Key Points
Question
Can a ward-based, age-friendly improvement program (“Eat Walk Engage”) reduce hospital-associated complications (delirium, functional decline, pressure injuries, falls, and incontinence) and length of stay for older adults admitted to acute medical and surgical wards?
Findings
In this pragmatic cluster randomized trial, which implemented and evaluated Eat Walk Engage in 4 hospitals, comprising 539 patients, there was no reduction in the composite primary outcome of any hospital-associated complication of older people (HAC-OP) (46% intervention vs 52% control) or median length of stay (6 days intervention vs 7 days control). Incident delirium was significantly lower in intervention wards (16% vs 31%), but other individual HAC-OPs were not reduced.
Meaning
Eat Walk Engage did not reduce the composite primary outcome of any HAC-OP, which affected almost half of older inpatients, although there was a significant reduction in delirium.
Abstract
Importance
Hospital-associated complications of older people (HAC-OPs) include delirium, hospital-associated disability, incontinence, pressure injuries, and falls. These complications may be preventable by age-friendly principles of care, including early mobility, good nutrition and hydration, and meaningful cognitive engagement; however, implementation is challenging.
Objectives
To implement and evaluate a ward-based improvement program (“Eat Walk Engage”) to more consistently deliver age-friendly principles of care to older individuals in acute inpatient wards.
Design, Setting, and Participants
This cluster randomized CHERISH (Collaboration for Hospitalised Elders Reducing the Impact of Stays in Hospital) trial enrolled 539 consecutive inpatients aged 65 years or older, admitted for 3 days or more to study wards, from October 2, 2016, to April 3, 2017, with a 6-month follow-up. The study wards comprised 8 acute medical and surgical wards in 4 Australian public hospitals. Randomization was stratified by hospital, providing 4 clusters in intervention and in control groups. Statistical analysis was performed from August 28, 2018, to October 17, 2021, on an intention-to-treat basis.
Intervention
A trained facilitator supported a multidisciplinary work group on each intervention ward to improve the care practices, environment, and culture to support key age-friendly principles.
Main Outcomes and Measures
Primary outcomes were incidence of any HAC-OP and length of stay. Secondary outcomes were incidence of individual HAC-OPs, facility discharge, 6-month mortality, and all-cause readmission. Outcomes were analyzed at the individual level, adjusted for confounders and clustering.
Results
A total of 265 participants on 4 intervention wards (124 women [46.8%]; mean [SD] age, 75.9 [7.3] years) and 274 participants on 4 control wards (145 women [52.9%]; mean [SD] age, 78.0 [8.2] years) were enrolled. The composite primary outcome of any HAC-OP occurred for 115 of 248 intervention participants (46.4%) and 129 of 249 control participants (51.8%) (intervention group: adjusted odds ratio, 1.07; 95% CI, 0.71-1.61). The median length of stay was 6 days (IQR, 4-9 days) for the intervention group and 7 days (IQR, 5-10 days) for the control group (adjusted hazard ratio, 0.96; 95% credible interval, 0.80-1.15). The incidence of delirium was significantly lower for intervention participants (adjusted odds ratio, 0.53; 95% CI, 0.31-0.90). There were no significant differences in other individual HAC-OPs, facility discharge, mortality, or readmissions.
Conclusions and Relevance
The Eat Walk Engage program did not reduce the composite primary outcome of any HAC-OP or length of stay, but there was a significant reduction in the incidence of delirium.
Trial Registration
anzctr.org.au Identifier: ACTRN12615000879561
This cluster randomized clinical trial evaluates a hospital ward–based improvement program (“Eat Walk Engage”) to more consistently deliver age-friendly principles to older individuals in acute inpatient wards.
Introduction
Poor outcomes of hospitalization, including death, longer stays, and increased care needs, increase with advancing age.1,2 These poorer outcomes result from a complex interplay between acute illness or injury, underlying vulnerability (frailty), and hospital systems that are poorly designed for older people.3,4,5 This interplay manifests as hospital-associated complications of older people (HAC-OPs), including delirium, hospital-associated disability, incontinence, falls, and pressure injuries.6,7 These common and distressing complications share risk factors and often coexist, and people with a greater number of HAC-OPs have longer stays, more facility discharges, and higher mortality.6,7 Previous interventions have usually focused on preventing a single complication, particularly delirium and hospital-associated disability. There is evidence that multicomponent interventions addressing fundamental principles of age-friendly care, including mobility, cognitive and social activities, nutrition and hydration, sleep, and pain management, significantly reduce the incidence of delirium8 and may reduce the incidence of falls9 and hospital-associated disability,10 suggesting that systematic attention to these fundamental principles could have an additive effect on HAC-OPs and mediate better outcomes for older people.11,12 However, these principles remain challenging to implement within complex health services.13,14
“Eat Walk Engage” is a ward-based improvement program designed to support more consistent delivery of the key principles of nutrition and hydration, mobility, and meaningful cognitive and social engagement, as well as improve multidisciplinary teamwork.15 Implementation is guided by the integrated Promoting Action on Research Implementation in Health Services framework,16 a well-recognized framework to support implementation of complex interventions in health care. A facilitator on each ward helps a local multidisciplinary working group improve the practices, environment, and culture to support the key principles. Pilot pre-post studies on 1 medical and 1 surgical ward demonstrated that the program was feasible and acceptable, improved care processes and teamwork, and was associated with shorter length of stay and lower incidence of delirium, functional decline, and facility discharge.15,17 This multisite effectiveness study aimed to compare patient-level outcomes, including HAC-OPs, length of stay, discharge destination, and 6-month readmissions and mortality, among older people on wards implementing Eat Walk Engage with those on wards not implementing the program. A concurrent implementation evaluation reporting ward-level processes will be reported separately.
Methods
Study Design
We evaluated this ward-based program using a cluster randomized clinical trial design to minimize contamination. Four hospitals each nominated 2 acute care wards, and participant allocation was determined by the ward they were admitted to through usual hospital processes. A statistician used a coin toss to allocate 1 ward in each participating hospital to implement the Eat Walk Engage program, so there were 4 wards (clusters) in the intervention group and 4 in the control group. Outcomes were compared between participants recruited on intervention wards vs control wards. The study protocol18 and analysis plan were prospectively registered (ACTRN12615000879561; trial protocol in Supplement 1 and analysis plan in Supplement 2). Implementation and research costs were funded by a formal partnership among government, health services, and academic institutions. Written informed consent was obtained from all participants (or appropriate surrogate decision maker) for data collection and telephone follow-up. The study received institutional ethics approval from the Royal Brisbane and Women's Hospital Human Research Ethics Committee and the Queensland University of Technology University Human Research Ethics Committee. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline extension for cluster trials.
Setting
This cluster randomized CHERISH (Collaboration for Hospitalised Elders Reducing the Impact of Stays in Hospital) trial was undertaken in 4 publicly funded hospitals in Queensland, Australia, including 2 academic metropolitan hospitals, an outer metropolitan hospital, and a regional hospital. The intervention was targeted to acute medical and surgical wards, where multidisciplinary clinical care for a range of conditions was provided by junior medical officers in training supervised by consultant medical or surgical specialists, allied health professionals aligned to medical teams and/or wards, and nursing staff with daytime ratios of 4 to 6 patients per nurse, supervised by a ward-based nurse unit manager. Two wards were selected in each hospital by consensus discussion between the research team and hospital leaders. Wards were eligible if at least 50% of inpatients were aged 65 years or older, there was perceived alignment with hospital priorities, and the nurse unit manager agreed to the ward’s participation in the study. Preimplementation cohort data were collected on all wards before randomization to test recruitment and measurement strategies and validate the composite primary outcome.7 Wards were randomized in December 2015, and implementation began in January 2016. Outcome measurement began in October 2016.
Participants
Participants were consecutive inpatients aged 65 years or older admitted to a study ward between October 2, 2016, and April 3, 2017, for at least 3 consecutive days. Patients with terminal or critical illness, with severe cognitive impairment without a surrogate decision maker, or who were unable to communicate in English were excluded. Patients who were discharged or transferred within 3 days or had previously enrolled in the trial were also excluded.
Intervention
Eat Walk Engage is a structured, ward-based improvement program. A nurse or allied health professional from within each hospital was employed for 2 days per week as a site facilitator, trained and mentored by 2 experienced facilitators. On each intervention ward, the site facilitator undertook structured assessments of context, patient experience interviews, and structured audits of care processes to inform opportunities for improvement (eTable 1 in Supplement 3). They engaged staff from the intervention ward to form a multidisciplinary working group that reviewed local interview and audit findings to prioritize areas for improvement aligned with the key principles and program goals (eTable 2 in Supplement 3; Table 1). The multidisciplinary working group included key stakeholders and champions (eg, nurse unit manager, nurse educator, physiotherapist, dietitian, and occupational therapist) who met for 1 hour per month and worked with the facilitator and other staff as required toward iterative improvements between meetings, a process that optimized staff buy-in and engagement. Improvements could be at an individual, team, and/or system level and were supported by employing and training a half-time multiprofessional assistant on each ward who could have tasks delegated from any team member. Interventions were iteratively implemented and adapted to the local context during the study period. Table 1 provides an example of interventions on 1 ward.
Table 1. Example Intervention Strategies Used on 1 Intervention Ward.
| Key principles | Program goals | Interventionsa |
|---|---|---|
| Adequate nutrition and hydration |
|
|
| Early and progressive mobility and functional independence |
|
|
| Meaningful cognitive and social engagement |
|
|
| Multidisciplinary team communication |
|
|
Abbreviations: EWE-MPA, Eat Walk Engage–multiprofessional assistant; MDWG, multidisciplinary working group.
Interventions were developed and adapted locally by the MDWG, and may have varied between wards and over time.
Outcomes and Measures
Primary outcomes were any HAC-OP and length of stay under the index care team. Any HAC-OP was a composite measure of hospital-associated delirium, hospital-associated disability, hospital-associated incontinence, fall, or pressure injury occurring during hospitalization.7 Delirium was assessed using the 3-minute diagnostic interview for Confusion Assessment Method (CAM)–defined delirium (3D-CAM)19 at admission, day 5, and day of discharge, plus structured clinical record review, and was defined as positive if either method had positive results.20 Hospital-associated delirium excluded participants with delirium in the first 24 hours of admission, who were considered prevalent cases. Hospital-associated disability was defined as any increase in the requirement for assistance with self-reported basic activities of daily living at hospital discharge compared with 2 weeks prior to admission, in-hospital death, or new long-term care placement. Hospital-associated incontinence was defined as any new urinary or fecal incontinence at hospital discharge compared with 2 weeks prior to admission, based on participant self-report, excluding any preexisting incontinence. Falls and pressure injuries occurring in the hospital were identified by participant self-report at admission, day 5, or hospital discharge, supplemented by medical record review. The primary outcome of any HAC-OP was defined as experiencing 1 or more of these complications and is associated with longer hospital stay, more frequent facility discharge, and 6-month mortality.7
Each individual HAC-OP (ie, hospital-associated delirium, disability, incontinence, falls, and pressure injuries) was compared between groups as secondary outcomes. Other secondary outcomes were facility discharge (to a rehabilitation facility, convalescent care, or new long-term care placement) obtained from the clinical record for all participants discharged alive from the index team admission; 6-month all-cause readmissions, obtained from the Queensland statewide public hospitals admissions database; and 6-month mortality, obtained from the statewide death registry.
A trained clinical research assistant at each hospital conducted a structured interview within 3 days of hospital admission and extracted specified variables from the medical record. Admission variables collected from the medical record included age, sex, comorbidities,21 whether the patient was living in the community or a long-term care facility, emergency or elective admission, and hospitalizations in the previous 6 months. The interview elicited information about health status currently and 2 weeks prior to admission, including the need for assistance in basic (bathing, dressing, mobility, transfers, toileting, and eating) and instrumental activities of daily living (cleaning, shopping, meal preparation, medication management, transport, finances, and using the telephone); urinary or fecal incontinence; depressive symptoms22; falls in the previous 6 months; and risk of malnutrition.23 Appropriate proxy respondents assisted with responses if required. Cognition was tested using the Short Portable Mental Status Questionnaire (a score <8 indicates cognitive impairment).24 We derived a frailty index (FI) from 39 baseline variables using validated methods.25
Research assistants received 2 days of training from 2 investigators (A.M.M. and P.M.), including pilot testing patient interviews, watching 3D-CAM training videos, and being observed performing the 3D-CAM for several patients. These 2 investigators conducted a reliability cross-check of data extraction on a convenience sample of 10 medical records for each ward to identify any areas of misinterpretation. Data collection was supported by a comprehensive data manual, and weekly telephone support by 2 investigators (A.M.M. and P.M.) and the data manager. Study data were collected and managed using the REDCap (Research Electronic Data Capture; Vanderbilt University) secure web-based application.26 Data were validated by the data manager, with queries raised for missing data or illogical responses. Research assistants were not informed of the hypothesis or ward allocations; however, blinding of patients, staff, and research assistants was not possible owing to the ward-based nature of the intervention.
Statistical Analysis
Statistical analysis was performed from August 28, 2018, to October 17, 2021, on an intention-to-treat basis. Outcome analyses were conducted at the individual patient level. Any HAC-OP was summarized as the percentage of participants in the intervention and control groups and modeled using logistic regression, adjusting for clustering within hospital (using random intercepts) and for patient characteristics of age, sex, activities of daily living dependency at admission, Short Portable Mental Status Questionnaire score at admission, Charlson Comorbidity Index score, and emergency vs elective admission. Outcome data were missing for 17 of 265 intervention participants (6.4%) and 25 of 274 control participants (9.1%). A planned sensitivity analysis was conducted using inverse variance weighting derived from logistic regression modeling of missing outcomes as an accepted alternative approach to imputation.27 A post hoc sensitivity analysis was also conducted, controlling for other patient covariates that differed between groups. Length of stay was reported as a median value and modeled using a bayesian survival model (time to discharge), censoring patients who died in the hospital and adjusting for clustering and patient characteristics as already described. A sensitivity analysis treated in-hospital mortality as a competing risk. A parametric survival model based on time to failure was modeled with a Weibull distribution, and estimates were reported as hazard ratios and 95% credible intervals. We used bayesian methods because they give a common-sense interpretation of statistical conclusions28; in particular, the 95% credible intervals have a high probability of containing the unknown difference between treatments. A priori power calculations determined that 500 participants would provide 80% power to detect a 30% relative reduction in any HAC-OP and 15% reduction in acute length of stay.18
Preplanned subgroup analyses of any HAC-OP and length of stay tested the interaction between the subgroup and treatment effect in the same adjusted models. Subgroups were age (<75 vs ≥75 years), frailty (FI: nonfrail, <0.25; mildly frail, 0.25-0.39; and severely frail, ≥0.40), and hospital site. For the secondary outcomes, logistic regression models were conducted for each individual HAC-OP and facility discharge, adjusting for hospital clustering and patient characteristics. Survival models of cumulative risk of death and all-cause readmission (with death as a competing risk) to 6 months after hospital discharge were adjusted for hospital clustering and patient characteristics. All statistical analyses were made using Rmarkdown with R, version 3.4.4 (R Group for Statistical Computing).29 The bayesian models were fitted using JAGS, version 4.2 (SourceForge).30 The complete R and JAGS code are openly available online.31
Results
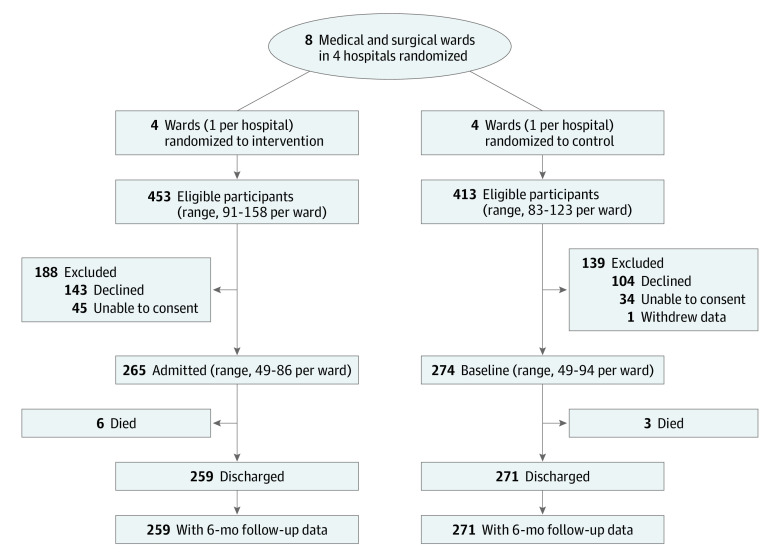
Eat Walk Engage was implemented in 4 hospitals (1 general medicine ward, 1 orthopedic ward, 1 general surgery ward, and 1 respiratory medicine ward). Control wards were matched for hospital (2 general medicine wards, 1 respiratory medicine ward, and 1 general surgery ward). Participant recruitment is summarized in the Figure. Research assistants screened 2020 older inpatients; 866 were eligible, and 539 were enrolled (62.2% of eligible; 265 in the intervention group [124 women (46.8%); mean (SD) age, 75.9 (7.3) years] and 274 in the control group [145 women (52.9%); mean (SD) age, 78.0 (8.2) years]) (Table 2).
Figure. Participant Flow.
Participants were clustered by ward, with 1 ward in each of 4 hospitals randomized to intervention and the other ward randomized to control.
Table 2. Characteristics of Participants.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Intervention (n = 265) | Control (n = 274) | |
| Age, mean (SD), y | 75.9 (7.3) | 78.0 (8.2) |
| Female | 124 (46.8) | 145 (52.9) |
| Living in community | 260 (98.1) | 258 (94.2) |
| Elective admission | 66 (24.9) | 26 (9.5) |
| Surgical procedure | 87 (32.8) | 29 (10.6) |
| Frailty index, mean (SD) | 0.2 (0.1) | 0.3 (0.2) |
| Charlson Comorbidity Index score, median (IQR) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) |
| Comorbidities | ||
| Hypertension | 152 (57.4) | 166 (60.6) |
| Chronic obstructive airway disease | 119 (44.9) | 102 (37.2) |
| Arthritis or rheumatic condition | 81 (30.6) | 83 (30.3) |
| Cancer | 76 (28.7) | 57 (20.8) |
| Arrhythmia | 68 (25.7) | 94 (34.3) |
| Type 1 or 2 diabetes | 56 (21.1) | 70 (25.5) |
| Peripheral vascular disease | 46 (17.4) | 39 (14.2) |
| Cerebrovascular disease | 34 (12.8) | 42 (15.3) |
| Heart failure | 30 (11.3) | 53 (19.3) |
| Previous myocardial infarction | 25 (9.4) | 40 (14.6) |
| No. of medications at admission, mean (SD) | 7.2 (4.4) | 8.1 (4.8) |
| Dependent in ≥1 basic ADL 2 wk before admission | 37 (14.0) | 81 (29.6) |
| Dependent in ≥1 instrumental ADL 2 wk prior to admission | 171 (64.5) | 194 (70.8) |
| Urinary incontinence 2 wk before admission | 40 (15.1) | 44 (16.1) |
| Fecal incontinence 2 wk before admission | 27 (10.2) | 32 (11.7) |
| Hospital admission in previous 12 mo | 113 (42.6) | 137 (50.0) |
| Fall in previous 6 mo | 88 (33.2) | 114 (41.6) |
| Malnutrition risk (Malnutrition Screening Tool score ≥2) | 96 (36.2) | 121 (44.2) |
| SPMSQ score at admission, mean (SD) | 8.2 (2.0) | 7.3 (2.4) |
Abbreviations: ADL, activities of daily living; SPMSQ, Short Portable Mental Status Questionnaire (a score of <8 represents cognitive impairment).
Participant characteristics are shown in Table 2 and detailed by ward in eTable 3 in Supplement 3. Data missing at baseline for cognition (n = 22 [4.1%]) were imputed using multivariate imputation by a chained equation.32 Overall, 305 participants (56.6%) were aged 75 years or older; 284 (52.7%) were nonfrail (FI, <0.25), 163 (30.2%) were mildly frail (FI, 0.25-0.39), and 92 (17.1%) were severely frail (FI, ≥0.40). Compared with the control group, the intervention group was younger (mean [SD] age, 75.9 [7.3] vs 78.0 [8.2] years) and less frail (mean [SD] FI, 0.2 [0.1] vs 0.3 [0.2]), with less functional impairment (dependence in ≥1 basic activities of daily living 2 weeks prior to admission, 37 [14.0%] vs 81 [29.6%]) and less cognitive impairment (mean [SD] Short Portable Mental Status Questionnaire score at admission, 8.2 [2.0] vs 7.3 [2.4]), and more elective admissions (66 [24.9%] vs 26 [9.5%]) (Table 2). All subsequent analyses controlled for age, sex, baseline cognitive and functional status, comorbidities, elective status, and hospital.
Any HAC-OP could be measured for 497 participants (92.2%). Discharge assessments were missing for 34 participants (6.3%) for functional status and for 54 participants (10.0%) for continence. Missingness was not significantly associated with intervention or patient characteristics. Overall, 244 of 497 participants (49.1%) experienced any HAC-OP, most commonly hospital-associated disability (166 of 505 [32.9%]), delirium (106 of 453 [23.4%]), and incontinence (60 of 446 [13.5%]). The proportion of intervention and control participants experiencing any HAC-OP and each individual HAC-OP are shown in Table 3. HAC-OPs occurred in 115 of 248 intervention participants (46.4%) and 129 of 249 control participants (51.8%) (intervention group: adjusted OR, 1.07; 95% CI, 0.71-1.61). There was a significant reduction in the incidence of hospital-associated delirium in the intervention group (adjusted OR, 0.53; 95% CI, 0.31-0.90), but no significant difference in any other HAC-OP. Sensitivity analyses accounting for missing outcome values using inverse variance methods and controlling for additional covariates (falls, malnutrition, previous hospitalization, and surgery) showed similar findings. In preplanned subgroup analyses, there were no significant interaction effects by age, frailty, or hospital site for any HAC-OP.
Table 3. Proportion of Patients Experiencing HAC-OPs.
| Complication | Patients, No./total No. (%) | Adjusted OR (95% CI)a | |
|---|---|---|---|
| Intervention group | Control group | ||
| Any HAC-OP | 115/248 (46.4) | 129/249 (51.8) | 1.07 (0.71-1.61) |
| Hospital-associated disabilityb | 80/252 (31.7) | 86/253 (34.0) | 1.23 (0.80-1.89) |
| Hospital-associated deliriumc | 37/233 (15.9) | 69/220 (31.4) | 0.53 (0.31-0.90) |
| Hospital-associated incontinenced | 30/225 (13.3) | 30/221 (13.6) | 1.25 (0.69-2.24) |
| Hospital-associated pressure injury | 18/265 (6.8) | 18/274 (6.6) | 1.56 (0.73-3.31) |
| Hospital-associated fall | 11/265 (4.2) | 12/274 (4.4) | 1.44 (0.57-3.60) |
Abbreviations: HAC-OP, hospital-associated complications of older people; OR, odds ratio.
From logistic regression models adjusted for hospital (cluster), age, sex, functional and cognitive status on admission, comorbidities, and elective status.
Disability outcome defined as any increase in the number of self-reported basic activities of daily living for which the patient required assistance at discharge compared with 2 weeks prior to admission, or in-hospital death or new long-term care placement; outcome excludes participants missing functional status at the time of hospital discharge.
Delirium outcome excludes participants with prevalent delirium (identified within 24 hours of admission).
Incontinence outcome defined as any self-reported urinary or fecal incontinence at hospital discharge that was not reported in the 2 weeks prior to hospital admission; outcome excludes participants with prevalent incontinence (in the 2 weeks prior to admission).
Data on length of stay were available for all participants. The median length of stay was 6 days in the intervention group (IQR, 4-9 days) and 7 days in the control group (IQR, 5-10 days). Bayesian model findings adjusted for hospital and patient characteristics did not show a difference in time to discharge (intervention group: hazard ratio, 0.96; 95% credible interval, 0.80-1.15), with an estimated adjusted mean difference in length of stay of 0.16 days (95% credible interval, −0.43 to 0.78 days). In preplanned subgroup analyses, there were no significant interaction effects for age or frailty subgroup, but there was significant variation in length of stay effect by site (eTables 4 and 5 in Supplement 3).
Nine participants died in the hospital (6 [2.3%] in the intervention and 3 [1.1%] in the control group), leaving 259 intervention and 271 control participants for discharge and posthospital outcome analysis. In the intervention group, 31 of 259 patients (12.0%) were discharged to facility care, 199 of 259 patients (76.8%) were discharged home (including return to usual long-term care), and 27 of 259 patients (10.4%) were transferred to another acute care ward; in the control group, 62 of 271 patients (22.9%) were discharged to facility care, 180 of 271 patients (66.4%) were discharged home, and 29 of 271 patients (10.7%) were transferred to another acute care ward. There was no significant difference in facility discharge (intervention group: adjusted OR, 0.79; 95% CI, 0.46-1.36).
During the following 6 months, 108 of 259 participants in the intervention group (41.7%) and 128 of 271 participants in the control group (47.2%) were readmitted to the hospital (intervention group: adjusted hazard ratio, 0.90; 95% credible interval, 0.70-1.19), and 38 of 259 participants in the intervention group (14.7%) and 52 of 271 participants in the control group (19.2%) died (adjusted hazard ratio, 0.90; 95% credible interval, 0.57-1.42).
Discussion
This prospective randomized clinical trial of 539 older adults hospitalized for at least 3 days on acute medical and surgical wards highlights the vulnerability of this patient group, with substantial hospital-associated harm and adverse outcomes. Almost half of participants developed 1 or more HAC-OPs, almost half experienced hospital readmission within 6 months, and 1 in 6 died within 6 months. The most common complications were delirium, affecting almost 1 in 4 participants, and hospital-associated disability, affecting 1 in 3 participants, consistent with reports from other health systems.33,34 Patients treated on wards implementing Eat Walk Engage were not less likely to develop any HAC-OP. They had significantly lower odds of developing delirium, but other HAC-OPs were not significantly different, and there were not significant reductions in length of stay, facility discharge, 6-month mortality, or 6-month readmission. The wide 95% CIs raise the possibility of benefit, no benefit, or harm, and the uncertainty in estimates is substantial.
Given the robust evidence base for risk factors and multicomponent intervention strategies for delirium prevention, the effectiveness of the program for delirium was not surprising. HAC-OPs share risk factors and may be mutually reinforcing6,7; there is emerging evidence that multicomponent interventions may play a role in reducing hospital-associated disability,10,12,35,36 but the evidence for effective interventions is generally less developed for HAC-OPs other than delirium. Causal pathways for HAC-OPs are complex,37 and intervention strategies may require further development and testing to reduce other HAC-OPs and improve outcomes. Contributing factors to our negative primary outcome may also include heterogeneous patient populations, the complexity of different implementation contexts, and/or insufficient adoption or adaptation of intervention strategies within the short time frame of this outcome evaluation.
Although delirium was a secondary outcome, the observed significant reduction is an important finding that confirms and extends findings from previous randomized and nonrandomized trials in medical and/or surgical wards.8,9,38 Our findings are consistent with the pooled relative risk reduction of 0.57 (95% CI, 0.46-0.71) reported in the most recent systematic review, which included 14 randomized clinical trials of multicomponent interventions for delirium.8 Only 1 previous randomized clinical trial has included both medical and surgical wards in multiple hospitals; the Prevention of Delirium feasibility cluster randomized clinical trial in the United Kingdom showed a similar relative reduction in the incidence of delirium, without reduction in length of stay or falls.39 Although other programs have had clear program logic addressing recognized delirium risk factors, and some have described program fidelity,10,40,41 we are aware of only 1 program that has used implementation theory to design and describe how the program is applied in practice.42 Implementation studies are recognized as an important direction for future delirium research.8 Our future implementation evaluation will provide ward-level analysis of process outcomes to better understand variability between sites.
The heterogeneity in wards, participants, and facilitators, as well as deliberate adaption of interventions to local context, are challenges to internal validity but also offer pragmatic, real-world strengths to our trial. These features enhance generalizability, but replication can be challenging when interventions are tailored to context, and it is difficult to provide a standardized description of potential interventions for differing settings. Nonetheless, we demonstrated that the program significantly reduced the incidence of delirium and was scalable and transferable across different wards with appropriate training and support. This provided evidence for health care decision makers within Queensland to invest continuing long-term funding in implementing the program, as part of a broader strategy to improve the hospital care of older people,43 and Eat Walk Engage has been implemented in 36 new wards in 20 hospitals since 2019.
Limitations
We acknowledge several study limitations. Cluster randomization is a strong design for ward-based interventions, but the small number of clusters resulted in differences between groups that may have favored the intervention group. This imbalance was mitigated by adjusting for important measured confounders in all analyses, but unmeasured confounders, including disease type and severity, and existing practice variations between wards may have influenced outcomes. Participants and research assistants could not be blinded, which may create reporting bias favoring the intervention. Delirium measurement relied on evidence in the medical record and the 3D-CAM, and there may have been misclassification bias between prevalent and incident cases of delirium, but this should not have affected the intervention and control groups differently. We report multiple outcomes in keeping with the logic model of our complex intervention, but our study was underpowered for secondary outcomes (discharge destination, readmissions, and mortality), which should be considered exploratory. We did not collect detailed racial and ethnic or socioeconomic data, and our study setting of public hospitals in large Australian population centers may not be generalizable to other settings. Although we enrolled almost two-thirds of eligible patients, those with more severe illness, greater frailty, and cognitive impairment may have been more likely to be excluded.
Conclusions
In this cluster randomized clinical trial, the Eat Walk Engage program did not reduce the composite outcome of any HAC-OP or reduce length of stay. There was a significant reduction in the incidence of delirium but not in other individual HAC-OPs. Continuing to learn about and improve this complex intervention program may enhance benefits as it expands to other hospitals.
Trial Protocol
Data Analysis Plan
eTable 1. Process Measures Used to Understand Current Care and Inform Improvements on Intervention Wards
eTable 2. Program Logic
eTable 3. Participant Characteristics by Ward
eTable 4. Length of Stay Difference Between Intervention and Control Participants by Preplanned Subgroups
eTable 5. Proportion of Intervention and Control Participants With Any of the Five Hospital-Associated Complications (HAC-OP), by Preplanned Subgroups
Data Sharing Statement
References
- 1.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451-458. doi: 10.1046/j.1532-5415.2003.51152.x [DOI] [PubMed] [Google Scholar]
- 2.Gajdos C, Kile D, Hawn MT, Finlayson E, Henderson WG, Robinson TN. Advancing age and 30-day adverse outcomes after nonemergent general surgeries. J Am Geriatr Soc. 2013;61(9):1608-1614. doi: 10.1111/jgs.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mudge AM, Hubbard RE. Management of frail older people with acute illness. Intern Med J. 2019;49(1):28-33. doi: 10.1111/imj.14182 [DOI] [PubMed] [Google Scholar]
- 4.Parke B, Chappell NL. Transactions between older people and the hospital environment: a social ecological analysis. J Aging Stud. 2010;24(2):115-124. doi: 10.1016/j.jaging.2008.09.003 [DOI] [Google Scholar]
- 5.Hubbard RE, Peel NM, Samanta M, Gray LC, Mitnitski A, Rockwood K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing. 2017;46(5):801-806. doi: 10.1093/ageing/afx081 [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780-791. doi: 10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mudge AM, McRae P, Hubbard RE, et al. Hospital-associated complications of older people: a proposed multicomponent outcome for acute care. J Am Geriatr Soc. 2019;67(2):352-356. doi: 10.1111/jgs.15662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton JK, Craig LE, Yong SQ, et al. Non-pharmacological interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2021;7(7):CD013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. doi: 10.1001/jamainternmed.2014.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YY, Yue JR, Xie DM, et al. Effect of the tailored, family-involved Hospital Elder Life Program on postoperative delirium and function in older adults: a randomized clinical trial. JAMA Intern Med. 2020;180(1):17-25. doi: 10.1001/jamainternmed.2019.4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulmer T, Mate KS, Berman A. The age-friendly health system imperative. J Am Geriatr Soc. 2018;66(1):22-24. doi: 10.1111/jgs.15076 [DOI] [PubMed] [Google Scholar]
- 12.de Foubert M, Cummins H, McCullagh R, Brueton V, Naughton C. Systematic review of interventions targeting fundamental care to reduce hospital-associated decline in older patients. J Adv Nurs. 2021;77(12):4661-4678. doi: 10.1111/jan.14954 [DOI] [PubMed] [Google Scholar]
- 13.Moore JE, Mascarenhas A, Marquez C, et al. ; MOVE ON Team . Mapping barriers and intervention activities to behaviour change theory for Mobilization of Vulnerable Elders in Ontario (MOVE ON), a multi-site implementation intervention in acute care hospitals. Implement Sci. 2014;9(1):160. doi: 10.1186/s13012-014-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greysen SR. Delirium and the “know-do” gap in acute care for elders. JAMA Intern Med. 2015;175(4):521-522. doi: 10.1001/jamainternmed.2014.7786 [DOI] [PubMed] [Google Scholar]
- 15.Mudge AM, McRae P, Cruickshank M. Eat Walk Engage: an interdisciplinary collaborative model to improve care of hospitalized elders. Am J Med Qual. 2015;30(1):5-13. doi: 10.1177/1062860613510965 [DOI] [PubMed] [Google Scholar]
- 16.Harvey G, Kitson A. PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci. 2016;11:33. doi: 10.1186/s13012-016-0398-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mudge AM, McRae P, Donovan PJ, Reade MC. Multidisciplinary quality improvement programme for older patients admitted to a vascular surgery ward. Intern Med J. 2020;50(6):741-748. doi: 10.1111/imj.14400 [DOI] [PubMed] [Google Scholar]
- 18.Mudge AM, Banks MD, Barnett AG, et al. CHERISH (Collaboration for Hospitalised Elders Reducing the Impact of Stays in Hospital): protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatr. 2017;17(1):11. doi: 10.1186/s12877-016-0399-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcantonio ER, Ngo LH, O’Connor M, et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161(8):554-561. doi: 10.7326/M14-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62(3):518-524. doi: 10.1111/jgs.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire–2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 23.Ferguson M, Capra S, Bauer J, Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15(6):458-464. doi: 10.1016/S0899-9007(99)00084-2 [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441. doi: 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 25.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 28.Gelman A, Carlin J, Stern H, Dunson D, Vehtari A, Rubin D. Bayesian Data Analysis. 3rd ed. Chapman and Hall/CRC; 2013. http://www.stat.columbia.edu/~gelman/book/. doi: 10.1201/b16018 [DOI] [Google Scholar]
- 29.The R Foundation. The R project for statistical computing. Accessed November 29, 2021. https://www.r-project.org/
- 30.Plummer M. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing. 2003;124(125.10):1-10. [Google Scholar]
- 31.GitHub. agbarnett/CHERISH. Accessed November 24, 2021. https://github.com/agbarnett/CHERISH
- 32.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 33.Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461. doi: 10.1016/j.jamda.2019.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibb K, Seeley A, Quinn T, et al. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing. 2020;49(3):352-360. doi: 10.1093/ageing/afaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen Y, Zisberg A, Chayat Y, et al. Walking for better outcomes and recovery: the effect of WALK-FOR in preventing hospital-associated functional decline among older adults. J Gerontol A Biol Sci Med Sci. 2019;74(10):1664-1670. doi: 10.1093/gerona/glz025 [DOI] [PubMed] [Google Scholar]
- 36.Reuben DB, Inouye SK, Bogardus ST Jr, Baker DI, Leo-Summers L, Cooney LM Jr. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48(12):1697-1706. doi: 10.1111/j.1532-5415.2000.tb03885.x [DOI] [PubMed] [Google Scholar]
- 37.Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63(1):55-62. doi: 10.1111/jgs.13193 [DOI] [PubMed] [Google Scholar]
- 38.Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview: the SENATOR project ONTOP series. PLoS One. 2015;10(6):e0123090. doi: 10.1371/journal.pone.0123090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young J, Green J, Farrin A, et al. A multicentre, pragmatic, cluster randomised, controlled feasibility trial of the POD system of care. Age Ageing. 2020;49(4):640-647. doi: 10.1093/ageing/afaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith J, Green J, Siddiqi N, et al. Investigation of ward fidelity to a multicomponent delirium prevention intervention during a multicentre, pragmatic, cluster randomised, controlled feasibility trial. Age Ageing. 2020;49(4):648-655. doi: 10.1093/ageing/afaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inouye SK, Baker DI, Fugal P, Bradley EH; HELP Dissemination Project . Dissemination of the Hospital Elder Life Program: implementation, adaptation, and successes. J Am Geriatr Soc. 2006;54(10):1492-1499. doi: 10.1111/j.1532-5415.2006.00869.x [DOI] [PubMed] [Google Scholar]
- 42.Godfrey M, Green J, Smith J, et al. Process of implementing and delivering the Prevention of Delirium system of care: a mixed method preliminary study. BMC Geriatr. 2019;20(1):1. doi: 10.1186/s12877-019-1374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clinical Excellence Queensland . Improving the quality, safety and care of older Queenslanders: inpatient care. Published 2019. Accessed July 26, 2021. https://clinicalexcellence.qld.gov.au/priority-areas/service-improvement/improving-quality-safety-and-care-older-queenslanders/inpatient
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Analysis Plan
eTable 1. Process Measures Used to Understand Current Care and Inform Improvements on Intervention Wards
eTable 2. Program Logic
eTable 3. Participant Characteristics by Ward
eTable 4. Length of Stay Difference Between Intervention and Control Participants by Preplanned Subgroups
eTable 5. Proportion of Intervention and Control Participants With Any of the Five Hospital-Associated Complications (HAC-OP), by Preplanned Subgroups
Data Sharing Statement