Abstract
Background:
After Emergency Use Authorization of the coronavirus disease 2019 (COVID-19) vaccines, guidance was provided by the Centers for Disease Control and Prevention that persons with an immediate allergic reaction to a messenger RNA (mRNA) COVID-19 vaccine should be evaluated by an allergist/immunologist before receipt of the second dose.
Methods:
In vaccinating health-care personnel, we referred those with significant reactions to allergy/immunology specialists so that they could safely receive the second dose.
Results:
We found that many reactions after the first dose were nonallergic but could be debilitating and a barrier to the second dose. We created a protocol of premedications to allow health-care personnel to safely receive their second mRNA COVID-19 vaccine dose.
Conclusion:
This protocol is adaptable and can be used in settings where allergy/immunology referral is not immediately available.
Keywords: COVID-19, mRNA vaccine, vaccine reactions, allergy evaluation
As the full extent of the coronavirus disease 2019 (COVID-19) pandemic was felt worldwide, the rapid development of vaccines against SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), which causes COVID-19, was launched. The first vaccines developed used messenger RNA (mRNA) vaccine technology to express the SARS-CoV-2 spike protein. In December 2020, Emergency Use Authorizations were issued by the U.S. Food and Drug Administration for Pfizer-BioNTech (Pfizer, New York, NY, USA; BioNTech, Mainz, Germany) and Moderna (Moderna, Cambridge, MA, USA) COVID-19 mRNA vaccines. Health-care personnel were among the first people to be vaccinated outside of clinical trials. Persons with an immediate allergic reaction to an mRNA COVID-19 vaccine or any of its components are at greater risk for anaphylaxis on re-exposure1,2 and should not receive another dose until evaluated by an allergist/immunologist.3 Our aim was to safely vaccinate eligible employees with both mRNA COVID-19 vaccine doses.
METHODS
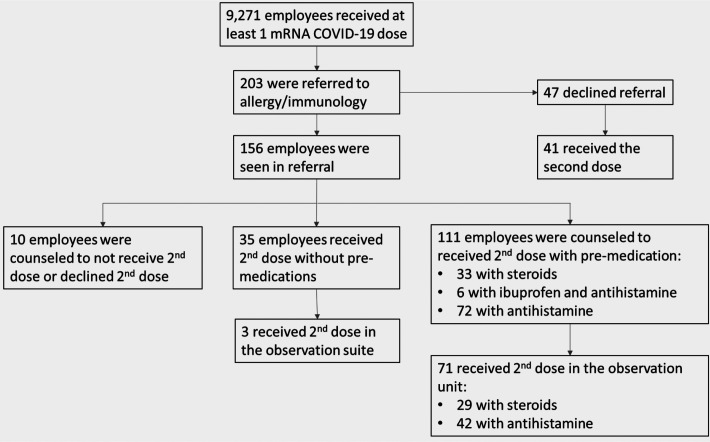
Employees at Baystate Health System (Springfield, Massachusetts) with an immediate allergic reaction or a severe debilitating reaction that interfered with usual activities after the first dose of an mRNA COVID-19 vaccine were referred to an allergist/immunologist. This project was conducted as part of a quality-improvement project and was deemed to not require institutional review board review by the Baystate Health System. From December 16, 2020, to February 16, 2021, 9271 employees received their first vaccine dose, with 2457 (26.5%) reporting reactions and 16 (0.2%) requiring transport to the emergency department. Most reactions (2200 [89.5%]) were consistent with expected vaccination effects or were related to anxiety; 203 employees (8.2%) were offered an allergy/immunology referral, with 156 (76.8%) being evaluated (Fig. 1). A total of 134 (85.9%) of those evaluated were women. Seventy-one (46.4%) (including 59 women [83.1%]) had nonallergic systemic symptoms, including fevers, chills, myalgias, malaise; and 44 (28.8%) (including 42 women [95.5%]) had allergic reactions, including pruritis, hives, and/or throat swelling. The remainder included injection-site reactions, vasovagal response, or asthma exacerbations. Among the 71 with nonallergic systemic reactions, 38 had previous COVID-19 infection, many of whom reported severe symptoms.
Figure 1.
Employees with reactions to the first coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine dose.
A modified protocol for an intravenous contrast anaphylactoid reaction was recommended to lessen severe systemic symptoms: 50 mg of prednisone given 13 hours, 7 hours, and 1 hour before the second dose as well as 5 mg of levocetirizine daily given 1 day before, the day of, and 1 week after the second dose. Levocetirizine was substituted for diphenhydramine to avoid drowsiness. This modified protocol was used because it effectively allows patients to receive a single dose of a drug to which they would otherwise have a severe reaction. Employees with mild-to-moderate systemic symptoms were pretreated with 400 mg of ibuprofen every 8 hours starting 2 hours before and continuing for 2 days after the second dose as well as 5 mg of levocetirizine daily given 1 day before, the day of, and 1 week after the second dose. Thirty-nine employees experienced delayed reactions, including rashes, pruritis, and hives, and were advised to pretreat with antihistamine with 5 mg of levocetirizine daily given 1 day before, the day of, and 1 week after the second dose. All pretreated employees were prescribed self-injectable epinephrine. Four employees declined to receive the second dose, and six were advised against receiving the second dose due to allergic reactions that required epinephrine (3), macular urticaria (1), facial numbness (1), and severe hives (1).
Employees who received steroids and those with concern for a non-anaphylactic allergic reaction were advised to receive the second dose in a monitored setting: a hospital observation unit with a 2-hour monitoring period. A rapid-response team, code cart, and anaphylaxis kits were immediately available. Fourteen of 70 employees (20%) vaccinated in the observation unit required intervention, including diphenhydramine (5 [35.7%]), acetaminophen (6 [42.9%]), ondansetron (2 [14.3%]), and intravenous fluids (2 [14.3%]). None required epinephrine or hospital admission.
One hundred and eleven employees received their second dose with premedications (Fig. 1); 35 employees did not require premedications. Seventy-two (64.9%) were premedicated with antihistamines, 42 of those in the observation unit. Six (5.4%) were premedicated with ibuprofen, none in the observation unit. Thirty-three (29.7%) were premedicated with steroids, 29 of those in the observation unit. Three employees had severe anxiety or concerns of syncope and received the second dose in the observation unit without premedications. All employees received their second dose within 6 weeks of receipt of the first dose, and the observation unit was fully staffed during this time frame to avoid delays beyond that point. Of the 33 employees premedicated with steroids, 24 agreed to qualitative testing for COVID-19 spike protein immunoglobulin G 2–3 weeks after the Pfizer-BioNTech and Moderna vaccines, respectively. All 24 tested were positive.
DISCUSSION
In this report, we described efforts to ensure that employees with reactions to their first mRNA vaccine dose could safely receive a second dose. The employees' anxiety was reduced, and we did not find evidence that steroids impaired spike protein antibody detection when using a qualitative assay. Although a protocol for using antihistamines for mild allergic reactions has been previously reported,4 to our knowledge, this was the first report that looked at the use of steroids to blunt severe systemic reactions. Although the Centers for Disease Control and Prevention recommends an allergy/immunology evaluation for people with an immediate allergic reaction to a COVID-19 mRNA vaccine or any of its components, it does not provide recommendations with regard to other reactions. We found that nonallergic systemic reactions were common and a potential barrier to future vaccine doses, similar to other reports.5 Where allergy/immunology evaluation is not readily available, this protocol can provide a framework to counsel people about safely getting a second dose or booster doses, if required. This framework is more important now in the setting of the delta variant, in which vaccine efficacy is significantly lower with only one dose of vaccine; 30.7% after just one dose compared with 88.0% after two doses.6
Postvaccine testing for employees premedicated with steroids only assessed immunoglobulin G levels, so we were unable to determine if the steroids lessened a cell-mediated immune response. We also only had a qualitative antibody assay available, so were unable to report antibody titers. This report only summarized our experience with mRNA COVID-19 vaccines and may not generalize to alternative COVID-19 vaccines.
CONCLUSION
This protocol provides a framework of pre-medications and monitored setting to allow people with a previous reaction to safely get a second dose or booster dose.
Footnotes
J. Bayuk reports personal fees from GSK, Astra-Zeneca, Takeda, Sanofi, CSL-Behring, and Grifols. The remaining authors have no conflicts of interest to declare pertaining to this article
No external funding sources reported
REFERENCES
- 1. CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021; 70:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine — United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. Available online at https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html; accessed 2/16/2021.
- 4. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021; 9:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelso JM. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann Allergy Asthma Immunol. 2021; 127:133–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernal JL, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Eng J Med. 2021; 385:585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]