Key Points
Question
What is the association between immunization with a third (booster) dose of BNT162b2 vaccine (Pfizer-BioNTech) and the incidence of SARS-CoV-2 infection among immunocompetent health care workers?
Findings
In this cohort study of 1928 health care workers in Israel who were previously vaccinated with a 2-dose series of BNT162b2, administration of a booster dose compared with not receiving one was significantly associated with lower risk of SARS-CoV-2 infection during a median of 39 days of follow-up (adjusted hazard ratio, 0.07).
Meaning
Among health care workers previously vaccinated with a 2-dose series of BNT162b2, administration of a booster dose compared with not receiving one was significantly associated with a lower rate of SARS-CoV-2 infection in short-term follow-up.
Abstract
Importance
Administration of a BNT162b2 booster dose (Pfizer-BioNTech) to fully vaccinated individuals aged 60 years and older was significantly associated with lower risk of SARS-CoV-2 infection and severe illness. Data are lacking on the effectiveness of booster doses for younger individuals and health care workers.
Objective
To estimate the association of a BNT162b2 booster dose with SARS-CoV-2 infections among health care workers who were previously vaccinated with a 2-dose series of BNT162b2.
Design, Setting, and Participants
This was a prospective cohort study conducted at a tertiary medical center in Tel Aviv, Israel. The study cohort included 1928 immunocompetent health care workers who were previously vaccinated with a 2-dose series of BNT162b2, and had enrolled between August 8 and 19, 2021, with final follow-up reported through September 20, 2021. Screening for SARS-CoV-2 infection was performed every 14 days. Anti–spike protein receptor binding domain IgG titers were determined at baseline and 1 month after enrollment. Cox regression with time-dependent analysis was used to estimate hazard ratios of SARS-CoV-2 infection between booster-immunized status and 2-dose vaccinated (booster-nonimmunized) status.
Exposures
Vaccination with a booster dose of BNT162b2 vaccine.
Main Outcomes and Measures
The primary outcome was SARS-CoV-2 infection, as confirmed by reverse transcriptase–polymerase chain reaction.
Results
Among 1928 participants, the median age was 44 years (IQR, 36-52 years) and 1381 were women (71.6%). Participants completed the 2-dose vaccination series a median of 210 days (IQR, 205-213 days) before study enrollment. A total of 1650 participants (85.6%) received the booster dose. During a median follow-up of 39 days (IQR, 35-41 days), SARS-CoV-2 infection occurred in 44 participants (incidence rate, 60.2 per 100 000 person-days); 31 (70.5%) were symptomatic. Five SARS-CoV-2 infections occurred in booster-immunized participants and 39 in booster-nonimmunized participants (incidence rate, 12.8 vs 116 per 100 000 person-days, respectively). In a time-dependent Cox regression analysis, the adjusted hazard ratio of SARS-CoV-2 infection for booster-immunized vs booster-nonimmunized participants was 0.07 (95% CI, 0.02-0.20).
Conclusions and Relevance
Among health care workers at a single center in Israel who were previously vaccinated with a 2-dose series of BNT162b2, administration of a booster dose compared with not receiving one was associated with a significantly lower rate of SARS-CoV-2 infection over a median of 39 days of follow-up. Ongoing surveillance is required to assess durability of the findings.
This cohort study estimates the association of a BNT162b2 (Pfizer-BioNTech) vaccine booster dose with SARS-CoV-2 infections among health care workers in Israel previously vaccinated with a 2-dose series of BNT162b2.
Introduction
The Pfizer-BioNTech BNT162b2 messenger RNA COVID-19 vaccine was found to be highly effective in preventing asymptomatic, symptomatic, and severe SARS-CoV-2 infection.1,2,3,4 Israel was among the first countries to achieve significant nationwide vaccination coverage, leading to rapid containment of SARS-CoV-2 transmission in the community.5,6 However, resurgence of COVID-19 cases predominated by the Delta variant was observed in June 20216,7 and raised concerns about waning immunity of the BNT162b2 vaccine. Declining protection of BNT162b2 against SARS-CoV-2 infection and hospitalization 4 months or more after full vaccination was demonstrated among individuals aged 60 years or older, in which those who completed a 2-dose series in March 2021 had a lower risk of SARS-CoV-2 infection compared with those vaccinated in January 2021.7 Similarly, analysis of US data of adults without immunocompromising conditions showed a significant decline in the effectiveness of the BNT162b2 vaccine against COVID-19 hospitalizations, from 91% to 72% more than 120 days after completion of vaccination.8 These findings are consistent with data showing time-dependent reduction in neutralizing antibody titers after vaccination.9,10
In response, on July 30, 2021, the Israeli ministry of health initiated BNT162b2 booster vaccination of persons older than 60 years11 and shortly thereafter expanded its recommendation to younger age groups. Analysis of nationwide data showed a lower risk of confirmed SARS-CoV-2 infection starting 12 days after booster vaccination among persons aged 60 years or older who received a booster dose12 compared with individuals vaccinated with 2 doses. Booster administration has also been shown to enhance immune response in immunosuppressed individuals.13 However, the effect of booster vaccination on younger adults and health care workers is unclear.
This study aimed to assess the association of booster vaccination with SARS-CoV-2 infection among health care workers at a large teaching hospital in Tel Aviv, Israel.
Methods
Study Design and Population
The COVI3 study was an investigator-initiated, prospective cohort study conducted at the Tel-Aviv Sourasky Medical Center, a tertiary medical center in Tel Aviv, Israel. Study population included health care workers (including employees, students, volunteers, and subcontractors) aged 18 years or older who received 2 doses of BNT162b2 vaccine at least 1 month before study enrollment. Participants who were immunocompromised, were taking biological or immunosuppressive drugs, were pregnant, or had documented past infection with SARS-CoV-2 were excluded.
Written informed consent was obtained from all participants at enrollment. Ethics approval and review according to the Declaration of Helsinki14 for this study were obtained from the hospital’s institutional review board. The study protocol is available in Supplement 1.
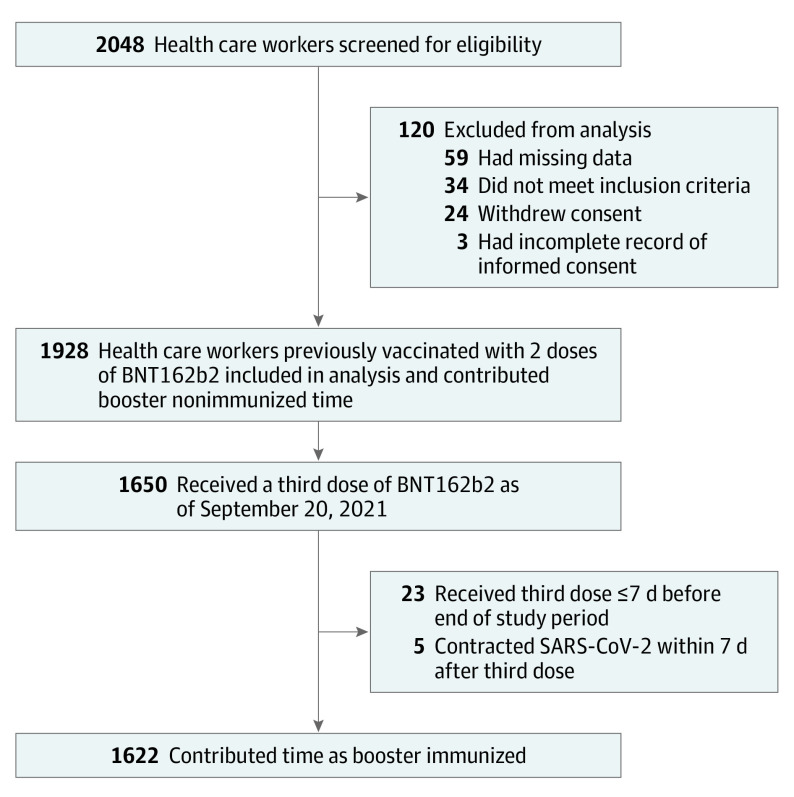
Study enrollment occurred between August 8 and 19, 2021. Results reported here include data collected up to September 20, 2021 (eFigure 1 in Supplement 2), with surveillance of participants planned to continue for 1 year after enrollment. The study was originally designed as an interventional study, offering a third dose of BNT162b2 to participants with anti–SARS-CoV-2 spike protein receptor binding domain (anti–S1-RBD) IgG levels below 5.5 index values, which was the median titer of the first 500 enrolled participants. However, shortly after study initiation the Israeli Ministry of Health recommended the administration of a booster dose to all health care workers, which mandated modifying the study design to a prospective cohort design. Therefore, timing of receipt of the booster dose was at the participants’ discretion and could have taken place at any point after enrollment in the study. This can be described most intuitively as a single cohort of health care workers in which exposure status could evolve over time, from unexposed to exposed, on booster immunization (Figure 1).
Figure 1. Study Population of Vaccinated Health Care Workers.
BNT162b2 vaccine is manufactured by Pfizer-BioNTech.
Data Collection
Data pertaining to demographics, employment sector and department, medical history including comorbid conditions, vaccination history, and medication use were obtained from the hospital’s information systems database and from a questionnaire completed by the participants at enrollment. Blood samples were obtained for anti–S1-RBD IgG levels at baseline and approximately 1 month after enrollment, which, for participants who opted to receive the booster dose, was at variable times after vaccination.
Participants were requested to undergo screening for SARS-CoV-2 infection with nasopharyngeal swab sampling and reverse transcriptase–polymerase chain reaction (RT-PCR) every 14 days regardless of symptom status. Reminders were sent by email and text messages to maximize screening compliance. Results of SARS-CoV-2 RT-PCR tests performed outside of study screening procedure (for example, because of symptoms or after exposure to a SARS-CoV-2–positive individual) were retrieved and analyzed.
Anti–S1-RBD IgG levels were determined with the ADVIA Centaur SARS-CoV-2 IgG assay (Siemens), which has an analytic measurement interval of 0.50 to 100.0 index values (eMethods in Supplement 2).
Participants who tested positive for SARS-CoV-2 during the study period underwent an interview to determine the presence of symptoms.
Definitions
On study enrollment, no participant had received a booster dose and thus all participants were initially considered booster nonimmunized. Participants were considered booster recipients on the day of booster dose administration, and then considered to have completed the booster immunization (ie, were booster immunized) once 7 days or more had elapsed since receipt of the third dose of BNT162b2, consistent with the Ministry of Health definition of fully vaccinated individuals after the second dose.
The risk of exposure to SARS-CoV-2 was classified according to work assignment at enrollment as high (persons working in emergency departments or dedicated COVID-19 wards), medium (persons working in internal medicine departments or who performed high-risk procedures), or low (other population subgroups).
Persons infected with SARS-CoV-2 were considered to be symptomatic if they reported new onset of any of the following: temperature greater than 37.6 °C, headache, sore throat, cough, dyspnea, rhinorrhea, diarrhea, myalgia, malaise, or loss of sense of taste or smell.
Study Outcomes
The primary outcome was RT-PCR–confirmed SARS-CoV-2 infection. Secondary outcomes included symptomatic and asymptomatic SARS-CoV-2 infection. Change in anti–S1-RBD IgG levels from baseline to follow-up was also analyzed among individuals who received a booster dose and those who did not, and follow-up P values were compared across groups.
Statistical Analysis
All continuous variables are displayed as mean (SD) for normally distributed variables or medians (IQR) for nonnormally distributed variables. Categorical variables are displayed as numbers (percentage) of participants within each group. Normally distributed continuous variables were compared with a t test, nonnormally distributed continuous variables with Wilcoxon rank sum test, and categorical variables with the χ2 test. Participants with missing data were excluded from all analyses.
Time-Dependent Cox Regression Analysis
To account for changing booster immunization status after enrollment in the study, a time-dependent Cox regression analysis was conducted, evaluating the hazard ratio associated with booster immunization status. Participants were defined as nonimmunized on enrollment in the study and as immunized at booster receipt date plus 7 days. To control for time from completion of the primary BNT162b2 series to booster receipt, a categorical variable was introduced, defining each participant as an “early” or “late” vaccine recipient (receipt of the second vaccine dose in January 2021 vs February to May 2021, respectively).
Participants were censored on event occurrence (ie, a confirmed SARS-CoV-2 infection) or at the end of the study period. To generate a model that was as parsimonious as possible, the effect associated with each covariate measured in the study was estimated in a preliminary Cox regression analysis by computing for each covariate a Cox model including only booster immunization status and the covariate in question as explanatory variables. Each of these models was tested against the basic model (that included only booster immunization status as an explanatory variable) with an analysis of variance test; only covariates that added a statistically significant contribution over the basic model were included in the final regression model, and all were tested with Schoenfeld tests to evaluate the assumption of hazard proportionality. A full list of covariates that were tested for significance is provided in eTable 1 in Supplement 2. When the association between booster immunization and the risk of symptomatic and asymptomatic SARS-CoV-2 infection was evaluated, the latter 2 were considered competing risks, censoring the asymptomatic cases at event occurrence and recomputing the Cox model for the symptomatic cases, and vice versa.
Cumulative incidence curves for the booster-immunized and booster-nonimmunized participants were estimated with the Kaplan-Meier method from the Cox regression model.
RT-PCR Test Density Over Time
RT-PCR test density (number of RT-PCR tests per 1000 participants) was computed separately for each day as follows: the number of RT-PCR tests performed among booster-immunized and booster-nonimmunized participants in a given day was divided by the number of participants in each group on that day.
Accounting for Baseline Anti–S1-RBD IgG Baseline Titers
A behavioral effect may have been introduced because participants were aware of their baseline anti–S1-RBD IgG titers and because the booster was initially offered only to those with “low” baseline anti–S1-RBD IgG titers (<5.5 index values) at the beginning of the study. To address this potential bias, a categorical covariate, stratifying the participants into low (<5.5 index values) or high baseline anti–S1-RBD IgG titer groups, was included in the Cox regression model.
Analysis of Anti–S1-RBD IgG Titers
Participants for whom follow-up serology measurement was available were categorized as booster recipients or nonrecipients. Statistical significance of between- and within-group differences in serology values were estimated with the Wilcoxon rank sum test.
Time From Booster Administration to Serologic Response
Discovery of the point at which a serologic response occurred after booster administration was conducted in a post hoc analysis by plotting the follow-up serology titers as a function of the time elapsed from booster dose administration. Serology results of participants tested before and after this point were evaluated with the Wilcoxon rank sum test for between- and within-group differences (baseline vs follow-up serology values).
Association Between Anti–S1-RBD IgG Titers and Incidence of Breakthrough Infections in Participants Vaccinated With 2 Doses
The cumulative fraction of SARS-CoV-2 infections in participants who did not receive the booster dose, across the range of baseline serology values in this group, was computed in a post hoc analysis by summing the number of SARS-CoV-2–positive participants in each baseline serology range (0 - i, where i is an integer in the range 0-100, by increments of 1) and dividing that by the total number of SARS-CoV-2–positive participants in this group. The same computation was conducted for the cumulative fraction of participants across the baseline serology range. Incidence rates within different ranges of baseline serology values were computed by dividing the number of SARS-CoV-2–positive cases by the cumulative follow-up time within the specified range. Statistical significance of the difference between incidence rates was estimated with the log-rank test.
All statistical analyses were performed with R version 4.0.3.15 All reported tests were 2 sided, and P < .05 was considered significant.
Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Results
Study Population
A total of 2048 health care workers were screened for eligibility, and 1928 were included in the primary analysis. Participants were excluded because of incompatibility with inclusion criteria, withdrawal of consent, and missing data on demographics, employment data, medical history, or baseline serology results (Figure 1). The median age of the cohort was 44 years (IQR, 36-52 years) and 1381 were women (71.6%). The median time from receipt of the second vaccine dose to study enrollment was 210 days (IQR, 205-213 days). Other characteristics of the study cohort are shown in Table 1. Overall, 1650 participants (85.6%) opted to receive the booster dose throughout the study period and 278 (14.4%) did not (eFigure 2 in Supplement 2). The median follow-up for the entire cohort was 39 days (IQR, 35-41 days), and the median follow-up in the booster-immunized state (ie, ≥7 days after receipt of booster) was 26 days (IQR, 21-29 days).
Table 1. Baseline Characteristics of the Study Population.
| No. (%) | |
|---|---|
| No. of participants | 1928 |
| Women | 1381 (71.6) |
| Men | 547 (28.4) |
| Age, median (IQR), y | 44 (36-52) |
| Age group, y | |
| <30 | 166 (8.6) |
| 30-39 | 533 (27.6) |
| 40-49 | 588 (30.5) |
| 50-59 | 537 (27.9) |
| ≥60 | 104 (5.4) |
| Marital status | |
| Married | 1148 (59.5) |
| Single | 589 (30.5) |
| Divorced | 172 (8.9) |
| Widowed | 19 (1.0) |
| No. of children, mean (SD) | 1.6 (1.3) |
| Employment sector | |
| Administration | 617 (32.0) |
| Nursing | 455 (23.6) |
| Medicine | 406 (21.1) |
| Health professions | 278 (14.4) |
| Research | 172 (8.9) |
| Estimated risk of exposure to SARS-CoV-2 | |
| Low | 1652 (85.7) |
| Medium | 60 (3.1) |
| High | 216 (11.2) |
| Height, mean (SD), cm | 167.4 (8.9) |
| Weight, mean (SD), kg | 70.8 (15.6) |
| BMI, mean (SD) | 25.2 (4.8) |
| Smoking history | |
| Never | 1259 (65.3) |
| Past | 358 (18.6) |
| Current | 311 (16.1) |
| No. of influenza vaccinations in past 3 y | |
| 0 | 384 (19.9) |
| 1 | 291 (15.1) |
| 2 | 399 (20.7) |
| 3 | 854 (44.3) |
| Hypercholesterolemia | 250 (13.0) |
| Hypertension | 194 (10.1) |
| Diabetes | 69 (3.6) |
| Pulmonary disease | 68 (3.5) |
| Liver disease | 24 (1.2) |
| Ischemic heart disease | 19 (1.0) |
| Solid malignancy in past 3 y | 17 (0.9) |
| Kidney disease | 13 (0.7) |
| Hematologic malignancy in past 3 y | 3 (0.2) |
| Hospitalized in the past 3 y | 86 (4.5) |
| Baseline serology, index value, median (IQR) | 5.8 (3.2-10.6) |
| Time from second dose to enrollment, median (IQR), d | 210 (205-213) |
| Second dose administration month, 2021 | |
| January | 1691 (87.7) |
| February | 140 (7.3) |
| March | 82 (4.2) |
| April | 13 (0.6) |
| May | 2 (0.1) |
| Surveillance time, median (IQR), d | 39 (35-41) |
| No. of RT-PCR tests per participant, median (IQR) | 2 (1-3) |
| No. of tests performed during the study period | |
| ≥1 | 1583 (82.1) |
| ≥2 | 1056 (54.8) |
| ≥3 | 488 (25.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); RT-PCR, reverse transcriptase–polymerase chain reaction.
Participants underwent 3552 SARS-CoV-2 RT-PCR tests during the study period, 1732 after booster immunization and 1820 before it. Overall, of the 1928 participants, 1583 (82.1%) underwent at least 1 RT-PCR test during the study period. There was no statistically significant difference between RT-PCR test densities in booster-immunized and booster-nonimmunized participants (median density, 22.3 vs 47.2 [IQR, 9.1-78.2 vs 11.1-74.5] per 1000 person-days, respectively; P = .30 for difference between groups, Wilcoxon rank sum test) (eFigure 3 in Supplement 2).
Primary Outcome
Association Between Booster Dose Administration and Incidence of SARS-CoV-2 Infection
A total of 44 SARS-CoV-2 infections occurred throughout the study period (incidence rate, 60.2 per 100 000 person-days) (eFigure 4 in Supplement 2). Of these, 31 infections (70.5%) were symptomatic and 13 (29.5%) were asymptomatic (eTable 2 in Supplement 2).
Five SARS-CoV-2 infections occurred in booster-immunized participants (incidence rate, 12.8 per 100 000 person-days) and 39 occurred in booster-nonimmunized participants (incidence rate, 116.1 per 100 000 person-days) (Figure 2).
Figure 2. Study Participants by Receipt of Booster Vaccination and Incidence of SARS-CoV-2 Infection.
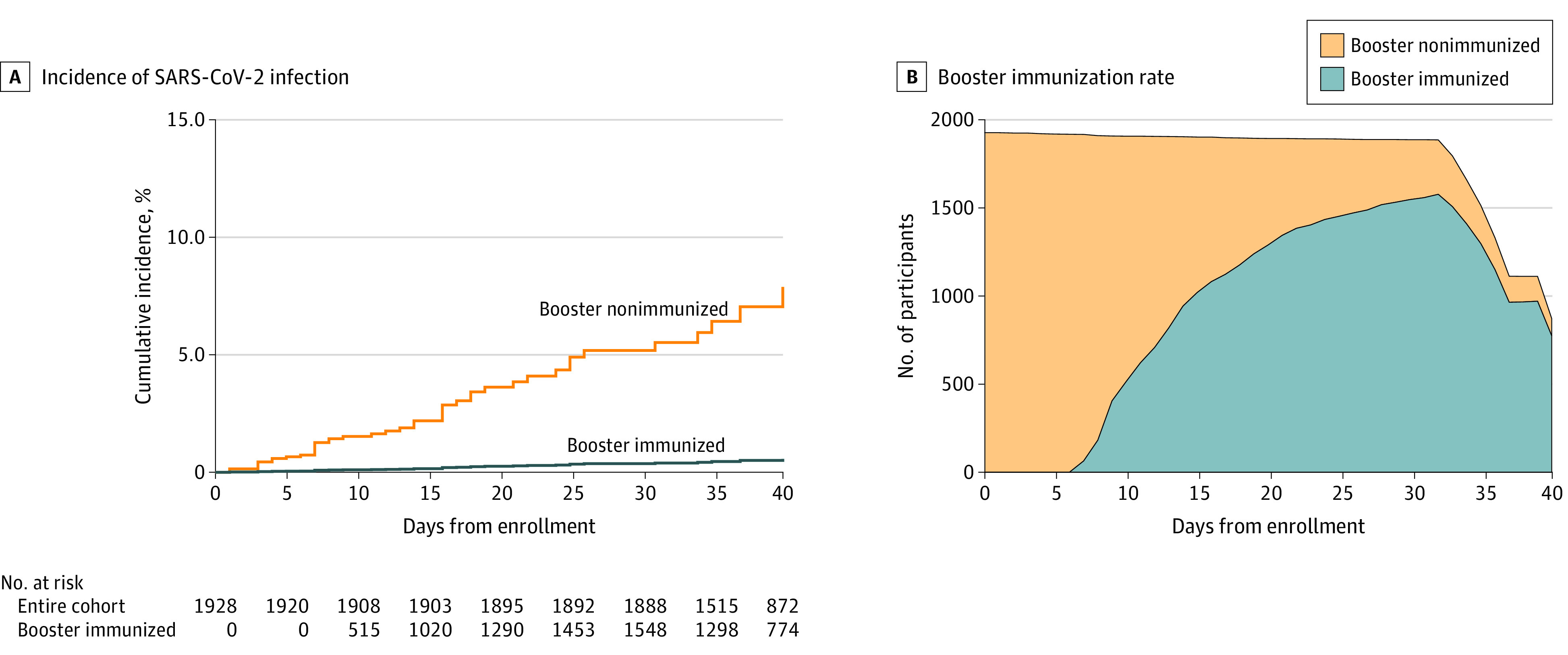
A, Cumulative incidence of SARS-CoV-2 infection among booster-immunized and nonimmunized participants throughout the study period.
B, Number of booster-immunized and booster-nonimmunized participants at each point throughout the study period.
Participants were considered booster immunized 7 days or more after receipt of the booster dose. SARS-CoV-2–confirmed cases were censored on the day of positive result.
Four covariates had a statistically significant effect in the preliminary covariate selection analysis and were included as explanatory variables in the final regression model (alongside booster immunization status): baseline serology result (≥5.5 or <5.5 index values), number of children, early or late receipt of the second vaccine dose, and number of RT-PCR tests in booster-immunized or booster-nonimmunized windows (a time-dependent covariate). None of the other covariates had a statistically significant effect on the results (eTable 1 in Supplement 2). All covariates, including those not included in the model, fulfilled the hazard proportionality assumption (eFigure 5 in Supplement 2). The hazard ratio of SARS-CoV-2 infection for booster-immunized vs booster-nonimmunized participants, as estimated by the time-dependent Cox regression analysis, was 0.07 (95% CI, 0.02-0.20) (Table 2).
Table 2. Results of Cox Multivariable Regression for Any SARS-CoV-2 Infection, Symptomatic Infection, and Asymptomatic Infection.
| Outcome evaluateda | Booster immunized | Booster nonimmunized | Covariate | Adjusted hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|---|---|---|
| No. of cases | Incidence rate per 100 000 person-days | No. of cases | Incidence rate per 100 000 person-days | ||||
| Any infection | 5 | 12.8 | 39 | 116.1 | Booster immunized vs booster nonimmunized | 0.07 (0.02-0.20) | <.001 |
| Surveillance time, 39 209 person-days |
Surveillance time, 33 590 person-days |
High (≥5.5 index values) vs low anti–S1-RBD IgG titers | 0.45 (0.24-0.86) | .02 | |||
| Month of second vaccine dose: Feb-May vs Jan | 0.11 (0.01-0.80) | .03 | |||||
| No. of children | 1.28 (1.04-1.57) | .02 | |||||
| No. of tests | 1.36 (1.16-1.60) | <.001 | |||||
| Symptomatic infection | 3 | 7.6 | 28 | 83.3 | Booster immunized vs booster nonimmunized | 0.07 (0.02-0.25) | <.001 |
| High (≥5.5 index values) vs low anti–S1 RBD IgG titers | 0.50 (0.24-1.07) | .07 | |||||
| Month of second vaccine dose: Feb-May vs Jan | 0.15 (0.02-1.14) | .07 | |||||
| No. of children | 1.22 (0.95-1.55) | .12 | |||||
| No. of tests | 1.33 (1.09-1.61) | .005 | |||||
| Asymptomatic infection | 2 | 5.1 | 11 | 32.7 | Booster immunized vs booster nonimmunized | 0.08 (0.01-0.48) | .006 |
| High (≥5.5 index values) vs low anti–S1 RBD IgG titers | 0.34 (0.10-1.19) | .09 | |||||
| Month of second vaccine dose: Feb-May vs Jan | 0 (0-∞) | .99 | |||||
| No. of children | 1.44 (0.99-2.09) | .06 | |||||
| No. of tests | 1.45 (1.09-1.92) | .01 | |||||
Abbreviation: anti–S1-RBD, anti–SARS-CoV-2 spike protein receptor binding domain.
Results of a multivariable Cox regression showing adjusted hazard ratios and 95% CIs for any infection, symptomatic infection, or asymptomatic SARS-CoV-2 infection. Variables that did not show a statistically significant contribution in univariable analysis were not included in the multivariable analysis (eTable 1 in Supplement 2).
Secondary Outcomes
Association Between Booster Dose Administration and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infection
Symptomatic disease occurred in 3 of 5 cases (60%) among booster-immunized participants and 28 of 39 cases (71.7%) among booster-nonimmunized participants (incidence rates, 7.7 vs 83.4 per 100 000 person-days, respectively) (eFigure 6 in Supplement 2). Asymptomatic infection occurred in 2 of 5 cases (40%) among booster-immunized participants and 11 of 39 cases (28.3%) among booster-nonimmunized participants (incidence rate, 5.1 vs 32.7 per 100 000 person-days, respectively) (eFigure 7 in Supplement 2). The adjusted hazard ratio for symptomatic and asymptomatic infection was 0.07 (95% CI, 0.02-0.25) and 0.08 (95% CI, 0.01-0.48), respectively (Table 2; eFigures 6 and 7 in Supplement 2).
Association Between Booster Dose Administration and Anti–S1-RBD IgG Titer
Follow-up serology was measured a median of 31 days (IQR, 28-34 days) after baseline measurement and was available for 1136 of the 1928 participants (58.9%; 60.3% of booster recipients and 50.7% of booster nonrecipients). Booster recipients had a higher proportion of men, participants with baseline titers less than 5.5 index values, and “early-vaccinated” participants compared with booster nonrecipients (eTable 3 in Supplement 2).
Median baseline levels of anti–S1-RBD IgG were 5.4 index values (IQR, 3.0-9.6 index values) for booster recipients and 9.3 index values (IQR, 5.4-19.0 index values) for nonrecipients (P < .001, Wilcoxon rank sum test).
For titers measured in booster nonrecipients, there was no statistically significant difference between baseline (shown earlier) and follow-up (11.1 index values [IQR, 6.2-31.1]; P = .70, Wilcoxon rank sum test). Conversely, the maximal value measured by the assay, 100 index values, was reached by 953 of 1021 (93.3%) booster recipients (P < .001 for difference between baseline and follow-up titers in booster recipients and P < .001 for difference between follow-up titers in booster recipients and nonrecipients, Wilcoxon rank sum test).
Post Hoc Analyses
Time From Booster Administration to Serologic Response
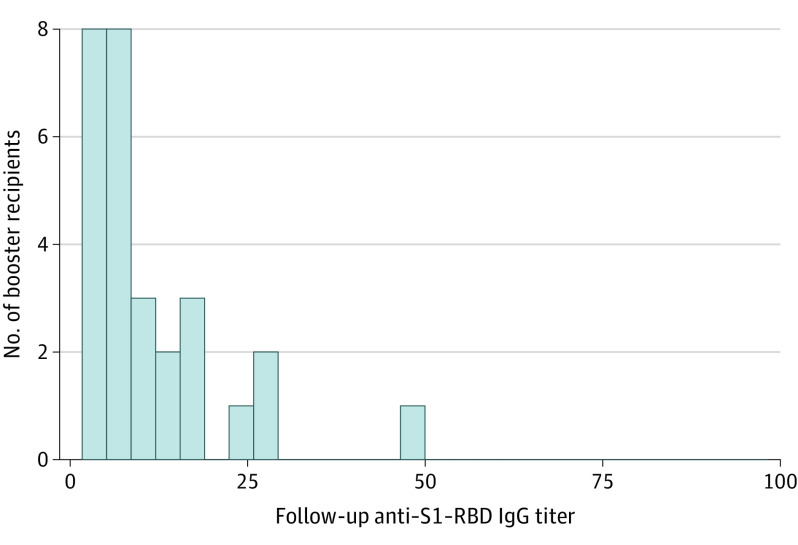
Some variation existed in the time elapsed between booster dose administration and follow-up serology sampling, which allowed assessment of the dynamics of serologic response to booster receipt. Among booster recipients for whom follow-up serology was measured between 5 and 42 days after the booster dose, 952 of 966 (98.6%) had reached the maximal anti–S1-RBD IgG level measurable by the assay used (100 index values) compared with 0 of 29 participants for whom follow-up serology was measured less than 5 days after booster administration (P < .001, χ2 test) (Figure 3). Furthermore, there was no statistically significant difference between baseline and follow-up serology titers in booster recipients for whom follow-up serology was measured less than 5 days after booster administration (P = .59, Wilcoxon rank sum test).
Figure 3. Distribution of Serology Values Obtained Within 5 Days of Booster.
Follow-up anti–spike protein receptor-binding domain (anti–S1-RBD) IgG titers for participants who were tested 0 to 4 days after receipt of the booster dose (n = 29). Among participants who received the booster dose and were tested 5 days or more after its receipt (n = 966), 98.6% (952/966) had reached the maximal anti–S1-RBD IgG level measurable by the assay used (100 index values).
Association Between Anti–S1-RBD IgG Titers and Incidence of Breakthrough Infections in Participants Vaccinated With 2 Doses
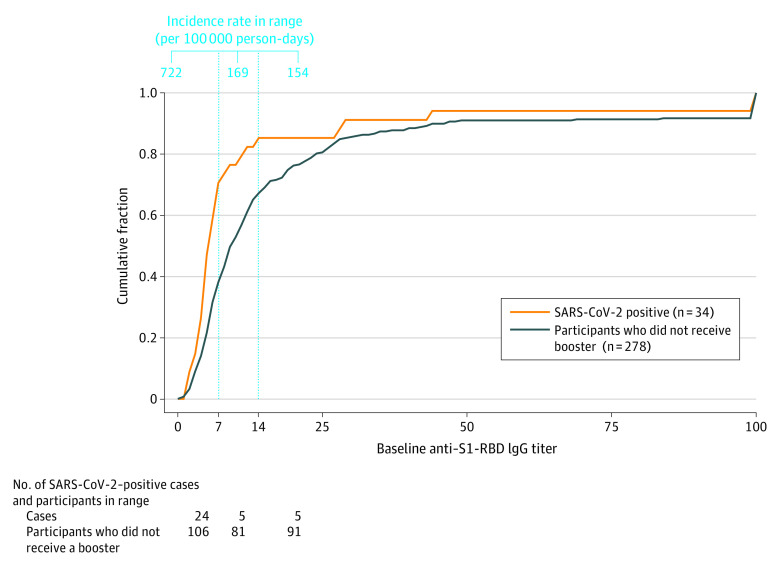
The association between baseline anti–S1-RBD IgG titers and incidence of SARS-CoV-2 infections was assessed in the group of participants who did not receive a booster dose (n = 278). Plotting the cumulative fraction of SARS-CoV-2–positive participants against the range of baseline serology values revealed 3 segments with distinct inclines (Figure 4). In the first segment (≤7 index values), the incidence rate was 722 per 100 000 person-days. In the second segment (7-14 index values), the incidence rate was 169 per 100 000 person-days. In the third segment (>14 index values), the incidence rate was 154 per 100 000 person-days. The difference in incidence rates between the segments was estimated by pairwise comparisons with the log-rank test and was found to be statistically significant for the comparison between segments 1 vs 2 (P = .003) and 1 vs 3 (P = .002) but not for the comparison of segments 2 vs 3 (P = .10). All P values were adjusted for multiple testing with the Holm method.
Figure 4. Breakthrough Infections in Vaccinated Participants Who Did Not Receive a Booster.
Cumulative fraction of the number of booster nonrecipients (n = 278) and SARS-CoV-2-positive individuals (via polymerase chain reaction [PCR] testing) within this group (n = 34) across the range of baseline anti–spike protein receptor-binding domain (anti–S1-RBD) IgG levels. This group of participants was further divided into 3 groups based on the 3 distinct incline segments of the PCR-positive curve (marked by the vertical lines). Incidence rates per 100 000 person-days in the first (≤7 index values), second (7-14 index values), and third (>14 index values) segments are shown.
Discussion
In this prospective cohort study of immunocompetent health care workers who were previously vaccinated with 2 doses of BNT162b2 messenger RNA vaccine, a booster dose was significantly associated with lower rates of symptomatic and asymptomatic SARS-CoV-2 infection at a median follow-up of 39 days. The adjusted hazard ratio for SARS-CoV-2 infection was 0.07 (95% CI, 0.02-0.20) compared with the protection conferred by a 2-dose regimen.
These findings are in line with the reduction in SARS-CoV-2–related hospitalizations across multiple age groups after booster administration reported in a large observational study in Israel,16 as well as with the reduction in SARS-CoV-2 infections observed after booster administration in persons older than 60 years reported in another Israeli nationwide study.12
Approximately 70% of SARS-CoV-2 infections in this cohort were symptomatic, similar to the proportion observed in other studies of vaccine breakthrough infection.17,18
An increase in anti–S1-RBD IgG antibody levels starting 5 days after booster vaccination was observed among booster recipients, whereas no such increase was observed in participants who did not receive the booster dose. This increase corresponds with an increase in anti-S1 antibodies observed after a third dose of BNT162b2 in a study of adults aged 60 years or older19 and after a third dose of mRNA-1273 vaccine (Moderna) in a cohort of kidney transplant recipients who did not have a serologic response to the first 2-dose regimen.20
A post hoc exploratory analysis of participants who did not receive a booster dose in this cohort revealed a greater incidence of SARS-CoV-2 infection in participants with baseline anti–S1-RBD IgG levels below 7 index values (approximately 153 binding antibody units/mL).21 There is currently no validated immune correlate of protection from SARS-CoV-2 infection. However, neutralizing antibody levels correlated with protection against SARS-CoV-2 infection and severe infection9,22 and an association between anti–S1-RBD IgG and neutralizing antibody levels after immunization with BNT162b2 have been previously reported.9,22,23,24 Because neutralization titers are not generally available at most clinical laboratories, more information is needed on clinical correlates of commercial immunoassays. Results of this study suggest that anti–S1-RBD IgG levels have potential usefulness for assessing waning immunity after BNT162b2 vaccination and should be validated in additional cohorts.
The strengths of this study include a prospective, investigator-initiated design, high SARS-CoV-2 incidence during the study period, a relatively homogenous population with a detailed data set that allowed robust analysis with adjustment for multiple confounders and correlation with anti–S1-RBD IgG levels, and periodic screening by RT-PCR of participants, which reduced testing imbalance between booster-immunized and booster-nonimmunized cohorts and allowed detection of asymptomatic infections. Moreover, this study addressed immunocompetent health care workers, a population not included in recent studies on the effect of booster vaccination.12
Limitations
This study has several limitations. First, the sample size was not powered to capture the effect of a booster dose on severe illness and hospitalization, especially in this cohort of immunocompetent individuals. Second, the low incidence of asymptomatic infection did not allow robust estimation of the association between booster administration and asymptomatic infection. The 14-day interval between tests, and that approximately 20% of the cohort did not undergo any RT-PCR test during the study period, may have caused some asymptomatic infections to be missed. However, the relatively high test density in this study and the similar rates of asymptomatic infections as observed in other reports17,18 do not support the presence of a such a detection bias. Third, this was an unblinded nonrandomized observational study, with participants aware of their baseline anti–S1-RBD IgG levels, which may have affected both their decision about whether and when to receive the booster dose and their health behavior throughout the study period. Some variability in the timing of second dose administration, study enrollment, and third dose administration was also present. However, the primary analysis included timing of second and third vaccine doses, baseline anti–S1-RBD IgG titers, and number of past influenza vaccines (a marker of health behavior) as model covariates to account for these confounders. Nevertheless, the presence of unknown confounders cannot be excluded. Fourth, neutralizing antibody levels and cellular immunity were not measured in this study, which limited the estimation of the immune response elicited by booster administration. Fifth, the short duration of follow-up does not allow conclusions to be drawn about the long-term effect of the booster dose. Sixth, our observations were made before Omicron emerged as a dominant variant of SARS-CoV-2, and therefore our results may not apply to that variant.
Conclusions
Among health care workers at a single center in Israel who were previously vaccinated with a 2-dose series of BNT162b2, administration of a booster dose compared with not receiving one was associated with a significantly lower rate of SARS-CoV-2 infection during a median of 39 days of follow-up. Ongoing surveillance is required to assess durability of the findings.
Study Protocol
eMethods
eTable 1. Candidate Covariates for Inclusion in the Cox Regression Analysis
eTable 2. Characteristics of SARS-CoV-2 positive participants (n=44)
eTable 3. Baseline Characteristics Stratified by Booster Dose Receipt Status
eFigure 1. SARS-CoV-2 Spread in Israel Throughout the Study Period
eFigure 2. Vaccine Uptake Over Time in Booster Cohort
eFigure 3. Daily PCR Test Density
eFigure 4. Cumulative Number of Positive PCR Tests Over Time From Study Initiation
eFigure 5. Cox Model Diagnostics
eFigure 6. Cumulative Incidence of Symptomatic SARS-CoV-2 Infections
eFigure 7. Cumulative Incidence of SARS-CoV-2 Asymptomatic Infection
eReference
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412-1423. doi: 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angel Y, Spitzer A, Henig O, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325(24):2457-2465. doi: 10.1001/jama.2021.7152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen B, Waitzberg R, Israeli A. Israel’s rapid rollout of vaccinations for COVID-19. Isr J Health Policy Res. 2021;10(1):6. doi: 10.1186/s13584-021-00440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus Pandemic (COVID-19). Our World in Data; 2020. Accessed January 6, 2021. https://ourworldindata.org/coronavirus [Google Scholar]
- 7.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Self WH, Tenforde MW, Rhoads JP, et al. ; IVY Network . Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337-1343. doi: 10.15585/mmwr.mm7038e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 10.Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397(10292):2331-2333. doi: 10.1016/S0140-6736(21)01290-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Israeli Ministry of Health . Indications for third dose of SARS-CoV-2 vaccine. Article in Hebrew. Accessed September 27, 2021. https://www.gov.il/BlobFolder/news/30072021-01/he/NEWS_Corona_3rd-and-moderna-30072021.pdf
- 12.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393-1400. doi: 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244-1246. doi: 10.1056/NEJMc2111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.R Project for Statistical Computing. R software version 4.0.3. https://www.r-project.org
- 16.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093-2100. doi: 10.1016/S0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474-1484. doi: 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K; HEROES-RECOVER Cohorts . Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance—eight US locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167-1169. doi: 10.15585/mmwr.mm7034e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliakim-Raz N, Leibovici-Weisman Y, Stemmer A, et al. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326(21):2203-2204. doi: 10.1001/jama.2021.19885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063-1065. doi: 10.1001/jama.2021.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siemens Healthineers. Understanding SARS-CoV-2 IgG immunity thresholds and the process of standardization. Accessed November 23, 2021. https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/b2406e708bf287c5/506564e9207f/Understanding-SARS-CoV-2-IgG-Immunity-Thresholds-and-the-Process-of-Standardization.pdf
- 22.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brochot E, Demey B, Touzé A, et al. Anti-spike, anti-nucleocapsid and neutralizing antibodies in SARS-CoV-2 inpatients and asymptomatic individuals. Front Microbiol. 2020;11:584251. doi: 10.3389/fmicb.2020.584251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irsara C, Egger AE, Prokop W, et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin Chem Lab Med. 2021;59(8):1453-1462. doi: 10.1515/cclm-2021-0214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
eMethods
eTable 1. Candidate Covariates for Inclusion in the Cox Regression Analysis
eTable 2. Characteristics of SARS-CoV-2 positive participants (n=44)
eTable 3. Baseline Characteristics Stratified by Booster Dose Receipt Status
eFigure 1. SARS-CoV-2 Spread in Israel Throughout the Study Period
eFigure 2. Vaccine Uptake Over Time in Booster Cohort
eFigure 3. Daily PCR Test Density
eFigure 4. Cumulative Number of Positive PCR Tests Over Time From Study Initiation
eFigure 5. Cox Model Diagnostics
eFigure 6. Cumulative Incidence of Symptomatic SARS-CoV-2 Infections
eFigure 7. Cumulative Incidence of SARS-CoV-2 Asymptomatic Infection
eReference