Learning objectives.
By reading this article, you should be able to:
-
•
Describe the physics of droplets, particles and aerosols.
-
•
Explain the mechanisms of natural droplet generation.
-
•
Define the recognised aerosol-generating procedures (AGPs).
-
•
State the risks of AGPs and the steps that can be taken to reduce these risks.
Key points.
-
•
Transmission of an infectious pathogen depends on the pathogen, the mode of transmission and the recipient.
-
•
Risks of transmission can usually be mitigated by taking appropriate precautions.
-
•
Few viruses are thought to be transmitted exclusively by a single route, so whilst airborne transmission is important, droplet and contact precautions remain crucial in mitigating risks.
-
•
Particles are defined by their diameter. Large droplets are generally >10 μm in diameter. Smaller droplets (<5 μm) are referred to as droplet nuclei, aerosols or airborne particles. Changes in properties related to diameter are likely to be a continuum rather than binary.
-
•
Aerosols are produced by high-velocity gas flow over a thin layer of liquid. High gas velocities can be generated by a patient (coughing and talking), a procedure or surgical equipment.
The novel coronavirus infectious disease 2019 (COVID-19) pandemic was not the first viral pandemic of this century and is unlikely to be the last.1 However, it has led to enormous changes in working practices and a renewed interest in aerosol-generating procedures (AGPs). Front-line healthcare workers (HCWs), especially those working in environments in which AGPs are performed, may be exposed to infectious pathogens. Understanding the basic science that describes the behaviours of droplets, particles and aerosols allows a better understanding of AGPs and how the risks associated with them can be mitigated. This article is primarily focused on transmission of viral infection via AGPs.
Routes of transmission
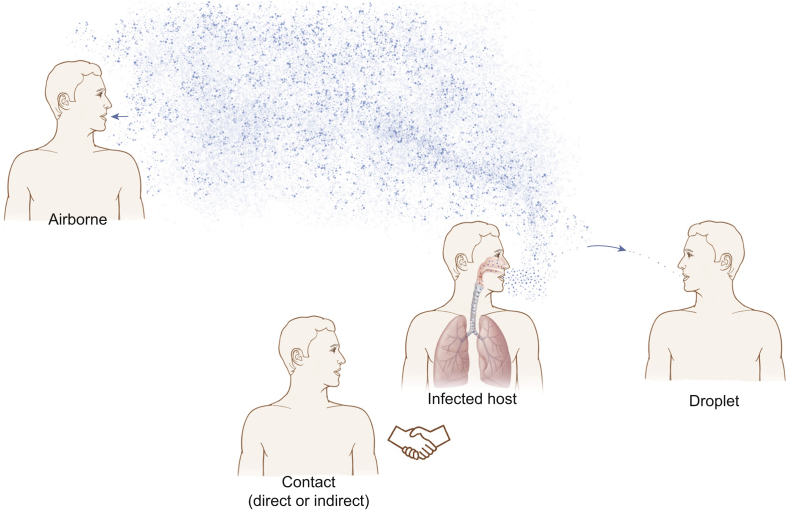
Respiratory infections are thought to be spread via three main mechanisms: droplets, contact (direct and indirect) and airborne (Fig. 1).
Fig 1.
Modes of transmission: airborne, contact (direct or indirect) and droplet.
Droplet spread refers to relatively large droplets ejected from an infected patient's mouth or nose that are transmitted directly to the recipient's mouth or nose. These larger droplets do not persist in the air and rapidly land on surfaces, causing contamination. Recommended personal protective equipment (PPE) for droplet transmission currently includes gloves, an apron and a fluid-resistant surgical mask (with or without eye protection).2
Contact spread can be direct (such as hand-to-hand contact) or indirect, which refers to contact with surfaces contaminated with respiratory droplets, known as fomites. These can be picked up (usually by the hands), and self-inoculation can occur via the nose or mouth.
Airborne spread refers to inhalation of smaller respiratory particles that are light enough to remain ‘suspended’ in the air and are hence able to be inhaled directly into the recipient's respiratory tract. Recommended PPE for airborne transmission currently includes gloves, a fluid-repellent long-sleeved gown, a filtering face piece (FFP) mask and eye protection.2
For COVID-19, the extent to which each mechanism contributes to transmission is uncertain. The majority of coronavirus transmission is thought to be caused by contact and droplet spread.2 A lesser degree is caused by airborne spread through an AGP or by natural droplet generation.
Respiratory droplets and aerosols
The WHO states that ‘aerosols are produced when an air current moves across the surface of a film of liquid, generating small particles at the air–liquid interface’.3 The particle size is thought to be inversely related to the velocity of gas hitting the interface. Procedures or events causing air to travel at high velocity over the respiratory mucosa risk producing droplets and aerosols.3
Large droplets with a diameter >10 μm are ejected from the respiratory tract of an infected individual during coughing or sneezing and potentially even whilst talking. These larger droplets are often defined as >10–20 μm diameter, are relatively heavy, follow a ballistic trajectory and are thought to travel only a short distance (<2 m) before falling to land on nearby surfaces. They are too heavy to remain ‘suspended’ in the air.2,4
Smaller droplets can be generated with higher gas velocities, their size being determined by the force and pressure involved. These smaller droplets (commonly quoted as <5–10 μm) are sometimes referred to as aerosols or droplet nuclei, and they are light enough to remain suspended in the air for long periods of time (minutes to hours). They behave similarly to ‘dust motes’ that float in airstreams. Aerosols can travel large distances before landing on a surface or evaporating completely; hence, airborne transmission of an infectious disease does not require face-to-face contact.5 The distance travelled and the length of time particles remain suspended in the air are determined by the particle size, settling velocity (defined as follows), relative humidity and airflow.3
The size distribution of respiratory droplets is determined by many factors, including environmental ventilation (airflow and opening windows and doors), ambient temperature and humidity. Droplet diameter has been reported to range between 0.5 and 1,000 μm, but this remains an area of controversy because of conflicting evidence. Respiratory droplets are generated in a high-relative-humidity environment in the airways. Upon exhalation into the lower-humidity ambient environment, they shrink by evaporation. This is a fast process, taking only seconds. The time taken for droplets to reach their equilibrium diameter is dependent on initial droplet size, composition, ambient temperature and relative humidity. Some estimates suggest that droplets may shrink to 50% of their original size.5
The speed at which a droplet falls with gravity is determined by its mass and is offset by drag or air resistance. Large droplets have a higher terminal settling velocity, so will tend to follow a ballistic trajectory and fall to the ground. Small particles and aerosols have a negligibly low terminal settling velocity; this allows them to remain suspended for minutes or hours (see Fig. 2).6
Fig 2.
Aerosol settling time over 1 metre as a function of particle size, assuming spherical particles with a density of 1000 kg m-3, an air temperature of 293.15 K, and an air pressure of 101.3 kPa (reproduced with kind permission of Prof Andrew Maynard).
Mechanisms of natural droplet generation
Although it is important to be aware of which clinical procedures can generate aerosols, there are many mechanisms of natural droplet generation that can provide a vector for transmission of infectious respiratory pathogens. Natural droplet generation is caused by expiratory episodes that include violent events (such as coughing or sneezing) and quiescent ones (such as talking, singing, breathing or laughing).5 These events are capable of generating large droplets, small droplets and aerosols.
The size of particles generated by these natural mechanisms has been extensively researched, but controversy remains because of the wide variation of scientific techniques.
Nicas and colleagues quote data suggesting that 99.9% of droplets contained within a cough are >8 μm.7 They quote three studies published between 1946 and 1997 that offer conflicting data.
Zayas and colleagues published a bench study in 2012 that used a laser diffraction system, which suggested that droplets <1 μm represented 97% of the total number of measured droplets contained in the cough aerosol of 45 healthy volunteers.8 Age, sex, weight and height had no statistically significant effect on the aerosol composition in terms of size and number of droplets.8
More recent work has highlighted the dichotomy between ‘large’ and ‘small’ particles as being arbitrary and inaccurate, and that study proposes a newer model for respiratory emissions. Although exhalations, sneezes and coughs contain mucosalivary droplets that follow short-range semiballistic emission trajectories, they are primarily made of a multiphase turbulent gas cloud that entrains ambient air and traps and carries within it clusters of droplets with a continuum of droplet sizes.9 This cloud may travel up to 8 m, persist for many minutes and alter local humidity for droplets, subsequently delaying their evaporation. Most agencies, including the WHO, have based guidance on traditional ‘large’ and ‘small’ droplet modelling, but further work may be required to clarify the issue.
Transmission of viruses
Transmission of an infectious pathogen from an infected host depends on many factors, including the pathogen (origin of pathogen within respiratory tree, virulence, infectious dose, pathogen load and pathogen inactivation rate defined as follows), transmission (droplet generation and size and mode of transmission) and the recipient (immune response and comorbidities).10 Different pathogens may shed their infectious load from different areas within the airways. As a result, droplets and aerosols from infectious patients may not originate from those areas of the respiratory tree with the highest pathogenic load. It is currently accepted that high loads of SARS CoV-2 virus are shed from the upper respiratory tract and oropharyngeal cavity.5,11 However, this appears to be at odds with the reported finding that there seems to be increased sensitivity for testing (with reverse transcriptase–polymerase chain reaction) for COVID-19 in samples from the lower respiratory tract compared with the upper respiratory tract.12
It is possible that the deposition site within the recipient is relevant to successful transmission of infection.5 Droplet size is one important factor for transmission of infectious pathogens. Droplets as aerosols <5 μm diameter may pass down small airways into the alveoli. Droplets <10 μm may pass below the glottis, whereas large droplets >20 μm diameter are too heavy to be inhaled, but they may still land on upper airway mucosa.4 Identification of the mode of transmission is essential for creating a strategy to control the spread of an epidemic, including the proper choice of PPE.5
The survival of pathogens within the droplet is determined biologically and contributes to the rate of inactivation of pathogens. Viral inactivation may be influenced by external factors. For example, ultraviolet radiation is known to increase the inactivation rate of influenza viruses.5
Many infectious pathogens (viruses, bacteria and fungi) can be transmitted through the airborne route.4 However, this article is focused on viral transmission. Viral bioaerosols usually originate from the respiratory tract.10 Which viruses remain virulent in this state is unclear.
Transmission of specific pathogenic viruses
The transmission of a virus is related its properties including the mode of transmission and the reproduction number (see Table 1).
Table 1.
Properties related to viral transmission.
| Virus | Important characteristics | Reproduction number (R0) | Mode of transmission | Vaccine available? |
|---|---|---|---|---|
| SARS (SARS-CoV-1)13 | Single-stranded RNA virus (coronavirus) | 2–4 | Likely airborne in addition to droplet and contact | No |
| COVID-19 (SARS-CoV-2)14 | 2.4–3.4 | Yes | ||
| MERS15 | 0.3–0.8 | Under development | ||
| Common cold16 | 2–3 | No | ||
| Influenza17 | Single-stranded RNA virus (Orthomyxoviridae) | 0.9–2.1 (strain dependent) | Mixed: likely airborne, droplet and contact | Yes |
| Ebola18 | Single-stranded RNA virus (Filoviridae), which causes viral haemorrhagic fever | 1.2–2.5 | Contact | Yes |
| Varicella zoster virus19 | DNA virus (Herpesviridae), which causes chickenpox | 10–12 | Mixed: airborne, droplet and contact | Yes |
| Measles20 | Single-stranded RNA virus (Paramyxoviridae) | 12–18 | Mixed: airborne, droplet and contact | Yes |
| Smallpox21 | Double-stranded DNA orthopoxvirus (Poxviridae) | 3.5–6 | Mixed: airborne, droplet and contact | Yes: eradicated; last case in 1977 |
-
(i)
Coronaviruses: SARS or SARS-CoV-1, Middle East respiratory syndrome (MERS) and COVID-19 (or SARS-CoV-2) are caused by single-stranded RNA viruses from the coronavirus family. A minority of relatively benign, seasonal common cold viruses are also coronaviruses, with the remainder being caused by rhinovirus, respiratory syncytial virus and parainfluenza. SARS emerged in 2003, MERS in 2012 and COVID-19 in 2019.
Several studies support the hypothesis of the airborne transmission of SARS. For SARS, MERS and the seasonal cold, it is likely that a proportion of transmission is airborne, in addition to droplet and contact spread.
Lower respiratory tract samples for SARS, MERS and COVID appear to offer a better diagnostic yield than upper respiratory tract samples, and symptomatic patients appear to have increased lower respiratory symptoms. This suggests that transmission must involve particles sufficiently small to travel down to the lower airways, such as aerosols.4 The exact transmission of COVID-19 is presumed to be airborne, droplet and contact. Further evidence is required to support this, but under experimental conditions SARS-CoV-2 was found to remain viable in aerosol for up to 3 h.22 Viral RNA has been detected in droplets <5 μm, and the virus has been shown to maintain infectivity in droplets of this size.23
-
(ii)
Influenza: a single-stranded RNA virus of the orthomyxoviridae family. It remains unclear whether human influenza viruses are spread by aerosol or large droplets. Numerous studies have detected influenza RNA molecules in exhaled and environmental air surrounding infectious patients, but more recent studies have questioned the viability of the virus in these air samples. It is likely that transmission is possible by both airborne and droplet spread, and a mixed picture of transmission, varying with the environment and circumstances, is realistic.4 Influenzae are currently the only common seasonal respiratory viruses for which there are licensed antiviral drugs and vaccines available.
-
(iii)
Measles: a single-stranded RNA virus from the paramyxoviridae family. Some evidence supports the possibility of airborne transmission.
-
(iv)
Chickenpox: caused by the varicella zoster virus, a DNA virus from the herpesviridae family. Evidence supports the possibility of airborne transmission.
-
(v)
Smallpox: double-stranded DNA orthopoxvirus from the poxviridae family. Literature supports the airborne transmission of this disease with one hospital outbreak affecting 17 patients spread over different floors.
-
(vi)
Ebola: a single-stranded RNA virus of the filoviridae family causing a viral haemorrhagic fever with high mortality. Four different ebola species are known with the most recent being an Ebola Zaire outbreak in West Africa between 2013 and 2016. Ebola has a low infectious dose, and animal studies have proved airborne spread is possible. Viral load in infected patients is very high, so all bodily fluids should be considered infectious.
Aerosol generation during airway management procedures
As discussed previously, the WHO definition of an AGP is ‘any medical procedure that can induce the production of aerosols of various sizes, including droplet nuclei’.3 Several agencies have produced guidance on what constitutes an AGP, but there does not appear to be a consensus.2 In 2012, Tran and colleagues conducted a systematic review of 10 studies (five case–control and five retrospective cohort studies).24 They assessed the risk of transmission of SARS to HCWs undertaking different procedures potentially capable of aerosol generation. Tracheal intubation was found to have consistently the highest association with transmission of SARS. Seven out of eight studies examined this procedure, demonstrating an increased association of viral transmission to HCWs (odds ratio 6.6; 95% confidence interval [CI]: 2.3–18.9). Also implicated were non-invasive ventilation (n=2 studies), tracheostomy (n=1) and manual ventilation before tracheal intubation (n=1). Other procedures were not found to have a statistically significant association with viral transmission on HCW.24 All of the 10 studies included in this systematic review were felt to be of ‘very low’ quality using the Grading of Recommendations Assessment, Development and Evaluation criteria, because of limitations in study design and imprecision. Crucially, the authors also acknowledged that for those studies included in the systematic review, they were unable to exclude transmission via other routes (droplet or contact), that they were small studies, lacking mandatory reporting of infections after AGPs and included many confounders (such as the same HCW performing manual ventilation and tracheal intubation). However, it is the only systematic review on the subject and subsequently has informed many policies on AGPs. The guidance published by Public Health England suggests multiple other procedures that should be considered as aerosol generating.25 The current AGPs guided by these sources are listed in Table 2.
Table 2.
Aerosol-generating procedures according to Tran and colleagues and Public Health England (PHE) 2020 guidance.24,25 ∗The evidence relating to suctioning is associated with ventilation. In line with a precautionary approach, open suctioning of the respiratory tract regardless of association with ventilation has been incorporated into the current PHE (COVID-19) AGP list. †Evidence trends towards positive association with increased risk of transmission but not statistically significant.24‡Current Resuscitation Council UK advice during the COVID-19 epidemic is to treat cardiopulmonary resuscitation as an AGP. However, PHE and New and Emerging Respiratory Virus Threats Advisory Group state clearly that chest compressions and defibrillation are not AGPs.
| Aerosol-generating procedures with increased risk of transmission of an infectious disease based on evidence from systematic review24 | Additional aerosol-generating procedures based on guidance from Public Health England 2020 | Relevant procedures with unclear risk |
|---|---|---|
|
|
|
Interpretation and practical application of this guidance remain challenging, with many agencies taking the most conservative approach to PPE during the COVID-19 pandemic. Counterarguments to this are that HCWs present for AGPs are at greater risk through the cumulative exposure of other job roles, instead of specifically through tracheal intubation. Furthermore, some argue that some of the procedures defined as AGPs, such as tracheal intubation, lack the high gas velocities required to generate an aerosol, unless there is coughing. Recent evidence quantifying aerosol generation during intubation, extubation, facemask ventilation and supraglottic airway use support this: these procedures have been shown to generate significantly fewer aerosols than a volitional cough.26, 27, 28 Other emerging evidence suggests that some respiratory therapies (non-invasive ventilation and high-flow nasal oxygen) may actually reduce the number of aerosols generated by a patient.29, 30 Despite this, at the time of publication there has been no change to national policy on AGPs and use of PPE.
Aerosol generation during other procedures
Several surgical procedures have been highlighted as being capable of generating aerosols. Examples include dental, orthopaedic and cardiothoracic surgeries. When high-powered electric tools are used, even blood-borne viruses (such as human immunodeficiency virus and hepatitis) can become airborne. Whether transmission of these viruses via this route is possible remains debatable, highlighting the point that an airborne pathogen may not always transmit infection.4
During the COVID-19 pandemic, endoscopy and colonoscopy were highlighted as having a risk of aerosol generation because of the possibility for faecal–oral spread of virus.10 Furthermore, surgery on the upper respiratory tract, including bronchoscopy and laryngoscopy, and surgery that uses the airway for operative access, such as transnasal skull base surgery, are deemed high risk because the anatomical site can potentially act as a reservoir with a high viral load.11 Surgery on the upper respiratory tract using high-speed drills, which promote aerosolisation of potentially infected mucous, was avoided or minimised at the start of the pandemic.11 However, with improvements in screening for COVID-19, this stance has been relaxed.
The majority of operative procedures occur in the operating theatre environment, and hence may benefit from enhanced ventilation or laminar flow systems. Aerosol suspension times, even for smaller droplet nuclei, can be greatly reduced in the presence of a significant downdraft.4 This supports an increased number of air changes per hour.
One paper reported that bed-making, tea trolley round, floor mopping, moving furniture, drug rounds, vacuum cleaner use, toilet use and cleaning a patient's room were found to produce aerosols. More recent evidence confirmed that aerosols can be produced by simple activities in theatre, such as opening and using certain equipment (gauze).26 This highlights the challenges faced when researching this area. Identifying aerosols containing respiratory viruses that are clinically relevant, and then establishing whether these aerosols pose any risk to HCWs and patients is crucial.
Relevance and risks to the anaesthetist and intensivist
Much of the existing evidence base on airborne transmission of infectious pathogens stems from the SARS epidemic. As a result, it may not be generalisable to other respiratory pathogens, such as COVID-19 or influenza.24 Emerging data suggest that one in 10 HCWs involved in tracheal intubation of a patient with confirmed or suspected COVID-19 will go on to develop laboratory-confirmed COVID-19 or symptoms requiring self-isolation or hospital admission.31 However, few viruses are thought to be transmitted exclusively by a single route, so although airborne transmission is important, droplet and contact precautions remain crucial in risk mitigation.
A comprehensive risk assessment should be the first step before any procedure, taking note of any known or suspected infectious disease.25 Risk stratification should consider the transmission risk from a patient to HCWs, but also the risk of increased morbidity and mortality to a patient who subsequently develops COVID-19 after major surgery. Risk stratification for surgery may place patients into three pathways: green (COVID-19 negative), amber (COVID-19 unknown) and red (COVID-19 positive).32 Using binomial modelling, it has been suggested that the risk of a COVID-19 encounter can be quantified as one in one for red, one in 2,000 for amber and one in 10,000 for green pathways.32 However, these rates will vary based on the background population prevalence and the false-negative rate for COVID-19 swab testing. False-negative rates have been reported to range widely (between 0.02 and 0.54) with a summary estimate of 0.13 (95% CI: 0.09–0.19).33
Personal protective equipment, including gloves, long-sleeved gowns, eye protection (goggles or a face-shield) and a face mask (N95 or FFP3 face mask), have been recommended for use during AGPs, such as intubation and extubation, but also during relevant surgical procedures.3 Some authorities have advocated the use of respirators for HCWs with any contact with patients with COVID-19.34 Although effective in preventing viral transmission, PPE carries a financial, waste management, human factor and ergonomics burden, and it is associated with increased problems with communication, vision, fine and gross motor skills.
The use of adequately ventilated single rooms, with as few staff present as is feasible, is also advocated.3,11 Videolaryngoscopy for tracheal intubation is recommended as first line, as it allows the intubator to remain at arm's length from the patient's airway. Evidence from the intubation of suspected or confirmed patients with COVID-19 suggests that the majority of intubations involved three staff members and videolaryngoscopy was used in 76% of cases, noting that the majority of cases were from UK, USA and Australia.31 Waiting for aerosols to clear before opening doors to the room is also a generally accepted practice, but the time required varies based on the ventilation properties of each room. A minimum of six total air changes per hour in ICU and 15 per hour in operating theatres is recommended by the Centers for Disease Control and Prevention.35 Each air change removes approximately 63% of air from the room, with >99% of air being exchanged after five air changes.
There are strong recommendations that respiratory hygiene is practised by all, such as covering the nose and mouth while coughing or sneezing with a mask, tissue or flexed elbow, followed by scrupulous hand hygiene, to reduce the dispersal of infectious pathogens.3,25 More recently, all patients and HCWs have been encouraged to wear face masks.
With appropriate risk mitigation in three key areas (PPE, risk stratification of patients and organisation of the environment), high-risk AGPs can be performed with a low risk to HCWs (Table 3).24
Table 3.
Suggested approach to minimise cross infection from AGPs.
| Environment | Procedure | Airborne personal protective equipment | Risk stratification |
|---|---|---|---|
|
|
|
|
Conclusions
The general quality of evidence surrounding AGPs is low, and there is an accepted research gap in this area. Although wearing PPE has resource, cost and human factor implications, it remains an effective way to prevent the transmission of airborne pathogens.
However, many questions remain unanswered. Should airborne PPE be worn when dealing with infectious patients who are coughing or sneezing (i.e. natural droplet generation)? Are all AGPs truly aerosol generating? Are the risks to HCWs more related to working in a high-risk environment and being exposed to droplet and contact transmission than aerosols themselves? Do we need to control airflow direction for AGPs? Further precision is also required to define the list of AGPs.
With so many unanswered questions, a pragmatic approach is required (Table 3). Although a precautionary approach is prudent, we must avoid the inappropriate use of PPE, particularly if supplies are limited. Whilst many procedures, activities and equipment may generate an aerosol, the subsequent risk of transmission of an infectious pathogen is not well understood.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Charlie PopeMBBCh FRCA FICM PGCE is a specialty registrar in anaesthesia and intensive care medicine at Southmead Hospital, Bristol, UK. He has an interest in medical education.
William Harrop-GriffithsMA MBBS FRCA FCAI is a professor at Imperial College, London, UK and a consultant anaesthetist at Imperial College Healthcare NHS Trust, London, UK. He is an elected council member of the Royal College of Anaesthetists and chair of its Clinical Quality and Research Board. He is an advisor to the Army and a past president of both the Association of Anaesthetists and the Tri Service Anaesthetic Society. His interests include regional anaesthesia, public speaking and grammatical nitpicking.
Jules BrownBSc MBChB MRCP FRCA DICM FICM is a consultant in anaesthesia and intensive care medicine at Southmead Hospital, Bristol, UK. He is an examiner for the Faculty of Intensive Care Medicine, and his past roles include being intensive care unit lead for infection prevention and control.
Matrix codes: 1A01, 1A03, 1C02, 2A01, 2C01, 2C02, 3A01, 3C00
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Carroll D., Morzaria S., Briand S., et al. Preventing the next pandemic: the power of a global viral surveillance network. BMJ. 2021;372:485. doi: 10.1136/bmj.n485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook T.M. Personal protective equipment during the COVID-19 pandemic—a narrative review. Anaesthesia. 2020;75:920–927. doi: 10.1111/anae.15071. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . 2014. Infection prevention and control of epidemic and pandemic prone acute respiratory infections in health care: WHO Guidelines.https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care Available from: [PubMed] [Google Scholar]
- 4.Tellier R., Li Y., Cowling B.J., et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drossinos Y., Stilianakis N.I. What aerosol physics tells us about airborne pathogen transmission. Aerosol Sci Technol. 2020;54:639–643. [Google Scholar]
- 6.Maynard A.D. 2020. How long do aerosol particles stay airborne?https://therealandrewmaynard.com/2020/07/17/how-long-do-aerosols-stay-airborne/ Available from: [Google Scholar]
- 7.Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zayas G., Chiang M., Wong E., et al. Cough aerosol in healthy participants: fundamental knowledge to optimize droplet-spread infectious respiratory disease management. BMC Pulm Med. 2012;12:11. doi: 10.1186/1471-2466-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020;323:1837–1838. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 10.Zemouri C., de Soet H., Crielaard W., et al. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castelnuovo P., Turri-Zanoni M., Karligkiotis A., et al. Skull-base surgery during the Covid-19 pandemic: the Italian skull base society recommendations. Int Forum Allergy Rhinol. 2020;10:963–967. doi: 10.1002/alr.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drame M., Teguo M.T., Proye E., et al. Should RT-PCR be considered a gold standard in the diagnosis of Covid-19? J Med Virol. 2020;92:2312–2313. doi: 10.1002/jmv.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Consensus document on the epidemiology of severe acute respiratory syndrome (SARS), Geneva. 2003. Department of communicable disease surveillance and response (technical report) [Google Scholar]
- 14.Billah M.A., Miah M.M., Khan M.N. Reproductive number of coronavirus: a systematic review and meta-analysis based on global level evidence. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kucharski A.J., Althaus C.L. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Euro Surveill. 2015;20:14–18. doi: 10.2807/1560-7917.es2015.20.25.21167. [DOI] [PubMed] [Google Scholar]
- 16.Freeman C. Magic formula that will determine whether Ebola is beaten. Telegraph. 2014 [Google Scholar]
- 17.Coburn B.J., Wagner B., Blower S. Modeling influenza epidemics and pandemics: insights into the future of swine flu (H1N1) BMC Med. 2009;7:30. doi: 10.1186/1741-7015-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Kerkhove M.D., Bento A.I., Mills H.L., Ferguson N.M., Donnelly C.A. A review of epidemiological parameters from Ebola outbreaks to inform early public health decision-making. Sci Data. 2015;2:150019. doi: 10.1038/sdata.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health Service Executive (Ireland) March 2020. Healthcare worker information: chapter 23 varicella zoster.https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter23.pdf [Google Scholar]
- 20.Guerra F.M., Bolotin S., Lim G., et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:e420–e428. doi: 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 21.Gani R., Leach S. Transmission potential of smallpox in contemporary populations. Nature. 2001;414:748–751. doi: 10.1038/414748a. [DOI] [PubMed] [Google Scholar]
- 22.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med April. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morawska L., Milton D.K. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran K., Cimon K., Severn M., et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Public Health England . 2020. COVID-19: infection prevention and control (IPS): guidance on infection prevention and control for seasonal respiratory infections including SARS-CoV-2.https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control Available from: [Google Scholar]
- 26.Brown J., Gregson F.K.A., Shrimpton A., et al. A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia. 2021;76:174–181. doi: 10.1111/anae.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrimpton A.J., Brown J.M., Gregson F.K.A., et al. Quantitative evaluation of aerosol generation during manual facemask ventilation. Anaesthesia. 2022;77:22–27. doi: 10.1111/anae.15599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrimpton A.J., Gregson F.K.A., Brown J.M., et al. A quantitative evaluation of aerosol generation during supraglottic airway insertion and removal. Anaesthesia. 2021;76:1577–1584. doi: 10.1111/anae.15542. [DOI] [PubMed] [Google Scholar]
- 29.Wilson N.M., Marks G.B., Eckhardt A., et al. The effect of respiratory activity, non-invasive respiratory support and facemasks on aerosol generation and its relevance to COVID-19. Anaesthesia. 2021;76:1465–1474. doi: 10.1111/anae.15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheikh S., Hamilton F.W., Nava G.W., et al. 02 November 2021. Are aerosols generated during lung function testing in patients and healthy volunteers? Results from the AERATOR study Thorax Published Online First. [DOI] [PubMed] [Google Scholar]
- 31.El-Boghdadly K., Wong D.J.N., Owen R., et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia. 2020;75:1437–1447. doi: 10.1111/anae.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakodkar P.S., Sivia D.S., Pandit J.J. Safety of aerosol-generating procedures in COVID-19 negative patients: binomial probability modelling of intubateCOVID registry data. Anaesthesia. 2020;75:1415–1419. doi: 10.1111/anae.15235. [DOI] [PubMed] [Google Scholar]
- 33.Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Centre for Disease Prevention and Control . 2021. Infection prevention and control and preparedness for COVID-19 in healthcare settings (sixth update)https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-covid-19-healthcare-settings Available from: [Google Scholar]
- 35.Centers for Disease Control and Prevention . 2003. Guidelines for environmental infection control in health-care facilities.https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html [updated July 2019]. Available from: [Google Scholar]