Abstract
Crimean–Congo haemorrhagic fever virus (CCHFV) is a tick-borne virus causing Crimean–Congo haemorrhagic fever (CCHF), a disease reported to have a high fatality rate in numerous countries. The virus is geographically widespread due to its vector, and numerous wild and domestic animals can develop asymptomatic infection. Serological and limited molecular evidence of CCHFV has previously been reported in Camelus dromedarius (the dromedary, or one-humped camel) in the United Arab Emirates (UAE). In this study, 238 camel samples were screened for CCHFV RNA where 16 camel samples were positive for CCHFV by RT-PCR. Analysis of full-length CCHFV genome sequences revealed a novel lineage in camels from the UAE, and potential reassortment of the M segment of the genome.
Keywords: camels, Crimean–Congo haemorrhagic fever (CCHF), UAE
Introduction
An orthonairovirus in the family Nairoviridae, Crimean–Congo haemorrhagic fever virus (CCHFV) is a vector-borne negative-sense RNA virus that causes severe viral haemorrhagic fever outbreaks. CCHFV has a tripartite genome consisting of the large (L) segment that minimally encodes for RNA-dependent RNA polymerase, the medium (M) segment for glycoprotein precursor proteins and the small (S) segment for the nucleocapsid, where CCHFVs have been reported to be highly variable [1]. With no vaccine or specific treatment available, the case fatality ratio for Crimean–Congo haemorrhagic fever (CCHF) in humans ranges from 10–40 % [2].
CCHFV infections have been reported in more than 30 countries across Africa, Asia and Europe, reflective of the broad geographical distribution of its natural reservoir and vector: ixodid ticks, predominantly of the genus Hyalomma. CCHFV persists over the ticks’ entire lifespan, allowing for vertical transmission, and proliferates through a tick–vertebrate–tick enzootic cycle. This results in a dynamic range of seroprevalence among various wild and domestic animals, including but not limited to cattle, goats, camels and sheep [3, 4].
Reported CCHFV seropositivity rates among camels in the United Arab Emirates (UAE) have ranged from 5.3–26 %, with an average of 13.4 % [3, 5–7]. Recently, CCHFV RNA prevalence was reported in Hyalomma dromedarii ticks from camels in the UAE and partial genome sequences were recovered [8]. Additionally, neighbouring countries such as Iran and Oman have reported CCHFV RNA prevalence ranging from 5.3–10.2 % in H. dromedarii ticks [9–11].
Human CCHF cases have been reported in the UAE since a hospital outbreak in 1979. In addition to sporadic cases, a 1994 outbreak and two 2010 cases revealed a connection with direct or indirect animal contact. In the latter two instances, more than half of the infected patients were livestock market, abattoir, or animal processing workers, with close contact with camels and other livestock prior to onset of disease [5, 12–14]. Anecdotal reports of CCHF have continued since 2010, suggesting continuance of disease transmission, further highlighting the need to closely monitor CCHF in both humans and animals in areas with close human–livestock interfaces.
Previously, we reported detecting CCHFV RNA in a subset of the Middle East respiratory syndrome coronavirus (MERS-CoV)-positive camels via metagenomic sequencing [15]. In the current study, we sought to better understand the prevalence of CCHFV among UAE camels and to obtain a full viral genome sequence.
Methods
Camel sampling
From 2015 to 2017, a total of 247 samples from 245 camels at an open-air livestock market, a commercial slaughterhouse and a public slaughterhouse in Abu Dhabi, UAE were sent to the US Centers for Disease Control and Prevention (CDC) as part of a MERS-CoV surveillance study. Nasal swabs were taken from camels at the open-air livestock market and lung or bronchial swabs were taken from camels with lung lesions at the slaughterhouses and pooled per every three camels. Of the available samples, a total of 238 camel samples were further screened for CCHFV.
RNA extraction and PCR screening of camel samples
A total of 224 nasal swabs, 7 pools of camel lung swabs and 7 pools of camel bronchial swabs, collected over a period of 3 years, were screened for the presence of CCHFV. Total RNA was extracted and purified using the EZ1 Virus Mini kit 2.0 (Qiagen, Hilden, Germany). RNA samples were screened for CCHFV by a broadly reacting semi-nested RT-PCR with consensus degenerate primers targeting a region of the L segment conserved across orthonairovirus genomes. Primary RT-PCR was conducted under the following parameters. RT step: 60 °C for 1 min with a decrease in temperature of 0.5 °C s−1 and 45 °C for 30 min. Initial denaturation at 94 °C for 2 min, followed by 40 cycles of 94 °C for 15 s, 50 °C for 30 s, 72 °C for 30 s, and a final extension of 72 °C for 7 min using the following primers: F1: ACAGGCATGGCAATACTICARCARYTNGC and R1: TGTATCGGGCCCCATTTIGTRTTRTCNCC. The subsequent PCR was conducted under the following parameters. Initial denaturation at 94 °C for 2 min, followed by 40 cycles of 94 °C for 15 s, 50 °C for 30 s, 72 °C for 30 s, and a final extension of 72 °C for 7 min using the following primers: F2: GCTTTCATGCAGTTTTAGCACCIAARGCNCA and R2: TGTATCGGGCCCCATTTIGTRTTRTCNCC. PCR products of expected size [243 nucleotides (nt)] were gel-extracted prior to bi-directional Sanger sequencing on an ABI3500 sequencer (Thermo Fisher Scientific, Waltham, MA, USA).
Full-genome sequencing
We generated small islands of reads throughout the three segments from a previous metagenomic sequencing study [15] and amplicon sequences through the semi-nested consensus degenerate RT-PCR as described above. These regions were then bridged using sequence-specific RT-PCR followed by Sanger sequencing on an ABI3500 sequencer (Thermo Fisher Scientific, Waltham, MA, USA). Sequences were cleaned and assembled using Sequencher 5.0 (Gene Codes, Ann Arbor, MI, USA), where ends and low-quality regions were trimmed manually. A consensus complete genome sequence of L, M and S segments was generated, and terminal nucleotide sequences were confirmed by RACE RT-PCR. Open reading frames (ORFs) were identified for genome annotation using the best matches from blastn/blastx as the reference.
Phylogenetic analysis
A representative collection of unique complete CCHFV genome sequences obtained from the National Center for Biotechnology Information’s (NCBI’s) GenBank were aligned using the muscle multiple sequence aligner with eight iterations [16]. The best-fit model (GTR+G+I, identified with jModelTest2) was selected to conduct phylogenetic analysis using the maximum-likelihood method with 1000 bootstraps [17]. All phylogenetic analysis was conducted with Geneious Prime (Biomatters, Inc., San Diego, CA, USA) and phylogenetic tree visualization was performed with FigTree 1.4.3 (University of Edinburgh, Edinburgh, UK) software.
Results and Discussion
From 2015–2017, camel samples (nasal swabs and respectively pooled bronchial and lung swabs) were collected from a total of three markets and slaughterhouses in Abu Dhabi, UAE as part of an ongoing camel surveillance study [18]. A total of 238 camel samples were tested for the presence of CCHFV RNA.
Of the 238 samples tested, 16 were PCR-positive for CCHFV. Sample type and camel origin are as follows: 2015, 11 nasal swabs (9 Oman, 2 UAE); 2016, 2 nasal swabs [1 UAE, 1 Kingdom of Saudi Arabia (KSA)]; 2017, 2 nasal swabs (2 UAE) and 1 lung swab pool (UAE). Alignment of the PCR amplicon sequences showed 99 % identity among the 16 samples; hence RNA extracted from 1 of the nasal swabs that had sufficient RNA was further processed to obtain a full genome sequence. Full-genome sequences from the S, M and L segments were obtained (S: MN901927, M: MN901926, L: MN901925). In addition, the available CCHF reads from a previous metagenomic sequencing study mapped well to full-genome sequences from this study, suggesting a potentially similar strain consistent with PCR amplicon sequences. Notably, among the 16 CCHFV-positive camels, 12 had previously been reported as positive for MERS-CoV RNA [13]. The impact of co-infection on either virus’s dynamics, if any, is yet to be understood.
The ORF of the camel sequences from the present study was compared with representative ORFs from human and tick isolates shown in the tree, respectively. Average percentage identity suggested that the greatest variation was in the M segment compared to the other two segments, for both human and tick isolate comparisons (Table 1). The greatest differences were observed in the mucin-like domain of the glycoprotein complex (aa 28 to 251), where the percentage difference ranged from 77.6–82.6 %.
Table 1.
Average amino acid (aa) identity from ORF comparison of the camel sequence from present study with representative CCHFV sequences of human or tick origin as shown on the phylogenetic tree
|
Camel vs human (%) |
Camel vs tick (%) |
|
|---|---|---|
|
S segment |
95.5 |
95.1 |
|
M segment |
70.3 |
72.7 |
|
L segment |
95.6 |
95.2 |
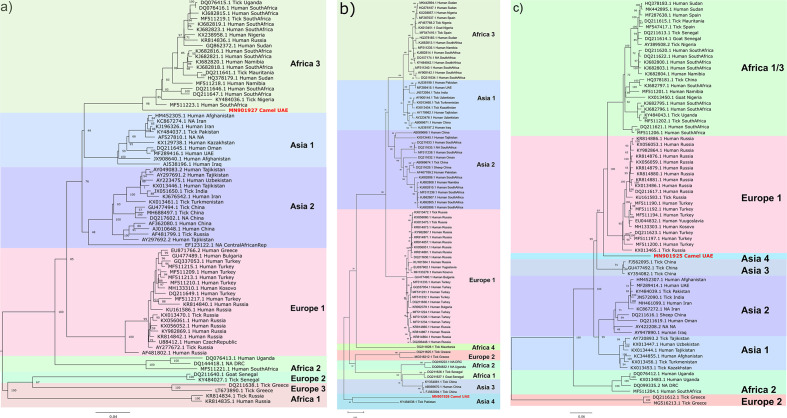
Phylogenetic analysis of the three segments suggests greater conservation in the S and L segments than in the M segment. S segment analysis suggests the current CCHFV sequence from camels was an outlier to the Africa 3 lineage, with the highest percentage nt identity at 89.8 % with a human CCHFV isolate from South Africa (KJ682821.1; Fig. 1a). M segment analysis suggests possible reassortment of the current sequence from camel and its nearest neighbour (76.7 % nt identity) from a tick in Pakistan (KY484038.1), forming a distinct lineage, Asia 4 (Fig. 1b). This echoes a frequent observation of greater reassortment in the M segment among tick vectors compared to mammalian hosts, due to vectors’ lifelong infection and potential to infect multiple hosts [19]. L segment analysis showed that the current sequence expanded the clade comprising the Africa 1/3 and Europe 1 lineages, with the highest percentage nt identity at 87.7 % with a human CCHFV isolate from Russia (KX013486.1; Fig. 1c). Since the S, M and L segments from this CCHFV genome isolated from a camel are not part of any previously defined lineages, in accordance with current CCHFV phylogeny, we assigned the camel genome to a new lineage termed Asia 4.
Fig. 1.
Maximum-likelihood tree for CCHFV by segment. Maximum-likelihood tree analysis conducted with unique complete CCHFV genome sequences obtained from the NCBI and the current study’s sequences. Sequences of complete segments were aligned with muscle aligner and maximum-likelihood analysis was conducted using the GTR+G+I model with 1000 bootstraps. Bootstrap values of 40 or above are noted. Bars indicate the nucleotide substitution per site. Tips are labelled with NCBI accession number, organism source and country. na indicates that information was not available. CCHFV sequences from camels sequenced in this study are indicated with red tips. (a) S segment, (b) M segment and (c) L segment.
While this paper was in preparation, partial CCHFV S- and M-segment sequences from H. dromedarii ticks and camels from the UAE were published separately. In addition to molecular detection, this study reported that 67 % of sampled camels had CCHFV antibodies, which further supports the view that camels in the region are commonly infected with CCHFV [8]. The partial sequences obtained by Camp et al. are almost identical to our current full-length sequences, which also have high sequence identity to previous partial sequences reported from camel ticks in Egypt [20], suggesting that virus strains belonging to the Asia 4 lineage might not only be prevalent in camels and camel ticks in the UAE . Future studies are required to not only demonstrate whether this lineage is also associated with camels from other countries of the Arabian Peninsula, Asia and Africa, but also better understand the viral transmission between camels and possibly other vertebrate hosts. The first complete sequence of CCHFV strain from the Asia 4 lineage will be instrumental for the development of specific RT-PCR assays for the identification of whether other animal hosts can be infected with similar strains and if camels or camel ticks are more prominent vectors of transmission to humans than previously suggested [20]. Comparison of the genome from this study with available human CCHFV isolate sequences from UAE and its neighbour, Oman, indicated that the current sequence belongs to the Asia 1 lineage. Current molecular diagnostics of CCHF typically rely on the amplification of conserved regions of the S segment, which should detect potential human infections with CCHFV strains related to the camel strain. However, more systematic sequencing efforts are warranted to better define the potential of camel- or camel tick-associated strains of CCHFV to cause disease in humans.
In addition to the importance of conducting whole-genome sequencing, the importance of regular surveillance of animals, including camels, with close human interaction cannot be stressed enough. Not only have recent UAE human CCHF cases been associated with camels, but camels have also previously been shown to harbour multiple viruses, including viruses such as Rift Valley fever virus, CCHFV and MERS-CoV that pose significant risks to public health. Here, we report the first molecular evidence of CCHFV in camels not only from nasal swabs, but also from molecular detection in lung swab samples. Human interaction with infected animals in closed environments such as abattoirs probably increases the potential for disease transmission; previous primary CCHFV cases in the UAE were associated with abattoirs. It is imperative that regular surveillance at animal markets in the context of One Health investigations be conducted so pathogens with zoonotic or anthroponotic potential can be detected and identified before outbreaks occur.
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
The opinions expressed by the authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: aa, amino acid; CCHF, Crimean-Congo haemorrhagic fever; CCHFV, Crimean-Congo haemorrhagiv fever virus; CDC, Centers for Disease Control and Prevention; L, large; M, medium; MERS-CoV, Middle East respiratory syndrome coronavirus; NCBI, National Center for Biotechnology Information; nt, nucleotides; ORF, open reading frame; S, small; UAE, United Arab Emirates.
References
- 1.Deyde VM, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. Crimean-Congo hemorrhagic fever virus genomics and global diversity. J Virol. 2006;80:8834–8842. doi: 10.1128/JVI.00752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, et al. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Spengler JR, Bergeron Éric, Rollin PE. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis. 2016;10:e0004210. doi: 10.1371/journal.pntd.0004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spengler JR, Bergeron Éric, Spiropoulou CF. Crimean-Congo hemorrhagic fever and expansion from endemic regions. Curr Opin Virol. 2019;34:70–78. doi: 10.1016/j.coviro.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AS, Maupin GO, Rollin PE, Noor AM, Shurie HHM, et al. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. 1997;57:519–525. doi: 10.4269/ajtmh.1997.57.519. [DOI] [PubMed] [Google Scholar]
- 6.Suliman HM, Adam IA, Saeed SI, Abdelaziz SA, Haroun EM, et al. Crimean Congo hemorrhagic fever among the one-humped camel (Camelus dromedaries) in central Sudan. Virol J. 2017;14:147. doi: 10.1186/s12985-017-0816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariner JC, Morrill J, Ksiazek TG. Antibodies to hemorrhagic fever viruses in domestic livestock in niger: Rift Valley fever and Crimean-Congo hemorrhagic fever. 1995;53:217–221. doi: 10.4269/ajtmh.1995.53.217. [DOI] [PubMed] [Google Scholar]
- 8.Camp JV, Kannan DO, Osman BM, Shah MS, Howarth B, et al. Crimean-Congo hemorrhagic fever virus endemicity in United Arab Emirates, 2019. Emerg Infect Dis. 2020;26:1019–1021. doi: 10.3201/eid2605.191414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedaghat MM, Sarani M, Chinikar S, Telmadarraiy Z, Moghaddam AS, et al. Vector prevalence and detection of Crimean-Congo haemorrhagic fever virus in Golestan Province, Iran. J Vector Borne Dis. 2017;54:353–357. doi: 10.4103/0972-9062.225841. [DOI] [PubMed] [Google Scholar]
- 10.Champour M, Chinikar S, Mohammadi G, Razmi G, Shah-Hosseini N, et al. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus detected from ticks of one humped camels (Camelus dromedarius) population in northeastern Iran. J Parasit Dis. 2016;40:110–115. doi: 10.1007/s12639-014-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Body MHH, Abdulmajeed HA, Hammad MH, Mohamed SA, Saif SA, et al. Cross-sectional survey of Crimean-Congo hemorrhagiv fever virus in the Sultanate of Oman. J Vet Med Anim Health. 2016;8:6 [Google Scholar]
- 12.Rodriguez LL, Maupin GO, Ksiazek TG, Rollin PE, Khan AS, et al. Molecular investigation of a Multisource outbreak of Crimeancongo hemorrhagic fever in the United Arab Emirates. 1997;57:512–518. doi: 10.4269/ajtmh.1997.57.512. [DOI] [PubMed] [Google Scholar]
- 13.Suleiman MN, Muscat-Baron JM, Harries JR, Satti AG, Platt GS, et al. Congo/Crimean haemorrhagic fever in Dubai. an outbreak at the Rashid Hospital. Lancet. 1980;2:939–941. [PubMed] [Google Scholar]
- 14.Mohamed Al Dabal L, Rahimi Shahmirzadi MR, Baderldin S, Abro A, Zaki A, et al. Crimean-Congo hemorrhagic fever in Dubai, United Arab Emirates, 2010: case report. Iran Red Crescent Med J. 2016;18:e38374. doi: 10.5812/ircmj.38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Khalafalla AI, Paden CR, Yusof MF, Eltahir YM, et al. Identification of diverse viruses in upper respiratory samples in dromedary camels from United Arab Emirates. PLoS One. 2017;12:e0184718. doi: 10.1371/journal.pone.0184718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgar RC. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusof MF, Queen K, Eltahir YM, Paden CR, Al Hammadi Z, et al. Diversity of middle East respiratory syndrome coronaviruses in 109 dromedary camels based on full-genome sequencing, abu dhabi, United Arab Emirates. Emerg Microbes Infect. 2017;6:1–10. doi: 10.1038/emi.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burt FJ, Paweska JT, Ashkettle B, Swanepoel R. Genetic relationship in southern African Crimean-Congo haemorrhagic fever virus isolates: evidence for occurrence of reassortment. Epidemiol Infect. 2009;137:7. doi: 10.1017/S0950268808001878. [DOI] [PubMed] [Google Scholar]
- 20.Chisholm K, Dueger E, Fahmy NT, Samaha HAT, Zayed A, et al. Crimean-congo hemorrhagic fever virus in ticks from imported livestock, Egypt. Emerg Infect Dis. 2012;18:181–182. doi: 10.3201/eid1801.111071. [DOI] [PMC free article] [PubMed] [Google Scholar]