Abstract
Background
Treatment of large bone defects and fracture healing complications (delayed and non-union) presents a substantial challenge for orthopaedic surgeons. Given that bone healing requires mechanical stability as well as a favourable biological microenvironment, orthobiologics such as Platelet-Rich Plasma (PRP) may have a significant clinical role to play.
Aims
To perform a systematic review of the available literature to assess the clinical effect of PRP, with or without other orthobiologics, on bone healing.
Method
Two independent reviewers performed the literature search based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Clinical studies of any evidence, assessing effect of PRP with or without other orthobiologics on bone healing, were included. A qualitative analysis was carried out on the clinical and radiological outcomes reported.
Result
27 articles with 1631 patients (mean age = 43.56, 57.1% male, mean follow-up = 17.27 months) were included in the qualitative. Of the 27 studies, 13 dealt with fracture complications (delayed or non-unions), 7 with acute fracture healing, 4 with tibial osteotomies and lengthening procedures and 3 with lumbar spine pathology. 18/27 studies showed a clinical benefit of PRP, 8/27 showed no significant effect, and 1/27 showed a worse outcome with PRP.
Conclusion
Our review suggests PRP may play a clinical role in bone healing but further randomised controlled trials (RCTs) using standardised outcomes should be performed to establish its efficacy.
Keywords: PRP, Platelet-rich plasma, Fracture healing, Non-union, Delayed union, Orthobiologic
1. Introduction
Bone and fracture healing is a multifactorial biological process relevant in all subspecialties of trauma & orthopaedic surgery, which when impaired can lead to delayed or non-union, with incidence of the latter reported at 5–10%.1, 2, 3 Along with causing significant morbidity (delayed functional recovery and loss of independence to the patient), these complications also carry major cost implications.4,5 The economic burden incurred by healthcare systems secondary to such fracture healing complications has been previously reported in the literature, with Dunlop et al. concluding from their 3908 patient study that there is a significant need to prioritise future research into preventing the above, as well as improving its management.6 In addition to delayed and non-union management, treatment of traumatic or pathological large bone defects remains a substantial challenge in orthopaedic surgery.7, 8, 9
Successful bone healing is determined by mechanical stability, as well as a favourable biological microenvironment. Fractures with compromised biology which will not go on to heal in a timely manner or at all, will therefore benefit from therapies that can augment the biologic potential at the fracture site, i.e. orthobiologics.1 Calcei et al. described orthobiologics as ‘a group of biological materials and substrates including bone grafts, bone graft substitutes, growth factors, cell-signalling proteins and cell-based therapies that promote bone, ligament, muscle and tendon healing’.10 Currently, bone autografts and allografts are considered the gold standard and are widely used as orthobiologic agents.1,9,11 However, various factors such as cost, donor site morbidity, chronic pain, graft harvesting complications, rejection, immunogenicity and infection transmission are major drawbacks of bone autografts and allografts and have necessitated development of alternative strategies.1,9,12,13
One of these strategies is Platelet Rich Plasma (PRP) – many studies have shown improved bone healing with platelet concentrations of up to 1,000,000/μL.10,14, 15, 16, 17 PRP is a preparation of autologous human plasma with supraphysiologic level of platelets and associated growth factors (at least 4-5x increase from baseline), produced from centrifugation of patient's own blood.1,10,15
Within the realm of trauma & orthopaedic surgery, PRP has been extensively studied and licensed for use in lateral epicondylitis and knee osteoarthritis with high-quality evidence.15 There is moderate-quality evidence supporting its use in plantar fasciitis, patellar tendinopathy and donor site pain in patellar tendon graft BTB ACL reconstruction.15
The clinical evidence for use in bone healing for acute fractures, large bone defects, delayed and non-unions is unfortunately, very limited, although there is a plethora of pre-clinical evidence supporting PRP in bone healing.1,4,9,10,15 To date, there have been two systematic reviews on this topic – Griffin et al. conducted the first study in 2012 (including 1 paper) and Roffi et al. conducted the second study in 2016 (45 preclinical papers, 19 clinical papers). Both studies concluded that it is difficult to establish the efficacy of PRP in bone healing given the limited studies available. Moreover, the studies that are available have significant heterogeneity in bone pathology, intervention and outcomes, that limit the ability to draw robust conclusions.4,9 Since 2016, there have been 8 clinical studies including 5 RCTs (>50% increase), which justifies this third systematic review assessing use of PRP in bone healing.
2. Aims
The aims and objectives of this systematic review were to perform a qualitative analysis to assess the clinical effect of PRP, with or without other orthobiologics, on bone healing. To this end, we included all studies describing PRP efficacy in bone healing including acute fractures, delayed unions, non-unions, osteotomies, tibial lengthening procedures and lumbar spine pathology.18
3. Methods
A systematic review of the literature was performed on the effect of PRP with or without other orthobiologics on bone healing. The search was carried out on the Cochrane Library and PubMed databases on November 20, 2019 with the following search terms: (Platelet-Rich Plasma OR PRP OR Platelet-rich therapy OR Platelet Concentrate OR Platelet Gel OR PRF) AND (Fracture OR Trauma OR Bone defect OR Non-union OR malunion).18
Inclusion criteria for the qualitative analysis was (1) Human clinical studies, (2) Written in English, (3) Published in a peer-reviewed journal, (4) Until December 2019, (5) With full text of studies available, (6) On the use of PRP or any platelet-rich therapy with or without other orthobiologics, (7) To treat acute fractures, delayed union, non-union, osteotomies, tibial lengthening procedures and lumbar spine pathology, and (8) Patients >18 years of age.18
Exclusion criteria for both analyses were (1) Any papers written in other languages, (2) Pre-clinical trials or basic science studies, (3) Systematic reviews, (4) Studies analysing application of PRP in non-bone pathology, (5) Studies analysing other applications of PRP in MSK pathology (e.g. PRP use in patellar tendon pathology), (6) Studies analysing application of PRP in any maxillofacial pathology, and (7) Studies with <10 participants.18
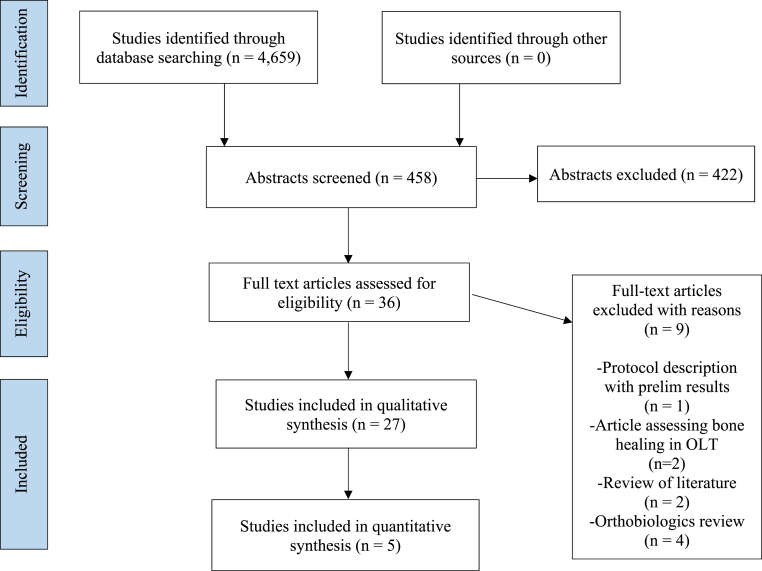
Two independent researchers (MSJ and ETH) conducted the screening and literature review process based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A senior third author was available to arbitrate any discrepancies. The articles were initially screened using the aforementioned inclusion and exclusion criteria by title and abstract to determine potential eligibility. A second screening of the potentially eligible studies was performed by reading through the full text and applying the previously described criteria again to determine study eligibility. Lastly, the references of all included studies and systematic reviews were manually screened to identify potential studies that met the inclusion criteria.18 A flowchart of the systematic review process is illustrated in Fig. 1.
Fig. 1.
PRISMA flowchart of paper selection process.
Given the paucity of literature on PRP and bone healing, we have kept our inclusion criteria broad and included the vertebral column along with the appendicular skeleton. Additionally, we have not limited our study to include trials that only assess PRP, we have allowed its use in conjunction with other orthobiologics. Lastly, we have kept our criteria for bone defects and injury broad as aforementioned to include acute fractures as well as impaired bone healing and bone defects secondary to osteotomies. We understand this will increase the heterogeneity of the studies included, however it is a limitation that we are aware of and believe is necessary to allow for a rounded analysis.
Pertinent information from the studies was extracted and collated using a pre-determined data sheet comprising study design, year of publication, type of study, number of cases, participant demographics, level of evidence, follow-up, type of surgery, PRP preparation, concomitant use of orthobiologics, control groups if present, clinical and radiological outcomes, results, and adverse effects. A qualitative analysis on this data was then carried out.
The level of evidence was based on criteria laid out by the Oxford Centre for Evidence-Based Medicine (Table 1). The risk of bias was assessed by using the Jadad 5-point scale (>3 = low risk of bias). As we have incorporated studies that look at radiological evidence of fracture healing, there is invariably a risk of error and bias in reading and reporting the radiographs. Consequently, any studies which required two consultant radiologists or orthopaedic surgeons reaching an agreement on patient improvement were deemed to have a low risk of bias. Another source of bias was participant selection bias. As this was a systematic review, it had the limitations and biases of all included studies.18
Table 1.
Levels of evidence.
| Level of Evidence | Type of Evidence |
|---|---|
| I | RCTs, systematic review/meta-analysis of RCTs |
| II | Cohort studies, systematic review of cohort studies |
| III | Case-Control studies, systematic review of case-control studies |
| IV | Case series, case reports, poor quality case-control or cohort studies |
| V | Expert opinion without critical appraisal/based on bench research |
4. Results
4.1. Literature search
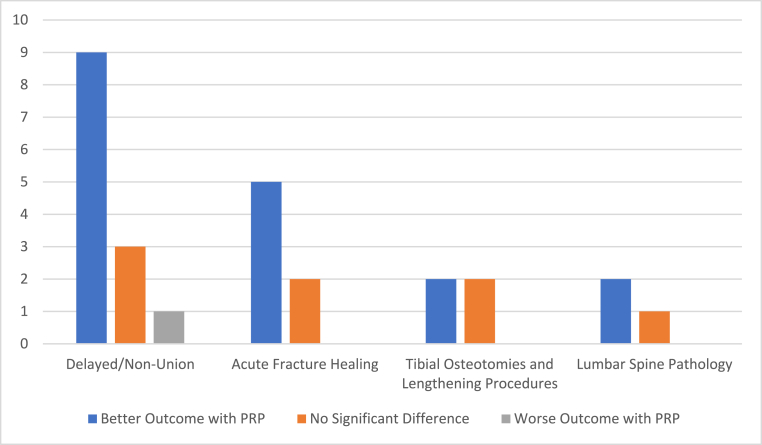
The initial literature search identified 4659 studies in total. 458 of these abstracts and titles were screened, with 36 full text articles then assessed for eligibility using the inclusion and exclusion criteria. 27 of these were included in the qualitative synthesis. 9 studies were excluded for various reasons: protocol description with preliminary results, assessment of bone healing in OLT, literature review and orthobiologics review. Of 27 studies included in the qualitative synthesis, 13 dealt with fracture complications (delayed or non-unions),19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 7 with acute fracture healing,32, 33, 34, 35, 36, 37, 38 4 with tibial osteotomies and lengthening procedures,39, 40, 41, 42 and 3 with lumbar spine pathology.43, 44, 45 Results of the qualitative synthesis are illustrated in Fig. 2.
Fig. 2.
Qualitative synthesis results.
4.2. Patient demographics & study characteristics
The 27 studies included 1631 patients (57.1% M, 42.9% F) with an average age of 43.56 years and mean follow-up of 17.27 months. There were 10 RCTs with a mean Risk of Jadad score of 2.56. Participant demographics are reported in Table 2.
Table 2.
Participant demographics.
| Mean | All Studies | Delayed/Non-Union | Acute Fracture Healing | Tibial Osteotomies/Lengthening Procedures | Lumbar Spine Pathology |
|---|---|---|---|---|---|
| Patients (n) | 1631 | 583 | 682 | 128 | 238 |
| Age (years) | 43.56 | 39.68 | 46.2 | 41.55 | 56.43 |
| Gender (% M) | 57.1% | 64.1% | 56.3% | 62.5% | 33.8% |
| Follow-up (mo) | 17.27 | 14.14 | 24.9 | 12.1 | 18.0 |
4.3. Management of fracture complications (delayed or non-union)
There were thirteen studies describing the role of PRP in management of delayed and non-union with 583 participants (mean age 39.68 years, 64.1% M and mean follow-up 14.14 months). Three out of the thirteen studies were RCTs and the other ten were case series. One RCT analysed 16 delayed unions in humeral shaft fractures treated with Open Reduction Internal Fixation (ORIF) and Iliac Crest Autograft (ICA) with PRP vs ORIF and ICA alone.22 Another RCT analysed 120 long bone non-unions treated with local PRP application (with or without bone graft) during revision surgery vs Bone Morphogenetic Protein-9 (BMP-7) application (with or without bone graft) during revision surgery.23 The last RCT analysed 40 long bone delayed unions treated with percutaneous autologous Platelet Concentrate (PC) injection into fracture site vs observation and follow-up.21 The remaining ten studies primarily analysed long bone (only 1 study assessing isolated non-union in ulna fractures) delayed or non-unions treated with PRP applied either during revision surgery or percutaneously.19,20,24, 25, 26, 27, 28, 29, 30, 31 A synopsis of the studies included is listed in Table 3.
Table 3.
Synopsis of all studies included in present study.
| Publication | Study Type (LoE) | Total Patients | Pathology | Therapeutic Protocol | Main Findings |
|---|---|---|---|---|---|
| Acosta Olivia et al. Arch Orthop Trauma Surg 201722 | RCT (I) | 16 | Delayed union of humeral shaft fractures | Fixation with ICA vs Fixation with ICA + PRP | PRP use promoted early bone consolidation |
| Bielecki T et al., Eur Surg Res 200825 |
Case Series (IV) | 32 | Long bone delayed and non-union | PRP injection at site of non-union | PRP was a valid strategy to obtain union |
| Calori et al., Injury. 200823 |
RCT (I) | 120 | Long bone non-union | Fixation with PRP±bone graft vs Fixation with BMP-7 ± bone graft | BMP-7 was significantly more effective than PRP in promoting bone healing |
| Chiang CC et al., J Trauma. 200726 |
Case Series (IV) | 12 | Long bone non-union | Local application of bone graft + PRP during revision surgery | There was a beneficial potential of PRP in treating non-unions |
| Dallari D et al., J bone Joint Surg Am. 200740 |
RCT (I) | 33 | High tibial osteotomy (HTO) to treat genu varum | Osteotomy with insertion of bone chips + PRP vs bone chips + PRP + BMC or bone chips into osteotomy gap site | PRP provided better outcomes + increased osteogenic potential of bone chips. Further beneficial effects with the addition of BMC |
| D'Elia et al. Cartilage 201041 | RCT (I) | 25 | HTO for varus deformity of the knee | HTO with PRP + BMAC vs HTO with ICA | PRP & BMAC did not show any advantage over ICA in HTO |
| Duramaz et al. Eur J Orthop Surg 201819 | Case Series (IV) | 29 | Long bone non-unions previously treated with IM Nails | Exchange IM Nail (EIN) + bone grafting vs percutaneous PRP application | PRP can be used as a safe and minimally invasive method of saving resources in long bone non unions EIN, with percutaneous PRP significantly affecting union rate |
| Galasso O et al., J Orthop Traumatol. 200827 |
Case Series (IV) | 22 | Long bone non-union | PRP applied locally during revision surgery | IM Nail and PRP produced comparable results with less complications |
| Golos J et al., Ortop Traumatol Rehabil. 201428 |
Case Series (IV) | 132 | Long bone delayed union | PRP injection at site of non-union | PRP was effective in treatment of long bone delayed union |
| Griffin XL et al., BMJ Open. 201334 |
RCT (I) | 200 | Intracapsular hip fracture | Internal fixation with or without local PRP application | No significant clinical effect in PRP group, except reduced LOS |
| Kubota et al. Spine J 201743 | RCT (I) | 134 | Posterolateral lumbar fusion surgery for degenerative spondylosis | PLF with bone graft with and without PRP | Patients treated with PRP showed a higher fusion rate, higher fusion mass and more rapid bone union |
| Lee Dh et al., Clin Orthop Relat Res.201442 |
RCT (I) | 20 | Tibial distraction osteogenesis for familial short stature | PRP and BMAC locally injected at osteotomy gap site | BMAC + PRP significantly improved bone healing in tibial distraction osteogenesis, allowing for earlier return to WB |
| Liebergall M et al., Mol. Ther.201335 |
RCT (I) | 24 | Distal tibial fractures | PRP and MSCs mixed with DBM and injected at the fracture site 3–6 weeks after primary surgery | The combination of PRP + MSCs + DBM was a safe therapeutic option and contributed to reduce the time of bone fusion |
| Malhotra R et al., Musculoskelet Surg. 201529 |
Case Series (IV) | 94 | Long bone non-union | PRP injection at the site of non-union | PRP was a safe and effective treatment for managing long bone non-unions |
| Mariconda M et al., J Orthop Trauma. 200820 |
Case Series (IV) | 20 | Long bone non-union | Platelet gel applied locally during revision surgery (external fixator) | No clinical usefulness of PRP reported |
| Namazi H et al., Orthop Tramatol Surg Res.201636 |
Case-Control (III) | 30 | Intra-articular distal radius fracture | CRPP with intra-articular PRP injection immediately after surgery | PRP had a significant effect on reduction of pain and functional recovery |
| Perbooms JC et al., Int Orthop. 201239 |
RCT (I) | 41 | HTO for medial knee OA | PRP mixed with bone chips and inserted into osteotomy gap site | PRP + allograft did not benefit bone healing |
| Rodriguez-Collazo ER et al., Strategies Trauma Limb Reconstr. 201532 |
Case Series (IV) | 20 | Bimalleolar ankle fractures in co-morbid patients | PRP and BMC mixed with DBM and injected locally at fracture site | External fixation + DBM, BMC and PRP promoted fracture healing |
| Samuel et al. Eur J Orthop Surg Traumatol 201721 | RCT (I) | 40 | Long bone delayed union | Percutaneous platelet concentrate (PC) injection at fracture site | Isolated percutaneous PC injection increased union rates |
| Samy AM Int orthop. 201533 |
RCT (I) | 60 | Femoral neck fracture | Internal fixation with or without local PRP application | Better radiological and clinical outcomes in PRP group |
| Sanchez M et al., J Orthop Trauma. 200930 |
Case Series (IV) | 15 | Long bone delayed and non-union | PRP membrane±bone graft locally applied during revision surgery (2 cases had PRP injection at the site of non-union without revision surgery) | PRP was clinically safe and enhanced the healing of non-hypertrophic non-unions |
| Say F et al. Acta Chir Orthop Traumatol Cech. 201431 |
Case Series (IV) | 20 | Lower limb long bone delayed and non-union | 3x weekly PRP injections at fracture site | PRP showed no additional healing potential, but may have a role to play in conservative management of delayed union |
| Singh et al. Chin J Traumatol 201737 | RCT (I) | 72 | Femoral shaft fractures | PRP gel and fibrin membrane applied locally during surgery (IM Nail) | PRP had no effect on femoral shaft fracture healing |
| Tarallo L et al., Eur J Orthop Surg Traumatol. 201224 |
Case Series (IV) | 10 | Isolated non-union of the ulna | Revision surgery with PRP and bone graft applied locally | High rate of clinical and radiological healing with biological augmentation |
| Tsai et al. J Spinal Disord Tech 200944 | RCT (I) | 67 | Adult isthmic spondylisthesis | Instrumented posterolateral lumbar fusion (PLF) with artificial bone expander and laminectomy autograft with and without platelet glue | Use of platelet glue cannot be proved to increase fusion rates in instrumented PLF |
| Tuakli-Wosornu et al. PM R 201645 | RCT (I) | 37 | Chronic lumbar discogenic pain refractory to conservative management | Intradiskal PRP injection vs contrast injection for lumbar degenerative disc disease | Patients who received intradiskal PRP had significantly improved function and pain scores vs control |
| Wei LC et al., J Orthop Res. 201238 |
Cohort Study (II) | 254 | Displaced intra-articular calcaneum fracture | PRP mixed with bone graft and applied locally during ORIF | PRP augmented allografts showed better radiological results than allografts alone |
All thirteen studies reported radiological outcomes and eleven of the thirteen studies reported clinical outcomes – subjective functional scores and/or clinical examination and complications and/or objective scores. Five of the thirteen studies compared PRP to a control – three showed better results with PRP, one did not show any difference and one showed a worse result with PRP.19, 20, 21, 22, 23 Of the eight non-comparative studies, six showed PRP to be effective in management of long bone fracture delayed and non-union, one showed no additional healing potential overall however suggested a potential role of PRP in conservative management of long bone delayed union, and one showed comparable results to previously reported in literature with fewer complications.24, 25, 26, 27, 28, 29, 30, 31
4.4. Management in acute fracture healing
There were seven studies describing the role of PRP in acute fracture healing with 682 participants (mean age 46.2 years, 56.3% M and mean follow-up 24.9 months). Four of these studies were RCTs, one was a case-control study, one was a cohort study and one was a case series. Two RCTs analysed the role of PRP in intracapsular hip fractures (Internal fixation with or without PRP injection), one in distal third tibial fractures (Surgical fixation with PRP, Mesenchymal Stem Cells (MSC) and Demineralised Bone Matrix (DBM) vs surgical fixation alone), and one in femoral shaft fractures (Intramedullary nailing with or without PRP injection/gel application).33, 34, 35,37 The case-control study analysed the role of PRP in intra-articular distal radius fractures treated with closed reduction and percutaneous pinning with or without intra-articular PRP injection.36 The cohort study analysed the role of PRP in displaced intra-articular calcaneum fractures treated with open reduction internal fixation with autograft vs with allograft vs with allograft and PRP.38 Lastly, the case-series analysed the role of PRP in co-morbid patients with bimalleolar ankle fractures treated with Ilizarov fixator and DBM vs Ilizarov fixator with DBM and concentrated Bone Marrow Aspirate (cBMA) and PRP.32 A synopsis of the studies is listed in Table 3.
Six out of the seven studies reported radiological outcomes and clinical outcomes – subjective functional scores and/or clinical examination and complications and/or objective scores. Five of the studies showed better outcomes with PRP application,32,33,35,36,46 one study showed no significant difference however did state the PRP group had reduced LOS,34 and one study showed no difference in outcomes with PRP.37
4.5. Management in tibial osteotomies and lengthening procedures
There were four studies describing the role of PRP in tibial osteotomies and lengthening procedures with 128 participants (mean age 41.55 years, 62.5% M and mean follow-up 12.1 months). Three of these studies were RCTs and one was a cohort study. One RCT analysed 28 patients with genu varum undergoing unilateral opening wedge high tibial osteotomy (HTO) with lypholised bone chips, Bone Marrow Stromal Cells (BMC) and PRP vs HTO with lypholised bone chips alone.40 Another RCT analysed 25 patients with varus deformity of the knee undergoing medial opening wedge HTO (MOWHTO) with PRP and Bone Marrow Aspirate (BMAC) vs MOWHTO with Iliac Crest Autograft (ICA).41 The last RCT analysed 40 patients with familial short stature undergoing tibial distraction osteogenesis with PRP and BMAC vs tibial distraction osteogenesis alone.42 The cohort study analysed 35 patients with medial compartment OA undergoing medial HTO with internal fixation, bone chips and PRP vs medial HTO with internal fixation and bone chips alone.39 A synopsis of the studies included is listed in Table 3.
All four studies reported radiological outcomes and two of the four studies reported clinical outcomes – subjective functional scores and/or clinical examination and complications and/or objective scores. One study showed PRP enhanced the osteogenic potential of bone chips and improved radiological outcomes,40 one study showed PRP significantly improved bone healing allowing for an earlier return to weightbearing,42 and two studies showed no benefit of using PRP.39,41
4.6. Management in lumbar spine pathology
There were three studies describing the role of PRP in lumbar spine pathology with 238 participants (mean age 56.43 years, 33.8% M and mean follow-up 18 months). All three were RCTs assessing various treatment options in patients with lumbar spine pathology. One RCT analysed 134 patients with degenerative spondylosis undergoing posterolateral fusion (PLF) with local bone graft (laminectomy bone) and PRP vs PLF with local bone graft alone.43 Another RCT analysed 67 patients with spondylisthesis undergoing PLF with local bone graft (laminectomy bone), artificial bone expander and Platelet Glue vs PLF with local bone graft and bone expander alone.44 The last RCT analysed 37 patients with chronic lumbar degenerative disc disease undergoing intradiscal PRP injection vs contrast injection.45 A synopsis of the studies included is listed in Table 3.
Two out of the three studies reported radiological outcomes and all three studies reported clinical outcomes – subjective functional scores and/or clinical examination and complications and/or objective scores. Two out of the three studies showed PRP improved outcomes,43,45 whilst one study showed no change in outcomes with or without Platelet Glue.44
5. Discussion
Following a continuously evolving and improving understanding of the bone healing process at a molecular level, favourable manipulation of the local fracture microenvironment via application of orthobiologics is increasingly becoming a therapeutic target and field of interest in orthopaedic surgery, particularly in limb reconstruction surgery.47, 48, 46, 49, 50 This is the third systematic review to date assessing the clinical role of PRP in bone healing. There is a plethora of pre-clinical evidence supporting its use, however this has not been reflected in clinical trials thus far.1,4,9,10,15 Our study suggests PRP may have a clinical role to play in the management of fracture healing (acute or delayed), however these results are not statistically significant. There is a need for further homogenous level 1 trials assessing PRP use, prepared according to agreed standardised protocol, in bone healing. This was the recommendation following the previous systematic review also, however despite this there have not been many new trials.
Our qualitative synthesis of 27 studies included 13 studies assessing PRP use in long bone delayed or non-union, 7 studies assessing acute fracture management, 4 studies assessing osteotomies and tibial lengthening procedures and 3 studies assessing lumbar spine pathology. Of these, there were 9 Level 1 RCTs. 16/27 studies assessed PRP in conjunction with other orthobiologics (autologous/bone allograft, BMAC, DBM, BMP-7, BMC, MSC), with only 11/27 studies assessing PRP as the sole orthobiologic, making it difficult to reliably conclude the efficacy of PRP. Overall, 18/27 studies reported a clinical benefit of PRP, 8/27 showed no significant effect, and only 1/27 showed a worse outcome with PRP.
There was significant heterogeneity in the outcome metrics used in the studies. Only 14/27 studies used time to union as an outcome metric with multiple other functional scores employed to assess clinical outcomes. Of these, only 8 were comparative trials (PRP group vs control group), with 5/8 reporting mean differences in time to union.
It is expected that almost 50% of the studies focused on delayed and non-union, as these two pathologies represent impaired bone healing and therefore it is imperative to optimise the fracture microenvironment to aid healing. Most studies included delayed or non-union in long bones, with just 2 studies focusing on one bone: isolated non-union of ulna and delayed union of humeral shaft fractures.22,24 7/13 studies analysed PRP alone, 5/13 analysed PRP along with bone graft (autograft or allograft) and 1/13 study analysed PRP compared to BMP-7. Our qualitative synthesis did suggest that PRP may have a clinical role to play in management of long bone delayed and non-union.
We had fewer trials focusing on spinal pathology than we expected. Whilst spinal fusion is common, the non-union rates reported in literature were alarmingly high at 5–45%.51, 52, 53, 54, 55 The use of orthobiologics in spinal fusion surgery is therefore paramount to obtaining a solid fusion. Historically, the use of iliac crest autograft was standard during spinal fusion surgery. However, this has decreased over time owing to the morbidity of the procedure being as high as 30%, with the most common complications being chronic donor site pain, fracture, haematoma, infection, increased operative time and costs.51,56, 57, 58, 59 This has prompted the development of bone graft substitutes, which are usually synthetic materials with osteoconductive properties, and need for further research to establish the evidence for the most appropriate orthobiologic in spinal fusion surgery.51 PRP has been studied in spinal fusion surgery before with studies reporting that it did not improve the spinal fusion rate.60, 61, 62 However, given its vast potential, safety and cost-effectiveness, it has been used as an analgaesic modality in intervertebral disc disease, facet joint disease and radiculopathy.51
The heterogeneity of the orthobiologics used in the various studies is reflective of the lack of evidence for a single most efficacious orthobiologic to date. The ability of these orthobiologics to improve bone healing is determined by 3 major properties: osteoinduction, osteoconduction and osteogenesis.10,63 Bray et al. reported autologous cancellous bone graft to be the only orthobiologic with significant osteogenic, osteoconductive and osteoinductive properties.64 The remainder of the orthobiologics have varying properties – DBM has osteoinductive with some osteoconductive properties, BMAC has good osteogenic with some osteoinductive properties, BMP has osteoinductive properties and PRP has good osteoinductive with some osteogenic properties.10,64
It must be noted that there is significant uncertainty in PRP composition and a lack of standardisation in PRP preparation. Depending on patient characteristics and preparation kits used, there are differences in leucocyte count, platelet count and growth factor concentration. To date, there is no literature to support any PRP injection protocol, with uncertainty surrounding optimal dosage and timing intervals.65
5.1. Limitations
As this was a systematic review, the limitations in all included studies were present in this review. There were many factors that limited the direct comparability of findings from individual studies – heterogeneity in PRP preparation, PRP activation, use of other orthobiologics along with PRP, bone pathology, anatomical placement and time of PRP application, intervention for given pathology and outcome metrics.
As we have incorporated studies that look at radiological evidence of fracture healing, there is invariably a risk of error and bias in reading and reporting the radiographs. Another source of bias was participant selection bias. Lastly, as it is already well established in literature, there is a publication bias – bias for publication of positive results only. This has been documented in the literature for decades, with recent evidence suggesting that this bias is increasing.66, 67, 68 Therefore, there may have been more studies with negative results which weren't reported in the current literature.
6. Conclusion
The current evidence suggests that PRP may play a clinical role in bone healing but further randomised controlled trials (RCTs) using standardised outcomes should be performed to establish its efficacy.
References
- 1.Emara K.M., Diab R.A., Emara A.K. Recent biological trends in management of fracture non-union. World J Orthopaed. 2015;6(8):623–628. doi: 10.5312/wjo.v6.i8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Childs S.G. Stimulators of bone healing. Biologic and biomechanical. Orthop Nurs. 2003;22(6):421–428. doi: 10.1097/00006416-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Heckman J.D., Sarasohn-Kahn J. The economics of treating tibia fractures. The cost of delayed unions. Bull Hosp Jt Dis. 1997;56(1):63–72. [PubMed] [Google Scholar]
- 4.Griffin X.L., Wallace D., Parsons N., Costa M.L. Platelet rich therapies for long bone healing in adults. Cochrane Database Syst Rev. 2012;7 doi: 10.1002/14651858.CD009496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaron R.K., Ciombor D.M., Simon B.J. Treatment of non-unions with electric and electromagnetic fields. Clin Orthop Relat Res. 2004;419:21–29. doi: 10.1097/00003086-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop S., Ekegren C.L., Edwards E.R., De Steiger R., Page R., Gabbe B.J. Hospital admissions and inpatient costs of non-union, delayed union and mal-union following long bone fracture. Value Health. 2016;19(7):A916. [Google Scholar]
- 7.Nair M.B., Varma H.K., Menon K.V., Shenoy S.J., John A. Reconstruction of goat femur segmental defects using triphasic ceramic-coated hydroxyapatite in combination with autologous cells and platelet-rich plasma. Acta Biomater. 2009;5(5):1742–1755. doi: 10.1016/j.actbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 8.He F., Chen Y., Li J., et al. Improving bone repair of femoral and radial defects in rabbit by incorporating PRP into PLGA/CPC composite scaffold with unidirectional pore structure. J Biomed Mater Res. 2015;103(4):1312–1324. doi: 10.1002/jbm.a.35248. [DOI] [PubMed] [Google Scholar]
- 9.Roffi A., Di Matteo B., Krishnakumar G.S., Kon E., Filardo G. Platelet-rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice. A systematic review. Int Orthop. 2016;41:221–237. doi: 10.1007/s00264-016-3342-9. [DOI] [PubMed] [Google Scholar]
- 10.Calcei J.G., Rodeo S.A. Orthobiologics for bone healing. Clin Sports Med. 2019;38(1):79–95. doi: 10.1016/j.csm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann G., Müller U., Löffler C., Wentzensen A., Moghaddam A. Therapeutic outcome in tibial pseudarthrosis: bone morphogenetic protein 7 (BMP-7) versus autologous bone grafting for tibial fractures. Unfallchirurg. 2007;110:931–938. doi: 10.1007/s00113-007-1347-y. [DOI] [PubMed] [Google Scholar]
- 12.Giordano A., Galderisi U., Marino I.R. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal Stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar M.R., Augat P., Shefelbine S.J., et al. Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials. 2006;27(9):1817–1823. doi: 10.1016/j.biomaterials.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Alves R., Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Append Disord. 2018;4(1):18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le A.D.K., Enweze L., DeBaun M.R., Dragoo J.L. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskel Med. 2018;11:624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. The. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 18.Jamal M.S. Orthopaedic Trauma Science MSc QMUL; 2019. Dissertation Project Plan. [Google Scholar]
- 19.Duramaz A., Ursavas H.T., Bilgili M.G., Bayrak A., Bayram B., Avlan M.C. Platelet-rich plasma versus exchange intramedullary nailing in treatment of long bone oligotrophic nonunions. Eur J Orthop Surg Traumatol. 2018;28(1):131–137. doi: 10.1007/s00590-017-2024-7. [DOI] [PubMed] [Google Scholar]
- 20.Mariconda M., Cozzolino F., Cozzolino A., D'Agostino E., Bove A., Milano C. Platelet gel supplementation in long bone nonunions treated by external fixation. J Orthop Trauma. 2008;22(5):342–345. doi: 10.1097/BOT.0b013e318172cea5. [DOI] [PubMed] [Google Scholar]
- 21.Samuel G., Menon J., Thimmaiah S., Behera G. Role of isolated percutaneous autologous platelet concentrate in delayed union of long bones. Eur J Orthop Surg Traumatol. 2018;28(5):985–990. doi: 10.1007/s00590-017-2077-7. [DOI] [PubMed] [Google Scholar]
- 22.Acosta-Olivo C., Garza-Borjon A., Simental-Mendia M., Vilchez-Cavazos F., Tamez-Mata Y., Pena-Martinez V. Delayed union of humeral shaft fractures: comparison of autograft with and without platelet-rich plasma treatment: a randomized, single blinded clinical trial. Arch Orthop Trauma Surg. 2017;137(9):1247–1252. doi: 10.1007/s00402-017-2736-5. [DOI] [PubMed] [Google Scholar]
- 23.Calori G.M., Tagliabue L., Gala L., D'Imporzano M., Peretti G., Albisetti W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39(12):1391–1402. doi: 10.1016/j.injury.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Tarallo L., Mugnai R., Adani R., Catani F. Treatment of the ulna non-unions using dynamic compression plate fixation, iliac bone grafting and autologous platelet concentrate. Eur J Orthop Surg Traumatol. 2012;22(8):681–687. doi: 10.1007/s00590-011-0902-y. [DOI] [PubMed] [Google Scholar]
- 25.Bielecki T., Gazdzik T.S., Szczepanski T. Benefit of percutaneous injection of autologous platelet-leukocyte-rich gel in patients with delayed union and nonunion. Eur Surg Res. 2008;40(3):289–296. doi: 10.1159/000114967. [DOI] [PubMed] [Google Scholar]
- 26.Chiang C.C., Su C.Y., Huang C.K., Chen W.M., Chen T.H., Tzeng Y.H. Early experience and results of bone graft enriched with autologous platelet gel for recalcitrant nonunions of lower extremity. J Trauma. 2007;63(3):655–661. doi: 10.1097/01.ta.0000219937.51190.37. [DOI] [PubMed] [Google Scholar]
- 27.Galasso O., Mariconda M., Romano G., et al. Expandable intramedullary nailing and platelet rich plasma to treat long bone non-unions. J Orthop Traumatol. 2008;9(3):129–134. doi: 10.1007/s10195-008-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gołos J., Waliński T., Piekarczyk P., Kwiatkowski K. Results of the use of platelet rich plasma in the treatment of delayed union of long bones. J. Orthopaed Traumatol & Rehabil. 2014;16(4):397–406. doi: 10.5604/15093492.1119617. [DOI] [PubMed] [Google Scholar]
- 29.Malhotra R., Kumar V., Garg B., et al. Role of autologous platelet-rich plasma in treatment of long-bone nonunions: a prospective study. Musculoskel Surg. 2015;99(3):243–248. doi: 10.1007/s12306-015-0378-8. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez M., Anitua E., Cugat R., et al. Nonunions treated with autologous preparation rich in growth factors. J Orthop Traumatol. 2009;23(1):52–59. doi: 10.1097/BOT.0b013e31818faded. [DOI] [PubMed] [Google Scholar]
- 31.Say F., Türkeli E., Bülbül M. Is platelet-rich plasma injection an effective choice in cases of non-union? Acta Chir Orthop Traumatol Cech. 2014;81(5):340–345. [PubMed] [Google Scholar]
- 32.Rodriguez-Collazo E.R., Urso M.L. Combined use of the Ilizarov method, concentrated bone Marrow aspirate (cBMA), and platelet-rich plasma (PRP) to expedite healing of bimalleolar fractures. Strat Trauma & Limb Reconstr. 2015;10(3):161–166. doi: 10.1007/s11751-015-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samy A.M. The role of platelet rich plasma in management of fracture neck femur: new insights. Int Orthop. 2016;40(5):1019–1024. doi: 10.1007/s00264-015-2844-1. [DOI] [PubMed] [Google Scholar]
- 34.Griffin X.L., Achten J., Parsons N.R., Costa M.L. Platelet-rich therapy in the treatment of patients with hip fractures: a single Centre, parallel group, participant-blinded, randomised controlled trial. BMJ Open. 2013;3(6) doi: 10.1136/bmjopen-2013-002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liebergall M., Schroeder J., Mosheiff R., et al. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther. 2013;21(8):1631–1638. doi: 10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namazi H., Mehbudi A. Investigating the effect of intraarticular PRP injection on pain and function improvement in patients with distal radius fracture. J Orthop Traumatol: Surg & Res. 2016;102(1):47–52. doi: 10.1016/j.otsr.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Singh R., Rohilla R., Gawande J., Sehgal P.K. To evaluate the role of platelet-rich plasma in healing of acute diaphyseal fractures of the femur. Chin J Traumatol. 2017;20(1):39–44. doi: 10.1016/j.cjtee.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei L.C., Lei G.H., Sheng P.Y., et al. Efficacy of platelet-rich plasma combined with allograft bone in the management of displaced intra-articular calcaneal fractures: a prospective cohort study. Orthopaed Res. 2012;30(10):1570–1576. doi: 10.1002/jor.22118. [DOI] [PubMed] [Google Scholar]
- 39.Peerbooms J.C., Colaris J.W., Hakkert A.A., et al. No positive bone healing after using platelet rich plasma in a skeletal defect. An observational prospective cohort study. Int Orthop. 2012;36(10):2113–2119. doi: 10.1007/s00264-012-1603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dallari D., Savarino L., Stagni C., et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone Marrow stromal cells. J Bone Joint Surg. 2007;89(11):2413–2420. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 41.D'Elia C.O., De Rezende M.U., Bitar A.C., et al. Comparison between platelet-rich plasma and autologous iliac grafts for tibial osteotomy. Cartilage. 2010;1(4):320–327. doi: 10.1177/1947603510376820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee D.H., Ryu K.J., Kim J.W., Kang K.C., Choi Y.R. Bone Marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin Orthop Relat Res. 2014;472(12):3789–3797. doi: 10.1007/s11999-014-3548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubota G., Kamoda H., Orita S., Yamauchi K., Sakuma Y., Oikawa Y. Platelet-rich plasma enhances bone union in posterolateral lumbar fusion: a prospective randomized controlled trial. Spine J. 2019;19(2) doi: 10.1016/j.spinee.2017.07.167. e34-0. [DOI] [PubMed] [Google Scholar]
- 44.Tsai C.H., Hsu H.C., Chen Y.J., Lin M.J., Chen H.T. Using the growth factors-enriched platelet Glue in spinal fusion and its efficiency. J Spinal Disord Tech. 2009;22:246–250. doi: 10.1097/BSD.0b013e3181753ae2. [DOI] [PubMed] [Google Scholar]
- 45.Tuakli-Wosornu Y.A., Terry A., Boachie-Adjei K., et al. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM&R: J Injur Funct Rehabil. 2016;8(1):1–10. doi: 10.1016/j.pmrj.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Giannoudis P.V., Psarakis S., Kanakaris N.K., Pape H.C. Biological enhancement of bone healing with bone morphogenetic protein-7 at the clinical setting of pelvic girdle non-unions. Injury. 2007;38(4):S43–S48. doi: 10.1016/s0020-1383(08)70008-1. [DOI] [PubMed] [Google Scholar]
- 47.Giannoudis P.V., Einhorn T.A., Marsh D. Fracture healing: a harmony of optimal biology and optimal fixation? Injury. 2007;38(4):S1–S2. doi: 10.1016/s0020-1383(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 48.Khan S.N., Bostrom M.P., Lane J.M. Bone growth factors. Orthopaed Clin North Am. 2000;31:375–388. doi: 10.1016/s0030-5898(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 49.Giannoudis P.V., Tzioupis C. Clinical applications of BMP-7: the UK perspective. Injury. 2005;36(3):S47–S50. doi: 10.1016/j.injury.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Kontakis G.M., Papadokostakis G.M., Alpantaki K., et al. Intramedullary nailing for non-union of the humeral diaphysis: a review. Injury. 2006;37:953–960. doi: 10.1016/j.injury.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 51.Rajnish R.K., Kumar V., Dhatt S.S. Orthobiologics in spine. J Postgrad Med Educ Res. 2018;52(2):83–87. [Google Scholar]
- 52.May V.R., Mauck W.R. Exploration of the spine for pseudarthrosis following spinal fusion in the treatment of scoliosis. Clin Orthop Relat Res. 1967;53:115–122. [PubMed] [Google Scholar]
- 53.O'Beirne J., O'Neill D., Gallagher J., Williams D.H. Spinal fusion for back pain: a clinical and radiological review. J Spinal Disord. 1992;5:32–38. doi: 10.1097/00002517-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Steinmann J.C., Herkowitz H.N. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80–90. [PubMed] [Google Scholar]
- 55.Zdeblick T.A. A prospective, randomized study of lumbar fusion: preliminary results. Spine. 1993;18(8):983–991. doi: 10.1097/00007632-199306150-00006. [DOI] [PubMed] [Google Scholar]
- 56.Arrington E.D., Smith W.J., Chambers H.G., Bucknell A.L., Davino N.A. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 57.Banwart J.C., Asher M.A., Hassanein R.S. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine. 1995;20(9):1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Fernyhough J.C., Schimandle J.H., Weigel M.C., Edwards C.C., Levine A.M. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17(12):1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Laurie S.W.S., Kaban L.B., Mulliken J.B., Murray J.E. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73(6):933–938. doi: 10.1097/00006534-198406000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Weiner B.K., Walker M. Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine. 2003;28(17):1968–1970. doi: 10.1097/01.BRS.0000083141.02027.48. [DOI] [PubMed] [Google Scholar]
- 61.Hee H.T., Majd M.E., Holt R.T., Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003;12(4):400–407. doi: 10.1007/s00586-003-0548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carreon L.Y., Glassman S.D., Anekstein Y., Puno R.M. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30(9):E243–E246. doi: 10.1097/01.brs.0000160846.85397.44. [DOI] [PubMed] [Google Scholar]
- 63.Egol K.A., Nauth A., Lee M., et al. Bone grafting: sourcing, timing, strategies, and alternatives. J Orthop Trauma. 2015;29(12):S10–S14. doi: 10.1097/BOT.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 64.Bray C.C., Walker C.M., Spence D.D. Orthobiologics in pediatric sports medicine. Orthopaed. Clin. North Am. 2017;48(3):334. doi: 10.1016/j.ocl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Hurley E.T., Fat D.L., Moran C.J., Mullet H. The efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: a meta-analysis of randoomized controlled trials. Am J Sports Med. 2019;47(3):753–761. doi: 10.1177/0363546517751397. [DOI] [PubMed] [Google Scholar]
- 66.Joober R., Schmitz N., Annable L., Boksa P. Publication bias: what are the challenges and can they be overcome? J Psychiatry Neurosci. 2012;37(3):149–152. doi: 10.1503/jpn.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ioannidis J.P. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jakobsen A.K., Christensen R., Persson R., et al. Open access publish-ing. And now, e-publication bias. BMJ. 2010;340:c2243. doi: 10.1136/bmj.c2243. [DOI] [PubMed] [Google Scholar]