Abstract
Background:
As the vaccination campaign in response to the coronavirus disease 2019 (COVID-19) pandemic continues, concerns with regard to adverse reactions to the vaccine remain. Although immediate hypersensitivity reactions have received much attention, delayed systemic urticarial reactions after vaccination can occur.
Objective:
To describe the clinical presentation, vaccine excipient skin testing results, and outcomes of subsequent COVID-19 vaccination in patients who experienced delayed systemic urticarial reactions after messenger RNA (mRNA) COVID-19 vaccination.
Methods:
This was a retrospective case series of 12 patients referred to the Mayo Clinics in Rochester, Minnesota, and Jacksonville, Florida, between January 19, 2021, and April 30, 2021, for evaluation of delayed systemic urticarial reactions after mRNA COVID-19 vaccination. Demographics, medical and allergic history, reaction details, vaccine excipient skin testing results (when performed), and the outcome after subsequent vaccination were collected for each patient.
Results:
The mean age of the patients was 52 years, all were white, and 9 (75%) were women. Half of the patients had a history of drug allergy, and one had a history of chronic spontaneous urticaria. Seven patients reacted to the Pfizer-BioNTech vaccine and five reacted to the Moderna vaccine. Seven patients developed symptoms between 8 and 24 hours after vaccination. Nine patients required antihistamines for treatment. The median time to symptom resolution was 4 days. Nine patients underwent allergist-directed COVID-19 vaccine excipient skin testing, all of which were negative. Ten patients chose to receive their next mRNA COVID-19 vaccine dose, and four patients experienced recurrent delayed urticaria.
Conclusion:
Delayed systemic urticarial reactions after mRNA COVID-19 vaccination were not life-threatening, could be treated with antihistamines, and were not predicted with vaccine excipient skin testing. They were not a contraindication to subsequent vaccination, although patients should be counseled with regard to the possibility of recurrence.
Keywords: COVID-19, urticaria, mRNA, vaccine, hypersensitivity, polyethylene glycol, excipient
In December 2020, the U.S. Food and Drug Administration issued Emergency Use Authorizations for two messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccines, from Pfizer-BioNTech (Pfizer Inc, New York, New York, BioNTech SE, Mainz, Germany) and from Moderna (Moderna Inc, Cambridge, Massachusetts), with > 300 million doses administered in the United States. Contraindications to mRNA vaccination include a history of immediate allergic reaction to a component or previous dose of an mRNA COVID-19 vaccine.1,2 Although much attention has been given to immediate hypersensitivity to the vaccines, delayed reactions, including delayed cutaneous eruptions, do occur and have been reported in clinical trials that led up to product licensure under Emergency Use Authorization.3,4 The mechanism of allergic reactions, both immediate and delayed, is currently unknown, although active and inactive vaccine components, e.g., polyethylene glycol, have been proposed as possible culprit antigens.5,6 The risk of a subsequent vaccination after an allergic reaction to an initial vaccine dose is also unclear, although previous reports have demonstrated safe vaccination after an immediate reaction to an mRNA vaccine dose.7–9 We report on 12 patients from two academic medical centers who had a delayed systemic urticarial eruption (>4 hours after vaccination) after a dose of an mRNA COVID-19 vaccine.
METHODS
A retrospective case series study was performed at the Mayo Clinics in Rochester, Minnesota, and Jacksonville, Florida, to describe the clinical characteristics and outcomes of patients with delayed systemic urticarial eruptions after COVID-19 vaccination. Between January 19, 2021, and April 30, 2021, 12 patients referred to the allergy clinic of the aforementioned institutions were evaluated and included in the study. A delayed reaction was defined as one with symptom onset > 4 hours after mRNA COVID-19 vaccination. A diagnosis of urticaria was made by the allergist based on historical features at the time of initial evaluation. Demographics, medical and allergic history, COVID-19 vaccine reaction details, COVID-19 vaccine excipient skin testing results, and outcomes after subsequent COVID-19 vaccination were extracted by manual chart review. Vaccine excipient skin testing was performed according to previously published methods, with the clinical need for testing determined by the provider at the time of the visit.7,10 This study was approved by Mayo Clinic Institutional Review Board.
RESULTS
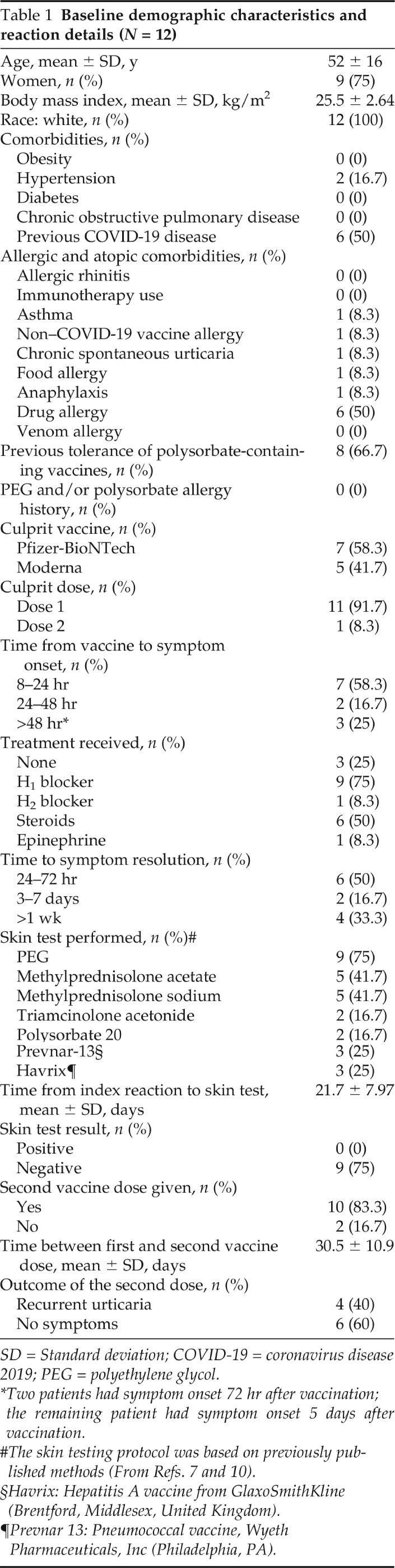
The clinical characteristics, reaction details, and outcome of subsequent vaccination are shown in Table 1. The mean age of the patients was 52 years, all were white, and 9 (75%) were women. Although half had a history of drug allergy, there otherwise was a minimal amount of allergic or nonallergic comorbidities, including only one patient with preexisting chronic spontaneous urticaria that was well controlled at the time of vaccination. None of the reactions represented anaphylaxis, and there were no significant noncutaneous symptoms. Seven patients had a reaction to the Pfizer-BioNTech vaccine; the other five reacted to the Moderna vaccine; and 11 of 12 had a reaction to their first dose. Seven patients developed symptoms between 8 and 24 hours after vaccination, two patients developed symptoms between 24 and 48 hours after vaccination, and the remaining three had symptoms >48 hours after vaccination (72 hours for two patients, 5 days for the remaining patient). Nine patients were treated with antihistamines, and six received oral systemic steroids in addition to antihistamines. The median time to symptom resolution was 4 days (interquartile range, 1–27 days). Nine patients underwent COVID-19 vaccine excipient skin testing, by using previously published methods, all of which were negative; however, no delayed intradermal reading was performed.7,10 Of the 11 patients who developed delayed urticaria with the first dose, 10 received their second dose, and 1 patient deferred additional doses. Two of these 10 were counseled to premedicate with oral antihistamines before their second dose, and neither developed recurrent urticaria after their second dose. In total, 4 of these 10 patients developed recurrent delayed urticaria. The time to symptom onset and time to symptom resolution were similar to each individual patient's index reaction to the first vaccine dose. All four were treated successfully with oral antihistamines.
Table 1.
Baseline demographic characteristics and reaction details (N = 12)
SD = Standard deviation; COVID-19 = coronavirus disease 2019; PEG = polyethylene glycol.
*Two patients had symptom onset 72 hr after vaccination; the remaining patient had symptom onset 5 days after vaccination.
§Havrix: Hepatitis A vaccine from GlaxoSmithKline (Brentford, Middlesex, United Kingdom).
¶Prevnar 13: Pneumococcal vaccine, Wyeth Pharmaceuticals, Inc (Philadelphia, PA).
DISCUSSION
This was one of the earliest case series that described delayed systemic urticarial reactions to the mRNA COVID-19 vaccines. Delayed cutaneous reactions have been previously reported by multiple groups, although the majority of these were large local reactions contiguous with the site of vaccination, which is the most common cutaneous adverse reaction reported in the nontrial literature.11–14 McMahon et al.13 published one of the earliest reports that detail cutaneous adverse reactions to the mRNA COVID-19 vaccines. In this report of 414 cutaneous adverse reactions, there were 23 instances of urticaria after Moderna COVID-19 vaccination (18 occurred > 24 hours after vaccination) and 17 instances of urticaria after Pfizer-BioNTech COVID-19 vaccination (16 occurred > 24 hours after vaccination).13 In the 18 patients who had urticaria after their first vaccine dose, 4 experienced recurrent urticaria with their second dose, which is slightly lower than the rate of 4 of 10 we report here.13 In addition, a large Italian study of 2740 patients reported 50 cutaneous adverse reactions after the mRNA COVID-19 vaccines and the viral vector COVID-19 vaccine from AstraZeneca (AstraZeneca PLC, Cambridge, England).15 Of these 50 reactions, 14 were urticarial in nature.15 Both of these large studies stress the ability to proceed with subsequent vaccination despite a history of a cutaneous adverse reaction.13,15 Urticaria occurred at a similar rate in both the placebo and the treatment groups in the original Moderna clinical trial (0.2%), although the time course of this is unclear.3
Although polyethylene glycol has been proposed as a possible culprit for immediate hypersensitivity reactions to the mRNA vaccines, this has not been proven, and the mechanism of reaction (both immediate and delayed) remains to be elucidated.5,6 The aforementioned reports with regard to delayed local cutaneous reactions both reported histopathologic data that was suggestive of T-cell–mediated reactions.11,12 An interesting observation in our cohort was the fact that half of the patients previously had COVID-19, as did three of the four patients who had recurrent delayed urticaria after their second vaccine dose. It could be hypothesized that these patients develop a memory T-cell response to a component of both the severe acute respiratory syndrome coronavirus 2 and the mRNA COVID-19 vaccine, viz., the spike protein. An alternative explanation could be that delayed T-cell responses to the vaccine components or excipients are to blame, in which case, patch testing or delayed intradermal testing to these components may be helpful.
Limitations of this study included its retrospective nature. In addition, the utility of premedication with antihistamines before a subsequent mRNA COVID-19 vaccine dose after experiencing a delayed urticarial reaction after a previous dose was not robustly evaluated. Also, our study design does not easily allow for determination of the mechanism of delayed urticarial reactions. Although a T-cell–mediated process detected by delayed intradermal skin testing may be hypothesized, the use of corticosteroids in the treatment of these reactions may limit this approach.
CONCLUSION
The most important point from this series is that all the patients who received a second dose after a first dose reaction were able to do so without serious life-threatening consequences. Although 4 of 10 patients did have recurrent urticaria after their second dose, delayed urticarial reactions are not a contraindication to future vaccine doses. Patients should be counseled that there is the potential for recurrent urticaria, which can safely be managed with antihistamines. This needs to be weighed against the likely greater risk of incomplete vaccination and contracting COVID-19, which can be life-threatening despite adequate treatment. In addition, the use of skin-prick and intradermal testing in evaluating delayed reactions is likely to be of little utility in either diagnosing or predicting the risk of recurrent symptoms on re-exposure, although delayed intradermal reading could prove useful in this regard. All these points should be considered when counseling and evaluating patients, with the goal being the safe and complete vaccination of the population as a whole.
Footnotes
The authors report no conflicts of interest pertaining to this article
No external finding sources reported
REFERENCES
- 1. Centers for Disease Control and Prevention. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States—Appendix B. Available online at https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html#Appendix-B; accessed December 24, 2020.
- 2. Turner PJ, Ansotegui IJ, Campbell DE, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021; 14:100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cabanillas B, Akdis C, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021; 76:1617–1618. [DOI] [PubMed] [Google Scholar]
- 6. Risma KA, Edwards KM, Hummell DS, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021; 147:2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pitlick MM, Sitek AN, Kinate SA, et al. Polyethylene glycol and polysorbate skin testing in the evaluation of coronavirus disease 2019 vaccine reactions: early report. Ann Allergy Asthma Immunol. 2021; 126:735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolfson AR, Robinson LB, Li L, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021; 9:3308–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arroliga ME, Dhanani K, Arroliga AC, et al. Allergic reactions and adverse events associated with administration of mRNA-based vaccines. A health-care system experience. Allergy Asthma Proc. 2021; 42:395–399. [DOI] [PubMed] [Google Scholar]
- 10. Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021; 9:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021; 384:1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnston MS, Galan A, Watsky K, et al. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021; 157:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021; 85:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun Q, Fathy R, McMahon DE, et al. COVID-19 vaccines and the skin: the landscape of cutaneous adverse reactions worldwide. Dermatol Clin. 2021; 39:653–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grieco T, Maddalena P, Sernicola A, et al. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: an observational study. Dermatol Ther. 2021; e15153. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]