Abstract
Bacillus coagulans (B. coagulans) have proven to be effective in improving the development of gut immunity and microbiome, and offering protection against pathogens, especially in young animals. The newborn chicks are highly vulnerable to the foodborne pathogenic Salmonella infections, leading to high mortality and economic loss. However, whether B. coagulans can protect young chickens from Salmonella-induced intestinal mucosal damage by modulating the development of intestinal epithelium remains unclear. In this study, B. coagulans with excellent anti-Salmonella property was selected and used. The results showed that B. coagulans alleviated the morphological damage, intestinal inflammation and body weight loss caused by Salmonella enteritidis (S. enteritidis) infections. B. coagulans significantly increased the crypt depth. Furthermore, the goblet cell loss and downregulating of mucin 2 induced by S. enteritidis were all relieved by B. coagulans treatment. Consistently, the expression of the related genes of Notch signaling pathway was also upregulated in the S. enteritidis group but inhibited by B. coagulans. In addition, B. coagulans improved the levels of immunoglobulin A, superoxide dismutase, total antioxidant capacity, and avian beta-defensin 2 in the intestinal mucosa. This study demonstrated that B. coagulans could regulate the development of intestinal epithelium, protect the intestinal barrier, thus relieve infections with S. enteritidis in chicks, which can be used as alternatives to antibiotics in poultry feed.
Key words: Bacillus coagulans, chick, goblet cell, intestinal mucosal barrier, Salmonella enteritidis
INTRODUCTION
Salmonella enteritidis (S. enteritidis) is one of the most frequently isolated foodborne pathogens, which has a wide range of hosts, including humans and various animals (Eng et al., 2015; Jajere, 2019). Over 2,500 Salmonella serotypes have been identified, and more than half of them belong to S. enteritidis, which causes death and great economic loss in poultry (Wang et al., 2020a; Ehuwa et al., 2021). Moreover, contaminated poultry products as carriers of S. enteritidis also pose serious threat to human health, which accounting for millions of foodborne illnesses and deaths per year (Sylejmani et al., 2016; Heredia and Garcia, 2018). In the past, various antibiotics were used in feed to treat and prevent Salmonella infections in poultry. However, the emergence of multiantibiotics-resistant Salmonella serotypes makes the infections harder to control, as reflected by increased severity and mortality in infected patients and animals (Eng et al., 2015; Jajere, 2019). Therefore, an alternative approach for controlling infections is urgently needed.
The use of probiotics has represented a promising approach to control varieties of diseases and Salmonella infections (Abdel-Daim et al., 2013). Supplementation of Enterococcus Faecium, Lactobacillus salivarius, Lactobacillus reuteri, and Bacillus coagulans (B. coagulans) in the feeds of chickens have been proven to be efficient in improving growth performance and in preventing the colonization of pathogens (Brisbin et al., 2015; Jha et al., 2020; He et al., 2021). B. coagulans is a lactic-acid producing bacterium with the properties of spore forming and microaerophilic, which allow it to better survive stomach acids and adapt to a low oxygen intestinal environment and inhibit the pathogens (Konuray and Erginkaya, 2018). Studies have shown that B. coagulans could improve feed conversion, intestinal morphology, strengthen immune responses and antagonize the pathogenic microbes (Zhen et al., 2018; Zhang et al., 2021). Although B. coagulans has a positive effect on gut health, it has rarely been reported before whether it can protect poultry from intestinal mucosal damage by modulating the development of intestinal epithelium.
The intestinal mucosal barrier plays a key role in separating harmful substances such as bacteria and toxins from the internal milieu (Turner, 2009). Microbiota-epithelial interactions in the early life window profoundly affect the establishment of intestinal barrier function and host immune system (Hughes et al., 2020; Yeşilyurt et al., 2021). Moreover, the early colonization of beneficial bacteria could promote the maturation of gut microbiota and competitive exclusion of enteric pathogens (Pickard et al., 2017). As the components of intestinal mucosal barrier, intestinal stem cells (ISCs) continuously proliferate and replace the damaged intestinal epithelial cells and are regulated by the Notch signaling pathway to differentiate into mucus-secreting goblet cells to maintain the intestinal mucosal barrier (Umar, 2010; Hou et al., 2017). Our previous studies have indicated that Lactobacillus modulated the ISCs to recover damaged intestinal mucosa (Hou et al., 2018). However, the interaction between B. coagulans and intestinal epithelium development and whether the interaction has the repairment effect on intestinal mucosal damage caused by S. enteritidis in young chickens are still unclear. In this study, a strain of B. coagulans with excellent antibacterial properties was selected. We showed that B. coagulans had a critical role in the proliferation and differentiation of young chicken intestinal epithelial cells to improve the intestinal mucosal damage by S. enteritidis.
MATERIALS AND METHODS
Assay of the Antimicrobial Activity
All the bacteria except B. coagulans used in this study are stored in our lab, and B. coagulans was kindly supplied by Wecare Probiotics Co., Ltd. The 8 probiotic strains numbered A, B, C, D, E, F, G and H were grown in de Man, Rogosa, and Sharpe medium (MRS) at 37°C, while S. pullorum, S. typhimurium, and S. enteritidis were cultured in Luria-Bertani (LB) medium at 37°C. Strain No. D, namely B. coagulans, was used in the animal experiments.
The antimicrobial activity of probiotics against S. pullorum, S. typhimurium, and S. enteritidis was determined using the well-diffusion assay (Lima et al., 2007). The overnight cultures of the indicator strains were used to inoculate LB agar medium (approximately 106 cells mL−1 of each indicator isolate), followed by embedding with sterilized Oxford cups into the LB agar plate. The Oxford cups were added 150 μL (108 CFU/mL) probiotics liquid culture or 150 μL MRS liquid medium as the negative control (N). The diameter of the inhibition zone was determined after 24 h incubation at 37°C. The area of the inhibition zone was calculated and then calibrated with the negative control values.
Animal Experimental Design
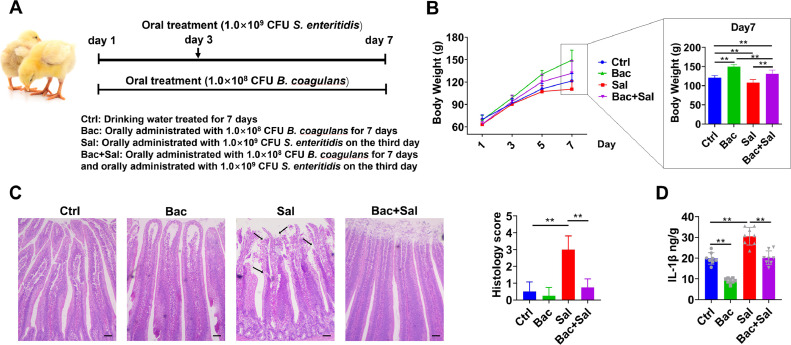
Animal care, slaughter and experimental procedures and design were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (PTA2020023). A total of 120 newly hatched Arbor acres broiler chickens were randomly divided into 4 groups: the control group (Ctrl), B. coagulans treatment group (Bac), S. enteritidis treatment group (Sal), and B. coagulans and S. enteritidis treatment group (Bac+Sal). Each group contained 3 replicates with 10 chicks per replicate. Chicks were orally administrated with 200 μL B. coagulans (1.0 × 108 CFU/mL) once a day, for a period of 7 d and were orally treated with 200 μL S. enteritidis (1.0 × 109 CFU/mL) on the third day. The details of chick experimental design are illustrated in Figure 2A. Water and diet were provided ad libitum. Fifteen chicks (5 chicks per pen) of each group were randomly selected for the document of body weight every 2 d. On the seventh day, 8 chicks (2 or 3 chicks per pen) of each treatment were randomly selected and euthanized, and the jejunum segments were sampled. All the data obtained by qRT-PCR and ELISA were accessed from these 8 chicks. And 6 chicks (2 chicks per pen) among the 8 chicks of each group were used for histomorphology observation.
Figure 2.
.B. coagulans ameliorates S. enteritidis-induced intestinal mucosal damage in chicks. (A) Newborn chicks were administrated with drinking water or B. coagulans (1.0 × 108 CFU/mL) suspended in drinking water once a day, for a period of 7 d. Chicks were orally administered with S. enteritidis (1.0 × 109 CFU/mL) on the third day. On the 7th day, chicks were sacrificed for subsequent experiments. (B) The body weight changes of chicks were monitored every 2 d; n = 15 per group. (C) Histopathological changes in jejunum tissues were examined by HE staining, and scoring was performed as described in the Materials and Methods (scale bar =50 μm). The areas marked by the arrows were the exfoliation of intestinal epithelial cells caused by S. enteritidis; n = 6 per group. (D) The concentration of IL-β in jejunum tissues was detected using an ELISA kit; n = 8 per group. Data are presented as the mean ± SDs. *P < 0.05, **P < 0.01.
Goblet cells in the jejunum segments were stained with periodic acid-Schiff (PAS) stain. The number of PAS+ cells and the crypts depth in jejunum were measured from 8 to 1 randomly selected villus and crypt, with one section per chicks, and were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD). Histopathological changes and scores were detected and quantified under light microscopy according to previous study (de Koning et al., 2006).
Detection of Cytokines and Proteins in Intestinal Mucosa
The jejunum tissues were collected, homogenized, and centrifuged. Then the supernatant was collected for cytokines level and intestinal antioxidant analysis. The levels of IL-1β and IgA were measured using the ELISA kits according to the manufacturer's protocol (Jiangsu Meibiao Biotechnology Co., Ltd, Yancheng, China). The levels of total antioxidant capacity (T-AOC) and superoxide dismutase (SOD) were determined using assay kits (Nanjing Jiancheng Institute of Bioengineering and Technology, Nanjing, China). Cytokine's content was standardized to the total protein in each sample. The total protein concentrations of jejunum tissues were measured by a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). The optical density of all reactions was measured in an ELISA reader (FC, Thermo).
Quantitative RT‑PCR
Total RNA from the jejunum was extracted with RNAiso Plus (Takara, Dalian, China) and quantified by measuring absorbance at 260 nm using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). One microgram of total RNA was treated for reverse transcription with a PrimeScript RT Reagent Kit (Takara, Dalian, China) in accordance with the manufacturer's instructions. Quantification of the target genes mucin 2 (Muc2), delta-like-1 (Dll1), Notch1, hairy and enhancer of split-1 (Hes1), Avian beta-defensin 2 (AvBD2) and a housekeeping gene (GAPDH) in cDNA samples was carried out by fluorometric real-time PCR using a 7500-fluorescence detection system (Applied Biosystems, Carlsbad, CA) and SYBR-Green PCR kits (Takara, Dalian, China). The thermal cycling conditions comprised 5 min at 95°C and then 40 cycles of 95°C for 10 s and 60°C for 34 s, followed by a standard melting curve analysis. The 2−ΔΔCt method was used to analyze gene expression levels (Livak and Schmittgen, 2001). The fold change value was calculated for a gene expressed in the experimental vs control condition. The primer sets listed in Table 1.
Table 1.
qPCR primer sequences.
| Product | Sequence (5’-3’) | Reference or accession no. |
|---|---|---|
| GAPDH | F: TGATGGTCCACATGGCATCC | NM_204305.1 |
| GAPDH | R: GGGAACAGAACTGGCCTCTC | |
| Muc2 | F: ATTGTGGTAACACCAACATTCATC | (Li et al., 2017) |
| Muc2 | R: CTTTATAATGTCAGCACCAACTTCTC | |
| AvBD2 | F: TTTCTCCAGGGTTGTCTTCG | (Ateya et al., 2019) |
| AvBD2 | R: AGCAGCTTCCGACTTTGATT | |
| Notch1 | F: CTCAACTGCCAGAACTTGGTG | XM_015279325.3 |
| Notch1 | R: CTGAGTTCCTGCAGAGATGAGC | |
| Hes1 | F: CACCGGAAGTCCTCCAAACC | NM_001005848.2 |
| Hes1 | R: GAGGTTCCTCAGGTGCTTCAC | |
| Dll1 | F: TGAACTACTGCACTCACCACAA | NM_204973.2 |
| Dll1 | R: TCGTTGATTTCAATCTCGCAGC |
Statistical Analysis
The results are expressed as the means ± SDs. The data were subjected to ANOVA after the determination of variance homogeneity by using SPSS 16.0 software. Significant differences among the means were determined by LSD post-tests at *P < 0.05, **P < 0.01, and ***P < 0.001, ns P >0.05, no difference.
RESULTS
The Screening for Prospective Probiotics Against Salmonella in Vitro
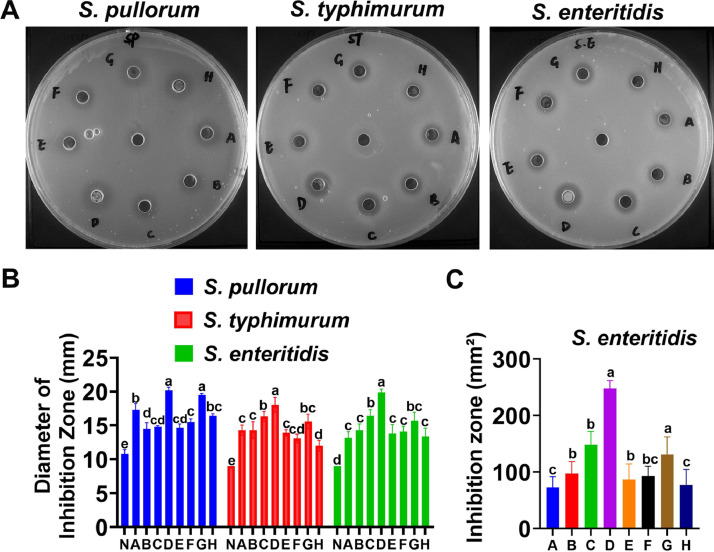
First, the antibacterial capacity of 8 probiotic strains (Strain no. A, B, C, D, E, F, G and H) were detected. The diameter of inhibition zone results showed that compared with negative control (N), most strains have the inhibiting effect on S. pullorum, S. typhimurium and S. enteritidis, whereas the inhibition zone of Strain no. D is clear and more prominent than the others, especially on the S. enteritidis (Figure 1A-C). The results suggested that Strain no. D, namely B. coagulans have the antibacterial potential.
Figure 1.
The screening for prospective probiotics against Salmonella in vitro. (A) LB agar plates containing S. pullorum, S. typhimurium and S. enteritidis. Probiotic isolates No. A, B, C, D, E, F, G, H are arranged clockwise in sequence on each plate, and the center plate is the negative control (N). (B-C), The diameter and area of the inhibition zone indicates the antibacterial activity of different isolates; n = 6 per group. Different letters indicate a significant difference (P < 0.05); otherwise, no difference.
B. coagulans Ameliorates S. enteritidis-Induced Intestinal Mucosal Damage in Chicks
The microorganisms are colonized outside the intestinal epithelial cells, which constitute as a physical barrier and act as the first line of defense against noxious luminal stimuli (Vancamelbeke and Vermeire, 2017; Schoultz and Keita, 2020). In this study, the newborn chicks were administrated with 1.0 × 108 CFU/mL B. coagulans for consecutive 7 d, followed by 1.0 × 109 CFU/mL S. enteritidis on the third day (Figure 2A). The results showed that B. coagulans had significantly improved effect on body weight gain and simultaneously inhibited the body weight loss caused by S. enteritidis, especially on 7 d old chicks (Figure 2B). Moreover, S. enteritidis infections caused severe pathological damages with the exfoliation of intestinal epithelial cells as well as the significantly higher levels of IL-1β, whereas B. coagulans could maintain the physiological integrity and relieve morphological damage of intestinal epithelium (Figure 2C,D).
The Inductive Effect of B. coagulans on Intestinal Proliferation and Differentiation
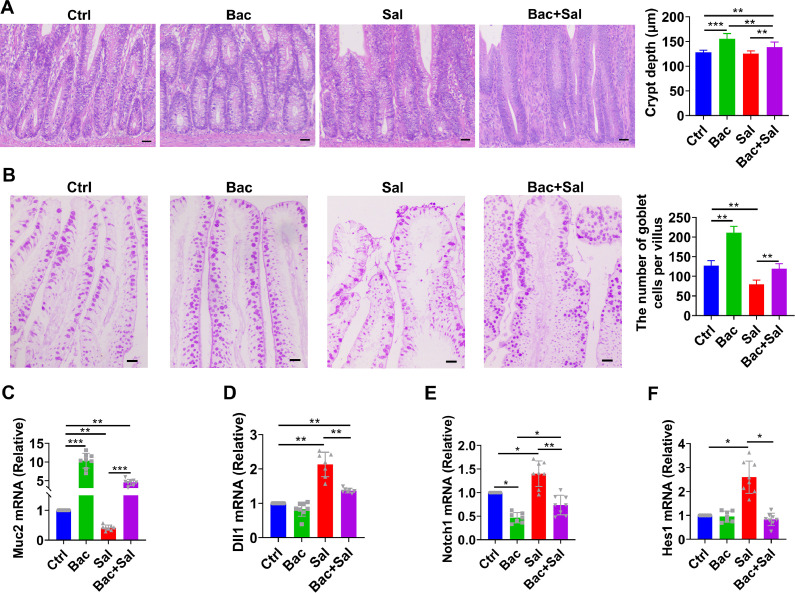
The ISCs in the crypt base can continuously proliferate and migrate to the villus to replace the damaged intestinal epithelial cells, and differentiate into goblet cells which secret mucus and antimicrobial peptides to guarantee the effective absorption of nutrients and resist enteropathogens invasion (Turner, 2009; Umar, 2010). Our results showed that the crypt depth was increased in B. coagulans treated groups (Bac, Bac+Sal) (Figure 3A). Interestingly, the number of pink secretory granules with the PAS staining in villus was also observed to populate after being treated with B. coagulans. In contrast, the number in S. enteritidis treated group decreased (Figure 3B), which meant B. coagulans could recover the goblet cells loss reduced by S. enteritidis. Meanwhile, B. coagulans upregulated the gene expression of Muc2 (Figure 3C). Furthermore, the gene expressions of the notch signaling pathway (Dll1, Notch1 and Hes1) related to goblet cells differentiation were upregulated by S. enteritidis, but were inhibited by being treated with B. coagulans together (Figure 3D–F).
Figure 3.
The inductive effect of B. coagulans on intestinal proliferation and differentiation. (A) The representative histology of jejunum showing crypt depth with HE staining in chicks; n = 6 per group. (B) Jejunum sections were stained with PAS stain and the graph shows the number of goblet cells per villus; n = 6 per group. (scale bar =50 μm). (C-F) Muc2, Dll1, Notch1, and Hes1 mRNA levels of homogenized jejunum in chicks were determined by quantitative RT-PCR and normalized to the expression of GAPDH; n = 8 per group. Data are presented as the mean ± SDs. *P < 0.05, **P < 0.01, ***P < 0.001.
Enhancement of Innate Mucosal Immunity and Intestinal Antioxidant Capacity With B. coagulans Against S. enteritidis
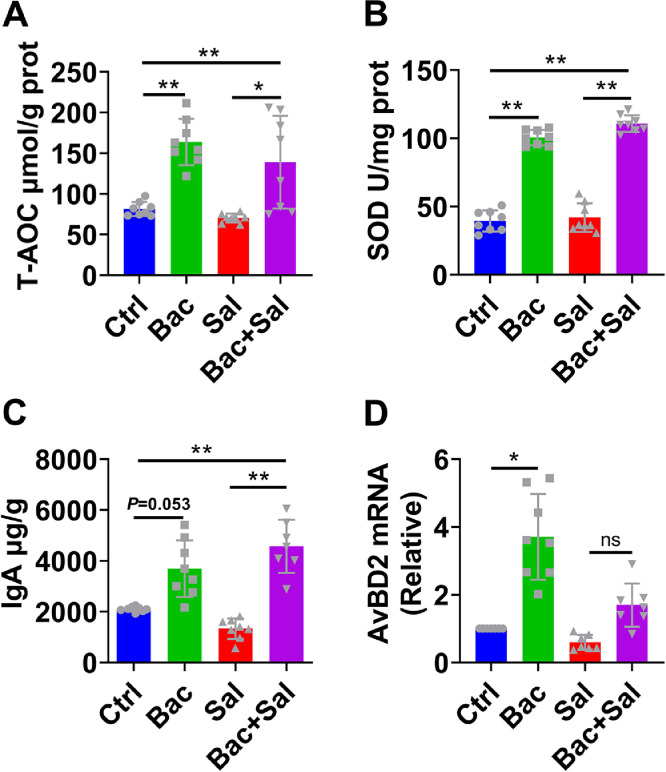
Intestinal innate immunity and antioxidant capacity are the critical parts of defense against invasion of pathogens (Kinnebrew and Pamer, 2012; Tian et al., 2017). Oxidative stress caused by noxious stimuli such as S. enteritidis is destructive to the GI tract, intestinal antioxidant defenses can counteract the adverse effects. We found that the levels of T-AOC and SOD in jejunum of B. coagulans treated groups were remarkably higher than the control or S. enteritidis treated group (Figure 4A,B), which indicated the improved intestinal antioxidant capacity. Moreover, our data indicated that B. coagulans could remarkably stimulate IgA secretion and increase the gene expression of AvBD2 in both Bac and Bac+Sal groups (Figure 4C,D). These 2 factors play the key role in antibacterial activities.
Figure 4.
Enhancement of innate mucosal immunity and intestinal antioxidant capacity with B. coagulans against S. enteritidis. (A,B), the levels of T-AOC and SOD in the jejunum tissues were determined using assay kits. (C) The concentration of IgA in jejunum tissues was detected using an ELISA kit. (D) AvBD2 mRNA level of homogenized jejunum in chicks was determined by quantitative RT-PCR and normalized to the expression of GAPDH; n = 8 per group. Data are presented as the mean ± SDs; ns P > 0.05 (not significant), *P < 0.05, **P < 0.01.
DISCUSSION
S. enteritidis is a leading pathogen that causes poultry enteric disease resulting in severe diarrhea (Eng et al., 2015). The pathogenic microorganisms are ingested in the gut through contact with contaminated food or water, then colonize, interact with the intestinal epithelial cells and breach them, then penetrate into the deep tissues, thus causing significant damage to the intestinal mucosal barrier (Pucciarelli and Garcia-del Portillo, 2017; Ehuwa et al., 2021). In young animals, especially the newly hatched chicks, are considered at high risk of infections, because of the immaturity of their immune system, underdeveloped gut microbiota and intestinal mucosal barrier (Neveling et al., 2020; Westrom et al., 2020). In the past, antibiotics were usually used to control the infections and diseases of Salmonella until the occurrence of multi-drug-resistant Salmonella strains and the problem about antibiotic residues, which makes the search for alternatives to antibiotics an urgent need (Eng et al., 2015; Jajere, 2019). One of the promising alternative control approaches is the possible beneficial use of probiotics against various pathogens. The intestine of a newly hatched chick is relatively sterile, the colonization of the probiotics in the GI tract begins immediately after birth and goes much more easily and smoothly (Haberecht et al., 2020). The colonization of probiotics can enhance intestinal health by preventing enteric pathogens from colonizing in the intestine, stimulating the development of healthy microbiota, increasing digestive capacity, lowering the pH, improving mucosal immunity and modulating the renewal and repairment of ISCs (Uyeno et al., 2015; Hou et al., 2018). B. coagulans is a kind of probiotic with the properties of lactic-acid producing, spores forming, and has the ability to maintain intestinal homeostasis, but has higher resistance against the environment than other types of probiotics such as Lactobacillus, which makes B. coagulans more suitable for industrial production and feed additive processing (Konuray and Erginkaya, 2018; Cao et al., 2020). However, there are few studies on B. coagulans in young chickens, especially on whether B. coagulans can protect poultry from intestinal mucosal damage with S. enteritidis infections. Most previously published papers have verified the probiotic effect of B. coagulans on feed digestibility and availability, growth performance, intestinal morphology, gut microbiota balance, among others (Hung et al., 2012; Li et al., 2018; Wu et al., 2018). However, the interaction between B. coagulans and intestinal epithelium development and whether the interaction has a repairment effect on intestinal mucosal damage caused by S. enteritidis in young chickens, are still unclear.
The probiotics usually prevent pathogens from growth and colonization to keep health, which defined the antibacterial property as one of the evaluation criterion of good probiotics (Guo et al., 2006). In this study, a strain of B. coagulans was selected to use in subsequent chickens’ study because of its inhibition zone area on the growth of Salmonella, especially on S. enteritidis which could reach as much as 250 mm2. In comparison, the inhibitory zone area of Bifidobacteria on growth of S. enteritidis is only 170 mm2 (Rahimifard, 2016). The results indicated that B. coagulans exerted a positive role in controlling S. enteritidis in vitro. As for the in vivo study in young chickens, B. coagulans could maintain the integrity of villus and alleviated the intestinal mucosal damage caused by S. enteritidis, including morphological damage, exfoliation of intestinal epithelial cells, alleviation of body weight loss, and intestinal inflammation. These results agreed with findings from others, showing that the use of B. coagulans had a protective effect in facing the challenge of pathogens. However, the underlying mechanism has nearly not been uncovered (Zhen et al., 2018).
Self-renewal and damage-repair in the intestinal epithelium cells is fueled by a population of ISCs in the crypt base that give rise to daughter or progenitor cells, which can subsequently differentiate into the mature cell types required for normal gut function, such as the mucus secreting-goblet cell, which is the vital part in intestinal mucosal barrier (Umar, 2010; Vancamelbeke and Vermeire, 2017). Lactobacillus could modulate ISCs and stimulate gut epithelium proliferation and self-renewal, manifested with the deeper intestinal crypts, thereby promoting the repairment of damage caused by adverse factors (Hou et al., 2017; Allahdo et al., 2018; Hou et al., 2018). Consistent with that, we found B. coagulans significantly increased the crypt depth, which possibly improved intestinal damage repairment capacity when facing infections. In addition, inhibition of the Notch pathway could direct ISCs differentiation into goblet cells and induce the secretion of mucus and antimicrobial peptides, which is of great significance to resist the damage caused by pathogens, heavy metals and other harmful substances (Kim and Khan, 2013; Xie et al., 2020). The previous study has shown that B. coagulans can restore the loss of goblet cells induced by S. enteritidis (Zhen et al., 2018). Our study supported these results and further found that S. enteritidis reduced the number of goblet cells by activating the Notch signaling pathway, while B. coagulans could inhibit the over-activation of the Notch signaling pathway, thus restoring the number of goblet cells, which explains the effect of B. coagulans against S. enteritidis infections.
Intestinal antioxidant capacity and innate mucosal immunity are the critical components of the intestinal mucosal barrier (Kurashima et al., 2013; Tian et al., 2017). Superoxide dismutase (SOD) and T-AOC, as the essential antioxidants, serve as an oxidative stress barrier in the intestine.
It was reported that Lactobacillus protected the intestinal barrier and attenuated oxidative stress in colitis by producing bacterial SOD (Nakagawa and Miyazaki, 2017). Metabolites of gut microbiota like GSH and butyrate are beneficial to intestinal health owing to their antioxidant properties (Wang et al., 2020b). In this study, we found that B. coagulans increased the levels of SOD and T-AOC, which could kill the harmful bacteria, as indicated in other results. Avian β-defensins and IgA play crucial roles in the innate immune response in chicks. B. coagulans increased AvBD2 and IgA expression, which may be necessary for protection against Salmonella infections in young chickens.
In summary, this study demonstrated that B. coagulans, with antibacterial activity, could relieve intestinal mucosal damage caused by S. enteritidis by inducing differentiation into goblet cells, which were modulated by Notch signaling pathways to strengthen the intestinal mucosal barrier in young chickens.
ACKNOWLEDGMENTS
This study was supported by the Chinese Academy of Agricultural Sciences [SKLVEB2019KFKT004]; Fundamental Research Funds for the Central Universities (JCQY201906), National Natural Science Foundation of China (31972631) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
DISCLOSURES
There are no conflicts of interest (financial, professional or personal) related to this manuscript.
REFERENCES
- Abdel-Daim A., Hassouna N., Hafez M., Ashor M.S., Aboulwafa M.M. Antagonistic activity of Lactobacillus isolates against Salmonella typhi in vitro. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/680605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahdo P., Ghodraty J., Zarghi H., Saadatfar Z., Kermanshahi H., Edalatian Dovom M.R. Effect of probiotic and vinegar on growth performance, meat yields, immune responses, and small intestine morphology of broiler chickens. Italian J. Anim. Sci. 2018;17:675–685. [Google Scholar]
- Ateya A.I., Arafat N., Saleh R.M., Ghanem H.M., Naguib D., Radwan H.A., Elseady Y.Y. Intestinal gene expressions in broiler chickens infected with Escherichia coli and dietary supplemented with probiotic, acidifier and synbiotic. Vet. Res. Commun. 2019;43:131–142. doi: 10.1007/s11259-019-09753-z. [DOI] [PubMed] [Google Scholar]
- Brisbin J.T., Davidge L., Roshdieh A., Sharif S. Characterization of the effects of three Lactobacillus species on the function of chicken macrophages. Res. Vet. Sci. 2015;100:39–44. doi: 10.1016/j.rvsc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Cao J., Yu Z.M., Liu W.Y., Zhao J.X., Zhang H., Zhai Q.X., Chen W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods. 2020;64 ARTN 103643. [Google Scholar]
- de Koning B.A., van Dieren J.M., Lindenbergh-Kortleve D.J., van der Sluis M., Matsumoto T., Yamaguchi K., Einerhand A.W., Samsom J.N., Pieters R., Nieuwenhuis E.E. Contributions of mucosal immune cells to methotrexate-induced mucositis. Int. Immunol. 2006;18:941–949. doi: 10.1093/intimm/dxl030. [DOI] [PubMed] [Google Scholar]
- Ehuwa O., Jaiswal A.K., Jaiswal S. Salmonella, food safety and food handling practices. Foods. 2021;10:907. doi: 10.3390/foods10050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng S.-K., Pusparajah P., Mutalib N.-S.Ab, Ser H.-L., Chan K.-G., Lee L.-H. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015;8:284–293. [Google Scholar]
- Guo X.H., Li D.F., Lu W.Q., Piao X.S., Chen X.L. Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Anton. Leeuw. Int. J. G. 2006;90:139–146. doi: 10.1007/s10482-006-9067-9. [DOI] [PubMed] [Google Scholar]
- Haberecht S., Bajagai Y.S., Moore R.J., Van T.T.H., Stanley D. Poultry feeds carry diverse microbial communities that influence chicken intestinal microbiota colonisation and maturation. Amb. Express. 2020;10 doi: 10.1186/s13568-020-01077-5. ARTN 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Liu X., Dong Y.Y., Lei J.Q., Ito K., Zhang B.K. Enterococcus faecium PNC01 isolated from the intestinal mucosa of chicken as an alternative for antibiotics to reduce feed conversion rate in broiler chickens. Microb. Cell Fact. 2021;20 doi: 10.1186/s12934-021-01609-z. ARTN 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia N., Garcia S. Animals as sources of food-borne pathogens: a review. Anim. Nutr. 2018;4:250–255. doi: 10.1016/j.aninu.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Ye L., Huang L., Yu Q. The research progress on intestinal stem cells and itts relationship with intestinal microbiota. Front. Immunol. 2017;8:599. doi: 10.3389/fimmu.2017.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Ye L., Liu H., Huang L., Yang Q., Turner J.R., Yu Q. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25:1657–1670. doi: 10.1038/s41418-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K.R., Schofield Z., Dalby M.J., Caim S., Chalklen L., Bernuzzi F., Alcon-Giner C., Gall G.Le, Watson A.J.M., Hall L.J. The early life microbiota protects neonatal mice from pathological small intestinal epithelial cell shedding. FASEB J. 2020;34:7075–7088. doi: 10.1096/fj.202000042R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung A.T., Lin S.-Y., Yang T.-Y., Chou C.-K., Liu H.-C., Lu J.-J., Wang B., Chen S.-Y., Lien T.-F. Effects of Bacillus coagulans ATCC 7050 on growth performance, intestinal morphology, and microflora composition in broiler chickens. Anim. Prod. Sci. 2012;52 [Google Scholar]
- Jajere S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World. 2019;12:504–521. doi: 10.14202/vetworld.2019.504-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Das R., Oak S., Mishra P. Probiotics (Direct-Fed Microbials) in poultry nutrition and their effects on nutrient utilization, growth and laying performance, and gut health: a systematic review. Animals-Basel. 2020;10 doi: 10.3390/ani10101863. ARTN 1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Khan W.I. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens. 2013;2:55–70. doi: 10.3390/pathogens2010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnebrew M.A., Pamer E.G. Innate immune signaling in defense against intestinal microbes. Immunol. Rev. 2012;245:113–131. doi: 10.1111/j.1600-065X.2011.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konuray G., Erginkaya Z. Potential use of Bacillus coagulans in the food industry. Foods. 2018;7 doi: 10.3390/foods7060092. ARTN 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima Y., Goto Y., Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur. J. Immunol. 2013;43:3108–3115. doi: 10.1002/eji.201343782. [DOI] [PubMed] [Google Scholar]
- Li C.L., Wang J., Zhang H.J., Wu S.G., Hui Q.R., Yang C.B., Fang R.J., Qi G.H. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2018;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li R.X., Liu G., Lv C.F., Mi Y.L., Zhang C.Q. Effect of melatonin on renewal of chicken small intestinal mucosa. Poult. Sci. 2017;96:2942–2949. doi: 10.3382/ps/pex085. [DOI] [PubMed] [Google Scholar]
- Lima E.T., Andreatti Filho R.L., Okamoto A.S., Noujaim J.C., Barros M.R., Crocci A.J. Evaluation in vitro of the antagonistic substances produced by Lactobacillus spp. isolated from chickens. Can. J. Vet. Res. 2007;71:103–107. [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Miyazaki T. Beneficial effects of antioxidative lactic acid bacteria. AIMS Microbiol. 2017;3:1–7. doi: 10.3934/microbiol.2017.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveling D.P., van Emmenes L., Ahire J.J., Pieterse E., Smith C., Dicks L.M.T. Effect of a multi-species probiotic on the colonisation of salmonella in broilers. Probiotics Antimicrob. Proteins. 2020;12:896–905. doi: 10.1007/s12602-019-09593-y. [DOI] [PubMed] [Google Scholar]
- Pickard J.M., Zeng M.Y., Caruso R., Nunez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucciarelli M.G., Garcia-del Portillo F. Salmonella intracellular lifestyles and their impact on host-to-host transmission. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.MTBP-0009-2016. ARTN MTBP-0009-2016. [DOI] [PubMed] [Google Scholar]
- Rahimifard N. Evaluation and comparison of the antimicrobial activity of Bifidobacteria bifidum and Bifidobacteria infantis as probiotic bacteria against Salmonella enterica serotype enteritidis. J. Bacteriol. Mycol.: Open Access. 2016;2:61–64. [Google Scholar]
- Schoultz I., Keita A.V. The Intestinal barrier and current techniques for the assessment of gut permeability. Cells. 2020;9:1909. doi: 10.3390/cells9081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylejmani D., Musliu A., Ramadani N., Sparagano O., Hamidi A. Associations between the level of biosecurity and occurrence of dermanyssus gallinae and Salmonella spp. in layer farms. Avian Dis. 2016;60:454–459. doi: 10.1637/11327-111415-Reg. [DOI] [PubMed] [Google Scholar]
- Tian T., Wang Z., Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Umar S. Intestinal stem cells. Curr. Gastroenterol. Rep. 2010;12:340–348. doi: 10.1007/s11894-010-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeno Y., Shigemori S., Shimosato T. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 2015;30:126–132. doi: 10.1264/jsme2.ME14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancamelbeke M., Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang H., Li T., Liu F., Cheng Y., Guo X., Wen G., Luo Q., Shao H., Pan Z., Zhang T. Characterization of Salmonella spp. isolated from chickens in Central China. BMC Vet. Res. 2020;16:299. doi: 10.1186/s12917-020-02513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen Y., Zhang X., Lu Y., Chen H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: a review. J. Funct. Foods. 2020;75 [Google Scholar]
- Westrom B., Arevalo Sureda E., Pierzynowska K., Pierzynowski S.G., Perez-Cano F.J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 2020;11:1153. doi: 10.3389/fimmu.2020.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Shao Y., Song B., Zhen W., Wang Z., Guo Y., Shahid M.S., Nie W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Sci. Biotechnol. 2018;9:9. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Jiang L., Wang M., Sun W., Yu S., Turner J.R., Yu Q. Cadmium ingestion exacerbates Salmonella infection, with a loss of goblet cells through activation of Notch signaling pathways by ROS in the intestine. J. Hazard Mater. 2020;391 doi: 10.1016/j.jhazmat.2020.122262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeşilyurt N., Yılmaz B., Ağagündüz D., Capasso R. Involvement of probiotics and postbiotics in the immune system modulation. Biologics. 2021;1:89–110. [Google Scholar]
- Zhang B., Zhang H., Yu Y., Zhang R., Wu Y., Yue M., Yang C. Effects of Bacillus coagulans on growth performance, antioxidant capacity, immunity function, and gut health in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen W.R., Shao Y.J., Gong X.Y., Wu Y.Y., Geng Y.Q., Wang Z., Guo Y.M. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poult.Sci. 2018;97:2654–2666. doi: 10.3382/ps/pey119. [DOI] [PubMed] [Google Scholar]