Highlights
-
•
Several chronic and acute pathological conditions are responsible of endothelial glycocalyx damage.
-
•
Role of heparanase on glycocalyx degradation.
-
•
HPSE dependent glycocalyx dysfunction conditions.
-
•
Heparanase inhibition as a therapeutic option to prevent glycocalyx degradation.
Keywords: Endothelium, Glycocalyx, Heparanase
Abstract
The surface of all animal cells is coated with a layer of carbohydrates linked in various ways to the outer side of the plasma membrane. These carbohydrates are mainly bound to proteins in the form of glycoproteins and proteoglycans and together with the glycolipids constitute the so-called glycocalyx. In particular, the endothelial glycocalyx that covers the luminal layer of the endothelium is composed of glycosaminoglycans (heparan sulphate -HS and hyaluronic acid -HA), proteoglycans (syndecans and glypicans) and adsorbed plasma proteins. Thanks to its ability to absorb water, this structure contributes to making the surface of the vessels slippery but at the same time acts by modulating the mechano-transduction of the vessels, the vascular permeability and the adhesion of leukocytes in thus regulating several physiological and pathological events. Among the various enzymes involved in the degradation of the glycocalyx, heparanase (HPSE) has been shown to be particularly involved. This enzyme is responsible for the cutting of heparan sulfate (HS) chains at the level of the proteoglycans of the endothelial glycocalyx whose dysfunction appears to have a role in organ fibrosis, sepsis and viral infection.
In this mini-review, we describe the mechanisms by which HPSE contributes to glycocalyx remodeling and then examine the role of glycocalyx degradation in the development of pathological conditions and pharmacological strategies to preserve glycocalyx during disease pathogenesis.
Introduction
The endothelium is defined histologically as a typical example of a simple paving (squamous) epithelium lining the blood and lymphatic vessels. It is formed by a single continuous layer of flattened polygonal cells joined and aligned according to the direction of the blood or lymphatic flow. Especially at the level of blood capillaries, the phenotype of endothelial cells can change according to the organs and the function they perform there [1]. As a proof of concept in the kidney, the endothelial cells of the glomerulus capillaries are fenestrated to allow blood filtration while those of the larger diameter vessels are characterized by the presence of occluding junctions between contiguous cells [2]. Like all epithelial lining tissues, endothelial cells also present a gelatinous layer of a glucidic nature called glycocalyx at the lumen level, which effectively constitutes an element of the vascular barrier [3]. Initially considered to be a simple protective barrier for cells, the glycocalyx has in recent years been recognized as one of the main barriers responsible for endothelial functions. It was also found that alterations of this structure are at the basis of some pathological situations such as sepsis, fibrosis, metabolic diseases and chronic cardiovascular and renal diseases [4], [5], [6], [7]. The purpose of this review is to describe not only the composition and function of the endothelial glycocalyx, but also the responsible mechanisms for its degradation by heparanase-1(HPSE) and then to discuss possible strategies aimed at safeguarding this cellular structure.
Endothelial glycocalyx structure and functions
The glycocalyx of vascular endothelial cells is mainly composed of side chains of glycosaminoglycans (GAGs) conjugated with the protein axis of proteoglycans linked to the plasma membrane (basically syndecans 1, 2 and 4 and glypican 1), by HA bound by its receptor, as well as from glycoproteins and adsorbed plasma proteins are attached [8], [9]. Structurally speaking, proteoglycans consist of a protein core to which long linear chains of negatively charged GAGs including heparan sulfate (HS), heparin (Hep), chondroitin sulfate (CS), keratan sulfate (KS) and dermatan sulfate (DS) [10]. Thanks to the numerous sulphate groups, the GAGs are negatively charged and this gives them the ability to bind considerable quantities of water, contributing to tissue hydration and mechanical resistance to compression. In addition, they can bind other molecules including growth factors, cytokines and enzymes with a storage function, thus protecting them from degradation [11]. They also help to create a gradient necessary for fluid transit and blood filtration in the kidney [12].
The core protein to which the GAGs of the endothelial glycocalyx bind belong to the group of transmembrane syndecans and to glypican-1 (GPC1) which binds to the membrane by means of GPI anchors. In particular, syndecans-1, -2, -4 (SDC1, SDC2, SDC4), have three attachment sites for HS in the N-terminal regions most distant from the cell surface while SDC1 also contains two additional sites for CS closer to the membrane [13]. The cytoplasmic tails of syndecans bind to various cytoskeletal proteins through connecting molecules helping to distribute pressure and shear blood forces over the entire endothelial cell [14]. GPC1 is the only glypican present in the endothelial glycocalyx and has three or four attachment sites exclusively for HS. GPC1 indirectly binds to the plasma membrane by anchoring with glycosylphosphatidylinositol (GPI) which localizes this proteoglycan at the level of the lipid rafts [13].
HA is the only non-sulfated GAG found mainly in the luminal part of the glycocalyx and which is synthesized on the cell surface by specific enzymes (HAS1, 2 and 3) [15]. Unlike HS and CS, it is a longer disaccharide polymer which does not covalently bind to any protein but is anchored to the cell membrane by specific surface receptors such as CD44. Although lacking in sulphate groups, HA contains numerous negative charges due to the presence of carboxylic groups which give it considerable hydration capacity [16].
Furthermore, at the endothelial glycocalyx level, glycoproteins with short oligosaccharide chains attached to their protein nucleus can also be found. These oligosaccharides are covered at their ends by molecules of sialic acid (SA), a 9-carbon monosaccharide that contributes to the net negative charge of the glycocalyx [17]. Other glycoproteins are considered to be part of the glycocalyx and include some important membrane receptors, including mechano-sensors and transducers such as selectins and integrins, and members of the immunoglobulin superfamily. Some plasma proteins such as albumin, antithrombin and alpha 1 acid glycoprotein can get trapped at GAG levels and thus further extend the glycocalyx layer [18], [19].
Mechanisms of glycocalyx degradation
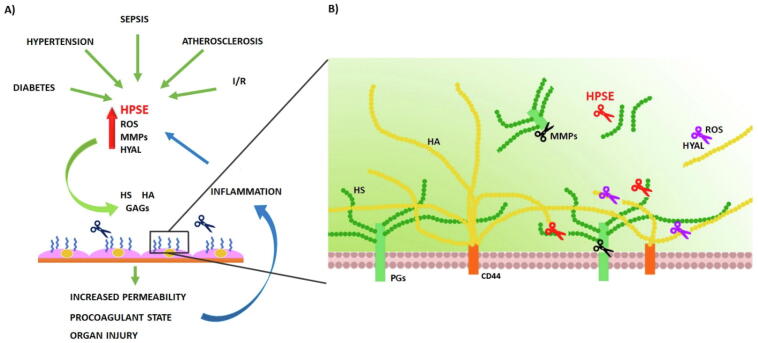
Several acute and chronic conditions such as sepsis, ischemia/reperfusion (I/R) injury, diabetes, trauma, atherosclerosis, hypertension, kidney and acute lung injury are characterized by endothelial glycocalyx degradation [20], [21], [22], [23], [24], [25]. Endothelial glycocalyx degradation determines the release of its components, i.e. HA, HS and/or proteoglycans (PGs) such as SDC1, into the bloodstream, where they can be measured to provide information about endothelial glycocalyx damage [26], [27], [28] since most of these parameters correlate with the severity of disease and mortality [23], [29]. The mechanisms prompting endothelial glycocalyx are various, and they impact each other fueling the degradation of the glycocalyx: reactive oxygen/nitrogen species (ROS/RNS), matrix metalloproteinases (MMPs), hyaluronidases (HYAL) and HPSE. (Fig. 1).
Fig. 1.
A) Several chronic and acute pathological conditions are responsible of endothelial glycocalyx damage. During sepsis, global and regional I/R, diabetes mellitus, hypertension, atherosclerosis a series of factors (HPSE, MMPs, HYAL and ROS) are produced and they contribute to glycocalyx shedding/depolymerization targeting HS, HA and proteoglycans. Glycocalyx dysfunction impairs the local microcirculation increasing procoagulant state leading to organ injury. Sub-endothelial damage together with cytokines released by glycocalyx shedding induce an inflammatory state which in a vicious loop sustain glycocalyx degradation. B) In detail, different glycocalyx components are degraded by different agents. HS is cleaved by HPSE, PGs are shedded by MMPs and HA is cleaved by HYAL and degraded by ROS.
Oxidative stress and ROS/RNS have a central role in endothelial glycocalyx degradation in an inflammatory context [30]. Two typical ROS components are peroxides and radicals; small molecules that are able to form chemical bond with DNA, lipids and proteins. ROS and RNS are also able to damage the GAGs in the glycocalyx by modifying the saccharide residues. The modified sugars are unstable and susceptible to hydrolytic cleavage which leads to the fragmentation of the polysaccharides. The unsulfated high-molecular weight polymer of HA are particularly vulnerable to chemical modifications and the derived low-molecular weight HA fragments have a pro-inflammatory activity sustaining, in turn, ROS production [31], [32], [33], [34], [35].
In physiological conditions, ROS is also essential for signaling mechanisms, regulating e.g. proliferation, differentiation and migration under physiological conditions [36] and their levels are controlled by antioxidant enzymes for ROS inactivation, such as SOD, catalases and glutathione peroxidases [37]. However, under pathological conditions such as atherosclerosis and I/R injury this balanced state is altered.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases, which cleave extracellular matrix components such as collagen, gelatin and elastin, thereby promoting vascular remodelling [38]. During vascular pathologies MMPs are mainly produced by inflammatory cells, but also by stimulated vascular smooth muscle cells [39]. Important activators of MMPs and inactivators of tissue inhibitors of MMPs (TIMPs) are ROS [40], but cytokines, shear stress, hypoxia and hormones may also lead to enhanced proteinase activity in atherosclerosis, hypertension, chronic venous disease, and aneurysm formation [41], [42].
MMPs degrade endothelial glycocalyx by cleaving the protein core of proteoglycans, such as syndecans [43]. Shedding of syndecans can represent a measure of glycocalyx degradation [44], [45], [46]. Specifically, SDC-1 is shed by MMP-2, MM-9, MT1-MMP and ADAM-17 and, SDC-2 is cleaved by MMP-9 [22], [47]. MMPs can also degrade chondroitin sulphate [48]. The action of MMPs reduces glycocalyx thickness [25] and causes the release of heparan sulphate and syndecans into the bloodstream sustaining thrombosis and endothelial inflammation [47], [49].
Another important constituent of endothelial glycocalyx is hyaluronic acid (HA), a linear, non-sulfate, negatively-charged glycosaminoglycan that consists of glucuronic acid and n-acetylglucosamine repeating units. HA within the glycocalyx binds non-covalently to the receptor CD44 [50]. The principal human hyaluronidases are HYAL1 and HYAL2 whose expression is increased in hyperglycemic and/or inflammatory settings as proved by several studies [51], [52], [53]. Another important regulator of hyaluronidases is shear stress which specifically increases HYAL4 expression which in turn downregulates NO expression [54]. The action of hyaluronidase on the glycocalyx produces low-molecular-weight HA fragments. High- and low-molecular-weight HA have very dissimilar properties. High-molecular weight HA enhances the barrier function of endothelial cells, while low-molecular weight HA is dangerous for endothelial cells, since it activates toll-like receptors 2 and 4, which triggers inflammation [47]. Low-molecular-weight HA fragments can also stimulate ROS production in a size-dependent manner and inducing the expression of VCAM-1 and CM-1, fueling inflammation and damage to endothelial cells [47].
Considering that between 50 % and 90% of the GAGs in the glycocalyx consist of HS [55], it is not difficult to predict how HPSE, an enzyme involved in the degradation of HS, can play a fundamental role in the turnover of the glycocalyx associated with different pathological situations.
The role of heparanase in glycocalyx degradation
Heparanase (HPSE) is defined as an endo-β-D-glucuronidase belonging to the group of glycosidic hydrolases or enzymes capable of processing carbohydrates. The cleavage site on which HPSE intervenes is the β 1,4 glycosidic bond between GlcA and GlcNS in the chains. Only a limited number of sites are cut by HPSE thus releasing 5–10 kDa fragments of HS (10–20 sugar units). The human gene encoding heparanase-1 (HPSE) has been mapped to chromosome 4q21.3 and can give rise to two mRNAs containing the same open reading module as a result of alternative splicing [56], [57]. Shortly thereafter, a second homologous gene with HPSE, named heparanase-2, was identified. It encodes the HPSE-2 protein which has an homology sequence of 40%. Unlike HPSE, this variant does not exhibit glycosidase activity but appears to have an inhibitory role against HPSE thus hypothesizing a role as a tumor suppressor [58].
HPSE is synthesized as a 65 kDa proenzyme which undergoes a post-translational cut, generating two subunits of 50 kDa and 8 kDa which are not covalently connected and which constitute the active form of the enzyme [59]. The glycosylation of HPSE is fundamental for its transport through the endoplasmic reticulum and the Golgi apparatus and its secretion [60]. The conversion and activation of the inactive pro-HPSE into the active dimeric enzyme then requires its reabsorption by endocytosis where at the lysosomal level the cathepsin L catalyzes the cut that originates the two subunits that form the mature enzyme. Mannose 6-phosphate and low-density lipoproteins receptors have been described as high-affinity receptors for HPSE while membrane HSPGs (in particular, syndecans) as low-affinity receptors [61]. In non-pathological tissues and in normal physiological conditions, HPSE usually shows low levels of protein expression limited mostly to keratinocytes, trophoblast, platelets and leukocytes [62]. On the contrary, in pathological conditions such as in tumor progression and metastasis, in inflammation, during the epithelial-mesenchymal transition (EMT) and in fibrosis there is a notable increase in expression for HPSE [63], [64], [65].
In the vascular endothelium it has been reported that the expression of HPSE is upregulated at the site of inflammation in multiple organs [65]. Its expression is up-regulated in endothelial cells by several factors: ROS [66], inflammatory cytokines [67], high glucose [68] and advanced glycosylation products [69]. HPSE is also released by inflammatory cells and platelets [70] within the vasculature.
HPSE can modulate glycocalyx damage in various ways: first, by degrading HS, HPSE controls the communication of endothelial cells with blood cells [71]. In fact, the degradation of heparan sulfate chains renders the proteoglycan core protein more accessible to enzymatic cleavage by MMPs and thrombin [72], [73]. By cleaving HS, HPSE also regulates vascular permeability [74], and makes adhesion molecules and cytokine receptors more accessible for binding and activation, thus propagating inflammation [75], [76], [77], [78]. Specifically, in inflammation, factors such as TNF-α promotes the activation of vascular endothelial cells by increasing P- and E-selectin expression. Subsequently, leukocytes begin to roll and attach to endothelial cells, and chemokines on endothelial cells start to activate followed by chemokine activation of the leukocytes [79], [80]. Following leucocyte attachment to the endothelial cell surface, they traverse into the interstitial tissue and inflict inflammatory damage to the organs [81]. The release of pro-inflammatory cytokines and growth factors linked to HS sustain oxidative stress with an additional fueling of inflammation [82]. Moreover, HS fragments released by HPSE activate toll-like receptor (TLR) 4 signaling [77] and concentrate growth factors and cytokines to an easier ligand recognition by cognate cell surface receptors [83]. TLR activation on macrophages activates Nuclear factor-kB (NF-kB) leading to expression of additional inflammatory cytokines (TNF-α, IL-1β, IL-8) [84]. The cytokines can also stimulate HPSE expression on endothelial cells [68] and potentiate release of ROS and MMPs [85].
HPSE also has non-enzymatic activity on endothelial cells through ERK activation and intracellular regulation of SDC-1 expression [86]. HPSE may contribute to MMP-9 and VEGF upregulation and trauma [87], [88], [89]. It has been established that an altered profile of circulating exosome levels is associated with the onset of critical illness [90], [91] and HPSE may sustain secretion of exosomes by activating the syndecan–syntenin–ALIX complex that engages the endosomal-sorting complex required for transport (ESCRT) [92], [93].
The shed glycocalyx layer prejudices intracellular production of endothelial nitric oxide synthase (eNOS) [94], [95]. Reduced eNOS activity diminishes nitric oxide (NO) production leading to impaired vascular reactivity. NO then promotes modifications in the exocytosis of Weibel–Palade bodies [96], [97] which favors the release of von Willebrand factor and angiopietin-2. In the end, these molecules activate circulating platelets and promote endothelial destabilization [98], [99].
HPSE also contributes to the pro-coagulant state by increasing tissue factor (TF) activity resulting in increased activation of the coagulation system [85]. Moreover, HPSE up-regulates TF expression in endothelial cells and the release of the protein tissue factor pathway inhibitor (TFPI) from the cell surface [100].
In summary, the consequences of HS cleavage lead to an activated and inflamed endothelium that results in tissue edema and hypoperfusion that sustain end-organ injury.
HPSE dependent glycocalyx dysfunction conditions
Glycocalyx dysfunction arises in response to mechanical cellular stress, endotoxins, inflammatory mediators, atrial natriuretic peptide, ischemia–reperfusion injury, free oxygen radicals and hyperglycemia, and also the novel corona-virus disease-2019 (COVID-19) [47], [101].
Glycocalyx degradation occurs in infective (sepsis) and non-infective (trauma) inflammation [102] and in these setting TNF-α is a central player [73]. Sepsis induces glycocalyx degradation and leads to delayed regeneration [103]. Afterwards, this setting also stimulates the recruitment and phenotype alterations of macrophages [104], [105] as well as leukocyte adhesion and focal vascular inflammation [74]. HPSE enzymatic activity and syndecan-1 shedding are increased in trauma [106], [107], [108] and sepsis [29], [109], [110] patients and they correlate with the degree of illness [44] and these results has been validated in a study of human and murine study [111]. Also, both septic patients with lung injury have increased levels of circulating HS [112] and sepsis-induced acute kidney injury (AKI) patients show augmented urinary HS [113] that may reflect increased HPSE levels due to the inflammatory state [114]. The central action of HPSE in sepsis is also confirmed by the fact that its inhibition in preclinical models mitigates lung injury and renal dysfunction [73], [115].
Hemorrhagic shock is one of the main responsible of endothelial glycocalyx derangement and endothelial injury, characterized by the disruption of junctional structures [116], [117]. Ischemia/reperfusion (I/R) event exerts a damage at two timepoints; firstly, glycocalyx damage arises during the hypoxic phase, and secondly during blood reperfusion. Consequently, endothelial cells become swollen and detached from the basement membrane. I/R injury also induces the release of a series of molecules (HPSE, histamine, cathepsin and oxygen-free radicals)which contribute to glycocalyx impairment [118], [119]. This evidence has also been confirmed by the fact that glycocalyx components, such as heparan sulfate and SDC-1, can be detected in soluble form in the plasma of patients undergoing major vascular surgery with global or regional ischemia [24].
Endothelial dysfunction is generally considered to be one of the initial events in atherosclerosis and HPSE also participates in this process. Inflammatory mediators present in atherosclerotic lesions lead to the upregulation of angiopoietin 2 by endothelial cells and foam macrophages; angiopoietin 2, in turn, increases the expression of HPSE, leading to the degradation of heparan sulfate [47], [120]. Then, shed HS fragments activate leukocytes and platelets, increase the expression of ICAM1 and VCAM1, and the consequent leucocyte adhesion and extravasation [47], [76], [121].
Alterations in the endothelial glycocalyx occur early in the onset of diabetes, a pathology in which the vasculature is disrupted globally[122], [123], [124]. HPSE has been deeply implicated in diabetes and its consequences [125]
The coronavirus disease-19 (COVID-19) pandemic has resulted from infection of human with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In the last two decades, other members of the coronavirus family have emerged: the severe acute respiratory syndrome coronaviruses (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) [126]. Globally, as of 13 October 2021, there have been 238,521,855 confirmed cases of COVID-19, including 4,863,818 deaths (World Health Organization).
The glycocalyx is an essential component of cell surfaces and it is the first barrier against all pathogens. Given its location, viruses and other pathogens have evolved to utilize glycocalyx components as attachment factors which facilitate their interaction with cell surface receptors [127]. Different tissues and cell types show differences in HS structure [128] and HS structure can vary between individuals and age groups [129]. These differences may contribute to tissue tropism from different pathogens. For example, several viruses use salicylic acids, while other viruses, such as coronaviruses, interact with heparan sulfate (HS) [130]. The angiotensin-converting enzyme 2 (ACE2), previously identified as the cellular receptor for SARS-CoV, also acts as a receptor of the new coronavirus (SARS-CoV-2) [131]. ACE2 is necessary but insufficient for infection, whereas interaction with both ACE2 and HS is required to initiate a productive infection [130]. Clausen et al 2020 have shown that the interaction of the receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein with cell surface HS promote a conformational change in an “open” active conformation that favors ACE2 binding. Competition studies, enzymatic removal of HS and genetic studies confirm that binding of HS to S protein enhances binding to ACE2 receptor [132].
After infection, replication and dissemination of SARS-CoV-2, COVID-19 patients primarily present common flu symptoms such as fever, muscle pain and cough. In severe cases, they have acute respiratory distress syndrome (ARDS) [133] and progression of illness can cause a multi-organ dysfunction in which a common complication is acute kidney injury (AKI) and proteinuria [134]. In COVID-19 patients, HPSE contribute to the progression of illness both for the disruption of barriers and also for cytokines storm that drive the massive inflammatory response. In fact, HPSE promotes endothelial barrier disruption, by degrading glycocalyx, an event that has been previously described both for ARDS and proteinuric diseases [7], [135]. In addition, HPSE stimulates the expression and release of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-8, IL-10 and TNF-α, that stimulate the recruitment of leukocytes [136], [137]. It has been demonstrated that HPSE levels are up-regulated in COVID-19 infected people and it is associated with disease severity [138]. In addition, it has been reported that the use of low molecular heparin can reduces HPSE activity in COVID-19 patients [138]. In line with increased HPSE activity, also plasma HS and IL-6 levels up-regulated [138]. Interestingly, viral dissemination is supported by high HPSE expression levels [139].
Heparanase inhibition as a therapeutic option to prevent glycocalyx degradation
As described so far, HPSE has been shown to play a fundamental role in promoting the degradation of the glycocalyx associated with various pathologies.
Experimental and clinical evidences have shown that HPSE can be a very promising therapeutic target, considering that this enzyme is unique in the mammalian genome and that its expression in physiological conditions is very low. In recent years, several classes of HPSE inhibitors have been developed ranging from monoclonal antibodies to polysulfated saccharides. The development of these compounds began with the observation that heparin had the ability to inhibit the activity of HPSE due to its competition with HS for binding with the enzyme [140] So, the use of heparin and related compounds such as low molecular weight heparin (LMWH) or ultra-fractionated heparin (UFH), is related to: competition with the cell surface HS binding site reducing viral infection, inhibition of circulating HPSE enzymatic activity, anti-inflammatory and anti-coagulative effects [132], [138], [141], [142]. In this regard, heparin have been the most widely used drugs for the treatments and prevention of endothelial cell disorders. On the other hand, the mimetics of heparan sulfate have a lower anticoagulant activity and a greater selectivity for HPSE than heparin, thus broadening the spectrum of the therapeutic activity of these compounds.
Currently, the only HPSE inhibitors that have reached the clinical trial stage belong to the class of polysaccharides and are: PI-88, PG545, Roneparstat and M402. These inhibitors have so far been used as potential anticancer compounds on the assumption that HPSE has been shown to play a key role in promoting the proliferation, angiogenesis and growth of tumor cells by acting both at the level of the tumor and its microenvironment [143]. Some of them have been shown to be effective in countering the neoplastic growth of some tumors but, more importantly, they have been shown to have a safety profile and good tolerability in prolonged treatments without particular side effects.
It will therefore be desirable that the next drugs aimed at inhibiting the activity of HPSE prove to have therapeutic efficacy not only in the vast field of cancer therapy but also in the treatment of other pathologies such as those affecting the vascular endothelium and for which HPSE is an important factor that determines the disease.
Funding
This work was supported by a grant provided by the University of Padova (BIRD192859/19).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.H.G. Augustin, G.Y. Koh, Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology, Science 357 (2017) 6353:eaal2379 10.1126/science.aal2379. [DOI] [PubMed]
- 2.Dumas S.J., Meta E., Borri M., et al. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nat. Rev. Nephrol. 2021;17:441–464. doi: 10.1038/s41581-021-00411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reitsma S., Slaaf D.W., Vink H., van Zandvoort M.A., Egbrink M.G. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchimido R., Schmidt E.P., Shapiro N.I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit. Care. 2019;23:16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia G., Aroor A.R., Jia C., Sowers J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865(7):1802–1809. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Broekhuizen L.N., Mooij H.L., Kastelein J.J., Stroes E.S., Vink H., Nieuwdorp M. Endothelial glycocalyx as potential diagnostic and therapeutic target in cardiovascular disease. Curr. Opin. Lipidol. 2009;20(1):57–62. doi: 10.1097/MOL.0b013e328321b587. [DOI] [PubMed] [Google Scholar]
- 7.Verma S.K., Molitoris B.A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015;35:96–107. doi: 10.1016/j.semnephrol.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Xie Y., Wong M., Barboza M., Lebrilla C.B. Comprehensive structural glycomic characterization of the glycocalyxes of cells and tissues. Nat. Protoc. 2020;15(8):2668–2704. doi: 10.1038/s41596-020-0350-4. [DOI] [PubMed] [Google Scholar]
- 9.Villalba N., Baby S., Yuan S.Y. The endothelial glycocalyx as a double-edged sword in microvascular homeostasis and pathogenesis. Front. Cell Dev. Biol. 2021;14(9) doi: 10.3389/fcell.2021.711003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karamanos N.K., Theocharis A.D., Piperigkou Z., Manou D., Passi A., Skandalis S.S., Vynios D.H., Orian‐Rousseau V., Ricard‐Blum S., Schmelzer C.E.H., Duca L., Durbeej M., Afratis N.A., Troeberg L., Franchi M., Masola V., Onisto M. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288(24):6850–6912. doi: 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- 11.Karamanos N.K., Piperigkou Z., Theocharis A.D., Watanabe H., Franchi M., Baud S., Brézillon S., Götte M., Passi A., Vigetti D., Ricard-Blum S., Sanderson R.D., Neill T., Iozzo R.V. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018;118(18):9152–9232. doi: 10.1021/acs.chemrev.8b00354. [DOI] [PubMed] [Google Scholar]
- 12.Curry F.-R. Microvascular solute and water transport. Microcirculation. 2005;12(1):17–31. doi: 10.1080/10739680590894993. [DOI] [PubMed] [Google Scholar]
- 13.Iozzo R.V., Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarbell J.M., Ebong E.E. The endothelial glycocalyx: a mechano-sensor and -transducer. Sci Signal. 2008;1(40):8. doi: 10.1126/scisignal.140pt8. [DOI] [PubMed] [Google Scholar]
- 15.Vigetti D., Viola M., Karousou E., De Luca G., Passi A. Metabolic control of hyaluronan synthases. Matrix Biol. 2014;35:8–1316. doi: 10.1016/j.matbio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang G., Tiemeier G.L., van den Berg B.M., Rabelink T.J. Endothelial glycocalyx hyaluronan- regulation and role in prevention of diabetic complications. Am. J. Pathology. 2020;190(4):781–790. doi: 10.1016/j.ajpath.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Fu B.M., Tarbell J.M. Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013;5(3):381–390. doi: 10.1002/wsbm.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldecoa C., Llau J.V., Nuvials X., Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care. 2020;10(1):85. doi: 10.1186/s13613-020-00697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Y.e., Adamson R.H., Curry F.-R., Tarbell J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306(3):H363–H372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancel L.M., Ebong E.E., Mensah S., Hirschberg C., Tarbell J.M. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis. 2016;252:136–146. doi: 10.1016/j.atherosclerosis.2016.07.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Huang X., Kong G., Xu H., Li J., Hao D., Wang T., Han S., Han C., Sun Y., Liu X., Wang X. Ulinastatin attenuates pulmonary endothelial glycocalyx damage and inhibits endothelial heparanase activity in LPS-induced ARDS. Biochem. Biophys. Res. Commun. 2016;478(2):669–675. doi: 10.1016/j.bbrc.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Iba T., Levy J.H. Derangement of the endothelial glycocalyx in sepsis. J. Thromb. Haemost. 2019;17(2):283–294. doi: 10.1111/jth.14371. [DOI] [PubMed] [Google Scholar]
- 23.Johansson P.I., Stensballe J., Rasmussen L.S., Ostrowski S.R. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 24.Rehm M., Bruegger D., Christ F., Conzen P., Thiel M., Jacob M., Chappell D., Stoeckelhuber M., Welsch U., Reichart B., Peter K., Becker B.F. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116(17):1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 25.Ramnath R., Foster R.R., Qiu Y., Cope G., Butler M.J., Salmon A.H., Mathieson P.W., Coward R.J., Welsh G.I., Satchell S.C. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: a contributor to endothelial cell glycocalyx dysfunction. FASEB J. 2014;28(11):4686–4699. doi: 10.1096/fj.14-252221. [DOI] [PubMed] [Google Scholar]
- 26.Marechal X., Favory R., Joulin O., Montaigne D., Hassoun S., Decoster B., Zerimech F., Neviere R. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock. 2008;29(5):572–576. doi: 10.1097/SHK.0b013e318157e926. [DOI] [PubMed] [Google Scholar]
- 27.Chen C., Chappell D., Annecke T., Conzen P., Jacob M., Welsch U., Zwissler B., Becker B.F. Sevoflurane mitigates shedding of hyaluronan from the coronary endothelium, also during ischemia/reperfusion: an ex vivo animal study. Hypoxia (Auckl) 2016;4:81–90. doi: 10.2147/HP.S98660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majerczak J., Grandys M., Duda K., Zakrzewska A., Balcerczyk A., Kolodziejski L., Szymoniak-Chochol D., Smolenski R.T., Bartosz G., Chlopicki S., Zoladz J.A. Moderate-intensity endurance training improves endothelial glycocalyx layer integrity in healthy young men. Exp. Physiol. 2017;102(1):70–85. doi: 10.1113/EP085887. [DOI] [PubMed] [Google Scholar]
- 29.Nelson A., Berkestedt I., Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol. Scand. 2014;58(1):36–43. doi: 10.1111/aas.12223. [DOI] [PubMed] [Google Scholar]
- 30.Rubio-Gayosso I., Platts S.H., Duling B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006;290(6):H2247–H2256. doi: 10.1152/ajpheart.00796.2005. [DOI] [PubMed] [Google Scholar]
- 31.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann. N. Y. Acad. Sci. 1999;893(1):13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 32.Moseley R., Waddington R.J., Embery G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. BBA. 1997;1362(2-3):221–231. doi: 10.1016/s0925-4439(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 33.Uchiyama H., Dobashi Y., Ohkouchi K., Nagasawa K. Chemical change involved in the oxidative reductive depolymerization of hyaluronic acid. J. Biol. Chem. 1990;265(14):7753–7759. [PubMed] [Google Scholar]
- 34.Šoltés L., Mendichi R., Kogan G., Schiller J., Stankovská M., Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7(3):659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 35.Safrankova B., Gajdova S., Kubala L. The potency of hyaluronan of different molecular weights in the stimulation of blood phagocytes. Mediat. Inflamm. 2010;2010 doi: 10.1155/2010/380948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown D.I., Griendling K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015;116(3):531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craige S.M., Kant S., Keaney J.F., Jr. Reactive oxygen species in endothelial function - from disease to adaptation. Circ. J. 2015;79(6):1145–1155. doi: 10.1253/circj.CJ-15-0464. [DOI] [PubMed] [Google Scholar]
- 38.Castro M.M., Rizzi E., Figueiredo-Lopes L., Fernandes K., Bendhack L.M., Pitol D.L., Gerlach R.F., Tanus-Santos J.E. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198(2):320–331. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Hobeika M.J., Thompson R.W., Muhs B.E., Brooks P.C., Gagne P.J. Matrix metalloproteinases in peripheral vascular disease. J. Vasc. Surg. 2007;45(4):849–857. doi: 10.1016/j.jvs.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 40.Rajagopalan S., Meng X.P., Ramasamy S., Harrison D.G., Galis Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Invest. 1996;98(11):2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q., Jin M., Yang F., Zhu J., Xiao Q., Zhang L. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y., Peng W., Raffetto J.D., Khalil R.A. Matrix metalloproteinases in remodeling of lower extremity veins and chronic venous disease. Prog. Mol. Biol. Transl. Sci. 2017;147:267–299. doi: 10.1016/bs.pmbts.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulivor A.W., Lipowsky H.H. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation. 2009;16(8):657–666. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 44.Nelson A., Berkestedt I., Schmidtchen A., Ljunggren L., Bodelsson M. Increased levels of glycosaminoglycans during septic shock. Shock. 2008;30:623–627. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 45.Steppan J., Hofer S., Funke B., Brenner T., Henrich M., Martin E., Weitz J., Hofmann U., Weigand M.A. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J. Surg. Res. 2011;165(1):136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 46.Lepedda A.J., Nieddu G., Piperigkou Z., Kyriakopoulou K., Karamanos N., Formato M. Circulating Heparan Sulfate Proteoglycans as Biomarkers in Health and Disease. Semin. Thromb. Hemost. 2021;47(03):295–307. doi: 10.1055/s-0041-1725063. [DOI] [PubMed] [Google Scholar]
- 47.Sieve I., Münster-Kühnel A.K., Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vasc. Pharmacol. 2018;100:26–33. doi: 10.1016/j.vph.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Bogner-Flatz V., Braunstein M., Ocker L.E., Kusmenkov T., Tschoep J., Ney L., Böcker W., Annecke T. On-the-scene hyaluronan and syndecan-1 serum concentrations and outcome after cardiac arrest and resuscitation. Mediators Inflamm. 2019;2019:1–8. doi: 10.1155/2019/8071619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsia K., Yang M.-J., Chen W.-M., Yao C.-L., Lin C.-H., Loong C.-C., Huang Y.-L., Lin Y.-T., Lander A.D., Lee H., Lu J.-H. Sphingosine-1-phosphate improves endothelialization with reduction of thrombosis in recellularized human umbilical vein graft by inhibiting syndecan-1 shedding in vitro. Acta Biomater. 2017;51:341–350. doi: 10.1016/j.actbio.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 50.Sladden T.M., Yerkovich S., Wall D., Tan M., Hunt W., Hill J., Smith I., Hopkins P., Chambers D.C. Endothelial glycocalyx shedding occurs during ex vivo lung perfusion: a pilot study. J Transplant. 2019;2019:1–12. doi: 10.1155/2019/6748242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieuwdorp M., Mooij H.L., Kroon J., Atasever B., Spaan J.A., Ince C., Holleman F., Diamant M., Heine R.J., Hoekstra J.B., Kastelein J.J., Stroes E.S., Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55(4):1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 52.Ikegami-Kawai M., Suzuki A., Karita I., Takahashi T. Increased hyaluronidase activity in the kidney of streptozotocin-induced diabetic rats. J. Biochem. 2003;134(6):875–880. doi: 10.1093/jb/mvg214. [DOI] [PubMed] [Google Scholar]
- 53.Leskova W., Pickett H., Eshaq R.S., Shrestha B., Pattillo C.B., Harris N.R. Effect of diabetes and hyaluronidase on the retinal endothelial glycocalyx in mice. Exp. Eye Res. 2019;179:125–131. doi: 10.1016/j.exer.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H., Zhu L., Chao Y., Gu Y., Kong X., Chen M., Ye P., Luo J., Chen S. Hyaluronidase2 (Hyal2) modulates low shear stress-induced glycocalyx impairment via the LKB1/AMPK/NADPH oxidase-dependent pathway. J. Cell. Physiol. 2018;233(12):9701–9715. doi: 10.1002/jcp.26944. [DOI] [PubMed] [Google Scholar]
- 55.S. Sarrazin, W.C. Lamanna, J.D. Esko. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. (2011) 3(7): a004952. [DOI] [PMC free article] [PubMed]
- 56.Petersonand S.B., Liu J. Multi-faceted substrate specificity of heparanase. Matrix Biol. 2013;32(5):223–227. doi: 10.1016/j.matbio.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Vlodavsky I., Friedmann Y., Elkin M., Aingorn H., Atzmon R., Ishai-Michaeli R., Bitan M., Pappo O., Peretz T., Michal I., Spector L., Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat. Med. 1999;5(7):793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 58.McKenzie E., Tyson K., Stamps A., Smith P., Turner P., Barry R., Hircock M., Patel S., Barry E., Stubberfield C., Terrett J., Page M. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem. Biophys. Res. Commun. 2000;276(3):1170–1177. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- 59.Levy-Adam F., Miao H.-Q., Heinrikson R.L., Vlodavsky I., Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem. Biophys. Res. Commun. 2003;308(4):885–891. doi: 10.1016/s0006-291x(03)01478-5. [DOI] [PubMed] [Google Scholar]
- 60.Simizu S., Ishida K., Wierzba M.K., Osada H. Secretion of heparanase protein is regulated by glycosylation in human tumor cell lines. J. Biol. Chem. 2004;279(4):2697–2703. doi: 10.1074/jbc.M300541200. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Zaken O., Shafat I., Gingis-Velitski S., Bangio H., Kelson I.K., Alergand T., Amor Y., Maya R.-Y., Vlodavsky I., Ilan N. Low and high affinity receptors mediate cellular uptake of heparanase. Int. J. Biochem. Cell Biol. 2008;40(3):530–542. doi: 10.1016/j.biocel.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vreys V., David G. Mammalian heparanase: what is the message? J. Cell Mol. Med. 2007;11(3):427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masola V., Zaza G., Gambaro G., Franchi M., Onisto M. Role of heparanase in tumor progression: Molecular aspects and therapeutic options. Semin. Cancer Biol. 2020;62:86–98. doi: 10.1016/j.semcancer.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Teixeira F.C.O.B., Götte M. Involvement of syndecan-1 and heparanase in cancer and inflammation. Adv. Exp. Med. Biol. 2020;1221:97–135. doi: 10.1007/978-3-030-34521-1_4. [DOI] [PubMed] [Google Scholar]
- 65.Masola V., Bellin G., Gambaro G., Onisto M. Heparanase: a multitasking protein involved in extracellular matrix (ECM) remodeling and intracellular events. Cells. 2018;7(12):236. doi: 10.3390/cells7120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kramer A., van den Hoven M., Rops A., Wijnhoven T., van den Heuvel L., Lensen J., van Kuppevelt T., van Goor H., van der Vlag J., Navis G., Berden J.H.M. Induction of glomerular heparanase expression in rats with adriamycin nephropathy is regulated by reactive oxygen species and the renin-angiotensin system. J. Am. Soc. Nephrol. 2006;17(9):2513–2520. doi: 10.1681/ASN.2006020184. [DOI] [PubMed] [Google Scholar]
- 67.Chen G., Wang D., Vikramadithyan R., Yagyu H., Saxena U., Pillarisetti S., Goldberg I.J. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43(17):4971–4977. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- 68.Han J., Mandal A.K., Hiebert L.M. Endothelial cell injury by high glucose and heparanase is prevented by insulin, heparin and basic fibroblast growth factor. Cardiovasc Diabetol. 2005;4:12. doi: 10.1186/1475-2840-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.An X.-F., Zhou L., Jiang P.-J., Yan M., Huang Y.-J., Zhang S.-N., Niu Y.-F., Ten S.-C., Yu J.-Y. Advanced glycation end-products induce heparanase expression in endothelial cells by the receptor for advanced glycation end products and through activation of the FOXO4 transcription factor. Mol. Cell. Biochem. 2011;354(1-2):47–55. doi: 10.1007/s11010-011-0804-7. [DOI] [PubMed] [Google Scholar]
- 70.Ishai-Michaeli R., Eldor A., Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990;1(11):833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pomin V.H. Sulfated glycans in inflammation. Eur. J. Med. Chem. 2015;92:353–369. doi: 10.1016/j.ejmech.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Ramani V.C., Pruett P.S., Thompson C.A., DeLucas L.D., Sanderson R.D. Heparan sulfate chains of syndecan-1 regulate ectodomain shedding. J. Biol. Chem. 2012;287(13):9952–9961. doi: 10.1074/jbc.M111.330803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manon-Jensen T., Multhaupt H.A.B., Couchman J.R. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013;280(10):2320–2331. doi: 10.1111/febs.12174. [DOI] [PubMed] [Google Scholar]
- 74.Garsen M., Rops A.L., Rabelink T.J., Berden J.H., van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol. Dial. Transplant. 2014 Jan;29(1):49–55. doi: 10.1093/ndt/gft410. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Barthel L., Zemans R.L., Bowman J.C., Koyanagi D.E., Yunt Z.X., Smith L.P., Cheng S.S., Overdier K.H., Thompson K.R., Geraci M.W., Douglas I.S., Pearse D.B., Tuder R.M. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012;18(8):1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald K.K., Cooper S., Danielzak L., Leask R.L., Sperandio M. Glycocalyx degradation induces a proinflammatory phenotype and increased leukocyte adhesion in cultured endothelial cells under flow. PLoS ONE. 2016;11(12):e0167576. doi: 10.1371/journal.pone.0167576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodall K.J., Poon I.K.H., Phipps S., Hulett M.D., Srinivasula S.M. Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS ONE. 2014;9(10):e109596. doi: 10.1371/journal.pone.0109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lever R., Rose M.J., McKenzie E.A., Page C.P. Heparanase induces inflammatory cell recruitment in vivo by promoting adhesion to vascular endothelium. Am. J. Physiol. Cell Physiol. 2014;306(12):C1184–C1190. doi: 10.1152/ajpcell.00269.2013. [DOI] [PubMed] [Google Scholar]
- 79.Singh A., Fridén V., Dasgupta I., Foster R.R., Welsh G.I., Tooke J.E., Haraldsson B., Mathieson P.W., Satchell S.C. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am. J. Physiol. Renal Physiol. 2011;300(1):F40–F48. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pahwa R., Nallasamy P., Jialal I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. Diabetes Compl. 2016;30(4):563–572. doi: 10.1016/j.jdiacomp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X., Sun D., Song J.W., Zullo J., Lipphardt M., Coneh-Gould L., Goligorsky M.S. Endothelial cell dysfunction and glycocalyx – a vicious circle. Matrix Biol. 2018;71–72:421–431. doi: 10.1016/j.matbio.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 82.Ilan N., Elkin M., Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol. 2006;38(12):2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Forsten-Williams K., Chu C.L., Fannon M., Buczek-Thomas J.A., Nugent M.A. Control of growth factor networks by heparan sulfate proteoglycans. Ann. Biomed. Eng. 2008;36(12):2134–2148. doi: 10.1007/s10439-008-9575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blich M., Golan A., Arvatz G., Sebbag A., Shafat I., Sabo E., Cohen-Kaplan V., Petcherski S., Avniel-Polak S., Eitan A., Hammerman H., Aronson D., Axelman E., Ilan N., Nussbaum G., Vlodavsky I. Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscler. Thromb. Vasc. Biol. 2013;33(2):e56–e65. doi: 10.1161/ATVBAHA.112.254961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nadir Y., Brenner B. Heparanase procoagulant activity in cancer progression. Thromb. Res. 2016;140(Suppl 1):S44–S48. doi: 10.1016/S0049-3848(16)30097-4. [DOI] [PubMed] [Google Scholar]
- 86.Chen L., Sanderson R.D., Chakravarti S. Heparanase regulates levels of syndecan-1 in the nucleus. PLoS ONE. 2009;4(3):e4947. doi: 10.1371/journal.pone.0004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lauhio A., Hastbacka J., Pettila V., Tervahartiala T., Karlsson S., Varpula T., Ruokonen E., Sorsa T., Kolho E. Serum MMP-8, -9 and TIMP-1 in sepsis: high serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol. Res. 2011;64(6):590–594. doi: 10.1016/j.phrs.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 88.van der Flier M., van Leeuwen H.J., van Kessel K.P., Kimpen J.L., Hoepelman A.I., Geelen S.P. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23(1):35–38. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 89.Ostrowski S.R., Sørensen A.M., Windeløv N.A., Perner A., Welling K.-L., Wanscher M., Larsen C.F., Johansson P.I. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: a prospective study. Scand J. Trauma Resusc Emerg. Med. 2012;20(1):27. doi: 10.1186/1757-7241-20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terrasini N., Lionetti V. Exosomes in critical illness. Crit. Care Med. 2017;45(6):1054–1060. doi: 10.1097/CCM.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 91.Real J.M., Ferreira L.R.P., Esteves G.H., Koyama F.C., Dias M.V.S., Bezerra-Neto J.E., Cunha-Neto E., Machado F.R., Salomão R., Azevedo L.C.P. Exosomes from patients with septic shock convey miRNAs related to inflammation and cell cycle regulation: new signaling pathways in sepsis? Crit. Care. 2018;22(1):68. doi: 10.1186/s13054-018-2003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thompson C.A., Purushothaman A., Ramani V.C., Vlodavsky I., Sanderson R.D. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 2013;288(14):10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roucourt B., Meeussen S., Bao J., Zimmermann P., David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25(4):412–428. doi: 10.1038/cr.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Florian J.A., Kosky J.R., Ainslie K., Pang Z., Dull R.O., Tarbell J.M. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 2003;93(10):e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 95.Ebong E.E., Lopez-Quintero S.V., Rizzo V., Spray D.C., Tarbell J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. (Camb). 2014;6(3):338–347. doi: 10.1039/c3ib40199e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsushita K., Morrell C.N., Lowenstein C.J. Sphingosine 1-phosphate activates Weibel-Palade body exocytosis. Proc. Natl. Acad. Sci. U.S.A. 2004;101(31):11483–11487. doi: 10.1073/pnas.0400185101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsushita K., Yamakuchi M., Morrell C.N., Ozaki M., O’Rourke B., Irani K., Lowenstein C.J. Vascular endothelial growth factor regulation of Weibel-Palade-body exocytosis. Blood. 2005;105(1):207–214. doi: 10.1182/blood-2004-04-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakayama T., Sato W., Yoshimura A., Zhang L.i., Kosugi T., Campbell-Thompson M., Kojima H., Croker B.P., Nakagawa T. Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am. J. Pathol. 2010;176(5):2198–2208. doi: 10.2353/ajpath.2010.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ju R., Zhuang Z.W., Zhang J., Lanahan A.A., Kyriakides T., Sessa W.C., Simons M. Angiopoietin-2 secretion by endothelial cell exosomes: regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. J. Biol. Chem. 2014;289(1):510–519. doi: 10.1074/jbc.M113.506899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nadir Y. Heparanase in the Coagulation System. Adv. Exp. Med. Biol. 2020;1221:771–784. doi: 10.1007/978-3-030-34521-1_33. [DOI] [PubMed] [Google Scholar]
- 101.Yamaoka-Tojo M. Vascular endothelial glycocalyx damage in COVID-19. Int. J. Mol. Sci. 2020;21(24):9712. doi: 10.3390/ijms21249712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kataoka H., Ushiyama A., Akimoto Y., Matsubara S., Kawakami H., Iijima T. Structural behavior of the endothelial glycocalyx is associated with pathophysiologic status in septic mice: an integrated approach to analyzing the behavior and function of the glycocalyx using both electron and fluorescence intravital microscopy. Anesth. Analg. 2017;125:874–883. doi: 10.1213/ANE.0000000000002057. [DOI] [PubMed] [Google Scholar]
- 103.Yang Y., Haeger S.M., Suflita M.A., Zhang F., Dailey K.L., Colbert J.F., Ford J.A., Picon M.A., Stearman R.S., Lin L., Liu X., Han X., Linhardt R.J., Schmidt E.P. Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am. J. Respir. Cell Mol. Biol. 2017;56(6):727–737. doi: 10.1165/rcmb.2016-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagy N., Freudenberger T., Melchior-Becker A., Röck K., Ter Braak M., Jastrow H., Kinzig M., Lucke S., Suvorava T., Kojda G., Weber A.A., Sörgel F., Levkau B., Ergün S., Fischer J.W. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation. 2010;122(22):2313–2322. doi: 10.1161/CIRCULATIONAHA.110.972653. [DOI] [PubMed] [Google Scholar]
- 105.Boels M.G., Avramut M.C., Koudijs A., Dane M.J., Lee D.H., van der Vlag J., Koster A., van Zonneveld A.J., van Faassen E., Gröne H.J., van den Berg B.M., Rabelink T.J. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65(8):2429–2439. doi: 10.2337/db15-1413. [DOI] [PubMed] [Google Scholar]
- 106.Rahbar E., Cardenas J.C., Baimukanova G., Usadi B., Bruhn R., Pati S., Ostrowski S.R., Johansson P.I., Holcomb J.B., Wade C.E. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. doi: 10.1186/s12967-015-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gonzalez Rodriguez E., Ostrowski S.R., Cardenas J.C., Baer L.A., Tomasek J.S., Henriksen H.H., Stensballe J., Cotton B.A., Holcomb J.B., Johansson P.I., Wade C.E. Syndecan-1: a quantitative marker for the endotheliopathy of trauma. J. Am. Coll. Surg. 2017;225(3):419–427. doi: 10.1016/j.jamcollsurg.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Gonzalez Rodriguez E., Cardenas J.C., Cox C.S., Kitagawa R.S., Stensballe J., Holcomb J.B., Johansson P.I., Wade C.E. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand. J. Trauma Resusc. Emerg. Med. 2018;26(1) doi: 10.1186/s13049-018-0565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hippensteel J.A., Uchimido R., Tyler P.D., Burke R.C., Han X., Zhang F., McMurtry S.A., Colbert J.F., Lindsell C.J., Angus D.C., Kellum J.A., Yealy D.M., Linhardt R.J., Shapiro N.I., Schmidt E.P. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit. Care. 2019;23(1):259. doi: 10.1186/s13054-019-2534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin L., Schmitz S., De Santis R., Doemming S., Haase H., Hoeger J., Heinbockel L., Brandenburg K., Marx G., Schuerholz T., van Griensven M. Peptide 19–2.5 inhibits heparan sulfate-triggered inflammation in murine cardiomyocytes stimulated with human sepsis serum. PLoS ONE. 2015;10(5):e0127584. doi: 10.1371/journal.pone.0127584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin L., De Santis R., Koczera P., Simons N., Haase H., Heinbockel L., Brandenburg K., Marx G., Schuerholz T., Stover C.M. The synthetic antimicrobial peptide 19–2.5 interacts with heparanase and heparan sulfate in murine and human sepsis. PLoS ONE. 2015;10(11):e0143583. doi: 10.1371/journal.pone.0143583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmidt E.P., Li G., Li L., Fu L.i., Yang Y., Overdier K.H., Douglas I.S., Linhardt R.J. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J. Biol. Chem. 2014;289(12):8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmidt E.P., Overdier K.H., Sun X., Lin L., Liu X., Yang Y., Ammons L.A., Hiller T.D., Suflita M.A., Yu Y., Chen Y., Zhang F., Cothren Burlew C., Edelstein C.L., Douglas I.S., Linhardt R.J. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2016;194(4):439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zaza G., Masola V., Granata S., Pontrelli P., Sallustio F., Gesualdo L., Gambaro G., Grandaliano G., Lupo A. Dialysis-related transcriptomic profiling: the pivotal role of heparanase. Exp. Biol. Med. (Maywood) 2014;239(1):52–64. doi: 10.1177/1535370213506678. [DOI] [PubMed] [Google Scholar]
- 115.Lygizos M.I., Yang Y., Altmann C.J., Okamura K., Hernando A.A., Perez M.J., Smith L.P., Koyanagi D.E., Gandjeva A., Bhargava R., Tuder R.M., Faubel S., Schmidt E.P. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol. Rep. 2013;1(6):e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Torres Filho I.P., Torres L.N., Salgado C., Dubick M.A. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am. J. Physiol. Heart Circ. Physiol. 2016;310(11):H1468–H1478. doi: 10.1152/ajpheart.00006.2016. [DOI] [PubMed] [Google Scholar]
- 117.Yini S., Heng Z., Xin A., Xiaochun M. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol. Scand. 2015;59(2):160–169. doi: 10.1111/aas.12418. [DOI] [PubMed] [Google Scholar]
- 118.Abassi Z., Hamoud S., Hassan A., Khamaysi I., Nativ O., Heyman S.N., Muhammad R.S., Ilan N., Singh P., Hammond E., Zaza G., Lupo A., Onisto M., Bellin G., Masola V., Vlodavsky I., Gambaro G. Involvement of heparanase in the pathogenesis of acute kidney injury: nephroprotective effect of PG545. Oncotarget. 2017;8(21):34191–34204. doi: 10.18632/oncotarget.16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Annecke T., Chappell D., Chen C., Jacob M., Welsch U., Sommerhoff C.P., Rehm M., Conzen P.F., Becker B.F. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br. J. Anaesth. 2010;104:414–421. doi: 10.1093/bja/aeq019. [DOI] [PubMed] [Google Scholar]
- 120.Rao G., Ding H.G., Huang W., Le D., Maxhimer J.B., Oosterhof A., van Kuppevelt T., Lum H., Lewis E.J., Reddy V., Prinz R.A., Xu X. Reactive oxygen species mediate high glucose-induced heparanase-1 production and heparan sulphate proteoglycan degradation in human and rat endothelial cells: a potential role in the pathogenesis of atherosclerosis. Diabetologia. 2011;54(6):1527–1538. doi: 10.1007/s00125-011-2110-z. [DOI] [PubMed] [Google Scholar]
- 121.Chappell D., Dörfler N., Jacob M., Rehm M., Welsch U., Conzen P., Becker B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34(2):133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 122.Desideri S., Onions K.L., Qiu Y., Ramnath R.D., Butler M.J., Neal C.R., King M.L.R., Salmon A.E., Saleem M.A., Welsh G.I., Michel C.C., Satchell S.C., Salmon A.H.J., Foster R.R. A novel assay provides sensitive measurement of physiologically relevant changes in albumin permeability in isolated human and rodent glomeruli. Kidney Int. 2018;93(5):1086–1097. doi: 10.1016/j.kint.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diebel L.N., Liberati D.M., Martin J.V. Acute hyperglycemia increases sepsis related glycocalyx degradation and endothelial cellular injury: a microfluidic study. Am. J. Surg. 2019;217(6):1076–1082. doi: 10.1016/j.amjsurg.2018.12.066. [DOI] [PubMed] [Google Scholar]
- 124.Schenning K.J., Anderson S., Alkayed N.J., Hutchens M.P. Hyperglycemia abolishes the protective effect of ischemic preconditioning in glomerular endothelial cells in vitro. Physiol. Rep. 2015;3(3) doi: 10.14814/phy2.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vlodavsky I., Iozzo R.V., Sanderson R.D. Heparanase: multiple functions in inflammation, diabetes and atherosclerosis. Matrix Biol. 2013;32(5):220–222. doi: 10.1016/j.matbio.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 126.Gordon D.E., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koehler M., Delguste M., Sieben C., Gillet L., Alsteens D. Initial step of virus entry: virion binding to cell-surface glycans. Annu Rev Virol. 2020;7(1):143–165. doi: 10.1146/annurev-virology-122019-070025. [DOI] [PubMed] [Google Scholar]
- 128.Silva J.C., Carvalho M.S., Han X., Xia K.e., Mikael P.E., Cabral J.M.S., Ferreira F.C., Linhardt R.J. Compositional and structural analysis of glycosaminoglycans in cell-derived extracellular matrices. Glycoconj. J. 2019;36(2):141–154. doi: 10.1007/s10719-019-09858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feyzi E., Saldeen T., Larsson E., Lindahl U., Salmivirta M. Age-dependent modulation of heparan sulfate structure and function. J. Biol. Chem. 1998;273(22):13395–13398. doi: 10.1074/jbc.273.22.13395. [DOI] [PubMed] [Google Scholar]
- 130.Milewska A., Zarebski M., Nowak P., Stozek K., Potempa J., Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014;88(22):13221–13230. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yan R., et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clausen T.M., Sandoval D.R., Spliid C.B., et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043–1057.e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., Hazzan A.D., Fishbane S., Jhaveri K.D. Northwell COVID-19 research consortium, & northwell nephrology COVID-19 research consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.LaRivière W.B., Schmidt E.P. The pulmonary endothelial glycocalyx in ARDS: a critical role for heparan sulfate. Curr. Top. Membr. 2018;82:33–52. doi: 10.1016/bs.ctm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Collins L.E., Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. J. Leukoc. Biol. 2019;105(1):81–92. doi: 10.1002/JLB.3RU0618-246R. [DOI] [PubMed] [Google Scholar]
- 137.Xie M., Li J.-P. Heparan sulfate proteoglycan – a common receptor for diverse cytokines. Cell. Signal. 2019;54:115–121. doi: 10.1016/j.cellsig.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 138.Buijsers B., Yanginlar C., de Nooijer A., Grondman I., Maciej-Hulme M.L., Jonkman I., Janssen N.A.F., Rother N., de Graff M., Pickkers P., Kox M., Joosten L.A.B., Nijenhuis T., Netea M.G., Hilbrands L., van de Veerdonk F.L., Duivenvoorden R., de Mast Q., van der Vlag J. Increased Plasma Heparanase Activity in COVID-19 Patients. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.575047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Agelidis A., Shukla D. Heparanase, heparan sulfate and viral infection. Adv. Exp. Med. Biol. 2020;1221:759–770. doi: 10.1007/978-3-030-34521-1_32. [DOI] [PubMed] [Google Scholar]
- 140.Cassinelli G., Naggi A. Old and new applications of non-anticoagulant heparin. Int. J. Cardiol. 2016;212(Suppl 1):S14–S21. doi: 10.1016/S0167-5273(16)12004-2. [DOI] [PubMed] [Google Scholar]
- 141.Billett H.H., Reyes-Gil M., Szymanski J., Ikemura K., Stahl L., Lo Y., Rahman S., Gonzalez-Lugo J.D., Kushnir M., Barouqa M., Golestaneh L., Bellin E. Anticoagulation in COVID-19: effect of enoxaparin, heparin, and apixaban on mortality. Thromb. Haemost. 2020;120(12):1691–1699. doi: 10.1055/s-0040-1720978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mycroft-West C.J., Su D., Pagani I., et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb. Haemost. 2020;120(12):1700–1715. doi: 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Masola V., Secchi M.F., Gambaro G., Onisto M. Heparanase as a target in cancer therapy. Curr. Cancer Drug Targets. 2014;14(3):286–293. doi: 10.2174/1568009614666140224155124. [DOI] [PubMed] [Google Scholar]