Abstract
This study aimed to evaluate the effects of dietary creatine nitrate (CrN) on growth performance, meat quality, energy status, glycolysis, and related gene expression of liver kinase B1/AMP-activated protein kinase (LKB1/AMPK) pathway in Pectoralis major (PM) muscle of broilers. A total of 240 male Arbor Acres broilers (28-day-old) were randomly allocated to one of 5 dietary treatments: the basal diet (control group), and the basal diets supplemented with 600 mg/kg guanidinoacetic acid (GAA), 300, 600, or 900 mg/kg CrN (identified as GAA600, CrN300, CrN600, or CrN900, respectively). We found that dietary GAA and CrN supplementation for 14 d from d 28 to 42 did not affect broiler growth performance, carcass traits, and textural characteristics of breast muscle. GAA600, CrN600, and CrN900 treatments increased pH24h and decreased drip loss of PM muscle compared with the control (P < 0.05). The PM muscles of CrN600 and CrN900 groups showed higher glycogen concentration and lower lactic acid concentration accompanied by lower activities of phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) (P < 0.05). Simultaneously, GAA600 and all CrN treatments increased concentration of muscle creatine, phosphocreatine (PCr) and ATP, and decreased AMP concentration and AMP/ATP ratio (P < 0.05). Meanwhile, the concentrations of muscle creatine, PCr, and ATP were increased linearly, while muscle AMP concentration and AMP/ATP ratio were decreased linearly and quadratic as the dose of CrN increased (P < 0.05). GAA600, CrN600, and CrN900 treatments upregulated mRNA expression of CreaT in PM muscle, and CrN600 and CrN900 treatments downregulated GAMT expression in liver and PM muscle compared with the control or GAA600 groups (P < 0.05). The mRNA expression of muscle LKB1, AMPKα1, and AMPKα2 was downregulated linearly in response to the increasing CrN level (P < 0.05). Overall, CrN showed better efficacy on strengthening muscle energy status and improve meat quality than GAA at the some dose. These results indicate that CrN may be a potential replacement for GAA as a new creatine supplement.
Key words: broiler, creatine nitrate, meat quality, energy status, glycolysis
INTRODUCTION
The demand for poultry meat has increased significantly in recent decades as a result of its low cost, more comprehensive nutrition, and convenient for further processing (Petracci et al., 2015). More importantly, an increasing number of consumers favor safe and high-quality poultry meat, which has become the focus of modern poultry industry (Kuźniacka et al., 2020). Previous research has established that age, breed-type, pre-slaughter stress, and rearing environment were the important factors influencing the meat quality of broilers (Bogosavljević-Bošković et al., 2012). Furthermore, diet is also one of the most important key factors affecting meat quality. Dietary supplementation with some exogenous amino acid derivatives, such as creatine monohydrate (CMH) and guanidinoacetic acid (GAA) have been suggested as an effective way to improve meat quality of broilers (Michiels et al., 2012; Zhang et al., 2017, 2019).
Creatine, also known as N-methyl guanidinoacetic acid, is a natural amino acid derivative. The endogenous synthesis of creatine can be divided into 2 steps: the first step occurs in the kidney and pancreas, where arginine and glycine synthesize GAA under the action of L-arginine-glycine amidinotransferase (AGAT); and the second step occurs in the liver, where GAA is methylated to creatine by guanidinoacetate methyltransferase (GAMT) (Brosnan et al., 2011; Longo et al., 2011). Therefore, GAA is a natural biosynthetic precursor of creatine and can be used as creatine source. Skeletal muscle cells accumulate creatine from the plasma via specific Na+-dependent creatine transporter (CreaT), and then creatine is phosphorylated by creatine kinase to produce phosphocreatine (PCr) for the temporary storage of energy (Wyss and Kaddurah-Daouk, 2000). When muscle ATP are overexpend, PCr can provide high-energy phosphate bond to ADP to regenerate ATP (Curt et al., 2015). Therefore, the creatine/PCr system serves as a rapidly mobilizable reserve of high-energy phosphate in skeletal muscle or brain cells that have high and variable energy demands to recycle ATP and maintain energy stability to a certain extent (Michiels et al., 2012; Zhang et al., 2017; Portocarero and Braun, 2021).
Postmortem muscle glycolysis is highly related to meat quality traits of broiler chickens. After being slaughtered, rapid anaerobic glycolysis and over accumulation of H+ and lactic acid in muscle always accompanied with meat quality deteriorating of broilers, especially in birds subjected to pre-slaughter stress (Hamilton et al., 2003; Zhang et al., 2014; Wang et al., 2017). AMP-activated protein kinase is a metabolic sensor that can sense changes in intracellular energy and regulate energy balance. Prior studies have noted that AMPK plays a significant role in regulating postmortem muscle glycolysis of animals (Shen et al., 2006; Xing et al., 2016; Zhang et al., 2017). Dietary supplementation with creatine additive CMH or its precursor GAA can improve the muscle reserves of creatine, PCr, and ATP, delay the occurrence of rapid muscle glycolysis and the accumulation of muscle lactic acid after slaughter, which help to improve meat quality of broilers (Nissen and Young, 2006; Zhang et al., 2014, 2019). CMH has been identified as the most common form of creatine supplements in the field of human sports medicine (Kreider et al., 2017). However, CMH has some limitations, such as its low solubility in water and the oral bioavailability of CMH was less than complete (Alraddadi et al., 2018).
As a precursor of creatine, GAA has been widely used to promote growth performance and muscle development, increase energy reserve and improve meat quality of broilers (Córdova-Noboa et al., 2018; DeGroot et al., 2019). The European Union authorized GAA as a nutritional additive for improving the performance at dosage of 600 to 1,200 mg/kg in chicken diets. Many previous studies have found that addition of 600 mg/kg GAA to broiler diet can strength the reserve of breast muscle energy substances by increasing the concentrations of creatine and PCr as well as the ATP/AMP ratio (Michiels et al., 2012; Zhang et al., 2019; Zarghi et al., 2020), increase breast meat yield and reduce the severity of wooden breast myopathy (Córdova-Noboa et al., 2018). However, exogenous GAA requires more methyl supply to synthesize creatine, which may limit the methyl required for other transmethylation reactions (Ibrahim et al., 2019). Creatine nitrate (CrN) is a novel form of creatine, which has greater solubility and muscle retention (Galvan et al., 2016). Moreover, compared with CMH and GAA, CrN has a lower cost. We, thus, hypothesized that dietary CrN has good potential as a substitute for GAA in poultry industry. To date, although the safety and efficacy of CrN to human has been verified (Joy et al., 2014; Dalton et al., 2017), little is known about of the effects of CrN on meat quality and muscle energy metabolism of broilers. Therefore, this study aims to evaluate the possibility effects of CrN as a substitute for GAA by investigating muscle energy status, glycolysis metabolism, and meat quality of broiler chickens fed diets supplementation with graded levels of CrN.
MATERIALS AND METHODS
Birds, Diets, and Experimental Design
All experimental procedures obtained ethical approval by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of 280 Arbor Acres male broiler chickens (28-day-old) with similar body weight (mean ± SD, 1,440.88 ± 4.40 g) were randomly allocated to one of 5 dietary treatments, with 6 replicate cages and 8 birds per cage (120 cm × 80 cm × 45 cm). These 5 dietary treatments included: the basal diet (control group), and the basal diet supplemented with 600 mg/kg GAA (identified as GAA600), 300, 600, or 900 mg/kg of CrN (identified as CrN300, CrN600, or CrN900, respectively). The experiment lasted 14 d from 28 to 42 d. Both GAA and CrN (purity ≥99%) were purchased from Tianjin Tiancheng Pharmaceutical Co., Ltd. (Tianjin, China). All birds were raised in three-tier wired cages and allowed access to feed and water in a temperature-controlled room maintained at 22°C during the trial period from 28 to 42 d. The basal diet formulation and nutritional levels are presented in Table 1. The diets were fed in pellet form. All birds were weighed at 28 and 42 d of age to calculate the average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR; feed: gain, g:g).
Table 1.
Basal diet formulation and nutritional values.
| Grower stage (22–42 d) | |
|---|---|
| Ingredient (%) | |
| Corn | 59.37 |
| Soybean meal | 31.90 |
| Soybean oil | 5.00 |
| Limestone | 1.23 |
| Dicalcium phosphate | 1.50 |
| L-Lysine⋅HCl | 0.11 |
| DL-Methionine | 0.27 |
| Salt | 0.30 |
| Vitamin premix 1 | 0.03 |
| Mineral premix 2 | 0.20 |
| 70% Choline chloride | 0.09 |
| Nutrient level (calculated, %) | |
| Metabolisable energy (MJ/kg) | 12.97 |
| Crude protein | 19.00 |
| Calcium | 0.90 |
| Total phosphorus | 0.56 |
| Available phosphorus | 0.35 |
| Lysine | 1.00 |
| Methionine | 0.46 |
| Methionine + cystine | 0.80 |
Vitamin premix provided per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 11 mg; menadione, 1.3 mg; thiamine, 2.21 mg; riboflavin, 7.8 mg; nicotinamide, 40 mg; calcium pantothenate, 16.5 mg; pyidoxine⋅HCl, 4 mg; biotin, 0.04 mg; folic acid, 1.2 mg; vitamin B12, 15 μg.
Mineral premix provided per kilogram of diet: iron, 80 mg; copper, 8 mg; manganese, 110 mg; zinc, 65 mg; iodine, 1.1 mg; selenium, 0.3 mg.
Slaughter and Sample Collection
On 42 d of age, 2 birds close to the average BW per replicate (cage) were selected. After electrically stunning in a salt-water bath (1% NaCl, wt/vol) with a constant voltage 50 V at 400 Hz for 5 s each bird, the chickens were then immediately slaughtered via exsanguination. Carcass weight was measured after defeathering. The head, neck, and feet were removed, and then the carcasses were eviscerated and weighted to determine the percentage of eviscerated yield. Dressing percentage was calculated by dividing the carcass weight by live body weight (BW). Abdominal fat (leaf fat surrounding the cloaca and abdominal fat surrounding the gizzard) and the breast and thigh muscle were removed and weighed to calculate their relative yield to eviscerated weight. Within 15 min, 2.0 g of liver and 3.0 g of muscle samples from the right Pectoralis major (PM) were put into RNase-free tables, snap-frozen in liquid nitrogen and stored at –80°C for further analysis. The entire left PM muscles were collected and stored at 4°C for meat quality measurements and texture profile analyses.
Meat Quality Measurements
The pH values of breast muscle at 45 min and 24 h postmortem were performed using a FiveGo pH Meter F2 (Mettler-Toledo AG, Analytical., Shanghai, China). The pH probe was inserted at an angle of 45° into the muscle directly and took the average value of 3 measurements as the final result (Zhang et al., 2014). At 24 h postmortem, the color parameters, including L* (lightness), a* (redness), and b* (yellowness) values, were measured by using a CR410 chroma meter (Konica Minolta Sensing Inc., Osaka, Japan). Each sample was measured 3 times at different positions, and the average value is taken as the final result. The drip loss, cooking loss, and shear force value of PM muscle at 24 h postmortem were determined as described previously (Zhang et al., 2014).
Texture Profile Analyses
The textural characteristics, including hardness (N), cohesiveness, springiness (mm), gumminess (N), and chewiness (mJ), were assessed by using a texture profile analyzer (TPA) (TMS-Pro, FTC, Sterling, VA) following the method according to Gurikar et al. (2014). Each cooked sample was cooled to room temperature and then cut into a cylinder with a diameter of 2 cm and a height of 1 cm by using a special sampler, which was placed on the sample determination platform of texture analyzer. The test settings of the texture analyzer were as follows: probe model P/50, pretest speed 2.0 mm/s, test speed 1.0 mm/s, post-test speed 1.0 mm/s, compression ratio 40%, time between two presses 5 s and load type auto −5 g.
Muscle Lactic Acid, Glycogen, and Glycolytic Potential Determination
The glycogen concentration in frozen muscle sample was determined as previously described (Zhang et al., 2014). The concentration of muscle lactic acid was measured by using a commercial diagnoses kit (Nanjing Jiancheng Biochemical Institute, Nanjing, China). Glycolytic potential (GP) was calculated according to the following formula: GP = 2 × [glycogen] + [lactic acid], and the result was expressed as μmol/g of lactic acid equivalent in wet muscle (Monin and Sellier, 1985).
Activity Analysis of Muscle Glycolytic Key Enzymes
Frozen muscle sample of exactly 0.50 g was homogenized in centrifuge tube containing 4.5 mL ice-cold physiological saline (0.75%) solution and centrifuged at 3,500 × g for 10 min at 4°C. The enzymes activities of hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), and lactate dehydrogenase (LDH) in the supernatant were measured spectrophotometrically with commercial diagnose kits (Nanjing Jiancheng Biochemical Institute).
Muscle Creatine, Phosphocreatine, and Adenosine Phosphate Determination
The concentrations of muscle creatine, PCr, ATP, ADP, and AMP were determined by HPLC method as previous described (Zhang et al., 2017). The frozen muscle samples were extracted by 5% perchloric acid for creatine and PCr extraction, and 7% perchloric acid for ATP, ADP, and AMP extraction. The supernatant used for creatine and PCr analysis was adjusted to a pH of 7.0 with 0.8 M K2CO3, and the supernatant used for adenosine phosphate analysis was adjusted to a pH of 6.5 with 1.03 M KOH. The final supernatant was filtered with a 0.45 μm membrane, and then 10 μL (for the determination of ATP, ADP, and AMP) or 20 μL (for the determination of creatine and PCr) of the relative sample solution was separated on an UltiMate 3000 HPLC system (Thermo Fisher Scientific, San Jose, CA) equipped with a Waters SunFire C18 column (250 mm × 4.6 mm id, 5 μm) at 25°C for creatine and PCr determination, and at 30°C for ATP, ADP and AMP determination. The mobile phase was a mixture of methyl cyanides and 29.4 mM KH2PO4 buffer (volume ratio = 2:98) for creatine and PCr determination and was a mixture of methanol and phosphate buffer (volume ratio = 13.5:86.5) for ATP, ADP and AMP determination. The flow rate was 1.0 mL/min. The standard samples of creatine-disodium salt, PCr-disodium salt, 5’-ATP-disodium salt, 5’-ADP sodium salt, and 5’-AMP sodium salt (Sigma-Aldrich, St. Louis, MO) were used to establish standard curves.
Total RNA Extraction and Real-Time Quantitative PCR Analysis
Total RNA from the frozen muscle sample was extracted using the RNAiso Plus reagent (Takara Biotechnology Co. Ltd., Dalian, China). Then, the concentration, quantity and quality of total RNA were tested with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE). Total RNA with the OD260/OD280 ratio at 1.8 to 2.0 was used for subsequent PCR reactions. The purified total RNA was reverse transcribed into cDNA with the primescript RT Master Mix kit (Takara Biotechnology Co. Ltd.), and the synthesized cDNA products were stored at −20°C until use. Real-time quantitative PCR (RT-qPCR) analyses were performed in the CFX ConnectTM Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) using SYBR Premix Ex Taq kits (Takara Biotechnology Co. Ltd.). The specific primer sequences used in the present study are listed in Table 2. The mRNA expression level of housekeeping gene (β-actin) was used to normalize the cycle threshold (Ct) values. The expression level of target genes relative to β-actin were calculated using the 2−ΔΔCt method and expressed as the relative fold change to the control group (Livak and Schmittgen, 2001).
Table 2.
Primer specific sequences used for RT-qPCR analysis.
| Genes | Primer sequence (5′-3′) | Amplicon size (bp) | Genbank identification |
|---|---|---|---|
| GAMT | F: CGTGAAGGGCAAATACAGCG | 146 | XM_040692773.1 |
| R: GGAAGGAGTAGTAGCGGCAC | |||
| CreaT | F: TGAACTACAAACCGCTGACG | 120 | JN628439.2 |
| R: GCTCGTAGATAACGGTGCAG | |||
| LKB1 | F: TGAGAGGGATGCTTGAATACGA | 138 | NM_001045833.1 |
| R: ACTTGTCCTTTGTTTCTGGGC | |||
| MO25α | F: CGTGTTTAAGGTGTTTGTAGCC | 245 | XM_015277058.3 |
| R: AGCAACTGCTGAATTTGGGT | |||
| STRADα | F: TAAACCCGAACGGATTAGGCG | 182 | NM_001305191.1 |
| R: TGCTGTCTGGGAGGAAGTTG | |||
| AMPKα1 | F: ATCTGTCTCGCCCTCATCCT | 125 | NM_001039603.1 |
| R: CCACTTCGCTCTTCTTACACCTT | |||
| AMPKα2 | F: GGGACCTGAAACCAGAGAACG | 215 | NM_001039605.1 |
| R: ACAGAGGAGGGCATAGAGGATG | |||
| β-actin | F: ATCCGGACCCTCCATTGTC | 120 | NM_205518.1 |
| R: AGCCATGCCAATCTCGTCTT |
Abbreviations: AMPKα1, adenosine 5′-monophosphate-activated protein kinase α1; AMPKα2, adenosine 5′-monophosphate-activated protein kinase α2; CreaT, creatine transporter; GAMT, S-adenosyl-L-methionine: guanidinoacetate N-methyltransferase; LKB1, liver kinase B1; MO25α, mouse protein 25α; STRADα, STE20-related adaptor α.
Statistical Analysis
Data were analyzed with the use of SPSS statistical software (Version 20.0 for windows, SPSS Inc., Chicago, IL) for one-way analysis of variance (ANOVA). The observed indexes were analyzed by the mean of 2 chickens per cage as a replicate except ADFI, ADG, and FCR were analyzed by the cage as a replicate (n = 6). Besides, orthogonal contrasts were used to examine the linear and quadratic effects in response to increasing the dietary supplementation of CrN among the control, CrN300, CrN600, and CrN900 groups. The results are presented as mean values and the standard error of the mean (SEM). A probability level of P < 0.05 was considered to indicate statistical significance.
RESULTS
Growth Performance
As exhibited in Table 3, no significant treatment differences were observed in ADFI, ADG, and FCR of birds during the trial period from 28 to 42 d (P > 0.05).
Table 3.
Effects of dietary graded creatine nitrate (CrN) supplementation on growth performance of broilers from 28 to 42 d of age.
| Items | Treatments1 |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GAA600 | CrN300 | CrN600 | CrN900 | ANOVA | Linear2 | Quadratic2 | ||
| Initial BW at 28 d (g/bird) | 1,438.00 | 1,442.50 | 1,440.00 | 1,441.50 | 1,441.88 | 0.80 | 0.422 | - | - |
| BW at 42 d (g/bird) | 2,798.50 | 2,826.57 | 2,839.50 | 2,841.86 | 2,856.04 | 15.60 | 0.840 | 0.300 | 0.719 |
| ADFI (g/ day⋅bird) | 169.24 | 174.58 | 175.82 | 176.62 | 175.41 | 1.76 | 0.722 | 0.311 | 0.359 |
| ADG (g/ day⋅bird) | 97.18 | 98.86 | 99.97 | 100.03 | 101.01 | 1.12 | 0.869 | 0.339 | 0.737 |
| FCR (feed:gain, g:g) | 1.74 | 1.77 | 1.76 | 1.77 | 1.74 | 0.01 | 0.649 | 0.925 | 0.222 |
The data are represented as the mean value and pooled SEM (n = 6).
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio.
Control, basal diet; GAA600, basal diet supplemented with 600 mg/kg guanidinoacetic acid (GAA); CrN300, CrN600, and CrN900, basal diets supplemented with 300, 600, and 900 mg/kg CrN, respectively.
Orthogonal polynomials were used to estimate the linear and quadratic effects of dietary CrN supplementation among the control, CrN300, CrN600, and CrN900 groups.
Carcass Traits, Meat Quality, and Textural Characteristics
In the present study, although dietary supplementation with 600 mg/kg of GAA, and 300, 600, or 900 mg/kg of CrN from 28 to 42 d had no significant effect on carcass traits of birds slaughtered at 42 d of market age (P > 0.05; Table 4). The thigh muscle yield was increased linearly in response to the increase in dietary CrN level (P < 0.05).
Table 4.
Effects of dietary graded creatine nitrate (CrN) supplementation on carcass traits of broilers.
| Items | Treatments1 |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GAA600 | CrN300 | CrN600 | CrN900 | ANOVA | Linear2 | Quadratic2 | ||
| Dressing percentage (%) | 95.19 | 94.65 | 95.52 | 95.05 | 94.98 | 0.11 | 0.183 | 0.196 | 0.288 |
| Eviscerated yield (%) | 78.56 | 78.29 | 79.81 | 79.91 | 79.77 | 0.24 | 0.082 | 0.054 | 0.108 |
| Breast muscle yield (%) | 25.32 | 25.65 | 25.37 | 26.09 | 26.15 | 0.27 | 0.801 | 0.101 | 0.977 |
| Thigh muscle yield (%) | 17.93 | 19.10 | 18.29 | 18.83 | 18.90 | 0.20 | 0.346 | 0.037 | 0.691 |
| Abdominal fat percentage %) | 1.71 | 1.53 | 1.69 | 1.59 | 1.52 | 0.05 | 0.619 | 0.128 | 0.807 |
The data are represented as the mean value and pooled SEM (n = 6).
Control, basal diet; GAA600, basal diet supplemented with 600 mg/kg guanidinoacetic acid (GAA); CrN300, CrN600, and CrN900, basal diets supplemented with 300, 600, and 900 mg/kg CrN, respectively.
Orthogonal polynomials were used to estimate the linear and quadratic effects of dietary CrN supplementation among the control, CrN300, CrN600, and CrN900 groups.
As shown in Table 5, the breast muscle of GAA600, CrN600, and CrN900 groups showed higher pH24h than were seen in the control group (P < 0.05). The pH24h was increased linearly in response to the increasing CrN supplementation level (P < 0.01). Moreover, compared with the control group, the drip loss of breast muscle in GAA600 and all CrN groups showed a significant reduction (P < 0.05). As the dose of CrN increased, the drip loss of breast muscle was decreased in linear and quadratic manners (P < 0.05). Meanwhile, chewiness and gumminess of the PM muscle were decreased linearly in response to the increase in dietary CrN level (P < 0.05).
Table 5.
Effects of dietary graded creatine nitrate (CrN) supplementation on meat quality and textural characteristics of the Pectoralis major muscle of broilers.
| Items | Treatments1 |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GAA600 | CrN300 | CrN600 | CrN900 | ANOVA | Linear2 | Quadratic2 | ||
| Meat quality | |||||||||
| pH45min | 6.17 | 6.20 | 6.23 | 6.19 | 6.22 | 0.01 | 0.630 | 0.427 | 0.561 |
| pH24h | 5.86b | 5.92a | 5.90ab | 5.93a | 5.95a | 0.01 | 0.015 | <0.001 | 0.556 |
| L* (lightness) | 42.54 | 42.58 | 42.78 | 42.72 | 44.37 | 0.28 | 0.217 | 0.086 | 0.252 |
| a* (redness) | 1.41 | 1.37 | 1.29 | 1.30 | 1.33 | 0.07 | 0.976 | 0.738 | 0.629 |
| b* (yellowness) | 3.13 | 3.27 | 3.72 | 3.77 | 3.67 | 0.12 | 0.291 | 0.118 | 0.147 |
| Drip loss (%) | 2.69a | 2.25b | 2.26b | 2.23b | 2.36b | 0.05 | 0.006 | 0.018 | 0.004 |
| Cooking loss (%) | 13.07 | 12.43 | 12.05 | 11.31 | 11.37 | 0.33 | 0.426 | 0.086 | 0.469 |
| Shear force (N) | 20.15 | 20.14 | 21.32 | 21.71 | 22.33 | 0.52 | 0.630 | 0.193 | 0.819 |
| Textural characteristics | |||||||||
| Hardness (N) | 22.26 | 21.44 | 21.28 | 20.40 | 20.84 | 0.62 | 0.923 | 0.357 | 0.571 |
| Cohesiveness (-) | 0.46 | 0.43 | 0.45 | 0.43 | 0.43 | 0.01 | 0.676 | 0.174 | 0.751 |
| Springiness (mm) | 2.85 | 2.88 | 2.74 | 2.90 | 2.65 | 0.05 | 0.488 | 0.301 | 0.676 |
| Gumminess (N) | 11.08 | 10.18 | 10.63 | 9.11 | 9.61 | 0.33 | 0.388 | 0.047 | 0.468 |
| Chewiness (mJ) | 31.47 | 28.06 | 29.94 | 24.68 | 24.87 | 0.90 | 0.057 | 0.001 | 0.594 |
Abbreviations: pH45 min, pH at 45 min postmortem; pH24h, pH at 24 h postmortem.
Different letters in the mean value of the same row indicate a significant difference (P < 0.05). The data are represented as the mean value and pooled SEM (n = 6).
Control, basal diet; GAA600, basal diet supplemented with 600 mg/kg guanidinoacetic acid (GAA); CrN300, CrN600, and CrN900, basal diets supplemented with 300, 600, and 900 mg/kg creatine nitrate (CrN), respectively.
Orthogonal polynomials were used to estimate the linear and quadratic effects of dietary CrN supplementation among the control, CrN300, CrN600, and CrN900 groups.
Concentrations of Muscle Glycogen, Lactic Acid, GP, and Activities of Muscle Glycolytic Enzymes
Both CrN600 and CrN900 treatments increased the concentrations of muscle glycogen and reduced the concentrations of lactic acid as compared with the control and CrN300 groups (P < 0.05; Table 6). The enzyme activities of PFK were lower in muscles of CrN600 and CrN900 treatments than were seen in the control, GAA600 and CrN300 treatments (P < 0.05). Moreover, the activities of muscle PK were lower in GAA600 and all CrN treatments, and the activities of muscle LDH were lower in all CrN supplementation groups than those were seen in the control group (P < 0.05). The muscle glycogen concentration was increased linearly (P < 0.01), and the concentration of lactic acid and activity of PFK were decreased linearly (P < 0.01) in response to the increasing CrN supplementation level. Meanwhile, the activities of PK and LDH were decreased in linear (P < 0.01) and quadratic (P < 0.05) manners as the dose of CrN increased.
Table 6.
Effects of dietary graded creatine nitrate (CrN) supplementation on glycolytic parameters and activities of glycolytic enzymes in Pectoralis major muscle of broilers.
| Items | Treatments1 |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GAA600 | CrN300 | CrN600 | CrN900 | ANOVA | Linear2 | Quadratic2 | ||
| Glycolytic parameters | |||||||||
| Glycogen (μmol/g) | 5.26b | 5.54b | 5.15b | 6.34a | 6.53a | 0.14 | 0.001 | <0.001 | 0.513 |
| Lactic acid (μmol/g) | 131.42a | 126.34ab | 130.79a | 125.43b | 125.56b | 0.85 | 0.042 | 0.010 | 0.844 |
| GP (μmol/g) | 141.94 | 137.42 | 141.08 | 138.11 | 138.62 | 0.81 | 0.321 | 0.132 | 0.717 |
| Activities of glycolytic enzymes | |||||||||
| HK (U/mg of protein) | 14.44 | 12.34 | 13.88 | 12.51 | 11.67 | 0.69 | 0.722 | 0.208 | 0.936 |
| PFK (U/mg of protein) | 56.93a | 53.59a | 52.80a | 47.61b | 45.66b | 0.92 | <0.001 | <0.001 | 0.480 |
| PK (U/g of protein) | 51.76a | 38.92b | 36.18b | 34.99b | 38.93b | 1.48 | 0.001 | 0.002 | 0.001 |
| LDH (U/mg of protein) | 4.30a | 3.61ab | 3.08b | 3.15b | 2.97b | 0.13 | 0.003 | 0.001 | 0.044 |
Abbreviations: GP, glycolytic potential (GP = 2 × [glycogen] + [lactic acid]); HK, hexokinase; LDH, lactate dehydrogenase; PFK, phosphofructokinase; PK, pyruvate kinase.
Different letters in the mean value of the same row indicate a significant difference (P < 0.05). The data are represented as the mean value and pooled SEM (n = 6).
Control, basal diet; GAA600, basal diet supplemented with 600 mg/kg guanidinoacetic acid (GAA); CrN300, CrN600, and CrN900, basal diets supplemented with 300, 600, and 900 mg/kg CrN, respectively.
Orthogonal polynomials were used to estimate the linear and quadratic effects of dietary CrN supplementation among the control, CrN300, CrN600, and CrN900 groups.
Concentrations of Muscle Creatine, PCr, ATP, ADP, and AMP
As shown in Table 7, GAA600 and all CrN treatments increased the concentrations of muscle creatine and ATP (P < 0.05), and decreased the concentration of muscle AMP and AMP/ATP ratio (P < 0.05). Moreover, the concentrations of PCr were higher in muscles of GAA600, CrN600, and CrN900 groups than were seen in the control group (P < 0.05). What's more, the muscle creatine concentration in CrN900 group was higher than that in the GAA600 group (P < 0.05). The concentrations of muscle creatine, PCr and ATP were increased linearly in response to the increasing CrN supplementation level (P < 0.01). Meanwhile, the muscle AMP concentration and AMP/ATP ratio were decreased linearly (P < 0.05) and quadratic (P < 0.05) in response to the increasing CrN supplementation level. There was no significant difference in concentrations of muscle PCr, ATP, AMP, and AMP/ATP ratio among GAA600 and all CrN treatments (P > 0.05).
Table 7.
Effects of dietary graded creatine nitrate (CrN) supplementation on energy status in Pectoralis major muscle of broilers.
| Items | Treatments1 |
SEM |
P value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GAA600 | CrN300 | CrN600 | CrN900 | ANOVA | Linear2 | Quadratic2 | ||
| Creatine (μmol/g) | 11.99c | 12.98b | 12.67b | 12.61b | 13.77a | 0.12 | <0.001 | <0.001 | 0.232 |
| PCr (μmol/g) | 1.50b | 2.01a | 1.72ab | 1.87a | 1.99a | 0.06 | 0.018 | 0.001 | 0.599 |
| PCr/Creatine ratio | 0.13 | 0.15 | 0.14 | 0.15 | 0.14 | 0.01 | 0.531 | 0.102 | 0.416 |
| ATP (μmol/g) | 1.20b | 1.93a | 1.99a | 1.96a | 1.98a | 0.09 | 0.022 | 0.009 | 0.046 |
| ADP (μmol/g) | 0.74 | 0.76 | 0.87 | 0.79 | 0.79 | 0.02 | 0.361 | 0.616 | 0.084 |
| AMP (μmol/g) | 0.35a | 0.23b | 0.24b | 0.25b | 0.26b | 0.01 | 0.021 | 0.036 | 0.041 |
| AMP/ATP ratio | 0.29a | 0.12b | 0.12b | 0.13b | 0.13b | 0.01 | <0.001 | <0.001 | <0.001 |
Abbreviations: ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; PCr, phosphocreatine.
Different letters in the mean value of the same row indicate a significant difference (P < 0.05). The data are represented as the mean value and pooled SEM (n = 6).
Control, basal diet; GAA600, basal diet supplemented with 600 mg/kg guanidinoacetic acid (GAA); CrN300, CrN600, and CrN900, basal diets supplemented with 300, 600, and 900 mg/kg CrN, respectively.
Orthogonal polynomials were used to estimate the linear and quadratic effects of dietary CrN supplementation among the control, CrN300, CrN600, and CrN900 groups.
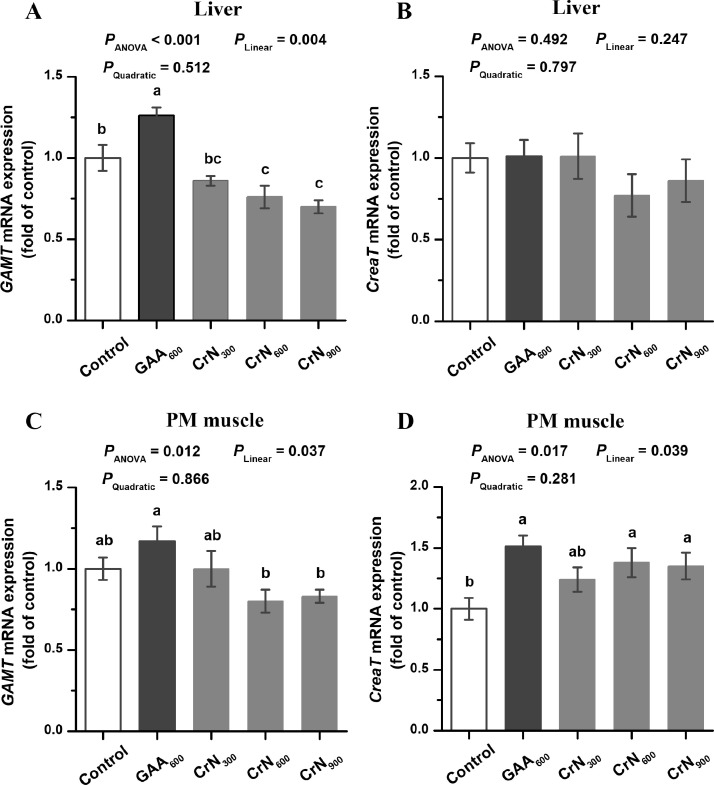
Relative Gene mRNA Expression of GAMT and CreaT in Liver and PM Muscle
As exhibited in Figure 1, CrN600 and CrN900 treatments downregulated the liver GAMT mRNA expression, and upregulated the muscle CreaT mRNA expression compared with the control group (P < 0.05). Birds in the GAA600 group showed higher the mRNA expression of liver GAMT and muscle CreaT than those in the control group (P < 0.05). Moreover, CrN600 and CrN900 treatments downregulated the relative mRNA expression of GAMT both in liver and PM muscle compared with the GAA600 treatment (P < 0.05). The mRNA expression levels of GAMT both in liver and PM muscle were decreased linearly in response to the increasing CrN supplementation level (P < 0.05). Meanwhile, the mRNA expression of muscle CreaT was up-regulated linearly with increasing dietary CrN level (P < 0.05).
Figure 1.
Effects of dietary graded creatine nitrate (CrN) supplementation on the relative mRNA expression for GAMT and CreaT in the liver and Pectoralis major (PM) muscle of broilers. The data are represented as the mean value ± SEM (n = 6). Means without a common letter (a, b or c) significantly differ (P < 0.05). Orthogonal polynomials were used to estimate the linear and quadratic effects of dietary CrN supplementation among the control, CrN300, CrN600, and CrN900 groups. Abbreviations: CreaT, creatine transporter; Control, basal diet; CrN300, CrN600, and CrN900, basal diets supplemented with 300, 600, and 900 mg/kg CrN, respectively; GAA600, basal diet supplemented with 600 mg/kg guanidinoacetic acid (GAA); GAMT, S-adenosyl-L-methionine: guanidinoacetate N-methyltransferase.
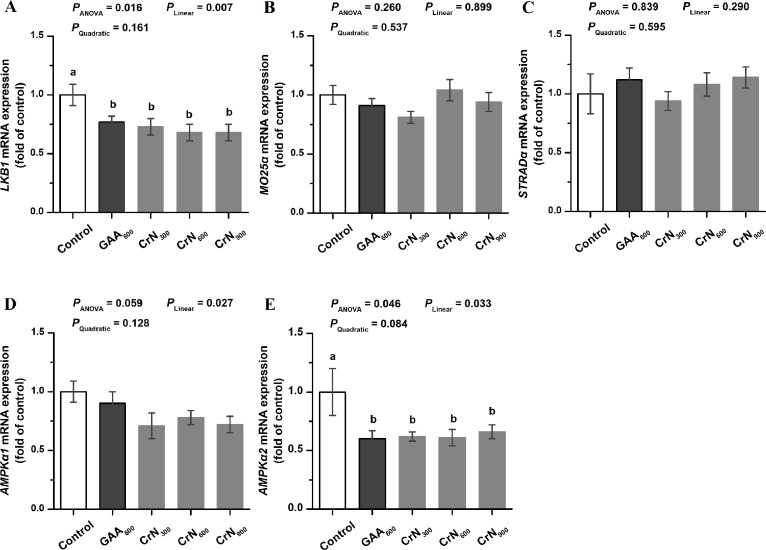
Relative Gene mRNA Expression of LKB1/AMPK Pathway in PM Muscle
According to the Figure 2, GAA600 and all CrN groups showed lower mRNA expression of LKB1 and AMPKα2 in PM muscle than those from the control group (P < 0.05). The mRNA expression levels of LKB1, AMPKα1, and AMPKα2 in PM muscle were downregulated linearly in response to the increasing CrN supplementation level (P < 0.05). There was no significant difference in the mRNA expression of MO25α and STRADα in muscle among all experimental treatments (P > 0.05).
Figure 2.
Effects of dietary graded creatine nitrate (CrN) supplementation on the relative mRNA expression of LKB1, MO25α, STRADα, AMPKα1 and AMPKα2 in Pectoralis major muscle of broilers. The data are represented as the mean value ± SEM (n = 6). Means without a common letter (a or b) significantly differ (P < 0.05). Orthogonal polynomials were used to estimate the linear and quadratic effects of dietary CrN supplementation among the control, CrN300, CrN600, and CrN900 groups. Abbreviations: AMPKα1, adenosine 5′-monophosphate-activated protein kinase α1; AMPKα2, adenosine 5′-monophosphate-activated protein kinase α2; Control, basal diet; CrN300, CrN600, and CrN900, basal diets supplemented with 300, 600, and 900 mg/kg CrN, respectively; GAA600, basal diet supplemented with 600 mg/kg guanidinoacetic acid (GAA); LKB1, liver kinase B1; MO25α, mouse protein 25α; STRADα, STE20-related adaptor α.
DISCUSSION
Some previous studies showed that dietary long-term (>35 d) supplementation with GAA at dose rate of 600 to 1,200 mg/kg can improve the feed conversion efficiency and increase body weight gain of broilers (Michiels et al., 2012; Córdova-Noboa et al., 2018; He et al., 2019), and even under stress condition (Majdeddin et al., 2020). Faraj et al. (2014) reported that dietary addition of 4 to 12 g/kg CMH for a trial period of 42 d can improve birds’ growth performance. In this study, dietary supplementation with 600 mg/kg of GAA, and 300 to 900 mg/kg of CrN for 14 d prior to slaughter had no significant effect on ADFI, ADG, FCR, and carcass traits of broilers. Similarly, earlier studies have shown that addition 600 and 1,200 mg/kg CMH or GAA to diets for 2 wk prior to slaughter had no significant effect on the growth performance and carcass traits of broilers (Zhang et al., 2014, 2019). These inconsistent results may be due to the differences in the types of creatine supplements, the level of dosage, bioavailability as well as the duration of trials.
Recently, consumers have become more critical toward meat quality when purchasing meat products. Generally, meat products with high quality traits are easy to be accepted by consumers. The pH is an important index to reflect meat quality. With the development of postmortem glycolysis, the contents of ATP and glycogen decreased continuously, and lactic acid accumulated continuously, resulting in the decrease of muscle pH, which finally leads to the degeneration of muscle protein (Ryu and Kim, 2005). In the present study, the muscle pH24h of the CrN600 and CrN900 groups was higher than those of the control and GAA groups, indicating that dietary addition of CrN could attenuate the rapid decline of muscle pH. Water-holding capacity (WHC) is also one important attribute reflecting meat quality as it can be directly used to evaluate the ability of fresh muscle maintains its own moisture (Pearce et al., 2011). Generally, inferior WHC can negatively affect the meat color, tenderness, flavor, and nutrients, and thus has important economic significance (Hughes et al., 2014). The results of this study showed that dietary supplementation with 600 mg/kg GAA, or 300, 600, and 900 mg CrN for 2 wk before slaughter significantly enhanced the WHC of breast muscle by reducing drip loss, which is consistent with previous report that addition of GAA or CMH to broiler diets at dose rate of 600 or 1,200 mg/kg enhanced the WHC of breast muscle (Zhang et al., 2017, 2019). During the transformation of muscle to meat, the postmortem glycolysis rate of muscle is closely related to the accumulation of lactate and H+, which subsequently affects multiple meat quality traits, such as pH, WHC, color, and tenderness (Bee et al., 2007). We thus further analyzed the muscle glycolytic parameters and activities of glycolytic enzymes in the PM muscle of broilers.
After the livestock and poultry are slaughtered, the blood flow stops, and the supply of oxygen and nutrients is cut off. In order to maintain short-term metabolic activities, muscle cells transform from aerobic respiration to anaerobic glycolysis to provide ATP. Glycogen is the main energy reserve of skeletal muscle. GP is an index to measure the content of carbohydrate compounds, including glycogen, glucose, and glucose-6-phosphate that can be converted into lactic acid in muscle (Monin and Sellier, 1985). In this study, higher glycogen concentration and lower lactic acid concentration in breast muscles was accompanied with higher pH24h and lower drip loss in birds of the CrN600 and CrN900 groups, suggesting that CrN could increase muscle pH and WHC by delaying postmortem glycolysis and reducing the production of lactic acid. More importantly, CrN showed better efficacy than GAA at the some dose. Some key enzymes of anaerobic metabolism mediate the glycolysis metabolic pathway, including HK, PFK, PK, and LDH. These enzymes catalyze the conversion of glucose to glucose-6-phosphate, glucose-6-phosphate to glucose-1,6-phosphate, phosphoenol pyruvate to pyruvate, and pyruvate to lactic acid under anaerobic condition, respectively (Scheffler and Gerrard, 2007; Nelson and Cox, 2008). High phosphorylation level of PK is conducive to rapid muscle glycolysis and rapid decline of pH, thus affecting meat quality (Huang et al., 2011). In the current study, muscle from CrN600 and CrN900 groups showed lower activities of PFK than that in the control and GAA600, and CrN300 groups. Meanwhile, the activities of muscle PK were lower in GAA600 and all CrN groups than those in the control group, and the activities of LDH decreased linearly in response to the increasing CrN supplementation level. These results indicated that dietary addition of CrN reduced the rate of glycolysis reaction and weakened lactic acid accumulation by inhibiting the activities of PFK, PK, and LDH in muscles.
Muscle creatine/PCr system is important for storing and transmitting phosphate-bound energy. When ATP level in muscle is lower than the threshold, PCr transfers its high-energy phosphorylation group to ADP to synthesize ATP again under the action of creatine kinase (Wallimann et al., 2011). ATP is a kind of high-energy phosphate compound, which can be transformed with ADP to store and release energy, thus ensuring the energy supply of various life activities of muscle cells (Cain et al., 1962). In addition, anaerobic glycolysis is also an important bioenergy system involved in the resynthesis of ATP, which provides energy for muscle activity (Wells et al., 2009). Previous studies have demonstrated that addition of 1,200 mg/kg CMH or GAA to the diet increased the concentrations of muscle creatine or PCr of broilers experienced 3-h pre-slaughter transport (Zhang et al., 2017, 2019; Zhang et al., 2021). Herein, we observed that GAA600, and all CrN groups increased the concentrations of muscle creatine and ATP, and reduced muscle AMP content and AMP/ATP ratio compared with control group. In addition, the concentration of muscle PCr in GAA600, CrN600, and CrN900 groups were higher than that in the control group. These findings suggest that GAA and CrN supplementation promoted muscle creatine and PCr loading, strengthened the buffer capacity of creatine/PCr pool in muscle, which could quickly afford adequate ATP for skeletal muscle cells to further attenuate glycolytic metabolism. These metabolic changes eventually delayed the muscle glycolysis, reduced the accumulation of lactic acid and the fast reduction of pH, and helped to improve meat quality. Under normal physiological conditions, GAA can be endogenously synthesized from arginine and glycine in kidney and pancreas under the catalysis of AGAT; after GAA transport to the liver, GAMT catalyzes the endogenous synthesis of creatine from S-adenosylmethionine and GAA (Wyss and Kaddurah-Daouk, 2000; Longo et al., 2011). CreaT is responsible for transport of endogenously synthesized and ingested creatine to muscle cells, which is the main way for tissues and cells to take up creatine (Wyss and Kaddurah-Daouk, 2000; Brault et al., 2003). In this study, GAA600, CrN600, and CrN900 group upregulated the gene expression of CreaT in PM muscle in comparison with the control group, suggesting that GAA and CrN supplementation improved the absorption of creatine by skeletal muscle cells via directly activate CreaT on the cell membrane. Zhang et al. (2019) reported that dietary supplementation with 600 and 1,200 mg/kg GAA upregulated the liver GAMT mRNA expression, and both the liver and muscle CreaT mRNA expression of birds. In this study, dietary GAA upregulated the mRNA expression of GAMT both in liver and muscle, but CrN supplementation linearly downregulated mRNA expression of GAMT both in liver and PM muscle. These results suggested that exogenous addition of GAA could promote the synthesis of creatine in the liver, but exogenous addition of CrN may inhibit self-synthesis of creatine via negative feedback on gene expression of GAMT. In spite of this, CrN supplementation linearly upregulated CreaT gene expression in PM muscle, promoted the absorption of creatine and the accumulation of PCr, which helps to improve cellular energy status of broiler muscle.
AMPK functions as a cell fuel gauge by sensing increased intracellular AMP/ATP ratio (Hardie, 2007). Therefore, AMPK is considered a central sensor of intracellular energy status to maintain cellular energy homeostasis. As a heterotrimeric protein, AMPK comprises of a catalytic α-subunit and 2 regulatory β- and γ- subunits (Hardie and Sakamoto, 2006). The α subunit has 2 isoforms, α1 and α2, which have different tissue expression. In mammals and chickens, AMPKα1 is ubiquitously expressed, whereas AMPKα2 is predominantly expressed in skeletal and cardiac muscle (Stapleton et al., 1996; Proszkowiec-Weglarz et al., 2006). AMPK is activated by upstream kinase LKB1, which phosphorylates AMPK at Thr172α Subunits (Woods et al., 2003). Ste20-related adaptor protein-α (STRAD-α) and mouse protein 25-α (MO25-α) are 2 accessory proteins of LKB1, MO25-α binds to the carboxyl terminal of STRAD-α, thereby stabilizing the association between STRAD and LKB1 (Boudeau et al., 2003). Previous reports pointed out that ATP content decreased and the AMP/ATP ratio increased in contracting muscle lead to activation of AMPK (Hardie and Carling, 1997). Some previous studies have shown that AMPK also plays a significant role in the regulation of postmortem muscle glycolysis (Du et al., 2005; Xing et al., 2016). In our current study, lower relative mRNA expression of LKB1 and AMPKα2 in PM muscle of GAA600 and CrN groups accompanied by lower AMP/ATP ratio and higher concentration of creatine, PCr, and ATP suggested dietary addition of exogenous GAA and CrN could promote muscle energy status and inhibit the activation of LKB1/AMPK pathway.
CONCLUSIONS
To our knowledge this study is the first to evaluate CrN effects in broilers. We found that graded supplementation with CrN at dose rate of 300 to 900 mg/kg to the broiler diets linearly promoted muscle energy status via strengthening the energy-buffering capacity of muscle creatine/PCr pool, linearly reduced muscle AMP/ATP ratio, and inhibited the activation of LKB1/AMPK pathway, which was conducive to improve meat quality by delaying postmortem glycolysis in muscle. Moreover, CrN showed better efficacy than GAA at the some dose. These results indicate that CrN may be a potential replacement for GAA as a new functional creatine supplement in poultry industry.
ACKNOWLEDGMENTS
This work was financed by the National Natural Science Foundation of China (32172753), the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2021]459), and Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents (2018).
DISCLOSURES
The authors declare that there are no conflicts of interest.
REFERENCES
- Alraddadi E.A., Lillico R., Vennerstrom J.L., Lakowski T.M., Miller D.W. Absolute oral bioavailability of creatine monohydrate in rats: debunking a myth. Pharmaceutics. 2018;10:31. doi: 10.3390/pharmaceutics10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee G., Anderson A.L., Lonergan S.M., Huff-Lonergan E. Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork. Meat Sci. 2007;76:359–365. doi: 10.1016/j.meatsci.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bogosavljević-Bošković S., Rakonjac S., Dosković V., Petrović M.D. Broiler rearing systems: a review of major fattening results and meat quality traits. Worlds Poult. Sci. J. 2012;68:217–228. [Google Scholar]
- Boudeau J., Baas A.F., Deak M., Morrice N.A., Kieloch A., Schutowski M., Prescott A.R., Clevers H.C., Alessi D.R. MO25α/β interact with STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault J.J., Abraham K.A., Terjung R.L. Muscle creatine uptake and creatine transporter expression in response to creatine supplementation and depletion. J. Appl. Physiol. 2003;94:2173–2180. doi: 10.1152/japplphysiol.01171.2002. [DOI] [PubMed] [Google Scholar]
- Brosnan J.T., Da Silva R.P., Brosnan M.E. The metabolic burden of creatine synthesis. Amino Acids. 2011;40:1325–1331. doi: 10.1007/s00726-011-0853-y. [DOI] [PubMed] [Google Scholar]
- Cain D.F., Infante A.A., Davies R.E. Chemistry of muscle contraction: adenosine triphosphate and phosphorylcreatine as energy supplies for single contractions of working muscle. Nature. 1962;196:214–217. doi: 10.1038/196214a0. [DOI] [PubMed] [Google Scholar]
- Córdova-Noboa H.A., Oviedo-Rondón E.O., Sarsour A.H., Barnes J., Ferzola P., Rademacher-Heilshorn M., Braun U. Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic acid. Poult. Sci. 2018;97:2479–2493. doi: 10.3382/ps/pey096. [DOI] [PubMed] [Google Scholar]
- Curt M.J.C., Voicu P.M., Fontaine M., Dessein A.F., Porchet N., Mention-Mulliez K., Dobbelaere D., Soto-Ares G., Cheillan D., Vamecq J. Creatine biosynthesis and transport in health and disease. Biochim. Open. 2015;119:146–165. doi: 10.1016/j.biochi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Dalton R.L., Sowinski R.J., Grubic T.J., Collins P.B., Coletta A.M., Reyes A.G., Sanchez B., Koozehchian M., Jung Y.P., Rasmussen C., Greenwood M., Murano P.S., Earnest C.P., Kreider R.B. Hematological and hemodynamic responses to acute and short-term creatine nitrate supplementation. Nutrients. 2017;9:1359. doi: 10.3390/nu9121359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot A.A., Braun U., Dilger R.N. Guanidinoacetic acid is efficacious in improving growth performance and muscle energy homeostasis in broiler chicks fed arginine-deficient or arginine-adequate diets. Poult. Sci. 2019;98:2896–2905. doi: 10.3382/ps/pez036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Shen Q.W., Zhu M.J. Role of beta-adrenoceptor signaling and AMP-activated protein kinase in glycolysis of postmortem skeletal muscle. J. Agric. Food Chem. 2005;53:3235–3239. doi: 10.1021/jf047913n. [DOI] [PubMed] [Google Scholar]
- Faraj H.A., Salih A.M., Hama K.O. The effect of different levels of creatine monohydrate on the performance and carcass characteristics of broiler chickens. Res. Opin. Anim. Vet. Sci. 2014;4:145–149. [Google Scholar]
- Galvan E., Walker D.K., Simbo S.Y., Dalton R., Levers K., Connor A.O, Goodenough C., Barringer N.D., Greenwood M., Rasmussen C., Smith S.B., Riechman S.E., Fluckey J.D., Murano P.S., Earnest C.P., Kreider R.B. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance. J. Int. Soc. Sports Nutr. 2016;13:12. doi: 10.1186/s12970-016-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurikar A.M., Lakshmanan V., Gadekar Y.P., Sharma B.D., Anjaneyulu A.S.R. Effect of meat chunk size, massaging time and cooking time on quality of restructured pork blocks. J. Food Sci. Technol. 2014;51:1363–1369. doi: 10.1007/s13197-012-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D.N., Miller K.D., Ellis M., McKeith F.K., Wilson E.R. Relationships between longissimus glycolytic potential and swine growth performance, carcass traits, and pork quality. J. Anim. Sci. 2003;81:2206–2212. doi: 10.2527/2003.8192206x. [DOI] [PubMed] [Google Scholar]
- Hardie D.G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie D.G., Carling D. The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur. J. Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hardie D.G., Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology. 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- He D., Yang L., Li J., Dong B., Lai W., Zhang L. Effects of guanidinoacetic acid on growth performance, creatine metabolism and plasma amino acid profile in broilers. J. Anim. Physiol. Anim. Nutr. 2019;103:766–773. doi: 10.1111/jpn.13081. [DOI] [PubMed] [Google Scholar]
- Huang H., Larsen M.R., Karlsson A.H., Pomponio L., Costa L.N., Lametsch R. Gel-based phosphoproteomics analysis of sarcoplasmic proteins in postmortem porcine muscle with pH decline rate and time differences. Proteomics. 2011;11:4063–4076. doi: 10.1002/pmic.201100173. [DOI] [PubMed] [Google Scholar]
- Hughes J.M., Oiseth S.K., Purslow P.P., Warner R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014;98:520–532. doi: 10.1016/j.meatsci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Ibrahim D., El Sayed R., Abdelfattah-Hassan A., Morshedy A.M. Creatine or guanidinoacetic acid? Which is more effective at enhancing growth, tissue creatine stores, quality of meat, and genes controlling growth/myogenesis in Mulard ducks. J. Appl. Anim. Res. 2019;47:159–166. [Google Scholar]
- Joy J.M., Lowery R.P., Falcone P.H., Mosman M., Vogel R.M., Carson L.R., Tai C., Choate D., Kimber D., Ormes J.A., Wilson J.M., Moon J.R. 28 days of creatine nitrate supplementation is apparently safe in healthy individuals. J. Int. Soc. Sports Nutr. 2014;11:60. doi: 10.1186/s12970-014-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider R.B., Kalman D.S., Antonio J., Ziegenfuss T.N., Wildman R., Collins R., Candow D.G., Kleiner S.M., Almada A.L., Lopez H.L. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017;14:18. doi: 10.1186/s12970-017-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuźniacka J., Banaszak M., Biesek J., Maiorano G., Adamski M. Effect of faba bean-based diets on the meat quality and fatty acids composition in breast muscles of broiler chickens. Sci. Rep. 2020;10:5292. doi: 10.1038/s41598-020-62282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the the 2– ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Longo N., Ardon O., Vanzo R., Schwartz E., Pasquali M. Disorders of creatine transport and metabolism. Am. J. Med. Genet. 2011;157:72–78. doi: 10.1002/ajmg.c.30292. [DOI] [PubMed] [Google Scholar]
- Majdeddin M., Braun U., Lemme A., Golian A., Kermanshahi H., De Smet S., Michiels J. Guanidinoacetic acid supplementation improves feed conversion in broilers subjected to heat stress associated with muscle creatine loading and arginine sparing. Poult. Sci. 2020;99:4442–4453. doi: 10.1016/j.psj.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademacher M., Dierick N.A., De Smet S. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 2012;91:402–412. doi: 10.3382/ps.2011-01585. [DOI] [PubMed] [Google Scholar]
- Monin G., Sellier P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: the case of the Hampshire breed. Meat Sci. 1985;13:49–63. doi: 10.1016/S0309-1740(85)80004-8. [DOI] [PubMed] [Google Scholar]
- Nelson D.L., Cox M.M. Pages 527–568 in Lehninger Principles of Biochemistry. 5th ed. W. H. Freeman and Company; New York, NY: 2008. Glycolysis, gluconeogenesis, and the pentose phosphate pathway. [Google Scholar]
- Nissen P.M., Young J.F. Creatine monohydrate and glucose supplementation to slow-and fast-growing chickens changes the postmortem pH in pectoralis major. Poult. Sci. 2006;85:1038–1044. doi: 10.1093/ps/85.6.1038. [DOI] [PubMed] [Google Scholar]
- Pearce K.L., Rosenvold K., Andersen H.J., Hopkins D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes - a review. Meat Sci. 2011;89:111–124. doi: 10.1016/j.meatsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Portocarero N., Braun U. The physiological role of guanidinoacetic acid and its relationship with arginine in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Richards M.P., Ramachandran R., McMurtry J.P. Characterization of the AMP-activated protein kinase pathway in chickens. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2006;143:92–106. doi: 10.1016/j.cbpb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Ryu Y.C., Kim B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Scheffler T.L., Gerrard D.E. Mechanisms controlling pork quality development: the biochemistry controlling postmortem energy metabolism. Meat Sci. 2007;77:7–16. doi: 10.1016/j.meatsci.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Shen Q.W., Means W.J., Thompson S.A., Underwood K.R., Zhu M.J., McCormick R.J., Ford S.P., Du M. Pre-slaughter transport, AMP-activated protein kinase, glycolysis, and quality of pork loin. Meat Sci. 2006;74:388–395. doi: 10.1016/j.meatsci.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Stapleton D., Mitchelhill K.I., Gao G., Widmer J., Michell B.J., Teh T., House C.M., Fernandez C.S., Cox T., Witters L.A., Kemp B.E. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Wang X., Li J., Cong J., Chen X., Zhu X., Zhang L., Gao F., Zhou G. Preslaughter transport effect on broiler meat quality and post-mortem glycolysis metabolism of muscles with different fiber types. J. Agric. Food. Chem. 2017;65:10310–10316. doi: 10.1021/acs.jafc.7b04193. [DOI] [PubMed] [Google Scholar]
- Wallimann T., Tokarska-Schlattner M., Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G.D., Selvadurai H., Tein I. Bioenergetic provision of energy for muscular activity. Paediatr. Respir. Rev. 2009;10:83–90. doi: 10.1016/j.prrv.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Woods A., Johnstone S.R., Dickerson K., Leiper F.C., Fryer L.G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Xing T., Xu X., Jiang N., Deng S. Effect of transportation and pre-slaughter water shower spray with resting on AMP-activated protein kinase, glycolysis and meat quality of broilers during summer. Anim. Sci. J. 2016;87:299–307. doi: 10.1111/asj.12426. [DOI] [PubMed] [Google Scholar]
- Zarghi H., Golian A., Yazdi F.T. Effect of dietary sulphur amino acid levels and guanidinoacetic acid supplementation on performance, carcase yield and energetic molecular metabolites in broiler chickens fed wheat-soy diets. Ital. J. Anim. Sci. 2020;19:951–959. [Google Scholar]
- Zhang B., Liu N., He Z., Song P., Hao M., Xie Y., Li J., Liu R., Sun Z. Guanidino-acetic acid: a scarce substance in biomass that can regulate postmortem meat glycolysis of broilers subjected to pre-slaughter transportation. Front. Bioeng. Biotechnol. 2021;8:1572. doi: 10.3389/fbioe.2020.631194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li J.L., Gao T., Lin M., Wang X.F., Zhu X.D., Gao F., Zhou G.H. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress. Animal. 2014;8:1955–1962. doi: 10.1017/S1751731114001906. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J.L., Wang X.F., Zhu X.D., Gao F., Zhou G.H. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers. Poult. Sci. 2019;98:3223–3232. doi: 10.3382/ps/pez052. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang X., Li J., Zhu X., Gao F., Zhou G. Creatine monohydrate enhances energy status and reduces glycolysis via inhibition of AMPK pathway in pectoralis major muscle of transport-stressed broilers. J. Agric. Food Chem. 2017;65:6991–6999. doi: 10.1021/acs.jafc.7b02740. [DOI] [PubMed] [Google Scholar]