Abstract
Background
The Whipple procedure in its current form owes its evolution to the groundbreaking and innovative work of giants in the field of surgery. From being a multistep procedure with high morbidity and mortality, it is now ubiquitously performed in a single setting, often offered via minimally invasive approaches. Training to perform this procedure is an arduous task, and different training paradigms vary significantly.
Objectives/Methods
The purpose of this paper is to share a standard method by which the surgeon can perform the Whipple procedure in a systematic manner. Using illustrations to make the steps clearer, the authors will postulate that an improvement in mean operative time can be realistically achieved by most pancreatic surgeons. The focus is also on presenting this complex procedure as reproducible and teachable techniques for trainees.
Conclusion
This illustrated review of the Whipple procedure as performed at our institution is intended to help facilitate a streamlined and stepwise progression through what is undoubtedly a challenging surgical procedure. Although the procedure described will not apply to all Whipple operations given the heterogeneity in anatomy and circumstances, our hope is that this will lead to a more efficient procedure and decreased operating room time and costs as well as provide a framework to teach and measure technical progress for surgical trainees.
Highlights
-
•
Whipple illustrations offer a reproducible and teachable technique for trainees.
-
•
Having a standardized and reproducible technique improves efficiency.
-
•
Improving OR times can help reduce the risk of morbidity and mortality.
INTRODUCTION
The Whipple procedure in its current form owes its evolution to the groundbreaking and innovative work of a multitude of physicians and surgeons. By "standing on the shoulders of giants," we now perform this procedure through open, laparoscopic, and robotic approaches, with an acceptable mortality rate of < 5% and improved perioperative morbidity. We have also noted improvement in intraoperative blood loss (1700 mL in the 1960s to less than 800 mL in the last 2 decades) and reduced operative times (438 minutes in 1970–1980 to 347 minutes in the 2000s) [[1], [2], [3]].
Many "Centers of Excellence" have been established to improve surgical outcomes associated with this procedure and to help establish standards of care. In fact, the American College of Surgeons has piloted a program focusing on high-risk gastrointestinal surgery, the Whipple procedure being one of the areas of focus [4].
The purpose of this paper is to share a standard method by which the surgeon can perform the Whipple procedure in a systematic manner. Using illustrations to make the steps clearer, the authors will postulate that the mean operative time of 197 minutes achieved by the senior author (DRJ) can be realistically reached by most pancreatic surgeons. This benefit can be added to the main goal of this procedure: a safe and oncologic resection of the cancer. The focus is on presenting the resection portion with key points made to help the surgeon avoid critical intraoperative issues and to present this complex procedure as reproducible and teachable techniques for trainees.
PERIOPERATIVE PREPARATION
Patients undergoing the Whipple procedure at our institution are prepared using an Enhanced Recovery After Surgery protocol. Patient is prepared by the anesthesiologist with appropriate intravenous access and monitoring devices, and induction of general anesthesia. Patient is placed in supine position with the right arm tucked. A Foley catheter and an orogastric tube are placed. The instrument setup includes an omni retractor, suction, electrocautery, harmonic scalpel, and the argon beam coagulator.
OPERATIVE STEPS
The procedure is begun with an upper midline incision. The viscera and abdominal cavity are thoroughly explored for any sign of metastatic disease. Moreover, the routine use of ultarsound in all cases will alert the surgeon to abherrant anatomy (especially the replaced right hepatic artery) and underestimated venous involvement (the right edge of the superior mesenteric vein [SMV] can be best assessed by ultrasound and may be underaccounted for by computed tomographic scan). A right to left medial visceral rotation is performed, elevating the duodenum and the pancreas off the inferior vena cava. The right mesocolon is now separated from the uncinate-mesocolic groove (Fig 1). The SMV is first identified in this fashion.
Fig 1.
Step 1: Right to left medial visceral rotation and dissection of the right mesocolon.
Note. Following a right to left medial visceral rotation (the Cattell–Braasch maneuver), the right mesocolon is dissected off the duodenum and head of pancreas. In this process, the SMV is identified early in the dissection, with the gastrocolic trunk of Henle.
Key Point: The SMV Can Be Found Outside the Lesser Sac, in an Area That Is Usually Not Involved With Tumor
The lesser sac is then entered through the gastrocolic ligament, and the stomach is retracted cephalad, often encircling the stomach with a penrose drain and attaching this to the self-retaining retractor. The authors' preference is to keep outside the right gastroepiploic vessels and use this as a key dissection plane. The omentum is left with the transverse colon, and this allows for less "bulk" in the lesser sac dissection. There may be concern regarding vascularity to the omentum, but the authors have not run into any issues of omental infaction. The right gastroepipolic vein is used as a key landmark and is followed to gastrocolic trunk of Henle, which leads again to the SMV (Fig 2, A and B]. Getting into the correct plane of the vein will allow for ensuring easy access to the SMV at the inferior border of the pancreas. The middle colic vein is preserved, if possible, and the right gastroepiploic vein is ligated and divided. The middle colic vein may need to be sacrificed in some circumstances. This prevents this vein from tearing and causing bleeding with retraction later in the case. The inferior end of the retropancreatic tunnel is begun at this point.
Fig 2.
A, Step 2: Dissection of the right gastroepiploic vein and superior mesenteric vein.
Note. The right gastroepiploic vein is now identified, dissected, and ligated. This allows further cephalad dissection of the SMV leading into the PV and inferior end of the retropancreatic tunnel. B, Operative exposure of SMV which is encircled with vessel loop. Thick arrow points to ligated stump of right gastroepiploic vein; thin arrow points to intersection between the SMV and inferior end of the retropancreatic tunnel.
Key Point: Follow the Right Gastroepiploic Vein to Find the SMV at the Inferior Border of the Pancreas
Attention is then turned to the portal dissection. The stomach is retracted caudally to expose the hepatoduodenal ligament and the superior border of the pancreas. The hepatic artery lymph node (station 8 lymph node) is excised, aiding in identification of the common hepatic artery (CHA) (Fig 3, A and B). It is critical to get in the correct plane of the vascular structure. The surgeon should see glistening white adventitia of the artery. Most surgeons are not in the correct plane here and are not deep enough in their dissection.
Fig 3.
A, Step 3: Entering the lesser sac and dissecting the station 8 lymph node. Note. Enter the lesser sac via pars flaccida. Identify the station 8 lymph node or hepatic artery lymph node and dissect it off the CHA underneath. Following identification of the CHA, dissect just caudal to it to identify the portal vein between the CHA and the superior border of the pancreas. B, Intraoperative picture demonstrating identification of the hepatoduodenal ligament (black arrow).
Key Point: The Station 8 Node Is Always at the Superior Border of the Pancreas and the Hepatic Artery. There Will Be No Hepatic Artery in This Location Only if There Is Altered Arterial Anatomy
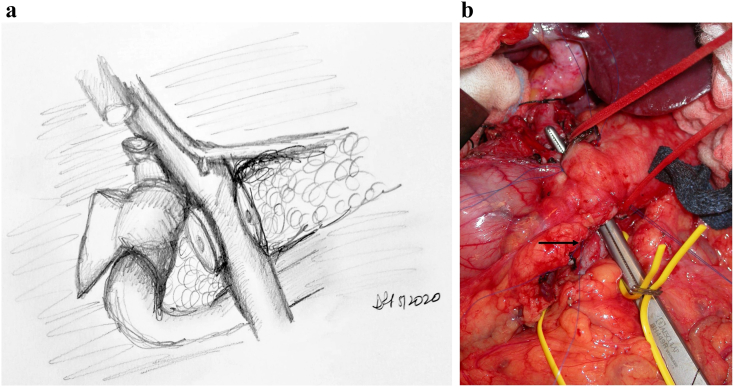
Dissection is carried along the hepatic artery in the hepatoduodenal ligament from the patient's left to right. This helps in identifying the gastroduodenal artery (GDA) and the portal vein (PV) posterior to it (Fig 4). The GDA is test-clamped and ligated. The authors' preference is to place a small metallic clip on the patient side of the ligature. This is to alert for any pseudoaneurym in the future: a computed tomographic angiogram that reveals the clip to have moved off the common hepatic aretery will alert the surgeon to a GDA pseudoaneurym even if there is no evidence of active extravasation. It is the authors' preference to tie the ligature flush with the comon hepatic artery; any interventional radiologic procedure for a GDA pseudoaneurysm will involve stent placement and not coils. Therefore, the authors believe that a GDA stump is not necessary for coil placement. The surgeon must be wary of a celiac or superior mesenteric artery (SMA) stenosis that can result in altered flow in the GDA. Test-clamping and ensuring that there is an excellent thrill in the proper hepatic artery are critical. Directly posteriorly to the hepatic artery lies the portal vein. Once identified, the superior end of the retropancreatic tunnel is begun. The portal vein can frequently be seen involved with the tumor; techniques for portal vein resection and reconstruction will not be discussed in this particular article.
Fig 4.
Step 4: Identification and dissection of the gastroduodenal artery and exposure of the portal vein. Note. Follow the CHA to identify the GDA and proper hepatic artery. Dissect and ligate the GDA, allowing further exposure of the PV and access to the superior end of the retropancreatic tunnel.
Key Point: The Portal Vein Lies Directly Behind the GDA Stump. It Is Important to Get in the Correct Plane of the Vein
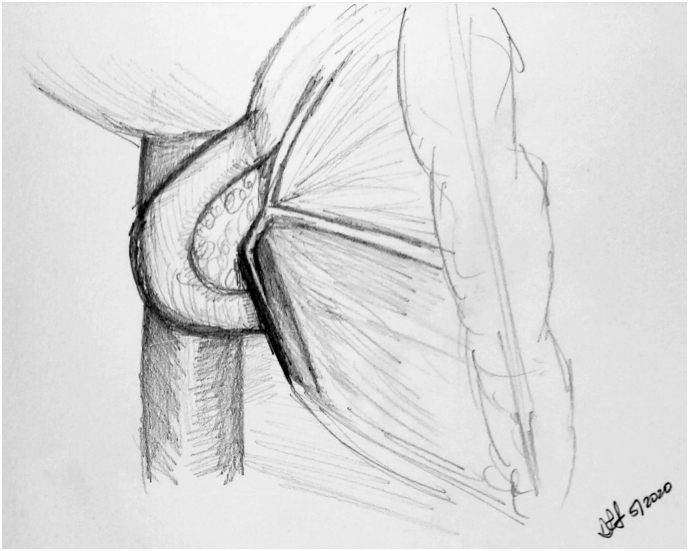
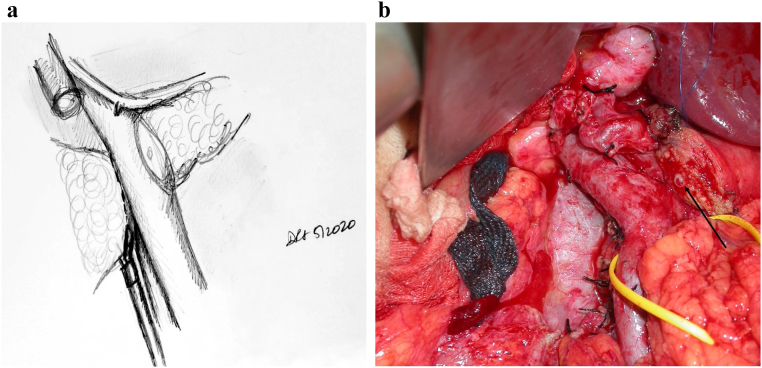
Dissection is now carried further to the patient's right. The bile duct is encircled and ligated after checking for replaced arterial anatomy. The lateral common bile duct node (station 12 lymph node) is excised to aid in this step. The stomach is transected using a stapling device, as the authors perform a standard Whipple most frequently. A peon is now used to complete the retropancreatic tunnel, and an umbilical tape is placed to provide easy access to this correct plane. In some circumstances, the surgeon may elect to divide the pancreas over a clamp as the tunnel is created. This can be a safer manner to watch the division of the pancreas over the vein without creating a blind tunnel. In some cases, a Whipple at the splenic artery procedure may be required [5]. The pancreas is now transected using an electrocautery device, over the peon (Fig 5, A and B). Some surgeons prefer that transection of the pancreas be performed with a knife to preserve blood supply to the duct and assist with duct identification; the decision is based on surgeon preference.
Fig 5.
A, Step 5: Transection of the pancreas, bile duct, stomach, and jejunum. Note. Having identified the PV, the pancreas and bile duct can now be safely transected. The stomach and jejunum are also transected, leaving behind the uncinate attached to the PV/SMV and the SMA. B, Intraoperative photograph demonstrating peon and umbilical tape placed through retropancreatic tunnel; division of the pancreas can now proceed. Arrow highlighting figure-of-8 Prolene used to control bleeding arcade vessels, which is the alternative method of controlling the bleeding to electrocautery.
In a poll performed at the Americas Hepato-Pancreato-Biliary Association meeting, more than 70% of pancreatic surgeons used electrocautery to divide the pancreas (personal communication). The authors believe that the key issue regarding blood supply centers around the lack of dissection of the pancreas stump off the splenic vein. This will preserve as much of the smaller arterial vessels that tend to be found in this location as possible.
The bleeding arcade vessels are dealt with using electrocautery. The reconstructive method involves ligation of these vessels with the through-and-throuigh 2-0 silk sutures that will "lasoo" the arcade vessels. The senior author has historically used figure-of-8 4-0 Prolene sutures prior to transection of the pancreas; this was when the classic two-layer capsule-to-serosa and duct-to-mucosa anastamosis was performed. With the change to the modified Blumgart technique, the senior author now uses cautery only to control these arcade vessels.
Key Point: Transecting the Stomach and the Bile Duct Prior to Transecting the Pancreas Allows for Easy Visualization of the Pancreas. In the Circumstance That the Tunnel Is Not Easy to Create, One Can Divide the Pancreas as the Tunnel Is Being Created
The jejunum is now transected using a stapling device. An energy device (harmonic scalpel preferred by the authors) is used to take the mesentery to the small bowel. It is critical to stay as close to the bowel as possible because wandering deeper into the mesentery can result in injury to the SMA. As the Cattell–Braasch maneuver has been performed, retracting all intestine toward the patient's head will allow easy access to the fourth portion of the duodenum. The energy device can be used to take this directly off the SMA and flip the whole specimen to the patient's right.
Key Point: Using Energy and Staying Close to the Bowel Wall Are Critical in Preventing an Injury to the SMA or Devascularizing a Large Area of Bowel
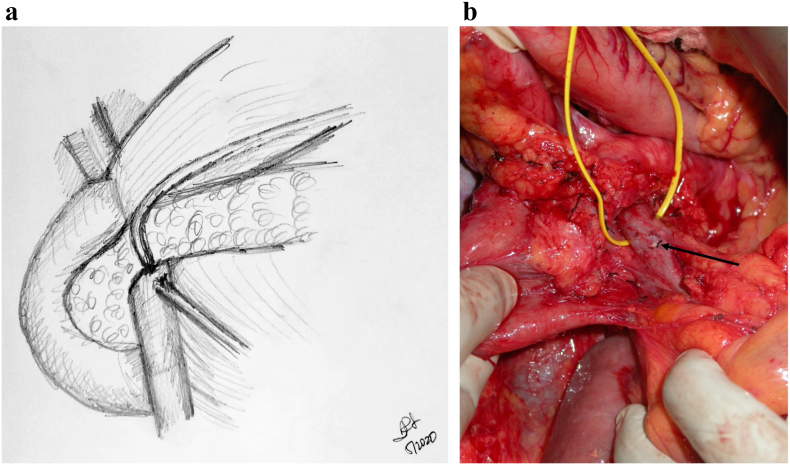
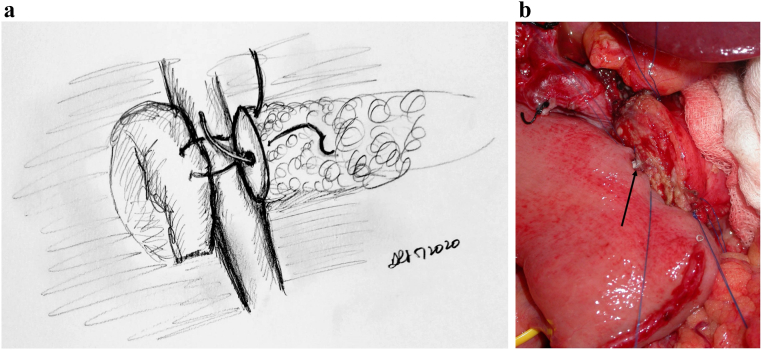
The head of pancreas is then rotated medially, and the SMA is dissected free from the mesopancreas (Fig 6, A and B), the shining posterior aspect to the pancreas that should not be violated during the Kocher process. This embryologic plane is a natural barrier to tumor violation and, as with the mesorectum, must be respected during this dissection. However, embryologically, the uncinate continues to the patient's left of the SMA, and at some point, this plane must be transected. This is similar to an "appropriate mesorectal dissection." Rotating the specimen toward the patient's right and incising into the plane from posteriorly allows for access to the SMA and allows for an SMA-first approach. Even if the plan is to detach the uncinate from an anterior approach, we encourage use of this posterior maneuver to allow for an easier anterior detachment. This method is in contrast to other SMA-first approaches including the medial uncinate approach, inferior infracolic approach, inferior supracolic, left posterior approach, and superior approach. The advantages of each of these approaches are best seen based on specific tumor location; in our experience, the approach outlined above has had the broadest application to operations performed by our group.
Fig 6.
A, Step 6: Transecting the uncinate and skeletonizing the SMA distally. Note. Dissection is then performed through the mesopancreas and the uncinate from the aorta, skeletonizing the SMA distally. A harmonic scalpel is then used to transect the uncinate off the SMA and SMV. B, Intraoperative photograph following pancreatic transection; black arrow pointing to pancreatic duct.
Key Point: Rotating the Specimen to the Patient's Right and Incising Directly Onto the SMA Will Allow for an Easier Detachment of the Specimen from the SMA
Lastly, the specimen is rotated toward the patient's right, and the uncinate is taken off the vein and artery using an energy device. To perform this efficiently, the surgeon on the right side of the patient should grasp the stomach and the proximal jejunum and retract them out of the abdomen. The surgeon should imagine "opening a book" to distract the specimen from the SMA and SMV. The energy device should stay as close to the SMA as possible. We prefer using a harmonic scalpel to achieve this. Oftentimes, we use large hemoclips that are placed at the SMA border for hemostasis.
Prior to gaining confidence with use of an energy device on the uncinate dissection, the senior author would perform the division of the uncinate from the SMA using clamps and ties. This method leaves more tissue on the SMA; the authors have evolved to feeling very comfortable with a direct dissection on the SMA using energy.
Some groups have advocated the use of staplers on the uncinate. The authors feel that this leaves tissue on the SMA that can affect margin status. The senior author uses this technique when performing a trauma-related PD.
Key Point: The Stomach and Transected Jejunum Should Be Retracted as if "Opening a Book," and the Energy Device Can Be Used to Take the Specimen Off the SMA
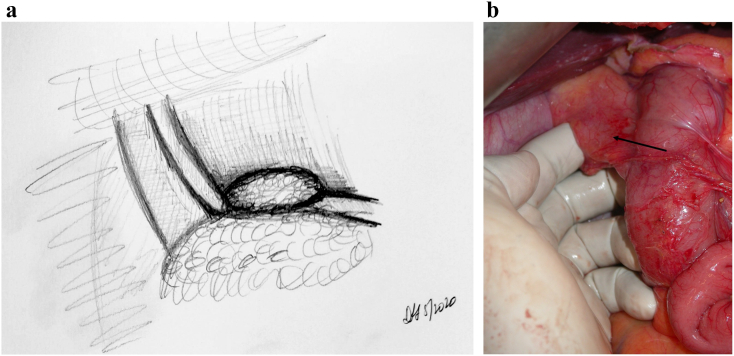
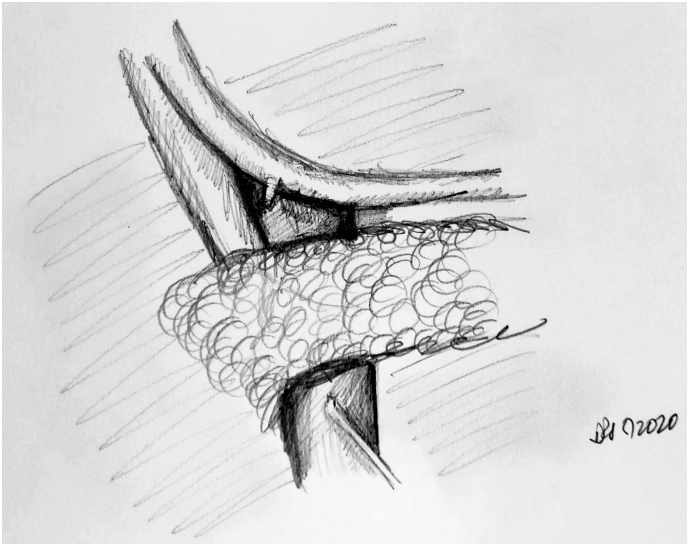
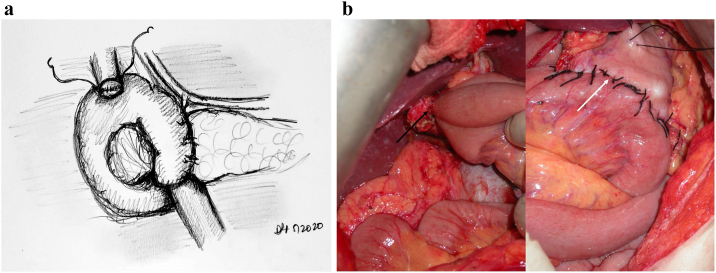
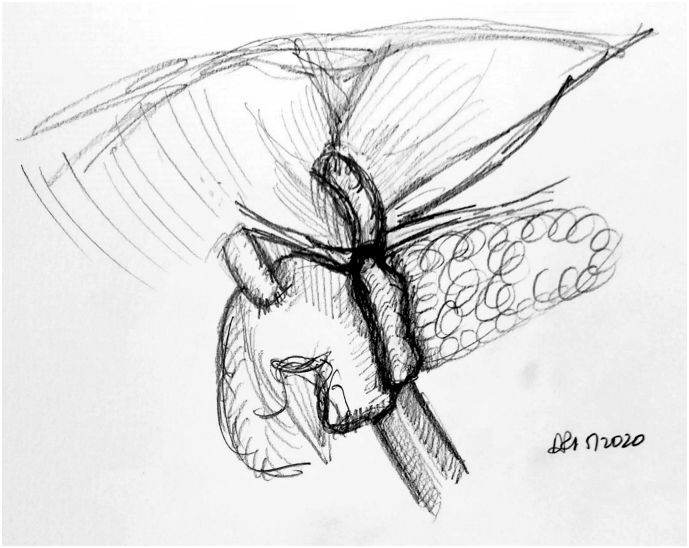
The jejunal reconstruction limb is brought either behind the SMA or through the mesocolon to begin reconstruction. The pancreatic duct is cannulated with a pediatric feeding tube that is left in place, and the pancreaticojejunostomy (PJ) is created in a two-layered, duct-to-mucosa fashion using Blumgart's technique using 2-0 silk and 5-0 Prolene sutures. Our group does not utilize externalization of the pancreatic stent, although some surgeons prefer this method to reduce the rate of pancreatic fistula. Data on stent placement at all are not clear, and the authors' preference to use a stent is related to the technical assistance that this provides. This is especially true in a teaching environment. The hepaticojejunostomy (HJ) is created using a single-layered, running fashion using 5-0 PDS suture. A stapled antecolic, retrogastric, and isoperistaltic gastrojejunostomy (GJ) is then created. A falciform flap is used to buttress the PJ [Fig 7, Fig 8, Fig 9). A drain is left in place encompassing both the HJ and the PJ. This marks the end of the procedure.
Fig 7.
A, Step 7: Modified Blumgart technique to create the pancreatojejunostomy. Note. Modified Blumgart technique using an outer buttressing layer of 2-0 silk and inner duct to mucosa using 5-0 Prolene is performed to create the pancreatojejunostomy. B, Intraoperative photograph demonstrating the alternative method of pancreatojejunostomy utilizing a two-layer anastomosis; arrow noting pediatric feeding tube cannulating the pancreatic duct.
Fig 8.
A, Step 8: Creation of the HJ and GJ.Note. HJ is created using a single-layered, running 5-0 polydioxanone suture. A stapled, antecolic, retrogastric, isoperistaltic GJ is then created.
B, Intraoperative photographs demonstrating completed HJ (black arrow) and GJ (white arrow).
Fig 9.
Step 9: Using the falciform ligament to cover the pancreatojejunostomy.
Note. The falciform ligament is passed posterior to the pancreatojejunostomy and sutured to itself, forming a layer of buttress and coverage for the pancreatojejunostomy.
DISCUSSION
The Whipple procedure has evolved since the first attempt at resection of the head of pancreas and duodenum in the late 19th to early 20th century [6]. Each iteration of the procedure has resulted in significantly improved operating room (OR) times as well as a decrease in postoperative morbidity and mortality. At the senior author's institution, the mean operating time for 220 Whipple procedures performed between June 2017 and August 2020 was 197 minutes, with a median time of 182 minutes.
Here, we propose and illustrate a standardized and easily reproducible operative strategy to approach the Whipple procedure, demonstrating that it is feasible to perform this surgery in an efficient manner and with significantly reduced operative times. Longer OR times are associated with an increased risk of surgical site infections, need for reintubation, longer hospital stay, and increased 30-day mortality [7]. By using this approach when possible, patients can receive the added benefits of reduced operating time. Patient circumstances, disease pathology, and variations in anatomy neccesitate alternative sequences and technique to create the most optimal and safest oncologic resection for each individual. As a result, not every patient will be able to undergo the procedure in the way described.
Another benefit of a standardized and easily reproducible operative procedure is the consistency with which this technique can be imparted to trainees. Breaking the procedure down into key definitive steps as the operation progresses will help surgical trainees attain graded autonomy during this procedure as they gain familiarity with each checkpoint.
In summary, this illustrated review of the Whipple procedure as performed at our institution is intended to help facilitate a streamlined and stepwise progression through what is undoubtedly a challenging surgical procedure. Our hope is that this will lead to a more efficient procedure and decreased OR time and costs as well as provide a framework to teach and measure technical progress for surgical trainees.
Author Contribution
Shankar Logarajah, MD: Writing – review & editing, Conceptualization, Visualization; Terence Jackson, MD: Writing – original draft, Conceptualization; Muhammad Darwish, MBBS: Writing – original draft; Kei Nagomoto, DO: Writing – original draft; Edward Cho, MD: Conceptualization; Houssam Osman, MD: Conceptualization, Supervision; D. Rohan Jeyarajah: Writing – original draft, Writing – review & editing, Conceptualization, Resources, Visualization, Supervision.
Conflict of Interest
None.
Funding Source
None.
Ethics Approval
Not applicable.
References
- 1.Howard J.M. History of pancreatic head resection—the evaluation of surgical technique. Am J Surg. 2007;194(4 SUPPL):S6–S10. doi: 10.1016/j.amjsurg.2007.05.029. [DOI] [Google Scholar]
- 2.Cameron J.L., Riall T.S., Coleman J., Belcher K.A. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-Del Castillo C., Morales-Oyarvide V., McGrath D., et al. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surg (United States) 2012;152(3 SUPPL):S56–S63. doi: 10.1016/j.surg.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheetz K.H., Dimick J.B., Nathan H. Centralization of high-risk cancer surgery within existing hospital systems. J Clin Oncol. 2019;37(34):3234–3242. doi: 10.1200/JCO.18.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strasberg S.M., Sanchez L.A., Hawkins W.G., Fields R.C., et al. Resection of tumors of the neck of the pancreas with venous invasion: the “Whipple at the Splenic Artery (WATSA)” procedure. J Gastrintest Surg. 2012;16(5):1048–1054. doi: 10.1007/s11605-012-1841-6. [DOI] [PubMed] [Google Scholar]
- 6.Are C., Dhir M., Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. HPB. 2011;13(6):377–384. doi: 10.1111/j.1477-2574.2011.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggino L., Liu J.B., Ecker B.L., Pitt H.A., Vollmer C.M. Impact of operative time on outcomes after pancreatic resection: a risk-adjusted analysis using the American College of Surgeons NSQIP database. J Am Coll Surg. 2018;226(5):844–857.e3. doi: 10.1016/j.jamcollsurg.2018.01.004. [DOI] [PubMed] [Google Scholar]