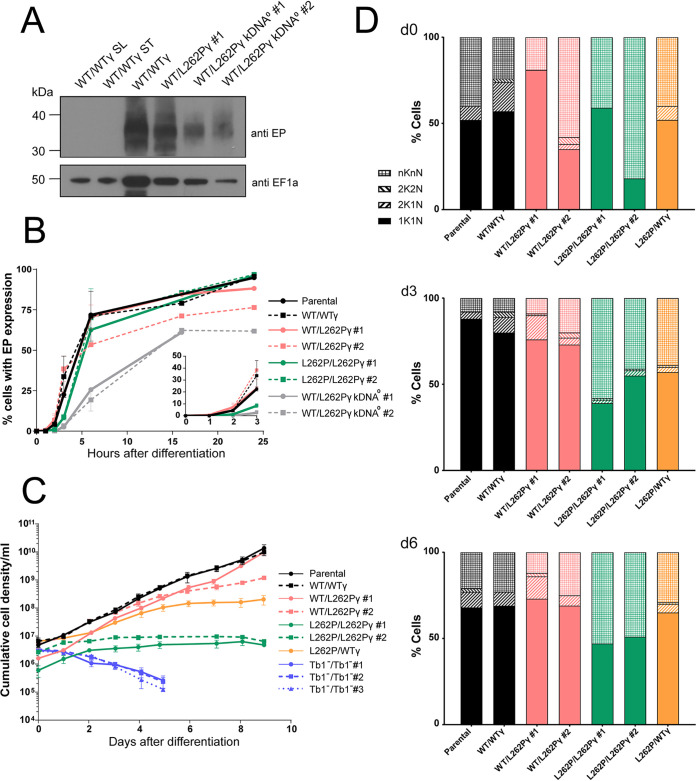
FIG 1.
Ability of kDNA0 cells and F1Fo-ATP synthase mutants to differentiate into PCF in vitro. (A) Western blot to assess EP procyclin expression. Stumpy cells were treated with 6 mM cis-aconitate (CA) and incubated at 27°C to induce differentiation. Cells were harvested after 24 h, 2 × 106 cells were loaded per lane of an SDS-PAGE gel, and blots were probed with anti-EP procyclin (showing an expected smear due to heterogenous nature of EP protein [top]) and anti-EF1α as a loading control (bottom). Slender WT/WTγ cells and stumpy WT/WTγ cells not exposed to CA were analyzed as negative controls for EP expression. (B) EP expression measured by flow cytometry. Stumpy cells were treated with 6 mM CA and incubated at 27°C at 0 h. At each time point, cells were fixed and stained with mouse anti-EP procyclin and anti-mouse IgG Alexa 488 antibodies. Ten thousand cells were analyzed per sample; n = 2. (Inset) Magnification of the time points taken between 0 and 3 h after differentiation. Note that for kDNA0 cells, numbers at 24 h represent large amounts of cell debris that stained positive for EP. No intact, motile cells were visible upon inspection by microscopy at this time point. (C) Growth of newly differentiated PCF T. brucei. Stumpy form cells were treated with 6 mM CA in HMI-9 medium for 24 h at 27°C. Cells were transferred to SDM80 medium at 27°C (0 h), and cell density was determined with a hemocytometer over 24 h. Counting was performed in triplicate with three separate blood cryostocks of each cell line thawed and differentiated at the same time. Error bars indicate standard deviations. kDNA0 cells were not included in this analysis due to their death within 24 h after induction of differentiation. (D) Cell cycle analysis of newly differentiated PCF T. brucei. Slides were prepared at day 0 (d0), d3, and d6 after differentiation and transfer into SDM80, and cells were blindly scored for cell cycle stage. Approximately 100 cells were assessed per strain and time point. The category nKnN includes zoids (i.e., cells with no nucleus), monsters (multiple nuclei), and dying (large, blurry, and/or fragmented nuclei) cells. kDNA0 and Tb1−/Tb1− cells were not included in this analysis due to their nonviability after differentiation.