Abstract
Using five diagnostic markers, we compared the types of 72 strains of methicillin-resistant Staphylococcus aureus (MRSA) isolated simultaneously from the nasal cavity, pharynx, and sputum from 24 patients. Almost identical MRSA types had colonized the nasal cavity and sputum from the same patient for 21 (88%) of the patients. We speculate that most MRSA organisms isolated in sputum are derived from the nasal cavity, while a few are derived from the pharynx.
Methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen and a major cause of nosocomial infections (18). MRSA strains easily colonize a host, particularly immunodeficient patients, and can cause a variety of serious infections (2, 13, 14). Pneumonia caused by MRSA often occurs after surgical wound infection or is ventilator associated and is a major factor associated with mortality (3, 7, 11). Previous studies indicated a high incidence of MRSA colonization in the upper respiratory tracts (URTs) of seriously ill patients which was associated with a high rate of pulmonary infections caused by MRSA (12). To our knowledge, differences between MRSA types colonizing the URT and those colonizing the bronchopulmonary tract have not yet been investigated. We examined the mechanism of URT infection caused by MRSA, with a particular emphasis on differences between MRSA types colonizing the nasal cavity, pharynx, and bronchopulmonary tract.
Our study was performed with 24 patients admitted to our hospital or affiliated hospitals between June 1997 and January 1998. In these patients, MRSA had colonized the nasal cavity, pharynx, and sputum simultaneously, without infection. Culture specimens were obtained by swabs from the anterior nares and the pharynx. Sputum samples were collected through tracheostomas in five patients and by suction tubes in the remaining patients. Each patient was asked to cough before such collection, and sterilized tubes were used just before suction in order to avoid contamination by the pharyngeal flora. Cultures were performed using TSA II medium (Becton Dickinson) supplemented with 5% rabbit blood agar and MRSA-selective medium (OPA-Staphylococcus agar) (Becton Dickinson) for 24 h at 37°C. Identification of S. aureus was based on the morphology of colonies and the use of a Staphylo-LA slide latex agglutination kit (Denka Seiken, Tokyo, Japan). MRSA organisms were identified by the oxacillin disk diffusion method (Kirby-Bauer) according to the guidelines of the National Committee for Clinical Laboratory Standards (9). We used five diagnostic markers (coagulase type, enterotoxin type, toxic shock syndrome toxin 1 [TSST-1] production, β-lactamase production, and pulsed-field gel electrophoresis [PFGE] pattern) to identify 72 strains of MRSA (24 from nasal cavities, 24 from pharynges, and 24 from sputa). Coagulase type was determined with a neutralizing reaction kit (Denka Seiken), and enterotoxin type and TSST-1 production were determined with a SET-reversed passive latex agglutination kit (Denka Seiken) and a TST-reversed passive latex agglutination kit (Denka Seiken), respectively. β-Lactamase production was measured with cefinase disks (Becton Dickinson). The reaction time of the cefinase test was approximately 1 h, and no induction was used. PFGE was performed as follows. MRSA isolates were grown overnight in brain heart infusion broth at 35°C, and PFGE with SmaI chromosomal digestion was performed to determine the genetic relatedness of the MRSA strains, using the Kitasato University-modified procedure of Bannerman et al. (1). For this purpose, 1 μl of lysostaphin (Wako) was added to 120 μl of cell suspension in Pett IV solution, and the suspension was mixed with an equal volume of 2% Incert agarose (FMC Bioproducts, Rockland, Maine) and cast into molds. Cells were lysed using 1 ml of lysis solution containing 1 μl of lysostaphin at 37°C for 1 h and then were treated with ES solution (0.25 M EDTA [pH 8.0] and 1% sarcosyl) containing 100 μg of proteinase K per ml at 50°C overnight. The DNA was then digested with 10 U of SmaI (Takara Shuzo Co., Shiga, Japan) at 30°C overnight. The CHEF Mapper pulsed-field electrophoresis system (Bio-Rad Life Science) was used for electrophoresis, with the potential set at 6 V/cm, switch times set at 0.47 and 63 s, and the run time set at 20 h 18 min. After staining with ethidium bromide, the band patterns were compared according to the criteria for bacterial strain typing described by Tenover et al. (17).
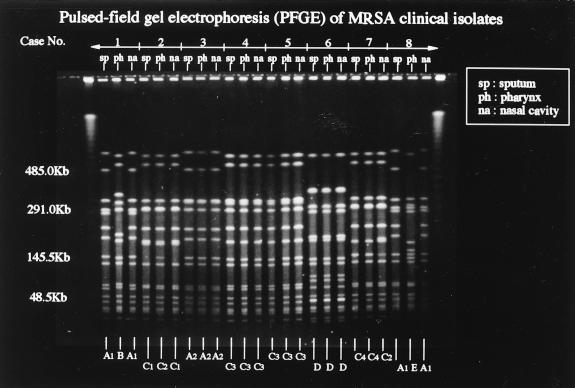
The most common underlying diseases in our patients (14 males and 10 females; mean age, 77.3 years) were cerebrovascular accidents in 14 (58%), chronic obstructive lung disease in 8 (33%), pressure wounds in 5 (21%), and malignant diseases in 5 (21%). Of 72 MRSA isolates, 69 were coagulase type II, and the remaining 3 were coagulase type VII; 68 of the coagulase type II strains were positive for enterotoxin C and TSST-1 production, and the remaining one was positive for enterotoxin A and negative for TSST-1 production. Furthermore, 41 of the MRSA isolates were β-lactamase positive, but the remaining isolates were negative. The patterns of coagulase type, enterotoxin type, TSST-1 production, and β-lactamase production for the MRSA isolates from the nasal cavity, pharynx, and sputum were identical in 22 (92%) of the patients. The patterns of enterotoxin type and TSST-1 production were different between the isolates from the sputum and nasal cavity and the one from the pharynx for case 8, and the pattern of β-lactamase production was different between the isolates from the sputum and pharynx and the one from the nasal cavity in case 11 (Table 1). Molecular typing demonstrated that the PFGE patterns of MRSA isolates from the nasal cavity and sputum were almost identical for 22 (92%) of the patients, although 2 (8%) of the patients showed differences in these patterns but had identical patterns from the pharynx and sputum (Table 1; Fig. 1).
TABLE 1.
Analysis of diagnostic markers in 72 MRSA clinical isolates
| Case no. | Sourcea | Coagulase type | Enterotoxin type | TSST-1 production | β-Lactamase production | PFGE pattern |
|---|---|---|---|---|---|---|
| 1 | SP, NA | II | C | + | − | A1 |
| PH | II | C | + | − | B | |
| 2 | SP, NA | II | C | + | + | C1 |
| PH | II | C | + | + | C2 | |
| 3 | SP, PH, NA | II | C | + | − | A2 |
| 4 | SP, PH, NA | II | C | + | − | C3 |
| 5 | SP, PH, NA | II | C | + | + | C3 |
| 6 | SP, PH, NA | VII | A | − | + | D |
| 7 | SP, PH | II | C | + | + | C4 |
| NA | II | C | + | + | C2 | |
| 8 | SP, NA | II | C | + | + | A1 |
| PH | II | A | − | + | E | |
| 9 | SP, PH | II | C | + | + | F1 |
| NA | II | C | + | + | G | |
| 10 | SP, PH | II | C | + | + | F2 |
| NA | II | C | + | + | F3 | |
| 11 | SP, PH | II | C | + | + | F1 |
| NA | II | C | + | − | F4 | |
| 12 | SP, PH, NA | II | C | + | − | F4 |
| 13 | SP, PH, NA | II | C | + | − | H |
| 14 | SP, PH, NA | II | C | + | + | F1 |
| 15 | SP, PH, NA | II | C | + | + | I |
| 16 | SP, PH, NA | II | C | + | + | J |
| 17 | SP, PH | II | C | + | + | K1 |
| NA | II | C | + | + | K2 | |
| 18 | SP, PH, NA | II | C | + | − | L1 |
| 19 | SP, PH | II | C | + | − | L2 |
| NA | II | C | + | − | L3 | |
| 20 | SP, PH, NA | II | C | + | + | L4 |
| 21 | SP, PH | II | C | + | − | M1 |
| NA | II | C | + | − | L4 | |
| 22 | SP, PH, NA | II | C | + | − | L1 |
| 23 | SP, PH, NA | II | C | + | + | M2 |
| 24 | SP, PH, NA | II | C | + | − | L1 |
SP, sputum; PH, pharynx; NA, nasal cavity (anterior nares).
FIG. 1.
Molecular typing by PFGE of SmaI-digested DNA from MRSA clinical isolates from eight patients. PFGE patterns are indistinguishable between MRSA from the nasal cavity and sputum but the pattern is different for MRSA from the pharynx for cases 1 and 8. PFGE patterns are indistinguishable between MRSA from the nasal cavity and sputum and closely related to that of MRSA from the pharynx for case 2. PFGE patterns are indistinguishable between MRSA from the pharynx and sputum but closely related to that of MRSA from the nasal cavity for case 7. The patterns of MRSA isolates from the nasal cavity, pharynx, and sputum are indistinguishable for cases 3, 4, 5, and 6.
MRSA infection reduces the chance of survival, particularly when it affects the lower respiratory tract (6). Previous studies have analyzed the relationship between bronchopulmonary infection caused by gram-negative bacilli and adherence of these bacteria to epithelial cells (8, 19). However, whether the MRSA type colonizing the URT is similar to the type colonizing the bronchopulmonary tract in the same patient remains to be determined. This issue is important with regard to the understanding of the route of infection and the design of preventative measures to protect against bronchopulmonary infections. Here we examined the type of MRSA colonizing the URT and compared it with the type detected in the sputum of the same patient in order to understand the process of development of bronchopulmonary infection. Our results demonstrated almost identical characteristics for MRSA organisms colonizing the nasal cavity and sputum from the same patient for 21 (88%) of 24 patients, although 3 (12%) showed differences between those and instead had MRSA organisms with similar characteristics colonizing the pharynx and sputum. These results suggest that most MRSA types isolated from sputum were derived from the nasal cavity but some types were derived from the pharynx and that these microorganisms occasionally cause bronchopulmonary infection. Mupirocin is the most effective antibiotic for the elimination of MRSA from the nasal passages (5, 15). Moreover, application of nasal mupirocin ointment is effective in reducing infections at surgical wounds and in decreasing the likelihood of bronchopulmonary tract infection (4, 16). However, cases of mupirocin-resistant MRSA infection have already been reported (10). In conclusion, our results demonstrated that the colonization of the URT by MRSA seems to be the first pathological process that ultimately leads to the development of MRSA bronchopulmonary infection. Therefore, early eradication of MRSA from the URT is important for the protection of patients against its spread to and colonization of the bronchopulmonary tract, particularly in susceptible patients, e.g., immunocompromised patients or those who are scheduled to have an operation.
Acknowledgments
We thank Akihiro Wada (Department of Bacteriology, Institute of Tropical Medicine, Nagasaki University), Chieko Shimauchi (Miyazaki Prefectural Nursing University), and Matsuhisa Inoue (Kitasato University School of Medicine) for their help in the completion of PFGE studies. We also thank F. G. Issa (Word-Medex, Sydney, Australia) for the careful reading and editing of the manuscript.
REFERENCES
- 1.Bannerman T L, Hancock G A, Tenover F C, Miller J M. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fierobe L, Decre D, Muller C, Lucet J C, Marmuse J P, Mantz J, Desmonts J M. Methicillin-resistant Staphylococcus aureus as a causative agent of postoperative intra-abdominal infection: relation to nasal colonization. Clin Infect Dis. 1999;29:1231–1238. doi: 10.1086/313454. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez C, Rubio M, Romero-Vivas J, Gonzalez M, Picazo J J. Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis. 1999;29:1171–1177. doi: 10.1086/313440. [DOI] [PubMed] [Google Scholar]
- 4.Harbarth S, Dharan S, Liassine N, Herrault P, Auckenthaler R, Pittet D. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1412–1416. doi: 10.1128/aac.43.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill R L R, Duckworth G J, Casewell M W. Elimination of nasal carriage of methicillin-resistant Staphylococcus aureus with mupirocin during a hospital outbreak. J Antimicrob Chemother. 1988;22:377–384. doi: 10.1093/jac/22.3.377. [DOI] [PubMed] [Google Scholar]
- 6.Ibelings M M, Bruining H A. Methicillin-resistant Staphylococcus aureus: acquisition and risk of death in patients in the intensive care unit. Eur J Surg. 1998;164:411–418. doi: 10.1080/110241598750004210. [DOI] [PubMed] [Google Scholar]
- 7.Iwahara T, Ichiyama S, Nada T, Shimokata K, Nakashima N. Clinical and epidemiologic investigations of nosocomial pulmonary infections caused by methicillin-resistant Staphylococcus aureus. Chest. 1994;105:826–831. doi: 10.1378/chest.105.3.826. [DOI] [PubMed] [Google Scholar]
- 8.Johanson W G, Jr, Woods D E, Chaudhuri T. Association of respiratory tract colonization with adherence of gram-negative bacilli to epithelial cells. J Infect Dis. 1979;139:667–673. doi: 10.1093/infdis/139.6.667. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 10.Rahman M, Noble W C, Cookson B. Mupirocin-resistant Staphylococcus aureus. Lancet. 1987;ii:387. doi: 10.1016/0140-6736(90)92667-7. [DOI] [PubMed] [Google Scholar]
- 11.Rello J, Torres A, Ricart M, Valles J, Gonzales J, Artigas A, Rodriguez-Roisin R. Ventilator-associated pneumonia by Staphylococcus aureus. Comparison of methicillin-resistant and methicillin-sensitive episodes. Am J Respir Crit Care Med. 1994;150:1545–1549. doi: 10.1164/ajrccm.150.6.7952612. [DOI] [PubMed] [Google Scholar]
- 12.Rikitomi N, Nagatake T, Sakamoto T, Matsumoto K. The role of MRSA (methicillin-resistant Staphylococcus aureus) adherence and colonization in the upper respiratory tract of geriatric patients in nosocomial pulmonary infections. Microbiol Immunol. 1994;38:607–614. doi: 10.1111/j.1348-0421.1994.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 13.Rubio M, Romero J, Corral O, Roca V, Picazo J J. Bacteremia by Staphylococcus aureus: analysis of 311 episodes. Enferm Infect Microbiol Clin. 1999;17:56–64. [PubMed] [Google Scholar]
- 14.Sakamoto T, Kikuchi K, Mineura K, Kowada M, Nakagomi O. MRSA meningitis in postoperative patients. Report of 4 cases. Jpn J Antibiot. 1990;43:1137–1142. [PubMed] [Google Scholar]
- 15.Sutherland R, Boon R J, Griffin K E, Masters P J, Slocombe B, White A R. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985;27:495–498. doi: 10.1128/aac.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talon D, Rouget C, Cailleaux V, Bailly P, Thouverez M, Barale F, Michel-Briand Y. Nasal carriage of Staphylococcus aureus and cross-contamination in a surgical intensive care unit: efficacy of mupirocin ointment. J Hosp Infect. 1995;30:39–49. doi: 10.1016/0195-6701(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 17.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenzel R P, Nettleman M D, Jones R N, Pfaller M A. Methicillin-resistant Staphylococcus aureus: implications for the 1990s and effective control measures. Am J Med. 1991;91(Suppl. 3B):221S–227S. doi: 10.1016/0002-9343(91)90372-5. [DOI] [PubMed] [Google Scholar]
- 19.Woods D E, Straus D C, Johanson W G, Jr, Bass J A. Role of salivary protease activity in adherence of gram-negative bacilli to mammalian buccal epithelial cells in vivo. J Clin Investig. 1981;68:1435–1440. doi: 10.1172/JCI110395. [DOI] [PMC free article] [PubMed] [Google Scholar]