Abstract
Enriching mitochondrial DNA (mtDNA) for sequencing entire mitochondrial genomes (mitogenomes) can be achieved by single long-range PCR. This avoids interference from the omnipresent nuclear mtDNA sequences (NUMTs). The approach is currently restricted to the use of samples collected from humans and ray-finned fishes. Here, we extended the use of single long-range PCR by introducing back-to-back oligonucleotides that target a sequence of extraordinary homology across vertebrates. The assay was applied to five hibernating rodents, namely alpine marmot, Arctic and European ground squirrels, and common and garden dormice, four of which have not been fully sequenced before. Analysis of the novel mitogenomes focussed on the prediction of mitochondrial-derived peptides (MDPs) providing another level of information encoded by mtDNA. The comparison of MOTS-c, SHLP4 and SHLP6 sequences across vertebrate species identified segments of high homology that argue for future experimentation. In addition, we evaluated four candidate polymorphisms replacing an amino acid in mitochondrially encoded subunits of the oxidative phosphorylation (OXPHOS) system that were reported in relation to cold-adaptation. No obvious pattern was found for the diverse sets of mammalian species that either apply daily or multiday torpor or otherwise cope with cold. In summary, our single long-range PCR assay applying a pair of back-to-back primers that target a consensus sequence motif of Vertebrata has potential to amplify (intact) mitochondrial rings present in templates from a taxonomically diverse range of vertebrates. It could be promising for studying novel mitogenomes, mitotypes of a population and mitochondrial heteroplasmy in a sensitive, straightforward and flexible manner.
Keywords: mitogenome, long-range PCR, back-to-back amplification primers, next-generation sequencing, mitochondrial-derived peptides
Introduction
Vertebrate mitochondria harbour a double-stranded circular mitogenome with a general size between 14 and 20 kb (Kolesnikov and Gerasimov, 2012). It is maternally inherited and exists in a highly polyploid state. The cellular copy number of mitochondrial DNA (mtDNA) ranges from ∼400 in peripheral blood mononuclear cells (O’Hara et al., 2019) over 6,000 or 7,000 copies in cells with high ATP requirement such as hepatocytes or cardiomyocytes (Miller et al., 2003; Yin et al., 2004), respectively, up to >200,000 in mammalian oocytes (Cree et al., 2008). Typically, the vertebrate mitogenome has a highly conserved organization with only 37 canonical genes that encode 13 of 83 protein subunits involved in electron transport and oxidative phosphorylation, 22 transfer RNAs (tRNAs), two ribosomal RNAs (rRNAs), a minor noncoding region (NCR) containing the origin of DNA replication for the light (guanine-poor) strand (O L ) and one longer NCR also referred to as the control region. The main NCR harbours the promoters for polycistronic transcription, one for each mtDNA strand, the replication origin of the heavy (guanine-rich) strand (O H ), a triple-stranded displacement-loop (D-loop) structure incorporating the mitochondrial single-stranded DNA molecule 7S-DNA and other prominent elements such as the conserved sequence boxes CSB1-3 or the termination-associated sequence (TAS (Gammage and Frezza, 2019)). Occasionally, variable numbers of tandem repeats (VNTRs; alias: minisatellites) are inserted within the main NCR (Lunt et al., 1998) or in closed proximity between two tRNA genes (Gorički and Trontelj, 2006; Shu et al., 2018). Short tandem repeats (STRs; alias: microsatellites), rarer cases of mtDNA length polymorphism, have also been reported for the main NCR (Sbisà et al., 1997; Feeroz et al., 2008).
Complexity is added to the genome by ‘dwarf’ short or small open reading frames (‘dwarf’ sORFs or smORFs) of ∼20 amino acids (Aspden et al., 2014). These “Russian doll genes” located in the rRNA genes MT-RNR1 and MT-RNR2 as well as in the protein-coding gene MT-CO1 encode a group of dwarf peptides termed MDPs. They comprise MOTS-c (mitochondrial ORF of the 12S rRNA type-c; (Lee et al., 2015), humanin (Hashimoto et al., 2001), the small humanin-like peptides 1 to 6 (SHLP1 to 6 (Cobb et al., 2016)), and gau microprotein (gene antisense ubiquitous in mtDNAs (Faure et al., 2011)). A unique feature of MDPs represents the combination of intra-mitochondrial transcription with cytoplasmic translation according to the nuclear genetic code (Cobb et al., 2016; Lee et al., 2016). In case of human MOTS-c, the “gold-standard” technology of liquid chromatography-mass spectrometry successfully detected the synthetic peptide used as positive assay control. However, the test failed to detect the circulating cell-free form of MOTS-c in human plasma samples irrespectively of the success in ELISA-based measurement (Knoop et al., 2019). MDPs regulate apoptosis and metabolic homeostasis, insulin sensitivity and obesity, reduce oxidative stress and inflammation, extend life expectancy, signal retrograde communication of mitochondria with the nucleus, protect against cancer and Alzheimer´s disease, show decreasing expression with age (Cobb et al., 2016; Kim et al., 2017; Lu et al., 2019) and bear considerable potential to be misused as a performance-enhancing drug in elite sports (Thevis and Schänzer, 2016). Considering their key functionalities in the human organism, the existence of orthologous MDPs in other vertebrate species is implicated.
Whole-genome sequencing (WGS) represents a straightforward approach for assembling the mitochondrial genome (Feldmeyer et al., 2010). Low-pass, shallow sequencing of a genome, termed ‘genome skimming’, can be used to assemble the entire mitogenome from <1% of the reads (Liu et al., 2016). Concomitantly, this shotgun sequencing approach delivers valuable genetic information about the high-copy fraction of the nuclear genome such as rRNA sequences, transposons and high-copy-number genes (Trevisan et al., 2019; Bohmann et al., 2020; Malukiewicz et al., 2021; Margaryan et al., 2021). Reliable mitogenome assembly can be obtained from genome skimming data (∼1 Gb) at an estimated cost of sequencing of 25 € per specimen (∼100 samples per lane, 200 × mtDNA depth; pricing of 2019 (Margaryan et al., 2021)). This would be equivalent with less than 17.3 € (20 $) per 1 Gb on Illumina’s NovaSeq 6,000 Sequencing System according to the current pricing.
However, this calculation does not consider the recent recommendation of the Vertebrate Genomes Project (www.vertebrategenomesproject.org) to assemble a mitogenome by combining WGS long reads for structural accuracy and WGS short reads for base calling accuracy (Formenti et al., 2021). The so-called ‘hybrid assembly’ helps to cope with the widespread presence of sequence duplications and repeats occurring in vertebrates mitogenomes, namely the (prevalent) short repeats of the NCR, but especially the (rarer) relatively large repetitive regions (Gibb et al., 2007; Satoh et al., 2016; Rhie et al., 2021). Third-generation approaches for long-read sequencing such as Oxford Nanopore technology or PacBio sequencing are options of choice to overcome this challenge (Kingan et al., 2019; Dhorne-Pollet et al., 2020). Moreover, the homology between NUMTs and mtDNA can confound the outcome of next generation sequencing (NGS) pipelines that align short reads of DNA to the reference genome based on sequence similarity (Maude et al., 2019). NUMTs represent a general evolutionary trend across vertebrate species (Puertas and González-Sánchez, 2020). They can occur at up to ∼1,000 integration sites (Calabrese et al., 2012; Dayama et al., 2014), vary in size from smaller fragments to one or several full-length mitochondrial integrations like in case of a bat species (Shi et al., 2017) or healthy humans (Lutz-Bonengel et al., 2021), share varying degrees of homology up to complete identity with parental mtDNA depending on the evolutionary time of the insertion event (Dayama et al., 2014; Wei and Chinnery, 2020).
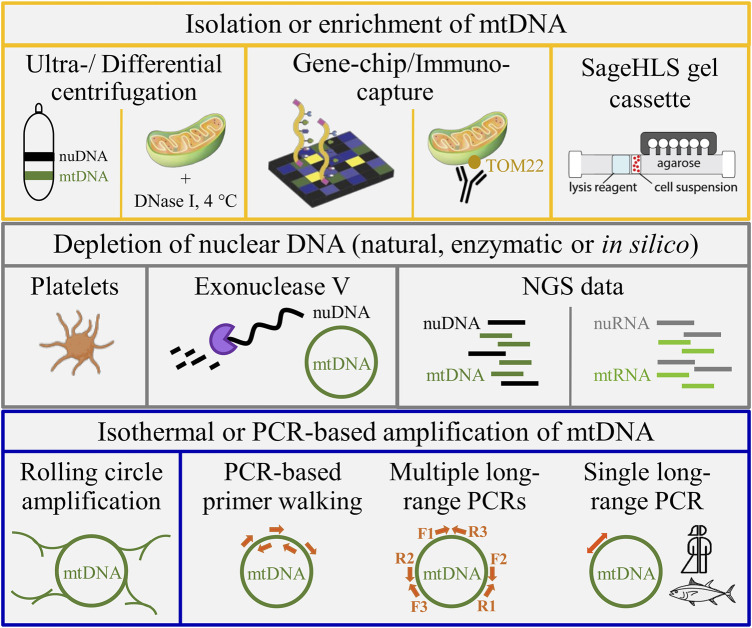
There are several ways for reducing the amount of undesired nuclear DNA (nuDNA) reads, thus solving the redundancy challenge in sequencing, mapping, and genotyping caused by NUMTs (Figure 1). Classically, contaminating nuDNA was depleted from a total extract of cellular DNA based on its different buoyant density using caesium chloride density gradient ultra-centrifugation (Carr and Griffith, 1987). While this approach is effective, there are several drawbacks such as the use of dangerous and toxic chemicals (CsCl and nucleic acid stain), the patience and dexterity to collect the stained mtDNA band, the force generated during the ultracentrifugation step and the expenditure of time. Time effectiveness and sample throughput can be increased by removing contaminating nuDNA by deoxyribonuclease I (DNase I)-treatment of native mitochondria obtained by differential and gradient centrifugations ((Chase and Pring, 1986; Zimmerman et al., 1988; Skubatz and Bendich, 1990); Figure 1). Nowadays, simpler and more straightforward extraction and enrichment of mtDNA without the need of (ultra)centrifugation can be achieved by immunocapture of mitochondria from a cell lysate (e.g., based on TOM22 (Franko et al., 2013)) or by electrophoretic separation of a cellular extract in a proprietary agarose gel cassette (Boles and Houde, 2019). Enzymatic linearization of the mitochondrial ring in combination with electrophoretic separation can be used to extract mtDNA from a mixture of total cellular DNA (Wiesner et al., 1991). However, lack of a priori knowledge on the (species-specific) restriction endonuclease that cuts only once in the respective circular mtDNA, e.g., restrictases AleI, BbvCI or PacI, argues against universal application of the method for sequencing novel mitogenomes. The size and conformation (supercoiling) similarities between conventional bacterial plasmids and mammalian mtDNA inspired introduction of rolling circle amplification (Tang and Hyman, 2005; Simison et al., 2006). The approach produced a ∼ 2,000-fold enrichment of mtDNA before NGS analysis (Quispe-Tintaya et al., 2013; Weissig and Edeas, 2015). Alternatively, interfering NUMTs can be excluded from the very start by targeting material naturally depleted of nuDNA such as thrombocytes (cellular mtDNA content of platelets: ∼1.6 copies (Urata et al., 2008; Wang et al., 2018)) or plasma that was recently reported to contain free-floating mitochondria (Al Amir Dache et al., 2020) and circular mtDNA derived from the haematopoietic system (Ma et al., 2019). The use of plasma DNA as natural source of nuclear depletion is hampered by the fact that direct NGS analysis would produce unnecessary nuDNA reads since extrachromosomal circular DNA was found to be released from tissues into the circulation (Kumar et al., 2017). This phenomenon impairs sequence analysis of circular mtDNA enriched by random-hexamer primed isothermal rolling circle amplification (Simison et al., 2006) and the enrichment of circular mtDNA by exonuclease V-based depletion of nuDNA (Gould et al., 2015; Zhu et al., 2017; Dhorne-Pollet et al., 2020). The latter likely explains the admixture of 70 to 41% of nuDNA reads following exonuclease depletion of linear DNA and phi29 polymerase-mediated isothermal amplification (Dhorne-Pollet et al., 2020). Another PCR-free pipeline for mtDNA enrichment and reduction of sequencing volume used a gene-capture chip that produced an up to 100-fold increase of the mtDNA fraction (Liu et al., 2016). The advent of RNA sequencing introduced another option for mitogenome assembly with less bias from NUMTs. Notably the Illumina platform has led to an explosion in the number of samples that can be studied at transcriptomic level at relatively low cost. Mitochondrial protein-coding and rRNA genes can be obtained from almost any transcriptomic sequencing experiment. When sequencing is performed in sufficient depth, even the complete mitogenome can be recovered (Moreira et al., 2015; Plese et al., 2019).
FIGURE 1.
Alternative approaches for mtDNA enrichment. Upper panel from left to right: CsCl gradient ultracentrifugation, isolation of native mitochondria with nucleolytic removal of surrounding nuclear DNA (nuDNA) contamination, mtDNA capture using a gene chip, TOM22-based immunocapture, and automated extraction of mtDNA in a proprietary agarose gel cassette (SageHLS™ system; Sage Science, Beverly, MA, United States). Central panel from left to right: natural depletion of nuDNA (platelet icon from www.biorender.com), enzymatic (exonuclease) depletion of nuDNA, and joint sequencing of nuDNA and mtDNA or nuclear RNA (nuRNA) and mitochondrial RNA (mtRNA) by NGS analysis (DNA or RNA sequencing, respectively). Bottom panel from left to right: rolling-circle amplification, primer walking, several long-range PCRs to cover the mitogenome (F & R: amplification primers), and single-amplicon long-range PCR with back-to-back primers that was extended within this study beyond application in humans (symbolized by the gender-neutral person icon) and ray-finned fishes (class Actinopterygii).
To avoid NUMT confounding biases, this study established an assay to sequence the entire mtDNA from a single amplicon produced by long-range PCR, a method that was used before for this purpose. Most long-range PCR approaches covered the entire mitogenome with one to three overlapping amplicons (Zhang et al., 2012; Burgstaller et al., 2014; Kloss-Brandstätter et al., 2015; Seneca et al., 2015; Paijmans et al., 2016; Deiner et al., 2017; Taylor et al., 2020). Nowadays, NGS-based determination of the entire mitogenome from a single long-range PCR product has become routine practice in human medicine, e.g., for diagnosing patients with clinically suspected mtDNA disease (Zhang et al., 2012; Luo et al., 2018). The approach was also successfully applied to address environmental DNA (eDNA)-related issues in ray-finned fishes (class Actinopterygii (Deiner et al., 2017)).
Here we extended the approach to various kinds of vertebrates including mammals, birds, reptiles, amphibians, and bony fishes. Templates from five hibernator rodents were used for testing the set of universal back-to-back primers capable of amplifying a whole mitogenome. Hibernation is characterized by multiday torpor as a highly efficient energy-saving strategy to survive predictable periods of low nutrient availability and cold ambient temperature. It is found in many mammals such as rodents, bears, hedgehogs, bats, marsupials (Heldmaier et al., 2004; Geiser et al., 2014) and even primates (Dausmann et al., 2004; Blanco et al., 2021). Other strategies used by endothermic mammals for coping with low temperature include increased heat production via shivering and non-shivering thermogenesis, reduction of heat loss from the body surface with the help of fur (Hart, 1956) and/or subcutaneous adipose tissue (Tattersall et al., 2012), or modifications of the blood system such as sequence changes of haemoglobin genes (Campbell et al., 2010), counter-current heat exchange (Ninomiya et al., 2011) or the heart’s pumping ability mediated by a missense mutation in the NRAP gene (Buggiotti et al., 2021). Mitogenome adaptations include polymorphisms in mitochondrial genes encoding subunits of the respiratory chain (Balloux et al., 2009; Awadi et al., 2021) and stem-loop formation(s) in the major NCR (Melo-Ferreira et al., 2014). Amino-acid changes associated with cold adaptation can lead to heat dissipation due to uncoupling between OXPHOS and ATP synthesis (Wijers et al., 2008). Here, we analysed the novel mitogenomes with special emphasis on these putative footprints of cold-adaptive evolution. Intraspecies mtDNA variants of individuals supposed to be not cold-adapted were selected for comparison. In addition, we addressed the evolutionary conservation of the MDPs predicted in silico.
Materials and Methods
Biological Material and Ethical Aspects
Phylogenetic categories in this article were derived from the current version of the NCBI Taxonomy database, a curated set of names and classifications that is regarded to be the phylogenetic taxonomy standard for GenBank's source organisms (DOI: 10.1093/nar/gkr1178; http://www.ncbi.nlm.nih.gov/taxonomy). Five rodent hibernators, Eliomys quercinus (garden dormouse), Marmota marmota (European marmot), Muscardinus avellanarius (hazel dormouse), Spermophilus citellus (European souslik) and Urocitellus parryii (Arctic ground squirrel) served as model species for this Genomic Assay Technology study (Table 1).
TABLE 1.
Target species of the long-range PCR assay amplifying vertebrate mtDNA with a pair of back-to-back primers.
| Latin (common) name | Geographic origin | Tissue | Storage temperature | Isolation kit a | Source |
|---|---|---|---|---|---|
| Eliomys quercinus (Garden dormouse) | Niederfischbach (Rhineland-Palatinate, Germany; 0°51′20″N, 7°52′40″E) | liver | −20°C | 2 | University of Veterinary Medicine (Vienna, Austria) |
| Marmota marmota (European marmot) | Schröcken (Vorarlberg, Austria; 47°15′00″N, 10°05′00″E) | liver | −20 °C | 2 | University of Veterinary Medicine (Vienna, Austria) |
| Muscardinus avellanarius (Hazel dormouse) | Mechelen (Flemish Region, Belgium; 51°01′40″N, 4°28′50″E) | tail | 4°C (in alcohol) | 2 | Non-governmental organization Natuurpunt (Mechelen, Belgium) |
| Spermophilus citellus (European suslik) | Bisamberg (Lower Austria, Austria; 48°19′15″N, 16°21′47″E) | heart | −20°C | 2 | University of Vienna (Austria) |
| Urocitellus parryii (Arctic ground squirrel) | Atigun River (Northern Alaska, United States; 68°27′N, 149°21′W) | liver | −20°C | 1 | Institute of Arctic Biology, University of Alaska Fairbanks (United States) |
Kit 1: QIAamp Fast DNA Tissue Kit (Qiagen, Hilden, Germany), kit 2: Tissue & Cell Genomic DNA Purification Kit (GeneMark, Taichung, Taiwan).
In addition, tissue of an individual of Cricetus cricetus (black-bellied hamster) deceased in the Vienna area (Austria) was used to increase resolution of phylogenetic reconstruction.
E. quercinus and S. citellus originated from breeding facilities that were approved by the Austrian government and the respective local authorities (BMBWF-68.205/0137-WF/V/3b/2014, MA22-1854/2012). Tissue of M. marmota was obtained from a local Austrian hunter in the course of regulated population-management activity. An individual of M. avellanarius was stored after its natural death by Natuurpunt Mechelen (Belgium), a non-governmental organisation. Animal trapping, housing, care and sampling related to U. parryii was carried out in accordance with approved IACUC protocols (#1081763 and #340270) through the University of Alaska Fairbanks and with state (ADF&G #17–100) and federal (BLM #F-94817) permits.
The procedures related to C. cricetus were performed in accordance with guidelines of the European Union (EU) for the protection of animals used for scientific purposes (Directive 2010/63/EU) and were approved by the ethics committee of the Faculty of Life Sciences, University of Vienna (2015-010), the Austrian Federal Ministry of Education, Science and Research (GZ: BMWFW-66.006/0013-WF/V/3b/2015), and the City of Vienna (MA22-2484/10, MA22-310/11).
The study was reviewed and approved by the board on animal ethics and experimentation of the Faculty of Life Science of the University of Vienna (#2021-009).
Deoxyribonucleic Acid Extraction and Single Long-Range Polymerase Chain Reaction With Pan-Vertebrate Back-To-Back Primers
DNA of the five hibernator rodents was isolated using silica-column-based procedures (Table 1). Universal back-to-back primers for single long-range PCR were derived from an alignment of representative mitogenomes of the five groups of the vertebrate phylum, i.e., bony fishes, amphibians, reptiles, birds and mammals (primers: 5′-TAC GTG ATC TGA GTT CAG ACC G and 5′-GTA GGA CTT TAA TCG TTG AAC AAA C; Figure 2). The 20-μL reaction for mitogenome amplification consisted of 1 × Phusion HF Buffer containing 1.5 mM MgCl2 (Thermo Fisher Scientific, Waltham, MA, United States), 0.2 mM of each dNTP (Solis Biodyne, Tartu, Estonia), 0.5 mM of additional MgCl2, 1 × Solution S (Solis Biodyne), 0.5 μM of each primer, 0.6 units Phusion Hot Start II DNA polymerase (Thermo Fisher Scientific) and ∼10 ng genomic DNA. The cycling conditions consisted of 98°C for 2 min, followed by 30 cycles of 95°C for 10 s, 66°C for 25 s and of 72°C for 500 s and final extension at 72°C for 5 min. Specificity of amplification was assessed by electrophoresis on a 0.8% agarose gel stained with GelGreen™ Nucleic Acid Stain (Biotium, Hayward, CA, United States) and run at 10 V/cm. The band of the expected size was purified using Hi Yield® Gel/PCR DNA Fragment Extraction Kit (SLG, Gauting, Germany).
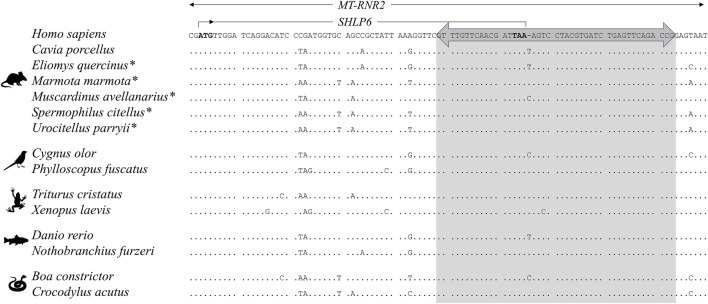
FIGURE 2.
Target sites of the universal back-to-back primers are located in the conserved region of MT-RNR2 (grey box) and include part of the SHLP6-encoding sORFs. Representatively, two species were selected for different classes of the subphylum Vertebrata. Asterisk: target species of this study; dot: identity with human sequence; hyphen: nucleotide gap; specific base: single-nucleotide polymorphism (SNP). Note that the back-to-back primers are completely homologous to the main mtDNA haplotypes of Homo sapiens (data not shown).
Next-Generation Sequencing, Bioinformatic Analysis and Annotation
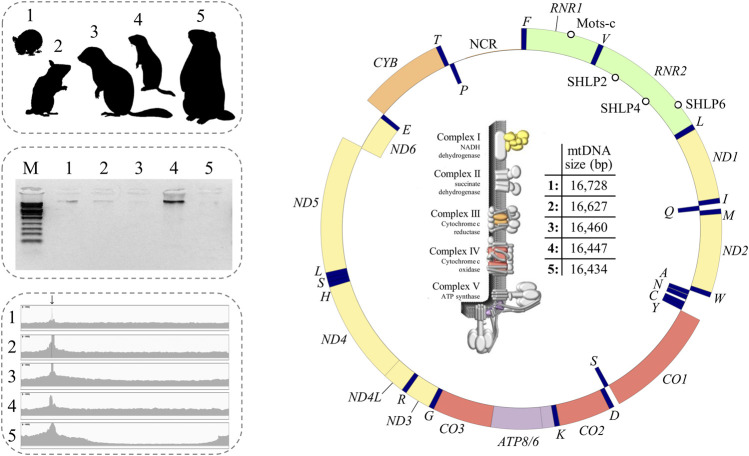
100 ng of the amplified long-range PCR product was used for library preparation with the NEBNext® Ultra II FS DNA Library Prep Kit for Illumina® and the NEBNext® Multiplex Oligos for Illumina® (Index Primers Set 1; New England Biolabs, Ipswich, United States) according to manufacturer’s instruction. Briefly, fragmentation was performed at 37°C for 6 min, adapters were tenfold diluted, and four cycles were run for PCR amplification before PCR clean-up and quality control. Libraries were quantified using the Quantus™ Fluorometer (Promega, Mannheim, Germany) and run on the DNA fragment analysis system BioAnalyzer 2100 (Agilent). Final libraries were pooled and sequenced on a MiSeq benchtop sequencer (Illumina, Eindhoven, Netherlands) with MiSeq Nano Reagent Kit v2 500 cycles chemistry in 2 × 250 mode at 10 pM with 5% PhiX control. FastQ raw data were used for data analysis. Sequencing reads were assembled using the genome assembly algorithm SPAdes (version 3.13.1; (Nurk et al., 2013); http://cab.spbu.ru/software/spades/). The coverage plots were visualized with the IGV desktop application (version 2.8.6; Figure 3 (Robinson et al., 2011)).
FIGURE 3.
Summary of this study including hibernator model species, electrophoretic separation of amplification products, sequence uniformity coverage plot, mitochondrial gene architecture, and a tabular view summarizing the slightly different mitogenome sizes (from left to right). The functional map of mtDNA depicts the similarity of gene architectures (identical gene order). The scheme of the circular mitogenome was constructed with the GenomeVx Web Browser ((Conant and Wolfe, 2008); http://wolfe.ucd.ie/GenomeVx/). The outside and inside of the ring indicate that the gene is encoded by the heavy and light chains of the mtDNA ring, respectively. Colour depicts assignation of a protein subunit to a particular complex of the electron transport chain (illustration kindly provided by Scott Lujan (Lujan et al., 2020)). Note that complex II is exclusively composed of nuclear subunits. Empty circles: MDPs. The tRNAs were designated by single-letter amino acid abbreviation. Origins of heavy and light strand replications and 7S-DNA are not shown.
Novel mitogenomes were annotated using the MITOS web server ((Bernt et al., 2013); http://mitos.bioinf.uni-leipzig.de/index.py).
Discovery of sORF-Encoding Mitochondrial-Derived Peptides
The sORFs of all known MDPs were determined using the Translate tool of the bioinformatics resource portal ExPASy ((Artimo et al., 2012); Swiss Institute of Bioinformatics, https://web.expasy.org/translate/). The prediction of MDPs was restricted to the rule that intra-mitochondrial transcription is combined with cytoplasmic translation according to the standard (nuclear) genetic code (Cobb et al., 2016; Lee et al., 2016). Considering the size range of the putative mitochondrial sORFome (9–40 amino acids (Miller et al., 2020)), an arbitrary cut-off of eight amino acids was applied as minimal sORF length.
Validation of Back-To-Back Primer’s Binding Sites by Bidirectional Sanger Amplicon Sequencing
Consensus primers adjacent to the back-to-back primers for mitogenome amplification were designed based on the mtDNA sequence assembled de novo (5′-GTT TAC GAC CTC GAT GTT G and 5′-TAG ATA GAA ACC GAC CTG G). Sequencing of the resulting PCR products was outsourced to a commercial service provider (LGC Genomics GmbH, Berlin, Germany). Electrochromatograms were assembled and aligned with CodonCode Aligner (version 7.1.2; CodonCode Corporation, Centerville, MA, United States).
Determination of the C. Cricetus Mitogenome Using Genome Skimming
To deeper illustrate the genetic relationship of our hibernator models, the mtDNA sequence of C. cricetus was determined de novo using genome skimming. Next-generation sequencing, bioinformatic analysis and annotation were performed as described (see above).
Phylogenetic Relationship Using Maximum Likelihood Analysis and Bayesian Inference
Nucleotide sequences of the entire mtDNA were retrieved from NCBI or added from own data, aligned using the CLC Sequence Viewer 8.0 software (Qiagen, Hilden, Germany). We excluded the major NCR following the recommendation that the control region of mtDNA should be avoided as a tool for constructing phylogenies or in dating evolutionary events (Slate and Phua, 2003). Since the pervasiveness of mtDNA recombination in animals is considered to be a very rare phenomenon (White et al., 2008; Duchêne et al., 2011; Yamamoto et al., 2020), linkage of mitochondrial regions was assumed meaning that they are expected to share the same phylogeny. Therefore, phylogenetic reconstruction was based on the three sets of coding sequences—protein-, rRNA- and tRNA-coding genes (Chan et al., 2010) without partitioning (Castañeda-Rico et al., 2020) using the maximum-likelihood (ML) approach performed in IQ-TREE (release 1.6.12 of August 15, 2019; (Nguyen et al., 2015; Kalyaanamoorthy et al., 2017; Hoang et al., 2018); http://iqtree.cibiv.univie.ac.at/). The best-fitting substitution model, the TIM2+F+I+G4 model, was selected according to the Bayesian Information Criterion using ModelFinder (Lanfear et al., 2012; Chernomor et al., 2016; Kalyaanamoorthy et al., 2017). Nodal support values were assessed with 1,000 bootstrap replicates. In parallel, we compared the bootstrap support values for the mtDNA sequence dataset partitioned into protein-coding, rRNA and tRNA genes using the best-fitting substitution models TIM2+F+I+G4, TIM2+F+I+G4 and TIM2+F+G4, respectively.
Alternatively, phylogenetic reconstruction of the dataset was assessed by Bayesian inference analysis using MrBayes version 3.2.7a ((Ronquist et al., 2012); http://nbisweden.github.io/MrBayes/) and the GTR + I + Γ model. Two simultaneous analyses were run. The Markov chain Monte Carlo analysis was performed with Markov chains for 10 million generations with trees sampled every 1,000 generations. Consensus topology and posterior probabilities value were produced after discarding (burn-in of 25%) the early resulting, typically poorer quality trees from the simulation.
The ML and Bayesian consensus trees were viewed and edited using the Interactive Tree of Life (iToL) version 6.3 ((Letunic and Bork, 2021); https://itol.embl.de/).
Results
Vertebrate Universal Back-To-Back Primers for Long-Range Polymerase Chain Reaction of the Mitogenome
This study extended the use of long-range PCR allowing to amplify the mitogenome in a single amplicon. A sequence segment that partly overlapped with the sORF of SHLP6 hosted by MT-RNR2 was found to be universally conserved across vertebrate mitogenomes and used as the binding site for the back-to-back primers (Figure 2). The approach was demonstrated for de novo determination of mitogenomes of the five hibernator rodents Eliomys quercinus, Marmota marmota, Muscardinus avellanarius, Spermophilus citellus and Urocitellus parryii (for common names see Table 1).
Next Generation Sequencing Analysis of the Amplicon Covering the Mitogenome
Single long-range PCR successfully produced the expected amplicon across the five target species (Figure 3) including E. quercinus and M. avellanarius that exhibited a single-base insertion at the binding site of one of the amplification primers (Figure 2). Their sequencing on a single MiSeq lane resulted in 408,245 ± 41,396 reads with a proportion of 95.7 ± 2.3% mitochondrial reads. Except for the obligatory peak at the primer binding site, coverage depth per base was similar across the genome (Figure 3). Analysis by Sanger sequencing confirmed the target sequence of the pan-vertebrate back-to-back primers.
Genome Skimming for C. Cricetus
Muscle DNA of the C. cricetus individual showed a proportion of 2.2% reads that aligned to the mitogenome (average coverage of mtDNA sequence: 51.9-fold). The mitogenome variant was 98.7% homologous to a Russian (RUS) haplotype (GenBank: NC_037,888.1 or MF034880 (Ding et al., 2020)). Further details are provided in Supplementary Table S1.
Phylogenetic Analysis
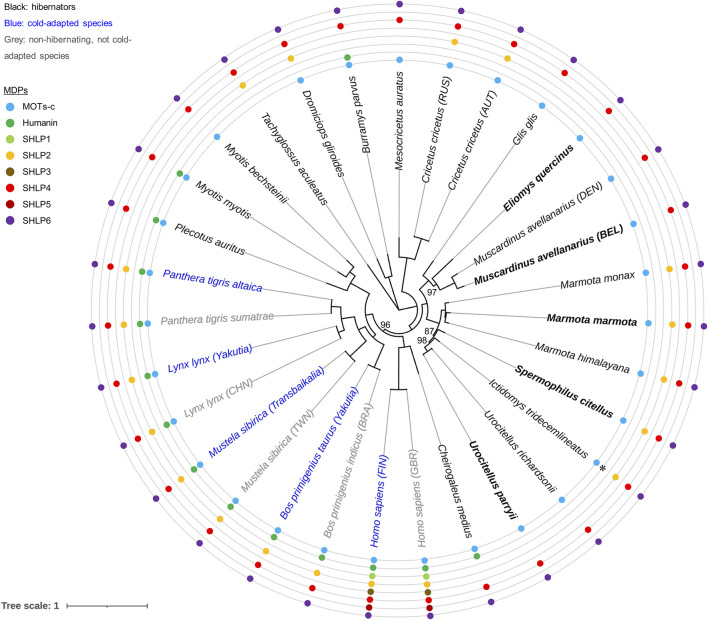
Phylogenetic reconstruction was performed to depict the relationship between the five rodent hibernators targeted in this study in relation to other rodents and representatives of hibernation or cold-adaptation phenotypes (Figure 4). Considering the highly conserved nature of mitogenomic architecture and sequence, we applied a single “grand alignment” strategy for the reconstruction of the NCR-depleted mitogenomes without partitioning. Congruent topologies were inferred by the ML and Bayesian frameworks supported by nodes with high or maximal posterior probability values (≥87%; Figure 4, Supplementary Figure S1). The common partitioning of the mitogenome into the three mtDNA gene sets, mRNA-, rRNA- and tRNA-coding genes, produced similar nodal support (for details see legend to Figure 4).
FIGURE 4.
Inference of evolutionary and mtDNA-based relationships among our five hibernator rodent models. Summary on predicted MDPs and ML phylogenetic reconstruction using NCR-depleted mitogenomes without partitioning. Rodent hibernators used for extending mitogenome enrichment based on single long-range PCR are highlighted in bold. Cricetus cricetus, a facultative hibernator (Weinhold, 2015), is represented by a Russian (RUS) haplotype (GenBank: NC_037,888.1 or MF034880 (Ding et al., 2020)) originating from Nalchik (republic Kabardino-Balkariya, southwestern Russia), and by an Austrian (AUT) haplotype determined by genome skimming within this project (GenBank: MF405145.2; sequence homology: 98.7%). Note that a second mitogenome was also available for the hazel dormouse (Danish (DEN) haplotype, mtDNA divergence: 9.1%). Bootstrap support measures were calculated from 1,000 tree replicates; only values below 100% are depicted. GenBank accession numbers of the presented mitogenomes are provided in Supplementary Table S4. Pairs of subspecies, intraspecies cohorts or haplogroups were selected to illustrate the different degrees of adaptation to cold temperatures (cattle, Eurasian lynx, human, Siberian weasel and tiger). Prediction of MDPs (specified by coloured circles) was restricted to the rule that intra-mitochondrial transcription is combined with cytoplasmic translation according to the standard (nuclear) genetic code (Cobb et al., 2016; Lee et al., 2016). Eight amino acids were applied as an arbitrary minimum for sORF size. The asterisk depicts the only deviation from this general rule—the experimental proof of humanin translation according to the vertebrate mitochondrial code in case of the thirteen-lined ground squirrel (Szereszewski and Storey, 2019). We note that this illustration neither specifies length and sequence differences of MDPs nor issues expression of MDPs from nuDNA that cannot be ruled out in principle. Names of countries are presented as three-letter country codes of the International Organization for Standardization: Brazil (BRA), China (CHN), Finland (FIN), Great Britain (GBR), Taiwan (TWN). The nucleotide alignment used for phylogenetic analysis is available as Supplementary File 1. Similar bootstrap support was obtained by partitioning the NCR-depleted mitogenome sequences into three sets of coding sequence (mRNA, rRNA and tRNA genes; values of 85–99 (n = 5) and 100).
Optimal bootstrap support was also obtained for two genetic geographies of M. avellanarius represented by the mtDNA of a Belgian individual (this study) and a Danish mitogenome that became available during the course of this work (GenBank accessions MT410887.1 or NC_050,264.1). The latter individual of M. avellanarius originated from Svendborg, a town in south-central Denmark (coordinates: 55°3′34″N, 10°36′30″E (Margaryan et al., 2021)). Its mitogenome differed slightly in nucleotide frequency and in the length of several genes (three protein-coding genes and five tRNAs) and of the mitogenome overall (Supplementary Table S2). However, the two individuals were considerably different in the nucleotide sequences of the mitochondrial MT-CYB gene and of the entire mitogenome (10.3 and 9.9%, respectively). A similar degree of dissimilarity was reported earlier for MT-CYB of two genetically distinct lineages of the hazel dormouse (∼11% (Mouton et al., 2017)). This was indicative of species recognition according to the Genetic Species Concept (threshold of MT-CYB divergence: > 11% (Bradley and Baker, 2001; Baker and Bradley, 2006)). The two sampling spots, Belgium and Denmark, match the two distinct genetic geographies of M. avellanarius located in Western Europe or Central-Eastern Europe and Anatolia, respectively, regarded to be cryptic species (Mouton et al., 2017).
Summarising, the complex phylogeographic structure with at least two lineages assumed for the cryptic M. avellanarius species is in line with our phylogenetic reconstruction.
Candidate Polymorphisms of mtDNA Genes
We focussed our analysis of candidate polymorphisms in mitochondrial OXPHOS genes to amino acid substitutions formerly associated with cold adaptation and living at high altitude (n = 4 and 1, respectively). To study their distribution across mammalian species, we composed three sets of species that either cope with cold by using a torpor-free strategy, by entering daily torpor or hibernation, or that occupy habitats with moderate or hot climates where cold adaptation is not regarded a key issue (each: n = 29; Supplementary Table S3). A unique pattern was not obvious for the candidate polymorphisms linked to minimum habitat temperature (MT-ATP6: 8,701G/A and MT-ND3: 10,398G/A of Homo sapiens, (Balloux et al., 2009); MT-ND4: codon 29 in hare species of the genus Lepus (Awadi et al., 2021)).
In addition to these signatures of cold adaptation, we analysed the occurrence of the high altitude-associated amino acid change Y30C/H located in MT-ND1, a polymorphism that affected a highly conserved amino acid of the gene. It was reported for three lineages of high-altitude Tibetans of the macrohaplogroup M (Y30H), but not high-altitude Tibetans of haplogroup N (Ji et al., 2012), and the gelada, the high-altitude Ethiopian monkey (Y30C; Wallace, 2015). Surprisingly, two sloth species (genus Bradypus), slowly moving mammals inhabiting moderate climatic zones, also harboured this change (Y30H; Supplementary Table S3). It is unclear whether this points to a partial functional overlap between the phenotypes of high-altitude adaptation and slowness of movement caused by the respiratory chain.
Mitochondrial-Derived Peptides and Their Potentially Translatable sORFs
During the annotation of the novel mitogenomes determined for our five hibernator models, special focus was attributed to the sequences encoding putative MDPs (Figure 4; Supplementary Figure S3; Supplementary Table S4).
In case of MOTS-c, the prediction indicated an evolutionarily conserved core of four amino acids, differences of peptide length and/or the occurrence of a downstream AUG start codon. No reasonable sORF size was predicted for the homologous nucleotide sequences sporadically encoding humanin, SHLP1, 3 and 5. In contrast, the predicted amino acid sequences and sizes of SHLP4 and 6 peptides were highly similar (Supplementary Figure S3). In case of SHLP4, the first twelve amino acids were identical among the target species. For M. marmota, M. avellanarius, S. citellus and U. parryii the prediction indicated a very short peptide of twelve amino acids. A longer one similar to the human SHLP4 was derived for E. quercinus. Both size variants were frequently seen across vertebrate phylogeny with slight variation of the longer sORF (Supplementary Figure S4). For SHLP6, two sORF size variants were observed, one with an oligopeptide of nine amino acids and the other one exhibiting the length of the human homologue (20 amino acids). In general, the pattern of the two SHLP6 size variants was consistently found across different vertebrate species (Supplementary Figure S4).
Evolutionary conservation of the gau protein was evaluated a decade ago (Faure et al., 2011). For all species that have been sequenced in this study, translation according to the nuclear genetic code returned no functional start codon (ATA instead of ATG). When the repertoire of mitochondrial start codon variants was extended, the translation yielded a dwarf sORF of 14 amino acids for the sequenced species as well as for human mtDNA (Supplementary Figure S5). Interestingly, except for the terminal amino acid the predicted dwarf peptide covered the synthetic peptide sequence used for raising the monoclonal antibody that was key to prove the colocalization of the gau protein with mitochondria (Faure et al., 2011). In this regard we note that the exact size of the gau protein remains an experimental issue to be solved.
The comparison of intraspecies haplotype pairs differing in the degree of adaptation to a cold climate (cattle, Eurasian lynx, human, Siberian weasel and tiger) yielded identical signatures of putative MDPs (Figure 4). Future research should increase cohort sizes for the phenotypes of hibernation, no hibernation and cold adaptation to assess the marker issue more comprehensively.
Discussion
Out of the various approaches for enriching mtDNA before mitogenome sequencing, this study favoured the use of back-to-back amplification primers considering its simplicity, throughput, universality, as well as flexibility and amount of template source. The assay for mitogenome amplification applicable for fresh or well-preserved blood and tissue samples can be helpful to assess issues of mitogenomics such as point and length heteroplasmy of clinical and forensic samples (Cagnin et al., 2009), evolutionary mechanisms of heteroplasmy (Tikochinski et al., 2020) or intraspecific phylogeography (Avise et al., 1987). Our assay holds promise for tissue samples preserved in ethylenediaminetetraacetic acid (EDTA) (Sharpe et al., 2020), or choline-based ionic liquids (Oosting et al., 2020) as well as for certain applications based on aquatic eDNA (Deiner et al., 2017; Ding et al., 2020). The proof that eDNA derived from macro-organisms inhabiting a water body remains intact at least at the size of vertebrate mitogenomes (∼16 kb) contradicts the former common view that eDNA from communities of living fishes in water is highly degraded (Bohmann et al., 2014). Currently, the assay’s full potential for eDNA that is shed by animals into water, moist soil or air via cells, hair, pieces of skin, mucus, gametes, urine, faeces, or free-floating, naked DNA etc., cannot be comprehensively assessed based on the available literature (Cagnin et al., 2009; Bylemans et al., 2018; Clare et al., 2021). Other types of templates derived from formalin-fixed and paraffin-embedded (FFPE) tissue (Guyard et al., 2017), museum samples, ancient and highly degraded material (e.g., ice cores, permafrost, fossil bones, faeces, etc.), or samples preserved for several years in ethanol will likely not be suited due to extensive DNA degradation (Merheb et al., 2019).
Our enrichment assay facilitates amplification with highest accuracy. Similar replication error rates have been reported for the current top-standard enzyme for long-range PCR, Q5® High-Fidelity DNA polymerase, and the phi29 (Φ29) DNA polymerase used in isothermal multiple-displacement amplification (error rates of 5.3 × 10−7 (Potapov and Ong, 2017) and 1 × 10−6 to 10−7 nucleotides (www.qiagen.com), respectively). Our assay is resistant against partial or single-copy NUMTs dispersed in the nuclear genome since they generate no double-stranded amplification product (Balciuniene and Balciunas, 2019). Notably, in case of nuclear mtDNA concatemers (Mega-NUMTs) amplification seems to be likely (Balciuniene and Balciunas, 2019). However, the rareness of their occurrence renders this phenomenon rather negligible.
For a mitoepigenetic perspective, single long-range PCR can identify restriction enzymes cutting the novel mitogenome of interest at a single site. Linearization of the circular mitogenome would facilitate mtDNA enrichment from total cellular DNA based on the different electrophoretic mobilities of covalently closed circular mtDNA versus endonuclease-digested nuDNA (Wiesner et al., 1991), thus, providing a mtDNA template for measuring epigenetic marks such as 5-methylcytosine, 5-hydroxymethylcystosine, 5-formylcytosine and 5-carboxylcytosine (Devall et al., 2016).
Accumulation of genetic changes in response to coping with cold is a historical and current topic of scientific interest. Enrichment of mtDNA by single long-range PCR seems promising to decipher mtDNA variants related to cold adaptation. For example, after early humans had migrated to colder climates, their chance of survival increased when their mtDNA was mutated leading to mitochondrial uncoupling of oxidative phosphorylation and ATP synthesis and heat dissipation (Wijers et al., 2008; Wallace, 2015). For example, amino acid replacements in mitochondrial subunits of OXPHOS complexes I or V were related to cold adaptation in man (Wijers et al., 2008) or monkey (Wallace, 2015).
There are various options to further extend the range of vertebrates for which an experimental proof of applicability for our mtDNA-enrichment assay is demonstrated concomitantly focussing on mtDNA markers for cold adaptation and/or the alteration of the time point when animals enter or leave hibernation. In cape hares (Lepus capensis) collected along a steep ecological gradient, polymorphisms in mitochondrially encoded OXPHOS subunits are significantly associated with mean annual temperature (Slimen et al., 2017). Likewise, climatic variation and/or introgression were suggested to be at the origin of such adaptation among eight Chinese hare species occupying different habitats ranging from the cold north of the interior mainland to the tropic island of Hainan (Awadi et al., 2021). Analysing these cohorts of hares with our approach would allow targeting the entire mitogenome instead of just several candidate genes. A similar field of possible application for the approach are the many shrew species distributed from cold to warmer habitats of the United States. The ability of the cold-adapted, but not hibernating shrews to survive in cold climates is evidenced by Vermont having eight kinds of shrews and Alaska ten, compared to Alabama with six and Florida with only three (Gibbons, 2019). Furthermore, it could also be addressed if there are changes in the mtDNA of thirteen-lined ground squirrels (Ictidomys tridecemlineatus) that are related to the different latitudes and climatic zones of their Northern American habitats and/or that contribute to the genetic variation driving the seasonal onset of its hibernation (Grabek et al., 2019). Currently, we discuss two other putative applications of our mtDNA-enrichment assay. First, we asked whether polymorphisms in mtDNA-encoded OXPHOS subunits might contribute to different adaptability to climate change-induced duration of hibernation in bats and, second, we would like to issue the hypothesis whether specific mtDNA mutations are accumulated in Japanese macaques (Macaca fuscata) to adapt to cold climates (Kawamoto et al., 2007). Japanese macaques can be stratified into two main mitogroups that show strong correspondence with geographic distribution (Kawamoto et al., 2007). The two subspecies of this non-human primate inhabit geographically separated and climatically different regions with cold to moderate up to subtropical climates (Aimi and Fooden, 2005). Whereas Northern and central habitats are covered by M. fuscata fuscata, the macaque of the Japanese mainland, Yakushima Island—the southern limit of Japanese macaque’s distribution, hosts the indigenous subspecies M. fuscata yakui.
The prediction of MDPs represents another issue when annotating novel mitogenomes. It is complicated by the facts that mammalian mitochondria actively import tRNAs (Rubio et al., 2008; Duchêne et al., 2009; Schneider, 2011), and that mitochondrial mRNAs can be exported into the cytoplasm and translated according to the standard genetic code of eukaryotic nuclear genomes (Sreekumar et al., 2016; Kim et al., 2018). These options and their combination could have implication for predicting the amino acid sequences of gau and other MDPs – an issue that might be addressed in future experiments. Likewise, the highly conserved predictions of SHLP4 and 6 as well as of a four amino acid-core in MOTS-c ((Lee et al., 2015); this study), a highly regulative peptide encoded by MT-RNR1 (Fuku et al., 2015; Lee et al., 2015, 2016; Kim et al., 2018; Benayoun and Lee, 2019; Lu et al., 2019), argue for research beyond the classical model species of human and mice. For humanin our extended range of target species confirmed the mosaic of absent and putatively functional sORFs reported earlier (Logan, 2017). Analogously, pseudogenization caused by a missing translational start, a premature stop codon and/or a frameshifting insertion/deletion mutation (Logan, 2017), might also explain why our five hibernating target species do not exhibit sORFs encoding SHLP1, 3 and 5.
The MDP’s role in bioenergetics and metabolism of mitochondria would implicate a connection to cold adaptation (Lu et al., 2019) and hibernation requiring cytoprotecting and oxidative stress reduction. Recently, the first experimental link between hibernation and MDPs was demonstrated for the Alzheimer’s survival peptide humanin (Szereszewski and Storey, 2019). Likewise, the functional similarities of other MDPs such as SHLP4 and 6 that play roles in proliferation and apoptosis (Cobb et al., 2016) might participate in reversing hibernation-associated mass reduction of tissues such as the gastrointestinal tract and the mucosa (Carey, 1990, 1992; Hume et al., 2002). Future research should also address whether mtDNA plays a role in the up to ∼20% skull shrinkage in the non-hibernating mole-like mammal Sorex araneus, the most common shrew species, that occurs before the hardships of winter and, come spring, returns to roughly normal size (Lázaro et al., 2017). Concluding, the relationship of some of the annotated MDPs to hibernation physiology could inspire and direct future studies.
Conclusion
This study provided experimental evidence for the option of mitogenome enrichment by single long-range PCR amplification extended from humans and ray-finned fishes to five rodent hibernator species. Our assay used a set of universal back-to-back oligonucleotides designed against a consensus motif of the vertebrate subphylum (bony fishes, amphibians, reptiles, birds, and mammals), and the size range reported for mitogenomes of vertebrate species (14–20 kb (Kolesnikov and Gerasimov, 2012)) that is well compatible with single long-range PCR, argue for the assay’s potential for application across an extended set of vertebrates beyond the hibernating rodent species analysed in this work.
We report the degree of nucleotide-sequence dissimilarity at the level of the complete mitogenome for two M. avellanarius individuals derived from Belgium and Denmark (9.9%). These locations match the genetically distinct lineages of M. avellanarius regarded to be a cryptic species (at least two species hidden under a single name). Future research on social communication, reproduction mechanisms or morphometrical differentiation, etc. may help gaining insight into possible adaptive differentiation across Europe and to delimit the species of the cryptic M. avellanarius species complex (Mouton et al., 2017) that is in line with our data.
The “mitochondrial Russian doll genes”–sORFs located in mitochondrial rRNA or protein-coding genes, represent promising targets of future in-silico analyses and experimental studies given the functional importance of the encoded micropeptides in retrograde signalling (Kim et al., 2018), cellular metabolism (Merry et al., 2020; Miller et al., 2020; Breton, 2021), but also hibernation (Szereszewski and Storey, 2019). A special focus on cold adaption and the various torpor-related phenotypes might be of interest.
Acknowledgments
We are grateful to the contributors of biological material (Juliana Giselbrecht: alpine marmot, Sara Wilbur & Brian Barnes: arctic ground squirrel, Karolina Hilse-Koller & Sylvain Giroud: garden dormouse, and Goedele Verbeylen: hazel dormouse). We thank Ingeborg Klymiuk (Core Facility Molecular Biology, ZMF Graz, Austria) for next-generation sequencing and the reviewers for constructive input. Support by Ashot Margaryan, Bernard Wallner, Ingeborg Klymiuk, Daniela Allmer, and Martin Hofer is gratefully acknowledged.
Funding Statement
This study was supported by resources of the University of Vienna.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repositories and accession number(s) can be found below: NCBI’s Nucleotide Database (https://www.ncbi.nlm.nih.gov/nucleotide/), accession numbers MN935776 to MN935780 and MF405145.2; NCBI’s Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra), accession number PRJNA748678.
Ethics Statement
The animal study was reviewed and approved by the board on animal ethics and experimentation of the Faculty of Life Science of the University of Vienna (#2021-009).
Author Contributions
Study conception: SE, RS, contribution of biological material: EM, conduction of experiments: SE, Bayesian phylogenetic reconstruction: HS, manuscript writing: SE, RS, proofreading: EM, HS, funding: EM. All authors agree to be held accountable for the content therein and approve the final manuscript version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.685806/full#supplementary-material
References
- Al Amir Dache Z., Otandault A., Tanos R., Pastor B., Meddeb R., Sanchez C., et al. (2020). Blood Contains Circulating Cell‐free Respiratory Competent Mitochondria. FASEB j. 34, 3616–3630. 10.1096/fj.201901917RR [DOI] [PubMed] [Google Scholar]
- Artimo P., Jonnalagedda M., Arnold K., Baratin D., Csardi G., de Castro E., et al. (2012). ExPASy: SIB Bioinformatics Resource portal. Nucleic Acids Res. 40, W597–W603. 10.1093/nar/gks400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspden J. L., Eyre-Walker Y. C., Phillips R. J., Amin U., Mumtaz M. A. S., Brocard M., et al. (2014). Extensive Translation of Small Open reading Frames Revealed by Poly-Ribo-Seq. eLife 3, 1–19. 10.7554/eLife.03528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise J. C., Arnold J., Ball R. M., Bermingham E., Lamb T., Neigel J. E., et al. (1987). Intraspecific Phylogeography: The Mitochondrial DNA Bridge between Population Genetics and Systematics. Annu. Rev. Ecol. Syst. 18, 489–522. 10.1146/annurev.es.18.110187.002421 [DOI] [Google Scholar]
- Awadi A., Ben Slimen H., Schaschl H., Schaschl F., Suchentrunk F. (2021). Positive Selection on Two Mitochondrial Coding Genes and Adaptation Signals in Hares (Genus Lepus) from China. BMC Ecol. Evo 21, 100. 10.1186/s12862-021-01832-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. J., Bradley R. D. (2006). Speciation in Mammals and the Genetic Species Concept. J. Mammalogy 87, 643–662. 10.1644/06-MAMM-F-038R2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balciuniene J., Balciunas D. (2019). A Nuclear mtDNA Concatemer (Mega-NUMT) Could Mimic Paternal Inheritance of Mitochondrial Genome. Front. Genet. 10, 518. 10.3389/fgene.2019.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloux F., Handley L.-J. L., Jombart T., Liu H., Manica A. (2009). Climate Shaped the Worldwide Distribution of Human Mitochondrial DNA Sequence Variation. Proc. R. Soc. B. 276, 3447–3455. 10.1098/rspb.2009.0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Slimen H., Schaschl H., Knauer F., Suchentrunk F. (2017). Selection on the Mitochondrial ATP Synthase 6 and the NADH Dehydrogenase 2 Genes in Hares (Lepus Capensis L., 1758) from a Steep Ecological Gradient in North Africa. BMC Evol. Biol. 17, 1–16. 10.1186/s12862-017-0896-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun B. A., Lee C. (2019). MOTS‐c: A Mitochondrial‐Encoded Regulator of the Nucleus. BioEssays 41, 1900046. 10.1002/bies.201900046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt M., Donath A., Jühling F., Externbrink F., Florentz C., Fritzsch G., et al. (2013). MITOS: Improved De Novo Metazoan Mitochondrial Genome Annotation. Mol. Phylogenet. Evol. 69, 313–319. 10.1016/j.ympev.2012.08.023 [DOI] [PubMed] [Google Scholar]
- Blanco M. B., Greene L. K., Schopler R., Williams C. v., Lynch D., Browning J., et al. (2021). On the Modulation and Maintenance of Hibernation in Captive dwarf Lemurs. Sci. Rep. 11, 5740. 10.1038/s41598-021-84727-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann K., Evans A., Gilbert M. T. P., Carvalho G. R., Creer S., Knapp M., et al. (2014). Environmental DNA for Wildlife Biology and Biodiversity Monitoring. Trends Ecol. Evol. 29, 358–367. 10.1016/j.tree.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Bohmann K., Mirarab S., Bafna V., Gilbert M. T. P. (2020). Beyond DNA Barcoding: The Unrealized Potential of Genome Skim Data in Sample Identification. Mol. Ecol. 29, 2521–2534. 10.1111/mec.15507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles C., Houde N. (2019). Targeted Mitochondrial DNA Extraction and Enrichment Using the SageHLS System. Application Note: SageHLS. Sage Science Ltd. Available at: https://sagescience.com/wp-content/uploads/2019/10/mtDNA-Application-Note-1.pdf .
- Bradley R. D., Baker R. J. (2001). A Test of the Genetic Species Concept: Cytochrome-B Sequences and Mammals. J. Mammalogy 82, 960–973. [DOI] [Google Scholar]
- Breton S. (2021). Mitochondrial Russian Doll Genes May Explain Some Discrepancies in Links between mtDNA Mutations and Mitochondrial Diseases. BioEssays 43, 2100104. 10.1002/bies.202100104 [DOI] [PubMed] [Google Scholar]
- Burgstaller J. P., Johnston I. G., Jones N. S., Albrechtová J., Kolbe T., Vogl C., Futschik A., Mayrhofer C., Klein D., Sabitzer S., Blattner M., Gülly C., Poulton J., Rülicke T., Piálek J., Steinborn R., Brem G. (2014). MtDNA segregation in heteroplasmic tissues is common in vivo and modulated by haplotype differences and developmental stage. Cell Rep. 2014 Jun 26; 7(6), 2031–2041. 10.1016/j.celrep.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggiotti L., Yurchenko A. A., Yudin N. S., vander Jagt C. J., Vorobieva N. v., Kusliy M. A., et al. (2021). Demographic History, Adaptation, and NRAP Convergent Evolution at Amino Acid Residue 100 in the World Northernmost Cattle from Siberia. Mol. Biol. Evol. 38, 3093–3110. 10.1093/molbev/msab078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylemans J., Furlan E. M., Gleeson D. M., Hardy C. M., Duncan R. P. (2018). Does Size Matter? An Experimental Evaluation of the Relative Abundance and Decay Rates of Aquatic Environmental DNA. Environ. Sci. Technol. 52, 6408–6416. 10.1021/acs.est.8b01071 [DOI] [PubMed] [Google Scholar]
- Cagnin S., Biscuola M., Patuzzo C., Trabetti E., Pasquali A., Laveder P., et al. (2009). Reconstruction and Functional Analysis of Altered Molecular Pathways in Human Atherosclerotic Arteries. BMC Genomics 10, 13. 10.1186/1471-2164-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F. M., Simone D., Attimonelli M. (2012). Primates and Mouse NumtS in the UCSC Genome Browser. BMC Bioinformatics 13, S15. 10.1186/1471-2105-13-S4-S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. L., Roberts J. E. E., Watson L. N., Stetefeld J., Sloan A. M., Signore A. v., et al. (2010). Substitutions in Woolly mammoth Hemoglobin Confer Biochemical Properties Adaptive for Cold Tolerance. Nat. Genet. 42, 536–540. 10.1038/ng.574 [DOI] [PubMed] [Google Scholar]
- Carey H. v. (1992). Effects of Fasting and Hibernation on Ion Secretion in Ground Squirrel Intestine. Am. J. Physiology-Regulatory, Integr. Comp. Physiol. 263, R1203–R1208. 10.1152/ajpregu.1992.263.6.r1203 [DOI] [PubMed] [Google Scholar]
- Carey H. v. (1990). Seasonal Changes in Mucosal Structure and Function in Ground Squirrel Intestine. Am. J. Physiology-Regulatory, Integr. Comp. Physiol. 259, R385–R392. 10.1152/ajpregu.1990.259.2.r385 [DOI] [PubMed] [Google Scholar]
- Carr S. M., Griffith O. M. (1987). Rapid Isolation of Animal Mitochondrial DNA in a Small Fixed-Angle Rotor at Ultrahigh Speed. Biochem. Genet. 25, 385–390. 10.1007/BF00554547 [DOI] [PubMed] [Google Scholar]
- Castañeda-Rico S., León-Paniagua L., Edwards C. W., Maldonado J. E. (2020). Ancient DNA from Museum Specimens and Next Generation Sequencing Help Resolve the Controversial Evolutionary History of the Critically Endangered Puebla Deer Mouse. Front. Ecol. Evol. 8, 94. 10.3389/fevo.2020.00094 [DOI] [Google Scholar]
- Chan Y.-C., Roos C., Inoue-Murayama M., Inoue E., Shih C.-C., Pei K. J.-C., et al. (2010). Mitochondrial Genome Sequences Effectively Reveal the Phylogeny of Hylobates Gibbons. PLoS ONE 5, e14419. 10.1371/journal.pone.0014419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase C. D., Pring D. R. (1986). Properties of the Linear N1 and N2 Plasmid-like DNAs from Mitochondria of Cytoplasmic Male-Sterile Sorghum Bicolor. Plant Mol. Biol. 6, 53–64. 10.1007/BF00021306 [DOI] [PubMed] [Google Scholar]
- Chernomor O., von Haeseler A., Minh B. Q. (2016). Terrace Aware Data Structure for Phylogenomic Inference from Supermatrices. Syst. Biol. 65, 997–1008. 10.1093/sysbio/syw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare E. L., Economou C. K., Faulkes C. G., Gilbert J. D., Bennett F., Drinkwater R., et al. (2021). eDNAir: Proof of Concept that Animal DNA Can Be Collected from Air Sampling. PeerJ 9, e11030. 10.7717/peerj.11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb L. J., Lee C., Xiao J., Yen K., Wong R. G., Nakamura H. K., et al. (2016). Naturally Occurring Mitochondrial-Derived Peptides Are Age-dependent Regulators of Apoptosis, Insulin Sensitivity, and Inflammatory Markers. Aging 8, 796–809. 10.18632/aging.100943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant G. C., Wolfe K. H. (2008). GenomeVx: Simple Web-Based Creation of Editable Circular Chromosome Maps. Bioinformatics 24, 861–862. 10.1093/bioinformatics/btm598 [DOI] [PubMed] [Google Scholar]
- Cree L. M., Samuels D. C., de Sousa Lopes S. C., Rajasimha H. K., Wonnapinij P., Mann J. R., et al. (2008). A Reduction of Mitochondrial DNA Molecules during Embryogenesis Explains the Rapid Segregation of Genotypes. Nat. Genet. 40, 249–254. 10.1038/ng.2007.63 [DOI] [PubMed] [Google Scholar]
- Dausmann K. H., Glos J., Ganzhorn J. U., Heldmaier G. (2004). Hibernation in a Tropical Primate. Nature 429, 825–826. 10.1038/429825a [DOI] [PubMed] [Google Scholar]
- Dayama G., Emery S. B., Kidd J. M., Mills R. E. (2014). The Genomic Landscape of Polymorphic Human Nuclear Mitochondrial Insertions. Nucleic Acids Res. 42, 12640–12649. 10.1093/nar/gku1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner K., Renshaw M. A., Li Y., Olds B. P., Lodge D. M., Pfrender M. E. (2017). Long‐range PCR Allows Sequencing of Mitochondrial Genomes from Environmental DNA. Methods Ecol. Evol. 8, 1888–1898. 10.1111/2041-210X.12836 [DOI] [Google Scholar]
- Devall M., Roubroeks J., Mill J., Weedon M., Lunnon K. (2016). Epigenetic Regulation of Mitochondrial Function in Neurodegenerative Disease: New Insights from Advances in Genomic Technologies. Neurosci. Lett. 625, 47–55. 10.1016/j.neulet.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhorne-Pollet S., Barrey E., Pollet N. (2020). A New Method for Long-Read Sequencing of Animal Mitochondrial Genomes: Application to the Identification of Equine Mitochondrial DNA Variants. BMC Genomics 21, 1–15. 10.1101/2019.12.20.884486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Zhou Q., Sun Y., Feoktistova N. Y., Liao J. (2020). Two Novel Cricetine Mitogenomes: Insight into the Mitogenomic Characteristics and Phylogeny in Cricetinae (Rodentia: Cricetidae). Genomics 112, 1716–1725. 10.1016/j.ygeno.2019.09.016 [DOI] [PubMed] [Google Scholar]
- Duchêne A.-M., Pujol C., Maréchal-Drouard L. (2009). Import of tRNAs and Aminoacyl-tRNA Synthetases into Mitochondria. Curr. Genet. 55, 1–18. 10.1007/s00294-008-0223-9 [DOI] [PubMed] [Google Scholar]
- Duchêne S., Archer F. I., Vilstrup J., Caballero S., Morin P. A. (2011). Mitogenome Phylogenetics: The Impact of Using Single Regions and Partitioning Schemes on Topology, Substitution Rate and Divergence Time Estimation. PLoS ONE 6, e27138. 10.1371/journal.pone.0027138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E., Delaye L., Tribolo S., Levasseur A., Seligmann H., Barthélémy R.-M. (2011). Probable Presence of an Ubiquitous Cryptic Mitochondrial Gene on the Antisense Strand of the Cytochrome Oxidase I Gene. Biol. Direct 6, 56. 10.1186/1745-6150-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeroz M. M., Hasan K., Hamada Y., Kawamoto Y. (2008). STR Polymorphism of mtDNA D-Loop in Rhesus Macaques of Bangladesh. Primates 49, 69–72. 10.1007/s10329-007-0047-0 [DOI] [PubMed] [Google Scholar]
- Feldmeyer B., Hoffmeier K., Pfenninger M. (2010). The Complete Mitochondrial Genome of Radix Balthica (Pulmonata, Basommatophora), Obtained by Low Coverage Shot Gun Next Generation Sequencing. Mol. Phylogenet. Evol. 57, 1329–1333. 10.1016/j.ympev.2010.09.012 [DOI] [PubMed] [Google Scholar]
- Fooden J., Aimi M. (2005). Systematic Review of Japanese Macaques, Macaca fuscata (Gray, 1870)/Jack Fooden, Mitsuru Aimi. Fildiana: Zoolog. 104, 1–198. 10.5962/bhl.title.3500 [DOI] [Google Scholar]
- Formenti G., Rhie A., Rhie A., Balacco J., Haase B., Mountcastle J., et al. (2021). Complete Vertebrate Mitogenomes Reveal Widespread Repeats and Gene Duplications. Genome Biol. 22, 120. 10.1186/s13059-021-02336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko A., Baris O. R., Bergschneider E., von Toerne C., Hauck S. M., Aichler M., et al. (2013). Efficient Isolation of Pure and Functional Mitochondria from Mouse Tissues Using Automated Tissue Disruption and Enrichment with Anti-TOM22 Magnetic Beads. PLoS ONE 8, e82392. 10.1371/journal.pone.0082392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuku N., Pareja‐Galeano H., Zempo H., Alis R., Arai Y., Lucia A., et al. (2015). The Mitochondrial derived Peptide MOTS-c: a Player in Exceptional Longevity? Aging Cell 14, 921–923. 10.1111/acel.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammage P. A., Frezza C. (2019). Mitochondrial DNA: The Overlooked Oncogenome? BMC Biol. 17, 1–10. 10.1186/s12915-019-0668-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F., Currie S. E., O'Shea K. A., Hiebert S. M. (2014). Torpor and Hypothermia: Reversed Hysteresis of Metabolic Rate and Body Temperature. Am. J. Physiology-Regulatory, Integr. Comp. Physiol. 307, R1324–R1329. 10.1152/ajpregu.00214.2014 [DOI] [PubMed] [Google Scholar]
- Gibb G. C., Kardailsky O., Kimball R. T., Braun E. L., Penny D. (2007). Mitochondrial Genomes and Avian Phylogeny: Complex Characters and Resolvability without Explosive Radiations. Mol. Biol. Evol. 24, 269–280. 10.1093/molbev/msl158 [DOI] [PubMed] [Google Scholar]
- Gibbons W. (2019). Why Do Shrews Travel through Snow? Ecoviews. Available at: https://archive-srel.uga.edu/outreach/ecoviews/ecoview190203.htm .
- Gorički Š., Trontelj P. (2006). Structure and Evolution of the Mitochondrial Control Region and Flanking Sequences in the European Cave Salamander Proteus anguinus . Gene 378, 31–41. 10.1016/j.gene.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Gould M. P., Bosworth C. M., McMahon S., Grandhi S., Grimerg B. T., LaFramboise T. (2015). PCR-free Enrichment of Mitochondrial DNA from Human Blood and Cell Lines for High Quality Next-Generation DNA Sequencing. PLoS ONE 10, e0139253. 10.1371/journal.pone.0139253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabek K. R., Cooke T. F., Epperson L. E., Spees K. K., Cabral G. F., Sutton S. C., et al. (2019). Genetic Variation Drives Seasonal Onset of Hibernation in the 13-lined Ground Squirrel. Commun. Biol. 2, 1–13. 10.1038/s42003-019-0719-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyard A., Boyez A., Pujals A., Robe C., Tran Van Nhieu J., Allory Y., et al. (2017). DNA Degrades during Storage in Formalin-Fixed and Paraffin-Embedded Tissue Blocks. Virchows Arch. 471, 491–500. 10.1007/s00428-017-2213-0 [DOI] [PubMed] [Google Scholar]
- Hart J. S. (1956). Seasonal Changes in Insulation of the Fur. Can. J. Zool. 34, 53–57. 10.1139/z56-007 [DOI] [Google Scholar]
- Hashimoto Y., Niikura T., Tajima H., Yasukawa T., Sudo H., Ito Y., et al. (2001). A rescue Factor Abolishing Neuronal Cell Death by a Wide Spectrum of Familial Alzheimer's Disease Genes and A. Proc. Natl. Acad. Sci. 98, 6336–6341. 10.1073/pnas.101133498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldmaier G., Ortmann S., Elvert R. (2004). Natural Hypometabolism during Hibernation and Daily Torpor in Mammals. Respir. Physiol. Neurobiol. 141, 317–329. 10.1016/j.resp.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Hoang D. T., Chernomor O., von Haeseler A., Minh B. Q., Vinh L. S. (2018). UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 35, 518–522. 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D., Beiglböck C., Ruf T., Frey-Roos F., Bruns U., Arnold W. (2002). Seasonal Changes in Morphology and Function of the Gastrointestinal Tract of Free-Living alpine Marmots (Marmota marmota). J. Comp. Physiol. B 172, 197–207. 10.1007/s00360-001-0240-1 [DOI] [PubMed] [Google Scholar]
- Ji F., Sharpley M. S., Derbeneva O., Alves L. S., Qian P., Wang Y., et al. (2012). Mitochondrial DNA Variant Associated with Leber Hereditary Optic Neuropathy and High-Altitude Tibetans. Proc. Natl. Acad. Sci. 109, 7391–7396. 10.1073/pnas.1202484109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B. Q., Wong T. K. F., von Haeseler A., Jermiin L. S. (2017). ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 14, 587–589. 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto Y., Shotake T., Nozawa K., Kawamoto S., Tomariichiro K.-i., Kawai S., et al. (2007). Postglacial Population Expansion of Japanese Macaques (Macaca fuscata) Inferred from Mitochondrial DNA Phylogeography. Primates 48, 27–40. 10.1007/s10329-006-0013-2 [DOI] [PubMed] [Google Scholar]
- Kim K. H., Son J. M., Benayoun B. A., Lee C. (2018). The Mitochondrial-Encoded Peptide MOTS-C Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metab. 28, 516–524.e7. 10.1016/j.cmet.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-J., Xiao J., Wan J., Cohen P., Yen K. (2017). Mitochondrially Derived Peptides as Novel Regulators of Metabolism. J. Physiol. 595, 6613–6621. 10.1113/JP274472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingan S., Heaton H., Cudini J., Lambert C., Baybayan P., Galvin B., et al. (2019). A High-Quality De Novo Genome Assembly from a Single Mosquito Using Pacbio Sequencing. Genes 10, 62. 10.3390/genes10010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss-Brandstätter A., Weissensteiner H., Erhart G., Schäfer G., Forer L., Schönherr S., et al. (2015). Validation of Next-Generation Sequencing of Entire Mitochondrial Genomes and the Diversity of Mitochondrial DNA Mutations in Oral Squamous Cell Carcinoma. PLoS ONE 10, e0135643. 10.1371/journal.pone.0135643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop A., Thomas A., Thevis M. (2019). Development of a Mass Spectrometry Based Detection Method for the Mitochondrion-Derived Peptide MOTS-C in Plasma Samples for Doping Control Purposes. Rapid Commun. Mass. Spectrom. 33, 371–380. 10.1002/rcm.8337 [DOI] [PubMed] [Google Scholar]
- Kolesnikov A. A., Gerasimov E. S. (2012). Diversity of Mitochondrial Genome Organization. Biochem. Mosc. 77, 1424–1435. 10.1134/S0006297912130020 [DOI] [PubMed] [Google Scholar]
- Kumar P., Dillon L. W., Shibata Y., Jazaeri A. A., Jones D. R., Dutta A. (2017). Normal and Cancerous Tissues Release Extrachromosomal Circular DNA (eccDNA) into the Circulation. Mol. Cancer Res. 15, 1197–1205. 10.1158/1541-7786.MCR-17-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R., Calcott B., Ho S. Y. W., Guindon S. (2012). PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 29, 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Lázaro J., Dechmann D. K. N., LaPoint S., Wikelski M., Hertel M. (2017). Profound Reversible Seasonal Changes of Individual Skull Size in a Mammal. Curr. Biol. 27, R1106–R1107. 10.1016/j.cub.2017.08.055 [DOI] [PubMed] [Google Scholar]
- Lee C., Kim K. H., Cohen P. (2016). MOTS-c: A Novel Mitochondrial-Derived Peptide Regulating Muscle and Fat Metabolism. Free Radic. Biol. Med. 100, 182–187. 10.1016/j.freeradbiomed.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Zeng J., Drew B. G., Sallam T., Martin-Montalvo A., Wan J., et al. (2015). The Mitochondrial-Derived Peptide MOTS-C Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance. Cel Metab. 21, 443–454. 10.1016/j.cmet.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Bork P. (2021). Interactive Tree of Life (iTOL) V5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 49, W293–W296. 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang X., Xie L., Tan M., Li Z., Su X., et al. (2016). Mitochondrial Capture Enriches mito‐DNA 100 Fold, Enabling PCR‐free Mitogenomics Biodiversity Analysis. Mol. Ecol. Resour. 16, 470–479. 10.1111/1755-0998.12472 [DOI] [PubMed] [Google Scholar]
- Logan S. I. (2017). Pseudogenization of the Humanin gene is common in the mitochondrial DNA of many vertebrates. Zool. Res. 38, 198–202. 10.24272/j.issn.2095-8137.2017.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Tang S., Xue C., Liu Y., Wang J., Zhang W., et al. (2019). Mitochondrial-Derived Peptide MOTS-C Increases Adipose Thermogenic Activation to Promote Cold Adaptation. Int. J. Mol. Sci. 20, 2456. 10.3390/ijms20102456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan S. A., Longley M. J., Humble M. H., Lavender C. A., Burkholder A., Blakely E. L., et al. (2020). Ultrasensitive Deletion Detection Links Mitochondrial DNA Replication, Disease, and Aging. Genome Biol. 21, 248. 10.1186/s13059-020-02138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt D. H., Whipple L. E., Hyman B. C. (1998). Mitochondrial DNA Variable Number Tandem Repeats (VNTRs): Utility and Problems in Molecular Ecology. Mol. Ecol. 7, 1441–1455. 10.1046/j.1365-294x.1998.00495.x [DOI] [PubMed] [Google Scholar]
- Luo S., Valencia C. A., Zhang J., Lee N.-C., Slone J., Gui B., et al. (2018). Biparental Inheritance of Mitochondrial DNA in Humans. Proc. Natl. Acad. Sci. USA 115, 13039–13044. 10.1073/pnas.1810946115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz-Bonengel S., Niederstätter H., Naue J., Koziel R., Yang F., Sänger T., et al. (2021). Evidence for Multi-Copy Mega-NUMTs in the Human Genome. Nucleic Acids Res. 49, 1517–1531. 10.1093/nar/gkaa1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M.-J. L., Zhang H., Jiang P., Sin S. T. K., Lam W. K. J., Cheng S. H., et al. (2019). Topologic Analysis of Plasma Mitochondrial DNA Reveals the Coexistence of Both Linear and Circular Molecules. Clin. Chem. 65, 1161–1170. 10.1373/clinchem.2019.308122 [DOI] [PubMed] [Google Scholar]
- Malukiewicz J., Cartwright R. A., Dergam J. A., Igayara C. S., Nicola P. A., Pereira L. M. C., et al. (2021). Genomic Skimming and Nanopore Sequencing Uncover Cryptic Hybridization in One of World’s Most Threatened Primates. Sci Rep. 11, 17279. 10.1038/s41598-021-96404-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaryan A., Noer C. L., Richter S. R., Restrup M. E., Bülow‐Hansen J. L., Leerhøi F., et al. (2021). Mitochondrial Genomes of Danish Vertebrate Species Generated for the National DNA Reference Database, DNAmark. Environ. DNA 3, 472–480. 10.1002/edn3.138 [DOI] [Google Scholar]
- Maude H., Davidson M., Charitakis N., Diaz L., Bowers W. H. T., Gradovich E., et al. (2019). NUMT Confounding Biases Mitochondrial Heteroplasmy Calls in Favor of the Reference Allele. Front. Cel Dev. Biol. 7, 1–7. 10.3389/fcell.2019.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Ferreira J., Vilela J., Fonseca M. M., da Fonseca R. R., Boursot P., Alves P. C. (2014). The Elusive Nature of Adaptive Mitochondrial DNA Evolution of an Arctic Lineage Prone to Frequent Introgression. Genome Biol. Evol. 6, 886–896. 10.1093/gbe/evu059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merheb M., Matar R., Hodeify R., Siddiqui S. S., Vazhappilly C. G., Marton J., et al. (2019). Mitochondrial DNA, a Powerful Tool to Decipher Ancient Human Civilization from Domestication to Music, and to Uncover Historical Murder Cases. Cells 8, 433. 10.3390/cells8050433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry T. L., Chan A., Woodhead J. S. T., Reynolds J. C., Kumagai H., Kim S.-J., et al. (2020). Mitochondrial-derived Peptides in Energy Metabolism. Am. J. Physiology-Endocrinology Metab. 319, E659–E666. 10.1152/ajpendo.00249.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B., Kim S.-J., Kumagai H., Mehta H. H., Xiang W., Liu J., et al. (2020). Peptides Derived from Small Mitochondrial Open reading Frames: Genomic, Biological, and Therapeutic Implications. Exp. Cel Res. 393, 112056. 10.1016/j.yexcr.2020.112056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. J., Rosenfeldt F. L., Zhang C., Linnane A. W., Nagley P. (2003). Precise Determination of Mitochondrial DNA Copy Number in Human Skeletal and Cardiac Muscle by a PCR-Based Assay: Lack of Change of Copy Number with Age. Nucleic Acids Res. 31, 61e–61. 10.1093/nar/gng060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D. A., Furtado C., Parente T. E. (2015). The Use of Transcriptomic Next-Generation Sequencing Data to Assemble Mitochondrial Genomes of Ancistrus Spp. (Loricariidae). Gene 573, 171–175. 10.1016/j.gene.2015.08.059 [DOI] [PubMed] [Google Scholar]
- Mouton A., Mortelliti A., Grill A., Sara M., Kryštufek B., Juškaitis R., et al. (2017). Evolutionary History and Species Delimitations: a Case Study of the Hazel Dormouse, Muscardinus Avellanarius. Conserv Genet. 18, 181–196. 10.1007/s10592-016-0892-8 [DOI] [Google Scholar]
- Nguyen L.-T., Schmidt H. A., von Haeseler A., Minh B. Q. (2015). IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 32, 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya H., Akiyama E., Simazaki K., Oguri A., Jitsumoto M., Fukuyama T. (2011). Functional Anatomy of the Footpad Vasculature of Dogs: Scanning Electron Microscopy of Vascular Corrosion Casts. Vet. Dermatol. 22, 475–481. 10.1111/j.1365-3164.2011.00976.x [DOI] [PubMed] [Google Scholar]
- Nurk S., Bankevich A., Antipov D., Gurevich A. A., Korobeynikov A., Lapidus A., et al. (2013). Assembling Single-Cell Genomes and Mini-Metagenomes from Chimeric MDA Products. J. Comput. Biol. 20, 714–737. 10.1007/978-3-642-37195-0_1310.1089/cmb.2013.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara R., Tedone E., Ludlow A., Huang E., Arosio B., Mari D., et al. (2019). Quantitative Mitochondrial DNA Copy Number Determination Using Droplet Digital PCR with Single-Cell Resolution. Genome Res. 29, 1878–1888. 10.1101/gr.250480.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosting T., Hilario E., Wellenreuther M., Ritchie P. A. (2020). DNA Degradation in Fish: Practical Solutions and Guidelines to Improve DNA Preservation for Genomic Research. Ecol. Evol. 10, 8643–8651. 10.1002/ece3.6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paijmans J. L. A., Fickel J., Courtiol A., Hofreiter M., Förster D. W. (2016). Impact of Enrichment Conditions on Cross-Species Capture of Fresh and Degraded DNA. Mol. Ecol. Resour. 16, 42–55. 10.1111/1755-0998.12420 [DOI] [PubMed] [Google Scholar]
- Plese B., Rossi M. E., Kenny N. J., Taboada S., Koutsouveli V., Riesgo A. (2019). Trimitomics: An Efficient Pipeline for Mitochondrial Assembly from Transcriptomic Reads in Nonmodel Species. Mol. Ecol. Resour. 19, 1230–1239. 10.1111/1755-0998.13033 [DOI] [PubMed] [Google Scholar]
- Potapov V., Ong J. L. (2017). Examining Sources of Error in PCR by Single-Molecule Sequencing. PLoS ONE 12, e0169774. 10.1371/journal.pone.0169774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertas M. J., González-Sánchez M. (2020). Insertions of Mitochondrial DNA into the Nucleus-Effects and Role in Cell Evolution. Genome 63, 365–374. 10.1139/gen-2019-0151 [DOI] [PubMed] [Google Scholar]
- Quispe-Tintaya W., White R. R., Popov V. N., Vijg J., Maslov A. Y. (2013). Fast Mitochondrial DNA Isolation from Mammalian Cells for Next-Generation Sequencing. BioTechniques 55, 133–136. 10.2144/000114077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. C., Lai R. W., Woodhead J. S. T., Joly J. H., Mitchell C. J., Cameron-Smith D., et al. (2021). MOTS-c Is an Exercise-Induced Mitochondrial-Encoded Regulator of Age-dependent Physical Decline and Muscle Homeostasis. Nat. Commun. 12, 1–11. 10.1038/s41467-020-20790-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A., McCarthy S. A., Fedrigo O., Damas J., Formenti G., Koren S., et al. (2021). Towards Complete and Error-free Genome Assemblies of All Vertebrate Species. Nature 592, 737–746. 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E. S., Getz G., et al. (2011). Integrative Genomics Viewer. Nat. Biotechnol. 29, 24–26. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., et al. (2012). MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 61, 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M. A. T., Rinehart J. J., Krett B., Duvezin-Caubet S., Reichert A. S., Soll D., et al. (2008). Mammalian Mitochondria Have the Innate Ability to Import tRNAs by a Mechanism Distinct from Protein Import. Proc. Natl. Acad. Sci. 105, 9186–9191. 10.1073/pnas.0804283105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T. P., Miya M., Mabuchi K., Nishida M. (2016). Structure and Variation of the Mitochondrial Genome of Fishes. BMC Genomics 17, 1–20. 10.1186/s12864-016-3054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbisà E., Tanzariello F., Reyes A., Pesole G., Saccone C. (1997). Mammalian Mitochondrial D-Loop Region Structural Analysis: Identification of New Conserved Sequences and Their Functional and Evolutionary Implications. Gene 205, 125–140. 10.1016/S0378-1119(97)00404-6 [DOI] [PubMed] [Google Scholar]
- Schneider A. (2011). Mitochondrial tRNA Import and its Consequences for Mitochondrial Translation. Annu. Rev. Biochem. 80, 1033–1053. 10.1146/annurev-biochem-060109-092838 [DOI] [PubMed] [Google Scholar]
- Seneca S., Vancampenhout K., Van Coster R., Smet J., Lissens W., Vanlander A., et al. (2015). Analysis of the Whole Mitochondrial Genome: Translation of the Ion Torrent Personal Genome Machine System to the Diagnostic Bench? Eur. J. Hum. Genet. 23, 41–48. 10.1038/ejhg.2014.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A., Barrios S., Gayer S., Allan-Perkins E., Stein D., Appiah-Madson H. J., et al. (2020). DESS Deconstructed: Is EDTA Solely Responsible for protection of High Molecular Weight DNA in This Common Tissue Preservative? PLoS ONE 15, e0237356–16. 10.1371/journal.pone.0237356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Xing Y., Mao X. (2017). The Little Brown Bat Nuclear Genome Contains an Entire Mitochondrial Genome: Real or Artifact? Gene 629, 64–67. 10.1016/j.gene.2017.07.065 [DOI] [PubMed] [Google Scholar]
- Shu L., Wanghe K., Wang J., Niu Y., Zhao K., Peng Z. (2018). The Complete Mitochondrial Genomes of Two Schizothoracine Fishes (Teleostei, Cypriniformes): A Novel Minisatellite in Fish Mitochondrial Genomes. J. Appl. Ichthyol. 34, 1028–1034. 10.1111/jai.13671 [DOI] [Google Scholar]
- Simison W. B., Lindberg D. R., Boore J. L. (2006). Rolling circle Amplification of Metazoan Mitochondrial Genomes. Mol. Phylogenet. Evol. 39, 562–567. 10.1016/j.ympev.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Skubatz H., Bendich A. J. (1990). “Isolation of Mitochondria and mtDNA,” in Plant Molecular Biology Manual (Dordrecht: Springer; ), 1–7. 10.1007/978-94-017-6953-2_1 [DOI] [Google Scholar]
- Slate J., Phua S. H. (2003). Patterns of Linkage Disequilibrium in Mitochondrial DNA of 16 Ruminant Populations. Mol. Ecol. 12, 597–608. 10.1046/j.1365-294X.2003.01765.x [DOI] [PubMed] [Google Scholar]
- Sreekumar P. G., Ishikawa K., Spee C., Mehta H. H., Wan J., Yen K., et al. (2016). The Mitochondrial-Derived Peptide Humanin Protects RPE Cells from Oxidative Stress, Senescence, and Mitochondrial Dysfunction. Invest. Ophthalmol. Vis. Sci. 57, 1238–1253. 10.1167/iovs.15-17053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szereszewski K. E., Storey K. B. (2019). Identification of a Prosurvival Neuroprotective Mitochondrial Peptide in a Mammalian Hibernator. Cell Biochem Funct 37, 494–503. 10.1002/cbf.3422 [DOI] [PubMed] [Google Scholar]
- Tang S., Hyman B. C. (2005). Rolling circle Amplification of Complete Nematode Mitochondrial Genomes. J. Nematol 37, 236–241. [PMC free article] [PubMed] [Google Scholar]
- Tattersall G. J., Sinclair B. J., Withers P. C., Fields P. A., Seebacher F., Cooper C. E., et al. (2012). Coping with thermal Challenges: Physiological Adaptations to Environmental Temperatures. Compr. Physiol. 2, 2151–2202. 10.1002/cphy.c110055 [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Kiesler K. M., Sturk-Andreaggi K., Ring J. D., Parson W., Schanfield M., et al. (2020). Platinum-quality Mitogenome Haplotypes from United States Populations. Genes 11, 1290. 10.3390/genes11111290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevis M., Schänzer W. (2016). Emerging Drugs Affecting Skeletal Muscle Function and Mitochondrial Biogenesis - Potential Implications for Sports Drug Testing Programs. Rapid Commun. Mass. Spectrom. 30, 635–651. 10.1002/rcm.7470 [DOI] [PubMed] [Google Scholar]
- Tikochinski Y., Carreras C., Tikochinski G., Vilaça S. T. (2020). Population-specific Signatures of Intra-individual Mitochondrial DNA Heteroplasmy and Their Potential Evolutionary Advantages. Sci. Rep. 10, 1–14. 10.1038/s41598-019-56918-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan B., Alcantara D. M. C., Machado D. J., Marques F. P. L., Lahr D. J. G. (2019). Genome Skimming Is a Low-Cost and Robust Strategy to Assemble Complete Mitochondrial Genomes from Ethanol Preserved Specimens in Biodiversity Studies. PeerJ 7, e7543. 10.7717/peerj.7543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urata M., Koga-Wada Y., Kayamori Y., Kang D. (2008). Platelet Contamination Causes Large Variation as Well as Overestimation of Mitochondrial DNA Content of Peripheral Blood Mononuclear Cells. Ann. Clin. Biochem. 45, 513–514. 10.1258/acb.2008.008008 [DOI] [PubMed] [Google Scholar]
- Wallace D. C. (2015). Mitochondrial DNA Variation in Human Radiation and Disease. Cell 163, 33–38. 10.1016/j.cell.2015.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Yang X., Zhang Y., Lin D., Li P., Zhang Z., et al. (2018). Platelet Mitochondrial Cytochrome C Oxidase Subunit I Variants with Benzene Poisoning. J. Thorac. Dis. 10, 6811–6818. 10.21037/jtd.2018.11.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Chinnery P. F. (2020). Inheritance of Mitochondrial DNA in Humans: Implications for Rare and Common Diseases. J. Intern. Med. 287, 634–644. 10.1111/joim.13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold U. (2015). European Action Plan for the Conservation of the Common Hamster. Hudson MA: Cricetus cricetus, L. 1758. [Google Scholar]
- Weissig V., Edeas M. (2015). Mitochondrial Medicine. Preface. Methods Mol. Biol. 1264, 1–480. 10.1007/978-1-4939-2257-4 [DOI] [PubMed] [Google Scholar]
- White D. J., Wolff J. N., Pierson M., Gemmell N. J. (2008). Revealing the Hidden Complexities of mtDNA Inheritance. Mol. Ecol. 17, 4925–4942. 10.1111/j.1365-294X.2008.03982.x [DOI] [PubMed] [Google Scholar]
- Wiesner R. J., Swift H., Zak R. (1991). Purification of Mitochondrial DNA from Total Cellular DNA of Small Tissue Samples. Gene 98, 277–281. 10.1016/0378-1119(91)90186-F [DOI] [PubMed] [Google Scholar]
- Wijers S. L. J., Schrauwen P., Saris W. H. M., van Marken Lichtenbelt W. D. (2008). Human Skeletal Muscle Mitochondrial Uncoupling Is Associated with Cold Induced Adaptive Thermogenesis. PLoS ONE 3, e1777. 10.1371/journal.pone.0001777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Sakaue S., Matsuda K., Murakami Y., Kamatani Y., Ozono K., et al. (2020). Genetic and Phenotypic Landscape of the Mitochondrial Genome in the Japanese Population. Commun. Biol. 3, 104. 10.1038/s42003-020-0812-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P. H., Lee H. C., Chau G. Y., Wu Y. T., Li S. H., Lui W. Y., et al. (2004). Alteration of the Copy Number and Deletion of Mitochondrial DNA in Human Hepatocellular Carcinoma. Br. J. Cancer 90, 2390–2396. 10.1038/sj.bjc.6601838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Cui H., Wong L.-J. C. (2012). Comprehensive One-step Molecular Analyses of Mitochondrial Genome by Massively Parallel Sequencing. Clin. Chem. 58, 1322–1331. 10.1373/clinchem.2011.181438 [DOI] [PubMed] [Google Scholar]
- Zhu J., Zhang F., Du M., Zhang P., Fu S., Wang L. (2017). Molecular Characterization of Cell-free eccDNAs in Human Plasma. Sci. Rep. 7, 1–11. 10.1038/s41598-017-11368-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E. G., Akins D. R., Planz J. v., Schurr M. J. (1988). A Rapid Procedure for Isolating Mitochondrial DNA. Gene Anal. Tech. 5, 102–104. 10.1016/0735-0651(88)90004-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repositories and accession number(s) can be found below: NCBI’s Nucleotide Database (https://www.ncbi.nlm.nih.gov/nucleotide/), accession numbers MN935776 to MN935780 and MF405145.2; NCBI’s Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra), accession number PRJNA748678.