Abstract
Objectives
Despite decades of research using animals to develop pharmaceutical treatments for patients who have had a stroke, few therapeutic options exist. The vast majority of interventions successful in preclinical animal studies have turned out to have no efficacy in humans or to be harmful to humans. In view of this, we explore whether there is evidence of a move away from animal models in this field.
Methods
We used an innovative methodology, the analysis of opinion papers. Although we took a systematic approach to literature searching and data extraction, this is not a systematic review because the study involves the synthesis of opinions, not research evidence. Data were extracted from retrieved papers in chronological order and analysed qualitatively and descriptively.
Results
Eighty eligible papers, published between 1979 and 2018, were identified. Most authors were from academic departments of neurology, neuroscience or stroke research. Authors agreed that translational stroke research was in crisis. They held diverse views about the causes of this crisis, most of which did not fundamentally challenge the use of animal models. Some, however, attributed the translational crisis to animal–human species differences and one to a lack of human in vitro models. Most of the proposed solutions involved fine-tuning animal models, but authors disagreed about whether such modifications would improve translation. A minority suggested using human in vitro methods alongside animal models. One proposed focusing only on human in vitro methods.
Conclusion
Despite recognising that animal models have been unsuccessful in the field of stroke, most researchers exhibited a strong resistance to relinquishing them. Nevertheless, there is an emerging challenge to the use of animal models, in the form of human-focused in vitro approaches. For the sake of stroke patients there is an urgent need to revitalise translational stroke research and explore the evidence for these new approaches.
Keywords: stroke, animal models, neuroscience, opinion
Introduction
Despite decades of research using animals to develop pharmaceutical treatments for patients who have had a stroke, few therapeutic options exist. The vast majority of interventions successful in preclinical animal studies have turned out to either have no efficacy in humans, as with NXY-0591 or calcium channel blockers,2 or to be harmful to humans, as with diaspirin,3 emlimobab,4 selfotel5 and tirilazad,6 all of which increased the risk of death when taken to clinical trials. The failed quest for a neuroprotective agent is infamous; of more than 1000 candidate neuroprotective drugs tested in animals, not one was found to benefit humans with stroke.7
Currently, the only effective options available to those with haemorrhagic stroke consist of controlling blood pressure and admission to a stroke unit. For ischaemic stroke, the main options include admission to a stroke unit, secondary prevention using antiplatelets (eg, aspirin), and recanalisation, either pharmaceutically with thrombolytics or mechanically with endovascular thrombectomy. Although more treatment options are available for ischaemic stroke, thrombolytics can only be given to selected patients who present to a centre of expertise within 4.5 hours post stroke. (Clinical trials of thrombolytics for stroke followed on from their success with heart attack; animal studies were conducted to establish dosing8 but did not play a direct role in clinical translation.9) Currently, 11%–12% of patients receive thrombolysis in England, Wales and Northern Ireland,10 but around half of those who receive the treatment remain dependent or die.11 Similarly, only about 10% of patients who have a stroke are estimated to be eligible for endovascular thrombectomy.12 Against this background, stroke remains the second leading cause of death worldwide and the second most common cause of disability-adjusted life years.13 Despite the decline in death rates and stroke incidence in most regions, stroke is still prevalent and disabling, and population growth and ageing may result in a greater absolute pool of people at risk of stroke.13 Clearly, there is still a pressing need to develop effective responses to stroke that will benefit more than a minority of patients.
Given the unmet need of stroke patients, and in view of translational disappointments, we explore whether there is evidence of a move away from animal models in the field of translational stroke research. Kuhn14 observed that when confronted with anomalies, scientists tend not to renounce their paradigm but modify their theory in order to eliminate any conflict. If an anomaly persists, he argues, the field will enter a period of crisis and debate and the anomaly will eventually be acknowledged; however, even if the first paradigm has gone badly astray, it will only be declared invalid if an alternate candidate is available to take its place. The failure to translate from animals to humans is certainly a significant anomaly for a field of preclinical translational research. At the same time, an alternate paradigm is emerging, in which preclinical researchers use human-focused (non-animal) in vitro methods (eg, human cells, tissues or organoids) to explore disease processes and test drugs. Here, we explore how preclinical stroke researchers concerned with developing pharmaceutical drugs for acute stroke are attempting to resolve the translational anomaly. In particular, we are interested in the extent to which these researchers are attempting to modify their use of animal models, or are moving towards more human-focused approaches. We explore this question by analysing opinion papers in the field, a novel methodology which to our knowledge is the first of its kind.
Aims
Our specific research questions are:
How have researchers using animal models of stroke responded to the poor ability of these models to translate into clinical benefit?
What do researchers attribute the translational problems to and what solutions do they propose?
Is there evidence of a move towards human-focused methods as a result of poor translation?
Methods
Design
Although we took a systematic approach to literature searching and data extraction, this is not a systematic review because the study involves the synthesis of opinions, not findings. We are neither generating new data by synthesising primary research data nor deriving new conclusions based on research evidence; our study simply provides a synthesis of opinions in the field, permitting insight into the range of views and debates occurring, as well as the direction of travel. Furthermore, the analysis of opinion should not be confused with evidence syntheses that use expert opinion as a substitute for findings where qualitative and quantitative evidence is lacking.15 Here, we use opinions in their own right, as a means of exploring the discourse around animal models of stroke.
Search strategies
We searched for opinion papers (eg, commentaries, editorials, viewpoints, special reports) about the use of animal models to develop pharmaceutical drugs for acute stroke, with no restrictions by date. Papers had to be written by researchers, either preclinical scientists conducting animal studies or clinical research scientists, working in this field. We excluded papers with a narrow, specific or technical focus, papers that did not focus specifically on stroke, papers about rehabilitation treatments or surgery, primary research, systematic reviews, conference abstracts and letters. We used a combination of hand-searching and simple electronic searches, having previously found this to be an effective strategy for locating elusive literature, such as qualitative studies or theoretical papers.16 17 For hand-searching, the first author searched two key journals in the field (Stroke, Translational Stroke Research) and the CAMARADES website (http://www.dcn.ed.ac.uk/camarades/), which includes opinion papers on preclinical stroke research. References of papers selected for full screening were scanned for further eligible papers. Papers found serendipitously were also included. The hand-searches identified 57 eligible papers (table 1), the full texts of which were screened by the second author to check eligibility.
Table 1.
Hand-searches
| Hand-searches | Number of eligible papers identified |
| Stroke journal | 30 |
| Reference checking | 12 |
| Translational Stroke Research journal | 7 |
| Serendipity | 4 |
| CAMARADES website | 2 |
| Google Scholar | 2 |
| Total | 57 |
Electronic searches were conducted using MEDLINE, Embase (table 2) and Web of Science in July 2018. The Web of Science search was as follows: TOPIC: (stroke OR cerebrovascular disease) AND TOPIC:(animal model* OR stroke model* OR experimental OR preclinical) AND TOPIC:(translation). DOCUMENT TYPES: (LETTER OR REVIEW OR EDITORIAL MATERIAL).
Table 2.
MEDLINE and Embase searches
| Number | Searches | MEDLINE (16 July 2018) | Embase (20 July 2018) |
| 1 | Stroke/ | 84 404 | 125 226 |
| 2 | Cerebrovascular Disorders/ | 45 250 | 25 542 |
| 3 | models, animal/ | 38 525 | 1 074 611 |
| 4 | Drug evaluation, Preclinical/ | 46 548 | 13 287 |
| 5 | stroke model.mp. | 1317 | 2067 |
| 6 | experimental.mp. | 1 200 603 | 1 180 327 |
| 7 | translation.mp | 114 811 | 153 494 |
| 8 | preclinical.mp. | 118 391 | 121 118 |
| 9 | 1 or 2 | 128 496 | 146 835 |
| 10 | 3 or 4 or 5 or 6 or 8 | 1 335 115 | 2 170 750 |
| 11 | 7 and 9 and 10 | 119 | 128 |
Four hundred and thirty-three papers were identified as being potentially relevant. The titles and abstracts of these 433 papers were screened independently by the two authors (80% agreement). Three hundred and twenty-nine papers were initially excluded and the remaining 104 were examined in greater detail. Duplicates were removed as each reviewer developed their shortlist. After discussion between the two reviewers, 30 were selected as potentially relevant, for which full publications were obtained and screened independently by the same two reviewers (77% agreement). Differences were resolved through discussion. On the final day of searching (19 September 2018), the electronic searches were rerun and a further search, restricted to 2018, was conducted using Google Scholar to check for very recent papers.
Data extraction
The papers were analysed chronologically and data relevant to the three research questions were extracted. Data were first extracted from the three earliest papers and a preliminary data extraction sheet was developed. As data were extracted from subsequent papers, the data extraction sheet was refined, expanded and agreed by the two authors. During this process, a further two papers were excluded: one was ineligible and the other was almost identical to another paper written by the same researcher.
Data analysis
The data were analysed qualitatively and descriptively. For the most part, we adopted a deductive approach whereby the analysis was guided by the research question. For example, a recommendation for improving translation was, “In preclinical stroke research animals with comorbidities, mixed gender, advanced age, etc. should play an important role to model the complexities of risk factors, patient profiles, and clinical situation as much as possible”. This was coded as “increase representativeness of animal samples” and organised within the larger category of “improve external validity”. However, researchers’ general observations and comments were also examined, allowing other themes to emerge from the data (inductive approach).
Results
Searches
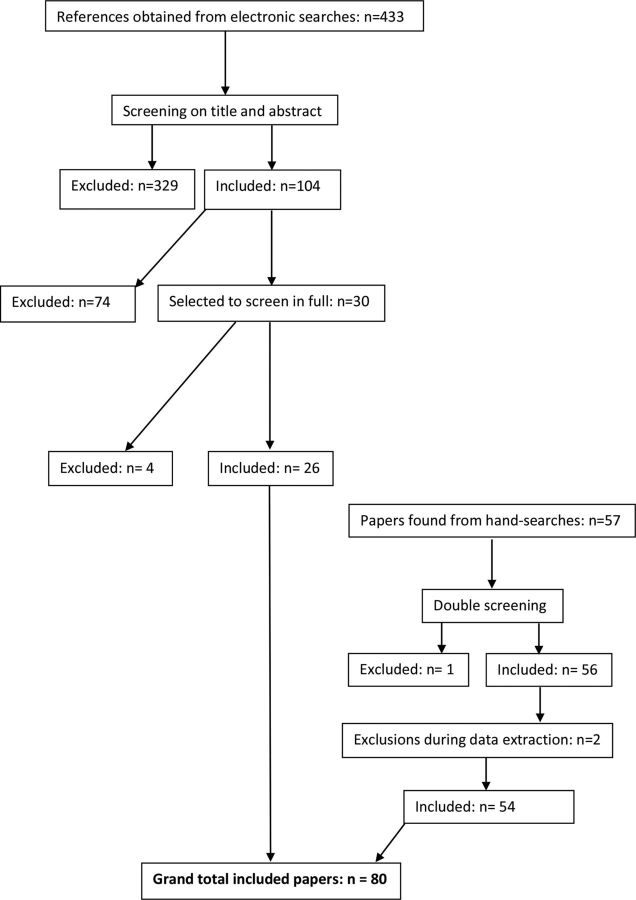
Eighty eligible papers were identified, 26 through electronic searches and 54 through hand-searches (figure 1). A list of the papers is provided in the associated dataset https://doi.org/10.5061/dryad.xpnvx0kb9.
Figure 1.
Flow chart showing searches.
Characteristics of authors and papers
Most authors were located in academic departments of neurology, neuroscience or stroke research (n=62). Seven were in general academic medical research institutions, eight were in a variety of other academic departments and three in pharmaceutical companies (table 3). They were mainly located in the USA (n=36), Germany (n=13), UK (n=10), Australia (n=8) and Canada (n=7), and all were involved in some capacity with the translation of preclinical stroke research. The papers were published between 1979 and 2018. The majority (n=47) discussed issues relating to animal models of stroke in general, while 31 focused on animal models of neuroprotective agents. Most papers discussed ischaemic stroke, but two (both published in 2018) considered animal models of haemorrhagic stroke.
Table 3.
Author information
| Authors | First author’s country | First author’s main institution | Department | |
| 1 | Waltz 197972 | USA | Dept of Neurology, Pacific Medical Center, San Francisco, CA | Neuro/stroke |
| 2 | Molinari 198820 | USA | George Washington University Medical Center, 2150 Pennsylvania Avenue NW, Washington, DC | General |
| 3 | Wiebers et al 199018 | USA | Dept of Neurology and Dept of Health Sciences Research, Mayo Clinic and Mayo Foundation, Rochester, Minnesota |
Neuro/stroke |
| 4 | Zivin and Grotta 1990 | USA | Dept of Neurosciences, University of California, San Diego |
Neuro/stroke |
| 5 | Millikan 199221 | USA | Dept of Neurology, Henry Ford Health Sciences Center, Detroit, Michigan |
Neuro/stroke |
| 6 | Hsu 199319 | USA | Division of Restorative Neurology and Human Neurobiology, Baylor College of Medicine, Houston, Texas | Neuro/stroke |
| 7 | Hunter et al 199543 | UK | Smith Kline Beecham Pharmaceuticals, The Pinnacles, Coldharbour Road, Harlow | Pharmaceutical company |
| 8 | del Zoppo 1995 | USA | Dept of Molecular and Experimental Medicine, Scripps Research Institute, La Jolla, California | Molecular/experimental |
| 9 | Grotta 1995 | USA | Dept of Neurology, University of Texas Medical School at Houston, Texas | Neuro/stroke |
| 10 | Ginsberg 1996 | USA | Cerebral Vascular Disease Research Center, Dept of Neurology, University of Miami School of Medicine, Miami, Florida | Neuro/stroke |
| 11 | del Zoppo 199860 | USA | Dept of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, California | Molecular/experimental |
| 12 | STAIR 199942 | USA | UMass/Memorial Healthcare, 119 Belmont St, Worcester, Massachusetts | General |
| 13 | Grotta 1999 | USA | University of Texas–Houston Medical School, 6431 Fannin, Houston, Texas |
General |
| 14 | DeGraba and Pettigrew 200069 | USA | Stroke Branch, National Institute of Neurologic Disorders and Stroke, National Institutes of Health, Bethesda, Maryland | Neuro/stroke |
| 15 | Gladstone et al 200264 | Canada | Cognitive Neurology and Stroke Research Unit, Sunnybrook, Toronto, Ontario | Neuro/stroke |
| 16 | Grotta 2002 | USA | Dept of Neurology, University of Texas–Houston Medical School, 6431 Fannin Street, Houston, Texas |
Neuro/stroke |
| 17 | Lees 2002 | UK | University of Glasgow, University Dept of Medicine and Therapeutics, Western Infirmary, Glasgow | General |
| 18 | Davis and Donnan 200274 | Australia | Dept of Neurology, Royal Melbourne Hospital, Parkville, Victoria | Neuro/stroke |
| 19 | Curry 2003 | USA | Dept of Pharmacology and Physiology, University of Rochester, and Stephen H. Curry Consulting, Rochester, New York | Pharmacology/physiology |
| 20 | Green et al 2003 | UK | AstraZeneca R&D Charnwood, Loughborough | Pharmaceutical company |
| 21 | Hoyte et al 200461 | Canada | Calgary Stroke Program, Dept of Clinical Neurosciences, University of Calgary, Calgary, Alberta | Neuro/stroke |
| 22 | Cheng et al 200471 | USA | Stroke Center, University of California School of Medicine, and University of California San Diego, La Jolla, California | Neuro/stroke |
| 23 | Fisher and Tatlisumak 2005 | USA | Dept of Neurology, University of Massachusetts Medical School, Worcester, Massachusetts |
Neuro/stroke |
| 24 | Kaste 200522 | Finland | Dept of Neurology, Helsinki University Central Hospital, University of Helsinki, Helsinki |
Neuro/stroke |
| 25 | Donnan and Davis 200523 | Australia | National Stroke Research Institute, Austin and Repatriation Medical Centre and University of Melbourne, Melbourne | Neuro/stroke |
| 26 | Dirnagl 2006 | Germany | Dept of Experimental Neurology, Center for Stroke Research, Humboldt-Universitat Berlin, Universitatsklinikum Charité, Berlin | Neuro/stroke |
| 27 | Sena et al 200744 | UK | Dept of Clinical Neurosciences, University of Edinburgh, Edinburgh | Neuro/stroke |
| 28 | Perez de la Ossa and Davalos 200724 | Spain | Stroke Unit, Dept of Neurosciences, Hospital Universitari Germans Trias i Pujol, Barcelona | Neuro/stroke |
| 29 | Savitz and Fisher 200725 | USA | Dept of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston |
Neuro/stroke |
| 30 | Green 200853 | UK | Institute of Neuroscience, School of Biomedical Sciences, Queen’s Medical Centre, University of Nottingham, Nottingham | Neuro/stroke |
| 31 | Donnan and Davis 200875 | Australia | Royal Melbourne Hospital, University of Melbourne, Parkville, Victoria | General |
| 32 | Röther 200826 | Germany | Dept of Neurology, Klinikum Minden, Academic Teaching Hospital, Hannover Medical School, Minden | Neuro/stroke |
| 33 | Hussain and Shuaib 200845 | Canada | Division of Neurology, Dept of Medicine, University of Alberta, Edmonton | Neuro/stroke |
| 34 | Donnan 2008 | Australia | National Stroke Research Institute, Austin Health, University of Melbourne, Waterdale Road, Heidelberg Heights | Neuro/stroke |
| 35 | Endres et al 2008 | Germany | Depts of Neurology and Experimental Neurology, Center for Stroke Research, Charité, Berlin | Neuro/stroke |
| 36 | Dirnagl and Macleod 200927 | Germany | Depts of Neurology and Experimental Neurology, Charité University Medicine, Berlin | Neuro/stroke |
| 37 | Ginsberg 200954 | USA | Dept of Neurology, University of Miami Miller School of Medicine, Miami, Florida | Neuro/stroke |
| 38 | Fisher et al 200946 | USA | Dept of Neurology, University of Massachusetts Medical School, Worcester, Massachusetts | Neuro/stroke |
| 39 | Bath et al 200947 | UK | Institute of Neuroscience, School of Biomedical Sciences, Queen’s Medical Centre, University of Nottingham, Nottingham | Neuro/stroke |
| 40 | Fisher et al 200946 | USA | Discovery Translational Medicine, Wyeth Research, 500 Arcola Rd, Collegeville, Pennsylvania | Pharmaceutical company |
| 41 | Macleod et al 200947 | UK | Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh | Neuro/stroke |
| 42 | Tymianski 201048 | Canada | Toronto Western Hospital Research Institute, Toronto, Ontario | General |
| 43 | Moskowitz 2010 | USA | Neuroscience Center, Radiology and Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts | Neuro/stroke |
| 44 | Ginsberg 2010 | USA | Dept of Neurology, University of Miami Miller School of Medicine, Miami, Florida | Neuro/stroke |
| 45 | Turner et al 201149 | USA | Dept of Neurology, University of California at Davis, Sacramento, California | Neuro/stroke |
| 46 | Fisher 2011 | USA | Dept of Neurology, UMASS/Memorial Healthcare, Worcester, Massachusetts | Neuro/stroke |
| 47 | Budincevic et al 201155 | Croatia | Dept of Neurology, University Hospital ‘Sveti Duh’, Sveti Duh 64, Zagreb |
Neuro/stroke |
| 48 | Cook and Tymianski 2011 | Canada | University of Toronto, Dept of Surgery, Toronto Western Research Institute Neuroprotection Laboratory, Toronto, Ontario | Neuro/stroke |
| 49 | Antonic et al 201259 | Australia | The National Stroke Research Institute, Florey Neuroscience Institutes, Heidelberg, Victoria | Neuro/stroke |
| 50 | Sutherland et al 201257 | UK | Acute Stroke Programme, Nuffield Dept of Clinical Medicine, University of Oxford, Oxford | Neuro/stroke |
| 51 | Lyden and Lapchak 201231 | USA | Dept of Neurology, Cedars-Sinai Medical Center, Los Angeles, California |
Neuro/stroke |
| 52 | Minnerup et al 201256 | Germany | Dept of Neurology, University of Münster, Albert-Schweitzer-Campus 1 to 48 149 Münster | Neuro/stroke |
| 53 | Howells et al 201229 | Australia | Florey Neuroscience Institutes, Melbourne Brain Centre, Heidelberg, Victoria | Neuro/stroke |
| 54 | Dirnagl and Fisher 201228 | Germany | Center for Stroke Research, Charité Universitätsmedizin, Berlin | Neuro/stroke |
| 55 | Dirnagl et al 201365 | USA | Dept of Neurology, University of Massachusetts Medical School, 119 Belmont Street, Worcester, Massachusetts | Neuro/stroke |
| 56 | Xu and Pan 201366 | China | Dept of Neurology, Nanfang Hospital, Southern Medical University, Guangzhou | Neuro/stroke |
| 57 | Greenberg 201334 | Canada | Buck Institute for Research on Aging, 8001 Redwood Blvd, Novato, California | Ageing research |
| 58 | Howells and Macleod 201350 | Australia | Florey Institute of Neuroscience and Mental Health, Heidelberg, Victoria | Neuro/stroke |
| 59 | Lapchak 201332 | USA | Dept of Neurology, Cedars-Sinai Medical Center, Los Angeles, California | Neuro/stroke |
| 60 | Dirnagl et al 201365 | Germany | Dept of Neurology and Experimental Neurology, Center for Stroke Research, Charité Universitätsmedizin Berlin | Neuro/stroke |
| 61 | Herson and Traystman 201433 | USA | Dept of Pharmacology, University of Colorado Denver, Anschutz Medical Campus, Aurora, Colorado | Pharmacology |
| 62 | Lo 2014 | USA | Depts of Neurology and Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown | Neuro/stroke |
| 63 | Dirnagl and Endres 201430 | Germany | Depts of Neurology and Experimental Neurology, Center for Stroke Research Berlin, Charité Universitätsmedizin, Berlin | Neuro/stroke |
| 64 | Neuhaus et al 201458 | UK | Acute Stroke Programme, Radcliffe Dept of Medicine, University of Oxford, Oxford |
Neuro/stroke |
| 65 | Howells et al 201411 | Australia | Florey Institute of Neuroscience and Mental Health, 245 Burgundy Street, Heidelberg, Victoria | Neuro/stroke |
| 66 | Boltze et al 201435 | Germany | Fraunhofer Institute of Cell Therapy and Immunology, University of Leipzig, Leipzig | Cell therapy |
| 67 | Dirnagl 2014 | Germany | Depts of Neurology and Experimental Neurology Charité, Center for Stroke Research Berlin, Charité, ExcellenceCluster NeuroCure—Universitätsmedizin Berlin, Berlin | Neuro/stroke |
| 68 | Offner 201437 | USA | Neuroimmunology Research R&D-31, Veterans Affairs Medical Center, 3710 SW Veterans Hospital Rd, Portland | Neuro/stroke |
| 69 | Sharp and Jickling 201468 | USA | University of California at Davis, MIND Institute, 2805 50th St, Sacramento, California | Neuro/stroke |
| 70 | Dirnagl 201638 | Germany | Dept of Experimental Neurology, Center for Stroke Research, Charité Universitätsmedizin Berlin | Neuro/stroke |
| 71 | Boltze and Ayata 201639 | Germany | Fraunhofer Research Institution for Marine Biotechnology, University of Lübeck, Mönkhofer Weg 239a, 23 562 Lübeck | Marine biotech |
| 72 | Boltze and Ayata 201639 | Germany | Fraunhofer Institute for Cell Therapy and Immunology, Leipzig |
Cell therapy |
| 73 | Neuhaus et al 201740 | UK | Acute Stroke Programme, Radcliffe Dept of Medicine, University of Oxford, Oxford | Neuro/stroke |
| 74 | Zerna et al 201741 | Canada | Calgary Stroke Program, Dept of Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Alberta | Neuro/stroke |
| 75 | Bosetti et al 201776 | USA | National Institute of Neurological Disorders and Stroke, National Institutes of Health | Neuro/stroke |
| 76 | Lapchak 2017 | USA | Dept of Neurology and Neurosurgery, Cedars-Sinai Medical Center, San Vicente Blvd, Los Angeles, California | Neuro/stroke |
| 77 | Marbacher 2017 | Switzerland | Kantonsspital Aarau, Aarau, Switzerland | General |
| 78 | Suzuki and Nakano 201867 | Japan | Dept of Neurosurgery, Mie University Graduate School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507 | Neuro/stroke |
| 79 | HEADS 201862 | USA | Division of Stroke, Dept of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts | Neuro/stroke |
| 80 | Bix et al 2018 | USA | Center for Advanced Translational Stroke Science, University of Kentucky, Lexington, Kentucky | Neuro/stroke |
| USA: 36; Germany: 13; UK: 10; Australia: 8; Canada: 7; Japan, Switzerland, China, Croatia, Spain, Finland: 1 each | Depts of neurology, neurosciences, neurosurgery, cerebrovascular disease, stroke research: 62; General academic medical research depts: 7; Pharmaceutical companies: 3; Molecular/experimental depts: 2; Cell therapy depts: 2; Pharmacology/physiology depts: 2; Marine biotechnology: 1; Ageing dept: 1 |
Research findings
First, we consider how researchers using animal models of stroke have responded to the poor ability of these models to translate to humans. Second, we examine what researchers attribute the translational problems to and what solutions they propose, including their views about whether improvements in the scientific rigour of animal studies would increase clinical translation. Third, we explore whether there is evidence of a move towards human-focused approaches as a result of poor clinical translation.
How have researchers using animal models of stroke responded to the poor ability of these models to clinically translate?
Almost 30 years ago, Wiebers et al18 predicted that overreliance on animal models would impede rather than advance progress in treating stroke, and Hsu,19 noting that interventions that worked in animals a decade earlier had failed to translate clinically, suggested this challenged the legitimacy of using animal models for stroke. At the same time, several early commentators warned about the dangers of raising expectations, cautioning that overselling ‘promising’ results would lead to the launch of expensive, but ultimately disappointing, clinical trials,19 20 while at the same time increasing scepticism about preclinical stroke research.21 Some suggested that advances in stroke medicine had occurred without the use of animal models, and that case selection and patient management in clinical trials had exerted the major impact on patient care.22 23
In 2007, the failure of the free-radical scavenger NXY-059 represented a huge setback in translational stroke research.1 NXY-059 was shown to be neuroprotective in several preclinical studies using rats and primates and indeed the first clinical trial (SAINT-I) found a reduction of global disability. However, SAINT-II failed to replicate the results of SAINT-I and NXY-059 was withdrawn from further development.24–26 Writing about the reasons for this failure, Dirnagl and Macleod27 suggested that the most troubling explanation—that animals do not model human stroke with sufficient fidelity to be useful—lacked evidence, but acknowledged that the field had not yet demonstrated proof of concept. Success, however, was not forthcoming and before long pharmaceutical companies started exiting28 and the concept of human neuroprotection was in doubt.29 30 Drawing parallels with the mythological King Sisyphus (condemned to push a large boulder to the top of a hill, only to have to continually repeat the exercise each time the boulder rolled to the bottom), Lyden and Lapchak noted that the repeated failures within translational stroke research were sapping the energy and enthusiasm of collaborators and funders.31 It was also observed that some prominent clinical trials had been halted due to lack of efficacy or significant adverse events.32
By 2014, it was acknowledged that widespread doubt now existed about the validity of using animal models as predictive tools in stroke, given the remarkable number of neuroprotective agents that had shown promise in animals but gone on to fail in humans.33 Similarly, Greenberg noted that although animal models of stroke had been available for over 50 years, few advances in the clinical treatment of acute stroke had occurred.34 The sense of crisis35 was deepened by a widely publicised article outside the field that demonstrated poor correlation between human and mouse genomic responses to acute inflammatory insults,36 with concern expressed for stroke researchers using mice to understand inflammation in human stroke.37
By 2016, it was accepted that bench-to-bedside translation in stroke had a ‘disappointing track record’38 and that neuroprotection in particular had been a ‘spectacular’39 or ‘notable’40 failure. Dirnagl38 noted that while stroke incidence, morbidity and mortality had decreased, and stroke units and recanalisation had benefited patients, none had been due to ‘bench-to-bedside’ translation. Zerna et al41 made a similar point with regard to recanalisation. Observing that more than 90% of patients who had an acute stroke still lacked treatment to limit injury or improve outcome, it was proposed that translational stroke research was now at a turning point39 and faced a ‘substantial transition’.41
What do researchers attribute translational problems to and what solutions do they propose?
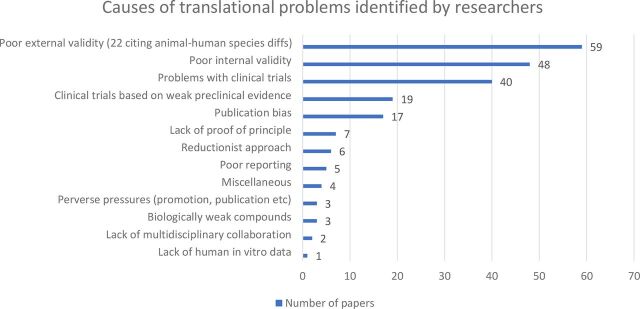
In terms of researchers’ views on the causes of translational problems, the majority fell into three categories: poor external validity of animal studies (n=59), poor internal validity of animal studies (n=48) and problems relating to clinical trials (n=40). In terms of poor external validity, researchers noted issues such as the inability of animal models to mimic human disease progression, difficulty recapitulating human risk factors, polypharmacy or comorbidity, the use of clinically irrelevant outcome measures and animal–human species differences. In terms of poor internal validity, researchers noted problems such as failure to control bias, lack of pre-trial sample size calculations, inappropriate analyses and poor physiological monitoring. With respect to problems relating to clinical trials, issues such as insufficient selectivity in targeting patients, underpowered trials and late administration of experimental drugs were identified. The associated dataset https://doi.org/10.5061/dryad.xpnvx0kb9 lists the subcategories of the three main explanations identified. Several other explanations for translational problems were identified, including proceeding to clinical trials on the basis of weak preclinical evidence (n-19) and publication bias (n=17). Only a small proportion of the responses cited causes that fundamentally challenged the use of animal models; one cited lack of human in vitro data and 22 within the category of poor external validity cited animal–human species differences (figure 2).
Figure 2.
Causes of translational problems identified by researchers.
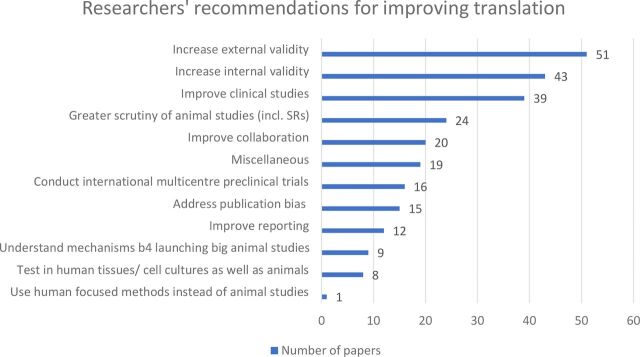
In terms of solutions, the most popular recommendation (suggested in 51 papers) was to improve external validity. Included within this category were recommendations such as making animal models more relevant to human stroke, making the timing, dose and route of drug administration more relevant to the human situation, and testing in a variety of animal models and species, including non-human primates (NHPs). The second most popular recommendation (n=43) was to increase internal validity, including taking measures to reduce the risk of bias and adhering to the STAIR guidelines42 for improving scientific rigour in preclinical stroke research. Thirty-nine papers recommended that clinical studies should be improved, including suggestions for better patient selection and the earlier administration of experimental drugs. Other proposals included greater scrutiny of animal studies by journal editors, reviewers and those planning clinical trials, together with systematic reviews and audits of animal studies (n=24), greater interdisciplinary collaboration, including between basic and clinical scientists and between academia and pharmaceutical companies (n=20), conducting international multicentre preclinical trials (n=16), tackling publication bias (n=15) and improving reporting (n=12). The associated dataset https://doi.org/10.5061/dryad.xpnvx0kb9 lists the subcategories of the main recommendations given. Again, only a small proportion of recommendations fundamentally challenged the use of animal models; eight papers suggested testing in human tissues/cell cultures as well as animals and one recommended that human-focused methods be used in place of animal studies (figure 3).
Figure 3.
Researchers’ recommendations for improving translation.
While improvements in the internal and external validity of animal studies were popular recommendations, there was disagreement about whether such improvements would increase clinical translation. Many were of the view that they would,43–49 with some arguing that the neuroprotection hypothesis, for example, had not yet been refuted because it had not so far been tested in sufficiently rigorous studies.33 48 50 There was a reluctance to relinquish the concept of neuroprotection51 even after the failure of the SAINT-II trial; the view was that with rigorous translational science, neuroprotection in humans would still be possible.28 29 50 52 There was a view that improvements in the scientific quality of preclinical studies, together with ongoing improvements in clinical trial design, would eventually lead to successful translation.33 As Dirnagl claimed, translational stroke research was not broken, its engine simply needed overhauling.38
Others, however, were more pessimistic. Some suggested that improvements in animal models or study design would not improve clinical translation to a sufficiently high degree,22 24 and it was noted that the failure of NXY-059, “a compound developed with close regard to the best currently established guidelines of preclinical and clinical methodology”, raised major questions about the value of animal models.53 Several pointed out that the STAIR guidelines had failed to improve clinical translation33 54–57 and that even perfect implementation of the guidelines might not lead to successful clinical trials58 because animal models of stroke might be too dissimilar to human stroke.33
Is there evidence of a move towards more human-focused approaches?
As noted above, some researchers’ views on the causes of poor translation challenged the animal model paradigm. Antonic et al59 suggested that poor rates of clinical translation could be due to a lack of human in vitro models and that more human-focused approaches might be necessary to make progress. Furthermore, 22 of the 59 papers that cited problems of external validity as causes of poor translation raised the issue of animal–human species differences, identifying key differences in terms of anatomy, physiology and disease manifestation, pharmacokinetics and treatment response, immune response and genetics (box 1).
Box 1. Animal–human species differences highlighted by researchers.
Variations in anatomy, physiology and disease manifestation
Differences between rodents and humans in vascular anatomy,18 collateral blood supply,52 60 blood flow and metabolic rate.55 Differences in neuroanatomy: rodents have small lissencephalic (smooth) brains with decreased cortical surface area to brain mass compared with humans who have large gyrencephalic brains with multiple folds of the cortical surface.52 59 Detailed architecture within cortex is different, with variations in functional maps and synaptic density.59 Rodents have relatively little white matter compared with humans.52 55 59 61 62 71 Differences between rodents and humans in stroke pathophysiology,38 63 70 histopathology,18 process of excitotoxicity,59 infarct size,64 72 location of ischaemic strokes,68 recovery from neurological deficits66 and possibly mechanisms of brain injury.63
Differences in pharmacokinetics and treatment response
Species variations in pharmacokinetics, drug dosage, side effects and efficacy,18 19 for example, administration of equipotent doses of urokinase or streptokinase mediates different thrombolytic responses in humans, chimpanzees and baboons, with greater differences between primates and non-primates.60 Hypoxia tolerance and reaction to neuroprotective agents probably differ between humans and rats.68 Some drugs might only work in humans.59
Differences in immune response
Genetic differences between animals and humans may affect immune responses and outcomes.69 Rodents and humans have different inflammatory and immune responses to cerebral ischaemia.49 60 70 Most animals used to model stroke (rats, mice, rabbits, sheep, macaques, baboons, rhesus monkeys) have greater percentage of lymphocytes compared with neutrophils in peripheral blood compared with humans, and rodents are more resistant to infections than humans after surgery.70
Genetic differences
Humans and rodents separated by up to 80 million years of evolution, so significant differences exist between the species.59 65 67 Although 90% of the gene order is conserved in mice and humans, and although the proportion of mouse genes without any homologue currently detectable in the human genome appears to be less than 1%, the generation of proteins from these genes differs significantly between the two species, indicating important differences in the way human and rodent cells respond to ischaemic stress.51 The expression of genes may vary significantly between rodents and humans despite genetic homology.68 71 While humans share 90% of their genome with rodents and have 93% homology with the rhesus macaque, a 10% difference implies that up to 3000 genes may be different and that even those genes with homology may have evolved different biochemistry and function. 59 Cannot assume that humans and rodents share identical molecular targets.59
However, while these 22 papers highlighted animal–human species differences—an issue that fundamentally challenges the animal model paradigm—most proposed solutions that involved continuing use of the existing paradigm: develop animal models that replicate human pathophysiology and human stroke more faithfully37 38 60–65; test in NHPs52 66 or unspecified ‘larger animal models’62 67; test in specific animal species49 68 or two different animal species52 61 64 69; address pharmacokinetics.19 One suggested focusing on the similarities70 and three did not propose any solution.55 71 72
Only 3 of the 22 papers proposed solutions that addressed the paradigm challenge presented by animal–human species differences. Wiebers et al,18 the only authors to completely dismiss the animal model paradigm (‘paradigm rejecters’), recommended focusing on approaches based on human biology instead of trying to perfect animal models. Two papers proposed what we call ‘paradigm bridging’ solutions (a term first coined by Ritzer73), that is, continuing use of animal models but alongside in vitro methods using human tissue.51 59 Donnan51 proposed that for promising neuroprotectants, it should be a requirement to use human in vitro methods before progressing to human in vivo studies. He observed that well-established in vitro models existed (in which tissue is subjected to hypoxic or ischaemic stress by placing it in a sealed chamber from which oxygen and/or glucose is removed) but noted that these almost universally used cell lines or slices from animals rather than humans. He also proposed using magnetic resonance with diffusion-weighted/perfusion-weighted imaging mismatch to ensure that neuroprotectants cross the blood–brain barrier in humans, as well as positron emission tomography (PET to determine whether the ischaemic penumbra is reached, before embarking on later phase human studies. His roadmap relies heavily on human-focused methods but maintains an initial phase of animal research. Antonic et al59 recommended using human in vitro methods to test prospective neuroprotective agents, noting that in vitro testing was considerably cheaper than in vivo testing. They proposed that drugs found to be effective in human in vitro systems should then be taken into preclinical animal experiments. In cases where drugs appear to work only in humans, they suggested that (in the absence of whole animal data) biological targeting and effect could be confirmed with PET using tracer quantities of the candidate drug. Antonic et al noted that since systemic and central inflammatory processes are a key facet of stroke biology and a source of potential therapeutic targets, an ability to test a range of human tissue (not just neurons) was important. They proposed using human embryonic stem cells, anticipating that as culture systems and tissue engineering evolved, it would be possible to develop increasingly realistic in vitro models of stroke. They emphasised that since humans were the target, there was little logic to using animal cells for in vitro testing, particularly since human cell cultures were available at similar cost.
Alongside Donnan51 and Antonic et al,59 six other papers proposed ‘paradigm bridging’ solutions, that is, proposed that tests should be performed in human tissues/cell cultures as well as in animal models, as a means of improving translation. Davis and Donnan74 and Donnan and Davis75 argued that proof-of-concept studies should be conducted in humans as a prelude to pivotal clinical trials, while Howells et al29 noted that testing drugs in vitro before embarking on in vivo research was an affordable technique that might enable a quarter of drugs to be excluded from further development. Neuhaus et al40 stated that in vitro models of stroke offered valuable mechanistic insight into potential neuroprotective candidates, were able to demonstrate the impact of oxygen and glucose deprivation on specific cell types, and provided valuable ‘target validation’ at the molecular level, while enabling more accurate deductions about causality as a consequence of being able to regulate conditions. By 2017, Bosetti et al76 anticipated that given the increased availability of human cell lines, human tissues, human organoids, induced pluripotent stem cell (iPSC) technologies and high-throughput assays, in vitro strategies (in combination with animal model data) would be increasingly prominent in future drug development strategies. Likewise, the HEADS consortium62 noted that emerging human-focused techniques (including primary adult microglial and astrocyte cultures, iPSCs, single-cell isolation from brain slices, and cell phenotyping after flow cytometry) could also be applied in intracerebral haemorrhage research.
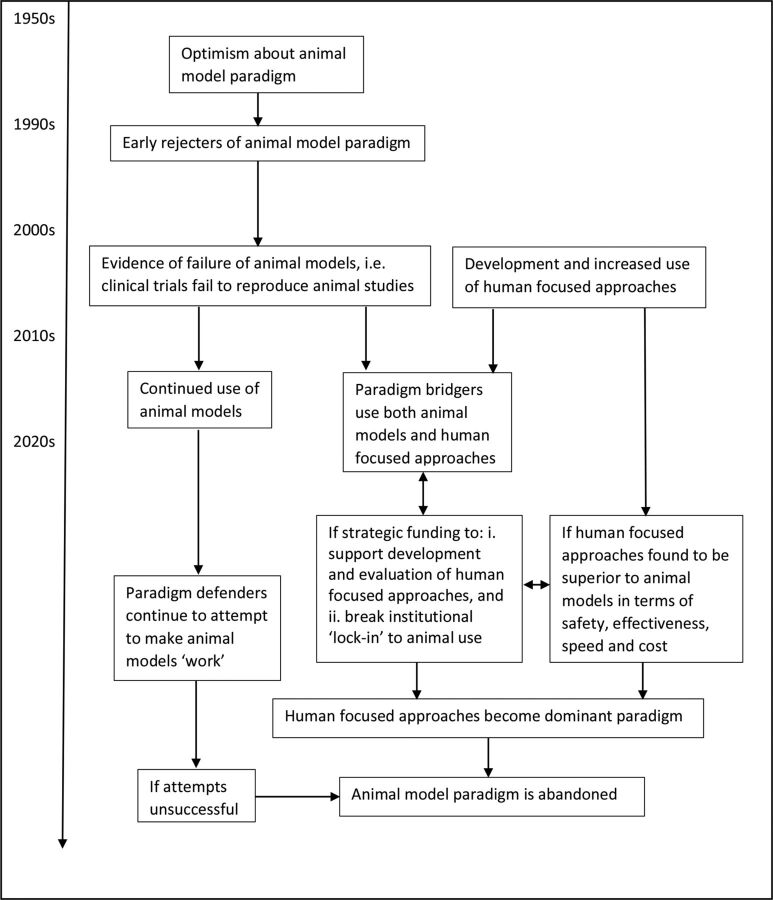
Is paradigm change likely?
With the increasing development of human-focused approaches and technologies in the field of stroke, alongside accumulating evidence of the translational failure of animal models, it seems likely that the proportion of paradigm bridgers will gradually increase. If human-focused approaches are found to be a safe and effective way forward in drug discovery and testing, and if they offer benefits in terms of speed and cost, they may eventually dominate translational stroke research. However, it seems probable that ‘paradigm defenders’ will continue for some time to try to make the animal model paradigm ‘work’ (figure 4).
Figure 4.
Paradigm change within translational stroke research.
Discussion
The systematic analysis of opinion papers is a novel methodology, and as we have shown, it can provide insight into the state of play within an academic discipline, including the range of views, the sorts of debates occurring and the direction in which the field appears to be heading. However, we recognise that papers published in established journals may not capture the full diversity of opinion within a field since they are likely to be biased towards ‘experts’ and possibly towards those with more conservative views. Nevertheless, if published opinions are more likely to be ‘establishment’ views, these probably give an accurate indication of where the field is heading and how soon it is likely to get there. Locating opinion papers can be challenging since they are not always easily identifiable and in this respect hand-searching played an important role. We feel confident that as a result of combining electronic and hand-searches, we did not miss any relevant papers; towards the end of the search process, we seemed to reach ‘saturation’ level, that is, we repeatedly came across the same papers without identifying any new ones.
We found universal agreement that translational stroke research was in crisis, as well as some robust questioning of animal models. At the same time, however, when it came to identifying the specific causes of this crisis, most researchers’ explanations did not fundamentally challenge the use of animal models, although some cited animal–human species differences and one cited a lack of human in vitro models. Similarly, most of the solutions proposed by researchers—even by those who had highlighted the problem of animal–human species differences—involved fine-tuning aspects of the existing paradigm (although there was some disagreement about whether such modifications would improve clinical translation). A small number of researchers proposed using human in vitro methods alongside animal models and one proposed using only human-focused methods. Although most advocated continued use of animal models then, there is evidence of an emerging challenge in the form of human-focused approaches.
As Kuhn14 noted, when confronted with anomalies within a paradigm, scientists tend not to renounce that paradigm but attempt to modify their theory instead. This may explain researchers’ dogged persistence with animal models despite decades of failure. Writing about the tenacity of systems of opinion within science, Fleck77 notes that contradictions may appear unthinkable and are often actively resisted, or remain ‘unseen’. Such contradictions within science have been referred to elsewhere as ‘uncomfortable knowledge’.78 Kuhn also observed that scientists tend to select problems that can be solved with theories, concepts and instruments close to those already in existence, rather than pursuing new phenomena or theories.14 This may help explain why only a minority of researchers in this study had so far begun to explore human-focused methods.
Researchers may also be reluctant to relinquish animal models due to ‘psychological lock-in’, the phenomenon of beliefs persevering in the face of contradictory evidence.79 As Frank suggests, for researchers using animal models, belief in the value of their work may have hardened as a result of being questioned about it on ethical grounds. In addition, because of the closed nature of animal research, scientists using animal models may not have been exposed to the usual diversity of academic opinion and debate, leading to beliefs and practices becoming entrenched. Furthermore, they may not perceive it to be in their interests to change; referring to ‘institutional lock-in’, Frank notes that a huge infrastructure perpetuates animal research within universities, whereby academic departments benefit from funding, professional associations, conferences and academic programmes devoted to animal research, all of which make it harder to embrace new approaches.79
Nevertheless, it seems appropriate that translational researchers consider new approaches. Animal models have been unsuccessful in the field of stroke and also in the fields of traumatic brain injury,80 motor neuron disease,81 inflammation,36 sepsis,82 central nervous system diseases,83 Alzheimer’s disease,84 85 arthritis,86 asthma,87 cancer,88 multiple sclerosis,89 myocardial infarction,90 Parkinson’s disease,90 type 1 diabetes91 and elsewhere,92 strongly suggesting that human-focused approaches might have relevance. A range of in vitro techniques has emerged and methods of culture and tissue engineering are continually evolving. With the increased availability of human cells and the development of new technologies such as microfluidic devices, it has been suggested that in vitro systems may improve the efficiency of clinical drug development and reduce drug attrition rates and their associated costs.93 94 The Medical Research Council has recently stated that it aims to fund exploration of emerging technologies such as 3D tissue models and organoids.95 Researchers using such technologies need to ensure that they attend to issues of internal and external validity, reporting and publication bias,96 otherwise they risk making the same mistakes as those conducting animal studies. Furthermore, even though the animal model paradigm has gone badly astray, Kuhn’s14 theory suggests that it will only be declared invalid if an alternate candidate is available to take its place—and an alternate candidate will only be seriously considered if it is valid, reliable and fit for purpose. Consequently, validity and reliability are of paramount importance in the development of new, human-focused approaches. The speed of any transition away from animal models will depend on the extent to which funding is redistributed, both to explore the evidence for new approaches and to break lock-in to the use of animal models. There is an urgent need to revitalise translational stroke research and pursue fresh approaches because the economic burdens of stroke are enormous97 and also because the personal costs for patients who had a stroke are huge.98–100
Conclusions
Despite frank acknowledgement that animal models have not been fruitful in the field of stroke, most researchers in the field exhibited a strong resistance to relinquishing this type of research. Nevertheless, there is evidence of an emerging challenge to the use of animal models, in the form of human-focused in vitro approaches. For the sake of stroke patients, whose needs have been unmet for so long, there is a pressing requirement to investigate the validity of these new approaches.
Footnotes
Correction notice: This article has been corrected since it was published Online First. Minor formatting has been added, and citations for supplementary files have been replaced with URLs.
Contributors: PP conceived the idea for the project, searched for the literature, analysed and interpreted the data. She wrote the first draft of the paper and revised several drafts before approving the final version. She agrees to be accountable for all aspects of the work. RR searched for the literature and contributed to data analysis. She reviewed and revised several drafts before approving the final version. She agrees to be accountable for all aspects of the work. Pound, Pandora Corresponding Author Conceptualization (Lead) Data curation (Lead) Formal analysis (Lead) Investigation (Lead) Methodology (Lead) Project administration (Lead) Validation (Equal) Writing-original draft (Lead) Writing-review & editing (Lead) Ram, Rebecca Formal analysis (Supporting) Investigation (Supporting) Validation (Equal) Writing-review & editing (Supporting).
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: Not applicable
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in Dryad data repository DOI https://doi.org/10.5061/dryad.xpnvx0kb9
This article was not formally preregistered. The data are available at https://datadryad.org/stash/dataset/doi:10.5061/dryad.xpnvx0kb9. The materials used are widely available.
Open materials: This article was not formally preregistered. The data are available at https://datadryad.org/stash/dataset/doi:10.5061/dryad.xpnvx0kb9. The materials used are widely available.
Open peer review: Prepublication and Review History is available online at http://dx.doi.org/10.1136/bmjos-2019-100041.
References
- 1.Shuaib A, Lees KR, Lyden P, et al. Nxy-059 for the treatment of acute ischemic stroke. N Engl J Med 2007;357:562–71. 10.1056/NEJMoa070240 [DOI] [PubMed] [Google Scholar]
- 2.Horn J, Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke 2001;32:570–6. 10.1161/01.str.32.2.570 [DOI] [PubMed] [Google Scholar]
- 3.Saxena R, Wijnhoud AD, Carton H, et al. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 1999;30:993–6. 10.1161/01.STR.30.5.993 [DOI] [PubMed] [Google Scholar]
- 4.Enlimomab Acute Stroke Trial Investigators . Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001;57:1428–34. 10.1212/WNL.57.8.1428 [DOI] [PubMed] [Google Scholar]
- 5.Davis SM, Lees KR, Albers GW, et al. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke 2000;31:347–54. 10.1161/01.str.31.2.347 [DOI] [PubMed] [Google Scholar]
- 6.Tirilazad International Steering Committee . Tirilazad for acute ischaemic stroke. Cochrane Database Syst Rev 2001;4. [DOI] [PubMed] [Google Scholar]
- 7.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006;59:467–77. 10.1002/ana.20741 [DOI] [PubMed] [Google Scholar]
- 8.Hill M. Personal communication. 2019.
- 9.Dirnagl U. Personal communication. 2019.
- 10.SSNAP (Sentinel Stroke National Audit Programme) . Annual Public Report 2018. SSNAP, School of Population Health and Environmental Sciences, King’s College London on behalf of the Intercollegiate Stroke Working Party, June, 2019. Available: https://www.strokeaudit.org/Documents/National/Clinical/Apr2017Mar2018/Apr2017Mar2018-AnnualReport.aspx [Accessed 2 Aug 2019].
- 11.Howells DW, Sena ES, Macleod MR. Bringing rigour to translational medicine. Nat Rev Neurol 2014;10:37–43. 10.1038/nrneurol.2013.232 [DOI] [PubMed] [Google Scholar]
- 12.McMeekin P, White P, James MA, et al. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur Stroke J 2017;2:319–26. 10.1177/2396987317733343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol 2019;18:417–8. 10.1016/S1474-4422(19)30030-4 [DOI] [PubMed] [Google Scholar]
- 14.Kuhn TS. The structure of scientific revolutions. Chicago, London: University of Chicago Press, 1962. [Google Scholar]
- 15.McArthur A, Klugarova J, Yan H, et al. Chapter 4: Systematic reviews of text and opinion. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute reviewer's manual. The Joanna Briggs Institute, 2017. https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- 16.Campbell R, Pound P, Morgan M, et al. Evaluating meta-ethnography: systematic analysis and synthesis of qualitative research. Health Technol Assess 2011;15:1–164. 10.3310/hta15430 [DOI] [PubMed] [Google Scholar]
- 17.Pound P, Campbell R. Locating and applying sociological theories of risk-taking to develop public health interventions for adolescents. Health Sociol Rev 2015;24:64–80. 10.1080/14461242.2015.1008537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiebers DO, Adams HP, Whisnant JP. Animal models of stroke: are they relevant to human disease? Stroke 1990;21:1–3. 10.1161/01.STR.21.1.1 [DOI] [PubMed] [Google Scholar]
- 19.Hsu CY. Criteria for valid preclinical trials using animal stroke models. Stroke 1993;24:633–6. 10.1161/01.STR.24.5.633 [DOI] [PubMed] [Google Scholar]
- 20.Molinari GF. Why model strokes? Stroke 1988;19:1195–7. 10.1161/01.STR.19.10.1195 [DOI] [PubMed] [Google Scholar]
- 21.Millikan C. Animal stroke models. Stroke 1992;23:795–7. 10.1161/01.STR.23.6.795 [DOI] [PubMed] [Google Scholar]
- 22.Kaste M. Use of animal models has not contributed to development of acute stroke therapies: pro. Stroke 2005;36:2323–4. 10.1161/01.STR.0000179037.82647.48 [DOI] [PubMed] [Google Scholar]
- 23.Donnan GA, Davis SM. Stroke drug development: usually, but not always, animal models. Stroke 2005;36. [DOI] [PubMed] [Google Scholar]
- 24.Pérez de la Ossa N, Dávalos A. Neuroprotection in cerebral infarction: the opportunity of new studies. Cerebrovasc Dis 2007;24:153–6. 10.1159/000107391 [DOI] [PubMed] [Google Scholar]
- 25.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the Saint trials. Ann Neurol 2007;61:396–402. 10.1002/ana.21127 [DOI] [PubMed] [Google Scholar]
- 26.Röther J. Neuroprotection does not work! Stroke 2008;39:523–4. 10.1161/STROKEAHA.107.494799 [DOI] [PubMed] [Google Scholar]
- 27.Dirnagl U, Macleod MR. Stroke research at a road block: the streets from adversity should be paved with meta-analysis and good laboratory practice. Br J Pharmacol 2009;157:1154–6. 10.1111/j.1476-5381.2009.00211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dirnagl U, Fisher M. International, multicenter randomized preclinical trials in translational stroke research: it's time to act. J Cereb Blood Flow Metab 2012;32:933–5. 10.1038/jcbfm.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howells DW, Sena ES, O'Collins V, et al. Improving the efficiency of the development of drugs for stroke. Int J Stroke 2012;7:371–7. 10.1111/j.1747-4949.2012.00805.x [DOI] [PubMed] [Google Scholar]
- 30.Dirnagl U, Endres M. Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 2014;45:1510–8. 10.1161/STROKEAHA.113.004075 [DOI] [PubMed] [Google Scholar]
- 31.Lyden P, Lapchak P. Sisyphus and translational stroke research. Sci Transl Med 2012;4:20. 10.1126/scitranslmed.3005083 [DOI] [PubMed] [Google Scholar]
- 32.Lapchak PA. Recommendations and practices to optimize stroke therapy: developing effective translational research programs. Stroke 2013;44:841–3. 10.1161/STROKEAHA.112.680439 [DOI] [PubMed] [Google Scholar]
- 33.Herson PS, Traystman RJ. Animal models of stroke: translational potential at present and in 2050. Future Neurol 2014;9:541–51. 10.2217/fnl.14.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg DA. Preclinical stroke research: gains and gaps. Stroke 2013;44:S114–5. 10.1161/STROKEAHA.113.002088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boltze J, Ayata C, Wagner D-C, et al. Preclinical phase III trials in translational stroke research: call for collective design of framework and guidelines. Stroke 2014;45. 10.1161/STROKEAHA.113.004148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013;110:3507–12. 10.1073/pnas.1222878110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Offner H. Modeling immunity and inflammation in stroke: don't be afraid of mice? Stroke 2014;45:e181–2. 10.1161/STROKEAHA.114.005642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dirnagl U. Thomas Willis Lecture: is translational stroke research broken, and if so, how can we fix it? Stroke 2016;47:2148–53. 10.1161/STROKEAHA.116.013244 [DOI] [PubMed] [Google Scholar]
- 39.Boltze J, Ayata C. Challenges and controversies in translational stroke research—an Introduction. Transl Stroke Res 2016;7:355–7. 10.1007/s12975-016-0492-4 [DOI] [PubMed] [Google Scholar]
- 40.Neuhaus AA, Couch Y, Hadley G, et al. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 2017;140:2079–92. 10.1093/brain/awx126 [DOI] [PubMed] [Google Scholar]
- 41.Zerna C, Hill MD, Boltze J. Towards improved translational stroke research: progress and perspectives of the recent National Institute of Neurological Disorders and Stroke Consensus Group meeting. Stroke 2017;48:2341–2. 10.1161/STROKEAHA.117.017906 [DOI] [PubMed] [Google Scholar]
- 42.Stroke Therapy Academic Industry Roundtable (STAIR) . Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999;30:2752–8. 10.1161/01.STR.30.12.2752 [DOI] [PubMed] [Google Scholar]
- 43.Hunter AJ, Green AR, Cross AJ. Animal models of acute ischaemic stroke: can they predict clinically successful neuroprotective drugs? Trends Pharmacol Sci 1995;16:123–8. 10.1016/S0165-6147(00)88999-3 [DOI] [PubMed] [Google Scholar]
- 44.Sena E, van der Worp HB, Howells D, et al. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 2007;30:433–9. 10.1016/j.tins.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 45.Hussain MS, Shuaib A. Research into neuroprotection must continue but with a different approach. Stroke 2008;39:521–2. 10.1161/STROKEAHA.107.494781 [DOI] [PubMed] [Google Scholar]
- 46.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009;40:2244–50. 10.1161/STROKEAHA.108.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macleod MR, Fisher M, O'Collins V, et al. Reprint: Good laboratory practice: preventing introduction of bias at the bench. J Cereb Blood Flow Metab 2009;29:221–3. 10.1038/jcbfm.2008.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tymianski M. Can molecular and cellular neuroprotection be translated into therapies for patients?: Yes, but not the way we tried it before. Stroke 2010;41:S87–90. 10.1161/STROKEAHA.110.595496 [DOI] [PubMed] [Google Scholar]
- 49.Turner RJ, Jickling GC, Sharp FR. Are underlying assumptions of current animal models of human stroke correct: from stairs to high hurdles? Transl Stroke Res 2011;2:138–43. 10.1007/s12975-011-0067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howells DW, Macleod MR. Evidence-based translational medicine. Stroke 2013;44:1466–71. 10.1161/STROKEAHA.113.000469 [DOI] [PubMed] [Google Scholar]
- 51.Donnan GA. A new road map for neuroprotection. Stroke 2008;39:242–8. [DOI] [PubMed] [Google Scholar]
- 52.Cook DJ, Tymianski M. Translating promising preclinical neuroprotective therapies to human stroke trials. Expert Rev Cardiovasc Ther 2011;9:433–49. 10.1586/erc.11.34 [DOI] [PubMed] [Google Scholar]
- 53.Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol 2008;153:S325–38. 10.1038/sj.bjp.0707594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke 2009;40:S111–4. 10.1161/STROKEAHA.108.528877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Budincevic H, Bielen I, Csiba L. Translational challenges of neuroprotection strategy in ischemic stroke. Transl Neurosci 2011;2:344–50. 10.2478/s13380-011-0041-2 [DOI] [Google Scholar]
- 56.Minnerup J, Sutherland BA, Buchan AM, et al. Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 2012;13:11753–72. 10.3390/ijms130911753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutherland BA, Minnerup J, Balami JS, et al. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke 2012;7:407–18. 10.1111/j.1747-4949.2012.00770.x [DOI] [PubMed] [Google Scholar]
- 58.Neuhaus AA, Rabie T, Sutherland BA, et al. Importance of preclinical research in the development of neuroprotective strategies for ischemic stroke. JAMA Neurol 2014;71:634–9. 10.1001/jamaneurol.2013.6299 [DOI] [PubMed] [Google Scholar]
- 59.Antonic A, Sena ES, Donnan GA, et al. Human in vitro models of ischaemic stroke: a test bed for translation. Transl Stroke Res 2012;3:306–9. 10.1007/s12975-012-0201-x [DOI] [PubMed] [Google Scholar]
- 60.del Zoppo GJ. Clinical trials in acute stroke: why have they not been successful? Neurology 1998;51:S59–61. 10.1212/WNL.51.3_Suppl_3.S59 [DOI] [PubMed] [Google Scholar]
- 61.Hoyte L, Kaur J, Buchan AM. Lost in translation: taking neuroprotection from animal models to clinical trials. Exp Neurol 2004;188:200–4. 10.1016/j.expneurol.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 62.Hemorrhagic Stroke Academia Industry (HEADS) Roundtable Participants . Basic and translational research in intracerebral hemorrhage: limitations, priorities, and recommendations. Stroke 2018;49:1308–14. 10.1161/STROKEAHA.117.019539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Zoppo GJ. Why do all drugs work in animals but none in stroke patients? 1. Drugs promoting cerebral blood flow. J Intern Med 1995;237:79–88. 10.1111/j.1365-2796.1995.tb01144.x [DOI] [PubMed] [Google Scholar]
- 64.Gladstone DJ, Black SE, Hakim AM, et al. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 2002;33:2123–36. 10.1161/01.str.0000025518.34157.51 [DOI] [PubMed] [Google Scholar]
- 65.Dirnagl U, Hakim A, Macleod M, et al. A concerted appeal for international cooperation in preclinical stroke research. Stroke 2013;44:1754–60. 10.1161/STROKEAHA.113.000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu S-Y, Pan S-Y. The failure of animal models of neuroprotection in acute ischemic stroke to translate to clinical efficacy. Med Sci Monit Basic Res 2013;19:37. 10.12659/msmbr.883750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki H, Nakano F. To improve translational research in subarachnoid hemorrhage. Transl Stroke Res 2018;9:1–3. 10.1007/s12975-017-0546-2 [DOI] [PubMed] [Google Scholar]
- 70.Sharp FR, Jickling GC. Modelling immunity and inflammation in stroke: differences between rodents and humans? Stroke 2014;45:178–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeGraba TJ, Pettigrew LC. Why do neuroprotective drugs work in animals but not humans? Neurol Clin 2000;18:475–93. 10.1016/S0733-8619(05)70203-6 [DOI] [PubMed] [Google Scholar]
- 70.Sharp FR, Jickling GC, Dirnagl U. Modeling immunity and inflammation in stroke: differences between rodents and humans? 10.1161/STROKEAHA.114.005639 [DOI] [PMC free article] [PubMed]
- 71.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx 2004;1:36–45. 10.1602/neurorx.1.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waltz AG. Clinical relevance of models of cerebral ischemia. Stroke 1979;10:211–3. 10.1161/01.STR.10.2.211 [DOI] [PubMed] [Google Scholar]
- 73.Ritzer G. Sociology: a multiple paradigm science. Boston: Allyn and Bacon, 1975. [Google Scholar]
- 74.Davis SM, Donnan GA. Neuroprotection: establishing proof of concept in human stroke. Stroke 2002;33:309–10. 10.1161/str.33.1.309 [DOI] [PubMed] [Google Scholar]
- 75.Donnan GA, Davis SM. Neuroprotection: still achievable in humans. Stroke 2008;39. 10.1161/STROKEAHA.107.494807 [DOI] [PubMed] [Google Scholar]
- 76.Bosetti F, Koenig JI, Ayata C, et al. Translational stroke research: vision and opportunities. Stroke 2017;48:2632–7. 10.1161/STROKEAHA.117.017112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fleck L. Genesis and development of a scientific fact. London: University of Chicago Press, 1981. [Google Scholar]
- 78.Rayner S. Uncomfortable knowledge: the social construction of ignorance in science and environmental policy discourses. Econ Soc 2012;41:107–25. 10.1080/03085147.2011.637335 [DOI] [Google Scholar]
- 79.Frank J, lock-in T. Technological lock-in, positive institutional feedback, and research on laboratory animals. Structural Change and Economic Dynamics 2005;16:557–75. 10.1016/j.strueco.2004.11.001 [DOI] [Google Scholar]
- 80.Pound P. The search for drug treatments after traumatic brain injury: problems with animal models. In: Johnson L, Fenton A, Shriver A, eds. Neuroethics and nonhuman animals. Springer, 2019. [Google Scholar]
- 81.Perrin S. Make mouse studies work. Nature 2014;507:423–5. [DOI] [PubMed] [Google Scholar]
- 82.Leist M, Hartung T. Inflammatory findings on species extrapolations: humans are definitely no 70-kg mice. Arch Toxicol 2013;87:563–7. 10.1007/s00204-013-1038-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geerts H. Of mice and men: bridging the translational disconnect in CNS drug discovery. CNS Drugs 2009;23:915–26. 10.2165/11310890-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 84.Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 2014;6. 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pippin JE, Cavanaugh SE, Pistollato F. Animal research for Alzheimer disease: failures of science and ethics. In: Herrmann K, Jayne K, eds. Animal experimentation. Working towards a paradigm change, 2019. [Google Scholar]
- 86.McNamee K, Williams R, Seed M. Animal models of rheumatoid arthritis: how informative are they? Eur J Pharmacol 2015;759:278–86. 10.1016/j.ejphar.2015.03.047 [DOI] [PubMed] [Google Scholar]
- 87.Mullane K, Williams M. Animal models of asthma: reprise or reboot? Biochem Pharmacol 2014;87:131–9. 10.1016/j.bcp.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 88.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014;6:114. [PMC free article] [PubMed] [Google Scholar]
- 89.Vesterinen HM, Sena ES, ffrench-Constant C, et al. Improving the translational hit of experimental treatments in multiple sclerosis. Mult Scler 2010;16:1044–55. 10.1177/1352458510379612 [DOI] [PubMed] [Google Scholar]
- 90.van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med 2010;7:1000245. 10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol 2004;4:989–97. 10.1038/nri1502 [DOI] [PubMed] [Google Scholar]
- 92.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 2004;3:711–6. 10.1038/nrd1470 [DOI] [PubMed] [Google Scholar]
- 93.Innovate UK . Non-animal technologies: new vision, strategy and roadmap for UK. Innovate UK: Emerging and enabling technologies, research and development, 2015. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/474558/Roadmap_NonAnimalTech_final_09Nov2015.pdf [Accessed 17 May 2019].
- 94.BioIndustry Association and the Medicines Discovery Catapult . State of the discovery nation 2018 and the role of the Medicines Discovery Catapult, 2018. Available: https://s3-eu-west-1.amazonaws.com/media.newmd.catapult/wp-content/uploads/2018/01/16220811/MDC10529-Thought-Leader_v10_Interactive_v1.pdf
- 95.Medical Research Council . Delivery Plan 2019, 2019. Available: https://www.ukri.org/files/about/dps/mrc-dp-2019/ [Accessed 6 Dec 2019].
- 96.Steckler T, Macleod M. Drug discovery and preclinical drug development—have animal studies really failed? BMJ Open Science Blog 2019. [Google Scholar]
- 97.Xu X-M, Vestesson E, Paley L, et al. The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J 2018;3:82–91. 10.1177/2396987317746516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pound P, Gompertz P, Ebrahim S. A patient-centred study of the consequences of stroke. Clin Rehabil 1998;12:338–47. 10.1191/026921598677661555 [DOI] [PubMed] [Google Scholar]
- 99.Pound P, Gompertz P, Ebrahim S. Illness in the context of older age: the case of stroke. Sociol Health Illn 1998;20:489–506. 10.1111/1467-9566.00112 [DOI] [Google Scholar]
- 100.Pound P, Bury M, Gompertz P, et al. Stroke patients' views on their admission to hospital. BMJ 1995;311:18–22. 10.1136/bmj.311.6996.18 [DOI] [PMC free article] [PubMed] [Google Scholar]