Key Points
Question
What is the benefit-risk balance associated with long-term treatment with intravenous edaravone as an add-on treatment to standard therapy with riluzole among patients with amyotrophic lateral sclerosis (ALS)?
Findings
In this multicenter, propensity score–matched cohort study of 324 patients with ALS, long-term intravenous edaravone treatment was feasible and was mainly well tolerated. However, disease progression rates, time to noninvasive ventilation, and survival probability did not significantly differ from matched patients with ALS receiving only standard therapy.
Meaning
These findings show that reatment with long-term intravenous edaravone in addition to standard therapy for patients with ALS is feasible and relatively safe but may not provide a clinically relevant benefit.
Abstract
Importance
Intravenous edaravone is approved as a disease-modifying drug for patients with amyotrophic lateral sclerosis (ALS), but evidence for efficacy is limited to short-term beneficial effects shown in the MCI186-ALS19 study in a subpopulation in which efficacy was expected.
Objective
To evaluate the long-term safety and effectiveness of intravenous edaravone therapy for patients with ALS in a real-world clinical setting.
Design, Setting, and Participants
Multicenter, propensity score–matched cohort study conducted between June 2017 and March 2020 at 12 academic ALS referral centers associated with the German Motor Neuron Disease Network. Of 1440 patients screened, 738 were included in propensity score matching. Final analyses included 324 patients with ALS comprising 194 patients who started intravenous edaravone treatment (141 received ≥4 consecutive treatment cycles; 130 matched) and 130 propensity score–matched patients with ALS receiving standard therapy. All patients had probable or definite ALS according to the El Escorial criteria, with disease onset between December 2012 and April 2019. Subgroups were defined by applying the MCI186-ALS19 study inclusion criteria to evaluate whether patients would have been considered eligible (EFAS) or ineligible (non-EFAS).
Exposures
Intravenous edaravone plus riluzole vs riluzole only.
Main Outcomes and Measures
Patient characteristics and systematic safety assessment for patients who received at least 1 dose of intravenous edaravone. Effectiveness assessment of edaravone was conducted among patients who received at least 4 treatment cycles compared with propensity score–matched patients with ALS who received only standard therapy. Primary outcome was disease progression measured by decrease in the ALS Functional Rating Scale–Revised (ALSFRS-R) score. Secondary outcomes were survival probability, time to ventilation, and change in disease progression before vs during treatment. To account for the matched design, patients receiving edaravone and their corresponding matched controls were regarded as related samples in disease progression analyses; stratification for propensity score quintiles was used for survival probability and time to ventilation analyses.
Results
A total of 194 patients started intravenous edaravone treatment; 125 (64%) were male, and the median age was 57.5 years (IQR, 50.7-63.8 years). Potential adverse effects were observed in 30 cases (16%), most notably infections at infusion sites and allergic reactions. Disease progression among 116 patients treated for a median of 13.9 months (IQR, 8.9-13.9 months) with edaravone did not differ from 116 patients treated for a median of 11.2 months (IQR, 6.4-20.0 months) with standard therapy (ALSFRS-R points/month, −0.91 [95% CI, −0.69 to −1.07] vs −0.85 [95% CI, −0.66 to −0.99]; P = .37). No significant differences were observed in the secondary end points of survival probability, time to ventilation, and change in disease progression. Similarly, outcomes between patients treated with edaravone and matched patients did not differ within the EFAS and non-EFAS subgroups.
Conclusions and Relevance
This cohort study using propensity score matching found that, although long-term intravenous edaravone therapy for patients with ALS was feasible and mainly well tolerated, it was not associated with any disease-modifying benefit. Intravenous edaravone may not provide a clinically relevant additional benefit compared with standard therapy alone.
This multicenter cohort study uses real-world clinical data to compare the long-term safety and effectiveness of intravenous edaravone plus riluzole therapy vs propensity score–matched controls receiving riluzole therapy alone among patients with amyotrophic lateral sclerosis.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that causes muscle weakness, atrophy, and eventually nutritional and respiratory failure leading to a fatal outcome.1 Median survival ranges from approximately 2 to 4 years from symptom onset.2 Riluzole has a small effect on survival3 and is currently the only disease-modifying drug approved by all major drug authorities. Hence, there is an urgent need for more effective therapies.
In 2006, edaravone (MCI186) was introduced into clinical ALS research4 after it slowed the decline of motor function in the Sod1 (superoxide dismutase-1) transgenic rodent model of ALS.5,6 Oxygen radical scavenging properties are suggested to provide a neuroprotective effect in patients with ALS as well as in patients after ischemic stroke.7,8 For patients with ALS, an infusion regimen was evaluated with a dosage of 60 mg/d administered intravenously in an alternating cycle of 10 of 14 days of treatment with 14 days off. Edaravone development for ALS was exclusively performed in Japan and included 547 Japanese patients with ALS in 2 randomized clinical trials (MCI186-ALS169 and MCI186-ALS1910), 2 extension studies (MCI186-ALS1711 and MCI186-ALS19 extension),12,13 and a small trial for patients with advanced disease (MCI186-ALS18).14
The first 3 trials failed to reach the primary end point; however, post hoc analysis of the MCI186-ALS16 trial revealed an efficacy-expected subpopulation (EESP)15 comprising patients with a maximum disease duration of 2 years, preserved respiratory function, minor functional impairment, and moderate disease progression. The MCI186-ALS19 trial enrolled only patients meeting the EESP criteria and found that edaravone slowed disease progression by approximately 30% after 24 weeks, measured by a decreased score on the ALS Functional Rating Scale–Revised (ALSFRS-R).10
In summary, evidence for the efficacy of edaravone in the treatment of patients with ALS is limited to short-term beneficial effects on disease progression observed in the MCI186-19 study in Japanese patients meeting the EESP criteria. Several major drug agencies, including the US Food and Drug Administration, approved edaravone for treatment of ALS without restricting the indication for an EESP.16,17 By contrast, the European Medical Agency assessed the evidence as too uncertain to warrant approval.18 In Germany, edaravone was available under special access conditions.
Real-world evidence studies have become a key part of evaluating new drugs19 and have been suggested during the European Medical Agency approval process to improve evidence for edaravone.18 In the present prospective multicenter cohort study, we evaluated the use of intravenous edaravone with respect to disease progression, survival probability, time to ventilation, safety, and burden of the treatment in actual clinical application.
Methods
Study Design and Participants
This propensity score–matched multicenter cohort study compares patients with ALS treated with intravenous edaravone plus the standard therapy of riluzole with propensity score–matched patients with ALS receiving only riluzole. The study was conducted between June 2017 and March 2020 in 12 academic referral centers of the German Motor Neuron Disease Network (MND-NET). Local ethics committees approved the study, and all patients gave written informed consent that was obtained in a manner consistent with the Declaration of Helsinki.20 No one received compensation or was offered any incentive for participating in this study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Edaravone was administered according to the infusion regimen used in the MCI186-ALS19 study. Infusions were conducted either by physicians in their medical practice or by nurses trained in the intravenous application under a physician’s delegation at home. Patients were treated in the frame of regular public health care; hence, therapeutic decisions and medical care during the treatment were carried out by the treating physicians and were not defined by a specific protocol.
Safety and Outcome Parameters
Safety was assessed among patients who received at least 1 dose of intravenous edaravone during the study period by evaluating potentially associated adverse effects and hospitalizations as well as by checking a disproportionate increase in disease progression and excess mortality.
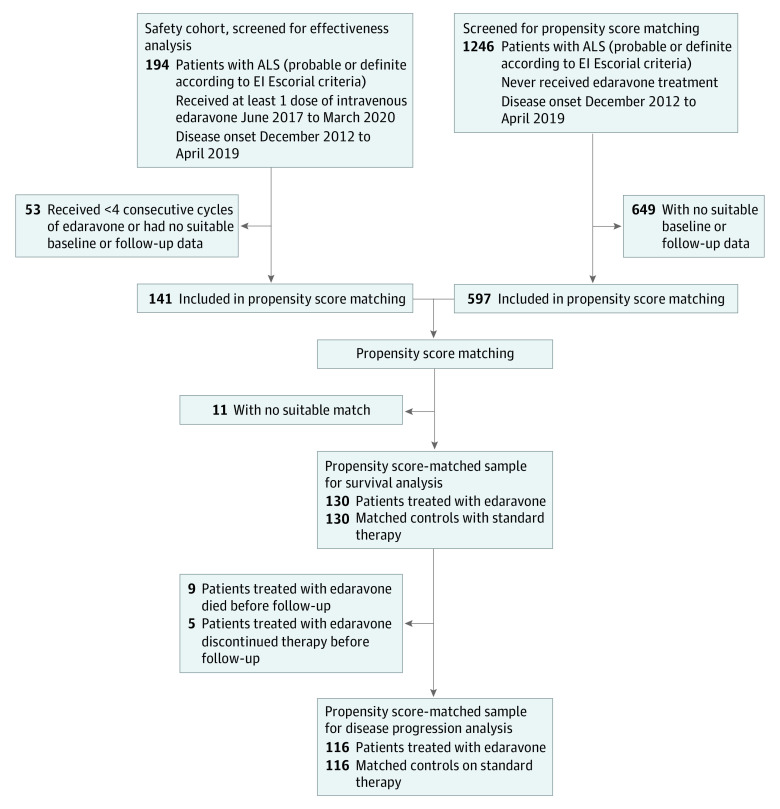
Patients who received at least 4 consecutive cycles of edaravone (16 weeks of treatment) were included in propensity score matching and effectiveness analyses. As potential matches, we assessed patients with ALS from the participating MND-NET centers with disease onset during the same period as patients receiving edaravone (from December 2012 to April 2019) but who had never been treated with edaravone. Patients receiving edaravone and control patients not receiving edaravone had to meet the El Escorial criteria for probable (including laboratory-supported) or definite ALS (Figure 1). Study baseline was the start of the edaravone treatment for patients receiving edaravone or the first onsite visit for control patients. Follow-up included the time between baseline and death, discontinuation of edaravone treatment, last patient visit, or the end of data collection (March 31, 2020).
Figure 1. Participant Flow Through Study Analyses.
ALS indicates amyotrophic lateral sclerosis.
Propensity score matching was conducted using the method described by Thoemmes21 with the following parameters: nearest-neighbor matching (1:1) with a caliper of 0.2; exact matching for site of disease onset; and covariates of age at onset, disease duration, and baseline ALSFRS-R score. Matching quality was evaluated by comparing baseline characteristics between patients receiving edaravone and matched control patients.
The primary outcome to evaluate the effectiveness of edaravone was disease progression during follow-up, measured by ALSFRS-R score points lost per month (ALSFRS-R treatment slope). The monthly decrease was computed via linear regression, including the ALSFRS-R score at baseline and all ALSFRS-R scores of the follow-up period.
Secondary outcomes were survival probability, ventilation-free survival (any use of noninvasive or invasive ventilation or death without ventilation), and the change in disease progression rates from before baseline to treatment, measured using the ALSFRS-R score. Disease progression rates before baseline (ΔFRS) relate to the score points lost since the onset of ALS computed using the formula ΔFRS = (ALSFRS-R Score − 48)/Disease Duration in Months, with 48 being the maximum ALSFRS-R score. The change in disease progression from before baseline to the treatment period was calculated by subtracting ΔFRS from ALSFRS-R treatment slopes.
EESP Subgroups
For each patient receiving edaravone, we assessed the fulfillment of the EESP criteria used as eligibility criteria in the MCI186-ALS19 study.10 At the time of the MCI186-ALS19 study enrollment, the criteria included (1) age between 20 and 75 years, (2) definite or probable ALS according to the revised El Escorial criteria,22 (3) maximum disease duration of 2 years, (4) a score of at least 2 points for each item of the ALSFRS-R and after a 12-week observation period between study enrollment and the start of the intervention, (5) preserved respiratory function (forced vital capacity ≥80%), and (6) ALSFRS-R score decreased by 1 to 4 points throughout the 12-week observation period.
To account for the nonexistence of a 12-week observation period in a real-world setting, we adjusted the EESP criteria and converted the allowed ALSFRS-R point loss during the observation period of the MCI186-ALS19 study to a monthly point loss before baseline (−0.33 to −1.33 points/month based on ΔFRS). For patients without forced vital capacity data at baseline, we alternatively evaluated preserved respiratory function by transcutaneous nocturnal capnography measures within reference ranges or the absence of clinical signs for respiratory insufficiency assessed by a full score in the respiratory subitems of the ALSFRS-R. Considering the disease progression that occurred in the MCI186-ALS19 study during the observation period (mean, −0.62 ALSFRS-R points/month10), we defined the following subgroups: (1) EFAS, which included patients potentially eligible for the MCI186-ALS19 study (ie, patients meeting 5 or 6 of 6 EESP criteria at treatment start); and (2) non-EFAS, which included patients ineligible for the MCI186-ALS19 study (ie, all other patients). Patients in the control group were assigned to the EFAS or non-EFAS subgroup in accordance with their matched patient who was receiving edaravone.
Treatment Adherence and Satisfaction
We evaluated the adherence to edaravone treatment by treatment time and assessed the treatment status (ongoing, discontinued, or died) in 3 monthly intervals. In case of treatment discontinuation, we assessed the reason. To analyze treatment satisfaction, we asked patients to complete the 9-item Treatment Satisfaction Questionnaire of Medication (TSQM-9) instrument,23 either the paper version or the online version for patients who participated using the APST (Ambulanzpartner Soziotechnologie) case management platform.24 The questionnaire was modified by leaving out the items “How satisfied or dissatisfied are you with the way the medication relieves your symptoms?” and “How satisfied or dissatisfied are you with the amount of time it takes the medication to start working?” because these items apply only to a limited extent to therapy with edaravone.
Statistical Analysis
We used SPSS, version 26.0 (SPSS Inc), with SPSS extension PSmatching3 for statistical analyses. In normally distributed items (tested with Kolmogorov-Smirnov statistics), mean (SD) values are reported, otherwise median (IQR) values are reported. All tests were performed at a level of significance of P = .05. Missing data were handled via pairwise deletion; if less than 95% of data points were available, we additionally reported the exact number. We used a 2-sided t test for either independent or related samples to compare normally distributed variables or the Mann-Whitney test (independent samples) and the Wilcoxon signed rank test (related samples) for nonnormally distributed variables. Categorical variables were compared using the χ2 test. To account for the matched design in the propensity score–matched sample, ALSFRS-R treatment slopes and slope changes for patients receiving edaravone and their corresponding matched controls were regarded as related samples; for survival probability and time to ventilation analyses, we used Kaplan-Meier plots with a log-rank test stratified for propensity score quintiles.25
Results
Patient Characteristics
Between June 2017 and March 2020, 194 patients with ALS had started intravenous edaravone treatment (125 men [64%] and 69 women [36%]) (eFigure 1 in Supplement 1). Taking each patient’s treatment time into consideration, we analyzed approximately 2400 edaravone treatment cycles corresponding to 190 treatment years.
At treatment start, patients receiving edaravone had a median disease duration of 16.5 months (IQR, 10.6-24.2 months) and a median age of 57.5 years (IQR, 50.7-63.8 years), and 186 patients (97%) were administered riluzole. The median ALSFRS-R score at therapy initiation was 37 (IQR, 32-42), and the median prebaseline disease progression was −0.58 points per month (IQR, −0.35 to −1.06 points per month) (Table 1). At analysis, 141 patients (73%) had received at least 4 cycles of edaravone. In 11 cases, the propensity score matching yielded no suitable control patient; thus, 130 patients were included in the outcome analyses (Figure 1 and eFigure 2 in Supplement 1). Baseline characteristics did not differ between patients treated with edaravone and matched controls, indicating proper matching quality (Table 2).
Table 1. Demographic and Clinical Characteristics of Patients Treated With Edaravone.
| Characteristic | No. (%) of patients | P value for EFAS vs non-EFAS | ||
|---|---|---|---|---|
| Total (N = 194) | EFAS (n = 64) | Non-EFAS (n = 130) | ||
| Baseline characteristics | ||||
| Sex | ||||
| Female | 69 (36) | 23 (36) | 46 (35) | .94 |
| Male | 125 (64) | 41 (64) | 84 (65) | |
| Symptom onset | ||||
| Spinal | 139 (71) | 47 (73) | 92 (71) | .88 |
| Bulbar | 52 (27) | 17 (27) | 35 (27) | |
| Unknown | 3 (2) | 0 | 4 (2) | |
| Age, median (IQR), y | ||||
| At disease onset | 55.6 (48.9 to 61.9) | 55.8 (48.6 to 61.2) | 55.6 (48.9 to 51.1) | .88 |
| At therapy start | 57.5 (50.7 to 63.8) | 57.3 (49.4 to 62.7) | 57.5 (51.1 to 64.4) | .69 |
| Disease duration at therapy start, mo | 16.5 (10.6 to 24.4) | 12.8 (10.0 to 17.6) | 19.1 (11.8 to 28.2) | <.001 |
| Receipt of riluzole | 186 (97) | 63 (98) | 123 (96) | .38 |
| ALSFRS-R score at start of therapy, median, (IQR), points | 37 (32 to 42) | 39.5 (36 to 42) | 34 (30 to 41) | <.001 |
| No. | 183 | 64 | 119 | |
| ΔFRS, median (IQR), points/mo | −0.58 (−0.35 to −1.06) | −0.61 (−0.46 to −0.93) | −0.58 (−0.29 to −1.22) | .83 |
| No. | 183 | 64 | 119 | |
| EESP criteria met | ||||
| Age between 20 and 75 y | 185 (95) | 64 (100) | 121 (93) | .04 |
| Definite or probable ALS | 194 (100) | 64 (100) | 130 (100) | >.99 |
| Maximum disease duration of 2 y | 144 (74) | 63 (98) | 81 (62) | <.001 |
| ALSFRS-R slope of −0.33 to −1.33 points/mo | 111 (57) | 58 (91) | 53 (41) | <.001 |
| ≥2 Points for each item of the ALSFRS-R | 41 (21) | 29 (45) | 12 (9) | <.001 |
| Preserved respiratory function | 115 (59) | 58 (91) | 57 (44) | <.001 |
| Follow-up | ||||
| Treatment duration, median (IQR), wk | 50.1 (26.1 to 78.7) | 62.0 (37.9 to 84.6) | 43.3 (24.7 to 78.0) | .03 |
| No. | 175 | 115 | 60 | |
| ALSFRS-R treatment slope, median (IQR), points/moa | −0.85 (−0.37 to −1.45) | −0.97 (−0.56 to −1.60) | −0.77 (−0.30 to −1.38) | .14 |
| No. | 134 | 53 | 82 | |
| Slope change, median (IQR), points/moa,b | −0.22 (−0.88 to 0.20) | −0.31 (−1.00 to 0.15) | −0.19 (−0.87 to 0.22) | .43 |
| No. | 131 | 53 | 78 | |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, ALS Functional Rating Scale–Revised; EESP, efficacy-expected subpopulation; ΔFRS, change in ALSFRS-R score slope between disease onset and baseline; EFAS, potentially eligible for the MCI186-ALS19 study; non-EFAS, ineligible for the MCI186-ALS19 study.
If received 4 or more consecutive cycles of edaravone.
Slope change is the ALSFRS-R treatment slope minus ΔFRS; negative values signify an acceleration of the ALSFRS-R score decrease.
Table 2. Patient Characteristics in the Propensity Score–Matched Sample.
| Characteristic | No. (%) of patients | |||||
|---|---|---|---|---|---|---|
| Totala | EFASa | Non-EFASa | ||||
| Edaravone (n = 130) | Matched controls (n = 130) | Edaravone (n = 52) | Matched controls (n = 52) | Edaravone (n = 78) | Matched controls (n = 78) | |
| Sex | ||||||
| Female | 48 (37) | 47 (36) | 19 (37) | 18 (35) | 29 (37) | 29 (37) |
| Male | 82 (63) | 83 (64) | 33 (63) | 34 (65) | 49 (63) | 49 (63) |
| Symptom onset | ||||||
| Spinal | 97 (75) | 97 (75) | 37 (71) | 37 (71) | 60 (77) | 60 (77) |
| Bulbar | 33 (25) | 33 (25) | 15 (29) | 15 (29) | 18 (23) | 18 (23) |
| Age, median (IQR), y | ||||||
| At disease onset | 56.1 (49.0 to 63.3) | 55.4 (48.0 to 62.3) | 55.8 (47.1 to 60.8) | 56.2 (52.1 to 62.4) | 56.2 (49.8 to 63.5) | 53.2 (47.6 to 62.6) |
| At baseline | 57.5 (50.5 to 64.5) | 56.7 (49.8 to 63.8) | 57.2 (47.8 to 62.6) | 57.8 (53.5 to 63.2) | 57.7 (51.8 to 64.8) | 54.9 (48.8 to 64.8) |
| Baseline, median (IQR) | ||||||
| Disease duration, mo | 16.4 (10.3 to 23.0) | 15.5 (10.1 to 24.3) | 12.9 (9.97 to 18.6) | 15.1 (8.24 to 25.2) | 19.0 (11.7 to 28.1) | 16.1 (10.7 to 22.9) |
| ALSFRS-R, score | 38 (32.7 to 42) | 39 (35 to 42) | 39.5 (36 to 42) | 39 (36 to 42.7) | 36 (30 to 42) | 38 (34 to 42) |
| ΔFRS, median (IQR), points/mo | −0.58 (−1.07 to −0.31) | −0.52 (−1.02 to −0.32) | −0.61 (−0.97 to −0.43) | −0.54 (−1.04 to −0.29) | −0.56 (−1.30 to −0.24) | −0.52 (−0.96 to −0.35) |
| Follow-up, median (IQR), mo | 12.7 (7.6 to 18.5) | 11.1 (6.4 to 19.2) | 14.9 (9.6 to 21.9) | 12.4 (6.2 to 22.2) | 10.3 (6.4 to 17.2) | 11.1 (6.4 to 18.0) |
Abbreviations: ALSFRS-R, ALS Functional Rating Scale-Revised; EFAS, potentially eligible for the MCI186-ALS19 study; ΔFRS, change in ALSFRS-R slope between disease onset and baseline; non-EFAS, ineligible for the MCI186-ALS19 study.
Data formally tested for the total population as well as the EFAS and non-EFAS subgroups, and no statistically significant differences were found between patients receiving edaravone and their matched controls.
EESP Criteria
At the start of treatment, 64 patients (33%) belonged to the EFAS group, including 16 patients (8%) meeting all 6 EESP criteria and 48 patients (25%) meeting 5 of 6 criteria; of patients meeting 5 criteria, most (35 patients) did not have at least 2 points for each ALSFRS-R item. This EESP criterion, which was critical for the entire cohort, was met by only 41 of 194 patients (21%). Comparing the baseline characteristics between patients in the EFAS subgroup and patients in the non-EFAS subgroup (Table 1), we found no significant differences in age, sex, site of symptom onset, and ΔFRS. By contrast, for patients in the EFAS subgroup, disease duration at treatment initiation was approximately 6 months shorter (median, 12.8 months [IQR, 10.0-17.6 months] vs 19.1 months [IQR, 11.8-28.2 months]; P < .001), the ALSFRS-R score at treatment start was 5.5 points higher (median, 39.5 points [IQR, 36.0-42.0 points] vs 34.0 points [IQR, 30.0-41.0 points]; P < .001), and respiratory function was mostly preserved (58 patients [91%] vs 57 patients [44%]; P < .001).
Effectiveness
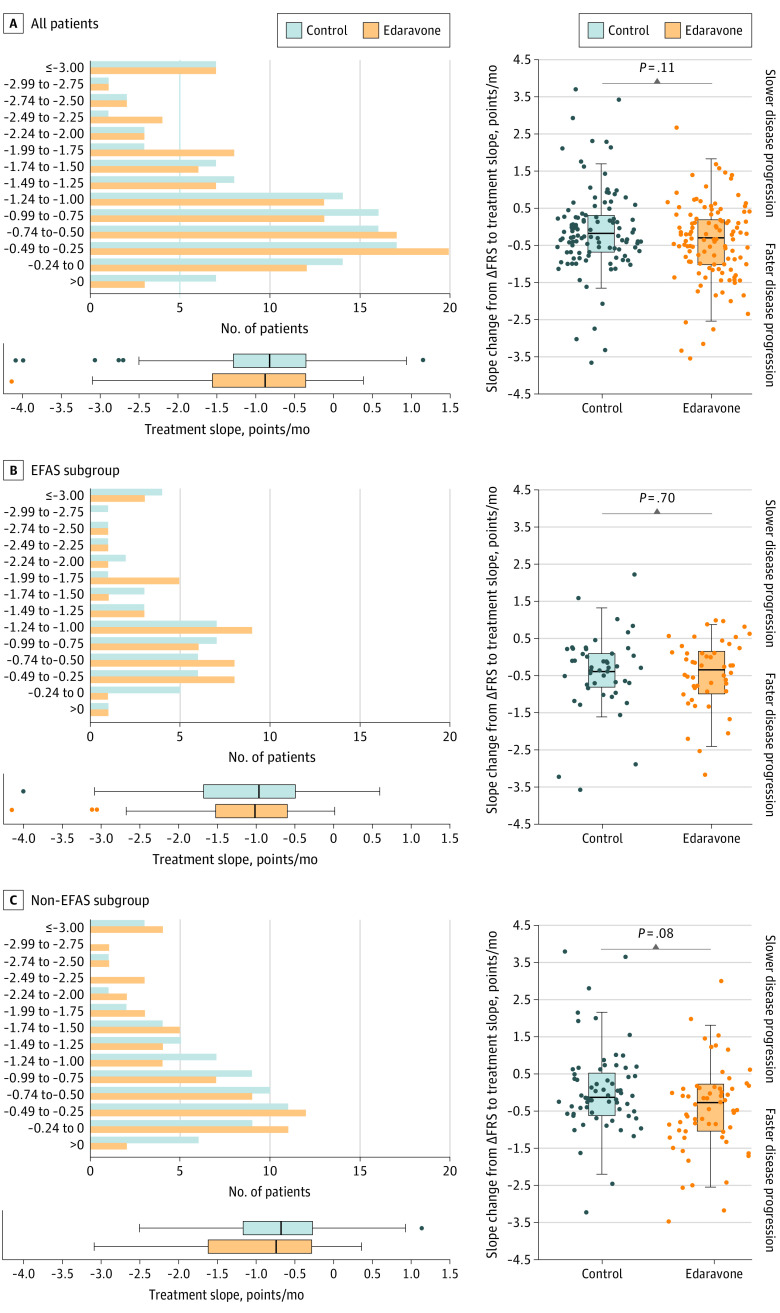
Disease progression during follow-up was −0.91 points/month (95% CI, −0.69 to −1.07 points/month) for 116 patients treated for a median of 13.9 months (IQR, 8.9-13.9 months) with edaravone compared with −0.85 points/month (95% CI, −0.66 to −0.99 points/month) for 116 control patients treated a median of 11.2 months (IQR, 6.4-20.0 months) with standard therapy, not a statistically significant difference (P = .37). In line with the results in the entire cohort, analyses of the primary end point in the EFAS and non-EFAS subgroups showed no significant differences (Figure 2).
Figure 2. Disease Progression in the Propensity Score–Matched Sample.
Left panels show treatment slope in amyotrophic lateral sclerosis (ALS) Functional Rating Scale–Revised (ALSFRS-R) score points per month during follow-up for all patients, EFAS subgroup, and non-EFAS subgroup. Box and whisker plots show median (central line), IQR (boxes), and 1.5 × IQR (whiskers), with individual points representing outliers. Right panels showing individual changes in ALSFRS-R slopes from ΔFRS (before baseline) to treatment slopes (follow-up) are displayed with box plots for groups (median, central line; IQR, boxes, and 1.5 × IQR, whiskers) overlaid with dots for single patients. Negative values signify faster disease progression during follow-up. Wilcoxon signed rank tests were used to compare treatment groups. ΔFRS indicates change in ALSFRS-R slope between disease onset and baseline; EFAS, subgroup of patients potentially eligible for the MCI186-ALS19 study; and non-EFAS, subgroup ineligible for the MCI186-ALS19 study.
For patients receiving edaravone who had both short- and long-term follow-up data, we compared the short-term ALSFRS-R score decrease (up to 24 weeks) with the decrease within the entire follow-up period to investigate a potential temporary slowdown of disease progression. No relevant difference was observed (first 24 weeks: −0.91 points/month [IQR, −1.73 to −0.27 points/month]; entire follow-up: −1.00 point/month [IQR, −1.50 to −0.56 points/month]; n = 62; P = .94; see the eAppendix in Supplement 1 for the full short-term analysis).
Supporting the primary outcome analysis results, we observed no significant differences when we compared the median change in disease progression before baseline (ΔFRS) with the median disease progression during treatment (edaravone, −0.31 points/month [95% CI, −0.15 to −0.51 points/month]; control, −0.19 points/month [95% CI, −0.08 to −0.15 points/month]; P = .11). Again, we found similar findings in the EFAS and non-EFAS subgroups (Figure 2).
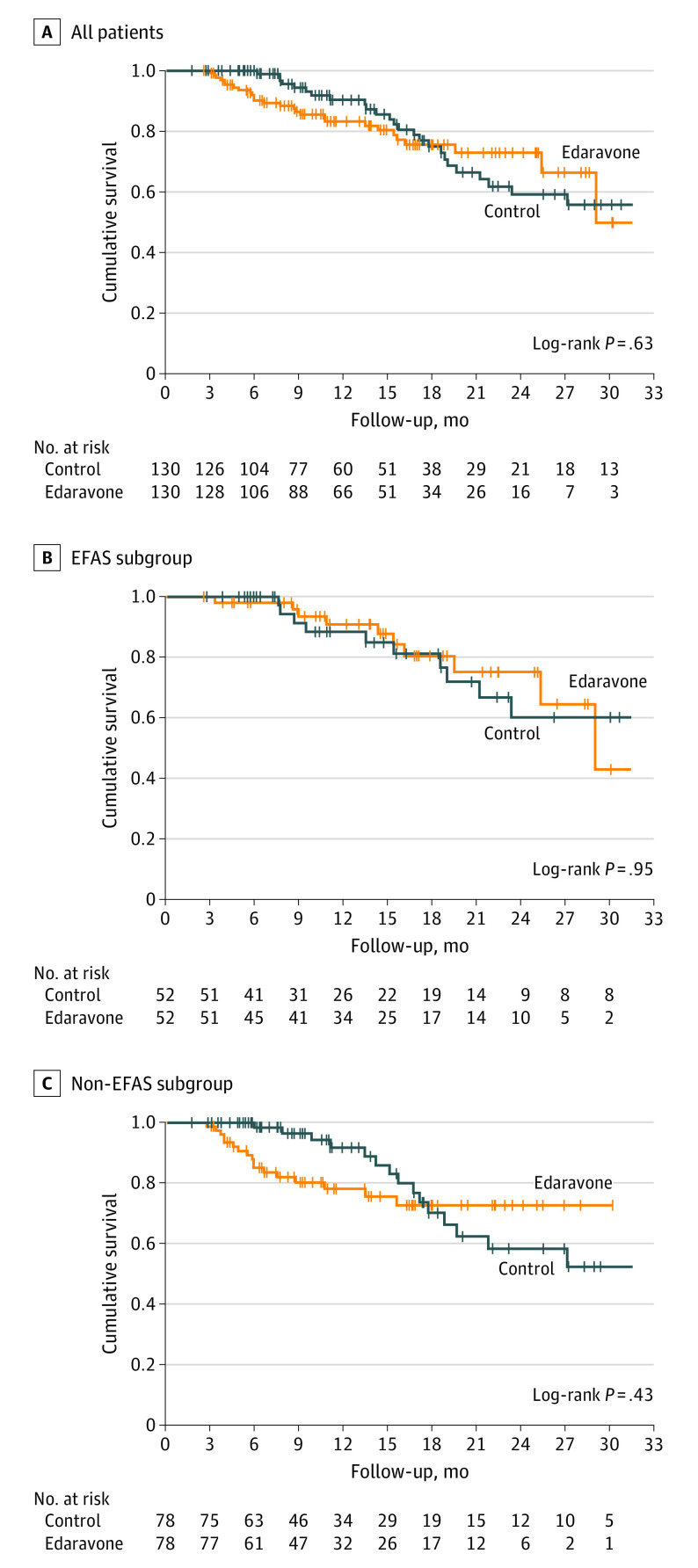
Consistent with the results for disease progression, survival probability after baseline (median follow-up, 11.4 months [IQR, 6.6-18.9 months]; n = 260; Figure 3) did not significantly differ between patients treated with edaravone and control patients. The survival probability 18 months after baseline was identical in the edaravone group (75%; SE, 4.8%) and in the control group (75%; SE, 5.4%). Furthermore, subgroup analyses for patients in the EFAS vs non-EFAS groups yielded no statistically significant differences.
Figure 3. Kaplan-Meier Plots for Survival Probability During Follow-up.
Panels show the propensity score–matched sample for survival probability analysis. If not deceased, control patients were censored at last visit. Patients receiving edaravone who discontinued therapy were censored at the time of discontinuation, and patients with ongoing follow-up were censored at the last patient contact. EFAS indicates potentially eligible for the MCI186-ALS19 study; non-EFAS, ineligible for the MCI186-ALS19 study.
In total, 62 patients (29%) started ventilation during follow-up, whereas 39 patients (15%) were already using ventilation at baseline. Supporting our survival probability data, the median ventilation-free survival after baseline (edaravone, 21.7 months [95% CI, 15.0-28.3 months]; control, 19.7 months [95% CI, 15.2-24.1 months]; P = .40; eFigure 3 in Supplement 1) did not significantly differ between patients treated with edaravone and patients receiving standard therapy alone—neither in all patients nor in the EFAS and non-EFAS subgroups.
Safety
Potential treatment-associated adverse effects were documented in 30 cases (16%); 20 events (67%) occurred in the first 24 weeks of treatment. Observed adverse events can be divided into 2 categories: (1) potential drug-associated events (n = 24; EFAS, 12 events [19%]; non-EFAS, 12 events [11%]; P = .07), which included allergic reactions (4 events), orthostatic dysregulation (4 events), fatigue (4 events), eczema or dermatitis (3 events), gait disturbance (2 events), and visual disturbance, hot flushes, general weakness, headache, elevated transaminases, intracranial bleeding under comedication of oral anticoagulation, shortness of breath (each 1 event) and (2) infusion-related events (n = 6; EFAS, 3 events; non-EFAS, 3 events; P = .40), which included port infections (5 events), and thrombophlebitis (1 event).
In 7 cases, adverse events were associated with hospitalization independent of ALS disease progression, mostly owing to port issues due to port infections. Allergic reactions were observed only in the first 24 weeks, whereas thrombophlebitis and port infections were observed both during the first 24 weeks and during long-term treatment. We did not observe any notable adverse events after the discontinuation of treatment.
Patients treated with edaravone did not show a disproportionate increase in disease progression (Figure 2) or excess mortality. Patients who died while receiving active treatment with edaravone (n = 33) had worse prognostic factors at baseline compared with patients who survived while receiving treatment with edaravone: faster disease progression before treatment (ΔFRS −0.85 points/month [IQR, −0.47 to −1.30 points/month] vs −0.52 points/month [IQR, −0.30 to −0.94 points/month]; P = .003), older age at disease onset (57.1 years [IQR, 52.4-67.2 years] vs 55.5 years [IQR, 48.0-61.7 years]; P = .04), and lower ALSFRS-R score (34.0 [IQR, 29.5-40.0] vs 38.0 [IQR, 32.8-42], P = .02); moreover, the proportion of patients with impaired respiratory function was slightly but not significantly higher (16 of 33 [49%] vs 50 of 140 [36%]; P = .16).
Treatment Adherence and Satisfaction
At analysis, 91 patients (47%) who started edaravone treatment had ongoing therapy, 51 patients (26%) discontinued treatment, 33 patients died (17%), and 19 patients (10%) were lost to follow-up. The treatment status did not differ between subgroups (P = .39). However, the median treatment time was longer in the EFAS subgroup (62.0 weeks; IQR, 37.9-84.6 weeks) than in the non-EFAS subgroup (43.3 weeks; IQR, 24.7-78.0 weeks; P = .03), which was caused by differences in patients who discontinued therapy or died (median 46.9 weeks [IQR, 25.1-63.3 weeks] vs 24.7 weeks [IQR, 14.8-37.3 weeks]; P = .005), whereas patients with ongoing therapy did not differ (median 74.0 weeks [IQR, 55.1-103.3 weeks] in the EFAS subgroup vs 71.2 weeks [IQR, 43.3-96.9 weeks] in the non-EFAS subgroup; P = .50) (eFigure 4 in Supplement 1). The reasons for discontinuation were heterogeneous: patient’s decision in 15 cases (29%), cost coverage by health insurance not extended in 9 cases (18%), adverse effects in 8 cases (16%), incapability of ensuring infusions in an outpatient setting in 3 cases (6%), and unknown reason in 16 cases (31%).
The median effectiveness (66.6% [IQR, 33.3%-83.3%]), convenience (61.1% [IQR, 38.9%-77.8%]), and global satisfaction (55.6% [IQR, 33.3%-76.4%]) scores for the modified TSQM-9 questionnaire (n = 68) were widely scattered, with each subscore rated in the upper half of the scale. Patients receiving edaravone who discontinued therapy showed lower global satisfaction, whereas their effectiveness and convenience scores were not significantly lower (eFigure 5 in Supplement 1). The TSQM-9 scores showed no significant differences between EFAS and non-EFAS subgroups.
Discussion
The main finding of this multicenter, propensity score–matched cohort study was that disease progression rates among patients with ALS receiving edaravone in addition to standard therapy (riluzole) did not differ from patients treated with standard therapy alone. This result did not depend on whether patients belonged to the subpopulation with expected drug efficacy with respect to the MCI186-ALS19 study. Although we primarily evaluated outcomes associated with long-term treatment, additional analyses in our study provided no evidence that edaravone treatment was associated with a temporary, short-term benefit.
Evidence for the efficacy of edaravone in long-term use is scarce, to our knowledge. The extension studies in the edaravone development program11,12,13 covered treatment for 48 weeks but did not include control patients because the original placebo group was switched to edaravone after 24 weeks. Consistent with our results, an observational study by Lunetta and colleagues26 found no benefit in disease progression for up to 12 months when they compared 197 patients who started treatment with edaravone with historical controls from the PRO-ACT (Pooled Resource Open-Access ALS Clinical Trials) database. However, the changes in medical care since data collection for historical controls and the differences in age and disease progression rates at baseline between patients who received edaravone and controls are limitations. Our study investigated propensity score–matched controls participating in a single observational study to achieve the best possible comparability and ensure identical medical care structures during follow-up. Propensity score matching replicates some characteristics of randomized clinical trials and can improve effectiveness analysis under nonrandomized conditions.27
Consistent with the key outcome of the present study, we observed no beneficial effects for survival probability associated with administration of edaravone. To date, survival data from randomized clinical trials are lacking, and retrospective data from smaller studies28,29,30 are contradictory. In line with our results, Vu and colleagues31 observed no significant differences for survival when they compared 96 older and mostly male patients in the US Department of Veterans Affairs who had a mean edaravone treatment time of 10 months with propensity score–matched controls who also had ALS. Our study provides survival data for long-term treatment with edaravone in a more general ALS cohort and, in addition, facilitates evaluating the subgroup with expected drug efficiency. Consistent with the results of our survival probability analyses, no benefit of edaravone treatment was observed for time to ventilation.
Our study reports adverse effects consistent with those observed in the edaravone development program and in the postmarketing experience and does not point toward more serious safety concerns for long-term treatment compared with short-term treatment. Our study population included European patients treated up to 32 months and a relevant number of patients in more advanced disease stages and with impaired respiratory function, a population that has rarely been evaluated. Although edaravone treatment was mainly well tolerated, allergic reactions and infections at the infusion site associated with hospitalization in single cases suggested a residual treatment risk, which, in terms of infusion-related events, was already suggested by previous work.32,33
Despite the burden of high-frequency infusions, patients were generally satisfied with edaravone treatment and were predominantly willing to adhere to long-term therapy. We found that treatment initiation in the early stages of disease was associated with significantly longer treatment adherence but not necessarily higher satisfaction. We also observed a significantly lower level of satisfaction among patients who discontinued edaravone therapy. Therefore, it appears that both functional status and therapy perception are crucial for the feasibility of long-term treatment with intravenous edaravone. Given the lack of highly effective therapies, there is ongoing demand for intravenous edaravone use among patients with ALS. Meanwhile, an oral formulation of edaravone has been developed but must first be assessed in clinical studies before it can replace the time-consuming intravenous application.
Limitations
The main limitation of our study is its observational design with nonrandomized treatment allocation. Although matching based on propensity scores replicates some characteristics of randomization, it cannot account for unknown confounders and thus does not yield the same level of evidence as randomized clinical trials.27 Genetic information was only partially available and therefore not incorporated in the matching process. Incomplete data acquisition for some patients may limit the capture of potential adverse effects, and heterogenous follow-up intervals may have influenced the analysis of disease progression rates. We adjusted the EESP criteria used in the MCI186-ALS19 study to account for the nonexistence of a 12-week observation period in a real-world setting by assigning patients who met 5 of 6 EESP criteria to the EFAS subgroup. Although baseline parameters confirmed that those patients were in the early stage of the disease, the subgroup may not exactly match the MCI186-ALS19 study population.
Conclusions
In summary, the results of our multicenter, propensity score–matched cohort study provide evidence for the feasibility and safety of long-term treatment with intravenous edaravone for patients with ALS. However, our effectiveness analyses do not support the association of edaravone treatment with a clinically relevant benefit on disease progression. The discrepancy with the observations made in the previous randomized, placebo-controlled trial could suggest that evaluating effectiveness using a short-term ALSFRS-R score decrease may have relevant limitations. In addition, our study indicated that edaravone in its current dosage and administration may not prolong time to ventilation or survival probability. Supported by additional real-world studies, our results raise doubts whether patients with ALS benefit from long-term intravenous edaravone treatment. This doubt is particularly important in light of the time-consuming and challenging intravenous application. Oral formulations of edaravone have been developed and are currently under investigation in clinical trials, creating an opportunity to both reduce the treatment-associated burden for patients and to further elucidate the efficacy of edaravone under randomized, placebo-controlled conditions.
eAppendix. Analysis of Potential Short-term Efficacy
eFigure 1. Treatment Evolution in the German Motor Neuron Disease Network
eFigure 2. Effects of Propensity Score Matching
eFigure 3. Ventilation-Free Survival
eFigure 4. Edaravone Treatment Adherence
eFigure 5. TSQM-9 Results in Patients Treated With Edaravone
Nonauthor Collaborators
References
- 1.Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377(2):162-172. doi: 10.1056/NEJMra1603471 [DOI] [PubMed] [Google Scholar]
- 2.Spittel S, Maier A, Kettemann D, et al. Non-invasive and tracheostomy invasive ventilation in amyotrophic lateral sclerosis: utilization and survival rates in a cohort study over 12 years in Germany. Eur J Neurol. 2021;28(4):1160-1171. doi: 10.1111/ene.14647 [DOI] [PubMed] [Google Scholar]
- 3.Bensimon G, Lacomblez L, Meininger V; ALS/Riluzole Study Group . A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330(9):585-591. doi: 10.1056/NEJM199403033300901 [DOI] [PubMed] [Google Scholar]
- 4.Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (phase II study). Amyotroph Lateral Scler. 2006;7(4):241-245. doi: 10.1080/17482960600881870 [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Wate R, Zhang J, et al. Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice. Exp Neurol. 2008;213(2):448-455. doi: 10.1016/j.expneurol.2008.07.017 [DOI] [PubMed] [Google Scholar]
- 6.Aoki M, Warita H, Mizuno H, Suzuki N, Yuki S, Itoyama Y. Feasibility study for functional test battery of SOD transgenic rat (H46R) and evaluation of edaravone, a free radical scavenger. Brain Res. 2011;1382:321-325. doi: 10.1016/j.brainres.2011.01.058 [DOI] [PubMed] [Google Scholar]
- 7.Ohta Y, Yamashita T, Nomura E, et al. Improvement of a decreased anti-oxidative activity by edaravone in amyotrophic lateral sclerosis patients. J Neurol Sci. 2020;415:116906. doi: 10.1016/j.jns.2020.116906 [DOI] [PubMed] [Google Scholar]
- 8.Ren Y, Wei B, Song X, et al. Edaravone’s free radical scavenging mechanisms of neuroprotection against cerebral ischemia: review of the literature. Int J Neurosci. 2015;125(8):555-565. doi: 10.3109/00207454.2014.959121 [DOI] [PubMed] [Google Scholar]
- 9.Abe K, Itoyama Y, Sobue G, et al. ; Edaravone ALS Study Group . Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi: 10.3109/21678421.2014.959024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe K, Aoki M, Tsuji S, et al. ; Writing Group; Edaravone (MCI-186) ALS 19 Study Group . Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi: 10.1016/S1474-4422(17)30115-1 [DOI] [PubMed] [Google Scholar]
- 11.Takahashi F, Takei K, Tsuda K, Palumbo J. Post-hoc analysis of MCI186-17, the extension study to MCI186-16, the confirmatory double-blind, parallel-group, placebo-controlled study of edaravone in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(sup1):32-39. doi: 10.1080/21678421.2017.1361442 [DOI] [PubMed] [Google Scholar]
- 12.Takei K, Tsuda K, Takahashi F, Palumbo J. Post-hoc analysis of open-label extension period of study MCI186-19 in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(sup1):64-70. doi: 10.1080/21678421.2017.1365372 [DOI] [PubMed] [Google Scholar]
- 13.Shefner J, Heiman-Patterson T, Pioro EP, et al. Long-term edaravone efficacy in amyotrophic lateral sclerosis: post-hoc analyses of Study 19 (MCI186-19). Muscle Nerve. 2020;61(2):218-221. doi: 10.1002/mus.26740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M, Akimoto M, Palumbo J, Sakata T. A double-blind, parallel-group, placebo-controlled, 24-week, exploratory study of edaravone (MCI-186) for the treatment of advanced amyotrophic lateral sclerosis (ALS) (P3.191). Neurology. 2016;86(16 suppl):P3.191. [Google Scholar]
- 15.The Edaravone (MCI-186) ALS 16 Study Group. A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(sup1):11-19. doi: 10.1080/21678421.2017.1363780 [DOI] [PubMed] [Google Scholar]
- 16.Al-Chalabi A, Andersen PM, Chandran S, et al. July 2017 ENCALS statement on edaravone. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(7-8):471-474. doi: 10.1080/21678421.2017.1369125 [DOI] [PubMed] [Google Scholar]
- 17.Yoshino H. Edaravone for the treatment of amyotrophic lateral sclerosis. Expert Rev Neurother. 2019;19(3):185-193. doi: 10.1080/14737175.2019.1581610 [DOI] [PubMed] [Google Scholar]
- 18.European Medicine Agencies. Withdrawal assessment report: Radicava. May 24, 2019. Accessed May 26, 2021. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-radicava_en.pdf
- 19.Milne CP, Cohen JP, Felix A, Chakravarthy R. Impact of postapproval evidence generation on the biopharmaceutical industry. Clin Ther. 2015;37(8):1852-1858. doi: 10.1016/j.clinthera.2015.05.514 [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Thoemmes F. Propensity score matching in SPSS. January 2012. Accessed May 26, 2021. https://ui.adsabs.harvard.edu/abs/2012arXiv1201.6385T
- 22.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299. doi: 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- 23.Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7(1):36. doi: 10.1186/1477-7525-7-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fürstenau D, Klein S, Vogel A, Auschra C. Multi-sided platform and data-driven care research. Electron Markets. Published online March 23, 2021. doi: 10.1007/s12525-021-00461-8 [DOI] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984; 79(387):516-524. doi: 10.1080/01621459.1984.10478078 [DOI] [Google Scholar]
- 26.Lunetta C, Moglia C, Lizio A, et al. ; EDARAVALS Study Group . The Italian multicenter experience with edaravone in amyotrophic lateral sclerosis. J Neurol. 2020;267(11):3258-3267. doi: 10.1007/s00415-020-09993-z [DOI] [PubMed] [Google Scholar]
- 27.Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int. 2016;113:597-603. doi: 10.3238/arztebl.2016.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada M, Yamashita S, Ueyama H, Ishizaki M, Maeda Y, Ando Y. Long-term effects of edaravone on survival of patients with amyotrophic lateral sclerosis. eNeurologicalSci. 2018;11:11-14. doi: 10.1016/j.ensci.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortuna A, Gizzi M, Bello L, et al. ; Edaravone Study Group . Safety and efficacy of edaravone compared to historical controls in patients with amyotrophic lateral sclerosis from North-Eastern Italy. J Neurol Sci. 2019;404:47-51. doi: 10.1016/j.jns.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 30.Abraham A, Nefussy B, Fainmesser Y, Ebrahimi Y, Karni A, Drory VE. Early post-marketing experience with edaravone in an unselected group of patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(3-4):260-263. doi: 10.1080/21678421.2019.1572191 [DOI] [PubMed] [Google Scholar]
- 31.Vu M, Tortorice K, Zacher J, et al. Assessment of use and safety of edaravone for amyotrophic lateral sclerosis in the Veterans Affairs health care system. JAMA Netw Open. 2020;3(10):e2014645. doi: 10.1001/jamanetworkopen.2020.14645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbull J. Is edaravone harmful? (a placebo is not a control). Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(7-8):477-482. doi: 10.1080/21678421.2018.1517179 [DOI] [PubMed] [Google Scholar]
- 33.Turnbull J. Reappraisal of an ALS trial: unaccounted procedural risk. Lancet Neurol. 2020;19(9):717-718. doi: 10.1016/S1474-4422(20)30265-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Analysis of Potential Short-term Efficacy
eFigure 1. Treatment Evolution in the German Motor Neuron Disease Network
eFigure 2. Effects of Propensity Score Matching
eFigure 3. Ventilation-Free Survival
eFigure 4. Edaravone Treatment Adherence
eFigure 5. TSQM-9 Results in Patients Treated With Edaravone
Nonauthor Collaborators