Abstract
Objectives
Intraoperative fluorescence imaging is currently used in a variety of surgical fields for four main purposes: assessing tissue perfusion; identifying/localizing cancer; mapping lymphatic systems; and visualizing anatomy. To establish evidence-based guidance for research and practice, understanding the state of research on fluorescence imaging in different surgical fields is needed. We evaluated the evidence on fluorescence imaging for perfusion assessments using the Idea, Development, Exploration, Assessment, Long Term Study (IDEAL) framework, which was designed for describing the stages of innovation in surgery and other interventional procedures.
Design
Narrative literature review with analysis of IDEAL stage of each field of study.
Setting
All publications on intraoperative fluorescence imaging for perfusion assessments reported in PubMed through 2019 were identified for six surgical procedures: coronary artery bypass grafting (CABG), upper gastrointestinal (GI) surgery, colorectal surgery, solid organ transplantation, reconstructive surgery, and cerebral aneurysm surgery.
Main outcome measures
The IDEAL stage of research evidence was determined for each specialty field using a previously described approach.
Results
196 articles (15 003 cases) were selected for analysis. Current status of research evidence was determined to be IDEAL Stage 2a for upper GI and transplantation surgery, IDEAL 2b for CABG, colorectal and cerebral aneurysm surgery, and IDEAL Stage 3 for reconstructive surgery. Using the technique resulted in a high (up to 50%) rate of revisions among surgical procedures, but its efficacy improving postoperative outcomes has not yet been demonstrated by randomized controlled trials in any discipline. Only one possible adverse reaction to intravenous indocyanine green was reported.
Conclusions
Using fluorescence imaging intraoperatively to assess perfusion is feasible and appears useful for surgical decision making across a range of disciplines. Identifying the IDEAL stage of current research knowledge aids in planning further studies to establish the potential for patient benefit.
Keywords: development study, device evaluation, health technology
Key messages.
What is already known about this subject?
Intraoperative fluorescence imaging has become widely used for assessing organ perfusion in a variety of surgical procedures.
What are the new findings?
Based on literature reviews, the IDEAL stage of fluorescence imaging for perfusion assessment could be determined in the following six surgical fields: coronary artery bypass grafting (Stage 2b), upper gastrointestinal surgery (2a), colorectal surgery (2b), solid organ transplantation (2a), reconstructive surgery (3), and cerebral aneurysm surgery (2b).
How might these results affect future research or surgical practice?
Identifying the IDEAL stage of current research knowledge would aid in planning further studies required for developing intraoperative fluorescence imaging into essential surgical tools contributing to better operative outcomes.
Introduction
Since the origin of surgical treatment, surgeons have needed “lights” to illuminate the surgical field brightly and clearly enough to identify lesions to be removed and the anatomy of surrounding tissues/organs. Recently, surgeons have also started using “fluorescence signals”, mainly in the near-infrared region invisible to the naked eye, for identifying biological structures, physiological function, and neoplastic transformation more clearly than naked-eye inspections or conventional white-light examinations.1 2
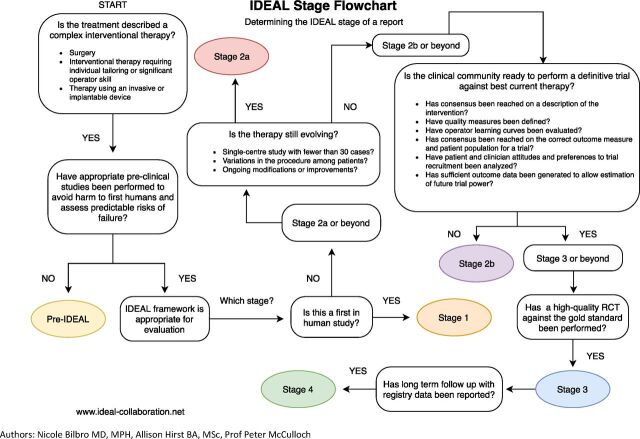
In response to the widening use of intraoperative fluorescence imaging, the International Society for Fluorescence Guided Surgery (ISFGS) was established in 2014, aiming to deepen communication between surgeons, researchers, and industry engineers.3 During discussions at the sixth annual meeting of the ISFGS in 2019, we launched a project to overview the development of intraoperative fluorescence imaging across surgical fields and applications, using the IDEAL framework4 5 to identify which indications for fluorescence-guided surgery were ready for a randomized controlled trial (RCT) and which preparatory studies were needed in the other fields to increase the feasibility and value of future RCTs. The IDEAL framework describes five stages—Idea, Development, Exploration, Assessment, Long-term follow-up—in the evaluation life-cycle of surgical techniques, which can be recognised for a given technique by the types of study already published in which the technique was assessed.6 The IDEAL recommendations set out study designs and reporting standards which aim to facilitate the rapid progress of research towards a definitive RCT (where possible) and beyond to surveillance studies.4–6 The stages and method for identifying them from the literature are summarised in figure 1, table 1.
Figure 1.
Flowchart of the ideal staging system. Cited from a webpage of the ideal collaboration: https://www.ideal-collaboration.net/wp-content/uploads/2021/04/IDEAL-Stages-Guidebook-Final.pdf. literature/. RCT, randomized controlled trial.
Table 1.
Breakdown of key considerations used to determine ideal Stage 6
| Characteristics of reports | Key issues addressed and content items | Key milestones for stage completion | |
| Idea (1) | One or very few reports Appears to be the earliest or near earliest report Only case reports or very small case series |
States that this is first in humans Detailed technical description |
Reports an intervention not previously used in human |
| Development (2a) | Small number of reports Reports from one or a few centers All reports have small number of patients Nearly all reports are case series |
Safety of procedure Short-term outcomes Discusses indications Discusses technical detail and may describe modifications |
Content and nature of reports suggest intervention technique has reached stability |
| Exploration (2b) | Increasing number of reports Patients per report Centers involved Some prospective collaborative studies (registries, audits, databases) |
Discusses procedural quality Discusses learning curves Comparison of outcomes with standard treatment Calls for an RCT to be done |
Reports suggest that consensus has been reached on optimal technique, indications and outcome measures |
| Assessment (3) | Reports of multicenter RCTs Quasi-experimental designs Stepped-wedge designs Case-matching studies Analysis of large data sets with risk adjustment |
Compares procedure with standard treatment | Reports document a high-quality RCT or other valid experimental comparison of the intervention compared with the current standard of care |
| Long-term (4) | Long-term cohort studies Retrospective case series Registries and databases Analyses of large administrative data sets No recent RCTs |
Reports long-term outcomes Identifies rare outcomes May analyze risk or prognostic factors May report on changing indications |
Ongoing reports of late or rare outcomes Which patients benefit most Whether indications are changing Variation in performance |
RCT, randomized clinical trial;.
In this project, fluorescence imaging applications were classified into (1) Perfusion assessment, (2) Cancer identification, (3) Lymphatic system identification, and (4) Anatomy visualization. Assessing tissue/organ perfusion is the oldest and most widely distributed of these applications. In 1997, Lund and Jogestrand7 reported in vivo fluorescence imaging techniques for the evaluation of regional cutaneous perfusion in occlusive arterial disease, based on pioneering work by Lange and Boyd8 published in 1942. In vivo fluorescence imaging using intravenous indocyanine green (ICG) has also been applied to ophthalmic fundus angiography9 and a range of other uses. Since the dawn of this century, the use of fluorescence imaging techniques has been reported for visualizing blood flow during reconstructive surgery (2002),10 coronary artery bypass grafting (CABG),11 cerebral aneurysm surgery (2003),12 solid organ transplantation (2004),13 and both colorectal (2010)14 and upper gastrointestinal (upper GI, 2011)15 surgery. In this study, the state of current research on the use of fluorescence imaging for assessing perfusion in each of the six above-listed surgical fields was evaluated, using the IDEAL framework to analyze relevant publications through 2019.
Methods
No ethical approval is required in this study without any interventions. A literature review was conducted, through December 31, 2019, of English-written publications in PubMed and Ovid/Medline reporting techniques and outcomes of intraoperative fluorescence imaging used to assess perfusion. For each surgical specialty area, specialist surgeons with experience of using fluorescence-guided surgery identified all relevant papers without selecting between them (online supplemental figure 1). The following keywords were used for both the title and abstract fields to identify potentially pertinent studies:
bmjsit-2021-000088supp007.pdf (752.8KB, pdf)
Coronary artery bypass grafting
(“fluorescence imaging” OR “fluorescent imaging” OR “near infrared imaging” OR “fluorescence angiography” OR “indocyanine green”) AND (“CABG” OR “coronary artery” OR “cardiac surgery”)
Upper GI surgery
(“fluorescence imaging” OR “fluorescent imaging” OR “near infrared imaging” OR “fluorescence angiography” OR “indocyanine green”) AND (“oesophagus” OR “stomach” OR “esophagectomy” OR “gastrectomy”)
Colorectal surgery
(“fluorescence imaging” OR “fluorescent imaging” OR “near infrared imaging” OR “fluorescence angiography” OR “indocyanine green”) AND (“colorectal” OR “colon” OR “rectal” OR “anterior resection” OR “sphincter”)
Solid organ transplantation
(“fluorescence imaging” OR “fluorescent imaging” OR “near infrared imaging” OR “fluorescence angiography” OR “indocyanine green”) AND (“transplantation” OR “graft” OR "donor ")
Reconstructive surgery
(“fluorescence imaging” OR “fluorescent imaging” OR “near infrared imaging” OR “fluorescence angiography” OR “indocyanine green”) AND (“plastic surgery” OR “flap” OR “reconstruction” OR “reconstructive”)
Cerebral aneurysm surgery
(“fluorescence imaging” OR “fluorescent imaging” OR “near infrared imaging” OR “fluorescence angiography” OR “indocyanine green”) AND (“neurosurgery” OR “aneurysm” OR “cerebral artery” OR “brain surgery”)
Inclusion criteria
Reports had to provide enough detail on fluorescence-guided surgery to allow readers to potentially reproduce the technique, and to report either technical or clinical (or both) outcomes of clinical use.
Exclusion criteria
Non-human preclinical studies, meta-analyses/systemic reviews, supplementary articles, and conference proceedings were excluded. We also excluded articles with a focus outside the stated target application and those with inadequate information on patients’ background, treatment, and/or outcomes to allow the evaluation of what was done to whom and with what outcome.
Analysis
The maturity stage of intraoperative fluorescence imaging for perfusion assessment was determined according to the IDEAL framework.4–6 Since the reports studied were not written in IDEAL style, the stage of the research field was determined by allocating studies to IDEAL stages retrospectively, using the following heuristic: Reports of only one or very few cases are classified as IDEAL Stage 1 (Idea). Outcomes of fluorescence imaging reported from case series and cohort studies were classified as IDEAL Stage 2a or Stage 2b (Exploration), with reports involving only a few centers (five or less) and with case series of <100 cases classed as Stage 2a, and larger studies as Stage 2b. Fluorescence imaging techniques evaluated in multicenter RCTs were considered Stage 3 (Assessment) and long-term evaluations using registries or databases were classified as Stage 4 (Long-term). The IDEAL stage proposed by the expert panels was determined after consensus formation in the observer panel about the overall state of the research field (online supplemental figure 1), rather than assigning the highest stage possible based on single studies.
Results
Overview
The research keywords, intended to include all reports related to intraoperative fluorescence imaging for perfusion assessments, identified 4268 articles for initial screening. After applying the inclusion and exclusion criteria, 196 articles, reporting on a total of 15 003 patients, were submitted for further evaluation (online supplemental figure 1).
The number of previous publications and number of reported cases are summarized, by year and by regional origin (Asia, Europe, North America, Oceana), in figures 2 and 3, respectively. The use of intraoperative fluorescence imaging to assess perfusion has been reported since the early 2000s for CABG, reconstructive surgery, and cerebral aneurysm surgery. The number of publications and reported cases seemed to peak in 2004–2005 for CABG, while publications still appear to be increasing in number in all the other five fields. Reports on the use of fluorescence imaging during solid organ transplantation were much fewer than for the other surgical fields.
Figure 2.
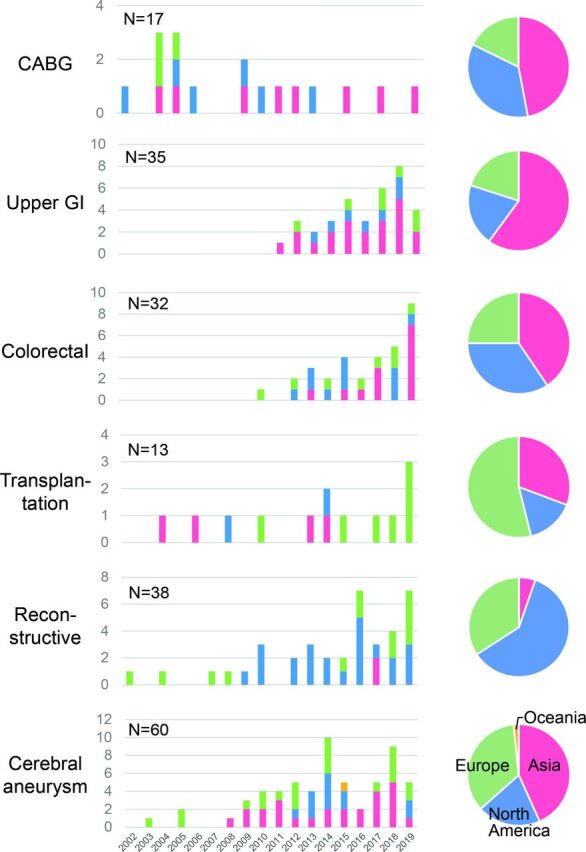
Time and regional trends of publications on intraoperative fluorescence imaging for perfusion assessment were summarized according to the six surgical procedures. CABG, coronary artery bypass grafting; GI, gastrointestinal.
Figure 3.
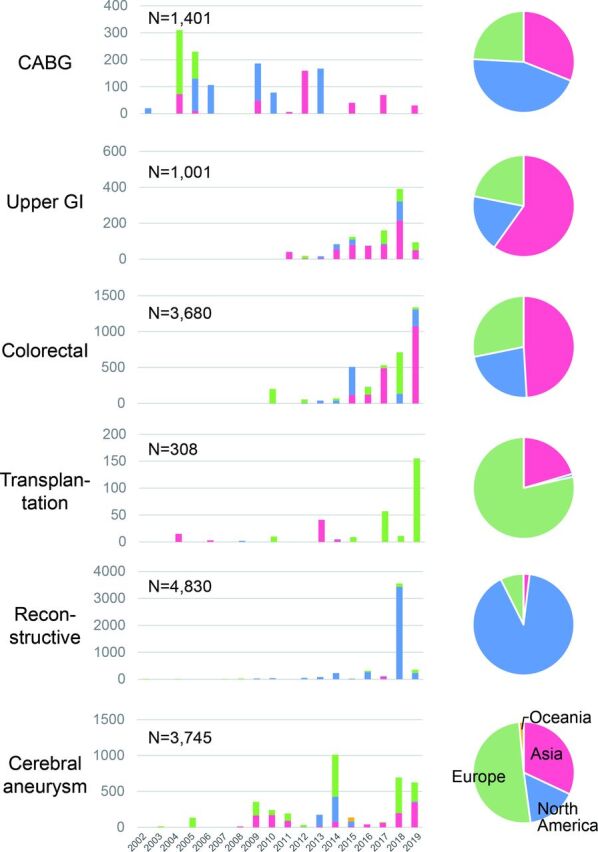
Time and regional trends of reported cases on intraoperative fluorescence imaging for perfusion assessment were summarized according to the six surgical procedures. CABG, coronary artery bypass grafting; GI, gastrointestinal.
Regional distribution of the previous publications also differed between the six applications. In upper GI surgery, the majority of reports came from Asian countries, while the USA and Europe dominated in the fields of reconstructive surgery and solid organ transplantation, respectively.
Summary of surveillance for each surgical field
Results on the literature surveillance for each surgical field are summarized, in terms of study design and imaging techniques (table 2), as well as the endpoint measures and additional information (table 3) used to determine each field’s IDEAL stage. Background data for each article are summarized in online supplemental tables 1–6.
Table 2.
Summary of previous publications on intraoperative fluorescence imaging for perfusion assessment in terms of study design and imaging techniques
| Applications | No. of publications | No. of reported cases (Max. sample size) |
Study design | Imaging techniques | IDEAL stage | |
| Fluorogenic agent | Frequently used dose (range) | |||||
| CABG | 17 | 1401 (200) | Small case series*, 9 (53%) Prospective studies, 15 (88%) Large multicenter study†, 0 (0%) RCT, 1 (6%) |
ICG (100%) | 1.25–2.5 mg recognized (35%) (0.625–5.0 mg) |
2b |
| Upper GI surgery | 35 | 1001 (86) | Small case series*, 31 (89%) Prospective studies, 12 (34%) Large multicenter study†, 0 (0%) RCT, 0 (0%) |
ICG (100%) | 2.5 mg IV (34%) (1.25–25 mg) |
2a |
| Colorectal surgery | 33 | 3718 (609) | Small case series*, 15 (45%) Prospective studies, 16 (48%) Large multicenter study†, 2 (6%) RCT, 0 (0%) |
ICG (100%) | 2.5–5 mg IV (31%) or 0.2–0.5 mg/kg IV (30%) (2.5–12.5 mg) |
2b |
| Solid organ transplantation | 13 | 308 (77) | Small case series*, 13 (100%) Prospective studies, 9 (69%) Large multicenter study†, 0 (0%) RCT, 0 (0%) |
ICG (100%) | 2.5–7.5 mg IV (38%) or 0.3–0.5 mg/kg IV (31%) (0.6–25 mg) |
2a |
| Reconstructive surgery | 38 | 4830 (3,315) | Small case series*, 23 (61%) Prospective studies, 17 (45%) Large multicenter study†, 0 (0%) RCT, 1 (3%) |
ICG (97%) | 5–12.5 mg IV (37%) or 0.5 mg/kg IV (13%) (1.5–30 mg) |
3 |
| Cerebral aneurysm surgery | 60 | 3745 (347) | Small case series*, 45 (75%) Prospective studies, 7 (12%) Large multicenter study†, 0 (0%) RCT, 0 (0%) |
ICG (98%) | 0.2–0.5 mg/kg IV (35%) or 25 mg IV (32%) (6–30 mg) |
2b |
*Retrospective or prospective studies including less than 100 cases in five or less centers, without a control arm.
†Prospective multicenter study including equal to or more than 100 cases.
CABG, coronary artery bypass grafting; GI, gastrointestinal; ICG, indocyanine green; IV, intravenous; RCT, randomized controlled trial.
Table 3.
Summary of previous publications on intraoperative fluorescence imaging for perfusion assessment in terms of endpoint measures and additional information
| Applications | No. of publications | Endpoint measures | Adverse effects of fluorescence imaging | Assessment of learning curve | Cost analysis | IDEAL stage | |||
| Main purpose | Imaging accuracy | Clinical impact, changes in intraoperative decision making | Significant advantages in postoperative outcomes (No. of publications) | ||||||
| CABG | 17 | Assessment of graft/anastomotic patency | 75%–100% success rate 50%–100%/100% sensitivity/specificity documented in 71% publications |
0.5%–7.2% documented in 71% publications |
– | None | 1 (6%) | 1 (6%) | 2b |
| Upper GI surgery | 35 | Assessment of remnant stomach perfusion | 50%–100% success rate documented in 54% publications |
23%–40% documented in 11% publications |
Less anastomotic leak (1) | None | 0 (0%) | 0 | 2a |
| Colorectal surgery | 33 | Assessment of colorectal perfusion to be anastomosed | 63%–100% success rate 100%/93% sensitivity/specificity documented in 39% publications |
3.7%–40% documented in 82% publications |
Less anastomotic leak (7)/stenosis (1) | None | 0 (0%) | 0 | 2b |
| Solid organ transplantation | 13 | Assessment of vessel patency and graft perfusion | 100% success rate documented in 69% publications |
0%–30% documented in 23% publications |
– | None | 0 (0%) | 0 | 2a |
| Reconstructive surgery | 38 | Assessment of graft perfusion | 71%–100% success rate 85%–100%/88%–100% sensitivity/specificity documented in 39% publications |
4.3%–70% documented in 21% publications |
Less postoperative complications (2), necrosis (8), infection (2) | None | 2 (5%) | 3 (8%) | 3 |
| Cerebral aneurysm surgery | 60 | Assessment of aneurysm closure and cerebral artery perfusion | 74%–100% documented in 82% publications |
4.4%–50% documented in 55% publications |
Less ischemic complications (1) | Sat O2 decrease (ICG, 1) and skin yellowing (fluorescein, 10) | 0 (0%) | 2 (3%) | 2b |
CABG, coronary artery bypass grafting; GI, gastrointestinal; ICG, indocyanine green.
bmjsit-2021-000088supp001.pdf (92.2KB, pdf)
bmjsit-2021-000088supp002.pdf (155.1KB, pdf)
bmjsit-2021-000088supp003.pdf (156.4KB, pdf)
bmjsit-2021-000088supp004.pdf (129.2KB, pdf)
bmjsit-2021-000088supp005.xlsx (24.5KB, xlsx)
bmjsit-2021-000088supp006.pdf (229.2KB, pdf)
Coronary artery bypass grafting
In this field, our search yielded 17 eligible reports, of which 9 (53%) were case series with no control arm, involving five or fewer centers and under 100 patients. There were no large multicenter prospective studies. There was one RCT.16 In total, 1401 cases were reported, the maximum number of patients in any study being 200 (online supplemental table 1).
Intravenously injected ICG was used in all studies, the most frequent dose being 1.25–2.5 mg (reported in 35% of publications). Acceptable success rate and imaging accuracy visualizing graft/anastomosis patency was reported in 71% of the publications, some of which indicated better diagnostic ability to evaluate graft patency than with transit-time flowmetry (TTF).17–19 In these reports, ICG fluorescence angiography enabled the modification of surgical procedures (primarily anastomosis revision) in 0.5%–7.2% of patients. However, the only reported RCT16 failed to identify any significant decrease in postoperative graft occlusion using ICG fluorescence angiography with TTF, relative to standard intraoperative management without intraoperative imaging. Only one study (6%) assessed the learning curve for the technique.20
Although consensus seemed to be reached on the imaging technique and outcome measures, suggesting that the technique is now stable, the one RCT failed to demonstrate any clinical benefit from ICG fluorescence angiography over conventional surgery. Thus, the field of intraoperative fluorescence imaging for CABG was considered to be in IDEAL stage 2b. A significant number of key questions requiring answers in Stage 2b have not yet been addressed, suggesting that a large collaborative cohort study or pilot study leading to an RCT might be useful before a new RCT is attempted.
Upper GI surgery
In this field, our search yielded 35 eligible reports, of which 31 (89%) were case series involving five or fewer centers and under 100 patients. There was no large multicenter study or randomized trial. In total 1001 cases had been reported, the maximum sample size being 86 cases (online supplemental table 2).
Intravenously injected ICG at a dose of 2.5 mg was mainly used to assess blood perfusion in the remnant stomach being used for reconstruction. Although this technique was associated with a 50%–100% success rate visualizing perfusion, leading to changes in the reconstruction procedure in up to 40% of patients, diagnostic accuracy—based on a quantitative analysis of fluorescence intensity—was rarely demonstrated. Whether ICG fluorescence imaging reduces postoperative anastomotic leaks remains unclear.
In this field, the lack of larger prospective cohort studies and RCTs, despite apparent consensus on technique, suggests that the evidence base is still at IDEAL Stage 2a, although it is now ready to proceed to Stage 2b.
Colorectal surgery
In this field, our search yielded 33 eligible reports, encompassing 3718 patients, among which 15 (45%) were case series involving five or fewer centers and under 100 patients. Two (6%) were larger multicenter prospective studies, involving 12 US centers21 and 4 European centers,22 respectively. There were no RCTs (online supplemental table 3).
ICG was injected intravenously at a dose of 2.5–5 mg or 0.2–0.5 mg/kg in the majority of studies, enabling visualization of blood perfusion in the colorectal and the ileal wall to be anastomosed in 63%–100% of cases. Application of this technique led surgeons to adjust the anastomotic site/method in 4%–40% of patients (rate reported in 80% of publications). This might lead to a lower rate of postoperative anastomotic leak/stenosis relative to non-randomized controls (reported in eight studies).
Current evidence on the use of ICG fluorescence imaging for colorectal surgery could be classified as IDEAL Stage 2b, because consensus on the methodology, expected effects, patient population, and main endpoint (postoperative anastomotic leak) has been reached, and seems sufficient to proceed to an RCT.
Solid organ transplantation
In this field, our search yielded 13 eligible reports, all case series involving five or fewer centers and under 100 patients. There was no larger multicenter study or randomized trial. A total of 308 cases was reported (online supplemental table 4).
The purpose of this technique is to assess vessel patency and graft perfusion, which was achieved following the intravenous injection of ICG (2.5–7.5 mg or 0.3–0.5 mg/kg) in approximately half of the studies. Perfusion also was assessed based on intensity and trends in fluorescence signals following ICG administration.
Although the safety and feasibility of this technique have been reported sufficiently, further prospective studies are needed to demonstrate the benefits of ICG fluorescence imaging in decision making during transplantation, and for postoperative graft function/survival. The nature of current studies places the technique in IDEAL Stage 2a. This suggests that larger collaborative prospective cohort studies should be done, once it is clear that the technique is now stable, before an RCT is attempted.
Reconstructive surgery
In this field, our search yielded 38 eligible reports, involving 4830 cases, two-thirds of them in a single retrospective database study in the USA (sample size 3315).23 Twenty-three studies (61%) were case series involving five or fewer centers and under 100 patients. No larger multicenter prospective studies were included. There was one RCT (online supplemental table 5).
Intravenous ICG was used in all but one study, the dose ranging from 5 mg to 12.5 mg. ICG fluorescence imaging enabled graft perfusion visualization in 71%–100% of cases, leading to the adjustment of reconstruction procedures in up to 70% of patients. For 12 studies, the authors suggested a possible decrease in postoperative complications, but this was not demonstrated in an internally valid manner against randomized controls. Quantitative analysis also was applied in 45% of the studies to determine cut-off values to predict postoperative complications. Learning curve and cost analyses also were available.
In the first double-blinded RCT reported in 2016,24 skin/subcutaneous hypoperfusion identified by ICG fluorescence imaging was associated with a significantly higher rate of wound infection (28% vs 9.4%), but flap modification based on the fluorescence images did not prevent wound-related complications. The current development status of this technique could be classified as IDEAL Stage 3, whereby further RCTs are needed to establish its efficacy improving postoperative complications and graft survival.
Cerebral aneurysm surgery
In this field, our search yielded 60 eligible reports encompassing a total of 3745 cases, among which 45 reports (75%) were case series involving five or fewer centers and under 100 patients. There was no larger multicenter study or randomized trial. The maximum sample size in any study was 347 cases (online supplemental table 6).
ICG was usually administered intravenously just before observation at a dose of 0.2–0.5 mg/kg or 25 mg. The main purpose of ICG fluorescence imaging was to identify neck remnants, incomplete occlusion, and/or branch stenosis or occlusion, as an alternative to conventional digital subtraction angiography. An acceptable success rate of target identification (74%–100%) was reported for 80% of the studies, resulting in modification of 4%–50% of the surgical procedures (clip repositioning). Another possible application of this technique was to confirm the patency of anastomotic vessels, based on the quantitative analysis of fluorescence intensity. A potential complication associated with ICG administration was reported in one patient (transiently decreased oxygen saturation for 1 min intraoperatively).25 Fluorescein was used as a fluorophore in three studies, with an adverse reaction (skin yellowing) documented in 10 patients.26 Two studies performed a cost analysis.
Despite the low proportion of prospective studies for this application, consensus on the methods and outcome measures of ICG fluorescence imaging seems to be established, probably because of the spread of surgical microscopy equipped with near-infrared imaging systems. The clinical impact of fluorescence imaging use should be evaluated by further large prospective collaborative studies (IDEAL Stage 2b) before an RCT is conducted.
Discussion
This review of intraoperative fluorescence imaging for perfusion assessments revealed that the number of publications differs by year and by geographic region across the different surgical fields. A recent increase in publications in digestive surgery reflects interest in the use of intraoperative perfusion assessments for anastomotic leak prevention. Our IDEAL framework analysis indicates that evidence for upper GI surgery is in Stage 2a, but ready to progress to Stage 2b, whereas in colorectal surgery it is at Stage 2b and ready to progress to Stage 3. Therefore, prospective, collaborative, cohort studies (to finalize consensus on methods and outcome measures for an RCT) are now appropriate for upper GI surgery, while colorectal surgery is now ready to proceed to one or more well-designed RCTs. For example, a multicenter RCT (IntAct) is currently underway for evaluating whether the assessment of anastomotic perfusion using ICG fluorescence imaging can minimize the incidence of anastomotic leak compared with conventional white-light laparoscopy.27 This difference in progression may be associated with the complicated vessel networks that exist in the stomach, making perfusion assessments during upper GI surgery more difficult than during colorectal surgery. Techniques enabling the systematic and quantitative evaluation of organ perfusion would be valuable, both in this field and that of solid organ transplantation (IDEAL 2a).
Reconstructive surgery has the oldest history of intraoperative fluorescence imaging use, since the first report in 2002,9 with an increasing number of recently published prospective studies, including one RCT.21 Few alternatives are available for real-time assessments of graft perfusion. Although its IDEAL stage could be considered ‘early Stage 3’, to date there have been no large, prospective studies (Stage 2b). Further well-designed prospective trials are needed to clarify whether perfusion assessments are effective at decreasing postoperative complications; however, prior Stage 2b exploratory studies would help to strengthen the design of these trials. Active introduction of this technique to regions outside the USA is also recommended to further disseminate its use, which could contribute to enhancing the safety of reconstructive surgery worldwide.
The first report on fluorescence angiography use during CABG was also published in 2002,10 but publications did not increase after peaking in 2004–2005, suggesting that this technique might be routinely used by only specialized surgeons. Early studies indicated that ICG fluorescence angiography enables graft revisions, but few studies demonstrated improved postoperative graft patency. Despite the presence of an RCT,15 this field lacks evidence from larger, prospective studies and is, therefore, just at the start of IDEAL Stage 2b. Larger collaborative cohort studies would strengthen the design of future RCTs, which are needed if this technique is to gain wider application. The advantages of ICG fluorescence angiography should also be evaluated in terms of cost, since it requires an additional investment to attain the necessary near-infrared imaging systems.
In the field of cerebral aneurysm surgery, the number and worldwide distribution of previous publications on fluorescence imaging for use assessing perfusion was remarkably high, relative to the five other surgical fields we evaluated, but there was a lack of high-level evidence indicating improved postoperative outcomes. No other techniques are widely available which enable the real-time visualization of blood flow around aneurysms during microscopic surgery. Fluorescence imaging is already widely disseminated and useful, at least, for surgical decision making. It is, therefore, unrealistic to create an appropriate control arm for an RCT. This is typical of situations wherein implementation has preceded evaluation. In such instances, remedial improvement of the evidence base can best be achieved through large, retrospective analyses of data extracted from registry systems and/or prospective clinical databases (IDEAL Stage 4).
The major advantage of using the IDEAL framework lies in the fact that it facilitates the planning of further studies to develop novel surgical procedures into standard techniques to be used more widely and effectively. In the present study, we did not make precise evaluations of research quality for each of the 196 articles, since some critical information tended to be missing especially in the reports in the earlier development phase. In contrast, the types of publication and the questions addressed to understand the progress of the overall field of research could be studied sufficiently. As a result, we were able to reach evidence-based consensus on the development stage of these fluorescence imaging techniques systematically by using questionnaires based on the IDEAL flowchart for stage assignment.
Overviewing IDEAL staging among applications of fluorescence imaging for perfusion assessment will be helpful in standardization and optimization of the imaging techniques, which have so far been developed independently in each surgical procedure. We are going to adopt the same approach with improving the survey protocol to elucidate the development stage of intraoperative fluorescence imaging for the remaining applications such as anatomy visualization, cancer localization, and lymph mapping. Lastly, delineation of regional trends in the development of fluorescence imaging will enable us to understand where further research and clinical application are needed and whether the technique is sufficiently distributed for designing international multicenter studies.
In summary, intraoperative fluorescence imaging has been used in numerous surgical fields for the assessment of perfusion, with the evaluation development status ranging from IDEAL Stage 2a to Stage 3. Although this technique is considered safe and feasible, and provides surgeons with beneficial information on blood perfusion for surgical decision making, prospective cohort studies and RCT remain necessary to evaluate its effectiveness enhancing surgical outcomes, also taking into account other issues like standardization of imaging techniques, cost and learning curves. The IDEAL stage analysis reported here has identified the preliminary work needed to optimize the design of such trials.
Acknowledgments
This work was endorsed by the International Society for Fluorescence Guided Surgery (ISFGS), based on discussion that occurred at the 6th Annual Meeting on February 16, 2018 in Miami, Florida.
Footnotes
Contributors: Study conception and design: TI, MCC. Acquisition of data: TI, DM, TC, OW, GD, SS-K, SDW, MA-G, LB, EC, CS, RC, FR, MC, AS, EV, MTe, MTa, KH, RMS, TP, YM, FM. Interpretation of data: MCC, MB, MD, NK, FD, KW, RJR. Drafting the manuscript: TI, KW. Critical revisions of the manuscript: MCC, DM, TC, OW, GD, SS-K, SDW, MA-G, LB, EC, CS, RC, FR, MC, AS, EV, MTe, MTa, KH, RMS, TP, YM, FM, MB, MD, NK, FD, KW, RJR. Final approval of the final version and agreement for submission: All authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: TI (associate editor), PMC (editor in chief), NK and RJR are editorial board members of BMJ Surgery, Interventions, & Health Technologies. The other authors have no competing interests to declare.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Dip FD, Ishizawa T, Kokudo N, eds. Fluorescence imaging for surgeons. Switzerland: Springer International Publishing, 2015. [Google Scholar]
- 2. Dip F, Boni L, Bouvet M, et al. Consensus conference statement on the general use of near-infrared fluorescence imaging and indocyanine green guided surgery: results of a modified delphi study. Ann Surg 2020. 10.1097/SLA.0000000000004412. [Epub ahead of print: 17 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The International society for fluorescence-guided surgery. Available: https://www.isfgs.org/
- 4. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the ideal recommendations. Lancet 2009;374:1105–12. 10.1016/S0140-6736(09)61116-8 [DOI] [PubMed] [Google Scholar]
- 5. Hirst A, Philippou Y, Blazeby J, et al. No surgical innovation without evaluation: evolution and further development of the ideal framework and recommendations. Ann Surg 2019;269:211–20. 10.1097/SLA.0000000000002794 [DOI] [PubMed] [Google Scholar]
- 6. Pennell CP, Hirst AD, Campbell WB, et al. Practical guide to the idea, development and exploration stages of the ideal framework and recommendations. Br J Surg 2016;103:607–15. 10.1002/bjs.10115 [DOI] [PubMed] [Google Scholar]
- 7. Lund F, Jogestrand T. Video fluorescein imaging of the skin: description of an overviewing technique for functional evaluation of regional cutaneous blood perfusion in occlusive arterial disease of the limbs. Clin Physiol 1997;17:619–33. 10.1046/j.1365-2281.1997.00057.x [DOI] [PubMed] [Google Scholar]
- 8. Lange K, Boyd LJ. The use of fluorescein to determine the adequacy of the circulation. Med Clin North Am 1942;26:943–52. 10.1016/S0025-7125(16)36467-7 [DOI] [Google Scholar]
- 9. Guyer DR, Puliafito CA, Monés JM, et al. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology 1992;99:287–91. 10.1016/S0161-6420(92)31981-5 [DOI] [PubMed] [Google Scholar]
- 10. Holm C, Mayr M, Höfter E, et al. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br J Plast Surg 2002;55:635–44. 10.1054/bjps.2002.3969 [DOI] [PubMed] [Google Scholar]
- 11. Rubens FD, Ruel M, Fremes SE. A new and simplified method for coronary and graft imaging during CABG. Heart Surg Forum 2002;5:141–4. [PubMed] [Google Scholar]
- 12. Raabe A, Beck J, Gerlach R, et al. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 2003;52:132–9. 10.1097/00006123-200301000-00017 [DOI] [PubMed] [Google Scholar]
- 13. Sekijima M, Tojimbara T, Sato S, et al. An intraoperative fluorescent imaging system in organ transplantation. Transplant Proc 2004;36:2188–90. 10.1016/j.transproceed.2004.09.001 [DOI] [PubMed] [Google Scholar]
- 14. Kudszus S, Roesel C, Schachtrupp A, et al. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 2010;395:1025–30. 10.1007/s00423-010-0699-x [DOI] [PubMed] [Google Scholar]
- 15. Shimada Y, Okumura T, Nagata T, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus 2011;8:259–66. 10.1007/s10388-011-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh SK, Desai ND, Chikazawa G, et al. The graft imaging to improve patency (GRIIP) clinical trial results. J Thorac Cardiovasc Surg 2010;139:e291:294–301. 10.1016/j.jtcvs.2009.09.048 [DOI] [PubMed] [Google Scholar]
- 17. Desai ND, Miwa S, Kodama D, et al. A randomized comparison of intraoperative indocyanine green angiography and transit-time flow measurement to detect technical errors in coronary bypass grafts. J Thorac Cardiovasc Surg 2006;132:585–94. 10.1016/j.jtcvs.2005.09.061 [DOI] [PubMed] [Google Scholar]
- 18. Handa T, Katare RG, Sasaguri S, et al. Preliminary experience for the evaluation of the intraoperative graft patency with real color charge-coupled device camera system: an advanced device for simultaneous capturing of color and near-infrared images during coronary artery bypass graft. Interact Cardiovasc Thorac Surg 2009;9:150–4. 10.1510/icvts.2008.201418 [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto M, Orihashi K, Nishimori H, et al. Efficacy of intraoperative hypereye medical system angiography for coronary artery bypass grafting. Surg Today 2015;45:966–72. 10.1007/s00595-014-1015-0 [DOI] [PubMed] [Google Scholar]
- 20. Desai ND, Miwa S, Kodama D, et al. Improving the quality of coronary bypass surgery with intraoperative angiography: validation of a new technique. J Am Coll Cardiol 2005;46:1521–5. 10.1016/j.jacc.2005.05.081 [DOI] [PubMed] [Google Scholar]
- 21. Jafari MD, Wexner SD, Martz JE, et al. Perfusion assessment in laparoscopic left-sided/anterior resection (Pillar II): a multi-institutional study. J Am Coll Surg 2015;220:82–92. 10.1016/j.jamcollsurg.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 22. Ris F, Liot E, Buchs NC, et al. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg 2018;105:1359–67. 10.1002/bjs.10844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chattha A, Bucknor A, Chen AD, et al. Indocyanine green angiography use in breast reconstruction: a national analysis of outcomes and cost in 110,320 patients. Plast Reconstr Surg 2018;141:825–32. 10.1097/PRS.0000000000004195 [DOI] [PubMed] [Google Scholar]
- 24. Wormer BA, Huntington CR, Ross SW, et al. A prospective randomized double-blinded controlled trial evaluating indocyanine green fluorescence angiography on reducing wound complications in complex abdominal wall reconstruction. J Surg Res 2016;202:461–72. 10.1016/j.jss.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 25. Lai LT, Morgan MK. Use of indocyanine green videoangiography during intracranial aneurysm surgery reduces the incidence of postoperative ischaemic complications. J Clin Neurosci 2014;21:67–72. 10.1016/j.jocn.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 26. Kakucs C, Florian I-A, Ungureanu G, et al. Fluorescein angiography in intracranial aneurysm surgery: a helpful method to evaluate the security of clipping and observe blood flow. World Neurosurg 2017;105:406–11. 10.1016/j.wneu.2017.05.172 [DOI] [PubMed] [Google Scholar]
- 27. Armstrong G, Croft J, Corrigan N, et al. Intact: intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Dis 2018;20:O226–34. 10.1111/codi.14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsit-2021-000088supp007.pdf (752.8KB, pdf)
bmjsit-2021-000088supp001.pdf (92.2KB, pdf)
bmjsit-2021-000088supp002.pdf (155.1KB, pdf)
bmjsit-2021-000088supp003.pdf (156.4KB, pdf)
bmjsit-2021-000088supp004.pdf (129.2KB, pdf)
bmjsit-2021-000088supp005.xlsx (24.5KB, xlsx)
bmjsit-2021-000088supp006.pdf (229.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.