. [DOI] [PubMed] [Google Scholar]
- Robinson D.A., Wei F., Wang G.D., Li P., Kim S.J., Vogt S.K., Muglia L.J., Zhuo M. Oxytocin mediates stress-induced analgesia in adult mice. J. Physiol. 2002 doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H.E., Freeman S.M., Spiegel L.L., Ren X., Terwilliger E.F., Young L.J. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland N.E., Dunn A.J. Effect of dexfenfluramine on metabolic and neurochemical measures in restraint-stressed ob/ob mice. Physiol. Behav. 1995;58:749–754. doi: 10.1016/0031-9384(95)00105-R. [DOI] [PubMed] [Google Scholar]
- Rukstalis M., French J.A. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm. Behav. 2005;47:1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J., Mayford M., Jeste D. Empathic fear responses in mice are triggered by recognition of a shared experience. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.Z., Crockett M.J. How serotonin shapes moral judgment and behavior. Ann. N. Y. Acad. Sci. 2013 doi: 10.1111/nyas.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.S., Lieberwirth C., Wang Z. Behavioral and physiological responses of female prairie voles (Microtus ochrogaster) to various stressful conditions. Stress. 2013 doi: 10.3109/10253890.2013.794449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.S., Tabbaa M., Lei K., Eastham P., Butler M.J., Linton L., Altshuler R., Liu Y., Wang Z. Local oxytocin tempers anxiety by activating GABAA receptors in the hypothalamic paraventricular nucleus. Psychoneuroendocrinology. 2016 doi: 10.1016/j.psyneuen.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.S., Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatr. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.S., Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm. Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman M.Q., Duque-Wilckens N., Trainor B.C. Complementary neural circuits for divergent effects of oxytocin: social approach versus social anxiety. Biol. Psychiatr. 2019 doi: 10.1016/j.biopsych.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterley T.L., Baimoukhametova D., Füzesi T., Zurek A.A., Daviu N., Rasiah N.P., Rosenegger D., Bains J.S. Social transmission and buffering of synaptic changes after stress. Nat. Neurosci. 2018 doi: 10.1038/s41593-017-0044-6. [DOI] [PubMed] [Google Scholar]
- Stowe J.R., Liu Y., Curtis J.T., Freeman M.E., Wang Z. Species differences in anxiety-related responses in male prairie and meadow voles: the effects of social isolation. Physiol. Behav. 2005;86:369–378. doi: 10.1016/j.physbeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Sun P., Smith A.S., Lei K., Liu Y., Wang Z. Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res. 2014 doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kiyokawa Y., Kodama Y., Arata S., Takeuchi Y., Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav. Brain Res. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Tubbiola M.L., Wysocki C.J. FOS immunoreactivity after exposure to conspecific or heterospecific urine: where are chemosensory cues sorted? Physiol. Behav. 1997;62:867–870. doi: 10.1016/S0031-9384(97)00254-0. [DOI] [PubMed] [Google Scholar]
- Valentino R.J., Lucki I., Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Aragona B.J. Neurochemical regulation of pair bonding in male prairie voles. Physiol. Behav. 2004;83:319–328. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- White C.A., McCombe P.A., Pender M.P. Microglia are more susceptible than macrophages to apoptosis in the central nervous system in experimental autoimmune encephalomyelitis through a mechanism not involving Fas (CD95) Int. Immunol. 1998;10:935–941. doi: 10.1093/intimm/10.7.935. [DOI] [PubMed] [Google Scholar]
- Williams J.R., Insel T.R., Harbaugh C.R., Carter C.S. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J. Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Williams J.R., Slotnick B.M., Kirkpatrick B.W., Carter C.S. Olfactory bulb removal affects partner preference development and estrus induction in female prairie voles. Physiol. Behav. 1992;52:635–639. doi: 10.1016/0031-9384(92)90390-N. [DOI] [PubMed] [Google Scholar]
- Windle R.J., Kershaw Y.M., Shanks N., Wood S.A., Lightman S.L., Ingram C.D. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. : Off. J. Soc. Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J., Jurek B. The interplay between oxytocin and the CRF system: regulation of the stress response. Cell Tissue Res. 2019 doi: 10.1007/s00441-018-2866-2. [DOI] [PubMed] [Google Scholar]
- Wotjak C.T., Ganster J., Kohl G., Holsboer F., Landgraf R., Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998 doi: 10.1016/S0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Wu B. Social isolation and loneliness among older adults in the context of COVID-19: a global challenge. Global Health Res. Policy. 2020;5:1–3. doi: 10.1186/s41256-020-00154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.A., Gobrogge K.L., Liu Y., Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.J., Lim M.M., Gingrich B., Insel T.R. Cellular mechanisms of social attachment. Horm. Behav. 2001 doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young S.N., Leyton M. The role of serotonin in human mood and social interaction: insight from altered tryptophan levels. Pharmacol. Biochem. Behav. 2002 doi: 10.1016/S0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Yuan L., Liu S., Bai X., Gao Y., Liu G., Wang X., Liu D., Li T., Hao A., Wang Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflammation. 2016;13:77. doi: 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.