Abstract
Objectives:
Systemic endothelial activation may contribute to sepsis-associated organ injury, including acute respiratory distress syndrome. We hypothesized that children with extrapulmonary sepsis with versus without acute respiratory distress syndrome would have plasma biomarkers indicative of increased endothelial activation and that persistent biomarker changes would be associated with poor outcome.
Design:
Observational cohort.
Setting:
Academic PICU.
Patients:
Patients less than 18 years old with sepsis from extrapulmonary infection with (n = 46) or without (n = 54) acute respiratory distress syndrome and noninfected controls (n = 19).
Interventions:
None.
Measurements and Main Results:
Endothelial (angiopoietin-1, angiopoietin-2, tyrosine kinase with immunoglobulin-like loop epidermal growth factor homology domain 2, vascular endothelial growth factor, soluble fms-like tyrosine kinase, von Willebrand factor, E-selectin, intercellular adhesion molecule, vascular cell adhesion molecule, thrombomodulin) and inflammatory biomarkers (C-reactive protein, interleukin-6, and interleukin-8) were measured from peripheral plasma collected within 3 days (time 1) of sepsis recognition and at 3–6 days (time 2) and 7–14 days (time 3). Time 1 biomarkers and longitudinal measurements were compared for sepsis patients with versus without acute respiratory distress syndrome and in relation to complicated course, defined as greater than or equal to two organ dysfunctions at day 7 or death by day 28. Angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, tyrosine kinase with immunoglobulin-like loop epidermal growth factor homology domain 2, vascular endothelial growth factor, von Willebrand factor, E-selectin, intercellular adhesion molecule, vascular cell adhesion molecule, thrombomodulin, endocan, C-reactive protein, interleukin-6, and interleukin-8 were different between sepsis and noninfected control patients at time 1. Among patients with sepsis, those with acute respiratory distress syndrome had higher angiopoietin-2/angiopoietin-1 ratio, vascular endothelial growth factor, vascular cell adhesion molecule, thrombomodulin, endocan, interleukin-6, and interleukin-8 than those without acute respiratory distress syndrome (all p < 0.003). Angiopoietin-2 and angiopoi-etin-2/angiopoietin-1 ratio remained higher in sepsis with versus without acute respiratory distress syndrome after multivariable analyses. Time 1 measures of angiopoietin-2, angiopoietin-2/-1 ratio, von Willebrand factor, and endocan were indicative of complicated course in all sepsis patients (all area under the receiver operating curve ≥ 0.80). In sepsis without acute respiratory distress syndrome, soluble fms-like tyrosine kinase decreased more quickly and von Willebrand factor and thrombomodulin decreased more slowly in those with complicated course.
Conclusions:
Children with extrapulmonary sepsis with acute respiratory distress syndrome had plasma biomarkers indicative of greater systemic endothelial activation than those without acute respiratory distress syndrome. Several endothelial biomarkers measured near sepsis recognition were associated with complicated course, whereas longitudinal biomarker changes yielded prognostic information only in those without sepsis-associated acute respiratory distress syndrome.
Keywords: acute respiratory distress syndrome, children, endothelium, pediatric, sepsis
Sepsis and acute respiratory distress syndrome (ARDS) are common causes of death and disability for critically ill children (1–4). Endothelial activation, common to the pathobiology of both sepsis and ARDS, permits pathogen recognition and augments the local immune response (5). However, at its extreme, endothelial activation can become dysfunctional, leading to increased vascular permeability, microangiopathy, and death via progressive multiple organ dysfunction syndrome (MODS).
Current ARDS therapies primarily target epithelial lung injury rather than endothelial dysfunction (6–9). Yet, for patients with sepsis-associated ARDS, endothelial dysfunction may represent a biologic mechanism linking these two conditions that could be targeted to prevent, ameliorate, or reverse lung injury. However, data about endothelial dysfunction are limited in children with sepsis-associated ARDS.
Prior reports of endothelial dysfunction in pediatric sepsis have demonstrated altered blood levels of biomarkers involved in regulation of vascular permeability, such as angiopoietin-1, angiopoietin-2, and their receptor, tyrosine kinase with immunoglobulin-like loop epidermal growth factor homology domain 2 (Tie-2) (10–15), vascular endothelial growth factor (VEGF), and its receptor soluble fms-like tyrosine kinase (sFLT) 16–18, endocan (17–20); those involved in neutrophil adhesion, such as intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), and E-selectin (21–24); and those involved in platelet activation, such as von Willebrand Factor (vWF) and soluble thrombomodulin (25–27). However, these studies have reported widely ranging biomarker levels in heterogeneous patient groups, and no study has examined a comprehensive biomarker profile of endothelial activation over time.
We sought to determine if the presence of ARDS in children with extrapulmonary sepsis is associated with severity of endothelial activation. We hypothesized that septic patients with ARDS would exhibit a biomarker profile indicative of increased endothelial activation compared with those without ARDS. We further hypothesized that persistent elevation of endothelial biomarkers would be associated with worse clinical outcome.
MATERIALS AND METHODS
Study Design and Population
We conducted a retrospective analysis of an observational cohort study of children with sepsis treated in the PICU of a single academic children’s hospital. Eligible patients had blood collected after enrollment in one of two parent studies between May 2014 and January 2018, had sufficient residual blood available to permit biomarker analysis, and had provided consent for use of residual blood for future research as part of parent study enrollment. The first parent study (28) enrolled critically ill children with sepsis, and the second parent study (29, 30) enrolled children with ARDS. Only patients who met a common set of eligibility criteria for the present study, which was separately approved by the Institutional Review Board with a waiver of informed consent, were considered for this analysis.
Sepsis and ARDS were defined according to the consensus criteria (International Pediatric Sepsis Consensus Conference [31] and modified Berlin [32] criteria) in use at the time the first patient was enrolled in either parent study. The parent sepsis study included patients less than 18 years old weighing greater than or equal to 7.5 kg with severe sepsis or septic shock defined as 1) at least two systemic inflammatory response syndrome criteria; 2) suspected or confirmed systemic infection; and 3) at least two organ system dysfunctions or cardiovascular dysfunction (31). Patients with WBC count less than 0.5×103/μL, known mitochondrial disorder, or unrepaired cyanotic congenital heart disease were excluded. The parent ARDS study included patients greater than 1 month old and less than 18 years old weighing at greater than or equal to 3 kg (to exclude children with perinatal respiratory failure) with ARDS defined using modified Berlin criteria including 1) acute respiratory failure within 7 days of known risk factor requiring mechanical ventilation; 2) PaO2/FIO2 less than or equal to 300 in two consecutive arterial blood gas samples drawn at least 2 hours apart with the patient receiving invasive or noninvasive positive end-expiratory pressure at least 5 cm H2O; and 3) bilateral infiltrates on chest radiograph (32). Patients with chronic home-based mechanical ventilation or unrepaired cyanotic congenital heart disease were excluded.
This analysis was limited to children with an extrapulmonary source of sepsis. The source of infection was determined a priori using established criteria (33, 34) with three-person adjudication of cases in which the source of infection was ambiguous after initial review. For patients who had been enrolled in both the sepsis and the ARDS parent studies, we analyzed the earliest blood samples collected. PICU patients without infection or organ dysfunction (e.g., patients having undergone airway or neurosurgical surgery) were included as controls. Patients with extrapulmonary sepsis were categorized as having ARDS if ARDS was present within 1 week of sepsis recognition.
Biomarkers
Blood was collected in an EDTA or lithium heparin tube within 72 hours (time 1 [T1]) of meeting criteria for severe sepsis, then again at days 3–6 (time 2 [T2]), and days 7–14 (time 3 [T3]). Samples were centrifuged within 30 minutes of collection at 3,000 G for 10 minutes, and aliquoted plasma was stored at –80°C. Plasma was thawed once at the time of biomarker measurement, which was performed at the Center for Cellular Immunotherapies at the University of Pennsylvania.
Candidate biomarkers representing different aspects of endothelial activation were identified a priori based on reports of diagnostic or prognostic utility in sepsis or ARDS, including angiopoietin-1, angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, Tie-2, VEGF, sFLT, endocan, ICAM, VCAM, E-selectin, vWF, and thrombomodulin (12–29,35,36). C-reactive protein (CRP), interleukin (IL)-6, and IL-8 were included as markers of systemic inflammation (37–39). Biomarkers were measured using a combination of commercially available assays (Life Technologies, Carlsbad, CA; EMD Millipore, Darmstadt, Germany), custom multiplex panels (R&D Systems, Minneapolis, MN), and enzyme-linked immunosorbent assay with human-specific reagents. Samples were run in duplicate, and the mean value was used as the measured biomarker concentration. To ensure accuracy and precision, standard curves calculated for each analyte were required to have R2 greater than 95%.
Data Collection
Clinical data, including demographics, comorbidities, and source of infection, were collected blinded to biomarker measurements and using the Research Data Capture system through the Children’s Hospital of Philadelphia (40) by a single investigator (J.E.W.) who reviewed all patient charts. Double-data entry was performed by a second investigator (K.G.) for a random 10% of patients to ensure accuracy of data collection, yielding a mean percent agreement of 92% (± 4% SD) across all data fields. Illness severity within 12 hours of PICU admission was summarized using the Pediatric Risk of Mortality (PRISM) III score (41).
Outcomes
The main outcomes were biomarker levels at T1. The main clinical endpoint was complicated course, defined as the presence of greater than or equal to two organ system dysfunctions on day 7 or death by day 28 following sepsis recognition (42).
Statistical Analyses
Analyses were performed using Stata version 14 (StataCorp, College Station, TX) and SAS (SAS Institute, Cary, NC). Data were summarized using medians with interquartile range for continuous variables and proportions for categorical variables. Continuous variables were compared using Wilcoxon rank sum test, and proportions were compared using chi-square or Fisher exact tests. We initially compared T1 biomarkers in septic patients with versus without ARDS then used multivariable regression to adjust for the effect of potential confounders. Confounders were identified a priori based on biologic plausibility, were tested for association with exposure and outcome variables, and were included in multivariable models if p value less than or equal to 0.1 in univariate analyses. Biomarkers were log transformed when used as outcomes.
The association between endothelial biomarkers and complicated course was determined by comparing T1 biomarkers in patients with versus without complicated course in multivariable logistic regression, subsequently stratified by ARDS status. We calculated the area under the receiver operating curve (AUROC) to predict complicated course by biomarkers that were different between groups. Because biomarker measurements were missing for 24 patients at T1 and 41 patients at T3, we used mixed-effect linear regression to model the mean change in biomarkers by complicated course, then repeated the analysis stratified by ARDS status. We tested the interaction of ARDS in the association of biomarkers with complicated course in multivariable models using a p value of less than or equal to 0.1 to indicate effect modification. Statistical significance was defined as a p value of less than 0.0033 after correction for 15 comparisons using Bonferroni correction (43).
RESULTS
Among 128 potentially eligible patients, 119 met all inclusion criteria, including 46 with extrapulmonary sepsis with ARDS, 54 with extrapulmonary sepsis without ARDS, and 19 controls (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F99; legend, Supplemental Digital Content 9, http://links.lww.com/CCM/F107). Patient characteristics are shown in Table 1. Patients across the three groups did not differ by age, sex, or race. Among the 100 patients with sepsis, those with ARDS were younger (p = 0.03) and had a higher prevalence of gastrointestinal comorbidity (p = 0.04) compared with those without ARDS. Septic patients with complicated course had a higher prevalence of cardiac comorbidity (p = 0.05). Therefore, age, cardiac comorbidity, and gastrointestinal comorbidity met criteria as potential confounders for evaluation in multivariable models. Although PRISM-III score was higher in sepsis patients than controls (p < 0.001) and in sepsis with versus without ARDS (p = 0.005), we did not include PRISM-III as a covariate in multivariable models because a component of the score is oxygenation, which, by definition was worse in patients with ARDS.
Table 1.
Patient Characteristics by Group, Including Demographics, Frequency of Baseline Comorbidities, and Extrapulmonary Sepsis Source
| Variable | Control (n = 19) | Sepsis With ARDS (n = 46) | Sepsis Without ARDS (n = 54) | p a |
|---|---|---|---|---|
| Age (yr), median (IQR) | 10 (3–13) | 5 (4–13) | 9 (7–16) | 0.03 |
| Sex, n (%) | 0.33 | |||
| Female | 10 (53) | 21 (46) | 30 (56) | |
| Male | 9 (47) | 25 (54) | 24 (44) | |
| Race, n (%) | 0.94 | |||
| Asian | 0 (0) | 2 (4) | 3 (6) | |
| Black or African American | 2 (11) | 10 (22) | 12 (22) | |
| Middle Eastern/Northern African | 0 (0) | 0 (0) | 1 (2) | |
| White | 15 (78) | 28 (61) | 30 (56) | |
| Other | 2 (11) | 6 (13) | 8 (15) | |
| Comorbidities,b n (%) | ||||
| None | 4 (21) | 9 (20) | 17 (31) | 0.25 |
| Cardiac | 0 (0) | 9 (20) | 7 (13) | 0.37 |
| Pulmonary | 0 (0) | 10 (22) | 10 (19) | 0.69 |
| Gastrointestinal | 0 (0) | 19 (41) | 12 (22) | 0.04 |
| Endocrine | 0 (0) | 7 (15) | 9 (17) | 0.84 |
| Renal | 0 (0) | 1 (2) | 6 (11) | 0.08 |
| Rheumatologic | 0 (0) | 3 (7) | 5 (9) | 0.62 |
| Immunologic | 0 (0) | 3 (7) | 1 (2) | 0.24 |
| Hematologic | 0 (0) | 4 (9) | 3 (6) | 0.54 |
| Oncologic | 5 (26) | 11 (24) | 9 (17) | 0.37 |
| Musculoskeletal | 5 (26) | 7 (15) | 4 (7) | 0.21 |
| Dermatologic | 0 (0) | 1 (2) | 2 (4) | 0.66 |
| Neurologic | 8 (42) | 18 (39) | 16 (30) | 0.32 |
| Immunosuppressedc | 0 (0) | 16 (35) | 19 (35) | 0.97 |
| Extrapulmonary sepsis source, n (%) | 0.97 | |||
| Blood | – | 18 (39) | 16 (30) | |
| Gastrointestinal | – | 12 (26) | 14 (26) | |
| Urinary | – | 5 (11) | 8 (15) | |
| CNS | – | 2 (4) | 4 (7) | |
| Skin | – | 2 (4) | 2 (4) | |
| Musculoskeletal | – | 2 (4) | 1 (2) | |
| Unknown | – | 5 (11) | 9 (17) | |
| Most frequent bacterial pathogens,d n (%) | ||||
| Escherichia coli | 4 (8) | 4 (7) | – | |
| Enterobacter cloacae | – | 2 (4) | 1 (2) | |
| Enterococcus faecalis | – | 0 (0) | 4 (7) | |
| Klebsiella pneumoniae | – | 2 (4) | 1 (2) | |
| Proteus mirabilis | – | 2 (4) | 0 (0) | |
| Pseudomonas aeruginosa | – | 0 (0) | 2 (4) | |
| Staphylococcus aureus (methicillin sensitive) | – | 2 (4) | 5 (9) | |
| Streptococcus pneumoniae | – | 1 (2) | 3 (6) | |
| Streptococcus pyogenes | – | 1 (2) | 5 (9) | |
| Pediatric Risk of Mortality-III score, median (IQR) | 0 (0–3) | 15 (10–24) | 11 (5–16) | 0.005 |
ARDS = acute respiratory distress syndrome, IQR = interquartile range.
p value reflects difference between sepsis with versus without ARDS. Significance is defined as p < 0.05.
Categories are not mutually exclusive because some patients had more than one baseline comorbidity.
Immunosuppressed patients included those with immune deficiencies and those receiving immunosuppressive therapies.
Most frequently occurring bacteria cultured from primary site of infection are listed as counts (n).
Dashes indicate not applicable.
T1 Biomarkers and ARDS
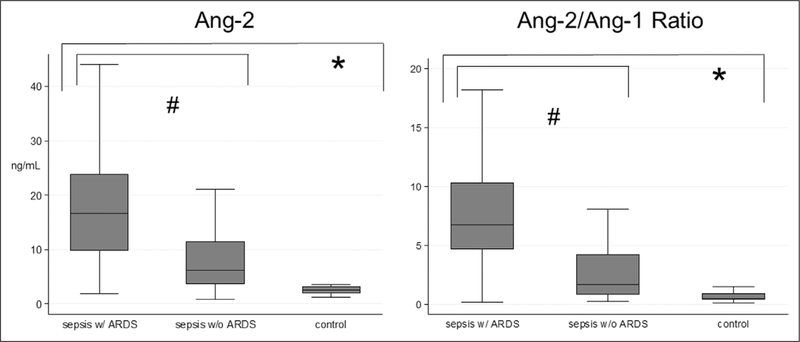
Endothelial and inflammatory biomarker levels measured at T1 for control and sepsis patients with and without ARDS are shown in Figure 1 and Supplemental Figure 2 (Supplemental Digital Content 2, http://links.lww.com/CCM/F100; legend, Supplemental Digital Content 9, http://links.lww.com/CCM/F107) and Supplemental Figure 3 (Supplemental Digital Content 3, http://links.lww.com/CCM/F101; legend, Supplemental Digital Content 9, http://links.lww.com/CCM/F107), with values shown in Supplemental Table 1 (Supplemental Digital Content 4, http://links.lww.com/CCM/F102). Angio-poietin-2, angiopoietin-2/angiopoietin-1 ratio, Tie-2, VEGF, vWF, E-Selectin, ICAM, VCAM, thrombomodulin, endocan, CRP, IL-6, and IL-8 were different between sepsis and control patients after correction for multiple comparisons (all p < 0.0033). Among patients with sepsis, those with ARDS had lower angiopoietin-1 and higher angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, VEGF, VCAM, thrombomodulin, endocan, IL-6, and IL-8 in unadjusted analyses after correction for multiple comparisons (all p < 0.0033). After adjusting for age and cardiac and gastrointestinal comorbidities, only angiopoietin-2 and angiopoietin-2/angiopoietin-1 ratio remained higher in patients with versus without ARDS (Fig. 1).
Figure 1.
Time 1 angiopoietin (Ang)-2 and Ang-2/Ang-1 ratio by group. Data are shown as boxplots with the horizontal line of each box representing the median, the ends of the box representing the 25th and 75th percentiles, and the whiskers representing the most extreme values within 1.5 times the interquartile range. Wilcoxon rank sum test was used to compare biomarkers between groups. *Significance defined as p value less than 0.0033 given multiple comparisons between controls versus all extrapulmonary sepsis. #Significance defined as p < 0.0033 given multiple comparisons between extrapulmonary sepsis with (w/) versus without (w/o) acute respiratory distress syndrome (ARDS).
T1 Biomarkers and Complicated Course
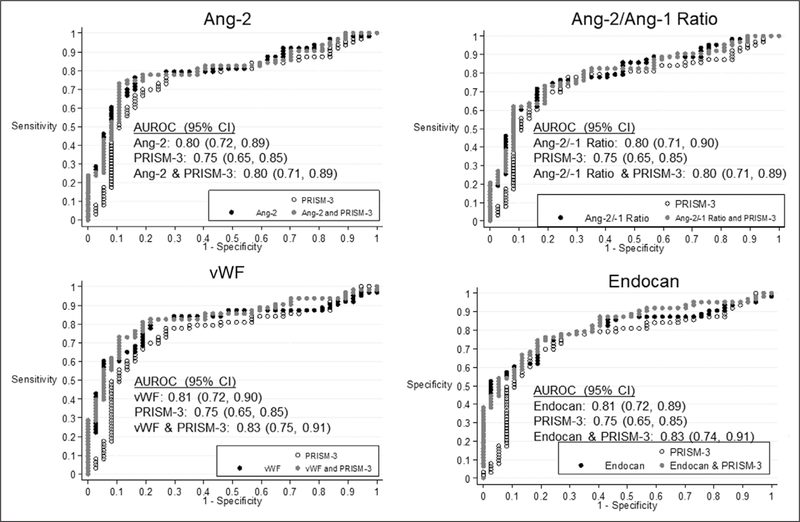
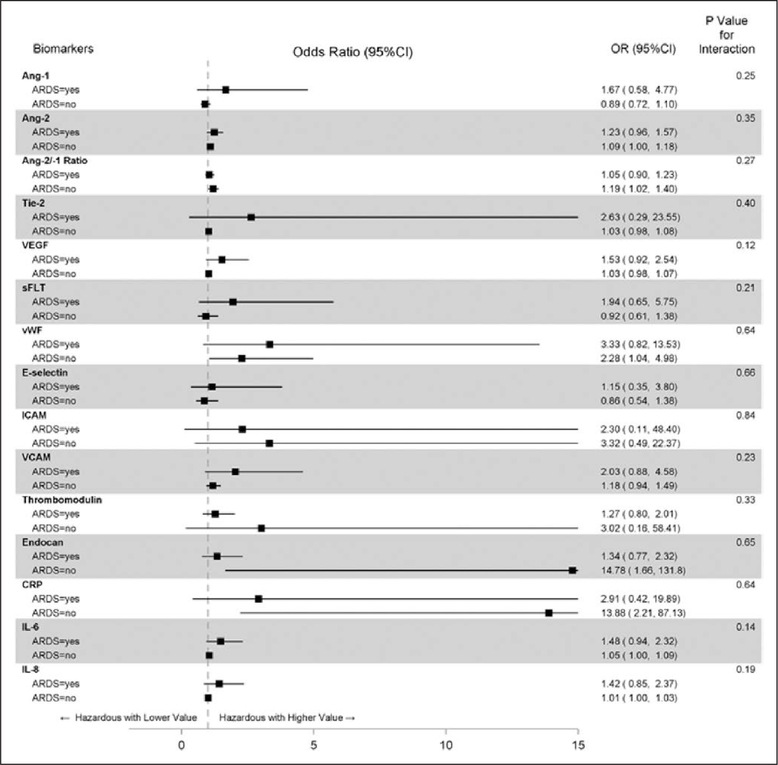
Among patients with sepsis, higher levels of angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, vWF, and endocan were associated with increased risk of complicated course after adjusting for age, cardiac and gastrointestinal comorbidities, and multiple comparisons (Supplemental Table 2, Supplemental Digital Content 5, http://links.lww.com/CCM/F103). Each of these T1 biomarkers yielded an AUROC greater than or equal to 0.80 to discriminate groups of sepsis patients with versus without complicated course. In the case of angiopoietin-2 and angio-poietin-2/angiopoietin-1 ratio, the biomarker alone predicted the outcome as well as the combination of the biomarker and PRISM-3, whereas for vWF and endocan, the AUROC associated with the combination of the biomarker and PRISM-III was 0.02 higher than the biomarker alone (Fig. 2). Effect modification by ARDS was not evident in the association of any T1 biomarker with complicated course, although trends (p < 0.3) toward an interaction were noted for angiopoietin-1, VEGF, sFLT, VCAM, IL-6, and IL-8 (Fig. 3; and Supplemental Table 2, Supplemental Digital Content 5, http://links.lww.com/CCM/F103).
Figure 2.
Receiver operating characteristic curves for Pediatric Risk of Mortality (PRISM)-3, selected time 1 (T1) biomarkers (angiopoietin [Ang]-2, Ang-2/Ang-1 ratio, von Willebrand factor [vWF], endocan), and the combination of PRISM-3 and T1 biomarkers to predict complicated course. Among patients with sepsis and after adjustment for age, cardiac, and gastrointestinal comorbidity, the area under the receiver operating characteristic curve (AUROC) for Ang-2 was 0.80 (95% CI, 0.72–0.89), for Ang-2/Ang-1 was 0.80 (95% CI, 0.71–0.89), for vWF was 0.81 (95% CI, 0.72–0.90), and for endocan was 0.81 (95% CI, 0.72–0.89) to predict complicated course. The AUROC for PRISM-3 alone (white) and the AUROC for the combination of PRISM-3 and any biomarker (gray) were not superior for prediction of complicated course compared with any biomarker alone (black).
Figure 3.
Odds of complicated course by time 1 (T1) biomarkers, stratified by acute respiratory distress syndrome (ARDS) status. Data are presented as adjusted odds ratio (OR) with 95% CIs for the association of T1 biomarkers with complicated course, stratified by ARDS status. A p value of less than or equal to 0.10 was defined a priori to indicate a significant difference in the odds of complicated course in sepsis patients with versus without ARDS. Ang = angiopoietin, CRP = C-reactive protein, ICAM = intracellular adhesion molecule, IL = interleukin, sFLT = soluble fms-like tyrosine kinase, Tie-2 = tyrosine kinase with immunoglobulin-like loop epidermal growth factor homology domain 2, VCAM = vascular adhesion molecule, VEGF = vascular endothelial growth factor, vWF = von Willebrand factor.
Longitudinal Change in Biomarkers and Complicated Course
Seventy-five of the 100 patients with sepsis had biomarkers measured at multiple timepoints. Of the 25 patients with biomarkers measured at only T1, blood was not available at T2 or T3 because of nonsurvival in five patients. Change in biomarkers by complicated course is shown in Supplemental Figure 4 (Supplemental Digital Content 6, http://links.lww.com/CCM/F104; and legend, Supplemental Digital Content 9, http://links.lww.com/CCM/F107). In multivariable analyses adjusted for age, cardiac, and gastrointestinal comorbidity, thrombomodulin (p = 0.01) and CRP (p = 0.04) decreased more slowly in sepsis patients with than without complicated course. sFLT decreased more quickly (p = 0.002) in sepsis patients with complicated course than patients without complicated course, in whom it increased. Because these analyses were considered exploratory and limited in sample size, significance was not adjusted for multiple comparisons. After stratifying by ARDS, longitudinal changes in biomarkers were not associated with complicated course in sepsis patients with ARDS. However, in sepsis patients without ARDS, vWF decreased more quickly (p = 0.03) and thrombomodulin decreased more slowly (p = 0.01) in those with versus without complicated course after adjustment for age, cardiac, and gastrointestinal comorbidity (Supplemental Table 3, Supplemental Digital Content 7, http://links.lww.com/CCM/F105). Sensitivity analysis comparing these results to those obtained after inclusion of 25 patients with only T1 biomarkers demonstrated no differences (Supplemental Table 4, Supplemental Digital Content 8, http://links.lww.com/CCM/F106).
DISCUSSION
In this study of critically ill children with extrapulmonary sepsis, those with compared with without ARDS exhibited a biomarker profile indicative of increased endothelial activation measured within 3 days of sepsis recognition. Peripheral blood plasma angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, vWF, and endocan measured within 3 days of sepsis recognition also predicted complicated course (AUROC ≥ 0.8) for this cohort of patients in multivariable analyses. Among all patients with sepsis, longitudinal changes were less strongly associated with complicated course, but sFLT exhibited a slight decrease over time in those with but not without complicated course, and thrombomodulin and CRP decreased more slowly in patients with complicated course. These longitudinal associations with complicated course were evident only in sepsis patients without ARDS.
Our finding that biomarkers indicating increased endothelial activation, including regulators of vascular permeability, neutrophil chemotaxis, coagulation, and inflammation, were elevated in patients with sepsis compared with controls is supported by multiple prior studies (13, 17, 24, 26, 36, 37). Whether derangement in endothelial biomarkers reflects a distinct pathobiology responsible for aspects of sepsis or ARDS physiology or simply indicate illness severity cannot be determined from this observational study. However, the stepwise increase in angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, VEGF, VCAM, thrombomodulin, endocan, IL-6, and IL-8 observed from controls to septic patients without ARDS to septic patients with ARDS suggests a contribution of endothelial dysfunction to the pathobiology of ARDS in extrapulmonary sepsis.
The presence of elevated angiopoietin-2 and angiopoietin-2/angiopoietin-1 ratio indicates a more severe systemic endotheliopathy in sepsis patients with versus without ARDS, consistent with prior data reported separately for patients with sepsis (12, 44–48) or ARDS (10, 14). Angiopoietin dysregulation has been noted in septic patients with pulmonary edema (15, 45, 49), some of whom develop ARDS. However, these studies have not separated pulmonary and extrapulmonary sources of infection. Our finding that elevated angiopoietin-2 and angiopoietin-2/angiopoietin-1 ratio was associated with ARDS in patients with extrapulmonary sepsis supports a possible role of endothelial dysfunction in sepsis-associated ARDS.
Our finding that complicated course was associated with biomarker derangements early in the course of illness is consistent with prior studies in both sepsis (13, 17, 50) and ARDS (10, 19, 51, 52). In our cohort, increased angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, vWF, and endocan early in sepsis predicted complicated course. Elevation in this cluster of proteins may reflect damage to the endothelial cell layer (53–55), a proposed initial step in the cascade toward MODS and death (5). Although ARDS status did not modify the effect of T1 biomarkers on complicated course, there were trends to suggest that angiopoietin-1, VEGF, sFLT, VCAM, IL-6, and IL-8 may be higher in patients with than without ARDS who had complicated course. Other biomarkers (angiopoietin-2, angiopoietin-2/angiopoietin-1 ratio, vWF, and endocan) were associated with complicated course in both ARDS and non-ARDS patients. Derangement in various biomarkers indicating endothelial activation suggests that endotheliopathy at sepsis onset may be relevant to complicated course in patients with sepsis, and even more so in sepsis patients with ARDS.
Complicated course was associated with a decrease in sFLT over time, consistent with experimental data suggesting a protective effect of sFLT, and therefore a deleterious effect of decreasing sFLT, in a murine sepsis model (56). Complicated course was also associated with prolonged elevation in thrombomodulin and CRP, consistent with prior studies reporting an association between this pattern of biomarker change and MODS in sepsis (26, 57, 58).
Our finding that complicated course was not associated with longitudinal changes in most biomarkers differs from prior studies showing an association between persistent biomarker elevation and worse outcomes in septic adults (18, 20, 47). One potential explanation is that our study was underpowered to detect this association. A possible biologic explanation is that, regardless of outcome, endotheliopathy peaks during the acute phase of illness then decreases independent of patient outcome. An alternative explanation is that endotheliopathy generally improved over time in this pediatric cohort, which had low mortality compared with adult studies (47), possibly reflecting age-related differences in physiology. The association of a slower decrease in vWF and thrombomodulin with complicated course only in patients without ARDS deserves more investigation and may be an artifact of inadequate power for this exploratory aim.
Our study has several limitations. First, although our sample size permitted observation of biomarker differences, power was reduced for stratified analyses. Second, because most patients had concurrent sepsis and ARDS, we were not able to differentiate between differences in biomarkers that predisposed to versus caused ARDS in patients with extrapulmonary sepsis. Third, determination of sepsis diagnosis and source of infection is subject to misclassification bias. Fourth, missing longitudinal biomarker measurements did not occur at random given that both rapid recovery and early death occurred. However, early death was rare, and sensitivity analysis produced similar results. The possibility of competing risks in longitudinal analyses was ameliorated by the use of complicated course, a composite of death, and persistent MODS. Finally, we relied on patient samples collected as part of two parent studies with overlapping, yet distinct, enrollment criteria that may have led to selection bias and could limit generalizability. Although our selection of endothelial and inflammatory biomarkers was guided by prior research, it was not exhaustive. Validation of our findings in a prospective cohort of children with extrapulmonary sepsis is necessary.
CONCLUSIONS
Children with extrapulmonary sepsis with ARDS exhibited a biomarker profile indicative of increased endothelial activation compared with children with sepsis without ARDS. Several endothelial biomarkers measured near-sepsis recognition were associated with complicated course, whereas longitudinal biomarker changes appeared less useful, yielding prognostic information only in those without sepsis-associated ARDS. Selected endothelial biomarker levels might be useful for predictive enrichment in clinical trials in children with sepsis with or without ARDS if a reliable point-of-care assay was available.
Supplementary Material
Acknowledgments
Supported, in part, by grants from National Heart, Lung, and Blood Institute T32 HL-00891 (supporting Dr. Whitney), Eunice Kennedy Shriver National Institute of Child Health and Human Development K12HD047349 (to Dr. Weiss), National Institute of General Medical Sciences K23GM110496 (to Dr. Weiss), and Department of Anesthesiology and Critical Care at the Children’s Hospital of Philadelphia. Drs. Whitney, Melenhorst, Teachey, Yehya, and Weiss received support for article research from the National Institutes of Health (NIH). Drs. Zhang and Melenhorst disclosed government work. Dr. Koterba’s institution received funding from the Children’s Hospital of Pennsylvania (CHOP) (her laboratory, a service center at the University of Pennsylvania, received a fee-for-service for performing the Luminex biomarker assays on patient samples, which covered reagents, supplies, and salary support). Dr. Chen’s institution received funding from CHOP, and she disclosed work for hire. Dr. Lacey’s institution received funding from Novartis (intellectual property in the area of chimeric antigen receptor-T celltherapy licensed by his institution). Dr. Melenhorst’s institution received funding from Novartis, Incyte, Parker Institute for Cancer Immunotherapy, Stand Up to Cancer, and the NIH. Dr. Teachey’s institution received funding from La Roche, and he received funding from Novartis and Amgen. Dr. Mensinger’s institution received funding from the Department of Critical Care Medicine and Anesthesiology at CHOP for 20% of her time to provide methods and statistics consulting for their faculty, residents, and fellows doing research. Dr. Yehya’s institution received funding from the NIH and Pfizer. Dr. Weiss’ institution received funding from National Institute of General Medical Sciences, and he received funding from Bristol Myers Squibb (member, advisory committee). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
This study was performed at the Children’s Hospital of Philadelphia.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Flori HR, Glidden DV, Rutherford GW, et al. : Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005; 171:995–1001 [DOI] [PubMed] [Google Scholar]
- 2.Balamuth F, Weiss SL, Neuman MI, et al. : Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014; 15:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss SL, Fitzgerald JC, Pappachan J, et al. ; Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Fernández Y, Azagra AM, de la Oliva P, et al. ; Pediatric Acute Lung Injury Epidemiology and Natural History (PED-ALIEN) Network: Pediatric acute lung injury epidemiology and natural history study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med 2012; 40:3238–3245 [DOI] [PubMed] [Google Scholar]
- 5.Johansson PI, Stensballe J, Ostrowski SR: Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care 2017; 21:1–7. DOI: 10.1186/s13054-017-1605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brower RG, Matthay MA, et al. ; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 7.Jouvet P, Thomas NJ, Willson DF, et al. : Pediatric acute respiratory distress syndrome: Consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015; 16:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khemani RG, Conti D, Alonzo TA, et al. : Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med 2009; 35:1428–1437 [DOI] [PubMed] [Google Scholar]
- 9.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An official American Thoracic Society/European Society of Intensive Care Medicine/society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 10.Zinter MS, Spicer A, Orwoll BO, et al. : Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol 2016; 310:L224–L231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Bhandari V, Giuliano JS Jr, et al. : Angiopoietin-1, angiopoietin-2 and bicarbonate as diagnostic biomarkers in children with severe sepsis. PLoS One 2014; 9:e108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melendez E, Whitney JE, Norton JS, et al. : Systemic angiopoietin-1/2 dysregulation in pediatric sepsis and septic shock. Int J Med Sci 2019; 16:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuliano JS Jr, Lahni PM, Harmon K, et al. : Admission angiopoietin levels in children with septic shock. Shock 2007; 28:650–654 [PMC free article] [PubMed] [Google Scholar]
- 14.Ong T, McClintock DE, Kallet RH, et al. : Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med 2010; 38:1845–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh SM, Mammoto T, Schultz A, et al. : Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 2006; 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickkers P, Sprong T, Eijk Lv, et al. : Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock 2005; 24:508–512 [DOI] [PubMed] [Google Scholar]
- 17.Mihajlovic DM, Lendak DF, Brkic SV, et al. : Endocan is useful biomarker of survival and severity in sepsis. Microvasc Res 2014; 93:92–97 [DOI] [PubMed] [Google Scholar]
- 18.Hsiao SY, Kung CT, Tsai NW, et al. : Concentration and value of endocan on outcome in adult patients after severe sepsis. Clin Chim Acta 2018; 483:275–280 [DOI] [PubMed] [Google Scholar]
- 19.Orbegozo D, Rahmania L, Irazabal M, et al. : Endocan as an early biomarker of severity in patients with acute respiratory distress syndrome. Ann Intensive Care 2017; 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauly D, Hamed S, Behnes M, et al. : Endothelial cell-specific molecule-1/endocan: Diagnostic and prognostic value in patients suffering from severe sepsis and septic shock. J Crit Care 2016; 31:68–75 [DOI] [PubMed] [Google Scholar]
- 21.Whalen MJ, Doughty LA, Carlos TM, et al. : Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 are increased in the plasma of children with sepsis-induced multiple organ failure. Crit Care Med 2000; 28:2600–2607 [DOI] [PubMed] [Google Scholar]
- 22.Schuetz P, Jones AE, Aird WC, et al. : Endothelial cell activation in emergency department patients with sepsis-related and non-sepsis-related hypotension. Shock 2011; 36:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassiliou AG, Mastora Z, Jahaj E, et al. : Does serum lactate combined with soluble endothelial selectins at ICU admission predict sepsis development? In Vivo 2015; 29:305–308 [PubMed] [Google Scholar]
- 24.Amalakuhan B, Habib SA, Mangat M, et al. : Endothelial adhesion molecules and multiple organ failure in patients with severe sepsis. Cytokine 2016; 88:267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Basset Abo El Ezz AA, Abd El Hafez MA, El Amrousy DM, et al. : The predictive value of Von Willebrand factor antigen plasma levels in children with acute lung injury. Pediatr Pulmonol 2017; 52:91–97 [DOI] [PubMed] [Google Scholar]
- 26.Lin JJ, Hsiao HJ, Chan OW, et al. : Increased serum thrombomodulin level is associated with disease severity and mortality in pediatric sepsis. PLoS One 2017; 12:e0182324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapru A, Calfee CS, Liu KD, et al. ; NHLBI ARDS Network: Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive Care Med 2015; 41:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss S, Zhang D, Bush J, et al. : Persistent mitochondrial dysfunction linked to prolonged organ dysfunction in pediatric sepsis. Crit Care Med 2019; 47:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yehya N, Thomas NJ, Meyer NJ, et al. : Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med 2016; 42:1137–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yehya N, Thomas NJ, Margulies SS: Circulating nucleosomes are associated with mortality in pediatric acute respiratory distress syndrome. Am J Physiol Cell Mol Physiol 2016; 42:1137–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 32.Ranieri VM, Rubenfeld GD, Thompson BT, et al. : Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 33.Horan TC, Andrus M, Dudeck MA: CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–332 [DOI] [PubMed] [Google Scholar]
- 34.Yehya N, Keim G, Thomas NJ: Subtypes of pediatric acute respiratory distress syndrome have different predictors of mortality. Intensive Care Med 2018; 44:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitney JE, Silverman M, Norton JS, et al. : Vascular endothelial growth factor and soluble vascular endothelial growth factor receptor as novel biomarkers for poor outcomes in children with severe sepsis and septic shock. Pediatr Emerg Care 2018; 00:1–5 [DOI] [PubMed] [Google Scholar]
- 36.Shapiro NI, Yano K, Okada H, et al. : A prospective, observational study of soluble FLT-1 and vascular endothelial growth factor in sepsis. Shock 2008; 29:452–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castelli GP, Pognani C, Cita M, et al. : Procalcitonin, C-reactive protein, white blood cells and SOFA score in ICU: Diagnosis and monitoring of sepsis. Minerva Anestesiol 2006; 72:69–80 [PubMed] [Google Scholar]
- 38.Feng M, Sun T, Zhao Y, et al. : Detection of serum interleukin-6/10/18 levels in sepsis and its clinical significance. J Clin Lab Anal 2016; 30:1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kellum JA, Kong L, Fink MP, et al. ; GenIMS Investigators: Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med 2007; 167:1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollack MM, Patel KM, Ruttimann UE: PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 42.Wong HR, Cvijanovich NZ, Anas N, et al. : Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 2015; 191:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonferroni CE: Teoria statistica delle classi e calcolo delle probabilità. Pubbl del R Ist Super di Sci Econ e Commer di Firenze 1936; 8:3–62 [Google Scholar]
- 44.Fang Y, Li C, Shao R, et al. : Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit Care 2015; 19:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, et al. : Circulating angiopoietin-2 levels in the course of septic shock: Relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med 2009; 35:1567–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mankhambo LA, Banda DL, Jeffers G, et al. ; IPD Study Group: The role of angiogenic factors in predicting clinical outcome in severe bacterial infection in Malawian children. Crit Care 2010; 14:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricciuto DR, dos Santos CC, Hawkes M, et al. : Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med 2011; 39:702–710 [DOI] [PubMed] [Google Scholar]
- 48.Alves BE, Montalvao SA, Aranha FJ, et al. : Imbalances in serum angiopoietin concentrations are early predictors of septic shock development in patients with post chemotherapy febrile neutropenia. BMC Infect Dis 2010; 10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. : Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008; 63:903–909 [DOI] [PubMed] [Google Scholar]
- 50.Hyseni A, Kemperman H, Lange DW De, et al. : Active von Willebrand factor predicts 28-day mortality in patients with systemic inflammatory response syndrome. Blood 2019; 123:16–20 [DOI] [PubMed] [Google Scholar]
- 51.Tsangaris I, Tsantes A, Vrigkou E, et al. : Angiopoietin-2 levels as predictors of outcome in mechanically ventilated patients with acute respiratory distress syndrome. Dis Markers 2017; 2017:6758721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin DB, Wiener-Kronish JP, Murray JF, et al. : Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest 1990; 86:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maisonpierre PC, Suri C, Jones PF, et al. : Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277:55–60 [DOI] [PubMed] [Google Scholar]
- 54.Kali A, Shetty KS: Endocan: A novel circulating proteoglycan. Indian J Pharmacol 2014; 46:579–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribes JA, Francis CW, Wagner DD: Fibrin induces release of von Willebrand factor from endothelial cells. J Clin Invest 1987; 79:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yano K, Liaw PC, Mullington JM, et al. : Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med 2006; 203:1447–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krafte-Jacobs B, Brilli R: Increased circulating thrombomodulin in children with septic shock. Crit Care Med 1998; 26:933–938 [DOI] [PubMed] [Google Scholar]
- 58.Mihajlovic DM, Lendak DF, Draskovic BG, et al. : Thrombomodulin is a strong predictor of multiorgan dysfunction syndrome in patients with sepsis. Clin Appl Thromb Hemost 2015; 21:469–474 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.