Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Maconellicoccus hirsutus (Hemiptera: Pseudococcidae), the pink hibiscus mealybug, for the EU. M. hirsutus is native to Southern Asia and has established in many countries in tropical and subtropical regions throughout the world. Within the EU, the pest has been reported from Cyprus and Greece (Rhodes). M. hirsutus is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072. It is highly polyphagous, feeding on plants assigned to 229 genera in 78 plant families, and shows some preference for hosts in the families Malvaceae, Fabaceae and Moraceae. Economically important crops in the EU such as cotton (Gossypium spp.), citrus (Citrus spp.), ornamentals (Hibiscus spp.), grapes (Vitis vinifera), soybean (Glycinae max), avocado (Persea americana) and mulberry trees (Morus alba) may be significantly affected by M. hirsutus. The lower and upper developmental temperature threshold of M. hirsutus on Hibiscus rosa‐sinensis are 14.5 and 35.0°C, respectively, with optimal female development estimated to be at 29.0°C. There are about 10 generations a year in the subtropics but as many as 15 may occur under optimal conditions. Plants for planting, fruits, vegetables and cut flowers provide potential pathways for entry into the EU. Climatic conditions in EU member states around the Mediterranean Sea and host plant availability in those areas are conducive for establishment. The introduction of M. hirsutus is expected to have an economic impact in the EU through damage to various ornamental plants, as already observed in Cyprus and Greece, and reduction in yield and quality of many significant crops. Phytosanitary measures are available to reduce the likelihood of entry and further spread. Some uncertainties include the area of establishment, whether it could become a greenhouse pest, impact, and the influence of natural enemies. M. hirsutus meets the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest.
Keywords: pink hibiscus mealybug, Hemiptera, pest risk, plant health, plant pest, Pseudococcidae, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Maconellicoccus hirsutus is one of a number of pests listed in Annex 1A to the Terms of Reference (ToR) (Section 1.1.2) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform European Commission decision making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
1.3. Additional information
This pest categorisation was initiated following the commodity risk assessment of Ficus carica plants from Israel performed by EFSA (EFSA PLH Panel, 2021), in which M. hirsutus was identified as a relevant non‐regulated EU pest which could potentially enter the EU on F. carica.
2. Data and methodologies
2.1. Data
2.1.1. Information on pest status from NPPOs
In the context of the commodity risk assessment of Ficus carica plants from Israel (EFSA PLH Panel, 2021), EFSA consulted (in April‐May 2020) the NPPOs where the pest is present, in order to have an updated information on the pest status. For the information on pest status in Cyprus and Greece, please see Section 3.2.2.
2.1.2. Literature search
A literature search on M. hirsutus was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.3. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.2.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the EU, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
2.2. Methodologies
The Panel performed the pest categorisation for M. hirsutus, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 to this Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests’ introduction have an economic or environmental impact on the EU territory? |
|
Available measures (Section 3.6 ) |
Are there measures available to prevent the entry into the EU such that the likelihood of introduction becomes mitigated? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met |
The Panel’s conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. While the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
|
Is the identity of the pest established, or has it been shown to produce consistent symptoms and/or to be transmissible? Yes, the identity of the pest is established and Maconellicoccus hirsutus (Green) is the accepted name. |
The pink hibiscus mealybug, also known as the hibiscus mealybug, Maconellicoccus hirsutus (Green, 1908) is an insect within the order Hemiptera, family Pseudococcidae. This species was initially described by Green in 1908 as Phenacoccus hirsutus from specimens collected on an undetermined shrub attended by ants in India (García Morales et al., 2016). Indeed, this species is likely to be native to southern Asia (Williams, 2004). Former scientific names include Maconellicoccus pasaniae, Maconellicoccus perforatus, Paracoccus pasaniae, Phenacoccus glomeratus, Phenacoccus hirsutus, Phenacoccus quaternus, Pseudococcus hibisci and Spilococcus perforatus (CABI, 2021). The genus Maconellicoccus includes eight described species (Williams, 1996; CABI, 2021). Detailed morphological descriptions, illustrations and keys to the eight species of the genus Maconellicoccus can be found in Williams (1996), Meyerdirk et al. (2001) and EPPO (2006). The EPPO code (Griessinger & Roy, 2015; EPPO, 2019) for this species is PHENHI (EPPO, 2021).
3.1.2. Biology of the pest
Adult females of M. hirsutus in Jordan appear in early February and show their highest abundance in mid‐July (Al‐Fwaeer et al., 2014). M. hirsutus reproduces parthenogenetically or sexually (Williams, 1996). Reproduction is mostly parthenogenetic in Egypt and the State of Bihar, India (Hall, 1921; Singh and Ghosh, 1970), while it is sexual in the Indian state of West Bengal (Ghose, 1971) and probably in the Caribbean (Williams, 1996). According to Bartlett (1978) and Mani (1989), an adult female lays 150–600 eggs over a period of about 1 week on the host plants. The eggs are laid in an ovisac, consisting of a mass of sticky wax filaments. Oviposition occurs mainly in the outer parts of the host, such as the growing points, buds and fruits, but in case of cold weather conditions the females search for shelter to oviposit (Meyerdirk et al., 2001). The lower and upper developmental temperature threshold of M. hirsutus on Hibiscus rosa‐sinensis are 14.5 and 35.0°C, respectively. The optimal developmental temperature for females was estimated to be 29.0°C (Chong et al., 2008). In warm, but unspecified conditions, it takes 5 weeks for a generation to be completed (Bartlett, 1978). Chong et al. (2008) stated that the generation time is 41 days at 25°C and 82 days at 20°C. In countries with a cool winter the species overwinters as eggs (Bartlett, 1978) or other stages in protected parts of the host plant or as eggs in the soil (Pollard, 1995). There are about 10 generations a year in the subtropics (Meyerdirk et al., 2001). However, under optimum conditions, there may be as many as 15 generations per year (Pollard, 1995).
There are three immature instars in the female and four in the male (EPPO, 2005). First instar nymphs are known as crawlers and are mobile. The crawlers prefer the apical and tender regions of the host. However, large populations of nymphs may also settle on the older plant parts including stems, leaves, petioles, roots, tubers and pods (Ghose, 1972). After locating a suitable feeding site on a host plant, nymphs settle to feed and develop. Later instars turn grey–pink and start to secrete white wax that covers their bodies (Chong et al., 2015). In heavy infestations white masses of wax concealing the insect may occur in axils and on twigs and stems (EPPO, 2006) (Figure 1). Female adults live for 19–28 days (Chong et al., 2008; Sahito et al., 2012; Negrini et al., 2017). Males have one pair of wings, but they are weak flyers, only live a day or two, and are not commonly observed (Chong et al., 2015).
Figure 1.

Maconellicoccus hirsutus: (A) adult female; (B) adult female covered in waxy filaments; (C) large infestation on hibiscus; (D) ovisacs in the crevices of Annona fruit; (E) distorted growth characteristic of plants infested by M. hirsutus; (F) hibiscus plant in Rhodes, severely damaged by M. hirsutus © Chris Malumphy
Key features of the biology of each life stage are summarised in Table 2.
Table 2.
Important features of the life history strategy of Maconellicoccus hirsutus
| Life stage | Phenology and relation to host | Other relevant information |
|---|---|---|
| Egg | Adult female lays 150–600 eggs in a sticky waxy ovisac. Oviposition occurs mainly on the outer areas of the host, including the buds and fruit | The eggs hatch in 6–9 days at temperatures between 25 and 35°C but it requires 16 days at 20°C. The lower and upper threshold for the eggs and the optimal developmental temperature were estimated at 14.5, 39.8 and 33.4°C, respectively (Chong et al., 2008) |
| First instar nymph | First instar nymphs are known as crawlers. They prefer the apical and tender parts of the host. However, large populations of nymphs may also settle on the older plant parts including stems, leaves, petioles, roots, tubers, and pods. After locating a suitable host plant, nymphs settle on the host to feed and develop | The crawlers disperse by walking to other parts of the host plant. They may also be transported by water, wind or animals |
| Later instar nymphs | Later instars start to secrete white wax that covers their bodies. There are three immature instars in the female and four in the male | White masses of wax concealing the insect may occur in axils and on twigs and stems. The nymphal development is affected by both temperature and host plant. At 25°C, the female nymphs need 23 and 26.6 days on H. rosa‐sinensis and Morus alba, respectively, to complete their development (Chong et al., 2008; Sahito et al., 2012). On H. rosa‐sinensis and at 27°C nymphal development was reported to last either 17.5 or 20.6 days (Chong et al., 2008; Negrini et al., 2017). Whereas at 30 and 20°C the female nymphal stages last 26.6 and 50.1 days, respectively. The lower and upper threshold and the optimal developmental temperature for female nymphs were estimated at 15.1, 35.0 and 28.8°C, respectively (Chong et al., 2008) |
| Adult | Males have one pair of wings, but they are weak flyers. Female adults live for 19–28 days (Chong et al., 2008; Sahito et al., 2012; Negrini et al., 2017) while males only 1 or 2 days and are not commonly observed (Chong et al., 2015) | M. hirsutus reproduces parthenogenetically or sexually. The lower and upper developmental temperature threshold on H. rosa‐sinensis were 14.5 and 35°C, respectively. The optimal developmental temperature for females was estimated to be 29°C |
3.1.3. Host range/species affected
There is a long list of host plants of M. hirsutus worldwide. The host range of M. hirsutus is broad with more than 229 plant genera from 78 plant families (García Morales et al., 2016). Appendix A provides the full list of plant species reported to be M. hirsutus hosts. Economically important crops in the EU such as cotton (Gossypium spp.), citrus (Citrus spp.), ornamentals (Hibiscus spp.), grapes (Vitis vinifera), soybean (Glycinae max), avocado (Persea americana) and mulberry trees (Morus alba) may be significantly affected by M. hirsutus. M. hirsutus has also been recorded on several rosaceous crops that are important in the EU, including apple (Malus domestica), apricot (Prunus armeniaca), peach (Prunus persica), pear (Pyrus communis) and plum (Prunus domestica), but there appears to be no economic impact recorded on these hosts.
3.1.4. Intraspecific diversity
No intraspecific diversity is reported for this species.
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes. There are methods available for detection, and morphological and molecular identification of M. hirsutus.
Detection
Careful visual examination of plants is an effective way for the detection of the insect. The white waxy covering of mealybug instars and white waxy filaments in the egg mass allow detection (Meyerdirk et al., 2001). The mealybugs themselves are in general visible, although they are hidden in the swollen growth. Male adults can also be caught using sticky cards baited with a sex pheromone which contains esters of lavandulyl and maconellyl and allow detection in areas of low density of the pest (Francis et al., 2007).
Symptoms
The main symptoms of M. hirsutus infestation are (Dufour and Léon, 1997; Sagarra and Peterkin, 1999; Kairo et al., 2000; Alleyne, 2004; Chong et al., 2015):
large quantities of honeydew
black sooty mould
leaf curling
shoot and leave malformation
fruit malformation
bunchy top appearance
premature senescence of flowers and foliage
heavy infestation may cause a complete defoliation of the plant, leading to their death
Identification
The identification of M. hirsutus requires microscopic examination of slide‐mounted adult females and verification of the presence of key morphological characteristics as given in Meyerdirk et al. (2001) and Williams (1996). Moreover, a key is available (EPPO, 2006) to distinguish M. hirsutus from other species of the genus. Molecular techniques for species identification have also been developed (Malausa et al., 2011; Abd‐Rabou et al., 2012).
Description (detailed morphological descriptions are available from Meyerdirk et al. (2001) and EPPO (2006))
The main morphological characteristics of M. hirsutus are:
The eggs are 0.3 mm long and initially orange, turning pink before hatching (Chong et al., 2015).
Crawlers 0.37 mm long (Aristizábal et al., 2012), pink and oval with antennae; they lack the waxy body coating (CABI, 2021).
Second instars average length 0.70 mm, third instars 1.1 mm and male fourth instar 1.1 mm (Aristizábal et al., 2012). Immature females and newly matured females have greyish‐pink bodies dusted with mealy white wax (CABI, 2021).
Mature adult females are wingless, elongate oval, slightly flattened in profile, 2.5–4 mm long, and their ovisacs cover most of the body. Body is greyish pink or occasionally purple, and covered with a thin white cotton like wax forming a protective ovisac for her eggs. The entire colony tends to become covered by white, waxy ovisac material (EPPO, 2005, 2006; Chong et al., 2015).
On microscopic examination of slide‐mounted females, the combination of nine‐segmented antennae, anal lobe bars, numerous large dorsal oral rim ducts on all parts of the body, and long, flagellate dorsal setae make the species fairly easy to recognize in parts of the world where other Maconellicoccus species do not occur. Males have one pair of very simple wings, long antennae, white wax filaments projecting posteriorly and lack mouthparts CABI (2021).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
M. hirsutus has established in many tropical and subtropical regions throughout the world in the past 100 years (Culik et al., 2013). It has a wide distribution which includes many countries in Africa, South Asia, Australia, Central America, South America, Caribbean and the southern part of North America (EPPO, 2021) (Figure 2). For a detailed list of countries where M. hirsutus is present, see Appendix B.
Figure 2.
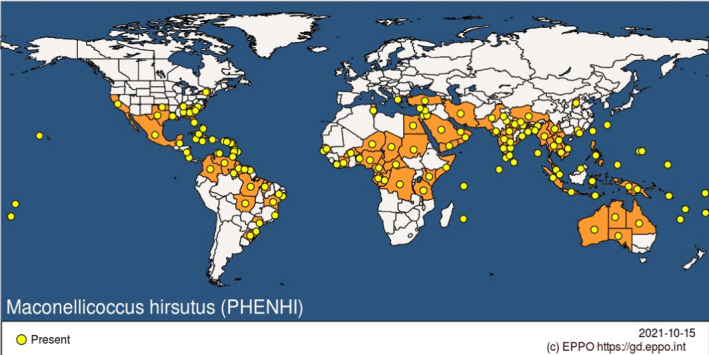
Global distribution of Maconellicoccus hirsutus (Source: EPPO Global Database accessed on 15/10/2021)
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
M. hirsutus has a restricted distribution in the EU. It is present in Greece and Cyprus.
The pest is widespread only in the island of Rhodes in southern eastern part of Greece. The pest is present, widespread and under official control in Cyprus (EPPO GD, online).
According to Miller et al. (2014), between 1995 and 2012 the species had been intercepted in USA ports in commodities originating from France and Italy. However, there are no records of the species from France and Italy. This has probably resulted from produce being imported to Europe from areas where the mealybug occurs and re‐exported to the USA.
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
M. hirsutus is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072.
3.3.2. Hosts or species affected that are prohibited from entering the Union from third countries
According to the Commission Implementing Regulation (EU) 2019/2072, Annex VI, introduction of several M. hirsutus hosts in the Union from certain third countries is prohibited (Table 3).
Table 3.
List of plants, plant products and other objects that are Maconellicoccus hirsutus hosts whose introduction into the Union from certain third countries is prohibited (Source Commission Implementing Regulation (EU) 2019/2072, Annex VI)
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN Code | Third country, group of third countries or specific area of third country | |
| 8. | Plants for planting of Chaenomeles Ldl., Crateagus L., Cydonia Mill., Malus Mill., Prunus L., Pyrus L. and Rosa L., other than dormant plants free from leaves, flowers and fruits |
ex 0602 10 90 ex 0602 20 20 ex 0602 20 80 ex 0602 40 00 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than: Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Turkey and Ukraine |
| 9. | Plants for planting of Cydonia Mill., Malus Mill., Prunus L. and Pyrus L. and their hybrids, and Fragaria L., other than seeds |
ex 0602 10 90 ex 0602 20 20 ex 0602 90 30 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries, other than: Albania, Algeria, Andorra, Armenia, Australia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canada, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, New Zealand, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Turkey, Ukraine, and United States other than Hawaii |
| 10. | Plants of Vitis L., other than fruits |
0602 10 10 0602 20 10 ex 0604 20 90 ex 1404 90 00 |
Third countries other than Switzerland |
| 11. | Plants of Citrus L., Fortunella Swingle, Poncirus Raf., and their hybrids, other than fruits and seed |
ex 0602 10 90 ex 0602 20 20 0602 20 30 ex 0602 20 80 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
|
| 18. |
Plants for planting of Solanaceae other than seeds and the plants covered by entries 15, 16 or 17 |
ex 0602 90 30 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than: Albania, Algeria, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Turkey and Ukraine |
| 20. | Growing medium as such, other than soil, consisting in whole or in part of solid organic substances, other than that composed entirely of peat or fibre of Cocos nucifera L., previously not used for growing of plants or for any agricultural purposes |
ex 2530 10 00 ex 2530 90 00 ex 2703 00 00 ex 3101 00 00 ex 3824 99 93 |
Third countries other than Switzerland |
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Comment on plants for planting as a pathway.
Yes. The pest has already entered the EU territory. The main pathways are plants for planting, fruits, vegetables and cut flowers.
Plants for planting, fruits, vegetables and cut flowers are the main pathways for entry of M. hirsutus (EPPO, 2005; Culik et al., 2013). It can also be associated with soil, which could however be considered as a closed pathway (Table 4).
Table 4.
Potential pathways for Maconellicoccus hirsutus into the EU 27
|
Pathways description (e.g. host/intended use/source) |
Life stage | Relevant mitigations [e.g. prohibitions (Annex VI), special requirements (Annex VII) or phytosanitary certificates (Annex XI) within Implementing Regulation 2019/2072] |
|---|---|---|
| Plants for planting | Eggs, nymphs and adults |
Plants for planting that are hosts of M. hirsutus, and are prohibited to import from third countries (Regulation 2019/2072, Annex VI), are listed in Table 3. The growing medium attached to or associated with plants, intended to sustain the vitality of the plants, are regulated in Regulation 2019/2072, Annex VII. Plants for planting from third countries require a phytosanitary certificate (Regulation 2019/2072, Annex XI, Part A) |
| Fruits, vegetables and cut flowers | Eggs, nymphs and adults |
Fruits, vegetables and cut flowers from third countries require a phytosanitary certificate to import into the EU (2019/2072, Annex XI, Part A). However, no requirements are specified for M. hirsutus. According to Regulation 2019/2072, Annex XI, Part C there is a list of plants which a phytosanitary certificate is not required for their introduction into the Union territory. M. hirsutus infests fruits that are included in that list (Ananas comosus and Musa spp.) |
| Soil | Eggs | Import of soil from third countries is prohibited (Regulation 2019/2072, Annex VI) |
The import of some host plants of M. hirsutus for planting from third countries is not allowed (Regulation 2019/2072, Annex VI), while there are many other hosts that can be imported to the EU with a phytosanitary certificate.
Vegetables, cut flowers and most fruits that are imported into the EU must have a phytosanitary certificate. However, pineapple (Ananas comosus) and banana (Musa spp.), which are hosts for M. hirsutus, are exempt by Regulation 2019/2072, Annex XI, Part C.
EU legislation (2019/2072) prohibits the import of soil from third countries so that pathway can be considered as closed.
Annual imports of M. hirsutus hosts from countries where the pest is known to occur are provided in Appendix C.
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As at 16/9/2021 (search date) there were two records of interceptions of M. hirsutus in the Europhyt and TRACES databases:
in 2008 on Colocasia sp. plants for planting imported from India
in 2018 on Annona squamosa fruits imported from Brazil
In the UK, a former member of the EU, there were more than 240 interceptions of M. hirsutus between 1994 and 2021, mostly on Annona squamosa fruits from India. M. hirsutus was also found on Annona fruits from Egypt, Indonesia, Kenya, Pakistan, Saint Lucia and Vietnam, and a range of fresh fruits and vegetables imported from Asia, Africa, and the Caribbean (Fera unpublished records). No action was taken against these findings.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes, in the EU countries of southern Europe the climate is suitable and there are many available hosts that can support establishment. Given that M. hirsutus occurs in Greece and has a wide distribution in Cyprus, it must have been able to transfer following entry.
3.4.2.1. EU distribution of main host plants
M. hirsutus is a polyphagous pest. The main hosts of the pest cultivated in the EU 27 between 2016 and 2020 are shown in Table 5. Among others, citrus, cotton, soybeans, grapes, pome fruits and stone fruits are highly economically important crops in the EU.
Table 5.
Crop area of Maconellicoccus hirsutus hosts in EU 27 in 1,000 ha (Eurostat accessed on 21/09/2021)
| Crop | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Citrus | 519.01 | 502.84 | 508.99 | 512.53 | 487.08 |
| Cotton | 301.34 | 326.12 | 345.64 | 361.78 | 349.94 |
| Soybeans | 831.18 | 962.39 | 955.40 | 907.91 | 939.86 |
| Grapes | 3,136.04 | 3,133.21 | 3,135.02 | 3,158,32 | 3,160.27 |
| Cucumbers | 32.33 | 31.81 | 32.65 | 33.69 | 33.15 |
| Bananas | 20.30 | 18.91 | 17.94 | 18.19 | 19.61 |
| Pome fruits | No data | 627.98 | 629.42 | 610.11 | 589.85 |
| Stone fruits | No data | 625.46 | 621.32 | 612.33 | No data |
| Avocados | 12.24 | 12.72 | 13.22 | 15.52 | 17.27 |
3.4.2.2. Climatic conditions affecting establishment
M. hirsutus occurs mainly in tropical and subtropical regions in Asia, Africa, Australia, and America. Moreover, it has also been recorded in Greece, Cyprus and Turkey, countries with a Mediterranean climate. According to the global Köppen‐Geiger climate zones (Kottek et al., 2006), M. hirsutus is present in countries with climate zones Aw (Equatorial savannah with dry winter), Am (Equatorial monsoon), Af (Equatorial rainforest, fully humid), BWh (Desert climate, hot desert), Bsh (Steppe climate, hot steppe) and Csa (warm temperate climate with dry hot summer). The lower and upper developmental temperature threshold of M. hirsutus on H. rosa‐sinensis is 14.5 and 35°C, respectively (Chong et al., 2008), temperatures that are relatively high. Figure 3 shows the World distribution of Köppen–Geiger climate types that occur in the EU and which occur in countries where M. hirsutus has been reported. Southern EU countries provide suitable climatic conditions that would support the establishment of M. hirsutus. There is uncertainty as to whether M. hirsutus could establish in the EU countries of central Europe. It is unlikely that the insect could establish in the northern EU, and if it did, the populations are likely to be small and have no impact. Countries and areas of the EU most suitable include Cyprus, Greece, Malta, Portugal, Spain, coastal areas of southern France, including Corsica, as well as southern Italy, including Sardinia and Sicily. There is a possibility that M. hirsutus could occur in glasshouses and on indoor plantings in cooler areas.
Figure 3.
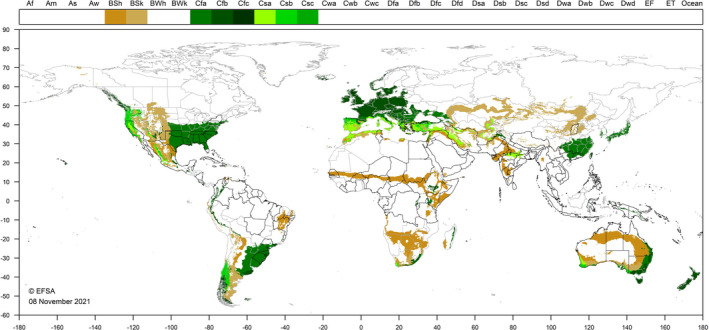
World distribution of Köppen–Geiger climate types that occur in the EU and which occur in areas where Maconellicoccus hirsutus has been reported
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
First instar nymphs are spread by crawling, wind, rainfall and on humans and animals. Overwintering eggs may be moved in soil. All stages may be moved over long distances in trade.
Comment on plants for planting as a mechanism of spread.
Plants for planting are one of the main pathways of spread of the pest over long distances.
First instar nymphs are active and spread by crawling, wind and rainfall. The sticky egg masses and mobile crawlers may also be carried to new areas on humans and other animals (Sagarra and Peterkin, 1999; EPPO, 2005; Culik et al., 2013). Moreover, overwintering eggs can be found in soil (Pollard, 1995) and spread through the soil attached to plants for planting and machinery.
The introduction of this pest to new territories over long distance is possible through the movement of infested plants for planting (e.g. fruit tree and ornamental nursery seedlings), and trade of infested fruit, vegetables, cut flowers or other plant products (Meyerdirk et al., 2001; CABI, 2021).
Plants for planting, fruits, vegetables and cut flowers are the main pathways of spread of the pest over long distances.
3.5. Impacts
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, if M. hirsutus established more widely in the EU, it would most probably have an economic impact on the host species of the pest.
The pest may seriously affect the commercial value of various ornamental plants and potentially have a high economic impact on crop production in the EU. M. hirsutus egests large quantities of honeydew, and as a result black sooty mould develops on the plants, which reduces the aesthetic value, normal growth and reproduction (Kairo et al., 2000; Chong et al., 2015). M. hirsutus also injects toxic saliva into the plant during feeding, which results in leaf curling, fruit malformation, bunchy top appearance (Figure 1E) and premature senescence of flowers and foliage (Dufour and Léon, 1997; Chong et al., 2015). Heavy infestations may cause a complete defoliation of the plant, leading to its death (Figure 1F) (Dufour and Léon, 1997; Sagarra and Peterkin, 1999). These impacts have been documented in city parks and gardens in Cyprus (Ülgentürk et al., 2015) and Greece (Milonas and Partsinevelos, 2017).
The potential annual economic impact of M. hirsutus to avocado (Persea americana), citrus (Citrus spp.), cotton (Gossypium hirsutum), peanut (Arachis hypogaea), soybean (Glycine max), nursery and vegetable crops was estimated at US$163 million in Florida or US$1.6 billion for the entire United States (Ranjan, 2006). In Egypt, M. hirsutus was reported to cause damage to Albizia lebbek, mulberry, Hibiscus spp., and cotton. In Africa, it was considered as a possible pest of cocoa. In India, Bangladesh and Pakistan it is a pest of cotton, mulberry and several fibre crop species. In India, it has also been considered to be a severe pest of grapes (Muralidharan and Badaya, 2000; Culik et al., 2013). When M. hirsutus was introduced in the Caribbean islands it became a very serious problem. Grenada reported economic losses of $3.5 to $10 million for the season 1996–1997 and Trinidad and Tobago estimated potential losses exceeding $125 million/year, if infestations continued to escalate (Meyerdirk et al., 2001). However, in many countries M. hirsutus is restricted to Hibiscus species and is not a serious pest, possibly because natural enemies effectively reduce its populations (Meyerdirk et al., 2001).
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impacts such that the risk becomes mitigated?
Yes. Although the existing phytosanitary measures identified in Section 3.3.2 do not specifically target M. hirsutus, they mitigate the likelihood of its entry into and spread within the EU (see also Section 3.6.1).
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2).
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 and 3.6.1.2.
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 6.
Table 6.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
|
Control measure/risk reduction option (Blue underline = Zenodo doc) |
RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Growing plants in isolation |
Description of possible exclusion conditions that could be implemented to isolate the crop from pests and if applicable relevant vectors. E.g. a dedicated structure such as glass or plastic greenhouses. Used to mitigate likelihood of infestation by specified pest in vicinity of growing site. Plants could be grown in glass or plastic structures |
Entry (reduce contamination/infestation)/spread |
| Chemical treatments on crops including reproductive material | Used to mitigate likelihood of infestation of pests susceptible to chemical treatments. Pesticide application for the control of M. hirsutus has been considered to be impractical (Culik et al., 2013). Some neonicotinoid and pyrethroid insecticides (e.g. imidacloprid, thiamethoxam, bifenthrin) as well as their mixture have provided encouraging results regarding the control of the pest (Castle and Prabhaker, 2011; Fatima et al., 2016). However, the use of some neonicotinoids for outdoor use in EU has been banned. Moreover, the natural wax coating covering the various stages of the insect protects it from pesticides (Meyerdirk et al., 2001) | Entry/establishment/impact |
| Chemical treatments on consignments or during processing |
Use of chemical compounds that may be applied to plants or to plant products after harvest, during process or packaging operations and storage. The treatments addressed in this information sheet are:
Used to mitigate likelihood of infestation of pests susceptible to chemical treatments. Eggs, nymphs and adults of M. hirsutus were susceptible to methyl bromide fumigations. A dose of 48 mg/litre methyl bromide at 21–26°C produced 100% mortality of all life stages (Zettler et al., 2002) |
Entry/spread |
| Physical treatments on consignments or during processing |
This information sheet deals with the following categories of physical treatments: irradiation/ionisation; mechanical cleaning (brushing, washing); sorting and grading, and; removal of plant parts (e.g. debarking wood). This information sheet does not address: heat and cold treatment (information sheet 1.14); roguing and pruning (information sheet 1.12). Used to mitigate likelihood of infestation of pests susceptible to physical treatments Washing, brushing and other mechanical cleaning methods can be used to reduce the prevalence of the pest in the consignments to be exported or to be planted |
Entry/spread |
| Cleaning and disinfection of facilities, tools and machinery |
The physical and chemical cleaning and disinfection of facilities, tools, machinery, transport means, facilities and other accessories (e.g. boxes, pots, pallets, palox, supports, hand tools). The measures addressed in this information sheet are: washing, sweeping and fumigation. Used to mitigate likelihood of entry or spread of soil borne pests |
Entry/spread |
| Limits on soil | Used to mitigate likelihood of entry or spread of M. hirsutus eggs in soil | Entry/spread |
| Soil treatment |
The control of soil organisms by chemical and physical methods listed below: a) Fumigation; b) Heating; c) Solarisation; d) Flooding; e) Soil suppression; f) Augmentative Biological control; g) Biofumigation Used to mitigate likelihood of presence of eggs in the soil |
Entry/establishment/impact |
| Heat and cold treatments |
Controlled temperature treatments aimed to kill or inactivate pests without causing any unacceptable prejudice to the treated material itself. The measures addressed in this information sheet are: autoclaving; steam; hot water; hot air; cold treatment Used to mitigate likelihood of infestation of pests susceptible to physical treatments. Hot water immersion treatment of fruits has been reported as an effective measure for disinfestation of fresh fruits. Effective temperature time combinations for control of M. hirsutus on fruits were 55 min at 47°C, 23 min at 48°C and 13 min at 49°C (Hara and Jacobsen, 2005) |
Entry/spread |
| Controlled atmosphere |
Treatment of plants by storage in a modified atmosphere (including modified humidity, O2, CO2, temperature, pressure). Used to mitigate likelihood of infestation of pests susceptible to modified atmosphere (usually applied during transport) hence to mitigate entry. Controlled atmosphere storage can be used in commodities such as fresh and dried fruits, flowers and vegetables |
Entry/spread (via commodity) |
| Post‐entry quarantine and other restrictions of movement in the importing country |
This information sheet covers post‐entry quarantine (PEQ) of relevant commodities; temporal, spatial and end‐use restrictions in the importing country for import of relevant commodities; Prohibition of import of relevant commodities into the domestic country. ‘Relevant commodities’ are plants, plant parts and other materials that may carry pests, either as infection, infestation, or contamination. Plants in PEQ are held in conditions that prevent the escape of pests; they can be carefully inspected and tested to verify they are of sufficient plant health status to be released, or may be treated, re‐exported or destroyed. Tests on plants are likely to include laboratory diagnostic assays and bioassays on indicator hosts to check whether the plant material is infected with particular pathogens |
Establishment/spread |
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 7.
Table 7.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Supporting measure | RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping |
Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5). The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques. Used to mitigate likelihood of infestation by specified pest at origin. Any shipments of fresh plant material from an infested country to another that is not infested should be examined thoroughly to detect M. hirsutus (CABI, 2021) |
Establishment/spread |
| Phytosanitary certificate and plant passport |
An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) a) export certificate (import) b) plant passport (EU internal trade) Used to attest which of the above requirements have been applied |
Entry/spread |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
M. hirsutus hide in cracks and crevices on the plant bark and in the calyx of fruits, making its detection, especially in early infestations and low population, difficult.
The high number of host plants and the wide distribution of M. hirsutus makes the inspections of all consignments imported from countries where the pest occurs difficult.
The natural wax coating covering the various stages of M. hirsutus protects it from treatments with contact insecticides.
3.7. Uncertainty
Uncertainty exists regarding the suitability of the climate of EU countries in central Europe for the establishment of M. hirsutus. However, its establishment in the southern EU countries is very likely since it has already been detected in Cyprus and Greece (Rhodes).
In many countries where climate is suitable, M. hirsutus is not a serious pest, largely due to natural enemies (Kairo et al., 2000), thus there is uncertainty on the magnitude of impact. For example, it is not known if, and how quickly, natural enemies such as the parasitoid Anagyrus kamali, will follow the spread of M. hirsutus in the EU.
The presence of M. hirsutus in France and Italy, implied by some interceptions in the USA, is uncertain (Miller et al., 2014). It is likely that the interceptions recorded in the US are on produce imported into the EU from other countries and reexported (see Section 3.2.2).
4. Conclusions
The criteria assessed by EFSA for consideration of M. hirsutus as a potential EU quarantine pest are met (Table 8).
Table 8.
The Panel’s conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel’s conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest ( 3.1 ) | The identity of the pest is established. Taxonomic keys based on morphology of female adults exist | None |
| Absence/presence of the pest in the EU ( 3.2 ) | The pest has a restricted distribution in the EU territory (Rhodes Island in Greece and Cyprus) | None |
| Regulatory status ( 3.3 ) | Maconellicoccus hirsutus is not regulated as a quarantine pest in the EU; the Cypriot NPPO is taking official action | None |
| Pest potential for entry, establishment and spread in the EU ( 3.4 ) |
Maconellicoccus hirsutus is able to enter into, become established, and spread within the EU territory. The main pathways are:
|
None |
| Potential for consequences in the EU ( 3.5 ) | The pests’ introduction could reduce the aesthetic value of various ornamental plants and the production of many crops | In many countries M. hirsutus is not a serious pest, possibly due to the climate being less favourable, and natural enemies reducing its population levels |
| Available measures ( 3.6 ) | There are measures available to prevent the entry, establishment and spread of M. hirsutus within the EU. Risk reduction options include the inspections and physical treatments on consignments of fresh plant material from infested countries and the production of plants for import into the EU in pest free areas (this could be difficult due to wide distribution of the pest) | Eradication and containment actions taken in the Caribbean (for example, restricting the movement of host plant material) were unsuccessful. There is uncertainty regarding how effective risk reduction measures would be in the EU |
| Conclusion ( 4 ) | The criteria assessed by EFSA for consideration as a potential quarantine pest are met | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | Establishment, impact, and natural enemies | |
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2018)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2018)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2018)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2018)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2018)
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2018)
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2018)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2018)
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2018)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2018)
Appendix A – Maconellicoccus hirsutus host plants/species affected
Source: EPPO Global Database (EPPO, online), García Morales et al. (2016) and other references.
| Host status | Host name | Plant family | Common name | Reference |
|---|---|---|---|---|
| Cultivated hosts | Abutilon indicum | Malvaceae | Country mallow | CABI (2021) |
| Acalypha hispida | Euphorbiaceae | Copperleaf | CABI (2021) | |
| Aegle marmelos | Rutaceae | Indian bael | Chong et al. (2015) | |
| Aglaonema | Araceae | Aglaonema | Chong et al. (2015) | |
| Albizia niopoides | Fabaceae | Guanacaste, monkey's earring | García Morales et al. (2016) | |
| Albizia saman | Fabaceae | Crow bean tree, monkey pod | García Morales et al. (2016) | |
| Allamanda | Apocynaceae | CABI (2021) | ||
| Allamanda cathartica | Apocynaceae | Yellow allamanda | CABI (2021) | |
| Alocasia cucullata | Araceae | Chinese taro | Chong et al. (2015) | |
| Alpinia | Zingiberaceae | Alpina (ginger and galangal) | Chong et al. (2015) | |
| Alpinia purpurata | Zingiberaceae | Red ginger | CABI (2021) | |
| Althaea | Malvaceae | Marshmallow | Chong et al. (2015) | |
| Amaranthus | Amatanthaceae | Amaranth | Chong et al. (2015) | |
| Abelmoschus esculentus | Malvaceae | Gumbo, lady's fingers, okra | EPPO GD (2021) | |
| Abelmoschus manihot | Malvaceae | Sunset musk mallow, sunset hibiscus, hibiscus manihot | García Morales et al. (2016) | |
| Ananas comosus | Bromeliaceae | Pineapple | EPPO GD (2021) | |
| Annona | Annonaceae | CABI (2021) | ||
| Annona cherimola | Annonaceae | Cherimoya, custard apple, graveola | EPPO GD (2021) | |
| Annona muricata | Annonaceae | Prickly custard apple | EPPO GD (2021) | |
| Annona reticulata | Annonaceae | Bullock's heart | CABI (2021) | |
| Annona squamosa | Annonaceae | Cachiman, Cuban sugar apple, sugar apple, sweetsop | EPPO GD (2021) | |
| Anthurium andraeanum | Araceae | Flamingo flower, flamingo lily, oilcloth flower, tail flower | EPPO GD (2021) | |
| Arachis hypogaea | Fabaceae | Groundnut, monkeynut, peanut | EPPO GD (2021) | |
| Aralia | Araliaceae | CABI (2021) | ||
| Artocarpus | Moraceae | Breadfruit trees | CABI (2021) | |
| Artocarpus altilis | Moraceae | Breadfruit | CABI (2021) | |
| Asparagus | Asparagaceae | CABI (2021) | ||
| Asparagus densiflorus | Liliaceae | Sprenger’s asparagus fern | Chong et al. (2015) | |
| Asparagus officinalis | Asparagaceae | Asparagus, garden asparagus, wild asparagus | EPPO GD (2021) | |
| Asparagus setaceus | Liliaceae | Asparagus fern | CABI (2021) | |
| Averrhoa carambola | Oxalidaceae | Caramba, carambola, Chinese gooseberry, country gooseberry, star fruit | EPPO GD (2021) | |
| Azadirachta indica | Meliaceae | Neem tree | CABI (2021) | |
| Basella alba | Basellaceae | Malabar spinach | García Morales et al. (2016) | |
| Bauhinia | Fabaceae | Camel's foot | CABI (2021) | |
| Bauhinia variegata | Fabaceae | Mountain ebony | CABI (2021) | |
| Begonia | Begoniaceae | Begonia | Chong et al. (2015) | |
| Beta | Chenopodiaceae | CABI (2021) | ||
| Beta vulgaris | Amaranthaceae | Beet | EPPO GD (2021) | |
| Bignonia | Bignoniaceae | CABI (2021) | ||
| Boehmeria | Urticaceae | CABI (2021) | ||
| Boehmeria nivea | Urticaceae | China grass, false nettle, ramie | EPPO GD (2021) | |
| Bougainvillea | Nyctaginaceae | CABI (2021) | ||
| Bougainvillea | Nyctaginaceae | Bougainvillea | Chong et al. (2015) | |
| Bougainvillea spectabilis | Nyctaginaceae | Great bougainvillea | Chong et al. (2015) | |
| Brassica oleracea | Brassicaceae | Cabbage, cauliflower | CABI (2021) | |
| Caesalpinia coriaria | Fabaceae | Divi‐divi | Chong et al. (2015) | |
| Caesalpinia pulcherrima | Fabaceae | Pride‐of‐Barbados | Chong et al. (2015) | |
| Cajanus cajan | Fabaceae | Bengal pea, cajan pea, Congo pea, dal, pigeon pea, red gram | EPPO GD (2021) | |
| Calliandra | Fabaceae | stick pea | Chong et al. (2015) | |
| Callistemon | Myrtaceae | Bottlebrush | Chong et al. (2015) | |
| Calostemma | Amatanthaceae | Wilcannia lily | Chong et al. (2015) | |
| Camaesyce (Euphorbia) hypericifolia | Euphorbiaceae | Graceful sandmat | Chong et al. (2015) | |
| Campsis (Tecoma) grandiflora | Bignoniaceae | Chinese trumpet vine | Chong et al. (2015) | |
| Cananga odorata | Annonaceae | Ilang‐ilang (kenanga) | Chong et al. (2015) | |
| Capsicum | Solanaceae | García Morales et al. (2016) | ||
| Capsicum annuum | Solanaceae | Bell pepper, chilli, paprika, red pepper, sweet pepper | EPPO GD (2021) | |
| Capsicum frutescens | Solanaceae | Bird chilli, bird pepper, cayenne pepper, chilli pepper, hot pepper | EPPO GD (2021) | |
| Carica papaya | Caricaceae | Papaw, papaya, pawpaw, tree melon | EPPO GD (2021) | |
| Carissa macrocarpa | Apocynaceae | Amatungulu (num‐num) | Chong et al. (2015) | |
| Cassia | Fabaceae | Cassia | Chong et al. (2015) | |
| Cassia javanica | Fabaceae | APPLE BLOSSOM (JAVA CASSIA) | Chong et al. (2015) | |
| Casuarina | Casuarinaceae | BEEFWOOD | CABI (2021) | |
| Catharanthus roseus | Apocynaceae | Madagascar periwinkle | Chong et al. (2015) | |
| Ceiba pentandra | Bombacaceae | Kapok | CABI (2021) | |
| Celosia argentea | Amatanthaceae | Cock’s comb | Chong et al. (2015) | |
| Centipede tongavine | Araceae | Chong et al. (2015) | ||
| Ceratonia | Fabaceae | CABI (2021) | ||
| Ceratonia siliqua | Fabaceae | Carob, carob tree, locust bean, locust tree, St John's bread | EPPO GD (2021) | |
| Cestrum nocturnum | Solanaceae | Night jessamine | Chong et al. (2015) | |
| Chrysanthemum | Asteraceae | Daisy | CABI (2021) | |
| Chrysanthemum coronarium | Asteraceae | Garland chrysanthemum | CABI (2021) | |
| Chrysothemis pulchella | Gesneriaceae | Squarestem | Chong et al. (2015) | |
| Cissus verticillata | Vitaceae | Possum grape vine | García Morales et al. (2016) | |
| Citrus | Rutaceae | EPPO GD (2021) | ||
| Citrus aurantiifolia | Rutaceae | Lime | CABI (2021) | |
| Citrus aurantium | Rutaceae | Bigarade, bitter orange, seville orange, sour orange | EPPO GD (2021) | |
| Citrus maxima | Rutaceae | Pummelo | CABI (2021) | |
| Citrus medica | Rutaceae | Citron | Chong et al. (2015) | |
| Citrus nobilis | Rutaceae | Tangor | Chong et al. (2015) | |
| Citrus paradisi | Rutaceae | Grapefruit, pomelo | EPPO GD (2021) | |
| Citrus reticulata | Rutaceae | Clementine, mandarin, tangerine | EPPO GD (2021) | |
| Citrus sinensis | Rutaceae | sweet orange | EPPO GD (2021) | |
| Clerodendrum aculeatum | Verbenaceae | Haggarbush | Chong et al. (2015) | |
| Clerodendrum infortunatum | Lamiaceae | CABI (2021) | ||
| Codiaeum | Euphorbiaceae | Codiaeum | Chong et al. (2015) | |
| Codiaeum variegatum | Euphorbiaceae | Garden croton | CABI (2021) | |
| Coffea | Rubiaceae | Coffee | CABI (2021) | |
| Coffea arabica | Rubiaceae | Arabian coffee | EPPO GD (2021) | |
| Coffea canephora | Rubiaceae | Congo coffee, robusta coffee | EPPO GD (2021) | |
| Colubrina arborescens | Rhamnaceae | Greenheart | Chong et al. (2015) | |
| Cordyline terminalis | Liliaceae | Ti plant, palm lily | Chong et al. (2015) | |
| Couroupita guianensis | Lecythidaceae | Cannonball tree | Chong et al. (2015) | |
| Crataegus | Rosaceae | Hawthorn | Chong et al. (2015) | |
| Crescentia cujete | Bignoniaceae | Calabash tree | Chong et al. (2015) | |
| Crotalaria | Fabaceae | CABI (2021) | ||
| Croton | Euphorbiaceae | Croton | Chong et al. (2015) | |
| Cucumis sativus | Cucurbitaceae | Cucumber, gherkin | EPPO GD (2021) | |
| Cucurbita | Cucurbitaceae | Pumpkin | CABI (2021) | |
| Cucurbita maxima | Cucurbitaceae | Giant pumpkin, marrow | EPPO GD (2021) | |
| Cucurbita moschata | Cucurbitaceae | Pumpkin | CABI (2021) | |
| Cucurbita pepo | Cucurbitaceae | Edible gourd, garden marrow, pumpkin, summer squash | EPPO GD (2021) | |
| Cydonia oblonga | Rosaceae | Quince | CABI (2021) | |
| Dahlia | Asteraceae | CABI (2021) | ||
| Delonix regia | Fabaceae | Flamboyant | CABI (2021) | |
| Dendrobium | Orchidaceae | Dendrobium orchid | Chong et al. (2015) | |
| Dieffenbachia | Araceae | Dieffenbachia | Chong et al. (2015) | |
| Dimocarpus longan | Sapindaceae | Longan | Chong et al. (2015) | |
| Diospyros kaki | Ebenaceae | Chinese date plum, Chinese persimmon, Japanese persimmon, kaki, persimmon | EPPO GD (2021) | |
| Dodonaea viscosa | Sapindaceae | Switch sorrel | CABI (2021) | |
| Dovyalis (Aberia) | Flacourtiaceae | Ceylon goose berry | Chong et al. (2015) | |
| Dracaena | Liliaceae | Dracaena (dragon tree) | Chong et al. (2015) | |
| Duranta | Verbenaceae | CABI (2021) | ||
| Duranta erecta | Verbenaceae | Golden dewdrops | Chong et al. (2015) | |
| Elaeagnus | Elaeagnaceae | Elaeagnus (oleaster) | Chong et al. (2015) | |
| Epipremnum pinnatum | Araceae | Centipede tonga vine | Chong et al. (2015) | |
| Eranthemum pulchellum | Acanthaeceae | Blue‐sage | Chong et al. (2015) | |
| Eriobotrya japonica | Rosaceae | Loquat | Chong et al. (2015) | |
| Eryngium foetidum | Apiaceae | Culantro, shadow beni, Mexican coriander | García Morales et al. (2016) | |
| Erythrina | Fabaceae | CABI (2021) | ||
| Erythrina corallodendron | Fabaceae | Coral erythrina | Chong et al. (2015) | |
| Erythrina crista‐galli | Fabaceae | Cry baby tree | Chong et al. (2015) | |
| Erythrina spp. | Fabaceae | CABI (2021) | ||
| Erythrina subumbrans | Fabaceae | December tree | CABI (2021) | |
| Erythrina variegata | Fabaceae | Flame tree, Indian coral tree, mountain ebony, tiger's claw | EPPO GD (2021) | |
| Euphorbia | Euphorbiaceae | Spurge | Chong et al. (2015) | |
| Euphorbia pulcherrima | Euphorbiaceae | Christmas flower, Christmas star, common poinsettia, lobster plant, Mexican flame‐leaf, painted leaf, poinsettia | EPPO GD (2021) | |
| Ficus | Moraceae | CABI (2021) | ||
| Ficus benghalensis | Moraceae | Banyan | CABI (2021) | |
| Ficus benjamina | Moraceae | Benjamin's fig, Java fig, small‐leaved rubber plant, tropical laurel, weeping fig, Benjamin tree | EPPO GD (2021) | |
| Ficus carica | Moraceae | Common fig, edible fig | EPPO GD (2021) | |
| Ficus elastica | Moraceae | Rubber plant | CABI (2021) | |
| Ficus laurifolia | Moraceae | CABI (2021) | ||
| Ficus obtusifolia | Moraceae | CABI (2021) | ||
| Ficus pertusa | Moraceae | CABI (2021) | ||
| Ficus platyphylla | Moraceae | CABI (2021) | ||
| Ficus pumila | Moraceae | Creeping fig | CABI (2021) | |
| Ficus racemosa | Moraceae | Cluster tree | CABI (2021) | |
| Ficus religiosa | Moraceae | Sacred fig tree | CABI (2021) | |
| Ficus semicordata | Moraceae | CABI (2021) | ||
| Flacourtis indica | Flacourtiaceae | Governor’s plum | Chong et al. (2015) | |
| Gerbera | Asteraceae | Gerbera | Chong et al. (2015) | |
| Glebionis coronaria | Asteraceae | Garland chrysanthemum, chrysanthemum greens, edible chrysanthemum | García Morales et al. (2016) | |
| Gliricidia sepium | Fabaceae | Gliricidia | CABI (2021) | |
| Glycine max | Fabaceae | Soybean | EPPO GD (2021) | |
| Glycosmis pentaphylla | Rutaceae | Orange berry, gin berry | García Morales et al. (2016) | |
| Cocos nucifera | Arecaceae | Common coconut palm | EPPO GD (2021) | |
| Colocasia | Araceae | CABI (2021) | ||
| Colocasia esculenta | Araceae | Chinese potato, cocoyam, dasheen, eddoe, Egyptian colocasia, elephant's‐ear, kalo, taro, wild taro, yam | EPPO GD (2021) | |
| Gossypium | Malvaceae | Cotton | CABI (2021) | |
| Gossypium arboreum | Malvaceae | Cotton, tree | CABI (2021) | |
| Gossypium herbaceum | Malvaceae | Short staple cotton | CABI (2021) | |
| Gossypium hirsutum | Malvaceae | American upland cotton, upland cotton | EPPO GD (2021) | |
| Grevillea | Proteaceae | CABI (2021) | ||
| Grevillea robusta | Proteaceae | Silk oak | Chong et al. (2015) | |
| Hamelia | Rubiaceae | Hamelia (firebush) | Chong et al. (2015) | |
| Helianthus annuus | Asteraceae | Common sunflower, sunflower | EPPO GD (2021) | |
| Hevea | Euphorbiaceae | García Morales et al. (2016) | ||
| Hevea brasiliensis | Euphorbiaceae | Brazilian rubber tree, para rubber, para rubber tree | EPPO GD (2021) | |
| Hibiscus boryanus | Malvaceae | García Morales et al. (2016) | ||
| Hibiscus | Malvaceae | Rose mallows | CABI (2021) | |
| Hibiscus acetosella | Malvaceae | African rosemallow | Chong et al. (2015) | |
| Hibiscus cannabinus | Malvaceae | Bombay hemp, Deccan hemp, kenaf | EPPO GD (2021) | |
| Hibiscus elatus | Malvaceae | Blue mahoe | CABI (2021) | |
| Hibiscus manihot | Malvaceae | Bele | CABI (2021) | |
| Hibiscus mutabilis | Malvaceae | Cotton rose | CABI (2021) | |
| Hibiscus rosa‐sinensis | Malvaceae | China rose, Chinese hibiscus, Chinese rose, Hawaiian hibiscus, rose mallow, rose of China, shoe‐black plant, shoe‐flower | EPPO GD (2021) | |
| Hibiscus sabdariffa | Malvaceae | Jamaica sorrel, red sorrel, roselle, tropical cranberry | EPPO GD (2021) | |
| Hibiscus schizopetalus | Malvaceae | Fringed hibiscus | CABI (2021) | |
| Hibiscus surattensis | Malvaceae | CABI (2021) | ||
| Hibiscus syriacus | Malvaceae | Shrubby althaea | CABI (2021) | |
| Hibiscus tiliaceus | Malvaceae | Coast hibiscus, hau tree, linden hibiscus, mahoe, mahoe tree, wild cotton tree | EPPO GD (2021) | |
| Holmskioldia sanguinea | Verbenaceae | Chinese hatplant | Chong et al. (2015) | |
| Jacaranda | Bignoniaceae | CABI (2021) | ||
| Jacaranda mimusifolia | Bignoniaceae | Black poui | Chong et al. (2015) | |
| Jasminum | Oleaceae | Jasmine | CABI (2021) | |
| Jasminum sambac | Oleaceae | Arabian jasmine | CABI (2021) | |
| Kalanchoe | Crassulaceae | Widow’s‐thrill | Chong et al. (2015) | |
| Kigelia | Bignoniaceae | Sausage tree | Chong et al. (2015) | |
| Lactuca sativa | Asteraceae | Garden lettuce, lettuce | EPPO GD (2021) | |
| Lagerstroemia speciosa | Lythraceae | Pride of India | Chong et al. (2015) | |
| Lantana | Verbenaceae | Lantana | Chong et al. (2015) | |
| Lantana camara | Verbenaceae | Lantana | CABI (2021) | |
| Leonotis | Lamiaceae | Lion’s ear | Chong et al. (2015) | |
| Manihot esculenta | Euphorbiaceae | Cassava, manioc, tapioca | EPPO GD (2021) | |
| Mangifera | Anacardiaceae | CABI (2021) | ||
| Mangifera indica | Anacardiaceae | Mango | EPPO GD (2021) | |
| Manilkara zapota | Sapotaceae | Bully tree, chapoti, chicle, chiku, marmalade plum, noseberry, sapodilla, sapodilla plum, sapota | EPPO GD (2021) | |
| Malpighia glabra | Malpighiaceae | Barbados cherry | EPPO GD (2021) | |
| Malus domestica | Rosaceae | Apple | EPPO GD (2021) | |
| Malus sylvestris | Rosaceae | Crab apple, wild apple, wild crab | EPPO GD (2021) | |
| Malvaviscus arboreus | Malvaceae | Wax mallow | CABI (2021) | |
| Medicago sativa | Fabaceae | Lucerne | CABI (2021) | |
| Melia azedarach | Meliaceae | Chinaberry tree | Chong et al. (2015) | |
| Melicocca bijugatus | Sapindaceae | Spanish lime | Chong et al. (2015) | |
| Mimosa | Fabaceae | Sensitive plants | CABI (2021) | |
| Mimosa caesalpiniifolia | Fabaceae | EPPO GD (2021) | ||
| Mimosa diplotricha | Fabaceae | Creeping‐sensitive plant | CABI (2021) | |
| Mimosa hostilis | Fabaceae | EPPO GD (2021) | ||
| Mimosa pigra | Fabaceae | Giant sensitive plant | CABI (2021) | |
| Mimosa pudica | Fabaceae | Sensitive plant | CABI (2021) | |
| Morus | Moraceae | Mulberry tree | CABI (2021) | |
| Morus alba | Moraceae | Silkworm mulberry, white mulberry | EPPO GD (2021) | |
| Morus nigra L. | Moraceae | Black mulberry | Chong et al. (2015) | |
| Murraya exotica | Rutaceae | Chinese box, orange jessamine | Chong et al. (2015) | |
| Murraya koenigii | Rutaceae | Curry leaf, karapincha | EPPO GD (2021) | |
| Murraya paniculata | Rutaceae | Orange jasmine, orange jessamine, china box, mock orange | García Morales et al. (2016) | |
| Musa | Musaceae | Banana | CABI (2021) | |
| Musa paradisiaca | Musaceae | Plantain | CABI (2021) | |
| Mussaenda | Rubiaceae | CABI (2021) | ||
| Myrtus communis | Myrtaceae | Myrtle | CABI (2021) | |
| Nephrolepis biserrata | Dryopteridaceae | Giant swordfern | Chong et al. (2015) | |
| Nephrolepis exaltata | Dryopteridaceae | Boston swordfern | Chong et al. (2015) | |
| Nerium oleander | Apocynaceae | Common oleander, oleander, rose bay | EPPO GD (2021) | |
| Pachystachys lutea | Acanthaeceae | Pachystachys, lollipop‐plant | Chong et al. (2015) | |
| Passiflora | Passifloraceae | Passionflower | CABI (2021) | |
| Passiflora caerulea | Passifloraceae | Bluecrown passionflower | Chong et al. (2015) | |
| Passiflora edulis | Passifloraceae | Passionfruit | CABI (2021) | |
| Passiflora quadrangularis | Passifloraceae | Giant granadilla | Chong et al. (2015) | |
| Pavonia | Malvaceae | Swampmallow | Chong et al. (2015) | |
| Peperomia pellucid | Piperaceae | Man‐to‐Man | Chong et al. (2015) | |
| Pereskia bleo | Cactaceae | Rose cactus | Chong et al. (2015) | |
| Persea americana | Lauraceae | Alligator pear, avocado, avocado pear, holly ghost pear | EPPO GD (2021) | |
| Petrea volubilis | Verbenaceae | Queen’s‐wreath | Chong et al. (2015) | |
| Phaseolus vulgaris | Fabaceae | Bush bean, climbing French bean, climbing kidney bean, field bean, flageolet bean, French bean, garden bean, green bean, haricot bean, kidney bean, pop bean, snap bean, string bean | EPPO GD (2021) | |
| Philodendron | Araceae | Philodendron | Chong et al. (2015) | |
| Phoenix dactylifera | Arecaceae | Common date palm, date palm | EPPO GD (2021) | |
| Phoenix sylvestris | Arecaceae | East Indian wine palm, silver date palm, wild date palm | EPPO GD (2021) | |
| Phyllanthus acidus | Euphorbiaceae | Tahitian gooseberry tree | Chong et al. (2015) | |
| Phyllanthus elsiae | Euphorbiaceae | CABI (2021) | ||
| Phyllanthus niruri | Euphorbiaceae | Seed‐under‐the‐leaf | CABI (2021) | |
| Plumbago auriculata | Plumbaginaceae | Cape leadwort | Chong et al. (2015) | |
| Portulaca grandiflora | Portulacaceae | Rose moss | CABI (2021) | |
| Portulaca oleracea | Portulacaceae | Common purslane, duckweed, little hogweed, pursley | García Morales et al. (2016) | |
| Portulaca pilosa | Portulacaceae | Kiss‐me‐quick, rimson‐flowered purslane, hairy pigweed, pink purslane, shaggy portulaca | García Morales et al. (2016) | |
| Prunus armeniaca | Rosaceae | Apricot | EPPO GD (2021) | |
| Prunus domestica | Rosaceae | European plum, garden plum, plum | EPPO GD (2021) | |
| Prunus persica | Rosaceae | Peach | EPPO GD (2021) | |
| Prunus salicina | Rosaceae | Japanese plum | CABI (2021) | |
| Psidium | Myrtaceae | Guava | CABI (2021) | |
| Psidium guajava | Myrtaceae | Common guava, guava, yellow guava | EPPO GD (2021) | |
| Punica granatum | Lythraceae | Pomegranate | EPPO GD (2021) | |
| Pyrus communis | Rosaceae | Common pear, pear | EPPO GD (2021) | |
| Quercus | Fagaceae | Oak | Chong et al. (2015) | |
| Rhododendron | Ericaceae | Azalea | CABI (2021) | |
| Ricinus communis | Euphorbiaceae | Castor‐oil plant, castor bean | EPPO GD (2021) | |
| Rivina humilis | Phytolacaceae | Rougeplant | Chong et al. (2015) | |
| Robinia pseudoacacia | Fabaceae | Black locust | CABI (2021) | |
| Rosa | Rosaceae | Rose | Chong et al. (2015) | |
| Russelia equisetiformis | Scrophulariaceae | Fountainbush | Chong et al. (2015) | |
| Saccharum officinarum | Poaceae | Sugarcane | CABI (2021) | |
| Salix | Salicaceae | Willows | CABI (2021) | |
| Schefflera | Araliaceae | Schefflera | Chong et al. (2015) | |
| Senna | Fabaceae | Senna | Chong et al. (2015) | |
| Senna siamea | Fabaceae | Yellow cassia | CABI (2021) | |
| Solanum aethiopicum | Solanaceae | African scarlet eggplant | CABI (2021) | |
| Solanum bicolor | Solanaceae | Chong et al. (2015) | ||
| Solanum lycopersicum | Solanaceae | Tomato | EPPO GD (2021) | |
| Solanum melongena | Solanaceae | Aubergine, eggplant | EPPO GD (2021) | |
| Spondias dulcis | Anacardiaceae | Otaheite apple | CABI (2021) | |
| Spondias purpurea | Anacardiaceae | Red mombin, purple mombin | CABI (2021) | |
| Stachytarpheta jamaicensis | Verbenaceae | Light‐blue snakeweed | Chong et al. (2015) | |
| Syngonium podophyllum | Araceae | American evergreen | Chong et al. (2015) | |
| Syzygium cumini | Myrtaceae | Black plum | CABI (2021) | |
| Syzygium malaccense | Myrtaceae | Malaysian apple | Chong et al. (2015) | |
| Tabebuia | Bignoniaceae | Trumpet‐tree | Chong et al. (2015) | |
| Tabebuia heterophylla | Bignoniaceae | Pink trumpet tree | CABI (2021) | |
| Tamarindus indica | Fabaceae | Tamarind | Chong et al. (2015) | |
| Tamarix | Tamaricaceae | Tamarisk | CABI (2021) | |
| Tecoma capensis | Bignoniaceae | Cape honeysuckle | Chong et al. (2015) | |
| Tecoma stans | Bignoniaceae | Yellow trumpetbush | Chong et al. (2015) | |
| Terminalia catappa | Combretaceae | Singapore almond | CABI (2021) | |
| Theobroma bicolor | Malvaceae | Bacao, Nicaraguan cocoa | EPPO GD (2021) | |
| Theobroma cacao | Malvaceae | Cacao, cocoa, common cacao, common cocoa | EPPO GD (2021) | |
| Theobroma grandiflorum | Malvaceae | Cupuassu | EPPO GD (2021) | |
| Thunbergia erecta | Acanthaeceae | Bush clockvine | Chong et al. (2015) | |
| Vinca minor | Apocynaceae | Common periwinkle, vinca | Chong et al. (2015) | |
| Vitis | Vitaceae | Rape | CABI (2021) | |
| Vitis vinifera | Vitaceae | Common grapevine, grapevine, European grape | EPPO GD (2021) | |
| Zea mays | Poaceae | Maize | CABI (2021) | |
| Ziziphus | Rhamnaceae | CABI (2021) | ||
| Ziziphus | Rhamnaceae | Jujube | Chong et al. (2015) | |
| Ziziphus jujuba | Rhamnaceae | Common jujube | CABI (2021) | |
| Ziziphus mauritiana | Rhamnaceae | Indian jujube | EPPO GD (2021) | |
| Ziziphus mucronata | Rhamnaceae | CABI (2021) | ||
| Ziziphus spina‐christi | Rhamnaceae | Christ's thorn jujube | CABI (2021) | |
| Wild weed hosts | Abutilon fruticosum | Malvaceae | Texas Indian mallow, pelotazo, sweet Indian mallow | García Morales et al. (2016) |
| Acacia | Fabaceae | Wattles | CABI (2021) | |
| Acacia acatlensis | Fabaceae | CABI (2021) | ||
| Acacia cochliacantha | Fabaceae | CABI (2021) | ||
| Acacia farnesiana | Fabaceae | Huisache | CABI (2021) | |
| Acacia hindsii | Fabaceae | CABI (2021) | ||
| Acacia nilotica | Fabaceae | Gum arabic tree | CABI (2021) | |
| Acalypha | Euphorbiaceae | Copperleaf | CABI (2021) | |
| Acalypha indica | Euphorbiaceae | Indian acalypha, Indian mercury, Indian copperleaf, Indian nettle, Three‐seeded mercury | García Morales et al. (2016) | |
| Acalypha wilkesiana | Euphorbiaceae | Copperleaf and Jacob’s coat | García Morales et al. (2016) | |
| Acanthus ilicifolius | Acanthaceae | Copperleaf | CABI (2021) | |
| Acharia | Limacodidae | CABI (2021) | ||
| Achyranthes aspera | Amaranthaceae | Devil's horsewhip | CABI (2021) | |
| Acokanthera | Apocynaceae | García Morales et al. (2016) | ||
| Aegiphila martinicensis | Lamiaceae | CABI (2021) | ||
| Albizia | Fabaceae | CABI (2021) | ||
| Albizia lebbeck | Fabaceae | Indian siris | CABI (2021) | |
| Angelica | Apiaceae | CABI (2021) | ||
| Anthurium | Araceae | CABI (2021) | ||
| Bauhinia forficata | Fabaceae | Brazilian orchid tree | García Morales et al. (2016) | |
| Bauhinia racemosa | Fabaceae | García Morales et al. (2016) | ||
| Bauhinia vahlii | Fabaceae | García Morales et al. (2016) | ||
| Biancaea decapetala | Fabaceae | Shoofly, Mauritius, Mysore thorn | García Morales et al. (2016) | |
| Bidens pilosa | Asteraceae | Beggar tick, bur marigold, butterfly needles | García Morales et al. (2016) | |
| Blighia sapida | Sapindaceae | Akee | Chong et al. (2015) | |
| Byttneria aculeata | Malvaceae | CABI (2021) | ||
| Calathea warszewiczii | Marantaceae | García Morales et al. (2016) | ||
| Calophyllum | Calophyllaceae | García Morales et al. (2016) | ||
| Carissa bispinosa | Apocynaceae | García Morales et al. (2016) | ||
| Cassia glauca | Fabaceae | García Morales et al. (2016) | ||
| Cassia renigera | Fabaceae | García Morales et al. (2016) | ||
| Cedrela odorata | Meliaceae | Spanish cedar | CABI (2021) | |
| Centrolobium paraense | Fabaceae | EPPO GD (2021) | ||
| Chenopodium album | Amaranthaceae | Goosefoot, green pigweed, lamb's quarters, wild spinach, fat‐hen, white goosefoot, pigweed | EPPO GD (2021) | |
| Clitoria ternatea | Fabaceae | Butterfly‐pea | CABI (2021) | |
| Coccoloba uvifera | Polygonaceae | Jamaica kino, platter leaf, sea grape, common sea grape | EPPO GD (2021) | |
| Combretum | Combretaceae | García Morales et al. (2016) | ||
| Corchorus | Tiliaceae | Jutes | CABI (2021) | |
| Corchorus capsularis | Tiliaceae | White jute | CABI (2021) | |
| Corchorus olitorius | Tiliaceae | Jute | CABI (2021) | |
| Cordia curassavica | Boraginaceae | Black sage or wild sage | García Morales et al. (2016) | |
| Cordia dichotoma | Boraginaceae | Indian cherry | CABI (2021) | |
| Cordyline fruticosa | Asparagaceae | Bongbush, cabbage palm, kiwi, palm lily, ti‐palm | García Morales et al. (2016) | |
| Cosmos | Asteraceae | EPPO GD (2021) | ||
| Crotalaria micans | Fabaceae | CABI (2021) | ||
| Croton flavens | Euphorbiaceae | García Morales et al. (2016) | ||
| Cyperus | Cyperaceae | García Morales et al. (2016) | ||
| Dalbergia | Fabaceae | Rosewoods | CABI (2021) | |
| Datura | Solanaceae | Jimsonweed (angel trumpet) | Chong et al. (2015) | |
| Daucus carota | Apiaceae | Queen Anne’s lace | Chong et al. (2015) | |
| Desmanthus virgatus | Fabaceae | False tamarind | CABI (2021) | |
| Dioscorea | Dioscoreaceae | García Morales et al. (2016) | ||
| Emilia | Asteraceae | García Morales et al. (2016) | ||
| Enterolobium | Fabaceae | CABI (2021) | ||
| Enterolobium cyclocarpum | Fabaceae | Ear pod tree | CABI (2021) | |
| Epipremnum aureum | Araceae | Golden pothos, Ceylon creeper, Hunter's robe, ivy arum | García Morales et al. (2016) | |
| Erythrina resinifera | Fabaceae | García Morales et al. (2016) | ||
| Erythrina speciosa | Fabaceae | García Morales et al. (2016) | ||
| Erythrina vespertilio | Fabaceae | García Morales et al. (2016) | ||
| Eugenia uniflora | Myrtaceae | Surinam cherry | CABI (2021) | |
| Euphorbia atoto | CABI (2021) | |||
| Euphorbia hypericifolia | Euphorbiaceae | Graceful spurge, golden spurge, and chickenweed | García Morales et al. (2016) | |
| Ficus amplissima | Moraceae | Indian Bat tree, Indian Bat fig, Pimpri | García Morales et al. (2016) | |
| Ficus lacor | Moraceae | García Morales et al. (2016) | ||
| Flacourtia indica | Flacourtiaceae | Governor's plum | García Morales et al. (2016) | |
| Gliricidia | Fabaceae | CABI (2021) | ||
| Gliricidia maculata | Fabaceae | CABI (2021) | ||
| Grevillea robusta | Proteaceae | Australian silky oak, silk oak, silk‐bark oak, silky oak | EPPO GD (2021) | |
| Grewia | Tiliaceae | CABI (2021) | ||
| Guazuma ulmifolia | Sterculiaceae | Bastard cedar | CABI (2021) | |
| Gymnanthemum urticifolium | Asteraceae | García Morales et al. (2016) | ||
| Haldina cordifolia | Rubiaceae | Heart‐leaf adina | García Morales et al. (2016) | |
| Heliconia | Heliconiaceae | EPPO GD (2021) | ||
| Hoya carnosa | Asclepiadaceae | Wax plant | CABI (2021) | |
| Inga | Fabaceae | García Morales et al. (2016) | ||
| Inga edulis | Fabaceae | Food inga, icecream bean, St John's bread | EPPO GD (2021) | |
| Inga ingoides | Fabaceae | CABI (2021) | ||
| Inga vera | Fabaceae | CABI (2021) | ||
| Ipomoea | Convolvulaceae | Morning glory | CABI (2021) | |
| Ipomoea batatas | Convolvulaceae | Sweet potato | EPPO GD (2021) | |
| Ixora | Rubiaceae | EPPO GD (2021) | ||
| Ixora chinensis | Rubiaceae | Flame of the woods, jungle flame, jungle geranium | EPPO GD (2021) | |
| Jatropha curcas | Euphorbiaceae | Barbados nut, purging nut, physic nut | EPPO GD (2021) | |
| Laportea aestuans | Urticaceae | West Indian woodnettle | García Morales et al. (2016) | |
| Lawsonia | Lythraceae | García Morales et al. (2016) | ||
| Lawsonia inermis | Lythraceae | Egyptian privet | CABI (2021) | |
| Leonotis nepetifolia | Lamiaceae | Christmas candlestick | García Morales et al. (2016) | |
| Leucaena | Fabaceae | CABI (2021) | ||
| Leucaena leucocephala | Fabaceae | Leucaena | CABI (2021) | |
| Lithocarpus | Fagaceae | Stone oak | Chong et al. (2015) | |
| Macaranga | Euphorbiaceae | CABI (2021) | ||
| Malachra alceifolia | Malvaceae | CABI (2021) | ||
| Malpighia | Malpighiaceae | CABI (2021) | ||
| Malpighia emarginata | Malpighiaceae | CABI (2021) | ||
| Malvaviscus conzattii | Malvaceae | CABI (2021) | ||
| Miconia cornifolia | Melastomataceae | García Morales et al. (2016) | ||
| Mikania cordata | Asteraceae | García Morales et al. (2016) | ||
| Mimosa tenuiflora | Fabaceae | García Morales et al. (2016) | ||
| Momordica charantia | Cucurbitaceae | Bitter gourd | CABI (2021) | |
| Montanoa grandiflora | Asteraceae | CABI (2021) | ||
| Mussaenda erythrophylla | Rubiaceae | Ashanti blood, red flag bush, red flag mussaenda | EPPO GD (2021) | |
| Nephelium lappaceum | Sapindaceae | Rambutan | EPPO GD (2021) | |
| Nerium indicum | Apocynaceae | García Morales et al. (2016) | ||
| Opuntia | Cactaceae | EPPO GD (2021) | ||
| Paritium | Malvaceae | García Morales et al. (2016) | ||
| Parkinsonia aculeata | Fabaceae | Mexican palo‐verde | CABI (2021) | |
| Parthenium hysterophorus | Asteraceae | Parthenium weed | CABI (2021) | |
| Persea | Lauraceae | CABI (2021) | ||
| Petiveria alliacea | Phytolaccaceae | García Morales et al. (2016) | ||
| Phyllanthus amarus | Euphorbiaceae | Gale of the wind, carry me seed, seed on the leaf | García Morales et al. (2016) | |
| Phyllanthus urinaria | Euphorbiaceae | Leafflower | García Morales et al. (2016) | |
| Piper tuberculatum | Piperaceae | García Morales et al. (2016) | ||
| Pithecellobium | Fabaceae | CABI (2021) | ||
| Pithecellobium caribaeum | Fabaceae | García Morales et al. (2016) | ||
| Plerandra elegantissima | Araliaceae | False aralia | ||
| Prosopis | Fabaceae | CABI (2021) | ||
| Prosopis cineraria | Fabaceae | Screw‐bean | CABI (2021) | |
| Prosopis laevigata | Fabaceae | CABI (2021) | ||
| Quisqualis | Combretaceae | CABI (2021) | ||
| Rosa obtusifolia | Rosaceae | García Morales et al. (2016) | ||
| Samanea saman | Fabaceae | Rain tree | CABI (2021) | |
| Schefflera actinophylla | Araliaceae | Octopus tree, Queensland umbrella tree, star leaf, umbrella tree | EPPO GD (2021) | |
| Schefflera pueckleri | Araliaceae | Mallet flower | García Morales et al. (2016) | |
| Schinus molle | Anacardiaceae | False pepper tree | García Morales et al. (2016) | |
| Schinus terebinthifolia | Anacardiaceae | Brazilian pepper tree | García Morales et al. (2016) | |
| Scoparia dulcis | Plantaginaceae | Licorice weed, goat weed, scoparia‐weed, sweet‐broom | García Morales et al. (2016) | |
| Senna italica | Fabaceae | Senegal senna | García Morales et al. (2016) | |
| Senna obtusifolia | Fabaceae | Sicklepod | García Morales et al. (2016) | |
| Senna polyphylla | Fabaceae | García Morales et al. (2016) | ||
| Senna sulfurea | Fabaceae | García Morales et al. (2016) | ||
| Senna surattensis | Fabaceae | Golden senna, foetid cassia, glaucous cassia, glossy shower | García Morales et al. (2016) | |
| Sesbania sesban | Fabaceae | Sesban, common sesban, Egyptian pea, Egyptian rattle pod | García Morales et al. (2016) | |
| Sida acuta | Malvaceae | Sida | CABI (2021) | |
| Solandra | Solanaceae | CABI (2021) | ||
| Solanum americanum | Solanaceae | Eastern black nightshade, glossy nightshade, West Indian nightshade, American black nightshade | EPPO GD (2021) | |
| Solanum donianum | Solanaceae | García Morales et al. (2016) | ||
| Solanum umbellatum | Solanaceae | CABI (2021) | ||
| Spondias | Anacardiaceae | Purple mombin | CABI (2021) | |
| Spondias mombin | Anacardiaceae | Golden apple, hog‐plum tree, yellow mombin | EPPO GD (2021) | |
| Spondias tuberosa | Anacardiaceae | Imbu | EPPO GD (2021) | |
| Synedrella nodiflora | Asteraceae | Synedrella | García Morales et al. (2016) | |
| Syzygium aqueum | Myrtaceae | Water apple | García Morales et al. (2016) | |
| Syzygium aromaticum | Myrtaceae | Clove | CABI (2021) | |
| Tabernaemontana divaricata | Apocynaceae | García Morales et al. (2016) | ||
| Talinum paniculatum | Talinaceae | Fame flower, Jewels‐of‐Opar, pink baby‐breath | EPPO GD (2021) | |
| Talipariti elatum | Malvaceae | Blue mahoe | García Morales et al. (2016) | |
| Tamarindus | Fabaceae | CABI (2021) | ||
| Tectona grandis | Lamiaceae | Common teak | EPPO GD (2021) | |
| Templetonia | Fabaceae | García Morales et al. (2016) | ||
| Tephrosia | Fabaceae | Hoary‐pea | CABI (2021) | |
| Teramnus labialis | Fabaceae | Blue wiss | CABI (2021) | |
| Terminalia | Combretaceae | García Morales et al. (2016) | ||
| Terminalia mantaly | Combretaceae | CABI (2021) | ||
| Terminalia neotaliala | Combretaceae | Madagascar almond tree | García Morales et al. (2016) | |
| Tetracera | Dilleniaceae | CABI (2021) | ||
| Theobroma speciosum | Malvaceae | EPPO GD (2021) | ||
| Thespesia | Malvaceae | CABI (2021) | ||
| Thespesia lampas | Malvaceae | CABI (2021) | ||
| Thespesia populnea | Malvaceae | Portia tree | CABI (2021) | |
| Tithonia diversifolia | Asteraceae | Mexican sunflower | CABI (2021) | |
| Tradescantia | Commelinaceae | García Morales et al. (2016) | ||
| Trema micrantha | Cannabaceae | Jamaican nettle tree, capulin | García Morales et al. (2016) | |
| Vachellia nilotica | Fabaceae | Gum arabic tree, babul, thorn mimosa, Egyptian acacia, thorny acacia | García Morales et al. (2016) | |
| Verbesina fastigiata | Asteraceae | CABI (2021) | ||
| Viburnum odoratissimum | Caprifoliaceae | Sweet viburnum | García Morales et al. (2016) | |
| Vigna mungo | Fabaceae | Black gram | García Morales et al. (2016) | |
| Vigna unguiculata | Fabaceae | Cowpea | García Morales et al. (2016) | |
| Volkameria aculeata | Lamiaceae | García Morales et al. (2016) | ||
| Xanthosoma | Araceae | Cocoyam | CABI (2021) | |
| Zinnia | Asteraceae | CABI (2021) |
Appendix B – Distribution of Maconellicoccus hirsutus
Distribution records based on EPPO Global Database (EPPO, online) and García Morales et al. (2016).
| Region | Country | Sub‐national (e.g. State) | Status | Reference |
|---|---|---|---|---|
| North America | Mexico | Present, restricted distribution | EPPO GD (2021) | |
| USA | Present, restricted distribution | EPPO GD (2021) | ||
| USA | Alabama | Present, no details | EPPO GD (2021) | |
| USA | California | Present, restricted distribution | EPPO GD (2021) | |
| USA | Florida | Present, few occurrences | EPPO GD (2021) | |
| USA | Georgia | Present, few occurrences | EPPO GD (2021) | |
| USA | Louisiana | Present, no details | EPPO GD (2021) | |
| USA | New York | Present, no details | EPPO GD (2021) | |
| USA | North Carolina | Present, no details | EPPO GD (2021) | |
| USA | Oklahoma | Present, no details | EPPO GD (2021) | |
| USA | South Carolina | Present, no details | EPPO GD (2021) | |
| USA | Tennessee | Present, no details | EPPO GD (2021) | |
| USA | Texas | Present, no details | García Morales et al. (2016) | |
| Central America | Belize | Present, no details | EPPO GD (2021) | |
| Costa Rica | Present, restricted distribution | EPPO GD (2021) | ||
| Guatemala | Absent, unreliable record | EPPO GD (2021) | ||
| Nicaragua | Present, restricted distribution | EPPO GD (2021) | ||
| Caribbean | Anguilla | Absent, unreliable record | EPPO GD (2021) | |
| Antigua and Barbuda | Present, no details | EPPO GD (2021) | ||
| Aruba | Present, no details | EPPO GD (2021) | ||
| Bahamas | Present, no details | EPPO GD (2021) | ||
| Barbados | Present, no details | EPPO GD (2021) | ||
| Cayman Islands | Present, no details | EPPO GD (2021) | ||
| Cuba | Present, no details | García Morales et al. (2016) | ||
| Dominica | Present, no details | EPPO GD (2021) | ||
| Dominican Republic | Absent, unreliable record | EPPO GD (2021) | ||
| Grenada | Present, restricted distribution | EPPO GD (2021) | ||
| Guadeloupe | Present, no details | EPPO GD (2021) | ||
| Haiti | Present, widespread | EPPO GD (2021) | ||
| Jamaica | Present, few occurrences | EPPO GD (2021) | ||
| Martinique | Present, no details | EPPO GD (2021) | ||
| Montserrat | Present, no details | EPPO GD (2021) | ||
| Netherlands Antilles | Present, no details | EPPO GD (2021) | ||
| Puerto Rico | Present, no details | EPPO GD (2021) | ||
| Saint Lucia | Present, no details | EPPO GD (2021) | ||
| St Kitts‐Nevis | Present, no details | EPPO GD (2021) | ||
| St Vincent and the Grenadines | Present, no details | EPPO GD (2021) | ||
| Saint Barthelemy | Present, no details | García Morales et al. (2016) | ||
| Saint Martin | Present, no details | García Morales et al. (2016) | ||
| Trinidad and Tobago | Present, no details | EPPO GD (2021) | ||
| Virgin Islands (British) | Present, no details | EPPO GD (2021) | ||
| Virgin Islands (US) | Present, no details | EPPO GD (2021) | ||
| South America | Brazil | Present, restricted distribution | EPPO GD (2021) | |
| Brazil | Alagoas | Present, no details | EPPO GD (2021) | |
| Brazil | Bahia | Present, restricted distribution | EPPO GD (2021) | |
| Brazil | Espirito Santo | Present, restricted distribution | EPPO GD (2021) | |
| Brazil | Maranhao | Present, no details | EPPO GD (2021) | |
| Brazil | Mato Grosso | Present, no details | EPPO GD (2021) | |
| Brazil | Para | Present, no details | EPPO GD (2021) | |
| Brazil | Pernambuco | Present, no details | EPPO GD (2021) | |
| Brazil | Rio Grande do Sul | Present, no details | EPPO GD (2021) | |
| Brazil | Roraima | Present, restricted distribution | EPPO GD (2021) | |
| Brazil | Santa Catarina | Present, no details | EPPO GD (2021) | |
| Brazil | Sao Paulo | Present, no details | EPPO GD (2021) | |
| Colombia | Present, restricted distribution | EPPO GD (2021) | ||
| French Guiana | Present, no details | EPPO GD (2021) | ||
| Guyana | Present, widespread | EPPO GD (2021) | ||
| Suriname | Present, restricted distribution | EPPO GD (2021) | ||
| Venezuela | Present, no details | EPPO GD (2021) | ||
| EU (27) | Cyprus | Present, widespread | EPPO GD (2021) | |
| Greece | Present, restricted distribution | EPPO GD (2021) | ||
| Africa | Algeria | Absent, invalid record | EPPO GD (2021) | |
| Benin | Present, no details | EPPO GD (2021) | ||
| Burkina Faso | Present, no details | EPPO GD (2021) | ||
| Cameroon | Present, no details | EPPO GD (2021) | ||
| Central African Republic | Present, no details | EPPO GD (2021) | ||
| Chad | Present, no details | EPPO GD (2021) | ||
| Congo | Present, no details | EPPO GD (2021) | ||
| Congo, Democratic republic of the | Present, no details | EPPO GD (2021) | ||
| Cote d'Ivoire | Present, no details | EPPO GD (2021) | ||
| Egypt | Present, no details | EPPO GD (2021) | ||
| Gabon | Present, no details | EPPO GD (2021) | ||
| Gambia | Present, no details | EPPO GD (2021) | ||
| Kenya | Present, no details | EPPO GD (2021) | ||
| Liberia | Present, no details | EPPO GD (2021) | ||
| Niger | Present, no details | EPPO GD (2021) | ||
| Nigeria | Present, no details | EPPO GD (2021) | ||
| Reunion | Present, no details | EPPO GD (2021) | ||
| Senegal | Present, no details | EPPO GD (2021) | ||
| Seychelles | Present, no details | EPPO GD (2021) | ||
| Socotra Island | Present, no details | García Morales et al. (2016) | ||
| Somalia | Present, no details | EPPO GD (2021) | ||
| Sudan | Present, no details | EPPO GD (2021) | ||
| Tanzania | Present, no details | EPPO GD (2021) | ||
| Tunisia | Present, restricted distribution | EPPO GD (2021) | ||
| Zambia | Absent, invalid record | EPPO GD (2021) | ||
| Zaire | Present, no details | García Morales et al. (2016) | ||
| Zanzibar | Present, no details | García Morales et al. (2016) | ||
| Asia | Bali | Present, no details | García Morales et al. (2016) | |
| Bangladesh | Present, no details | EPPO GD (2021) | ||
| Brunei Darussalam | Present, no details | EPPO GD (2021) | ||
| Cambodia | Present, no details | EPPO GD (2021) | ||
| China | Present, restricted distribution | EPPO GD (2021) | ||
| China | Aomen (Macau) | Present, no details | EPPO GD (2021) | |
| China | Guangdong | Present, no details | EPPO GD (2021) | |
| China | Guangxi | Present, no details | García Morales et al. (2016) | |
| China | Shanxi | Present, no details | EPPO GD (2021) | |
| China | Xianggang (Hong Kong) | Present, no details | EPPO GD (2021) | |
| China | Xizhang | Present, no details | EPPO GD (2021) | |
| China | Yunnan | Present, no details | EPPO GD (2021) | |
| China | Hong Kong | Present, no details | García Morales et al. (2016) | |
| India | Present, widespread | EPPO GD (2021) | ||
| India | Andaman and Nicobar Islands | Present, no details | EPPO GD (2021) | |
| India | Andhra Pradesh | Present, no details | EPPO GD (2021) | |
| India | Assam | Present, no details | EPPO GD (2021) | |
| India | Bihar | Present, no details | EPPO GD (2021) | |
| India | Delhi | Present, no details | EPPO GD (2021) | |
| India | Gujarat | Present, no details | EPPO GD (2021) | |
| India | Karnataka | Present, no details | EPPO GD (2021) | |
| India | Kerala | Present, no details | EPPO GD (2021) | |
| India | Madhya Pradesh | Present, no details | EPPO GD (2021) | |
| India | Maharashtra | Present, no details | EPPO GD (2021) | |
| India | Odisha | Present, no details | EPPO GD (2021) | |
| India | Punjab | Present, no details | EPPO GD (2021) | |
| India | Tamil Nadu | Present, no details | EPPO GD (2021) | |
| India | Telangana | Present, no details | EPPO GD (2021) | |
| India | Tripura | Present, no details | EPPO GD (2021) | |
| India | Uttar Pradesh | Present, no details | EPPO GD (2021) | |
| India | West Bengal | Present, no details | EPPO GD (2021) | |
| Indonesia | Present, widespread | EPPO GD (2021) | ||
| Indonesia | Flores | Present, no details | García Morales et al. (2016) | |
| Indonesia | Irian Jaya | Present, no details | EPPO GD (2021) | |
| Indonesia | Java | Present, no details | EPPO GD (2021) | |
| Indonesia | Lombok | Present, no details | García Morales et al. (2016) | |
| Indonesia | Nusa Tenggara | Present, no details | EPPO GD (2021) | |
| Indonesia | Sulawesi | Present, no details | EPPO GD (2021) | |
| Indonesia | Sumatra | Present, no details | EPPO GD (2021) | |
| Iran | Present, no details | EPPO GD (2021) | ||
| Israel | Present, few occurrences | EPPO GD (2021) | ||
| Japan | Present, restricted distribution | EPPO GD (2021) | ||
| Japan | Ryukyu Archipelago | Present, no details | EPPO GD (2021) | |
| Jordan | Present, no details | EPPO GD (2021) | ||
| Laos | Present, no details | EPPO GD (2021) | ||
| Lebanon | Present, no details | EPPO GD (2021) | ||
| Malaya | Present, no details | García Morales et al. (2016) | ||
| Malaysia | Present, no details | EPPO GD (2021) | ||
| Malaysia | West | Present, no details | EPPO GD (2021) | |
| Maldives | Present, no details | EPPO GD (2021) | ||
| Myanmar | Present, no details | EPPO GD (2021) | ||
| Nepal | Present, no details | EPPO GD (2021) | ||
| Oman | Present, no details | EPPO GD (2021) | ||
| Pakistan | Present, no details | EPPO GD (2021) | ||
| Philippines | Present, no details | EPPO GD (2021) | ||
| Saudi Arabia | Present, no details | EPPO GD (2021) | ||
| Singapore | Present, no details | EPPO GD (2021) | ||
| Sri Lanka | Present, no details | EPPO GD (2021) | ||
| Taiwan | Present, no details | EPPO GD (2021) | ||
| Thailand | Present, no details | EPPO GD (2021) | ||
| Turkey | Present, no details | EPPO GD (2021) | ||
| United Arab Emirates | Present, no details | EPPO GD (2021) | ||
| Vietnam | Present, no details | EPPO GD (2021) | ||
| Yemen | Present, no details | EPPO GD (2021) | ||
| Oceania | Australia | Present, no details | EPPO GD (2021) | |
| Australia | Northern Territory | Present, no details | EPPO GD (2021) | |
| Australia | Queensland | Present, no details | EPPO GD (2021) | |
| Australia | South Australia | Present, no details | EPPO GD (2021) | |
| Australia | Western Australia | Present, no details | EPPO GD (2021) | |
| Fiji | Present, no details | EPPO GD (2021) | ||
| Guam | Present, no details | EPPO GD (2021) | ||
| Micronesia | Present, restricted distribution | EPPO GD (2021) | ||
| New Caledonia | Present, no details | EPPO GD (2021) | ||
| Northern Mariana Islands | Present, no details | EPPO GD (2021) | ||
| Palau | Present, no details | EPPO GD (2021) | ||
| Papua New Guinea | Present, no details | EPPO GD (2021) | ||
| Samoa | Present, no details | EPPO GD (2021) | ||
| Solomon Islands | Present, no details | EPPO GD (2021) | ||
| Tonga | Present, no details | EPPO GD (2021) | ||
| Tuvalu | Present, no details | EPPO GD (2021) | ||
| USA | Hawaii | Present, no details | EPPO GD (2021) | |
| Vanuatu | Present, no details | EPPO GD (2021) |
Appendix C – Import data
Table C.1: Fresh or dried citrus (CN code: 0805) imported in 100 kg into the EU (27) from regions where Maconellicoccus hirsutus is known to occur (Source: Eurostat accessed on 22/9/2021)
| COUNTRY | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Australia | 3,279.84 | 1,284.38 | 644.97 | 10,645.40 | 2,733.47 |
| Bangladesh | 227.61 | 229.58 | 159.67 | 322.42 | 1,183.66 |
| Brazil | 864,863.09 | 903,432.95 | 900,907.24 | 822,134.46 | 902,354.68 |
| Burkina Faso | 78.14 | 148.57 | 103.95 | 38.95 | 53.52 |
| Cameroon | 10.48 | 0.20 | |||
| China | 827,840.57 | 1,084,857.27 | 1,024,163.15 | 1,108,595.22 | 1,098,691.70 |
| Colombia | 44,825.37 | 79,400.99 | 123,887.46 | 136,914.85 | 172,413.40 |
| Costa Rica | 4,700.31 | 921.32 | 704.93 | 231.20 | 461.60 |
| Cuba | 7,165.74 | 3,863.97 | 4,438.14 | 3,422.11 | 556.03 |
| Dominica | 865.67 | 193.34 | 57.65 | 76.50 | 78.69 |
| Egypt | 1,931,586.64 | 2,246,998.88 | 2,643,272.02 | 2,206,932.71 | 2,850,742.72 |
| Guyana | 24.00 | ||||
| Haiti | 207.41 | 176.53 | 72.10 | 31.00 | 248.29 |
| Hong Kong | 0.00 | 2.27 | 1.00 | ||
| India | 246.80 | 1.00 | 449.63 | 88.51 | 254.95 |
| Indonesia | 566.73 | 555.70 | 779.35 | 836.73 | 864.54 |
| Iran | 1,533.22 | 1,218.52 | 1,208.01 | 2,174.22 | 1,882.74 |
| Jamaica | 3,633.97 | 3,325.11 | 675.68 | 2,409.55 | 1,646.87 |
| Israel | 799,118.49 | 969,403.62 | 824,601.66 | 812,738.57 | 878,713.15 |
| Jordan | 1.17 | 0.00 | 3.79 | 1.40 | 11.80 |
| Japan | 352.58 | 417.44 | 270.73 | 319.24 | 162.50 |
| Kenya | 8.80 | 34.56 | |||
| Laos | 51.94 | 2.10 | 20.23 | ||
| Lebanon | 503.21 | 1,504.91 | 7.46 | 7.28 | 3.19 |
| Malaysia | 4.18 | 39.02 | 83.45 | 7.71 | |
| Mexico | 570,402.80 | 553,818.66 | 589,021.12 | 443,743.54 | 349,628.56 |
| Nepal | 1,170.00 | ||||
| Nigeria | 0.03 | 0.10 | 200.00 | ||
| Pakistan | 2.45 | 0.59 | |||
| Philippines | 0.20 | 7.71 | 0.10 | ||
| Somalia | 490.30 | 193.21 | 367.52 | 514.30 | 342.10 |
| Sudan | 2.10 | 20.58 | |||
| Taiwan | 157.49 | 0.01 | |||
| Tanzania | 179.90 | 190.01 | 144.12 | 35.95 | 75.50 |
| Thailand | 426.42 | 1,283.13 | 659.74 | 624.93 | 194.87 |
| Tunisia | 175,010.90 | 172,515.76 | 125,258.30 | 133,950.15 | 75,620.02 |
| Turkey | 2,569,671.58 | 2,026,980.05 | 3,149,386.85 | 2,102,077.48 | 2,573,806.18 |
| United States | 301,229.06 | 231,210.47 | 185,706.99 | 177,755.45 | 148,845.72 |
| Venezuela | 744.08 | 2,216.36 | 681.07 | ||
| Viet Nam | 28,649.46 | 46,738.17 | 70,934.07 | 73,964.35 | 63,730.13 |
Table C.2: Cotton linters (CN code: 140420) imported in 100 kg into the EU (27) from regions where Maconellicoccus hirsutus is known to occur (Source: Eurostat accessed on 22/9/2021)
| COUNTRY | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Benin | 400.00 | 294.95 | 608.38 | 132.94 | 87.99 |
| Brazil | 13,493.54 | 57,840.63 | 68,605.72 | 50,783.56 | 57,176.03 |
| China | 1,530.80 | 10.00 | 44.83 | 102.75 | 188.29 |
| Egypt | 1.47 | ||||
| India | 1,136.10 | 589.38 | 487.65 | 735.71 | 2,148.17 |
| Indonesia | 27.55 | 5.38 | |||
| Iran | 3.93 | ||||
| Turkey | 40,881.83 | 115,022.78 | 88,098.66 | 82,852.55 | 81,157.09 |
| United States | 56,181.45 | 32,472.85 | 16,629.25 | 7,933.06 | 19,150.08 |
| Viet Nam | 0.21 | 0.34 |
Table C.3: Fresh or dried bananas (CN code: 0803) imported in 100 kg into the EU (27) from regions where Maconellicoccus hirsutus is known to occur (Source: Eurostat accessed on 22/9/2021)
| COUNTRY | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Bangladesh | 174.66 | 79.85 | 72.75 | 38.05 | 35.64 |
| Brazil | 149,108.03 | 26,855.08 | 59,677.31 | 104,909.74 | 98,434.39 |
| Cameroon | 2,521,882.41 | 2,341,539.74 | 1,791,447.01 | 1,520,648.04 | 1,579,456.86 |
| China | 252.64 | 188.73 | 390.56 | 545.74 | 854.93 |
| Colombia | 10,120,590.13 | 11,594,479.46 | 11,282,545.88 | 11,524,355.75 | 12,193,049.39 |
| Costa Rica | 9,662,138.79 | 9,663,219.69 | 10,125,330.57 | 9,405,488.40 | 10,342,372.80 |
| Cuba | 1.28 | ||||
| Egypt | 42.98 | 0.18 | 146.87 | ||
| India | 515.19 | 445.99 | 571.13 | 607.74 | 1,418.91 |
| Indonesia | 0.01 | 37.27 | 14.72 | 64.17 | |
| Iran | 0.09 | 2.86 | 12.33 | ||
| Israel | 2.10 | 0.75 | |||
| Kenya | 1.90 | 0.72 | 6.15 | 11.23 | 14.95 |
| Malaysia | 8.02 | ||||
| Mexico | 516,367.97 | 558,896.47 | 348,905.62 | 239,173.11 | 141,492.42 |
| Nigeria | 0.72 | 2.04 | 2.50 | 0.84 | 6.35 |
| Pakistan | 2.60 | 49.70 | |||
| Philippines | 2,480.90 | 11,415.47 | 1,674.92 | 2,160.35 | 1,240.80 |
| Saudi Arabia | 5.00 | ||||
| Singapore | 0.06 | 0.12 | |||
| Taiwan | 0.15 | ||||
| Tanzania | 28.02 | 11.93 | 33.68 | 34.24 | 34.74 |
| Thailand | 550.44 | 674.34 | 603.32 | 526.15 | 334.58 |
| Turkey | 202.06 | 210.60 | 0.14 | ||
| United States | 7.00 | 6.37 | 1.54 | 6.32 | 10.37 |
| Viet Nam | 276.26 | 178.84 | 190.96 | 210.11 | 142.71 |
| Zambia | 0.72 |
Table C.4: Fresh grapes (CN code: 080610) imported in 100 kg into the EU (27) from regions where Maconellicoccus hirsutus is known to occur (Source: Eurostat accessed on 22/9/2021)
| COUNTRY | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Australia | 2.95 | 0.50 | |||
| Bangladesh | 1.05 | 0.50 | |||
| Brazil | 194,152.79 | 249,279.81 | 271,987.56 | 196,465.22 | 228,095.15 |
| China | 0.00 | 6.00 | 0.03 | ||
| Colombia | 0.00 | 381.30 | 669.12 | 186.96 | |
| Egypt | 330,565.57 | 404,801.23 | 429,994.87 | 442,798.85 | 462,890.07 |
| India | 640,933.67 | 827,467.67 | 722,802.04 | 950,910.96 | 733,881.71 |
| Iran | 2,158.50 | 366.00 | 399.80 | ||
| Israel | 13,169.16 | 7,165.09 | 6,397.33 | 318.24 | 1,080.90 |
| Japan | 4.84 | 1.19 | 1.17 | 1.15 | 20.67 |
| Kenya | 186.96 | ||||
| Mexico | 358.96 | 186.71 | 184.62 | ||
| Thailand | 0.37 | 0.14 | 0.16 | 0.87 | |
| Tunisia | 657.82 | 239.62 | 40.60 | 192.00 | |
| Turkey | 298,205.16 | 375,776.41 | 227,616.42 | 272,447.02 | 287,021.27 |
| United States | 1,714.93 | 8,868.74 | 4,413.37 | 1,866.20 | 1,072.48 |
| Zambia | 0.28 | 0.03 |
Table C.5: Fresh or dried avocados (CN code: 080440) imported in 100 kg into the EU (27) from regions where Maconellicoccus hirsutus is known to occur (Source: Eurostat accessed on 22/9/2021)
| COUNTRY | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Brazil | 44,357.36 | 71,040.50 | 68,697.61 | 78,673.73 | 48,183.82 |
| Cameroon | 133.50 | 173.54 | 221.30 | 259.38 | 205.93 |
| China | 193.97 | 35.28 | 1.23 | 0.04 | |
| Colombia | 152,115.55 | 210,139.60 | 251,050.33 | 387,367.23 | 663,149.95 |
| Costa Rica | 21.56 | 9.98 | 428.45 | 686.40 | |
| Cuba | 109.09 | 73.94 | 41.53 | 131.08 | 34.33 |
| Egypt | 211.20 | 5.35 | 4.58 | 79.92 | 363.95 |
| India | 0.04 | 2.06 | 0.52 | 0.06 | |
| Israel | 301,123.91 | 424,267.97 | 370,378.23 | 437,318.01 | 345,663.97 |
| Kenya | 228,426.16 | 243,947.31 | 404,593.87 | 346,231.90 | 435,309.11 |
| Malaysia | 0.03 | 47.04 | |||
| Mexico | 503,687.52 | 445,611.06 | 463,741.28 | 767,878.48 | 716,205.77 |
| Nigeria | 1.06 | 3.15 | 3.18 | 0.51 | |
| Tanzania | 26,823.05 | 25,773.58 | 55,517.16 | 60,480.96 | 50,769.74 |
| Thailand | 3.68 | 9.76 | 9.66 | 9.06 | 3.39 |
| Turkey | 213.41 | 477.05 | 1,530.93 | 2,172.09 | 1,864.65 |
| United States | 8,819.53 | 1.19 | 2,546.86 | 0.02 | 4.66 |
| Viet Nam | 1.00 | 0.05 | |||
| Zambia | 53.68 |
Table C.6: Fresh tamarinds, cashew apples, lychees, jackfruit, sapodillo plums, passion fruit, carambola and pitahaya (CN code: 08109020) imported in 100 kg into the EU (27) from regions where Maconellicoccus hirsutus is known to occur (Source: Eurostat accessed on 22/9/2021)
| COUNTRY | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Australia | 12.50 | ||||
| Bangladesh | 140.15 | 222.55 | 291.61 | 206.12 | 382.00 |
| Brazil | 49.36 | 147.37 | 368.88 | 966.63 | 1,220.26 |
| Cameroon | 41.84 | 100.53 | 38.52 | 92.00 | 46.11 |
| China | 314.75 | 287.38 | 1,112.11 | 1,014.77 | 823.41 |
| Colombia | 69,743.63 | 72,656.37 | 83,639.84 | 89,847.31 | 90,741.20 |
| Costa Rica | 9.11 | 3.52 | 0.13 | 18.62 | |
| Egypt | 13.79 | 39.05 | |||
| Hong Kong | 9.66 | ||||
| India | 324.19 | 621.75 | 1,095.12 | 1,168.69 | 754.33 |
| Indonesia | 103.20 | 333.37 | 297.72 | 246.67 | 463.60 |
| Iran | 6.25 | 1.75 | 0.50 | 3.88 | |
| Israel | 2,943.37 | 2,919.30 | 1,061.09 | 1,125.92 | 594.86 |
| Kenya | 714.44 | 221.45 | 603.11 | 481.00 | 697.14 |
| Malaysia | 15,348.23 | 14,205.33 | 13,879.92 | 14,235.96 | 7,849.69 |
| Mexico | 543.90 | 212.78 | 1,295.08 | 669.87 | 2,331.91 |
| Nigeria | 0.00 | 1.91 | 3.09 | ||
| Pakistan | 2.22 | 3.34 | 8.17 | ||
| Philippines | 9.78 | 14.26 | 0.88 | ||
| Singapore | 9.00 | 8.48 | |||
| Taiwan | 11.92 | 10.59 | 25.97 | 8.97 | |
| Tanzania | 0.35 | 1.27 | 8.77 | 4.52 | |
| Thailand | 9,774.93 | 10,279.68 | 12,461.38 | 14,900.21 | 10,138.74 |
| Turkey | 8.61 | 18.92 | 23.40 | ||
| United States | 3.97 | 3.00 | 0.07 | 0.02 | |
| Viet Nam | 33,078.82 | 38,428.61 | 44,070.83 | 52,846.33 | 45,652.75 |
| Zambia | 631.60 | 4,568.50 | 3,526.04 | 3,087.70 |
Table C.7: Fresh or dried pineapples (CN code: 08043000) imported in 100 kg into the EU (27) from regions where Maconellicoccus hirsutus is known to occur (Source: Eurostat accessed on 19/11/2021)
| COUNTRY | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| Algeria | 0.00 | 0.01 | |||
| Aruba | 0.00 | ||||
| Australia | 0.00 | 0.00 | 0.01 | ||
| Bahamas | 0.00 | ||||
| Belize | 0.00 | ||||
| Benin | 29,484.88 | 9,456.56 | 8,065.08 | 7,481.67 | 12,849.58 |
| Brazil | 1,522.02 | 1,272.34 | 484.83 | 639.05 | 280.66 |
| Burkina Faso | 145.92 | 19.68 | 3.57 | ||
| Cameroon | 38,878.76 | 39,301.85 | 30,633.74 | 23,825.83 | 13,811.36 |
| China | 69.90 | 25.05 | 9.91 | 62.65 | 42.74 |
| Colombia | 64,893.82 | 123,462.45 | 91,067.04 | 53,663.49 | 42,136.78 |
| Congo | 0.00 | 2.87 | 3.40 | ||
| Congo, Democratic Republic of | 0.78 | 2.56 | 0.85 | 0.07 | |
| Costa Rica | 6,095,312.66 | 6,832,249.09 | 7,693,551.48 | 7,543,050.71 | 6,650,975.31 |
| Côte d’Ivoire (Ivory Coast) | 202,205.93 | 255,038.72 | 220,581.56 | 244,175.93 | 203,552.53 |
| Cuba | 10,645.21 | 4,382.57 | 3,838.50 | 1,998.42 | 976.85 |
| Dominican Republic | 29,667.00 | 15,582.31 | 19,723.37 | 20,566.35 | 20,525.91 |
| Egypt | 201.60 | 28.16 | |||
| Fiji | 0.00 | ||||
| Gabon | 0.00 | ||||
| Grenada | 0.00 | ||||
| Guatemala | 229.74 | 40.08 | 64.03 | 282.50 | |
| Guinea | 17.35 | 98.34 | 83.45 | 72.90 | 19.95 |
| Guyana | 0.00 | 22.00 | |||
| India | 186.71 | 17.99 | 75.85 | 11.52 | 1.00 |
| Indonesia | 0.24 | 543.77 | 0.09 | 2.50 | |
| Iran, Islamic Republic of | 0.00 | 0.01 | 0.00 | ||
| Israel | 2.81 | 0.20 | 0.01 | ||
| Jamaica | 0.00 | ||||
| Japan | 0.02 | 0.00 | |||
| Jordan | 0.00 | 36.00 | |||
| Kenya | 761.13 | 745.19 | 2,147.97 | 23,799.06 | |
| Lao People’s Democratic Republic (Laos) | 0.00 | ||||
| Lebanon | 0.16 | 0.00 | 5.05 | ||
| Libya | 0.00 | ||||
| Malaysia | 13.60 | 5.00 | 2.40 | ||
| Maldives | 0.00 | ||||
| Mexico | 1,268.22 | 2,957.94 | 773.74 | 142.42 | 174.97 |
| Nicaragua | 0.00 | ||||
| Nigeria | 0.54 | 0.95 | 0.13 | 0.24 | 0.01 |
| Oman | 0.00 | ||||
| Pakistan | 0.00 | ||||
| Palau | 0.00 | ||||
| Philippines | 93.71 | 114.23 | 183.83 | 86.03 | 566.04 |
| Saudi Arabia | 0.00 | 0.45 | 0.17 | ||
| Singapore | 0.20 | 0.00 | 0.29 | ||
| Sri Lanka | 1,774.66 | 5,755.44 | 4,125.57 | 2,675.19 | 2,636.02 |
| Suriname | 0.00 | ||||
| Taiwan | 0.00 | 0.07 | 0.05 | ||
| Thailand | 10,183.30 | 11,093.21 | 9,505.48 | 8,056.49 | 8,828.72 |
| Trinidad and Tobago | 0.00 | ||||
| Tunisia | 0.05 | 0.00 | 0.01 | 0.03 | |
| Turkey | 0.00 | 25.20 | 0.04 | ||
| United Arab Emirates | 0.00 | 0.02 | |||
| United States | 69.72 | 56.66 | 22.03 | 28.28 | 57.29 |
| Venezuela, Bolivarian Republic of | 0.15 | 0.00 | 0.19 | 0.04 | |
| Viet Nam | 91.31 | 65.87 | 9.88 | 20.20 | 2.18 |
| Virgin Islands, British | 0.00 | ||||
| Virgin Islands, United States | 0.00 | ||||
| Zambia | 0.00 |
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Gregoire J‐C, Malumphy C, Antonatos S, Kertesz V, Maiorano A, Papachristos D and MacLeod A, 2022. Scientific Opinion on the pest categorisation of Maconellicoccus hirsutus . EFSA Journal 2022;20(1):7024, 45 pp. 10.2903/j.efsa.2022.7024
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00490
Panel members: Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to acknowledge the contribution of Caterina Campese and Oresteia Sfyra to this opinion.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © Courtesy of Cris Malumphy
Adopted: 25 November 2021
References
- Abd‐Rabou S, Shalaby H, Germain JF, Ris N, Kreiter P and Malausa T, 2012. Identification of mealybug pest species (Hemiptera: Pseudococcidae) in Egypt and France, using a DNA barcoding approach. Bulletin of Entomological Research, 24, 1–9. [DOI] [PubMed] [Google Scholar]
- Al‐Fwaeer M, Abu‐Obeid I, Al‐Zyoud F, Abo‐Alosh A and Halaybeh M, 2014. Population Dynamics of the hibiscus mealybug Maconellicoccus hirsutus Green (Hom., Pseudococcidae) and its parasitoid on guava trees in Madaba‐Jordan. International Journal of Agriculture and Forestry, 4, 171–177. [Google Scholar]
- Alleyne JC, 2004. Controlling a dangerous pest‐pink hibiscus mealybug. Newsletter of the University of Florida ‐ IFAS Extension ‐ Pinellas County Extension, 38, 2–3. [Google Scholar]
- Aristizábal LF, Mannion C, Bergh C and Arthurs S, 2012. Life history of pink hibiscus mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae) on three Hibiscus rosa‐sinensis cultivars. Florida Entomologist, 95, 89–94. [Google Scholar]
- Bartlett BR, 1978. Pseudococcidae. In: Clausen CP (ed.). Introduced parasites and predators of arthropod pests and weeds: a world review. Agriculture Handbook no. 480. USDA, Washington, US. pp. 137–170. [Google Scholar]
- CABI (Centre for Agriculture and Biosciences International) , 2021. Available online: www.cabi.org [Accessed: 15 September 2021]
- Castle SJ and Prabhaker N, 2011. Field evaluation of two systemic neonicotinoid insecticides against pink hibiscus mealybug (Maconellicoccus hirsutus (Green)) on mulberry trees. Journal of Pest Science, 84, 363–371. [Google Scholar]
- Chong JH, Roda AL and Mannion CM, 2008. Life history of the mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae), at constant temperatures. Environmental Entomology, 37, 323–332. [DOI] [PubMed] [Google Scholar]
- Chong JH, Aristizabal LF and Arthurs SP, 2015. Biology and management of Maconellicoccus hirsutus (Hemiptera: Pseudococcidae) on ornamental plants. Journal of Integrated Pest Management, 6, 5. [Google Scholar]
- Culik MP, Fornazier MJ, dos Santos Martins D, Zanuncio JS, Ventura JA, Peronti ALBG and Zanuncio JC, 2013. The invasive mealybug Maconellicoccus hirsutus: lessons for its current range expansion in South America and invasive pest management in general. Journal of Pest Science, 86, 387–398. [Google Scholar]
- Dufour BP and Léon J, 1997. Informe de mission de informacion sobre el control de la cochinilla rosada del hibisco (Maconellicoccus hirsutus Green) en la region del Caribe. IICA ‐ El Salvador. 22 pp. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. Available online: 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen-Schmutz K, Di Serio F, Jacques M-A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas-Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H-H, van der Werf W, Civera AV, Yuen J, Zappalá L, Battisti A, Mas H, Rigling D, Mosbach-Schulz O, and Gonthier P, 2021. Scientific Opinion on the commodity risk assessment of Ficus carica plants from Israel. EFSA Journal 2021;19(1):6353, 249 pp. Available online: 10.2903/j.efsa.2021.6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martínez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2005. OEPP/EPPO, data sheets on quarantine pests Maconellicoccus hirsutus . Bulletin OEPP/EPPO Bulletin, 35, 413–415. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2006. OEPP/EPPO, diagnostics Maconellicoccus hirsutus . Bulletin OEPP/EPPO, Bulletin, 36, 167–169. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2019. EPPO codes. Available online: https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO (European and Mediterranean Plant Protection Organization) , 2021. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 20 September 2021]
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 15/10/2021]
- FAO (Food and Agriculture Organization of the United Nations) , 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome. 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014‐04‐30_201405121523‐494.65%20KB.pdf [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) , 2018. International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. Revised version adopted CPM 13, April 2018. FAO, Rome. Available online: https://www.ippc.int/en/publications/621/ [Google Scholar]
- Fatima S, Hussain M, Shafqat S, Faheem Malik M, Abbas Z, Noureen N and Ane N, 2016. Laboratory evaluation of different insecticides against hibiscus mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Scientifica, 2016, 9312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A, Bloem KA, Roda AL, Lapointe SL, Zhang A and Onokpise O, 2007. Development of trapping methods with a synthetic sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Florida Entomologist, 90, 440–446. [Google Scholar]
- García Morales G, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, 2016. ScaleNet: a literature‐based model of scale insect biology and systematics. Database. 10.1093/database/bav118. Available online: http://scalenet.info [Accessed: 20 September 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose SK, 1971. Morphology of various instars of both sexes of the mealy‐bug, Maconellicoccus hirsutus . Indian Journal of Agricultural Sciences, 41, 602–611. [Google Scholar]
- Ghose SK, 1972. Biology of the mealybug, Maconellicoccus hirsutus (Green) (Pseudococcidae, Hemiptera). Indian Agriculturalist, 16, 323–332. [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Hall WJ, 1921. The hibiscus mealy bug (Phenacoccus hirsutus). Bulletin Ministry of Agriculture Egypt Technical and Scientific Service Entomological Section, 17, 1–28. [Google Scholar]
- Hara AH and Jacobsen CM, 2005. Hot water immersion for surface disinfestations of Maconellicoccus hirsutus (Homoptera: Pseudococcidae). Journal of Economic Entomology, 98, 284–288. [DOI] [PubMed] [Google Scholar]
- Kairo MTK, Pollard GV, Peterkin DD and Lopez VF, 2000. Biological control of the hibiscus mealybug, Maconellicoccus hirsutus Green (Hemiptera: Pseudococcidae) in the Caribbean. Integrated Pest Management Reviews, 5, 241–254. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Malausa T, Fenis A, Warot S, Germain JF, Ris N, Prado E, Botton M, Vanlerberghe‐Masutti F, Sforza R, Cruaud C, Couloux A and Kreiter P, 2011. DNA markers to disentangle complexes of cryptic taxa in mealybugs (Hemiptera: Pseudococcidae). Journal of Applied Entomology, 135, 142–155. [Google Scholar]
- Mani M, 1989. A review of the pink mealybug – Maconellicoccus hirsutus . Insect Science and Its Application, 10, 157–167. [Google Scholar]
- Meyerdirk DE, Warkentin R, Attavian B, Gersabeck E, Francis A, Adams J and Francis G, 2001. Biological control of pink hibiscus mealybug project manual, USDA, Washington. [Google Scholar]
- Miller D, Rung A, Parikh G, Venable G, Redford AJ, Evans GA and Gill RJ, 2014. Scale insects. Edition 2. USDA APHIS Identification Technology Program (ITP), Fort Collins, CO. Available online: http://idtools.org/id/scales/ [Accessed 1 October 2021] [Google Scholar]
- Milonas PG and Partsinevelos GK, 2017. The pink hibiscus mealybug Maconellicoccus hirsutus (Green) (Hemiptera: Pseudococcidae) in Greece. Hellenic Plant Protection Journal, 10, 80–83. [Google Scholar]
- Muralidharan CM and Badaya SN, 2000. Mealy bug (Maconellicoccus hirsutus) (Pseudococcidae: Hemiptera) out break on herbaceum cotton (Gossypium herbaceum) in Wagad cotton belt of Kachchh. Indian Journal of Agricultural Sciences, 70, 405–706. [Google Scholar]
- Negrini M, de Morais EGF and Zanuncio JC, 2017. Biology of Maconellicoccus hirsutus (Hemiptera: Pseudococcidae) on Hibiscus rosa‐sinensis . Revista Agro@mbiente On‐line, 11, 336–346. [Google Scholar]
- Pollard GV, 1995. Pink or hibiscus mealybug in the Caribbean. CARAPHIN News, 12, 1–2. [Google Scholar]
- Ranjan R, 2006. Economic impacts of pink hibiscus mealybug in Florida and the United States. Stochastic Environmental Research and Risk Assessment, 20, 353–362. [Google Scholar]
- Sagarra LA and Peterkin DD, 1999. Invasion of the Caribbean by the hibiscus mealybug, Maconellicoccus hirsutus Green (Homoptera: Pseudococcidae). Phytoprotection, 80, 103–113. [DOI] [PubMed] [Google Scholar]
- Sahito HA, Soomro RB, Tapur MA, Memon SA and Dhiloo KH, 2012. Biology of mulberry mealybug, Maconellicoccus hirsutus (Green) in laboratory conditions. Basic Research Journal of Agricultural Science and Review, 1, 11–18. [Google Scholar]
- Singh MP and Ghosh SN, 1970. Studies on Maconellicoccus hirsutus causing ‘bunchy top’ in mesta. Indian Journal of Science and Industry, A4, 99–105. [Google Scholar]
- Ülgentürk S, Kaydan MB and Hocaali Şişman S, 2015. New scale insect (Hemiptera: Coccomorpha) records for the Turkish Republic of Northern Cyprus. Türkiye Entomoloji Bülteni, 5, 59–68. [Google Scholar]
- Williams DJ, 1996. A brief account of the hibiscus mealybug Maconellicoccus hirsutus, a pest of agriculture and horticulture, with descriptions of two related species from southern Asia. Bulletin of Entomological Research, 86, 617–628. [Google Scholar]
- Williams DJ, 2004. Mealybugs of Southern Asia. The Natural History Museum, Kuala Lumpur: Southdene SDN. BHD. 896 pp. [Google Scholar]
- Zettler JL, Follett PA and Gill RF, 2002. Susceptibility of Maconellicoccus hirsutus (Homoptera: Pseudococcidae) to Methyl Bromide. Journal of Economic Entomology, 95, 1169–1173. [DOI] [PubMed] [Google Scholar]


