Abstract
Introduction:
Hepatic artery infusion pump (HAIP) chemotherapy is a specialized therapy for patients with unresectable colorectal liver metastases (uCRLM). Its effectiveness was demonstrated from high volume center, with uncertainty regarding the feasibility and safety at other centers. Therefore, we sought to assess the safety and feasibility of HAIP for management of uCRLM at other centers.
Methods:
We conducted a multicenter retrospective cohort study of patients with uCRLM treated with HAIP from January 2003 to December 2017 at six North American centers initiating HAIP program. Outcomes included safety and feasibility of HAIP chemotherapy.
Results:
We identified 154 patients with HAIP insertion and median age of 54 (48–61) years. The burden of disease was >10 intra-hepatic metastatic foci in 59 (38.3%) patients. Patients received at least one-cycle of systemic chemotherapy prior to HAIP insertion. Major complications occurred in 7 (4.6%) patients during their hospitalization and 13 (8.4%) patients developed biliary sclerosis during follow-up. 148 patients (96.1%) received at least one-dose of HAIP chemotherapy with a median of 5 (4–7) cycles. 78 patients (56.5%) had complete or partial response and 12 (7.8%) received a curative liver resection.
Conclusion:
HAIP programs can be safely and effectively initiated in previously inexperienced centers with good response.
Keywords: colorectal liver metastasis, Hepatic Artery Infusion Pump chemotherapy, liver metastases treatment
INTRODUCTION
Among patients with colorectal cancer, 30% will develop liver metastases during the course of their disease.1 Of those patients, 60% will present with metastases only to the liver.1 Surgical resection of colorectal liver metastases (CRLM) remains the only curative treatment option available but only a minority of patients present with initially resectable disease.2,3 Therefore, disease control is key to optimize prognosis.
Despite significant progress made in systemic chemotherapy over the last decades in the treatment of CRLM, these treatments infrequently lead to cure offering a median survival of 22–24 months and a disease progression-free survival of 10 months.4 The response rate to second-line systemic chemotherapies diminishes to 10–30%.5–7 As a result, alternative treatment strategies such as regional delivery of chemotherapy through hepatic arterial infusion pump (HAIP) have emerged as an effective treatment option to control disease progression in patients with uCRLM.8
HAIP is a catheter-based delivery of continuous chemotherapy into the hepatic artery using a subcutaneous pump. The driving principal is that CRLM derive their blood supply from the hepatic artery while normal hepatocytes are predominantly perfused through the portal vein.9 This allows targeted delivery of chemotherapy to the liver metastases while sparing the normal liver parenchyma. The intra-arterial administration of chemotherapeutic agents with high first-pass hepatic extraction rate and short half-life, such as floxuridine (FUDR), limits systemic side effects and increases FUDR exposure within the liver up to 400-fold compared to systemic administration.10
Previous work across multiple studies demonstrated promising results of HAIP combined with systemic chemotherapy compared to systemic chemotherapy alone for uCRLM.11–15 Despite encouraging results, HAIP has been confined to few high volume specialized centre in North America due to concerns of safety and feasibility at other centres.16 Several newer HAIP programs led by surgical and medical oncologists with training in HAIP insertion and treatment, have emerged at several specialized institutions in North America. We performed a multicenter retrospective analysis of patients with uCRLM who received HAIP chemotherapy to examine HAIP feasibility and safety at previously inexperienced centers.
METHODS
Study Design
We conducted a multicenter, single-arm, retrospective cohort analysis of data collected from North American centers, that established relatively new HAIP programs. The centers included: Sunnybrook Health Sciences Hospital, Ontario, Canada; Washington University School of Medicine, Missouri, United States; Cleveland Clinic, Ohio, United States; Oregon Health & Science University Knight Cancer Institute, Oregon, United States; Rutgers, New Jersey, United States; The Ohio State University Wexner Medical Center, Ohio, United States. This study was approved by the Research Ethics Board or Institutional Review Board of each center.
Participating centers were all large tertiary centers with access to multidisciplinary care which include Hepatopancreatobiliary (HPB) trained Surgical Oncologists, Medical Oncologists, Interventional Radiologists and nurses trained in administration of HAIP chemotherapy as well as management of HAIP related surgical and chemotherapy complications.
Population
Adult patients, ≥18 years old, from January 1st 2003 to December 31st 2017, with histologically or radiographically confirmed uCRLM at the time of HAIP insertion were included in this study. Patients were selected for HAIP at the discretion of the treating physician and the institutional multidisciplinary tumor board. In general, patients were considered for HAIP if they had uCRLM with no or limited extrahepatic disease. The first patients that received HAIP for uCRLM in each center were included in this analysis.
Training
Participating surgeons and medical oncologists in this study had either completed their training at Memorial Sloan-Kettering Cancer Center (MSKCC), New York, United States, which has over 20-years of experience in HAIP implantation, or completed extra training through workshops which included supervised HAIP insertions, detailed protocols, and instructions on surgical pump implantation and management.
Work-up
Preoperative imaging included cross-sectional imaging with computed tomography (CT) and/or magnetic resonance imaging (MRI) of the abdomen and pelvis and CT of the chest. Patients were evaluated with a CT angiogram for nonstandard arterial anatomy. Fluorodeoxyglucose–positron emission tomography (PET) scans and MRI were used selectively at the discretion of the treating physicians.
Hepatic intra-arterial pump insertion and treatment
Pre-operatively a CT angiogram was performed to evaluate for non-standard arterial anatomy. Laparotomy was performed to place the pump subcutaneously in the abdominal wall. The catheter tip was positioned at the origin of the gastroduodenal artery as previously described.17,18 Bilobar hepatic perfusion and lack of extrahepatic perfusion were confirmed by both intra-operative dye testing and postoperative technetium-99-labeled nuclear medicine scanning. All collateral branches distal to the catheter and proximal to the liver were ligated to avoid extrahepatic perfusion.17,18 Patients with extrahepatic perfusion were evaluated angiographically and aberrant branches were embolized before initiation of treatment. The pump implanted during the study duration is same as the pump used at MSKCC (Codman 3000).
The HAIP was filled with heparinized saline solution approximately every 2 weeks until the start of HAIP chemotherapy to prevent thrombosis of the catheter. Patients were evaluated every 2 weeks when the pump was filled. FUDR was administered based on the MSKCC regimen (0.12mg/kg/day) as previously described every 4 weeks with heparin saline refills every 2 weeks between cycles.19,20 Patients were evaluated in clinic when the pump was refilled and their liver function tests (LFTs) were reviewed while on treatment. FUDR dose modifications were made following previous established algorithms that uses liver function tests and bilirubin to guide dose reductions and holding treatment.21 Dexamethasone was added to FUDR infusion when patients demonstrated any concerns for biliary toxicity.22 Patients who did not complete HAIP treatment due to toxicity or pump failure were included in this database.
Systemic chemotherapy prior to HAIP chemotherapy and during HAIP was provided to patients and was determined by the treating medical oncologist following the regional guidelines based on patients’ comorbidities, past chemotherapy and disease burden.
Variables
Clinical and pathologic data were collected retrospectively on all patients included in the study from their hospital medical health records. Variables collected include patient demographics (age and gender), primary disease location, characteristics of CRLM (size, number), HAIP insertion date, hepatic artery anatomy (conventional, unconventional), postoperative complications (Clavien-Dindo classification),23 time to HAIP chemotherapy initiation and number of cycles administered, pump related complications, systemic chemotherapy if given, length of stay in hospital, tumor response, disease progression, liver resection, and death. Primary disease was considered right-sided tumor if localized proximal to the splenic flexure and left sided tumor if localized at or distal to the splenic flexure. The size and total number of CRLM was obtained from the most recent imaging available prior to HAIP insertion.
Outcomes
The primary outcome of interest in this study was to assess the safety and feasibility of HAIP for management of uCRLM. Safety was assessed through examining the 30-days postoperative complications including the general complications (not related to HAIP) and HAIP related complications. Feasibility was assessed through examining the proportion of patients who successfully received at least one cycle of HAIP chemotherapy, time to initiation of HAIP chemotherapy and number of HAIP chemotherapy cycles administered.
Secondary outcomes of interested included tumor response rate according to response evaluation criteria in solid tumors (RECIST) criteria,24 overall survival (OS), disease progression free survival, and liver resection.
Statistical analysis
Continuous variables were presented as mean and standard deviation if normally distributed or median and inter-quartile range (IQR) in cases of non-normal distribution. Assessment of normality was performed with Shapiro-Wilk test and visual histograms. Categorical variables were presented as frequencies and proportion.
Disease progression-free survival was defined as the period of time from time of HAIP insertion until time of locoregional or distant metastatic disease progression or time of death or censored at last follow-up. OS was calculated as the period of time from of HAIP insertion to the time of death or censored at last follow-up. Kaplan-Meier method was used to present the results.
Statistical analysis was performed with SAS University Edition version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Characteristics of patients with HAIP insertion
During the study period 154 patients with uCRLM underwent HAIP insertion at six centers (table 1). The median follow-up duration from time of HAIP insertion to last known follow up or death was 19.5 months (IQR 10.5–31). The median age was 54 (IQR 48–61), 86 (55.8%) were male, and 145 (94.2%) patients had no background liver disease. Left-sided colon cancer was more common in this cohort (n=116, 75.3%) than right-sided colon cancer. Prior to HAIP insertion, 88 (57.1%) patients received resection of their primary colorectal cancer (CRC) disease while 47 (30.5%) had their CRC resected at the time of HAIP insertion. The remainder of the patients either had their disease resected at a later time or their disease remained in situ.
Table 1:
Patient demographics and baseline disease characteristics
| Variable | |
|---|---|
| Sites, n (%) | |
| Sunnybrook Health Sciences Centre | 31 (20.1%) |
| The Ohio State University | 60 (39.0%) |
| Rutgers University | 21 (13.6%) |
| Oregon Health and Science University | 11 (7.1%) |
| Cleveland Clinic | 13 (8.4%) |
| Washington University School of Medicine | 18 (11.7%) |
|
| |
| Age, median (IQR) | 54 (48–61) |
|
| |
| Gender, n (%) | |
| Male | 86 (55.8%) |
|
| |
| Liver disease, n (%) | |
| None | 145 (94.16%) |
| Non-alcoholic steatohepatitis (NASH) | 2 (1.3%) |
| Chemotherapy-associated Steatohepatitis (CASH) | 1 (0.65%) |
| Other | 6 (3.9%) |
|
| |
| Primary tumor, n (%)* | |
| Right-sided colon cancer | 37 (24.2%) |
| Left-sided colon cancer | 116 (75.8%) |
|
| |
| Primary tumor stage, n (%) | |
| T0 | 3 (2.0%) |
| T1 | 1 (0.7%) |
| T2 | 13 (8.4%) |
| T3 | 94 (61.0%) |
| T4 | 35 (22.7%) |
| Missing | 8 (5.2%) |
|
N0 |
26 (16.9%) |
| N1 | 68 (44.2%) |
| N2 | 52 (33.3%) |
| Missing | 8 (5.2%) |
|
| |
| Primary tumor grade, n (%) | |
| Low | 124 (80.5%) |
| High | 13 (8.4%) |
| Missing | 17 (11.0%) |
|
| |
| Primary CRC resection, n (%) | |
| Prior to HAIP insertion | 88 (57.1%) |
| At the time of HAIP insertion | 47 (30.5%) |
| Not resected | 16 (10.4%) |
| Missing | 3 (2.0%) |
|
| |
| Number of CRLM, n (%) | |
| 1 and <5 | 57 (37.0%) |
| ≤5 and <10 | 30 (19.5%) |
| ≥10 | 59 (38.3%) |
| Missing | 8 (5.2%) |
|
| |
| Largest tumor size, median (IQR)** | 3.1 cm (2.0–5.3) |
|
| |
| Number of systemic chemotherapy cycles prior to HAIP, median (IQR) | 10 (6–12) |
IQR: interquartile range, n: number, HAIP: hepatic artery infusion pump, CRC: colorecta cancer, CRLM: colorectal liver metastases
missing n=1
missing n=9
The majority of patients in this study had a primary CRC disease stage T3 and N1 and low-grade tumor pathology. Patients had a high burden of CRLM such that 59 (38.3%) patients had ≥ 10 intra-hepatic metastatic foci with the largest tumor median size of 3.1 cm (IQR 2.0–5.3). All patients received at least one cycle of systemic therapy prior to HAIP insertion. The median number of cycles of chemotherapy received prior to HAIP was 10 (IQR 6–12) with 87.0% of patients receiving a FOLFOX- or FOLFIRI-based regimen. Two centers contributed 12 patients before 2010 and there were no differences in patient or tumor characteristics in patients that received HAIP treatment before and after 2010.
Operative safety and outcomes
The operative and post-operative safety outcomes are summarised in table 2. The median operative time for HAIP insertion with or without concurrent liver or colorectal resection was 257 minutes (IQR 191–383) and intraoperative estimated blood loss (EBL) was 200 cc (IQR 100–400). In the post-operative period, 52 (33.8%) patients had non-HAIP related complications and 22 (14.3%) patients had HAIP insertion-related complications with six patients experiencing more than one complication. HAIP complications were classified as pump pocket complications (9.1%), vascular perfusion complications (6.5%) and catheter occlusion or dislodgement (1.9%). Major complications (≥Clavien-Dindo 3b) occurred in 7 (4.5%) patients during their hospitalization. At last follow up, 17 patients had developed complications related to HAIP pump, most frequently, biliary sclerosis (n=13). Only 6 patients (3.9%) did not receive HAIP due to the listed complications.
Table 2:
Operative safety outcomes of HAIP procedure
| Variable | |
|---|---|
| Operative time, median (IQR)* | 257 minutes (191–383) |
|
| |
| Estimated blood loss, median (IQR)* | 200 cc (100–400) |
|
| |
| Non HAIP related complications, n (%) | 50 (32.5%) |
| Wound infection | 16 (10.4%) |
| Urinary tract infection/obstruction | 3 (2.0%) |
| Pneumonia/respiratory failure | 5 (3.3%) |
| Other | 18 (11.7%) |
| Ileus | 8 (5.2%) |
|
| |
| Acute pump related complications, n (%) | 22 (14.3%)** |
| Pump Pocket | 14 (9.1%) |
| Infection | 7 (4.5%) |
| Hematoma | 2 (1.3%) |
| Migration | 2 (1.3%) |
| Unspecified | 2 (1.3%) |
| Hepatic arterial system | 10 (6.5%) |
| Thrombosis | 2 (1.3%) |
| Incomplete perfusion | 4 (2.6%) |
| Bleeding | 4 (2.6%) |
| Catheter (occlusions or dislodgement) | 3 (1.9%) |
|
| |
| Clavien-Dindo grade, n (%) | 54 (35.1%) |
| 1 | 8 (5.2%) |
| 2 | 31 (20.1%) |
| 3a | 8 (5.2%) |
| 3b | 2 (1.3%) |
| 4 | 5 (3.3%) |
|
| |
| Length of stay, median (IQR) | 5 days (4–7) |
|
| |
| Long term HAIP complications, n (%) | 17 (11.0%) |
| Biliary Sclerosis | 13 (8.4%) |
| Others | 4 (2.6%) |
| Duodenal ulcer | 1 (0.6%) |
| Delayed pump infection | 2 (1.3%) |
| Pump flipped requiring reoperation | 1 (0.6%) |
IQR: interquartile range, n: number, HAIP: Hepatic artery infusion pump
missing n=1
complications will not add up to n=22 because 6 patients developed two complications
Feasibility outcomes
Summary of feasibility outcomes are presented in table 3. One-hundred and forty-eight patients (96.1%) received at least one cycle of HAIP chemotherapy with a median of 5 cycles of chemotherapy (IQR 4–7). The most common cause of patients not receiving HAIP chemotherapy was arterial thrombosis or non-functional pump. The time to initiation of HAIP chemotherapy was 20 days (IQR 15–31). The HAIP chemotherapy provided was Floxuridine (FUDR) in 148 (95.5%) patients, one of whom also received mitomycin. Concomitant with HAIP chemotherapy 81 (52.6 %) patients also received systemic therapy. Of those patients that received HAIP and systemic chemotherapy, the median number of HAIP chemotherapy cycles was 6 (IQR 4–8) and time to HAIP chemotherapy initiation was 18.5 months (IQR 16–28).
Table 3:
Operative feasibility outcomes of HAIP procedure
| Variable | |
|---|---|
| Number of cycles, median (IQR)* | 5 cycles (4–7) |
|
| |
| Time of HAIP chemotherapy initiation, median (IQR)** | 20 days (15–30.5) |
|
| |
| HAIP Chemotherapy provided, n (%) | |
| Floxuridine (FUDR) | 147 (95.5%) |
| Oxaliplatin | 1 (0.7%) |
| Pump not functional | 6 (3.9%) |
|
| |
| Systemic therapy during HAIP treatment, n (%) | 82 (53.2%) |
| FOLFIRI based | 35 (22.7%) |
| FOLFOX based | 18 (11.7%) |
| 5-FU alone | 5 (3.2%) |
| Irinotecan based other than FOLFIRI | 10 (6.5%) |
| Others | 14 (9.1%) |
IQR: interquartile range, n: number, HAIP: Hepatic artery infusion pump
missing n=11
missing n=6
Secondary outcomes
At the end of follow up, 87 (56.5%) patients had a complete or partial response (table 4). Twelve patients (7.8%) ultimately received a liver resection with curative intent with a median time of 7.9 months (IQR 5.5–11.4) to resection. The 1- and 3-year disease progression free survival of patients with complete or partial response was 33.0% and 4.1% while the 1- and 3- year OS was 92.7% and 56.0%, respectively (figure 1).
Table 4:
Disease response and recurrences outcomes
| Variable | |
|---|---|
| Response rate, n(%) | |
| Complete response | 28 (18.2%) |
| Partial response | 50 (38.3%) |
| Stable disease | 36 (23.4%) |
| Progressive disease | 27 (17.5%) |
| Missing | 4 (2.6%) |
|
| |
| Liver resection, n (%) | 12 (7.8%) |
|
| |
| Time to liver resection, median (IQR) | 7.9 months (5.5–11.4) |
|
| |
| Disease progression, n (%) | |
| Yes | 128 (83.1%) |
| No | 19 (12.3%) |
| Missing | 7 (4.6%) |
|
| |
| Site of disease progression, n (%) | 128 (83.2%) |
| Intra-hepatic | 49 (31.82%) |
| Extra-hepatic | 72 (46.8%) |
| Both | 7 (4.6%) |
IQR: interquartile range, n: number
Figure 1.
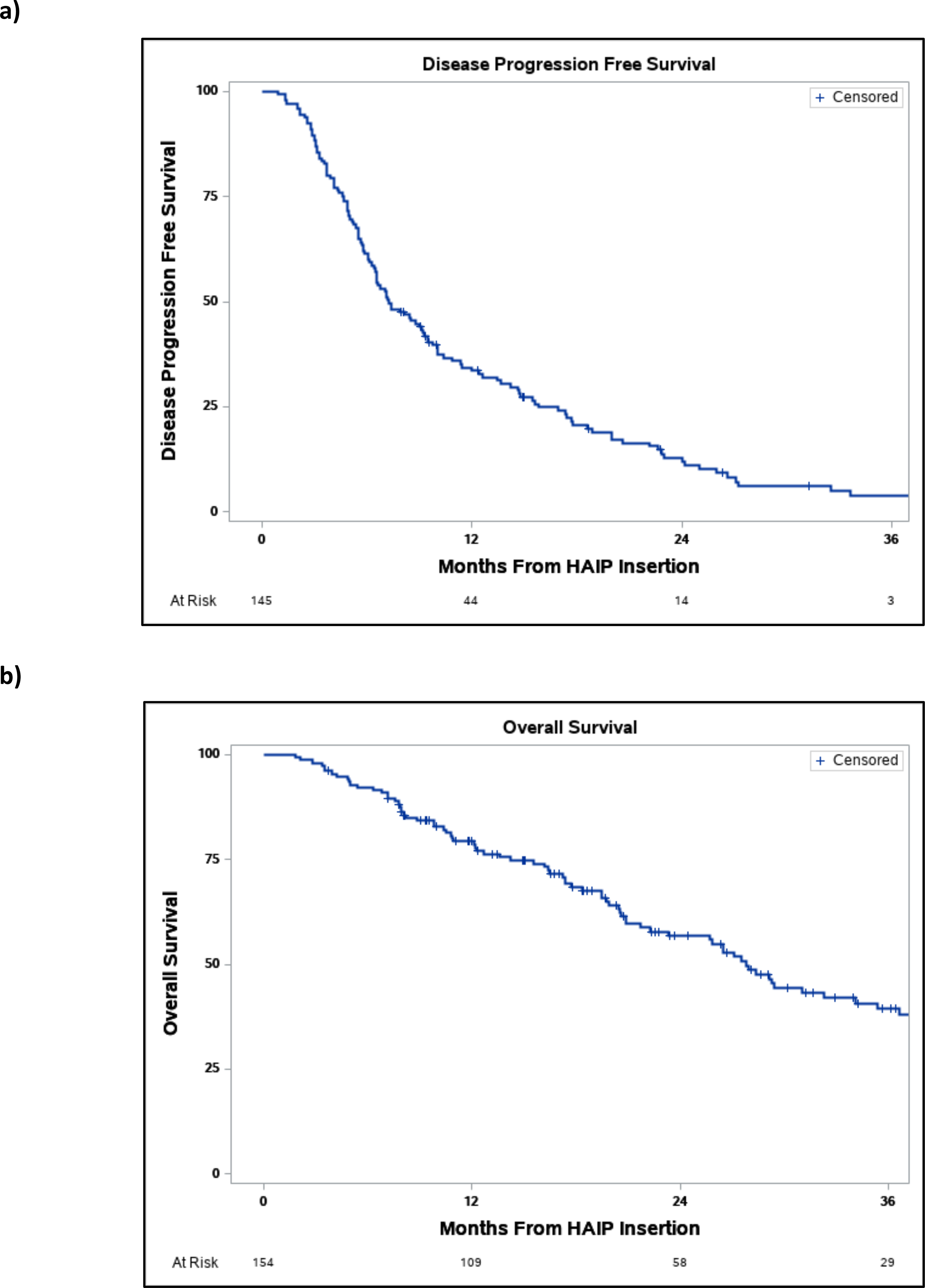
a) Disease free progression survival b) overall survival of patients from time of HAIP insertion.
One-hundred and twenty-eight (87.1%) patients experienced extra-hepatic or intra-hepatic disease progression and 84 (54.6%) patients died at the end of follow-up. The 1- and 3-year disease progression free survival was 33.1% and 4.1% and the 1- and 3-year OS survival was 78.9% and 39.3% with a median overall survival of 19.5 months (IQR 10.5–31) from HAIP placement.
DISCUSSION
To date, the majority of HAIP data is generated from a high-volume North American center with promising results. Herein, we performed a multicenter retrospective analysis, to demonstrate the feasibility and safety of HAIP insertion and chemotherapy administration in North American centers with relatively newly instituted HAIP programs. We demonstrated safety as only 14.3% of patients developed post-operative complications related to HAIP. Furthermore, we demonstrated feasibility since 96.1% of patients received HAIP chemotherapy within 20 days after HAIP insertion and a median number of 5 cycles received. Two centers contributing twelve patients performed HAIP treatment prior to 2010. Once those patients were excluded from the analysis the feasibility and safety outcomes were unchanged (data not shown).
We emphasize that these participating centers are all highly specialized centers where the delivery of care and follow-up is performed through a multidisciplinary team in an organized approach.16,25 First, at those centers, Surgical Oncologists, Oncologists, Radiation Oncologists, Interventional Radiologists, nurses and pharmacists are available and aware of HAIP treatment protocols, potential complications and management. Second, all treatment decisions were made at the institutional tumor board meetings through discussions amongst Surgical Oncologists, Oncologists and Radiation Oncologists. Lastly, all participating Surgical Oncologists and Oncologists received training in HAIP insertion and post-operative care. After discharge, patients were followed by Surgical Oncologists or Oncologist in collaboration with nurses and pharmacists to monitor for medication dosing and complication.
Patients included in this cohort have several characteristics indicative of poor outcomes; patients had a high burden of CRLM where 38.3% of patients had ≥ 10 intra-hepatic metastatic foci with the largest tumor median size of 3.1 cm (IQR 2.0–5.3) and 77.5% had node positive primary disease. All patients were previously treated and had received at least one cycle of systemic chemotherapy with a median of 10 (6–12) cycles prior to HAIP insertion. Yet, the response rate to HAIP chemotherapy was 56.5% with a 1- and 3-year disease progression-free survival of 33.1% and 4.1% respectively. The 1- and 3-year OS were 78.9% and 39.3% respectively with a median OS of 19.5 months (IQR 10.5–31). This data is comparable to previous results for patients that failed first line systemic chemotherapy prior to HAIP chemotherapy.25–29 It is challenging to make direct comparisons with other cohorts given that the patients included in this study have failed at least one line of systematic chemotherapy prior to starting HAIP chemotherapy. Nevertheless, this response rate remains better than response rates observed after second-line systemic therapy 10–30%.5–7
The majority of patients (87.6%) had their primary CRC disease resected prior to or at the time of HAIP insertion, however only 8% of patients received a potentially curative CRLM resection after HAIP chemotherapy. Although not examined in this study due to database limitations, our practise is to resect the primary CRC of asymptomatic and symptomatic patients to prevent complications such as bleeding, perforation or obstruction.30–32 Additionally, primary disease resections at the time of HAIP insertion adds little morbidity to patients postoperatively.33
Our data demonstrates that 32.5% of patients had a non-HAIP related post-operative complication and 14.3% had HAIP-related post-operative complications with 3.9% patients experiencing more than one complication. Only 4.6% of patients had a major post-operative complication (Clavien-Dindo ≥ 3b). Of patients with complications, only 6 patients were precluded from HAIP chemotherapy administration. These results are promising and consistent with post-operative complications of previous studies examining pump-related complications after HAIP placement (10–31.1%).25,33–36 Other post-operative outcomes including mean blood loss (490–724ml), mean operative time (241–260 mins), and length of stay in hospital (8–9days) were comparable to this study.33–35 This successful insertion and delivery of HAIP chemotherapy requires synchronous coordination of care amongst all involved specialists to ensure close observation to identify complications, intervene in a timely manner, monitor for chemotherapy toxicity to adjust the dose accordingly, and follow up to assess disease progression and monitor long-term outcomes.
Biliary sclerosis remains an important complication secondary to HAIP chemotherapy because of the potential need for biliary stent insertion. After a median follow up of 19.5 months (IQR 10.5–31), we demonstrate that in total 11.0% of patients developed a HAIP-related complication in the long term follow up. Of the entire cohort, 8.4% of developed biliary sclerosis. Due to database limitations we were unable to report the management of patients that developed biliary sclerosis, and this would require a separate formal investigation. However, others have similarly demonstrated that amongst the long term complications, the rate of biliary sclerosis was 5.5–8%.8,37,38 This highlights the importance of participating centers equipped for identification and management of biliary sclerosis.
Previous work demonstrated promising results of HAIP combined with systemic chemotherapy compared to systemic chemotherapy alone for uCRLM across multiple studies.11–15,25 This regimen was shown to achieve disease control or convert patients to resectable disease at higher rates than any second line systemic chemotherapy.11–15 However, the integration of HAIP into practise have been limited. This is mostly due to the required multidisciplinary expertise of Surgical Oncologists, Medical oncologists, Pharmacists, Nurses and Interventional Radiologist. Here we demonstrate that with the appropriate training, expertise and infrastructure, it is feasible for highly specialized centers to initiate HAIP programs safely. This will open the avenue of HAIP utilization in multiple treatment settings in addition to uCRLMS such as in the adjuvant setting after liver disease resection,17 or for management of cholangiocarcinoma.39
The main strength of this analysis is the multicenter nature of several lower-volume newly instituted centers interested in providing patients the benefits to HAIP chemotherapy. Furthermore, the data collected provides a ‘real life’ feasibility and safety assessment from those centers. There were limitations to our study which include the nature of any observational multi-institutional retrospective study; lack of granular details for management of post-operative complications, variation in systemic chemotherapy over time and between centers, and the small sample size collected from six centers across 15 years. Nevertheless, this data remains promising for centers with interest in participating in future HAIP chemotherapy trials as a modality for management of CRLM.
CONCLUSION
In summary, we demonstrate that HAIP treatment of uCRLM is safe and feasible in highly specialized multidisciplinary centers with new HAIP programs. Initiation and expansion of HAIP programs should be done in a systematic, organized and multidisciplinary approach.
Synopsis:
We performed a multicenter retrospective analysis to examine Hepatic artery infusion pump (HAIP) chemotherapy feasibility and safety at previously inexperienced centers. We demonstrate that HAIP treatment of patients with unresectable colorectal liver metastases is safe and feasible in highly specialized, multidisciplinary centers with newly established low volume HAIP programs.
Funding:
There was no funding source for this study.
Footnotes
Conflict of Interest:
DC is founder of Z53 Therapeutics Inc.
Data Availability Statement:
Data available on request due to privacy/ethical restrictions
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017. doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 2.Ko YJ, Karanicolas PJ. Hepatic arterial infusion pump chemotherapy for colorectal liver metastases: An old technology in a new era. Curr Oncol. 2014. doi: 10.3747/co.21.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.R. A. Chemotherapy and surgery: New perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008. doi: 10.1200/JCO.2008.17.7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peeters M, Price TJ, Cervantes A, et al. Final results from a randomized phase 3 study of FOLFIRI ± panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. 2014. doi: 10.1093/annonc/mdt523 [DOI] [PubMed] [Google Scholar]
- 6.Rothenberg ML, Eckardt JR, Kuhn JG, et al. Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol. 1996. doi: 10.1200/JCO.1996.14.4.1128 [DOI] [PubMed] [Google Scholar]
- 7.Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: Interim results of a phase III trial. J Clin Oncol. 2003. doi: 10.1200/JCO.2003.11.126 [DOI] [PubMed] [Google Scholar]
- 8.KEMENY N Intrahepatic or Systemic Infusion of Fluorodeoxyuridine in Patients with Liver Metastases from Colorectal Carcinoma. Ann Intern Med. 1987;107(4):459. doi: 10.7326/0003-4819-107-4-459 [DOI] [PubMed] [Google Scholar]
- 9.Bierman HR, Byron RL, Kelley KH, Grady A. Studies on the blood supply of tumors in man. iii. vascular patterns of the liver by hepatic arteriography in vivo. J Natl Cancer Inst. 1951. doi: 10.1093/jnci/12.1.107 [DOI] [PubMed] [Google Scholar]
- 10.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983. doi: 10.5555/uri:pii:0093775483900052 [DOI] [PubMed] [Google Scholar]
- 11.Allen-Mersh TG, Glover C, Fordy C, Mathur P, Quinn H. Randomized trial of regional plus systemic fluorinated pyrimidine compared with systemic fluorinated pyrimidine in treatment of colorectal liver metastases. Eur J Surg Oncol. 2000. doi: 10.1053/ejso.1999.0924 [DOI] [PubMed] [Google Scholar]
- 12.Kemeny NE, Huitzil Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009. doi: 10.1200/JCO.2008.20.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Angelica MI, Correa-Gallego C, Paty PB, et al. Phase ii trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer conversion to resection and long-term outcomes. Ann Surg. 2015. doi: 10.1097/SLA.0000000000000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pak LM, Kemeny NE, Capanu M, et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J Surg Oncol. 2018. doi: 10.1002/jso.24898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemeny NE, Gonen M, Sullivan D, et al. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol. 2001. doi: 10.1200/JCO.2001.19.10.2687 [DOI] [PubMed] [Google Scholar]
- 16.Karanicolas PJ, Metrakos P, Chan K, et al. Hepatic arterial infusion pump chemotherapy in the management of colorectal liver metastases: Expert consensus statement. Curr Oncol. 2014. doi: 10.3747/co.21.1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemeny N, Huang Y, Cohen AM, et al. Hepatic Arterial Infusion of Chemotherapy after Resection of Hepatic Metastases from Colorectal Cancer. N Engl J Med. 1999;341(27):2039–2048. doi: 10.1056/NEJM199912303412702 [DOI] [PubMed] [Google Scholar]
- 18.Kemeny N, Daly J, Oderman P, et al. Hepatic artery pump infusion: Toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol. 1984. doi: 10.1200/JCO.1984.2.6.595 [DOI] [PubMed] [Google Scholar]
- 19.Ammori JB, Kemeny NE. Regional Hepatic Chemotherapies in Treatment of Colorectal Cancer Metastases to the Liver. Semin Oncol. 2010. doi: 10.1053/j.seminoncol.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 20.Power DG, Kemeny NE. The role of floxuridine in metastatic liver disease. In: Molecular Cancer Therapeutics.; 2009. doi: 10.1158/1535-7163.MCT-08-0709 [DOI] [PubMed] [Google Scholar]
- 21.Lewis HL, Bloomston M. Hepatic Artery Infusional Chemotherapy. Surg Clin North Am. 2016. doi: 10.1016/j.suc.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Kemeny N, Conti JA, Cohen A, et al. Phase II study of hepatic arterial floxuridine, leucovorin, and dexamethasone for unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 1994. doi: 10.1200/JCO.1994.12.11.2288 [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 25.Creasy JM, Napier KJ, Reed SA, et al. Implementation of a Hepatic Artery Infusion Program: Initial Patient Selection and Perioperative Outcomes of Concurrent Hepatic Artery Infusion and Systemic Chemotherapy for Colorectal Liver Metastases. Ann Surg Oncol. 2020. doi: 10.1245/s10434-020-08972-y [DOI] [PubMed] [Google Scholar]
- 26.Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008. doi: 10.1245/s10434-007-9581-7 [DOI] [PubMed] [Google Scholar]
- 27.Rougier P, Loplonche A, Huguier M, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: Long-term results of a prospective randomized trial. J Clin Oncol. 1992. doi: 10.1200/JCO.1992.10.7.1112 [DOI] [PubMed] [Google Scholar]
- 28.Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colore. J Clin Oncol. 2000. doi: 10.1200/jco.2000.18.2.243 [DOI] [PubMed] [Google Scholar]
- 29.Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: A multicentre randomised trial. Lancet. 2003. doi: 10.1016/S0140-6736(03)12388-4 [DOI] [PubMed] [Google Scholar]
- 30.Eisenberger A, Whelan RL, Neugut AI. Survival and symptomatic benefit from palliative primary tumor resection in patients with metastatic colorectal cancer: A review. Int J Colorectal Dis. 2008. doi: 10.1007/s00384-008-0456-6 [DOI] [PubMed] [Google Scholar]
- 31.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage iv colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010. doi: 10.1007/s00268-009-0366-y [DOI] [PubMed] [Google Scholar]
- 32.Anwar S, Peter MB, Dent J, Scott NA. Palliative excisional surgery for primary colorectal cancer in patients with incurable metastatic disease. Is there a survival benefit? A systematic review. Color Dis. 2012. doi: 10.1111/j.1463-1318.2011.02817.x [DOI] [PubMed] [Google Scholar]
- 33.Brajcich BC, Bentrem DJ, Yang AD, et al. Short-Term Risk of Performing Concurrent Procedures with Hepatic Artery Infusion Pump Placement. Ann Surg Oncol. 2020. doi: 10.1245/s10434-020-08938-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: An institutional experience of 544 consecutive cases. J Am Coll Surg. 2005. doi: 10.1016/j.jamcollsurg.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 35.Buisman FE, Grünhagen DJ, Homs MYV, et al. Adjuvant Hepatic Arterial Infusion Pump Chemotherapy After Resection of Colorectal Liver Metastases: Results of a Safety and Feasibility Study in The Netherlands. Ann Surg Oncol. 2019. doi: 10.1245/s10434-019-07973-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buisman FE, Homs MYV, Grünhagen DJ, et al. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases - the multicenter randomized controlled PUMP trial. BMC Cancer. 2019. doi: 10.1186/s12885-019-5515-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito K, Ito H, Kemeny NE, et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: Incidence, clinical features, and risk factors. Ann Surg Oncol. 2012. doi: 10.1245/s10434-011-2102-8 [DOI] [PubMed] [Google Scholar]
- 38.Daly JM, Kemeny N, Oderman P, Botet J. Long-term Hepatic Arterial Infusion Chemotherapy: Anatomic Considerations, Operative Technique, and Treatment Morbidity. Arch Surg. 1984. doi: 10.1001/archsurg.1984.01390200054013 [DOI] [PubMed] [Google Scholar]
- 39.Cercek A, Boerner T, Tan BR, et al. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination with Systemic Gemcitabine and Oxaliplatin in Patients with Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020. doi: 10.1001/jamaoncol.2019.3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions


