Abstract
Age‐associated diseases are rising to pandemic proportions, exposing the need for efficient and low‐cost methods to tackle these maladies at symptomatic, behavioral, metabolic, and physiological levels. While nutrition and health are closely intertwined, our limited understanding of how diet precisely influences disease often precludes the medical use of specific dietary interventions. Caloric restriction (CR) has approached clinical application as a powerful, yet simple, dietary modulation that extends both life‐ and healthspan in model organisms and ameliorates various diseases. However, due to psychological and social‐behavioral limitations, CR may be challenging to implement into real life. Thus, CR‐mimicking interventions have been developed, including intermittent fasting, time‐restricted eating, and macronutrient modulation. Nonetheless, possible side effects of CR and alternatives thereof must be carefully considered. We summarize key concepts and differences in these dietary interventions in humans, discuss their molecular effects, and shed light on advantages and disadvantages.
Keywords: caloric restriction, fasting, healthspan, intermittent fasting, lifespan, time‐restricted eating
Subject Categories: Autophagy & Cell Death, Metabolism
Can dietary modulations promote better health? The current article comprehensively reviews key concepts of dietary interventions, their clinical application and the beneficial and disadvantageous effects of caloric restriction and fasting on human health.
Glossary
- Alternate day fasting (ADF)
Alternate day fasting (ADF) is defined by an alternating sequence of fasting days (zero calorie intake) and eating days (ad libitum food consumption)
- Alternate day modified fasting (ADMF)
Like ADF, but allows <25% calorie intake on fasting days
- AMP‐activated protein kinase (AMPK)
A phylogenetically conserved nutrient‐ and energy‐sensing enzyme. AMPK becomes activated by events that increase the AMP/ATP ratio (e.g., exercise, fasting)
- Caloric Restriction (CR)
CR involves the reduction in calorie intake over a given period, while maintaining adequate levels of macro‐ and micronutrients
- Caloric restriction mimetics (CRM)
CRMs are pharmacologically active compounds that mimic some anti‐aging effects of CR
- Circulating metabolome
The metabolome is a highly dynamic system and an important entity during CR and fasting. Moreover, it serves signaling functions, transports fuels, and contributes to the systematic health benefits during CR and IF
- Fasting‐mimicking diet (FMD)
The FMD is an eating regimen defined by a specific macro‐ and micronutrient composition that aims at inducing fasting‐like benefits
- Healthspan
Healthspan is the period of life that individuals spend in good health without any aging‐related chronic diseases or disabilities
- Intermittent Fasting (IF)
IF refers to many different fasting regimes, which combine phases of restricted calorie intake and ad libitum consumption
- Ketone Bodies (KBs)
KBs (β‐hydroxybutyrate, acetoacetate, and acetone) are compounds produced by the liver upon fasting or caloric restriction. KBs serve as fuel and signaling molecules under low glucose levels
- Lean body mass
The term “lean body mass” refers to non‐adipose tissue mass
- Mechanistic Target of Rapamycin (mTOR)
mTOR regulates fundamental processes like eukaryotic cell growth, protein synthesis, or autophagy as well as the metabolism by transducing environmental inputs, including nutrient availability. Deregulation of mTOR signaling is implicated in the progression of different diseases as well as in the aging process
- Metabolic switch
The metabolic switch upon fasting is the point of negative energy balance, at which liver glycogen stores are depleted. It is characterized by lipid metabolization, free fatty acid usage, and production of KBs
- Starvation
Starvation is a pathological state that results from a severe or total lack of nutrients
- Time‐restricted eating (TRE)
TRE describes the time‐limited consumption of calories during a period of 6–8 hours per day
Introduction
According to the World Health Organization, life expectancy has been continuously increasing during the past decades (World Health Organization, 2019), although the pace of this increase seems to slow down globally (Cardona & Bishai, 2018). This development is accompanied by a rise in age‐associated diseases, including obesity, cardiovascular diseases (CVDs), type 2 diabetes, neurodegenerative diseases, and cancer. Thereby, our healthspan, defined as the life time without significant age‐related disease burden, increases at a slower pace than lifespan, resulting in more life years suffering from one or multiple diseases (World Health Organization, 2019). This is not only detrimental at a personal level but is also severely challenging health care systems.
Dietary interventions for healthy aging
A major goal of aging research is to extend healthspan and delay the onset of age‐associated frailty and diseases. This concept has been termed “compression of morbidity” (Fries, 1980), or also “healthy aging,” and integrates various research fields to address the molecular and physiological modulation of aging, social well‐being, physical and cognitive fitness, etc. (Longo et al, 2015, 2021; Campisi et al, 2019; de Cabo & Mattson, 2019; Daniel, 2020; Eckstrom et al, 2020; Sharda et al, 2020). Theoretically, health‐ and life‐prolonging interventions can take different routes (Figure 1): (i) increasing maximal lifespan, while not affecting the ratio of healthy to unhealthy years; (ii) increasing healthspan only, without affecting maximal lifespan; and (iii) increasing both health‐ and maximal lifespan. On the other hand, (iv) increased disease burden affects health‐ and/or lifespan negatively (Figure 1). While genetic approaches in model organisms have revealed many molecular instances of aging control, their implementation in humans presents yet practical challenges and ethical limitations. However, recent advances in the field of gene editing via CRISPR‐Cas9‐technologies may soon become relevant, at least in some age‐associated diseases (Caobi et al, 2020). Non‐genetic health‐promoting interventions based on dietary modulation are less invasive and closer to broad‐scale application in health care. In fact, nutrition is a crucial determinant of aging. While chronic hypernutrition (as experienced in many western countries) leads to detrimental consequences, including increased incidence of overweight and obesity, malnutrition and undernutrition can evoke starvation effects, decreasing health and negatively impacting lifespan.
Figure 1. Theoretical concepts underlying dietary healthy aging intervention.
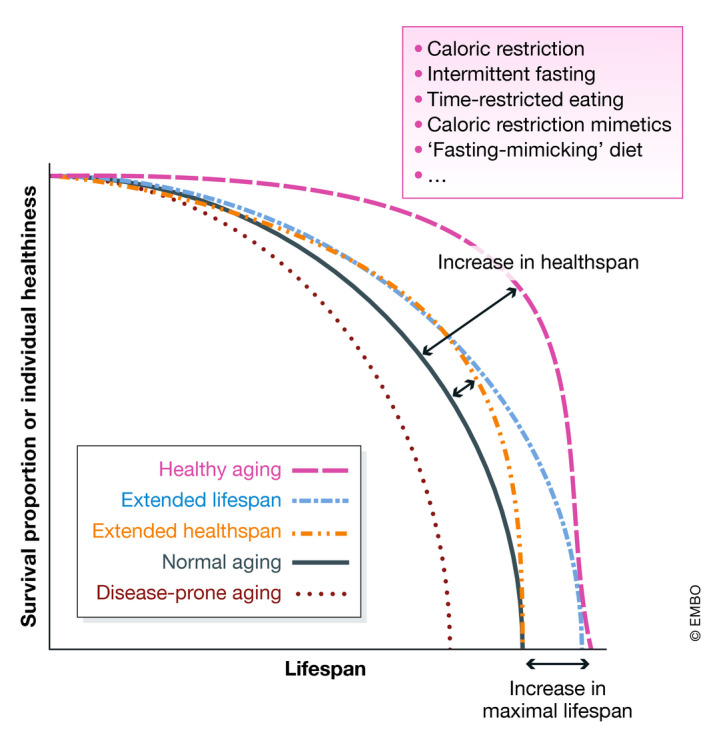
Different courses of aging, and how dietary approaches might influence the curves. Y‐axis shows survival proportions of a population and theoretical, individual healthiness. X‐axis shows the relative lifespan.
Dietary interventions in aging commonly target various outcomes related to aging as a whole. (i) In model organisms, extension of health‐ and lifespan of wild‐type or disease models is the most substantial outcome to be measured. In interventional clinical studies, this is yet an unreachable measure, but indirect measures of aging, such as epigenetic clocks, are emerging as methods to indirectly quantify potential anti‐aging interventions in humans (Horvath, 2013; Fitzgerald et al, 2021). (ii) Secondly, such interventions are preventive, i.e., they can delay the onset or progress of age‐related and generally non‐communicable diseases and avert obesity from hypernutrition. (iii) Finally, anti‐aging interventions can be revertive, i.e., ameliorate persisting symptoms of and tackle manifest age‐associated diseases and obesity.
A case for caloric restriction and fasting
Among the existing dietary anti‐aging interventions, the sustained reduction in caloric intake without malnutrition (calorie restriction, CR) is probably the most robust and best studied form of geroprotective dietary intervention in model organisms (Fontana, 2009; Green et al, 2021). In fact, Clive McCay and colleagues noted already nearly nine decades ago that CR could prolong the lifespan of rats (McCay et al, 1935). In humans, reduced calorie intake without malnutrition has been repeatedly shown to elicit systemic health benefits (Most et al, 2017a), e.g., upon natural CR as observed on the Okinawan island (Willcox et al, 2006), the Comprehensive Assessment of the Long‐term Effects of Reducing Energy Intake (CALERIE) studies (Rickman et al, 2011), the CR with optimal nutrition (CRON) society, the Biosphere 2 experiment (Walford et al, 2002), and a long‐term cohort of alternate day fasting (ADF) followers (Stekovic et al, 2019). Besides CR, different forms of intermittent fasting (IF) have gained scientific and public interest for their broad health‐promoting properties (Michalsen & Li, 2013; Longo et al, 2021). Interestingly, virtually all extant religions have incorporated recurring fasting rituals, e.g., the Lent before Easter (Christianity) or the fasting month of Ramadan (Islam).
Besides the caloric element, macronutrient content and balance represent additional important denominators in health‐promoting dietary interventions (Simpson et al, 2015; Solon‐Biet et al, 2015). Thus, dietary constitution might be equally important as calorie content (Wilder et al, 2013; Simpson et al, 2015; Green & Lamming, 2019). Industrial ultra‐processing of food also promotes excessive calorie consumption and weight gain (Hall et al, 2019), emphasizing the importance of a diet’s underlying calorie sources.
Scope of the review
In this non‐systematic review, we summarize molecular targets, physiological effects, and potential uses of CR and various forms of IF in clinical settings. Furthermore, we highlight potential caveats of these interventions and provide an outlook on novel, less invasive CR‐mimicking dietary interventions.
Common types of CR and fasting
Besides CR, several health‐promoting dietary interventions have been developed, with often vaguely defined terminology, the most common of which are briefly described below (Figure 2).
Caloric Restriction (CR) defines the reduction in calorie intake over a given period without malnutrition, sufficiently providing macro‐ and micronutrients. Typical levels of CR in mice and rats range from 10 to 50% (Mitchell et al, 2016; Acosta‐Rodríguez et al, 2017), while in humans most studies aim at 10–25% CR per day (Redman & Ravussin, 2011; Most et al, 2017b; Trepanowski et al, 2017). In rodent studies, CR is often achieved by restricting feeding windows and/or providing pre‐weighted amounts of food every day, which may be rapidly consumed by the animals (Acosta‐Rodríguez et al, 2017), thus possibly mixing effects of CR and IF. In humans, CR without significant temporal alterations of daily eating patterns can be achieved by reducing meal sizes. Of note, CR is often synonymously used with the term dietary restriction (DR), although DR rather describes the more specific restriction of macronutrients (e.g., proteins, carbohydrates, and amino acids) instead of overall food intake (Katewa & Kapahi, 2010).
-
Intermittent Fasting (IF) is a loosely defined term that applies to different rhythmic, or recurring, arhythmic fasting regimens, in which calorie reduction is not achieved by reducing meal sizes, but rather by skipping one or multiple consecutive meals. Thus, all types of IF combine phases of restricted calorie intake and ad libitum consumption. However, if experimentally not controlled, craving‐induced overcompensation during the limited mealtimes may result in no or only minor calorie restriction.
Alternate Day Fasting, ADF, describes a form of IF, in which participants fast every other day (resulting in periodic fasting windows of ca. 36 h). Fasting days are followed by ad libitum eating days, most commonly with no restrictions. Fasting days may allow no calorie intake (Heilbronn et al, 2005), or—if performed more mildly—a baseline calorie intake of, e.g., 25% (Varady et al, 2009; Trepanowski et al, 2017; Gabel et al, 2019). In the latter case, the term alternate day modified fasting (ADMF) may be used (Anton et al, 2018). In non‐obese adults, ADF can result in CR of around 35%, as the calorie loss is not fully compensated on eating days (Stekovic et al, 2019). ADF without net energy reduction is possible (Templeman et al, 2021), but likely impracticable outside study settings.
Periodic Fasting (PF), often used synonymously with IF, describes a type of arrhythmic IF. The most prominent PF intervention is the so‐called “5:2” scheme (Scholtens et al, 2020), in which 2 days a week are calorie‐restricted to a minimal level, either sequentially or spread throughout the week. Other PF patterns might describe fasting phases of multiple consecutive days during a given timeframe (e.g., 5 consecutive fasting days per month).
Time‐Restricted Eating (TRE), in model organisms often termed time‐restricted feeding (TRF), describes the restriction of daily calorie intake to a consecutive time window of 6–8 h (Hatori et al, 2012; Gabel et al, 2018; Regmi & Heilbronn, 2020). TRE can be achieved by either skipping a specific meal (breakfast or dinner) or by compressing the meals into a narrow time window. In either case, TRE may be classified into early (e.g., skipping dinner) and late (e.g., skipping breakfast), depending on the first meal consumed. Compared to other IF protocols, TRE has a much higher frequency of fasting–eating cycles, thus shorter fasting windows, and may influence circadian rhythms more profoundly.
Long‐term fasting, or very‐low‐calorie diet, is a loosely defined term often used for long‐lasting (more than 2 days, up to several weeks), very low calorie intake protocols (< 1,000 calories/day), which are often the basis for experimental, therapeutic, or lifestyle fasting applications (Wilhelmi de Toledo et al, 2020b).
Other Fasting/CR‐mimicking dietary interventions comprise various experimental avenues that aim at eliciting (some) CR‐like molecular and physiological effects, such as the fasting‐mimicking diet (FMD; Brandhorst et al, 2015; Choi et al, 2016a; Wei et al, 2017), ketogenic diets (Ludwig, 2020), or low‐carbohydrate/fat diets (Kim, 2021).
CR mimetics (CRMs) are pharmacologically active compounds that mimic some CR‐like effects on cells and organisms (Lane et al, 1998; Ingram & Roth, 2021). Examples include spermidine, rapamycin, metformin, or 2‐deoxy‐glucose (Mariño et al, 2014; Madeo et al, 2019; Ingram & Roth, 2021). Some of these candidates are under intense clinical investigation (Hofer et al, 2021). Depending on upstream or downstream molecular targets of CRMs, these compounds might provoke a broad or narrow selection of CR‐associated effects.
Figure 2. Different concepts of caloric restriction and fasting applications that have transitioned to clinical trials.
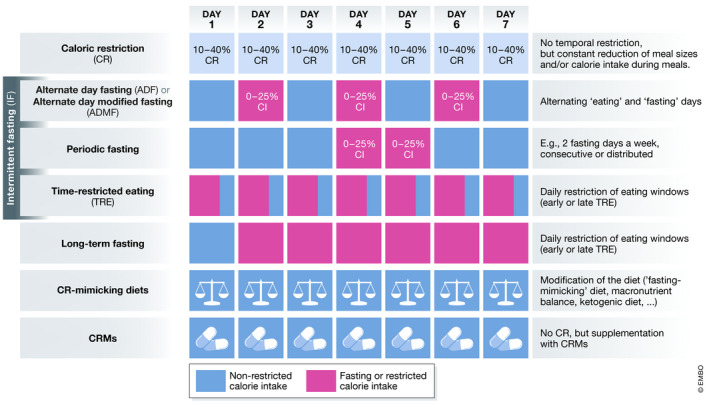
Blue = non‐restricted calorie intake, magenta = fasting or restricted calorie intake, CI = calorie intake, CRM = CR mimetic.
Cellular and molecular events during CR and fasting
The principal cellular mechanisms in response to nutrient availability are conserved from prokaryotes to primates and ensure survival under fasting conditions (Longo & Mattson, 2014). Here, we summarize key events and molecular players during fasting and CR in mammals, and which contribute to the systemic health‐promoting properties of these interventions (Figure 3).
Figure 3. Key molecular events during CR and fasting at the cellular and metabolic levels.
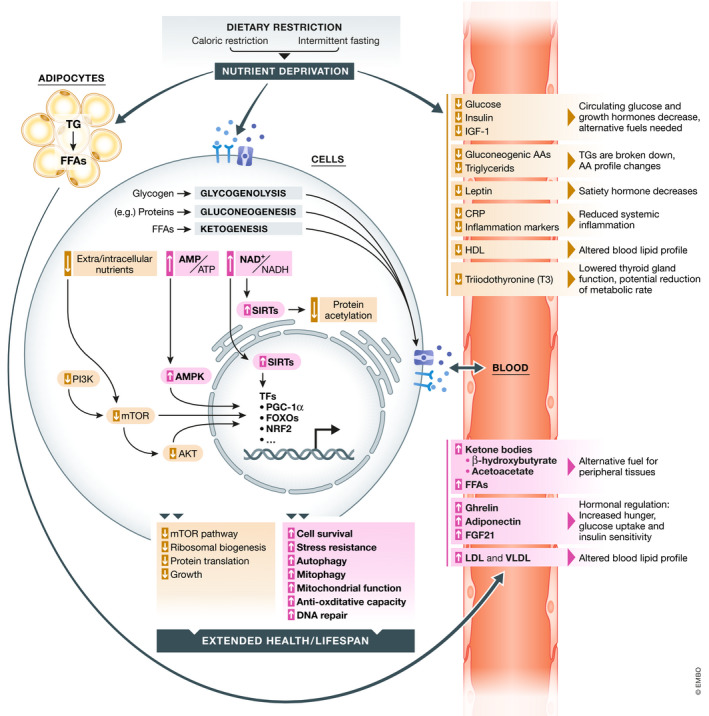
CR and fasting act on multiple levels, eliciting an increase in cellular and organismal multi‐stress resistance, which leads to systemic health benefits. AKT = protein kinase B/PKB, AMPK = AMP‐activated protein kinase, AMP/ATP = ratio of adenosine monophosphate to adenosine triphosphate, BCAA = branched‐chain amino acid, FFA = free fatty acid, FGF21 = fibroblast growth factor 21, FOXO = forkhead box O, IGF‐1 = insulin‐like growth factor, (V)LDL = (very) low‐density lipoprotein, mTOR = mechanistic target of rapamycin, NAD+/NADH = ratio of oxidized to reduced nicotinamide adenine dinucleotide, NRF2 = nuclear factor erythroid 2‐related factor 2, PARP1 = Poly [ADP‐ribose] polymerase 1, PGC‐1α = peroxisome proliferation‐activated receptor gamma co‐activator 1α, TG = triglycerides.
A metabolic switch ensures energy supply upon fasting
The fasting state is characterized by a metabolic switch from glucose dependency to lipid metabolization, free fatty acid (FFA) usage, and production of ketone bodies (KBs; b‐hydroxybutyrate, acetoacetate, and acetone) in the liver (although astrocytes might also produce KBs to fuel neurons; Guzmán & Blázquez, 2004). The occurrence and magnitude of this switch greatly depend on the specific intervention studied. Usually, it starts gradually after 12–36 h of food abstinence, depending on baseline hepatic glycogen storage, activity levels, and other factors. This event is marked by an increase in KBs that may continue up to several days until reaching a plateau (Anton et al, 2018; Steinhauser et al, 2018). During continuous CR with regular meals throughout the day, this switch is less likely turned on and/or dampened, depending on the duration and level of CR.
Cellular processes and molecular targets affected by CR and fasting
Both during fasting episodes and CR, macro‐ and micronutrients are less available to cells and tissues. Thus, several pathways are similarly involved in mediating CR and IF effects. Most of the molecular events that govern cellular and molecular responses to nutrient deprivation have been studied in liver and muscle cells and may partially differ in other specialized cell types. All cells sense the availability of micro/micronutrients and react to either increased or decreased availability through various interconnected pathways, including those controlled by the serine/threonine protein kinase mechanistic target of rapamycin (mTOR) and AMP‐activated protein kinase (AMPK). mTOR is part of two complexes (mTORC1 and 2) that are both regulated by a wide variety of environmental signals and integrate those into cellular changes (Cornu et al, 2013; González & Hall, 2017; Liu & Sabatini, 2020). They are most prominently activated in response to amino acids and growth factors. AMPK, on the other hand, is activated upon low energy conditions and stimulates ketogenesis, fatty acid oxidation, glucose uptake, and inhibition of lipogenesis in various cell types (González et al, 2020). Accordingly, reduced glucose levels or diminished protein and amino acid availability—as induced via CR or fasting—lead to activation of AMPK and shutdown of mTOR, resulting in a reduction in protein synthesis and ribosomal biogenesis, as well as activation of autophagy. Likewise, low carbohydrate levels, on their part, are transduced via a decrease in insulin and IGF‐1 (insulin‐like growth factor 1), and reduced PI3K and AKT signaling. As a cellular consequence, mechanisms to cope with (nutritional) stress are upregulated via a regulatory network of transcription factors. These include processes such as DNA repair, autophagy, mitophagy, and oxidative stress defense, among others. From bottom up, the wide range of cellular alterations provoked by (periodic) nutrient depletion is believed to enhance cellular survival, reduce cellular senescence, improve organ function, counteract age‐associated deteriorations, decrease systemic problems, such as chronic inflammation, and consequently prolong organismal health‐ and lifespan.
Ultimately, CR‐ and fasting‐related cellular processes are regulated by a network of transcription factors. Among them are FOXO1 (forkhead box O1), which regulates gluconeogenesis, glycogenolysis and the cell cycle, as well as redox‐sensitive NRF2 (nuclear factor erythroid 2‐related factor 2), which activates a myriad of cytoprotective genes. In addition, sirtuins (SIRTs) are NAD+‐dependent histone deacetylases with multiple targets, also outside the nucleus, whose activity is tightly linked to the energy status (Haigis & Sinclair, 2010). For instance, in the liver, SIRT1 is stimulated during the metabolic switch and activates AMPK. SIRT1 also activates the transcription factors PPAR‐α (peroxisome proliferator‐activated receptor), which is important for hepatic lipid metabolism as well as ketogenesis, and PGC‐1α (peroxisome proliferation‐activated receptor gamma co‐activator), a master regulator of mitochondrial biogenesis. Active PGC‐1α coordinates enhanced mitochondrial function, while parallelly decreasing lipolysis and inhibiting gluconeogenesis, among many other effects. Intriguingly, during CR, SIRT1 has been found to be upregulated in various tissues of mammalian models and in the skeletal muscle of humans (Cantó & Auwerx, 2009; Rahman & Islam, 2011). Other sirtuin paralogs may also be relevant during the nutrient‐deprived states. For instance, SIRT3 regulates the activity of diverse mitochondrial enzymes and liver ketogenesis. Importantly, SIRT activity depends on NAD+, allowing SIRTs to sense the dietary status. In fact, an increased ratio of NAD+ to NADH (leading to SIRT activation) represents an important cellular event in the fasting state. Similarly, an increased ratio of AMP to ATP (also leading to AMPK activation) is also a characteristic event that mirrors the energy balance shift upon CR and fasting (Yang et al, 2007; Hasenour et al, 2013).
Another important cellular consequence of nutrient depletion is autophagy, a conserved intracellular recycling program that clears dysfunctional organelles and proteins. Its activity generally decreases during aging (Barbosa et al, 2019), and its dysregulation may contribute to neurodegenerative diseases, CVDs, and cancer (Schiattarella & Hill, 2016; Abdellatif et al, 2018; Yun & Lee, 2018; Barbosa et al, 2019; Corti et al, 2020; Park et al, 2020). In turn, the induction of autophagy by pharmacological (e.g., CRMs), genetic, or dietary (e.g., fasting and CR) means has been causally linked to health‐ and lifespan extension (Jia & Levine, 2010; Hansen et al, 2018; Madeo et al, 2019). Autophagy is activated and regulated via a complex regulatory network that involves the AMPK‐mediated control of mTOR, SIRTs, and protein acetylation. Deacetylation often favors autophagic processes and, in mice and humans, acute fasting has been shown to reduce acetylation of cytoplasmic and nuclear proteins, which depends on the availability of acetyl‐CoA (Pietrocola et al, 2017). Thus, many cellular and metabolic effects of fasting and CR converge on autophagy induction (Madeo et al, 2019).
Changes in the circulating metabolome during acute fasting and CR, and maintenance of energy supply
The circulating metabolome during CR and fasting phases crucially contributes to the systemic health benefits, serves signaling functions, and transports fuel. The metabolome is a highly dynamic system that gradually changes upon duration and level of nutrient deprivation. Steinhauser et al (2018) identified distinct classes of metabolites that behave differently over a fasting period of 10 days in humans. Metabolic changes during CR will share many of these fasting‐associated changes, depending on length and magnitude of calorie deprivation.
One of the most prominent energetic reactions to fasting and drastic CR is lipid utilization. As outlined above, this results in increased blood stream levels of KBs and FFAs as alternative fuel types when glucose levels become low. Prior to lipid utilization, glucose is released from hepatic glycogen stores via glycogenolysis for usage in non‐hepatic tissue. Further glucose can be sourced from gluconeogenesis using glucogenic amino acids after protein breakdown, mainly in the liver and some other tissues. During the first days of fasting, branched‐chain amino acids are elevated in the blood stream. Subsequently, acetyl‐CoA from beta‐oxidation using adipocyte‐derived FFAs (released from triacyl‐ and diacylglycerols into the blood stream) build the basis for KB production in the liver. KBs are then released into the blood stream, reaching millimolar concentrations in the fasting state, and can be utilized by target organs/cells to obtain acetyl‐CoA via ketolysis to feed into the tricarboxylic acid cycle for ATP generation. This is especially important for energy‐demanding tissues like muscle and brain (Cahill, 2006). Notably, FFAs and KBs also harbor signaling functions on molecular targets like hormones and transcription factors with health‐promoting properties (Zechner et al, 2017). Interestingly, in model organisms, the sole supplementation of KBs can prolong health‐ and lifespan on its own (Camberos‐Luna et al, 2016; Veech et al, 2017).
Among other factors that usually show elevated blood stream levels upon fasting and CR is ghrelin (“hunger hormone”), which provokes the typical hunger sensation (Müller et al, 2015). This goes alongside with increased concentrations of the adipose tissue‐secreted hormone adiponectin, which increases glucose uptake, insulin sensitivity, and fatty acid oxidation. Another crucial player in the fasting response is the liver‐derived FGF21 (fibroblast growth factor 21), which stimulates glucose uptake in adipocytes among other effects (Geng et al, 2020).
On the contrary, several factors are depleted in the blood stream. These include insulin, IGF‐1, glucose, gluconeogenic amino acids, and triglycerides. As a counterplayer to ghrelin, leptin, a peptide hormone that inhibits hunger sensation, decreases. Furthermore, inflammatory markers and markers of oxidative stress decrease, including C‐reactive protein (CRP), a hepatic protein involved in the inflammatory acute‐phase response (Collet et al, 2017; Anton et al, 2018; Steinhauser et al, 2018). Importantly, these observations have been made in rodent as well as in human CR and fasting studies. This decrease in inflammatory markers may contribute to the systemic health‐promoting effects upon longer CR and IF studies, as seen in in model organisms and humans. Furthermore, cardiovascular blood markers change profoundly, including decreased triglycerides, elevated LDL species, and decreased HDL, contributing to the improvement of the cardiovascular system observed in CR and IF studies.
Several observational and interventional studies have shown reduced thyroid gland function in CR and IF cohorts. In agreement, a reduction in energy expenditure is often reported in CR studies, although these findings have been controversially discussed (Mitchell et al, 2017). In rodent studies, core body temperature (which is regulated by the thyroid gland) is often reported to decrease during CR and weight loss, and housing at thermoneutrality reverses some of CR’s (metabolic) effects (Guijas et al, 2020), arguing for some level of causal connection. In humans, plasma levels of the thyroid hormone triiodothyronine (T3) decrease in a non‐pathological range upon ADF (Stekovic et al, 2019) and CR (Soare et al, 2011; Fontana et al, 2016; Redman et al, 2018), a potential sign of slowed aging and metabolic rate (Rozing et al, 2010; Jansen et al, 2015). In line, energy expenditure and metabolic rate have been shown to decrease upon CR, even when adjusted for weight loss, although the outcome on body core temperature is mixed (Martin et al, 2007; Jiménez Jaime et al, 2015; Most et al, 2017a; Redman et al, 2018). Data from IF studies on these parameters are scarce and to date show no consistent changes (Lessan & Ali, 2019; Stekovic et al, 2019).
CR and IF prolong the lifespan of model organisms
The assessment of how CR and IF may affect aging has to be framed within the complexity of the aging process itself. The nine “hallmarks of aging” were defined to reflect this complexity as a deteriorating process that affects organisms at all levels (López‐Otín et al, 2013). They include, for instance, mitochondrial dysfunction, loss of proteostasis, cellular senescence, and telomere shortening. Importantly, all of the latter have been shown to be counteracted by CR and IF in model organisms. In agreement with this, CR and IF interventions prolong health‐ and/or lifespan of yeast, worms, flies, and rodents. Non‐human primates also benefit from CR (30%), as shown in the University of Wisconsin study with rhesus monkeys, which reported improved survival and health (Colman et al, 2009, 2014). In contrast, a similar study conducted at the National Institute on Aging showed no lifespan benefits, although health parameters improved nearly significantly (Mattison et al, 2012). Beyond lifespan extension, it is the “compression of morbidity” which co‐defines the success of an anti‐aging intervention, and many age‐associated disorders that are ameliorated by CR in models have also been shown to be impacted in human studies (reviewed in (Green et al, 2021)). These include obesity, CVDs, cancer incidence, and type 2 diabetes, among others. Similar observations, although to a lesser extent, were made in human IF studies (Longo et al, 2021). All of the above discussed effects on cellular and organismal levels can contribute to the anti‐aging properties of CR and IF.
Brief summary
The sum of metabolic, transcriptional, and proteomic alterations, but also those of the microbiota (briefly discussed later in the review) that are evoked by CR and fasting, result in increased stress resistance, enhanced cell and DNA repair, autophagy induction, and enhanced mitochondrial function. As a result, these alterations may impact several aspects of cellular and organismal health that lead to prolonged health‐ and lifespan in models and systemic health promotion in humans (Figure 3). However, the exact timescale of these effects and how they can be medically employed in a targeted manner are yet largely uncharted areas.
Comparing CR and fasting interventions
To date, it is yet not fully understood if the respective effects observed upon mere CR and fasting can be seen as fully independent from each other. In fact, as many classic rodent CR studies achieve CR by reducing feeding time windows, effects in these studies may be elicited by both the CR and increased fasting phases. At the same time, there has been much debate whether intermittent energy restriction (IF) may harbor superior effects compared to continuous restriction (CR). For instance, rodents fed isocaloric, high‐fat, and high‐sucrose diets show delayed disease onset during aging and are largely protected from metabolic disturbances and weight gain when subjected to IF (Anson et al, 2003; Hatori et al, 2012; Sherman et al, 2012; Chaix et al, 2014, 2019; Kim et al, 2019; Mitchell et al, 2019). This suggests a partial uncoupling of IF‐induced health benefits from CR, highlighting the importance of periodic fasting episodes.
In humans, only few studies have addressed the challenge of isocaloric diets upon fasting or CR studies, as several intrinsic limitations impede such study designs. One study found no differences between calorie‐matched CR and ADMF (Trepanowski et al, 2017), similar to a further study that compared CR vs. zero‐calorie ADF (Catenacci et al, 2016). A small‐scale study with normal‐weighted participants compared isocaloric diets consumed in three meals or one meal and found a subtle reduction in body weight and fat mass as well as an increase in blood pressure and blood lipids in the 1 meal/day group (Stote et al, 2007). Most of the other blood parameters, as well as heart rate and body temperature, remained unchanged. A similar study found no differences in fasting levels of BDNF, insulin, leptin, ghrelin, and adiponectin, among others, but for the 1 meal/day group observed a delayed response in the oral glucose tolerance test, performed in the morning (Carlson et al, 2007). Moreover, a recent work compared 3 weeks of continuous CR (25%) with ADF (0 calories on fasting days and 150% on eating days, resulting in a theoretical net CR of 25%) and ADF without energy restriction (0 calories during fasting days and 200% calories on eating days; Templeman et al, 2021). Interestingly, weight loss was comparable between CR and 150%‐ADF, but the latter caused an unfavorable greater loss of lean mass. ADF without energy restriction, on the other hand, resulted in an attenuated weight loss (ca. −0.5 kg vs. −1.6 to −1.7 kg in the other groups; Templeman et al, 2021). Overall, ADF had no significant, superior benefits on metabolic parameters or body composition in this study. In line, 3 months of PF in overweight and obese adults do not provoke improved outcomes on circulating biomarkers, weight or fat loss when compared to a continuous, matched CR intervention (20%) (Schübel et al, 2018). On the other hand, PF for 4 months (2 fasting days/week during the first 3 months, 1 fasting day/week during the 4th month), and allowing some calories on fasting days resulted in improved insulin sensitivity and greater fat loss in overweight women, when compared to continuous CR (Harvie et al, 2013). Whether weight loss rates differ between intermittent and continuous types of energy restriction is still under research, but growing evidence points to equal rates when duration and overall CR are matched (Harvie & Howell, 2017; Trepanowski et al, 2017). Meta‐analyses and systematic reviews support this notion (Varady, 2011; Cioffi et al, 2018). Altogether, the body of evidence available so far warrants further direct comparison studies between CR and IF.
The timing of eating windows, especially during TRE, may also influence the health outcome. Meta‐analyses have shown that breakfast skipping is associated with increased CVD risk and all‐cause mortality (Ofori‐Asenso et al, 2019; Chen et al, 2020). This suggests superiority of early eating windows during TRE, which in general has been shown to broaden health benefits (Longo & Panda, 2016). In line, a recent study of 3 months of late TRE (eating window 12 pm to 8 pm) found no significant improvements in weight loss, fasting insulin levels, fat mass glucose, or blood lipid levels (Lowe et al, 2020). Moreover, a 6‐week study of TRE without weight loss in non‐obese adults resulted in no significant change in cardiovascular parameters (Martens et al, 2020), suggesting that in humans, weight reduction might be a required factor for enhancing cardiovascular and metabolic health. The effectivity of TRE might thus largely depend on study settings, cohort descriptions, and especially timing of the eating windows. However, this has not been investigated in direct comparative studies, and there may be strong individual differences (e.g., with regard to the chronotype).
In summary, while in rodent studies, which are experimentally well controllable, some health‐promoting effects of IF can be uncoupled from CR and weight reduction; the data from human studies are far less clear on this topic. For now, little evidence exists that in humans, intermittent CR harbors superior effects over continuous CR. This conclusion might change with new studies reported rapidly. However, the outcomes of such comparative studies are to be implemented into the body of evidence while taking into account the similarities and differences in duration and specific intervention protocols, adherence rates, as well as the metabolic and nutritional constitution of the cohorts. Ultimately, for everyday life, it might be less important, which dietary intervention elicits superior effects in clinically controlled settings rather than what dietary intervention the individual patient is capable and willing to follow.
Physiological effects and potential clinical applications of fasting and CR
Pre‐clinical and clinical trials suggest a broad range of potential application areas for CR and fasting interventions. However, the individual response to calorie deprivation, the optimal level, and duration for specific clinical settings and other determinants of clinical effectiveness often remain to be clarified. Thus, apart from weight management, specific CR interventions are not yet routinely recommended for specific pathological conditions. Based on pre‐clinical and human studies, we here outline possible future areas of clinical application for CR and IF protocols (Figure 4).
Figure 4. Human organs affected by CR and fasting and selected health benefits.
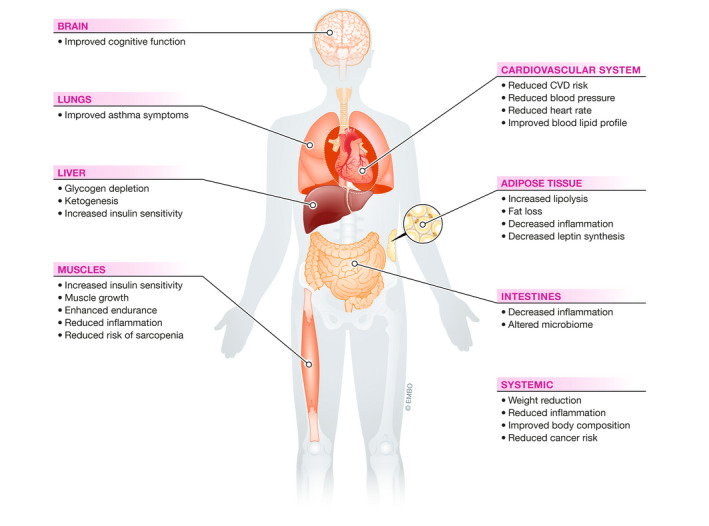
Evidence from amounting clinical trials point towards systemic health benefits of CR and IF in humans. A selection of key observations and important metabolic events is presented.
Obesity and weight reduction
Overweight and obesity are implicated in many complex disease states, including cancer, CVDs, and metabolic diseases, negatively impact quality of life and aggravate other diseases (Lee & Dixon, 2017). Despite large‐scale efforts by public health systems to reduce obesity cases, they are still at all‐time highs, with more than 2 billion people affected worldwide (González‐Muniesa et al, 2017). IF and CR are at the forefront of tackling obesity, with safety generally accepted for weight loss management in overweight cohorts (Harvie & Howell, 2017). Thereby fat tissue commonly decreases at a higher rate than lean mass (e.g., ADF in Stekovic et al, 2019; CR in Most et al, 2018). Typical weight reduction rates of normal weighted to overweight cohorts in clinical trials are −3 to −5% after 4 weeks of ADF and up to −7% after 6 months of CR or ADMF (Johnstone, 2015; Trepanowski et al, 2017). The rate of weight loss during complete fasting is estimated at 0.9 kg/day during the first days, which then continuously slows down (Brandhorst & Longo, 2016; Lessan & Ali, 2019). After an initial weight‐loss phase over 6–12 months, bodyweight usually stabilizes during long‐term CR interventions in normal‐ to overweight cohorts under study settings (Das et al, 2007; Dorling et al, 2020).
Type 2 diabetes and metabolic syndrome
Continuously elevated insulin and glucose blood levels are hallmarks of type 2 diabetes and pre‐diabetes, and both CR and IF have been shown to reduce insulin levels (Gabel et al, 2019). Likewise, markers of systemic inflammation and oxidative stress, two drivers of aging and metabolic diseases, decrease, while immune competency is largely maintained (Meydani et al, 2016; Stekovic et al, 2019; Wilhelmi de Toledo et al, 2020a). However, conflicting data exist on fasting‐induced insulin sensitivity, showing either improvement (ADMF, PF, Furmli et al, 2018; eTRF, Sutton et al, 2018) or no effect (ADMF, Trepanowski et al, 2017) in diverse cohorts. Study design, duration, cohort descriptions, dietary counseling, general diet composition, and other factors might obscure a consistent outcome on insulin parameters. Fasting might also act preventively on diabetes risk (TRE, Wilkinson et al, 2020): for instance, in a cohort of overweight older patients (66 ± 10 years), those who periodically fasted had lower prevalence of diabetes (Horne et al, 2012). In addition, various fasting regimes show significant visceral fat reduction, which has been linked to increased pre‐diabetes and type 2 diabetes risk (Neeland et al, 2012).
However, at this time, IF in diabetic patients is under controversial debate (Barnosky et al, 2014; Horne et al, 2020). Harsh fasting interventions, for instance, bear the risk of hypoglycemia, a major risk for diabetic patients, which might be less problematic in CR protocols, for which a higher number of trials has been published, showing promising outcomes on insulin parameters (Larson‐Meyer et al, 2006; Most et al, 2017a).
Cardiovascular diseases
CR and IF improve cardiac function and cardiovascular parameters in rodents and humans. For instance, blood pressure, CRP, TNF‐alpha and ‐beta levels, and heart rate are generally lower, as exemplified in a study of long‐term CR followers (Meyer et al, 2006). Similar observations were made for a long‐term ADF cohort (Stekovic et al, 2019) and in shorter interventional studies for nearly all dietary interventions discussed in this review. Both IF and CR improve the blood lipid profile (reduced levels of triglycerides and HDL, increased LDL and VLDL) and other CVD‐relevant factors in non‐obese and obese cohorts (Most et al, 2017a, 2018; Trepanowski et al, 2017; Francesco et al, 2018; de Cabo & Mattson, 2019). Fasting length and baseline characteristics, such as BMI, age, and glucose levels, have an impact on blood pressure effects (long‐term fasting, Grundler et al, 2020). Overall, the impact of dietary regimens on the cardiovascular system may lower long‐term CVD risk, at least in healthy individuals, as shown for ADF (Stekovic et al, 2019) and CR (Lefevre et al, 2009) and mirrored by the low CVD rate in CR societies and the Okinawan people (Willcox & Willcox, 2014).
Inflammatory diseases and the immune system
Systemic inflammation is central to aging processes, interferes with health in multiple ways, and may be a result of chronic excess calorie consumption (Franceschi et al, 2018; Flanagan et al, 2020). CR has been shown to reduce inflammatory processes in rodents, primates, and humans (Flanagan et al, 2020). IF shows similar outcomes in humans (Patterson & Sears, 2017), although the effects have not been systemically studied. Consistently, small‐scale studies have associated IF with improved symptoms of asthma (Johnson et al, 2007), multiple sclerosis, and arthritis (Müller et al, 2001; Fitzgerald et al, 2018). Extreme CR with malnutrition might elicit negative effects on immune function (Most et al, 2017a). Moderate CR without malnutrition as well as long‐term IF with proper dietary compositions reportedly seem safe regarding baseline immune function (CR, Most et al, 2017b; ADF, Stekovic et al, 2019; TRE, Faris et al, 2012; CR, Meydani et al, 2016), while acute challenges by infections and susceptibility toward infections may yield different immune responses upon CR and IF, hence medical supervision is advised.
Cancer
CR delays and diminishes the occurrence of induced and spontaneous cancer throughout aging in mice (Weindruch, 1992; Brandhorst & Longo, 2016) and rhesus monkeys (Colman et al, 2009; Mattison et al, 2017). Similar observations have been made for IF in rodents. In humans, CR may have a similar impact as suggested by the reduced cancer rates of Okinawan people (Kagawa, 1978; Willcox et al, 2007). Extended fasting durations (> 13 h) during nighttime have been associated with reduced breast cancer risk (Marinac et al, 2015, 2016). Collectively, CR and IF seem to bear anti‐neoplastic potential, but decisive clinical trials are rare and urgently needed.
Additionally, CR, IF, and short‐term fasting might render tumorous cells vulnerable for chemotherapeutic treatment (Brandhorst & Longo, 2016; de Cabo & Mattson, 2019), as evidenced in cell culture (Safdie et al, 2012) and rodent models (Safdie et al, 2012; Pietrocola et al, 2016) of different cancer types. This selective sensitization may follow a dual impact on cancer cells (e.g., through impaired proliferation due to reduced nutrient availability) and immunogenicity (e.g., through enhanced immunosurveillance via T lymphocytes). In accordance, a small‐scale study in humans reported general safety and tolerability of short‐term fasting (up to 140 h prior to and/or 56 h after chemotherapy) as a complementary treatment to chemotherapy, reducing its side effects while not preventing chemotherapeutic tumor reduction (Safdie et al, 2009). Other studies have corroborated these safety findings (reviewed in de Groot et al, 2019), warranting further research into the molecular basis and applicability of combining chemotherapy with various forms of fasting (Brandhorst & Longo, 2016; de Groot et al, 2019).
Neurological diseases and cognitive function
The effects of CR and IF on neuronal cells and tissue are well established in animal models, promoting, e.g., increased stress resistance, autophagy induction, DNA repair, and mitochondrial function (Martin et al, 2006; Liu et al, 2019). In animal models of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, CR and IF can delay the onset and progression of the disease phenotypes (Mattson et al, 2017), evoking molecular, physiological, and cognitive improvements (Wang et al, 2005; Halagappa et al, 2007). 3xTg‐AD mice, a common model of Alzheimer’s disease, maintained for 1 year on either ADF or 40% CR, performed better in behavioral and cognitive tasks, compared to ad libitum‐fed animals (Halagappa et al, 2007). Moreover, different studies have shown that CR reduced levels of Aβ and Tau accumulation in the brains of disease models (Patel et al, 2005). A 6‐month study applying 30% CR to a primate model of Parkinson´s disease found significantly higher levels of locomotor activity in the intervention group, along with reduced dopamine and dopamine metabolites depletion as well as increased survival of dopaminergic neurons in the substantia nigra (Maswood et al, 2004).
Only few clinical trials have been conducted in this realm: For instance, a 3‐month 30% CR study found significant improvements in an elderly cohort, correlating with reduced insulin and CRP levels (Witte et al, 2009). The same group corroborated the findings in a cohort of obese women and also detected increased gray matter volume (Prehn et al, 2017), inter alia in the hippocampus, an area known to be affected by aging (Bettio et al, 2017). The cognitive improvements were found only during the weight loss phase (8 weeks of CR), and not after a 4‐week isocaloric weight maintenance phase, although the weight loss persisted (−10%) (Prehn et al, 2017). Consistently, obese patients with mild cognitive impairment showed improvements after a 12‐month‐long CR intervention (Horie et al, 2016). The effects of CR on memory function seem to apply to non‐obese cohorts as well, as a multi‐center study found spatial working memory improvements in healthy volunteers after 12 and 24 months of CR (Leclerc et al, 2020).
Ischemic injuries and surgical procedures
Pre‐clinical evidence suggests that IF and CR can protect different tissue types from ischemic injury and surgical damage (Mitchell et al, 2010; Jongbloed et al, 2014; Rickenbacher et al, 2014; Rohrbach et al, 2014; Guo et al, 2018; Rojas‐Morales et al, 2019; Zhang et al, 2019). This is supported by first clinical trials. A multi‐center study found that dietary pre‐operative weight loss reduces post‐operative complications in obese patients undertaking bariatric surgery (Van Nieuwenhove et al, 2011). Similar effects have been proposed for other surgery types, such as vascular procedures (Mitchell et al, 2013).
Microbiome‐associated diseases
The human microbiome has a decisive role in health and disease, and dysregulated microbiota are associated with CVDs, inflammatory bowel disease, atopic asthma, behavioral disorders, obesity, type 2 diabetes, and autoimmune diseases (Shreiner et al, 2015; Durack & Lynch, 2019). The gut microbiome is intrinsically linked to the human diet and metabolome. Accordingly, it is very susceptible to dietary modification, although the exact relationships remain unsatisfactorily understood (Durack & Lynch, 2019).
IF and CR impact the composition and quantity of the microbiome. For instance, CR decreases the ratio of Firmicutes/Bacteroidetes and increases Lactobacilli in the rat microbiome. Some effects are already reversed 1 week after switching back to ad libitum feeding, arguing for a highly flexible diet–microbiome axis (Tanca et al, 2018). It is believed, that upon CR, the abundance of probiotic strains (e.g., Lactobacillus and Bifidobacterium) increases while that of pro‐inflammatory strains decreases (Zheng et al, 2018). As for IF regimens, a trial found decreased abundance of Fusobacterium, a strain associated with colorectal cancer, after 7 days of fasting (He et al, 2019). A 10‐day study of very low calorie fasting found reduced abundance of Lachnospiraceae and Ruminococcaceae, which are known to degrade polysaccharides, and increase levels of Bacteroidetes and Proteobacteria (Mesnage et al, 2019). These changes were partly reversed 4 days after ending the study and returned to baseline composition 3 months later.
In turn, differences in the microbiome might affect the effectiveness of CR or IF. For example, higher pre‐intervention abundance of Akkermansia muciniphila in obese people was associated with improved CR outcome (glucose homeostasis, insulin sensitivity, body fat distribution, and blood lipids; Dao et al, 2016). Interestingly, microbiota‐free mice do not experience the same extent of CR‐mediated effects (e.g., body weight and fat loss) (Wang, et al, 2018). Thus, as a determinant of health and disease, the diet–microbiome–host axis emerges as an important factor in shaping CR and fasting‐induced outcomes (Grajeda‐Iglesias et al, 2021).
Exercise and muscle growth/maintenance
In mice, CR has been shown to (counterintuitively) ameliorated age‐induced muscle loss (sarcopenia), although confounding effects, such as increased spontaneous behavior, could also have influenced this observation (van Norren et al, 2015). A small‐scale clinical trial found endurance and strength improvements upon TRF (4 h eating windows on 4 days a week) during 8 weeks of workout (3 days a week) when compared to non‐TRF training. Lean mass was retained and no adverse effects on training efficacy were observed (Tinsley et al, 2017). However, CR has been reported to have adverse effects on muscle strength in older cohorts (Weiss et al, 2007). Hence, more clinical trials are needed to evaluate the physiological effects of combining CR, IF, and exercise on different muscles under varying conditions (Zouhal et al, 2020).
Age and associated diseases
Pre‐clinical data show robust effects of CR and IF on health‐ and lifespan across species (Fontana, 2009; Most et al, 2017a; Francesco et al, 2018; Madeo et al, 2019). In humans, involuntary phases of CR have been reported with two notable examples (WWI in Denmark for 2 years and WWII in Oslo/Norway for 4 years), in which malnutrition was avoided by dietary plans causing a 30% death rate reduction compared to pre‐war times (Hindhede, 1920; Strøm et al, 1951). On the Japanese island of Okinawa, inhabitants naturally consumed up to 17% less calories than the rest of Japan, resulting in a markedly increased life expectancy (Willcox et al, 2007). Notably, dietary changes due to Westernization abolished this increase, already within the timeframe of one generation. Importantly, most of the age‐associated diseases, some of which have been briefly discussed above, are ameliorated by CR and IF in rodents and humans.
Pre‐clinical studies have shown that the age of onset of IF and CR interventions can influence the effects on health‐ and lifespan. In mice, the lifespan‐extending effects of IF (Goodrick et al, 1990) and CR (Duriancik et al, 2018; Vaughan et al, 2018) are diluted when the regime is introduced later in life. Therefore, future clinical trials and long‐term observational studies, which also consider the age of onset, are necessary to clarify whether any of the discussed dietary interventions lead to increased health‐ and/or lifespan in real‐life, self‐adherent CR and IF populations.
Considerations and potential caveats of CR and fasting in humans
CR and IF may generally be problematic for minors, very old people, pregnant or breastfeeding mothers, anorectic, or very lean persons, those with bone (density) loss, as well as for special disease groups.
Hunger, adherence, and mental health during CR and fasting as points to consider
Most people will experience various magnitudes of hunger when cutting calories. A complex regulatory system involving hormones that communicate between the gastrointestinal tract and the brain defines the balance between appetite and satiety. On the one hand, the peptide hormone ghrelin provokes a feeling of hunger via the hypothalamus. Recently, Acyl‐CoA‐binding protein (ACBP) has been proposed to be another phylogenetically conserved appetite‐stimulatory “hunger factor,” which is secreted upon starvation and triggers food intake and obesity (San‐Pedro et al, 2019; Charmpilas et al, 2020; Madeo et al, 2020). On the other hand, adipocyte‐derived leptin counteracts ghrelin’s actions by binding to the LepR receptor in the hypothalamus, provoking satiety. The subjective experience of hunger is often said to decrease with the length of CR and fasting periods, which is in agreement with theories that the ghrelin/leptin system can dynamically adapt to different metabolic and environmental statuses (Davis, 2018). Obesity is linked to dysregulation of this system and resistance to these hormones (Cui et al, 2017). The hunger feelings reported in IF and CR studies include a wide range from little/none to considerable/strong (ADF, Heilbronn et al, 2005; two‐day fasting, Solianik & Sujeta, 2018; ADMF, Johnson et al, 2007) with some studies explicitly noting the absence of hunger feelings in the majority of probands (long‐term fasting, Anton et al, 2009; Wilhelmi de Toledo et al, 2019). This discrepancy in the literature is likely due to differences in assessing hunger parameters, but also due to different intervention protocols, durations, pre‐intervention dietary behaviors, metabolic states, and motivations, which may affect hunger perception. However, to our knowledge, a systematic analysis of hunger perception and its development over time or its dependency on environmental and nutrition factors for CR or IF is yet missing. Also, it remains unclear whether hunger perceptions may be needed for some of the interventions’ effects.
The adherence rate to different types of CR and IF co‐defines their potential for medical uses. In long‐term CR trials, compliance rates decline over time, and factors such as individual/group counseling, regular monitoring, and diet tracking improve compliance (Flanagan et al, 2020). In the CALERIE‐2 study, adherence dropped markedly after 20 weeks of targeted 25% CR (Dorling et al, 2020). Reported dropout rates in clinical studies range between single‐digit percentages to 40%. A 1‐year study with obese probands found 38% dropouts in ADMF, 29% in CR, and 26% in the ad libitum control group (Trepanowski et al, 2017). It is yet unclear, whether intermittent or continuous forms of CR provoke better compliance rates. This likely depends on cohort characteristics and study settings, and may be improved by dietary counseling availability, food provisions, and individual monitoring of study participants.
Severe and malnourished CR has been shown to cause psychological stress, depression, and a detrimental impact on mental and sexual health. Instead, controlled CR or fasting with adequate nutritional compositions is not generally reported to have negative outcomes on quality of life, mood, or other psychological parameters (Velthuis‐te Wierik et al, 1994; Martin et al, 2016; Wilhelmi de Toledo et al, 2019). A study of long‐term fasting reported a gradual rise in emotional and physical well‐being (Wilhelmi de Toledo et al, 2019). However, the discriminators among positive, neutral, and negative psychological outcomes are highly individual and subject to the intervention’s magnitude and length. Thus, psychological monitoring should be sought through extended phases of CR or fasting, and should be mandatory for individuals who are endangered or susceptible to depression or other mental diseases.
Lean mass loss, weight regain, and potential bone density issues during and after CR and IF
Loss of lean mass usually occurs during CR and IF, but at a slower rate than fat tissue (Trepanowski et al, 2017). Observations of a long‐term cohort of ADF followers did not reveal differences in body composition to a weight‐matched, fasting‐naive control group (Stekovic et al, 2019). The CALERIE studies showed reductions in lean mass in both young and older participants, while a negative impact on muscle strength was only observed in older groups performing CR (Weiss et al, 2007; Larson‐Meyer et al, 2010). Controversially, a recent late‐TRE study found no significant decrease in fat mass, but in lean mass (Lowe et al, 2020). Overall, further research in this area is needed, especially in elderly cohorts at risk for age‐associated sarcopenia (Walston, 2012). Complementary exercise is believed to halt some of the lean mass loss that occurs during long‐term CR or IF and may promote fat reduction (Keenan et al, 2020), which may be a viable option in clinically subscribed dietary interventions.
Follow‐up data on weight regain are rare with mixed outcomes. Upon re‐assessing energy demand after 6 months of CR or ADF, modest weight regain was reported in both groups (Trepanowski et al, 2017). Importantly, during the CALERIE trial, the participants did not develop eating disorders or tendencies toward binge eating (Williamson et al, 2008), which could potentially annulate long‐term health benefits and weight loss in overweight patients.
Data on changes in bone mass and/or density due to sustained CR seem contradictory. In non‐obese, healthy participants, ADF was reported to have no effect on bone mass or density in a 4‐week RCT or in a cohort of self‐adherent ADF followers, who performed the intervention for several months (Stekovic et al, 2019). Likewise, 6‐month CR did not decrease bone mass in the CALERIE‐1 study (Redman et al, 2008), whereas CALERIE‐2, lasting for 2 years, did find a significant decrease in some areas (Villareal et al, 2016). The intervention magnitude and length, nutritional composition, complementary exercise, and the age of the participants likely have a big impact on bone health parameters. Future long‐term studies on IF and CR need to address the effects on bone health in more detail, especially in elderly cohorts, and the possible use of nutrient supplements to support bone structure.
The immune system during CR and IF
In mice, chronic CR increases the susceptibility to infections, especially of viral nature (Gardner, 2005; Kristan, 2008). In humans, the basic cellular immune profile does not differ significantly between long‐term ADF and control groups (Stekovic et al, 2019), while inflammatory markers may even be reduced. One study found slightly reduced immune cell counts after Ramadan fasting (Faris et al, 2012), while another 6‐month CR study found improved T‐cell function (Ahmed et al, 2009). Hence, future studies need to address the effects of CR and IF on the immune system in real‐life settings, especially in elderly populations and under acute pathogenic challenge.
CR with malnutrition and starvation
Correctly applied CR and IF, per definition, exclude malnutrition. In clinical trials, this can be prevented by dietary counseling, pre‐defined meals, and/or monitoring of dietary behavior. In non‐trial private endeavors, dietitians should be consulted before undertaking interventions. Due to ethical considerations, malnutrition and starvation clinical studies remain questionable. Insights have been gained from observational studies: In the “Minnesota Starvation Experiment,” extreme CR (40%) with improper dietary composition (e.g., insufficient protein, vegetable and fruit intake) and increased physical activity in young men over 6 months resulted in approx. 25% weight loss (2/3 fat, 1/5 fat‐free mass), chronic fatigue, lower limb edema, severe emotional distress, depression, confusion, apathy, suicidal thoughts, and libido loss. Most of the effects were normalized upon refeeding (KEYS et al, 1950). Furthermore, malnourished, extreme CR may negatively impact reproductivity and fecundity, steroid production, ovarian function, and immune function (Most et al, 2017b) (Ulijaszek, 1996).
Several early studies report adverse events in long‐term fasting of obese patients, although it was noted that many of those are absent upon minimal supplementation with carbohydrate and/or protein (Br Med J, 1978). These adversities include decreased bone density, acidosis, liver dysfunction, menses dysregulation, baldness, edema, nausea, headaches, fatigue, depression, kidney failure, cardiac problems, and even death, among others (Harrison & Harden, 1966; Hermann & Iversen, 1968; Ross et al, 1969; Rooth & Carlström, 1970; Munro & Duncan, 1972; Br Med J, 1978; Devathasan & Koh, 1982). A recent analysis found few adverse events for prolonged water‐only fasting (median 7 days, range 2–28 days) and concluded that it is, in principle, safe for multiple purposes (Finnell et al, 2018). Modern (short‐term) IF or CR studies under well‐nourished conditions that sufficiently provide micro‐ and macronutrients, usually show no strong adverse effects.
Alternative approaches to CR and fasting
Fasting and CR may be unsuitable in certain settings and, as mentioned above, for specific groups, including people of advanced age, with multiple co‐morbidities or minors. Furthermore, real‐life adherence of eligible participants to these interventions might be lower without trial‐associated counseling and motivational support. Thus, increasing efforts aim at developing alternative approaches that elicit (some) fasting‐ or CR‐like effects without the need to reduce calorie intake. One of them is bariatric surgery, which aims at weight loss and comorbidities management (Benaiges et al, 2015; Wolfe et al, 2016). Another, less invasive approach is the concept of pharmacological supplementation with CRMs, which have been extensively reviewed elsewhere (Ingram et al, 2006; Lee & Min, 2013; Mariño et al, 2014; Madeo et al, 2019; Hofer et al, 2021; Ingram & Roth, 2021).
Specific types of dietary modulation also represent alternative avenues for persons who cannot adhere to CR or IF protocols. These include, among others, the fasting‐mimicking diet (FMD; Brandhorst et al, 2015; Choi et al, 2016b), ketogenic diets (Sampaio, 2016; Boison, 2017; Ludwig, 2020), as well as very low calorie (Kim, 2021), low‐carbohydrate, or low‐fat diets (Kim, 2021).
The FMD, for instance, is commercially available as a low‐calorie, ‐sugar, and ‐protein diet that contains high levels of unsaturated fats and micronutrients (Brandhorst et al, 2015; Choi et al, 2016a; Wei et al, 2017). In both pre‐clinical and clinical studies, it has shown promising effects on health parameters, including overall body weight, body fat, trunk fat, IGF‐1, blood pressure, blood lipids, and CRP. Thereby, higher risk subgroups (by means of weight, blood pressure, or blood lipid profile) generally benefited more than lower‐risk groups (Wei et al, 2017). In pre‐clinical studies, it was effective in ameliorating age‐induced weight gain, improving cognitive function and locomotion, elevating adult neurogenesis, and increasing median lifespan of mice (Brandhorst et al, 2015). In agreement with its low‐calorie basis, the FMD has been shown to significantly increase blood KB levels (Brandhorst et al, 2015).
Initially developed as a treatment option for epilepsy, ketogenic diets share similarities with the FMD and other low‐carbohydrate diets. To shift the metabolism to a ketogenic state, they pair a drastic reduction in carbohydrates with an increase in fat (> 70%), forcing the body to produce KBs. Ketogenic diets are being studied for a range of maladies, including cancer, CVDs, diabetes, obesity, neurological diseases, multiple sclerosis, and others (Bahr et al, 2020; Ludwig, 2020; Ferrere et al, 2021). They are generally believed to be safe (Ludwig, 2020) if done in an intermitted fashion and if formulated well, and hold promise for clinical translation. Similar to IF and CR, ketogenic diets can induce autophagy and extend health‐ and lifespan in model organisms (Roberts et al, 2017; Wang, et al, 2018). Many of these effects may eventually originate from the increase in KBs, which per se have autophagy‐inducing and lifespan‐prolonging effects (Edwards et al, 2014; Camberos‐Luna et al, 2016; Veech et al, 2017; Han et al, 2020; Torres‐Esquivel et al, 2020).
While all herein discussed alternative approaches show promising results, future research will establish their long‐term effects and possible pitfalls, which will ultimately decide their clinical feasibility. It will be of great interest to see whether combining IF or CR with CRMs might induce synergistic metabolic effects. Variations in timing of the pharmacological/dietary supplementation might yield interesting new insights, e.g., giving a substance during fasting or ad libitum eating to constantly and timely modulate various molecular pathways (autophagy, ketone metabolism, mTOR activity, etc.). If supplemented during eating periods, CRMs could constantly shift the metabolism toward a fasting‐like state, minimizing the effort to cut calories. If taken during fasting periods, non‐caloric supplements that per se induce autophagy or ketogenesis might synergistically elevate a fasting response. A further prospect is to develop individual programs for patients based on their (health) goals and personal genetic or microbial setting, which will likely be more manageable by combining knowledge from all aforementioned alternatives.
Conclusion and emerging questions
Over the recent years, IF and CR have gained scientific, medical, and public attention due to their potentially broad health benefits. In preclinical studies, fasting and CR have been shown to prolong life‐ and healthspan, induce autophagy, and ameliorate symptoms of various diseases, such as CVDs, type 2 diabetes, neurodegenerative diseases, cancer, or ischemic injuries. However, transition into the clinics has been slow. Importantly, most clinical trials in the field last for a few weeks to several months, but long‐term and follow‐up trials are required to evaluate the effects and safety in the long run. Furthermore, the great majority of IF and CR trials have focused on overweight, metabolically compromised and/or middle‐aged cohorts. Thus, a generalization of the findings to the wider population remains difficult. Still, as summarized herein, the current understanding is that the downsides are relatively small for most people and the potential benefits may well outweigh the disadvantages.
Important questions remain partly unanswered: Are there molecular and physiological benefits of one intervention type over another when calories are matched, apart from individual preferences? For IF, which are the molecular improvements beyond mere weight loss? How do circadian rhythm and eating/fasting time patterns interact? Can we maximize the effectiveness by personalizing fasting or CR? Which baseline characteristics influence the outcome/response to fasting and CR? Can we support medical treatments (e.g., chemotherapy, surgeries) by applying fasting or CR? How do recurring fasting or CR phases affect the likelihood to develop metabolic or age‐associated diseases?
Eventually, we might see health care systems incorporating IF‐ and CR‐like therapies into routine care. First steps toward this development are emerging with scientific suggestions for therapeutical application (de Cabo & Mattson, 2019), mirrored by the rising popularity of wellness and/or rehabilitation centers that provide medical counseling during special fasting applications (Wilhelmi de Toledo et al, 2019). On a different line, deepening our understanding of the molecular basis of fasting and CR will help discover and develop novel CRMs (Madeo et al, 2019) that might overcome some of the application obstacles in humans. Since many fasting‐ and CR‐affected pathways also play major roles in the process of aging, research in these areas is extending our understanding of healthy aging and the underlying physiological and molecular foundations.
Author contributions
SJH and FM conceptualized the review. SJH wrote the manuscript, and SJH, DC‐G and MIM designed the figures. SJH, DC‐G, MIM and FM contributed to the editing and proofreading of the final draft.
Conflict of interest
F.M. and D.C.‐G. are scientific cofounders of Samsara Therapeutics, a company that develops novel pharmacological autophagy inducers. F.M. has equity interests in and is advisor of TLL, The Longevity Labs GmbH. D.C.‐G. has equity interests in TLL, The Longevity Labs GmbH. S.J.H and M.I.M. declare that they have no conflict of interest.
Pending issues.
Determination of molecular and physiological differences between CR and different forms of IF in humans.
Do controlled IF and CR interventions change emerging indirect markers of aging, e.g., epigenetic clocks, in humans?
Long‐term clinical trials and follow‐up studies to establish the effects of CR and IF on the development and progression of metabolic‐ or age‐associated diseases in different human cohorts.
More clinical trials concerning neurodegenerative disorders (e.g., in early‐onset AD patients), CVDs, and other age‐associated diseases.
More research on the real‐life applicability of CR and IF as supportive measures before or after medical treatments, such as surgeries or chemotherapies.
Understanding the impact of circadian rhythm on eating/fasting patterns.
Which molecular and physiological effects observed in IF studies can be uncoupled from weight loss/CR in humans?
Acknowledgments
F.M. is grateful to the Austrian Science Fund FWF (SFB LIPOTOX F3007 & F3012, DK‐MCD W1226, as well as grants P29203, P29262, P27893, and P31727) and the Austrian Federal Ministry of Education, Science and Research, as well as the University of Graz for grants “Unkonventionelle Forschung‐InterFast and Fast4Health” as well as “flysleep” (BMWFW‐80.109/0001‐WF/V/3b/2015). We acknowledge the support of the field of excellence BioHealth, NAWI Graz, and the BioTechMed‐Graz flagship project “EPIAge.”
EMBO Mol Med (2022) 14: e14418.
See the Glossary for abbreviations used in this article.
Contributor Information
Sebastian J Hofer, Email: sebastian.hofer@uni-graz.at.
Frank Madeo, Email: frank.madeo@uni-graz.at.
References
- Abdellatif M, Sedej S, Carmona‐Gutierrez D, Madeo F, Kroemer G (2018) Autophagy in cardiovascular aging. Circ Res 123: 803–824 [DOI] [PubMed] [Google Scholar]
- Acosta‐Rodríguez VA, de Groot MHM, Rijo‐Ferreira F, Green CB, Takahashi JS (2017) Mice under caloric restriction self‐impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab 26: 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN (2009) Calorie restriction enhances T‐cell–mediated immune response in adult overweight men and women. J Gerontol A Biol Sci Med Sci 64A: 1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP (2003) Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA 100: 6216–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton SD, Han H, York E, Martin CK, Ravussin E, Williamson DA (2009) Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet 22: 141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton SD, Moehl K, Donahoo WT, Marosi K, Lee S, Mainous AG III, Leeuwenburgh C, Mattson MP (2018) Flipping the metabolic switch: understanding and applying health benefits of fasting. Obesity 26: 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr LS, Bock M, Liebscher D, Bellmann‐Strobl J, Franz L, Prüß A, Schumann D, Piper SK, Kessler CS, Steckhan N et al (2020) Ketogenic diet and fasting diet as Nutritional Approaches in Multiple Sclerosis (NAMS): protocol of a randomized controlled study. Trials 21: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MC, Grosso RA, Fader CM (2019) Hallmarks of aging: an autophagic perspective. Front Endocrinol 9: 790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnosky AR, Hoddy KK, Unterman TG, Varady KA (2014) Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res 164: 302–311 [DOI] [PubMed] [Google Scholar]
- Benaiges D, Goday A, Pedro‐Botet J, Más A, Chillarón JJ, Flores‐Le Roux JA (2015) Bariatric surgery: to whom and when? Minerva Endocrinol 40: 119–128 [PubMed] [Google Scholar]
- Bettio LEB, Rajendran L, Gil‐Mohapel J (2017) The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev 79: 66–86 [DOI] [PubMed] [Google Scholar]
- Boison D (2017) New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 30: 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi I, Wei M, Cheng C, Sedrakyan S, Navarrete G, Dubeau L, Yap L, Park R, Vinciguerra M et al (2015) A periodic diet that mimics fasting promotes multi‐system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22: 86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Longo VD (2016) Fasting and caloric restriction in cancer prevention and treatment. Recent Results Cancer Res 207: 241–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo‐San Pedro JM, Sica V, Martins I, Pol J, Loos F, Maiuri MC, Durand S, Bossut N, Aprahamian F, Anagnostopoulos G et al (2019) Acyl‐CoA‐binding protein is a lipogenic factor that triggers food intake and obesity. Cell Metab 30: 754–767 [DOI] [PubMed] [Google Scholar]
- Cahill G (2006) Fuel metabolism in starvation. Ann Rev Nutr 26: 1–22. [DOI] [PubMed] [Google Scholar]
- Camberos‐Luna L, Gerónimo‐Olvera C, Montiel T, Rincon‐Heredia R, Massieu L (2016) The ketone body, β‐hydroxybutyrate stimulates the autophagic flux and prevents neuronal death induced by glucose deprivation in cortical cultured neurons. Neurochem Res 41: 600–609 [DOI] [PubMed] [Google Scholar]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E (2019) From discoveries in ageing research to therapeutics for healthy ageing. Nature 571: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Auwerx J (2009) Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab 20: 325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caobi A, Dutta RK, Garbinski LD, Esteban‐Lopez M, Ceyhan Y, Andre M, Manevski M, Ojha CR, Lapierre J, Tiwari S et al (2020) The impact of CRISPR‐Cas9 on age‐related disorders: from pathology to therapy. Aging Dis 11: 895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona C, Bishai D (2018) The slowing pace of life expectancy gains since 1950. BMC Public Health 18: 151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Rumpler WV et al (2007) Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal‐weight middle‐aged men and women. Metabolism 56: 1729–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, Martin B, MacLean PS, Melanson EL, Troy Donahoo W (2016) A randomized pilot study comparing zero‐calorie alternate‐day fasting to daily caloric restriction in adults with obesity. Obesity 24: 1874–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Manoogian ENC, Melkani GC, Panda S (2019) Time‐restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr 39: 291–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S (2014) Time‐restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20: 991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmpilas N, Ruckenstuhl C, Sica V, Büttner S, Habernig L, Dichtinger S, Madeo F, Tavernarakis N, Bravo‐San Pedro JM, Kroemer G (2020) Acyl‐CoA‐binding protein (ACBP): a phylogenetically conserved appetite stimulator. Cell Death Dis 11: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang B, Ge Y, Shi H, Song S, Xue W, Li J, Fu K, chen X, Teng W et al (2020) Association between skipping breakfast and risk of cardiovascular disease and all cause mortality: a meta‐analysis. Clin Nutr 39: 2982–2988 [DOI] [PubMed] [Google Scholar]
- Choi I, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross A, Morgan T et al (2016a) A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep 15: 2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross A, Morgan T et al (2016b) A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep 15: 2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, Contaldo F, Pasanisi F, Ghigo E, Bo S (2018) Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta‐analysis of randomized controlled trials. J Transl Med 16: 371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet T‐H, Sonoyama T, Henning E, Keogh JM, Ingram B, Kelway S, Guo L, Farooqi IS (2017) A metabolomic signature of acute caloric restriction. J Clin Endocrinol Metab 102: 4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW et al (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325: 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM (2014) Caloric restriction reduces age‐related and all‐cause mortality in rhesus monkeys. Nat Commun 5: 3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M, Albert V, Hall MN (2013) mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev 23: 53–62 [DOI] [PubMed] [Google Scholar]
- Corti O, Blomgren K, Poletti A, Beart PM (2020) Autophagy in neurodegeneration: new insights underpinning therapy for neurological diseases. J Neurochem 154: 354–371 [DOI] [PubMed] [Google Scholar]
- Cui H, López M, Rahmouni K (2017) The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol 13: 338–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel KM (2020) Best practices for promoting healthy aging. Clin Geriatr Med 36: 713–718 [DOI] [PubMed] [Google Scholar]
- Dao MC, Everard A, Aron‐Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L et al (2016) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436 [DOI] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH et al (2007) Long‐term effects of 2 energy‐restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1‐y randomized controlled trial. Am J Clin Nutr 85: 1023–1030 [DOI] [PubMed] [Google Scholar]
- Davis J (2018) Hunger, ghrelin and the gut. Brain Res 1693: 154–158 [DOI] [PubMed] [Google Scholar]
- de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381: 2541–2551 [DOI] [PubMed] [Google Scholar]
- de Sampaio LPB (2016) Ketogenic diet for epilepsy treatment. Arq Neuropsiquiatr 74: 842–848 [DOI] [PubMed] [Google Scholar]
- Devathasan G, Koh C (1982) Wernicke’s encephalopathy in prolonged fasting. Lancet 320: 1108–1109 [DOI] [PubMed] [Google Scholar]
- Dorling JL, Das SK, Racette SB, Apolzan JW, Zhang D, Pieper CF, Martin CK (2020) Changes in body weight, adherence, and appetite during 2 years of calorie restriction: the CALERIE 2 randomized clinical trial. Eur J Clin Nutr 74: 1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack J, Lynch SV (2019) The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med 216: 20–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriancik DM, Tippett JJ, Morris JL, Roman BE, Gardner EM (2018) Age, calorie restriction, and age of calorie restriction onset reduce maturation of natural killer cells in C57Bl/6 mice. Nutr Res 55: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrom E, Neukam S, Kalin L, Wright J (2020) Physical activity and healthy aging. Clin Geriatr Med 36: 671–683 [DOI] [PubMed] [Google Scholar]
- Edwards C, Canfield J, Copes N, Rehan M, Lipps D, Bradshaw PC (2014) D‐beta‐hydroxybutyrate extends lifespan in C. elegans . Aging 6: 621–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris MA‐IE, Kacimi S, Al‐Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK, Salem ML (2012) Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res 32: 947–955 [DOI] [PubMed] [Google Scholar]
- Fasting and obesity (1978) Br Med J 1: 673 [PMC free article] [PubMed] [Google Scholar]
- Ferrere G, Tidjani Alou M, Liu P, Goubet A‐G, Fidelle M, Kepp O, Durand S, Iebba V, Fluckiger A, Daillère R et al (2021) Ketogenic diet and ketone bodies enhance the anticancer effects of PD‐1 blockade. JCI Insight 6: e145207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell JS, Saul BC, Goldhamer AC, Myers TR (2018) Is fasting safe? A chart review of adverse events during medically supervised, water‐only fasting. BMC Complement Altern Med 18: 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KN, Hodges R, Hanes D, Stack E, Cheishvili D, Szyf M, Henkel J, Twedt MW, Giannopoulou D, Herdell J et al (2021) Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial. Aging 13: 9419–9432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KC, Vizthum D, Henry‐Barron B, Schweitzer A, Cassard SD, Kossoff E, Hartman AL, Kapogiannis D, Sullivan P, Baer DJ et al (2018) Effect of intermittent vs. daily calorie restriction on changes in weight and patient‐reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord 23: 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan EW, Most J, Mey JT, Redman LM (2020) Calorie restriction and aging in humans. Annu Rev Nutr 40: 105–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L (2009) The scientific basis of caloric restriction leading to longer life. Curr Opin Gastroenterol 25: 144–150 [DOI] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, Klein S, Bhapkar M, Rochon J, Ravussin E et al (2016) Effects of 2‐year calorie restriction on circulating levels of IGF‐1, IGF‐binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell 15: 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018) Inflammaging: a new immune‐metabolic viewpoint for age‐related diseases. Nat Rev Endocrinol 14: 576–590 [DOI] [PubMed] [Google Scholar]
- Francesco AD, Germanio CD, Bernier M, de Cabo R (2018) A time to fast. Science 362: 770–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF (1980) Aging, natural death, and the compression of morbidity. N Engl J Med 303: 130–135 [DOI] [PubMed] [Google Scholar]
- Furmli S, Elmasry R, Ramos M, Fung J (2018) Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep 2018: bcr2017221854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA (2018) Effects of 8‐hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 4: 345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Cienfuegos S, Kalam F, Varady KA (2019) Differential effects of alternate‐day fasting versus daily calorie restriction on insulin resistance. Obesity 27: 1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM (2005) Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci 60: 688–694 [DOI] [PubMed] [Google Scholar]
- Geng L, Lam KSL, Xu A (2020) The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol 16: 654–667 [DOI] [PubMed] [Google Scholar]
- González A, Hall MN (2017) Nutrient sensing and TOR signaling in yeast and mammals. EMBO J 36: 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A, Hall MN, Lin S‐C, Hardie DG (2020) AMPK and TOR: the yin and yang of cellular nutrient sensing and growth control. Cell Metab 31: 472–492 [DOI] [PubMed] [Google Scholar]
- González‐Muniesa P, Mártinez‐González M‐A, Hu FB, Després J‐P, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA (2017) Obesity. Nat Rev Dis Primers 3: 1–18 [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N (1990) Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev 55: 69–87 [DOI] [PubMed] [Google Scholar]
- Grajeda‐Iglesias C, Durand S, Daillère R, Iribarren K, Lemaitre F, Derosa L, Aprahamian F, Bossut N, Nirmalathasan N, Madeo F et al (2021) Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging 13: 6375–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CL, Lamming DW (2019) Regulation of metabolic health by essential dietary amino acids. Mech Ageing Dev 177: 186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CL, Lamming DW, Fontana L (2021) Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol 10.1038/s41580-021-00411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot S, Pijl H, van der Hoeven JJM, Kroep JR (2019) Effects of short‐term fasting on cancer treatment. J Exp Clin Cancer Res 38: 209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundler F, Mesnage R, Michalsen A, Wilhelmi de Toledo F (2020) Blood pressure changes in 1610 subjects with and without antihypertensive medication during long‐term fasting. J Am Heart Assoc 9: e018649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijas C, Montenegro‐Burke JR, Cintron‐Colon R, Domingo‐Almenara X, Sanchez‐Alavez M, Aguirre CA, Shankar K, Majumder E‐W, Billings E, Conti B et al (2020) Metabolic adaptation to calorie restriction. Sci Signal 13: eabb2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Rousselle T, Li J (2018) Caloric restriction increases the resistance of aged heart to myocardial ischemia/reperfusion injury. Diabetes 67(Supplement 1): 153 [Google Scholar]
- Guzmán M, Blázquez C (2004) Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids 70: 287–292 [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5: 253–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VKM, Guo Z, Pearson M, Matsuoka Y, Cutler RG, LaFerla FM, Mattson MP (2007) Intermittent fasting and caloric restriction ameliorate age‐related behavioral deficits in the triple‐transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 26: 212–220 [DOI] [PubMed] [Google Scholar]
- Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V et al (2019) Ultra‐processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab 30: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y‐M, Ramprasath T, Zou M‐H (2020) β‐hydroxybutyrate and its metabolic effects on age‐associated pathology. Exp Mol Med 52: 548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Rubinsztein DC, Walker DW (2018) Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 19: 579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MT, Harden R (1966) The long‐term value of fasting in the treatment of obesity. Lancet 288: 1340–1342 [DOI] [PubMed] [Google Scholar]
- Harvie M, Howell A (2017) Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects—A narrative review of human and animal evidence. Behav Sci 7: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S et al (2013) The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 110: 1534–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenour CM, Berglund ED, Wasserman DH (2013) Emerging role of AMP activated protein kinase in endocrine control of metabolism in the liver. Mol Cell Endocrinol 366: 152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong E, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick J et al (2012) Time‐restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high‐fat diet. Cell Metab 15: 848–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yin J, Lei J, Liu F, Zheng H, Wang S, Wu S, Sheng H, McGovern E, Zhou H (2019) Fasting challenges human gut microbiome resilience and reduces Fusobacterium. Med Microecol 1–2: 100003 [Google Scholar]
- Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E (2005) Alternate‐day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr 81: 69–73 [DOI] [PubMed] [Google Scholar]
- Hermann LS, Iversen M (1968) Death during therapeutic starvation. Lancet 2: 217 [DOI] [PubMed] [Google Scholar]
- Hindhede M (1920) The effect of food restriction during war on mortality in Copenhagen. JAMA 74: 381–382 [Google Scholar]
- Hofer SJ, Davinelli S, Bergmann M, Scapagnini G, Madeo F (2021) Caloric restriction mimetics in nutrition and clinical trials. Front Nutr 8: 628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie NC, Serrao VT, Simon SS, Gascon MRP, dos Santos AX, Zambone MA, del Bigio de Freitas MM, Cunha‐Neto E, Marques EL, Halpern A et al (2016) Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J Clin Endocrinol Metab 101: 1104–1112 [DOI] [PubMed] [Google Scholar]
- Horne BD, Grajower MM, Anderson JL (2020) Limited evidence for the health effects and safety of intermittent fasting among patients with type 2 diabetes. JAMA 324: 341–342 [DOI] [PubMed] [Google Scholar]
- Horne BD, Muhlestein JB, May HT, Carlquist JF, Lappé DL, Bair TL, Anderson JL (2012) Relation of routine, periodic fasting to risk of diabetes mellitus, and coronary artery disease in patients undergoing coronary angiography. Am J Cardiol 109: 1558–1562. [DOI] [PubMed] [Google Scholar]
- Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14: R115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Roth GS (2021) Glycolytic inhibition: an effective strategy for developing calorie restriction mimetics. Geroscience 43: 1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R (2006) Calorie restriction mimetics: an emerging research field. Aging Cell 5: 97–108 [DOI] [PubMed] [Google Scholar]
- Jansen SW, Akintola AA, Roelfsema F, van der Spoel E, Cobbaert CM, Ballieux BE, Egri P, Kvarta‐Papp Z, Gereben B, Fekete C et al (2015) Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Sci Rep 5: 11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Levine B (2010) Autophagy and Longevity: Lessons from C. elegans . In Protein Metabolism and Homeostasis in Aging, Tavernarakis N (ed), pp 47–60. Boston, MA: Springer US; [DOI] [PubMed] [Google Scholar]
- Jiménez Jaime T, Leiva Balich L, Barrera Acevedo G, de la Maza Cave MP, Hirsch Birn S, Henríquez Parada S, Rodríguez Silva J, Bunout Barnett D (2015) Effect of calorie restriction on energy expenditure in overweight and obese adult women. Nutr Hosp 31: 2428–2436 [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun D‐H, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S et al (2007) Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 42: 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone A (2015) Fasting for weight loss: an effective strategy or latest dieting trend? Int J Obes 39: 727–733 [DOI] [PubMed] [Google Scholar]
- Jongbloed F, de Bruin RWF, Pennings JLA, Payán‐Gómez C, van den Engel S, van Oostrom CT, de Bruin A, Hoeijmakers JHJ, van Steeg H, IJzermans JNM et al (2014) Preoperative fasting protects against renal ischemia‐reperfusion injury in aged and overweight mice. PLoS One 9: e100853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y (1978) Impact of westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med 7: 205–217 [DOI] [PubMed] [Google Scholar]
- Katewa SD, Kapahi P (2010) Dietary restriction and aging, 2009. Aging Cell 9: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan S, Cooke MB, Belski R (2020) The effects of intermittent fasting combined with resistance training on lean body mass: a systematic review of human studies. Nutrients 12: 2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A, Brožek J, Henschel A, Mickelsen O, Taylor HL, Simonson E, Skinner AS, Wells SM, Drummond JC, Wilder RM et al (1950) The biology of human starvation NED‐new edition. University of Minnesota Press; [Google Scholar]
- Kim JY (2021) Optimal diet strategies for weight loss and weight loss maintenance. J Obes Metab Syndr 30: 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Lee JH, Yeung J‐H, Das E, Kim RY, Jiang Y, Moon JH, Jeong H, Thakkar N, Son JE et al (2019) Thermogenesis‐independent metabolic benefits conferred by isocaloric intermittent fasting in ob / ob mice. Sci Rep 9: 2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan DM (2008) Calorie restriction and susceptibility to intact pathogens. Age 30: 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS (1998) 2‐Deoxy‐D‐glucose feeding in rats mimics physiologic effects of calorie restriction. J Anti‐Aging Med 1: 327–337 [Google Scholar]
- Larson‐Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E (2006) Effect of calorie restriction with or without exercise on insulin sensitivity, β‐cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson‐Meyer DE, Redman L, Heilbronn LK, Martin CK, Ravussin E (2010) Caloric restriction with or without exercise: the fitness versus fatness debate. Med Sci Sports Exerc 42: 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc E, Trevizol AP, Grigolon RB, Subramaniapillai M, McIntyre RS, Brietzke E, Mansur RB (2020) The effect of caloric restriction on working memory in healthy non‐obese adults. CNS Spectr 25: 2–8 [DOI] [PubMed] [Google Scholar]
- Lee PC, Dixon J (2017) Medical devices for the treatment of obesity. Nat Rev Gastroenterol Hepatol 14: 553–564 [DOI] [PubMed] [Google Scholar]
- Lee S‐H, Min K‐J (2013) Caloric restriction and its mimetics. BMB Rep 46: 181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E et al (2009) Caloric restriction alone and with exercise improves CVD risk in healthy non‐obese individuals. Atherosclerosis 203: 206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessan N, Ali T (2019) Energy metabolism and intermittent fasting: the Ramadan perspective. Nutrients 11: 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Sabatini DM (2020) mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21: 183–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cheng A, Li Y‐J, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D et al (2019) SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun 10: 1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown‐Borg HM, Caruso C, Curiel TJ, Cabo R, Franceschi C, Gems D et al (2015) Interventions to slow aging in humans: are we ready? Aging Cell 14: 497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Di Tano M, Mattson MP, Guidi N (2021) Intermittent and periodic fasting, longevity and disease. Nat Aging 1: 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19: 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Panda S (2016) Fasting, circadian rhythms, and time restricted feeding in healthy lifespan. Cell Metab 23: 1048–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153: 1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DA, Wu N, Rohdin‐Bibby L, Moore AH, Kelly N, Liu YE, Philip E, Vittinghoff E, Heymsfield SB, Olgin JE et al (2020) Effects of time‐restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the treat randomized clinical trial. JAMA Intern Med 180: 1491–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS (2020) The ketogenic diet: evidence for optimism but high‐quality research needed. J Nutr 150: 1354–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Carmona‐Gutierrez D, Hofer SJ, Kroemer G (2019) Caloric restriction mimetics against age‐associated disease: targets, mechanisms, and therapeutic potential. Cell Metab 29: 592–610 [DOI] [PubMed] [Google Scholar]
- Madeo F, Tavernarakis N, Pedro JMB‐S, Kroemer G (2020) ACBP is an appetite stimulator across phylogenetic barriers. Cell Stress 4: 27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, Patterson RE (2015) Prolonged nightly fasting and breast cancer risk: findings from NHANES (2009–2010). Cancer Epidemiol Biomarkers Prev 24: 783–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinac CR, Nelson SH, Breen CI, Hartman SJ, Natarajan L, Pierce JP, Flatt SW, Sears DD, Patterson RE (2016) Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol 2: 1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño G, Pietrocola F, Madeo F, Kroemer G (2014) Caloric restriction mimetics: natural/physiological pharmacological autophagy inducers. Autophagy 10: 1879–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, Richey JJ, Johnson SA, Ziemba BP, Wang Y et al (2020) Short‐term time‐restricted feeding is safe and feasible in non‐obese healthy midlife and older adults. GeroScience 42: 667–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Mattson MP, Maudsley S (2006) Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res Rev 5: 332–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH et al (2016) Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults. JAMA Intern Med 176: 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E (2007) Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 15: 2964–2973 [DOI] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA et al (2004) Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. PNAS 101: 18171–18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM (2017) Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 8: 14063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M et al (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489: 318–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Longo VD, Harvie M (2017) Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39: 46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA (1935) The effect of retarded growth upon the length of life span and upon the ultimate body size: one figure. J Nutr 10: 63–79 [PubMed] [Google Scholar]
- Mesnage R, Grundler F, Schwiertz A, Le Maho Y, Wilhelmi de Toledo F (2019) Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J Nutr Sci 8: e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M et al (2016) Long‐term moderate calorie restriction inhibits inflammation without impairing cell‐mediated immunity: a randomized controlled trial in non‐obese humans. Aging 8: 1416–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L (2006) Long‐term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 47: 398–402 [DOI] [PubMed] [Google Scholar]
- Michalsen A, Li C (2013) Fasting therapy for treating and preventing disease – current state of evidence. Forsch Komplementmed 20: 444–453 [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Beckman JA, Nguyen LL, Ozaki CK (2013) Reducing elective vascular surgery perioperative risk with brief preoperative dietary restriction. Surgery 153: 594–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Müller C, de Jong M et al (2010) Short‐term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 9: 40–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, Ikeno Y, Anderson RM, Ingram DK, de Cabo R (2019) Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab 29: 221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal‐Matute J, Scheibye‐Knudsen M, Fang E, Aon M, González‐Reyes JA, Cortassa S, Kaushik S, Gonzalez‐Freire M, Patel B et al (2016) Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 23: 1093–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SE, Tang ZhanHui, Kerbois C, Delville C, Derous D, Green CL, Wang Y, Han JJD, Chen L, Douglas A et al (2017) The effects of graded levels of calorie restriction: VIII. Impact of short term calorie and protein restriction on basal metabolic rate in the C57BL/6 mouse. Oncotarget 8: 17453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Gilmore LA, Smith SR, Han H, Ravussin E, Redman LM (2018) Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction‐induced weight loss and weight loss maintenance. Am J Physiol Endocrinol Metab 314: E396–E405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, Fontana L (2017a) Calorie restriction in humans: an update. Ageing Res Rev 39: 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most J, Tosti V, Redman LM, Fontana L (2017b) Calorie restriction in humans: an update. Ageing Res Rev 39: 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, de Toledo FW, Resch KL (2001) Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand J Rheumatol 30: 1–10 [DOI] [PubMed] [Google Scholar]
- Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F et al (2015) Ghrelin. Mol Metab 4: 437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro JF, Duncan LJ (1972) Fasting in the treatment of obesity. Practitioner 208: 493–498 [PubMed] [Google Scholar]
- Neeland IJ, Turer AT, Ayers CR, Powell‐Wiley TM, Vega GL, Farzaneh‐Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA (2012) Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 308: 1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofori‐Asenso R, Owen AJ, Liew D (2019) Skipping breakfast and the risk of cardiovascular disease and death: a systematic review of prospective cohort studies in primary prevention settings. J Cardiovasc Dev Dis 6: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kang J‐H, Lee S (2020) Autophagy in neurodegenerative diseases: a hunter for aggregates. Int J Mol Sci 21: 3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE (2005) Caloric restriction attenuates Aβ‐deposition in Alzheimer transgenic models. Neurobiol Aging 26: 995–1000 [DOI] [PubMed] [Google Scholar]
- Patterson RE, Sears DD (2017) Metabolic effects of intermittent fasting. Annu Rev Nutr 37: 371–393 [DOI] [PubMed] [Google Scholar]
- Pietrocola F, Demont Y, Castoldi F, Enot D, Durand S, Semeraro M, Baracco EE, Pol J, Bravo‐San Pedro JM, Bordenave C et al (2017) Metabolic effects of fasting on human and mouse blood in vivo . Autophagy 13: 567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F, Pol J, Vacchelli E, Rao S, Enot D, Baracco E, Levesque S, Castoldi F, Jacquelot N, Yamazaki T et al (2016) Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30: 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela A‐M, Fabian S, Grittner U, Spranger J et al (2017) Caloric restriction in older adults—differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex 27: 1765–1778 [DOI] [PubMed] [Google Scholar]
- Rahman S, Islam R (2011) Mammalian Sirt1: insights on its biological functions. Cell Commun Signal 9: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Ravussin E (2011) Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal 14: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E & Pennington Comprehensive Assessment of Long‐Term Effects of Reducing Intake of Energy (CALERIE) Research Team (2008) Calorie restriction and bone health in young, overweight individuals. Arch Intern Med 168: 1859–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Smith SR, Burton JH, Martin CK, Il'yasova D, Ravussin E (2018) Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab 27: 805–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi P, Heilbronn LK (2020) Time‐restricted eating: benefits, mechanisms, and challenges in translation. iScience 23: 101161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenbacher A, Jang JH, Limani P, Ungethüm U, Lehmann K, Oberkofler CE, Weber A, Graf R, Humar B, Clavien P‐A (2014) Fasting protects liver from ischemic injury through Sirt1‐mediated downregulation of circulating HMGB1 in mice. J Hepatol 61: 301–308 [DOI] [PubMed] [Google Scholar]
- Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK (2011) The CALERIE Study: Design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials 32: 874–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez‐Casado E, Koike S, Knotts TA et al (2017) A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 26: 539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach S, Aslam M, Niemann B, Schulz R (2014) Impact of caloric restriction on myocardial ischaemia/reperfusion injury and new therapeutic options to mimic its effects. Br J Pharmacol 171: 2964–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas‐Morales P, Tapia E, León‐Contreras JC, González‐Reyes S, Jiménez‐Osorio AS, Trujillo J, Pavón N, Granados‐Pineda J, Hernández‐Pando R, Sánchez‐Lozada LG et al (2019) Mechanisms of fasting‐mediated protection against renal injury and fibrosis development after ischemic acute kidney injury. Biomolecules 9: 404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooth G, Carlström S (1970) Therapeutic fasting. Acta Medica Scandinavica 187: 455–463 [DOI] [PubMed] [Google Scholar]
- Ross SK, Macleod A, Ireland JT, Thomson WS (1969) Acidosis in obese fasting patients. Br Med J 1: 380–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozing MP, Westendorp RGJ, de Craen AJM, Frolich M, Heijmans BT, Beekman M, Wijsman C, Mooijaart SP, Blauw G‐J, Slagboom PE et al (2010) Low serum free triiodothyronine levels mark familial longevity: the leiden longevity study. J Gerontol A Biol Sci Med Sci 65A: 365–368 [DOI] [PubMed] [Google Scholar]
- Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, Conti PS, Chen TC, Longo VD (2012) Fasting enhances the response of glioma to chemo‐ and radiotherapy. PLoS One 7: e44603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD (2009) Fasting and cancer treatment in humans: a case series report. Aging 1: 988–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiattarella GG, Hill JA (2016) Therapeutic targeting of autophagy in cardiovascular disease. J Mol Cell Cardiol 95: 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens EL, Krebs JD, Corley BT, Hall RM (2020) Intermittent fasting 5:2 diet: What is the macronutrient and micronutrient intake and composition? Clin Nutr 39: 3354–3360 [DOI] [PubMed] [Google Scholar]
- Schübel R, Nattenmüller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, Schlett CL, von Stackelberg O, Johnson T, Nabers D et al (2018) Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr 108: 933–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharda N, Wong S, White H (2020) The role of prevention in healthy aging. Clin Geriatr Med 36: 697–711 [DOI] [PubMed] [Google Scholar]
- Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O (2012) Timed high‐fat diet resets circadian metabolism and prevents obesity. FASEB J 26: 3493–3502 [DOI] [PubMed] [Google Scholar]
- Shreiner AB, Kao JY, Young VB (2015) The gut microbiome in health and in disease. Curr Opin Gastroenterol 31: 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Couteur DGL, Raubenheimer D (2015) Putting the balance back in diet. Cell 161: 18–23 [DOI] [PubMed] [Google Scholar]
- Soare A, Cangemi R, Omodei D, Holloszy JO, Fontana L (2011) Long‐term calorie restriction, but not endurance exercise, lowers core body temperature in humans. Aging 3: 374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solianik R, Sujeta A (2018) Two‐day fasting evokes stress, but does not affect mood, brain activity, cognitive, psychomotor, and motor performance in overweight women. Behav Brain Res 338: 166–172 [DOI] [PubMed] [Google Scholar]
- Solon‐Biet SM, Mitchell SJ, de Cabo R, Raubenheimer D, Couteur DGL, Simpson SJ (2015) Macronutrients and caloric intake in health and longevity. J Endocrinol 226: R17–R28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser ML, Olenchock BA, O’Keefe J, Lun M, Pierce KA, Lee H, Pantano L, Klibanski A, Shulman GI, Clish CB et al (2018) The circulating metabolome of human starvation. JCI Insight 3: e121434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J et al (2019) Alternate day fasting improves physiological and molecular markers of aging in healthy, non‐obese humans. Cell Metab 30: 462–476 [DOI] [PubMed] [Google Scholar]
- Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK et al (2007) A controlled trial of reduced meal frequency without caloric restriction in healthy, normal‐weight, middle‐aged adults. Am J Clin Nutr 85: 981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm A, Jensen RA, Oslo MD, Oslo MD (1951) Mortality from circulatory diseases in Norway 1940–1945. Lancet 257: 126–129 [DOI] [PubMed] [Google Scholar]
- Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM (2018) Early time‐restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27: 1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanca A, Abbondio M, Palomba A, Fraumene C, Marongiu F, Serra M, Pagnozzi D, Laconi E, Uzzau S (2018) Caloric restriction promotes functional changes involving short‐chain fatty acid biosynthesis in the rat gut microbiota. Sci Rep 8: 14778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeman I, Smith HA, Chowdhury E, Chen Y‐C, Carroll H, Johnson‐Bonson D, Hengist A, Smith R, Creighton J, Clayton D et al (2021) A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med 13: eabd8034 [DOI] [PubMed] [Google Scholar]
- Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW (2017) Time‐restricted feeding in young men performing resistance training: A randomized controlled trial. Eur J Sport Sci 17: 200–207 [DOI] [PubMed] [Google Scholar]
- Torres‐Esquivel C, Montiel T, Flores‐Méndez M, Massieu L (2020) Effect of β‐hydroxybutyrate on autophagy dynamics during severe hypoglycemia and the hypoglycemic coma. Front Cell Neurosci 14: 547215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, Gabel K, Freels S, Rigdon J, Rood J et al (2017) Effect of alternate‐day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med 177: 930–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulijaszek SJ (1996) Relationships between undernutrition, infection, and growth and development. Hum Evol 11: 233–248 [Google Scholar]
- Van Nieuwenhove Y, Dambrauskas Z, Campillo‐Soto A, van Dielen F, Wiezer R, Janssen I, Kramer M, Thorell A (2011) Preoperative very low‐calorie diet and operative outcome after laparoscopic gastric bypass: a randomized multicenter study. Arch Surg 146: 1300–1305 [DOI] [PubMed] [Google Scholar]
- van Norren K, Rusli F, van Dijk M, Lute C, Nagel J, Dijk FJ, Dwarkasing J, Boekschoten MV, Luiking Y, Witkamp RF et al (2015) Behavioural changes are a major contributing factor in the reduction of sarcopenia in caloric‐restricted ageing mice. J Cachexia Sarcopenia Muscle 6: 253–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA (2011) Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev 12: e593–e601 [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, Klempel MC (2009) Short‐term modified alternate‐day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr 90: 1138–1143 [DOI] [PubMed] [Google Scholar]
- Vaughan KL, Kaiser T, Peaden R, Anson RM, de Cabo R, Mattison JA (2018) Caloric restriction study design limitations in rodent and nonhuman primate studies. J Gerontol A Biol Sci Med Sci 73: 48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT (2017) Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 69: 305–314 [DOI] [PubMed] [Google Scholar]
- Velthuis‐te Wierik EJ, van den Berg H, Schaafsma G, Hendriks HF, Brouwer A (1994) Energy restriction, a useful intervention to retard human ageing? Results of a feasibility study. Eur J Clin Nutr 48: 138–148 [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M et al (2016) Effect of two‐year caloric restriction on bone metabolism and bone mineral density in non‐obese younger adults: a randomized clinical trial. J Bone Miner Res 31: 40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T (2002) Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2‐year period. J Gerontol A Biol Sci Med Sci 57: B211–B224 [DOI] [PubMed] [Google Scholar]
- Walston JD (2012) Sarcopenia in older adults. Curr Opin Rheumatol 24: 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B‐H, Hou Q, Lu Y‐Q, Jia M‐M, Qiu T, Wang X‐H, Zhang Z‐X, Jiang Y (2018) Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol‐kindled seizures. Brain Res 1678: 106–115 [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR et al (2005) Caloric restriction attenuates β‐amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J 19: 1–18 [DOI] [PubMed] [Google Scholar]
- Wang S, Huang M, You X, Zhao J, Chen L, Wang L, Luo Y, Chen Y (2018) Gut microbiota mediates the anti‐obesity effect of calorie restriction in mice. Sci Rep 8: 13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S et al (2017) Fasting‐mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med 9: eaai8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R (1992) Effect of caloric restriction on age‐associated cancers. Exp Gerontol 27: 575–581 [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger‐May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO (2007) Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise‐induced weight loss. J Appl Physiol 1985: 634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder SM, Couteur DGL, Simpson SJ (2013) Diet mediates the relationship between longevity and reproduction in mammals. Age 35: 921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S, Michalsen A (2019) Safety, health improvement and well‐being during a 4 to 21‐day fasting period in an observational study including 1422 subjects. PLoS One 14: e0209353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmi de Toledo F, Grundler F, Goutzourelas N, Tekos F, Vassi E, Mesnage R, Kouretas D (2020a) Influence of long‐term fasting on blood redox status in humans. Antioxidants 9: 496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmi de Toledo F, Grundler F, Sirtori CR, Ruscica M (2020b) Unravelling the health effects of fasting: a long road from obesity treatment to healthy life span increase and improved cognition. Ann Med 52: 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S et al (2020) Ten‐hour time‐restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 31: 92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M (2007) Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest‐lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci 1114: 434–455 [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC (2014) Caloric Restriction, CR mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr Opin Clin Nutr Metab Care 17: 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M (2006) Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology 7: 173–177 [DOI] [PubMed] [Google Scholar]
- Williamson DA, Martin CK, Anton SD, York‐Crowe E, Han H, Redman L, Ravussin E (2008) Is caloric restriction associated with development of eating‐disorder symptoms? Results from the CALERIE trial. Health Psychol 27: S32–S42 [DOI] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Flöel A (2009) Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA 106: 1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BM, Kvach E, Eckel RH (2016) Treatment of obesity: weight loss and bariatric surgery. Circ Res 118: 1844–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2019) World health statistics 2019: monitoring health for the SDGs: sustainable development goals. Geneva, Switzerland: World Health Organization; [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming D, Souza‐Pinto NC, Bohr VA, Rosenzweig A et al (2007) Nutrient‐sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130: 1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CW, Lee SH (2018) The roles of autophagy in cancer. Int J Mol Sci 19: 3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Madeo F, Kratky D (2017) Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18: 671–684 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang W, Gao X, Zhao Y, Chen D, Xu N, Pu H, Stetler RA, Gao Y (2019) Preconditioning with partial caloric restriction confers long‐term protection against grey and white matter injury after transient focal ischemia. J Cereb Blood Flow Metab 39: 1394–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang S, Jia W (2018) Calorie restriction and its impact on gut microbial composition and global metabolism. Front Med 12: 634–644 [DOI] [PubMed] [Google Scholar]
- Zouhal H, Saeidi A, Salhi A, Li H, Essop MF, Laher I, Rhibi F, Amani‐Shalamzari S, Ben Abderrahman A (2020) Exercise training and fasting: current insights. Open Access J Sports Med 11: 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]


