Abstract
Simple Summary
The microbial community that inhabits specific areas of the body, developing a symbiotic relationship with the host, is termed the microbiota. The intestinal microbiota plays a pivotal role in different physiological processes and is influenced by many factors, including nutrition. Goji berries are a popular nutraceutical product that have been proposed as a dietary supplement in some livestock species, including rabbits, but their effects on the composition of the microbiota have never been investigated. This study evaluated the effects of Goji berry supplementation on the microbiota of different digestive tracts (stomach, duodenum, jejunum, ileum, caecum and colon) of the rabbit, using a modern method of analysis. Our results suggest that Goji berries could modulate the microbiota of the rabbit’s digestive tract increasing the growth of beneficial bacteria, such as Ruminococcaceae, Lachnospiraceae, Lactobacillaceae, and particularly, the genus Lactobacillus. These findings suggest that Goji berries could be used to produce innovative feeds for rabbits, although further studies are necessary to evaluate their impact on productive performance, gut immune system maturation, as well as resistance to gastrointestinal disorders.
Abstract
Goji berries show health benefits, although the possible mechanisms of action, including compositional changes in the gut microbiome, are still not fully understood. The aim of this study was to evaluate the effect of Goji berry supplementation on microbiota composition and metabolites in the digestive tracts of rabbits. Twenty-eight New Zealand White rabbits were fed with a commercial feed (control group, C; n = 14) or the same diet supplemented with 3% of Goji berries (Goji group, G; n = 14), from weaning (35 days old) until slaughter (90 days old). At slaughter, samples from the content of the gastrointestinal tracts were collected and analyzed by Next Generation 16S rRNA Gene Sequencing to evaluate the microbial composition. Ammonia and lactic acid were also quantified in caecum. Results showed differences in microbiota composition between the groups for two phyla (Cyanobacteria and Euryarchaeota), two classes (Methanobacteria and Bacilli), five orders, fourteen families, and forty-five genera. Ruminococcaceae (p < 0.05) and Lachnospiraceae (p < 0.01) were more abundant in G than in C group. Lactobacillaceae also showed differences between the two groups, with Lactobacillus as the predominant genus (p = 0.002). Finally, Goji berry supplementation stimulated lactic acid fermentation (p < 0.05). Thus, Goji berry supplementation could modulate gastrointestinal microbiota composition and caecal fermentation.
Keywords: Goji fruit, intestinal bacterial community, caecum, lactic acid, ammonium, rabbit
1. Introduction
Goji berries, the fruits of the Lycium barbarum plant, are often used in traditional Chinese medicine for their nutritional and therapeutic properties, and are also widespread as supplementation in Western diets [1,2]. Their health benefits are associated with biologically active compounds, including polysaccharides, carotenoids, polyphenols, amino acids, ascorbic acid, and unsaturated fatty acids [3], although their mechanisms of action are still not fully understood. Recent evidence has shown that the fruit could modulate the gut microbiota and thus have a role in the prevention and treatment of several gastrointestinal diseases in mice [4,5], rats [6] and humans [7]. Recently, Goji berries have also been proposed as a dietary supplement for some livestock species, with the dual aim of improving productive performance and product quality [8,9,10]. In rabbits, Goji berry supplementation seems to improve reproductive [11] and productive performances, [12] energy metabolism [13], and meat quality [14,15] in a dose-dependent manner, but its effects on gut microbiota have not yet been investigated.
The microbiota represents a complex ecosystem of microorganisms which inhabits specific niches of the body and plays important roles in physiological processes developing symbiotic relationships with the host [16]. The intestinal microbiota is involved in the digestion and absorption of nutrients, maturation and stimulation of the immune system, as well as protection against pathogenic infections [17]. The bacterial microbiota composition along the gastrointestinal tract of adult rabbits fed with a commercial diet has recently been characterized [18]. This study showed interesting differences among the various sections of the digestive system in bacterial richness and diversity [18]. Within the same species, however, bacterial community composition of the gastrointestinal tract can be influenced by several factors, including nutrition [13]. Goji supplementation could therefore induce favorable changes in the intestinal microbiota of the rabbit with beneficial effects on health and productive performance, as seen in other animal species and humans [4,5,6,7,8,9,10].
The rabbit is a very interesting species because it can be a pet, livestock or animal model. Both in pet and farmed rabbits, the digestive system is a common site of diseases that are often associated with changes in intestinal microbiota [19,20]. In particular, the peri-weaning period is the most critical physiological phase as the diet transition induces changes in the gut microbiota increasing sensitivity to gastrointestinal pathogens [21]. Antibiotics are commonly used to control intestinal infections; however, according to recommendations of the European Union, this practice should be reduced [22,23]. An innovative strategy to limit the incidence of gastrointestinal disorders could be the use of specific feeds for pet and farmed rabbits integrated with nutraceutical products such, as Lycium barbarum fruit, to favor the growth of a beneficial gut microbiota. Understanding the effects of Goji berry supplementation on the intestinal microbiota can therefore have important implications for the health of rabbits. The rabbit could be also considered as an animal model for diet-induced changes in gut microbiota, as it has already been used for studies exploring the effect of nutrition on productive [24,25,26], reproductive [27,28], and immunological traits [29,30].
The aim of this study was to investigate the effect of Goji berry supplementation on the microbiota composition of the different tracts of the digestive system (stomach, duodenum, jejunum, ileum, caecum, and colon) in the rabbit. For this purpose, the microbiota of all the sections of the digestive apparatus was analyzed using Next Generation 16S rRNA Gene Sequencing. In addition, metabolites from bacterial fermentation in the caecum (lactic acid and ammonia) were also quantified.
2. Materials and Methods
2.1. Animals and Samples Collection
The experimental trial was conducted in the facilities of the Faculty of Veterinary Medicine of the Agricultural University of Tirana, Tirana, Albania.
The rabbits were maintained under the supervision of a responsible veterinarian and in accordance with the Directive 2010/63/EU regarding the protection of animals kept for farming purposes. The lowest number of rabbits necessary to obtain reliable results was used for the trial.
According to dietary treatment, 28 New Zealand White male rabbits were randomly assigned into two groups from weaning (35 days of age) until slaughter (90 days of age): control group (n = 14 animals, C), fed with a commercial pellet, and Goji group (n = 14 animals, G), fed with the same feed of the C group supplemented with 3% of Goji berries (Gianluca Bazzica, Foligno, Italy) before pelleting (Table 1). At weaning the average body weight was 875 ± 115 g and 893 ± 135 in C and G groups, respectively. Feeds from the same batches were previously used in other experiments [11,13,14,15].
Table 1.
Feed formulation and chemical composition (as fed) of control group and Goji group diet.
| Ingredients | Unit | Diet | |
|---|---|---|---|
| Control | Goji | ||
| Wheat bran | % | 30.0 | 29.0 |
| Dehydrated alfalfa meal | % | 42.0 | 41.0 |
| Barley | % | 9.5 | 9.0 |
| Sunflower meal | % | 4.5 | 4.2 |
| Rice bran | % | 4.0 | 3.9 |
| Soybean meal | % | 4.0 | 3.9 |
| Calcium carbonate | % | 2.2 | 2.2 |
| Cane molasses | % | 2.0 | 2.0 |
| Dicalcium phosphate | % | 0.7 | 0.7 |
| Vitamin-mineral premix 1 | % | 0.4 | 0.4 |
| Soybean oil | % | 0.4 | 0.4 |
| Salt | % | 0.3 | 0.3 |
| Goji berries | % | - | 3.0 |
| Chemical composition | |||
| Crude Protein | % | 15.74 | 15.66 |
| Ether extract | % | 2.25 | 2.47 |
| Ash | % | 9.28 | 9.25 |
| Starch | % | 16.86 | 16.99 |
| NDF | % | 38.05 | 37.49 |
| ADF | % | 19.54 | 19.01 |
| ADL | % | 4.01 | 3.98 |
| Digestible Energy 2 | MJ/Kg | 10.3 | 10.3 |
1 Per kg diet: vitamin A 11,000 IU; vitamin D3 2000 IU; vitamin B1 2.5 mg; vitamin B2 4 mg; vitamin B6 1.25 mg; vitamin B12 0.01 mg; alpha-tocopherol acetate 50 mg; biotine 0.06 mg; vitamin K 2.5 mg; niacin 15 mg; folic acid 0.30 mg; D-pantothenic acid 10 mg; choline 600 mg; Mn 60 mg; Fe 50 mg; Zn 15 mg; I 0.5 mg; Co 0.5 mg. 2 NDF: Neutral Detergent Fiber; ADF: Acid Detergent Fiber; ADL: Acid Detergent Lignin. Estimated by Maertens et al. [31].
Rabbits were bred in single cages and maintained at a temperature range between 18 and 21 °C, relative humidity of 60%, and with a photoperiod of 16 h of light. Throughout the entire trial, water and feed were provided ad libitum.
At the slaughterhouse, the gastrointestinal tract was immediately removed from each rabbit. The content of the different digestive tract sections from each animal (stomach, duodenum, jejunum, ileum, caecum, and colon) were collected separately in 15 mL sterile tubes and then stored at −80 °C until examination. Each sample was analyzed individually. The average body weights (±standard error) at weaning were 875 ± 55 g and 893 ± 75, while at slaughter, they were 2310 ± 82 g and 2357 ± 82 g in C and G groups, respectively.
2.2. Microbiota Evaluation—Genomic Sequencing
2.2.1. DNA Extraction
Using the commercial QIAamp PowerFecal Pro DNA Kit (Qiagen, Hilden, Germany), the bacterial DNA was extracted from each sample of intestinal contents following the manufacturer’s protocol. DNA quality and quantity were checked using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA, and the obtained DNA was stoked at −20 °C until use.
2.2.2. 16S Ribosomal RNA (rRNA) Gene Sequencing
Bacterial DNA was amplified using primers described in the literature [32] which target the V3-V4 hypervariable regions of the 16S rRNA gene. All the PCR amplifications were performed in 25 µL volumes per sample. A total of 12.5 µL of KAPA HIFI Master Mix 2× (Kapa Biosystems, Inc., Wilmington, MA, USA) and 0.2 µL of each primer (100 µM) were added to 2 µL of genomic DNA (5 ng/µL). Blank controls (no DNA template added to the reaction) were also performed. A first amplification step was performed in an Applied Biosystem 2700 thermal cycler (ThermoFisher Scientific, Waltham, MA, USA). The samples were denatured at 95 °C for 3 min, followed by 25 cycles with a denaturing step at 98 °C for 30 s, annealing at 56 °C for 1 min, and extension at 72 °C for 1 min, with a final extension at 72 °C for 7 min. The amplicons were then cleaned with Agencourt AMPure XP (Beckman, Coulter Brea, CA, USA), and libraries were prepared following the 16S Metagenomic Sequencing Library Preparation Protocol (Illumina, San Diego, CA, USA). The libraries obtained were quantified using Real Time PCR with KAPA Library Quantification Kits (Kapa Biosystems, Inc., Wilmington, MA, USA), pooled in equimolar proportion, and then sequenced in one MiSeq (Illumina, San Diego, CA, USA) run with 2 × 250-base paired-end reads.
2.2.3. Sequence Analysis
The reads obtained by the 16S rRNA sequencing were analyzed as previously described [18]. One rabbit from the Goji group and two samples, both from G diet (caecum intestinal tract), were removed because they had a total number of counts <100.
2.2.4. Alpha and Beta Diversity Indices
To assess the microbial diversity of the different rabbit gastrointestinal tracts the alpha (within-) and beta (across-) diversities were used. These indices were estimated starting from the OTU table, after filtering with more than 50 total counts, distributed in at least five samples. Besides the number of observed OTUs directly, within-sample microbial richness, diversity, and evenness were estimated using Chao1 and ACE (abundance-based coverage estimator) for richness, Shannon, Simpson, and Fisher’s alpha for diversity [33,34], and Simpson E and Pielou’s J (Shannon’s evenness) for evenness [35]. The Bray–Curtis dissimilarity [36] was used to quantify the across-sample microbiota diversity. Prior to the calculation of these metrics, the OTU counts were normalized for uneven sequencing depth by cumulative sum scaling (CSS) [37]. Details of these analyses can be found in Biscarini et al. [38].
2.2.5. Software
The QIIME 1.9 pipeline [39] was utilized both to analyze the reads obtained from 16S rRNA gene sequencing and to estimate most diversity indices. Own Python (https://github.com/filippob/Rare-OTUs-ACE.git, accessed on 15 November 2021) and R (https://github.com/filippob/sampleBasedRarefaction, accessed on 15 November 2021) scripts were used to estimate the ACE index and sample-based rarefaction. The figures were generated with the ggplot2 R package [40]. The R environment for statistical computing [41] was used to perform the additional data handling and statistical analysis.
2.3. Lactic Acid and Ammonia Quantification
For the analysis of bacterial metabolites (lactic acid and ammonia), 1 g of caecal content was diluted in 1 mL of 1 M perchloric acid and 8 mL of distilled water. After homogenization, tubes were centrifuged for 10 min at 5000 rpm, and the supernatant was transferred to 2 mL Eppendorf tube and frozen at −20 °C until metabolite quantification. The spectrophotometric method for biological fluids was used for lactic acid determination in accordance with Pryce et al. [42]. Ammonia concentration was detected in line with Patton et al. [43]. Spectrophotometer was set at 565 nm and 660 nm respectively (Shimadzu Corporation UV-2550, Kyoto, Japan). All chemicals were purchased from Sigma Chemical Co (St. Louis, MO, USA).
2.4. Statistical Analysis
Differences in alpha diversity indexes between treatments at various taxonomic levels along the rabbit’s gastrointestinal tract were tested with a linear model that took into account the hierarchical structure of within-subject nested data (consecutive sections of the gastrointestinal tract belonging to individual rabbits). The model had the following form:
| yijkt = µ + rabbitj + treatmentk + anatomic regiont(j) + eijkt | (1) |
where yijkt is the alpha diversity index value for record i from rabbit j with treatment k and anatomic region t, µ is the intercept, rabbitj is the systematic effect of the individual rabbits, treatmentk is the treatment effect (Goji vs. control), anatomic regiontk(j) is the effect of the anatomic region of the gastrointestinal tract nested within rabbitj, and eijkt is the residual.
| Var(y) = Sigma + Iσe2 |
where Sigma is a block diagonal matrix, with 1 s on the diagonal and the covariances σij between records within rabbits in the off-diagonal block elements, I is the identity matrix, and σe2 is the residual variance.
A simplified version of Model (1) was used to evaluate differences between Goji and control samples; in particular, where the anatomic region effect was dropped and data from all gastrointestinal sections were analyzed jointly to evaluate the effect of Goji supplementation on the overall rabbit gut microbiota.
For Bray–Curtis dissimilarities (beta diversity), differences along the digestive tract were tested non-parametrically using the permutational analysis of variance approach (999 permutations; [33]).
3. Results
3.1. Sequencing Results
The microbiota structure of the gastrointestinal tract of C and G groups was characterized by a total of 6,122,359 and 7,156,769 high quality reads (after filtering), respectively, with a mean of 75,584 ± 38,864 reads for C and 90,592 ± 33,296 reads for G group. The evaluation of the sample-based and sequence-based rarefaction curves suggested that the depth of coverage was sufficient to describe the biological diversity within the samples (Figure S1).
3.2. Taxonomic Composition of Gut Microbiota along the Rabbit Gastrointestinal Tract of C and G Groups
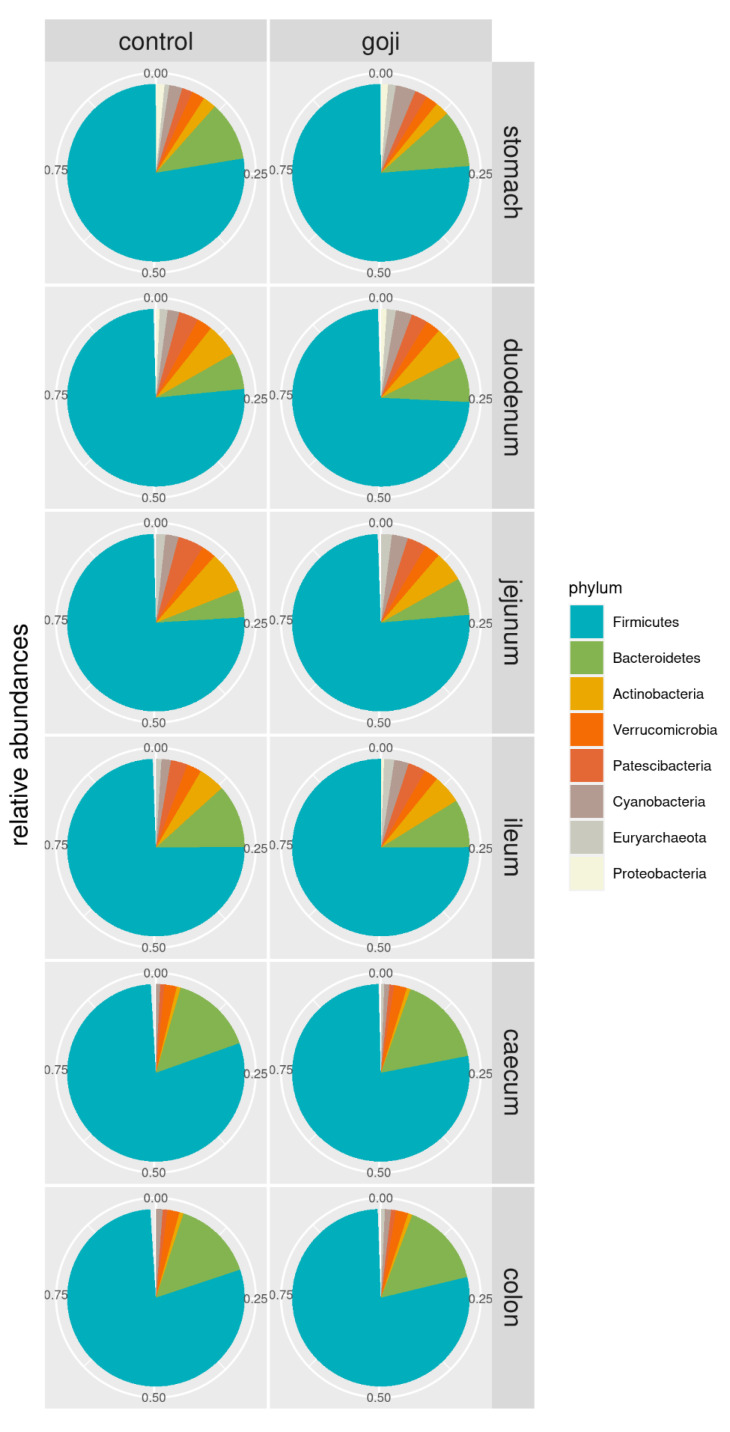
Phylum relative abundances distribution along the gastrointestinal tract of C and G groups are summarized in Figure 1. Significative differences were found in microbiota composition between the experimental groups for two phyla, two classes, five orders, fourteen families, and forty-five genera (Table S1). Firmicutes represented the main phylum in all sections of the digestive tract, especially in the most distal portions of caecum and colon (77–79% of total bacteria) for both groups, and Bacteroidetes the second (14–16% of total bacteria). The caecum and colon of rabbits treated with Goji berries showed differences regarding the abundance of Bacteroidetes (16%) compared to the control group (14%) although these were not statistically significant. As regards other phyla, Actinobacteria was present in the upper part of the gastrointestinal tract. In the jejunum, its relative abundance was higher in C than G group (7.5% for C vs. 5.5% for G group), while in the ileum the percentages were opposite (4.8% for C vs. 5.2% for G group); as with the Bacteroidetes, the differences regarding Actinobacteria were not significant. On the other hand, at the phylum level, Cyanobacteria and Euryarchaeota, the latter belonging to kingdom Archaea, were statistically different (p = 0.034 and p = 0.004, respectively) between the experimental groups, with higher relative abundances in the upper part of the gastrointestinal tract in G group.
Figure 1.
Pie-chart of phylum relative abundances in control and Goji-treated rabbits along the gastrointestinal tract. For the analyses, 14 and 13 samples were used for the control and Goji groups, respectively.
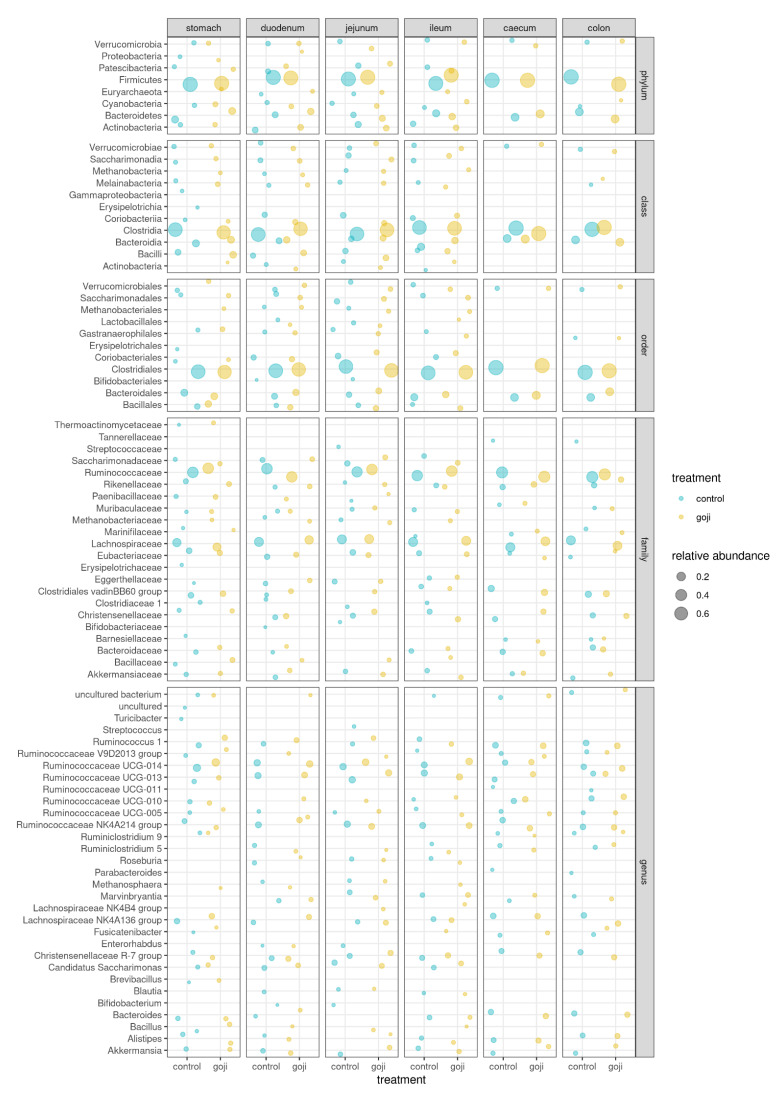
Moreover, Clostridia represented the major class in all anatomic regions, while Ruminococcaceae and Lachnospiraceae were the most abundant families in the Goji group (Figure 2).
Figure 2.
Bubble chart of relative abundances of all taxa (≥1%) in the microbiota of the digestive tract of rabbits, grouped by taxonomic level. Control (blue = 14 rabbits) and Goji (yellow = 13 rabbits) experimental groups. The size of the bubble is proportional to the relative abundance, with 0.2, 0.4 and 0.6 hallmarks, as shown in the legend.
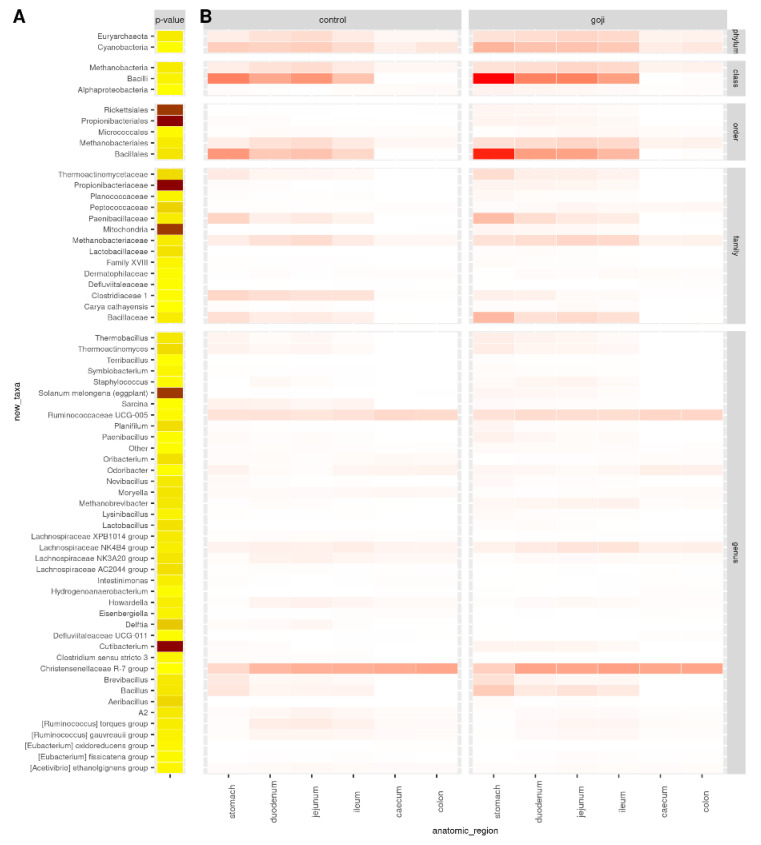
Figure 3 shows the comparison of the relative abundances of significant OTUs between treatments and along the rabbit’s gastrointestinal tract. As shown in Table S1 and Figure 3, there were significant differences between the groups; Bacillales were predominant (p = 0.0032) in the G group, and Bacillus was the major genus in the stomach (p = 0.0036). Ruminococcaceae UCG-005, Lachnospiraceae NK4B4 group, and Christensenellaceae R-7 group were genera detected in all the digestive tracts with statistically significant different results between the groups. As reported in Table S1, the Lactobacillaceae family was significantly different (p = 0.0018) between the groups with Lactobacillus as the predominant genus in G group compared to C group.
Figure 3.
Significantly different OTUs. OTU significantly different between treatments from analysis of variance based on normalized counts: p-values (A) and counts per group and anatomic region of the rabbit digestive tract (B). p-value < 0.05 was used as cut-off. Darker colours indicate lower p-values (A) or higher counts (B). p-values are in the range 10−15–0.049, from dark brown to light yellow. For the analyses, 14 and 13 samples were used for the control and Goji groups, respectively.
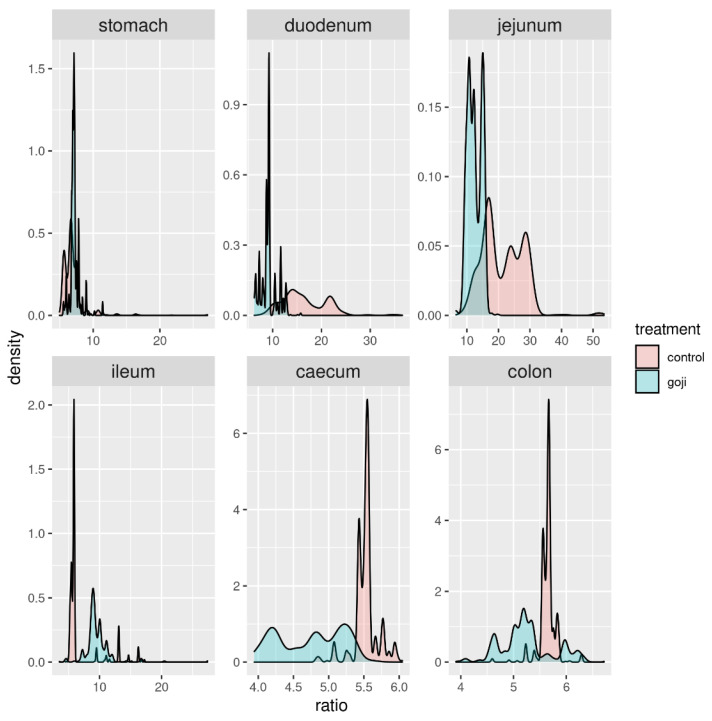
3.3. F/B Ratio
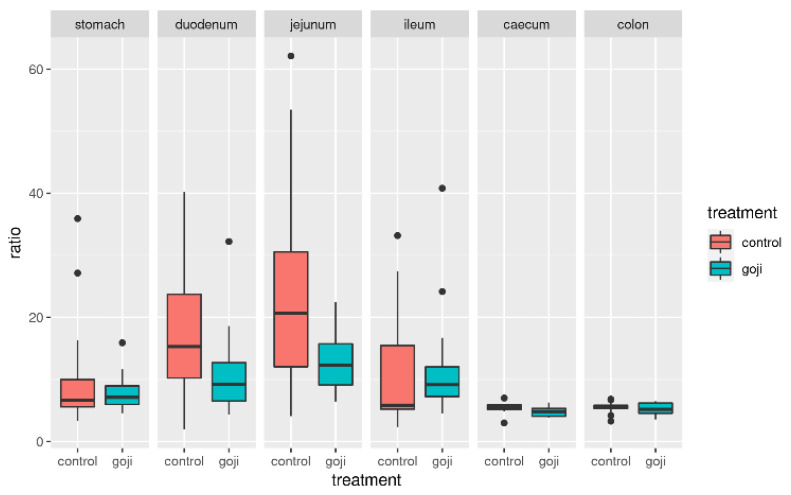
The Firmicutes: Bacteroidetes (F:B) ratio followed a clear pattern along the rabbit’s digestive tract starting at around 10 in the stomach, increasing clearly in the duodenum and jejunum, and finally decreasing again in the caecum and colon. The F:B ratio appeared to be significantly lower in the G group (Figure 4), in the duodenum (p = 0.0176) and jejunum (p = 0.000049). This was confirmed by bootstrapping (1000 replicates resampled with replacement from the original data, Figure 5), which provided further statistical support of the significance of F:B differences between G and C groups in the duodenum, jejunum and, slightly less so, in the ileum.
Figure 4.
Distribution of the F:B ratio (Firmicutes to Bacteroidetes) in control and Goji-treated groups along the gastrointestinal tract. For the analyses, 14 and 13 samples were used for the control and Goji groups, respectively.
Figure 5.
Distribution of the F:B ratio (x-axis) along the digestive tract in Goji-treated (blue) and control (red) rabbits from 1000 bootstrapping replicates of the data. For the analyses, 14 and 13 samples were used for the control and Goji groups, respectively.
3.4. Alpha Diversity Index—Treatment by Region
Table 2 reports the values for the alpha diversity indexes estimated in the rabbits’ gastrointestinal tract, in the two groups. Alpha diversity indexes were significantly different between treatments in the last portion of the digestive tract (Figure S2): six indexes were significantly different in the jejunum (ACE, Fisher’s alpha, observed n. of OTUs, Shannon and Simpson diversity), two in the ileum (Equitability and Simpson E), three in the caecum (Chao1, ACE, Fisher’s alpha), and two in the colon (Equitability and Simpson E).
Table 2.
Alpha diversity indices along the digestive tract of rabbits in the two experimental groups (14 controls and 13 Goji-treated; two more samples, both from caecum intestinal G diet were removed because they had a total number of read counts < 100). * indicates significant difference (p < 0.05) between control and Goji groups.
| Group | Anatomic Portion | N | Chao1 | Ace | Fisher Alpha | Observed OTUS | Shannon | Simpson | Equitability | Simpson E |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Stomach | 14 | 378.115 | 380.038 | 163.32 | 335.786 | 7.779 | 0.993 | 0.973 | 0.747 |
| Goji | Stomach | 13 | 320.39 | 318.117 | 135.593 | 300.923 | 7.926 | 0.995 | 0.976 | 0.766 |
| Control | Duodenum | 14 | 279.333 | 274.565 | 120.298 | 268.143 | 7.806 | 0.995 | 0.976 | 0.764 |
| Goji | Duodenum | 13 | 329.489 | 325.595 | 142.572 | 312.462 | 7.999 | 0.995 | 0.975 | 0.750 |
| Control | Jejunum | 14 | 205.000 * | 205.000 * | 85.734 * | 205.000 * | 7.427 * | 0.993 * | 0.979 | 0.787 |
| Goji | Jejunum | 13 | 306.591 * | 305.284 * | 130.950 * | 287.000 * | 7.878 * | 0.995 * | 0.975 | 0.750 |
| Control | Ileum | 14 | 341.365 | 345.910 | 152.660 | 327.286 | 8.034 | 0.995 | 0.975 * | 0.749 * |
| Goji | Ileum | 13 | 410.027 | 396.468 | 169.947 | 355.000 | 8.149 | 0.996 | 0.968 * | 0.700 * |
| Control | Caecum | 14 | 683.149 * | 640.207 * | 274.313 * | 534.714 | 8.734 | 0.997 | 0.965 | 0.674 |
| Goji | Caecum | 11 | 553.633 * | 555.73 * | 235.517 * | 494.909 | 8.642 | 0.997 | 0.966 | 0.687 |
| Control | Colon | 14 | 621.580 | 616.796 | 271.393 | 529.929 | 8.731 | 0.997 | 0.966 * | 0.682 * |
| Goji | Colon | 13 | 656.245 | 638.687 | 265.692 | 543.385 | 8.744 | 0.997 | 0.964 * | 0.666 * |
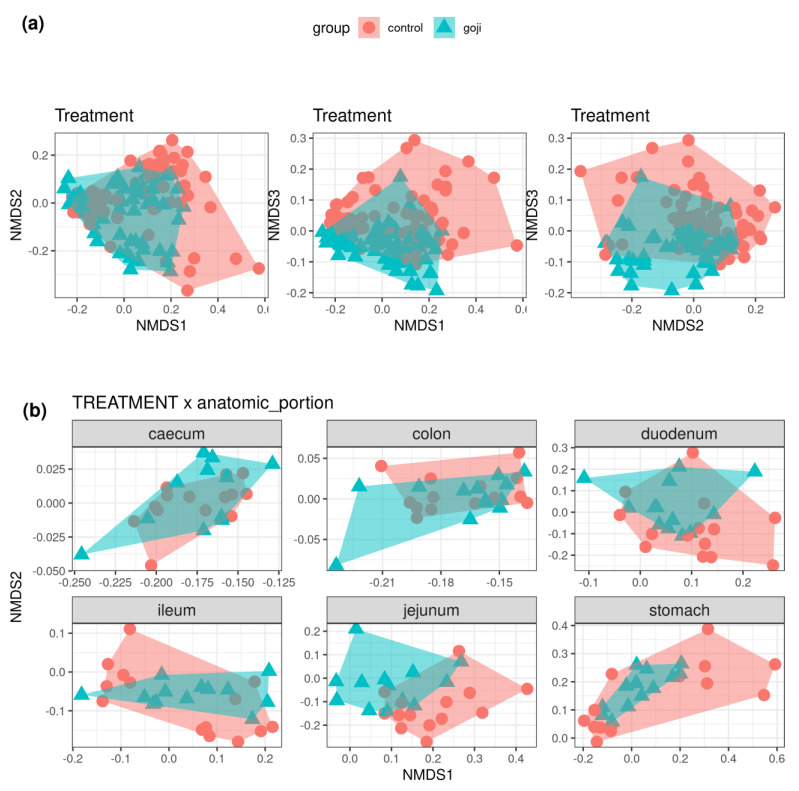
3.5. Beta Diversity Index (Clustering Treatment X Anatomic Portion)
Figure 6a shows the clustering of samples (C and G groups) from Bray–Curtis dissimilarities (first three dimensions from non-metric multidimensional scaling). The distance between groups were significantly different (p < 0.01) from permutational multivariate analysis of variance (PERMANOVA, 999 permutations). This difference appeared to vary along the gastrointestinal tract, with jejunum, caecum, and colon showing the clearest differences, while the two groups mostly overlapped in the stomach, duodenum, and ileum (Figure 6b: first two NMDS dimensions only).
Figure 6.
(a): Non-metric multidimensional scaling plot of Bray-Curtis dissimilarities estimated from the OTU table. The plots show the first three NMDS dimensions (from left to right: dimensions one and two, one and three, two and three). Control samples in red circles, Goji-treated samples in blue triangles. (b): First two dimensions from the non-metric dimensional scaling of Bray-Curtis dissimilarities between control and Goji-treated samples along the digestive tract of rabbits. For the analyses, 14 and 11–13 samples were used for the control and Goji groups, respectively.
3.6. Caecal Lactic Acid and Ammonia Quantification
Regarding lactic acid quantification, G group showed a higher concentration than C group, suggesting higher bacterial activity (3.91 ± 1.59 and 1.01 ± 1.22 mmol/kg in C and G groups, respectively; p = 0.033). No significant differences in ammonia concentration were detected between the two groups (5.81 ± 2.22 and 5.89 ± 1.81 mmol/kg in C and G groups, respectively; p = 0.305).
4. Discussion
Diet is one of the main factors affecting the composition of the microbiota in the digestive tract due to the relation between nutrients and microbial populations [44]. The bacterial populations inhabiting the different gastrointestinal compartments of the rabbit have been previously described [18]. For the first time, this study investigated the effect of Goji berry supplementation on microbiota composition in the different tracts of the digestive system and on caecal bacterial fermentations of adult rabbits.
The results of the present study showed a prevalence of Firmicutes in all the anatomic tracts in both experimental groups. This phylum is classified as the most efficient cellulose degrader [45] and it plays a fundamental role in rabbit digestion. Similar results were reported by both Cotozzolo et al. [18] and Arazzuria et al. [46]. This result was also supported by other studies investigating not only the caecal microbiota of rabbits [47,48] but also the gastrointestinal content and feces of both wild and domestic rabbits [49]. This is a common condition not only in hindgut fermenters, such as rabbits, but also in ruminants and monogastric animals [50].
Bacteroidetes was the second most abundant phylum, especially in the large intestine (caecum and colon tracts), and was slightly predominant in the G group. This phylum, not significantly different between the two groups and along the digestive tracts, is known for its role in the stimulation of gut-associated lymphoid tissue [46,48]. The abundance of Bacteroidetes is in accordance with what was already observed by Cotozzolo et al. [18] on the rabbit gastrointestinal microbiota and by Crowley et al. [49] on both domestic and wild rabbits. A further analysis of our samples with a shotgun metagenomic or metatranscriptomic approach, combined with immunological assays, could provide more information about the role of this relevant phylum in gut immunity.
Regarding other phyla, Verrucomicrobia were found in all sections, while Actinobacteria and Proteobacteria were found in the stomach and small intestine. Although with low levels in the core microbiome, the Euryarchaeota phylum, belonging to the kingdom Archea, was statistically different between the two groups, with higher levels in the G group in all the digestive tracts. All species of this phylum were taxonomically assigned to the methanogenic genus Methanobrevibacter [51]. Though this phylum is not very common in the intestinal microflora of some species, such as horses and pigs [18], it is often found in the human gut with the role of increasing polysaccharide digestion by consuming the end products of bacterial fermentation [52].
Clostridia, anaerobic Gram-positive bacteria present in the intestinal microbiota of human, mouse, chicken, and pig, represented the major class in all anatomic regions, in accordance with Velasco-Galilea and co-workers [51]; they are prevalent, cellulose-degrading symbiotic microorganisms, helping the rabbit for plant material digestion [51].
The families of Ruminococcaceae and Lachnospiraceae were present in all anatomic parts, and both were higher in the G group. Ruminococcaceae are usually prevalent in healthy rabbits [53], while Lachnospiraceae is known to be associated with a decrease of mortality [54]. These two families appear to have an important role in fiber digestion, in particular of peptose and cellulose [55], and are significant producers of short-chain fatty acids [56]. Moreover, as previously reported [4], in mice a diet with Goji supplementation promotes butyrate-producing bacteria, including Lachnospiraceae and Ruminococcaceae families, preventing colitis; their high levels in the digestive apparatus also allow protective and beneficial effects towards different diseases, such as diabetes and heart disease [57].
Lactobacillaceae was another family that showed significant differences between the two groups, although present in small quantities. Within this family, Lactobacillus was the predominant genus. Lactobacilli are rare in the rabbit intestine, occupying less than 1% of the total intestinal bacteria [58], and their function in gut health is not fully understood. A recent study has shown that the total intestinal bacteria from rabbits tends to induce a higher inflammatory level than the total intestinal bacteria from chickens or pigs [59], probably because of the low abundance of Lactobacilli in the rabbit’s intestine. Thus, the higher Lactobacillus abundance in rabbits supplemented with Goji could play a protective role against inflammatory diseases. Components of commensal bacteria can alleviate intestinal inflammation by regulating the expression of both pro-inflammatory and anti-inflammatory factors. Kawashima et al. [60] reported that bacterial double-stranded RNA, abundant in Lactobacillus bacteria, showed a regulatory function by triggering anti-inflammatory factor IFN-β production and inhibiting pro-inflammatory factors production.
The F:B ratio was at around 10 in the stomach, then increased in the duodenum and jejunum, and subsequently progressively decreased from the ileum to the caecum and colon. The F:B ratio appeared to be lower in the G group, significantly so in the duodenum and jejunum, less so in the ileum, caecum and colon, as also shown by the bootstrapping analysis. Studies in human microbiota and in animal models, have reported that the F:B ratio was directly related to body weight modifications and in particular to obesity [61]. In obese people, the population of Firmicutes shows an elevated proportion with a reduced Bacteroidetes population; this unbalance causes an altered F:B ratio [62]. Additionally, a strong correlation between the F:B ratio and milk fat yield has been observed in dairy cattle [63]. In previous studies, feed supplementation in livestock has been reported to alter the F:B ratio in the gut microbiota (e.g., grape pomace supplementation in cattle [38]). Further studies could investigate the link between Goji intake, F:B ratio and lipid metabolism in rabbits.
The alpha diversity results revealed higher microbial richness and diversity in bacterial composition independently from the treatments in the large intestine. That was an expected result because, as already demonstrated in other livestock species, the microbial densities (and also diversity) along the gastrointestinal tract is maximal in the fermenting compartments [64]. Indeed, Cotozzolo et al. found alpha diversity of the cecum and colon to be significantly higher than for the other compartments of the rabbit gastrointestinal tract [18]. As previously reported [18], this variability, typical of colon and caecum tracts, is due essentially to their physiological functions, such as fermentation of cellulose with production of volatile fatty acids (VFA) and their absorption for energy production. Goji berry supplementation caused higher microbial richness, especially in the jejunum, ileum, and colon tracts, where six indexes were significantly different in the jejunum (ACE, Fisher’s alpha, observed n. of OTUs, Shannon and Simpson diversity), two in the ileum (Equitability and Simpson E), three in the caecum (Chao1, ACE, Fisher’s alpha), and two in the colon (Equitability and Simpson E). In particular, the principal families involved in the microbial richness were Ruminococcaceae and Lachnospiraceae, as well as Lactobacillus spp. These conditions could guarantee greater resilience toward dysbiosis in the gut microbiota, which is necessary to maintain homeostasis and, in turn, the healthy status of the gastrointestinal system [65]. The beta diversity was greatly influenced by Goji treatment, especially in caecum and colon tracts, which play fundamental roles in the digestion of fermenter animals, such as rabbits. Conversely, less influence of this treatment was found in the stomach, duodenum, and ileum tracts.
The differences in microbiota composition are due to the environmental conditions, such as pH modifications, along the gastrointestinal tract. In adult rabbits, the principal substrates for caecal microorganisms are polysaccharides and protein. Caecal microorganisms ferment available nutrients, converting them to metabolites (e.g., short-chain VFA, ammonia, H2, CH4, CO2) and compounds that are incorporated into microbial cells [66]. Our results for caecal bacterial fermentations indicate that Goji berry supplementation did not influence proteolytic activity and ammonia production. On the other hand, Goji supplementation stimulated lactic acid fermentation, indicating changes in the intestinal microbiota in favor of specific bacterial populations. The caecum represents the main site of fermentative activity in the rabbit due to the presence of an abundant microbial flora [1]. Rabbits produce large amounts of VFA and lactate by fermentation of dietary carbohydrates, such as xylan and pectin, in the hindgut [67,68,69]. Lactobacilli are strong producers of lactic acid and, for this reason, can compete against pathogenic bacteria [70]. Regarding Goji berry supplementation, several authors [71,72] have confirmed the beneficial effects of this integration on the physiology and health of the gut acting on the intestinal microbiota composition of human and mice. Castrica et al. [15] reported that the incorporation of 3% w/w of Goji berries in the rabbit diet was able to increase the Lactobacilli population in rabbit meat.
This is a preliminary study on the effect of Goji berry supplementation on gastrointestinal microbiota of the rabbit and, although its practical implications are currently limited, it may represent a starting point for future exploratory research. Further experimental trials could be addressed to evaluate whether caecal fermentative activities (VFA production) could be affected by changes in microbial community composition. Moreover, evaluation of digestive efficiency by performing an in vivo digestibility trial could integrate the study of the microbiota composition of the rabbit. Finally, it could be interesting to evaluate the impact of microbiota modification on the maturation and activity of the immune system, as well as on resistance to infectious diseases, animal welfare and the productive performance of the rabbit.
5. Conclusions
The present study demonstrated that Goji berry supplementation can modulate gastrointestinal microbiota composition and caecal fermentations of the rabbit. In particular, Lycium barbarum fruit increased the growth of the phylum Bacteroidetes as well as of Ruminococcaceae, Lachnospiraceae, and Lactobacillus in the caecum and colon, and as a consequence, lactic acid production. The mechanism of absorption and integration of the bioactive molecules contained in the fruit, and their influence on the microbiota population, should be investigated to appropriately use Goji berries’ probiotic properties. The use of this natural compound needs to be further studied for its implications for both commercial performance and animal resistance to infection, as its supplementation could reduce the incidence of health problems in livestock and consequent antibiotic treatments.
Acknowledgments
The authors gratefully acknowledge the collaboration and support of Giovanni Migni.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani12010121/s1: Figure S1: Evaluation of the sample-based and sequence-based bacterial rarefaction curves to check the depth of coverage; Figure S2: p-values for the difference in alpha diversity indices between control and Goji-treated rabbits, along the gastrointestinal tract; Table S1: Significantly different OTUs between Goji-treated and control rabbits in the gut microbiota (p < 0.05).
Author Contributions
Conceptualization, L.M., D.V. and G.B.; data curation, P.C., F.B., L.M., B.C., O.B. and M.C.; formal analysis, G.C., F.B., L.M., M.L.M., O.B. and G.B.; funding acquisition, P.C., M.L.M., B.C., D.V. and G.B.; investigation, G.C., E.C., A.M., M.C., M.S. and S.A.; methodology, P.C., E.C., B.C., A.M. and S.A.; project administration, L.M., M.L.M., A.M. and G.B.; resources, F.R., M.L.M., D.V. and G.B.; software, F.B. and L.M.; supervision, P.C., O.B., D.V., M.S. and G.B.; validation, P.C., F.B., F.R. and G.B.; visualization, F.B., B.C. and A.Q.; writing—original draft, P.C., G.C., F.B., E.C., L.M., F.R., M.L.M., A.Q., S.A. and G.B.; writing—review and editing, G.C., L.M., A.M., A.Q., S.A. and G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by FAR 2019 of the University of Sassari.
Institutional Review Board Statement
The Albanian Ministry of Agriculture and Rural Development, National Authority of Veterinary and Plants protection authorized the protocol of the experimental trial (prot. 824/2021).
Data Availability Statement
The data presented in this study are available in the article and Supplementary materials. Further information is available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yao X., Peng Y., Xu L.J., Li L., Wu Q.L., Xiao P.G. Phytochemical and biological studies of lycium medicinal plants. Chem. Biodivers. 2011;8:976–1010. doi: 10.1002/cbdv.201000018. [DOI] [PubMed] [Google Scholar]
- 2.Sun W., Shahrajabian M.H., Cheng Q. Therapeutic Roles of Goji Berry and Ginseng in Traditional Chinese. J. Nutr. Food Secur. 2019;4:293–305. doi: 10.18502/jnfs.v4i4.1727. [DOI] [Google Scholar]
- 3.Magiera S., Zaręba M. Chromatographic determination of phenolic acids and flavonoids in Lycium barbarum L. and evaluation of antioxidant activity. Food Anal. Methods. 2015;8:2665–2674. doi: 10.1007/s12161-015-0166-y. [DOI] [Google Scholar]
- 4.Kang Y., Yang G., Zhang S., Ross C.F., Zhu M.J. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-Deficient Mice. Mol. Nutr. Food Res. 2018;62:e1800535. doi: 10.1002/mnfr.201800535. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y., Yan Y., Chen D., Ran L., Mi J., Lu L., Jing B., Li X., Zeng X., Cao Y. Modulating effects of polysaccharides from the fruits of: Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019;10:3671–3683. doi: 10.1039/C9FO00638A. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh S.Y., Lian Y.Z., Lin I.H., Yang Y.C., Tinkov A.A., Skalny A.V., Chao J.C.J. Combined Lycium babarum polysaccharides and C-phycocyanin increase gastric Bifidobacterium relative abundance and protect against gastric ulcer caused by aspirin in rats. Nutr. Metab. 2021;18:4. doi: 10.1186/s12986-020-00538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pap N., Fidelis M., Azevedo L., do Carmo M.A.V., Wang D., Mocan A., Pereira E.P.R., Xavier-Santos D., Sant’Ana A.S., Yang B., et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021;42:167–186. doi: 10.1016/j.cofs.2021.06.003. [DOI] [Google Scholar]
- 8.Chen J., Long L., Jiang Q., Kang B., Li Y., Yin J. Effects of dietary supplementation of Lycium barbarum polysaccharides on growth performance, immune status, antioxidant capacity and selected microbial populations of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020;104:1106–1115. doi: 10.1111/jpn.13247. [DOI] [PubMed] [Google Scholar]
- 9.Bai X., Yan X., Xie L., Hu X., Lin X., Wu C., Zhou N., Wang A., See M.T. Effects of pre-slaughter stressor and feeding preventative Chinese medicinal herbs on glycolysis and oxidative stability in pigs. Anim. Sci. J. 2016;87:1028–1033. doi: 10.1111/asj.12537. [DOI] [PubMed] [Google Scholar]
- 10.Chen H., Guo B., Yang M., Luo J., Hu Y., Qu M. Response of Growth Performance, Blood Biochemistry Indices, and Rumen Bacterial Diversity in Lambs to Diets Containing Supplemental Probiotics and Chinese Medicine Polysaccharides. Front. Vet. Sci. 2021;8:656. doi: 10.3389/fvets.2021.681389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andoni E., Curone G., Agradi S., Barbato O., Menchetti L., Vigo D., Zelli R., Cotozzolo E., Ceccarini M.R., Faustini M., et al. Effect of Goji Berry (Lycium barbarum) Supplementation on Reproductive Performance of Rabbit Does. Animals. 2021;11:1672. doi: 10.3390/ani11061672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menchetti L., Vecchione L., Filipescu I., Petrescu V.F., Fioretti B., Beccari T., Ceccarini M.R., Codini M., Quattrone A., Trabalza-Marinucci M., et al. Effects of Goji berries supplementation on the productive performance of rabbit. Livest. Sci. 2019;220:123–128. doi: 10.1016/j.livsci.2018.12.016. [DOI] [Google Scholar]
- 13.Menchetti L., Curone G., Andoni E., Barbato O., Troisi A., Fioretti B., Polisca A., Codini M., Canali C., Vigo D., et al. Impact of goji berries (Lycium barbarum) supplementation on the energy homeostasis of rabbit does: Uni- and multivariate approach. Animals. 2020;10:2000. doi: 10.3390/ani10112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menchetti L., Brecchia G., Branciari R., Barbato O., Fioretti B., Codini M., Bellezza E., Trabalza-Marinucci M., Miraglia D. The effect of Goji berries (Lycium barbarum) dietary supplementation on rabbit meat quality. Meat Sci. 2020;161:108018. doi: 10.1016/j.meatsci.2019.108018. [DOI] [PubMed] [Google Scholar]
- 15.Castrica M., Menchetti L., Balzaretti C.M., Branciari R., Ranucci D., Cotozzolo E., Vigo D., Curone G., Brecchia G., Miraglia D. Impact of dietary supplementation with goji berries (Lycium barbarum) on microbiological quality, physico-chemical, and sensory characteristics of rabbit meat. Foods. 2020;9:1480. doi: 10.3390/foods9101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 17.Bagóné Vantus V., Kovacs M., Zsolnai A. The rabbit caecal microbiota: Development, composition and its role in the prevention of digestive diseases—A review on recent literature in the light of molecular genetic methods. Acta Agrar. Kvar. 2014;18:55–65. [Google Scholar]
- 18.Cotozzolo E., Cremonesi P., Curone G., Menchetti L., Riva F., Biscarini F., Marongiu M.L., Castrica M., Castiglioni B., Miraglia D., et al. Characterization of bacterial microbiota composition along the gastrointestinal tract in rabbits. Animals. 2020;11:31. doi: 10.3390/ani11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reusch B. Rabbit gastroenterology. Vet. Clin. N. Am. Exot. Anim. Pract. 2005;8:351–375. doi: 10.1016/j.cvex.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H.-J., Yang W.-Y., Wang C.-Y. Advances in Biological Sciences Research, Proceedings of the 2017 2nd International Conference on Biological Sciences and Technology (BST 2017), Zhuhai, China, 17–19 November 2017. Volume 6. Atlantis Press; Amsterdam, The Netherlands: 2017. The Review on the Function of Intestinal Flora and the Regulatory Effects of Probiotics on the Intestinal Health of Rabbits. Advances in Biological Sciences Research (ABSR) [Google Scholar]
- 21.Chevance A., Moulin G. Suivi des Ventes de Médicaments Vétérinaires Contenant des Antibiotiques en France en 2007. AFSSA-ANMV; Javené, France: 2009. pp. 1–44. [Google Scholar]
- 22.Nogacka A.M., Salazar N., Arboleya S., Suárez M., Fernández N., Solís G., de los Reyes-Gavilán C.G., Gueimonde M. Early microbiota, antibiotics and health. Cell. Mol. Life Sci. 2018;75:83–91. doi: 10.1007/s00018-017-2670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall B.M., Ochieng D.J., Levy S.B. Commensals: Underappreciated reservoir of antibiotic resistance. Microbe. 2009;4:231–238. doi: 10.1128/microbe.4.231.1. [DOI] [Google Scholar]
- 24.Rommers J.M., Boiti C., Brecchia G., Meijerhof R., Noordhuizen J.P.T.M., Decuypere E., Kemp B. Metabolic adaptation and hormonal regulation in young rabbit does during long-term caloric restriction and subsequent compensatory growth. Anim. Sci. 2004;79:255–264. doi: 10.1017/S1357729800090111. [DOI] [Google Scholar]
- 25.Menchetti L., Brecchia G., Canali C., Cardinali R., Polisca A., Zerani M., Boiti C. Food restriction during pregnancy in rabbits: Effects on hormones and metabolites involved in energy homeostasis and metabolic programming. Res. Vet. Sci. 2015;98:7–12. doi: 10.1016/j.rvsc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Paredes E., Ródenas L., Martínez-Vallespín B., Cervera C., Blas E., Brecchia G., Boiti C., Pascual J.J. Effects of feeding programme on the performance and energy balance of nulliparous rabbit does. Animal. 2012;6:1086–1095. doi: 10.1017/S1751731111002643. [DOI] [PubMed] [Google Scholar]
- 27.Brecchia G., Menchetti L., Cardinali R., Castellini C., Polisca A., Zerani M., Maranesi M., Boiti C. Effects of a bacterial lipopolysaccharide on the reproductive functions of rabbit does. Anim. Reprod. Sci. 2014;147:128–134. doi: 10.1016/j.anireprosci.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Boiti C., Canali C., Brecchia G., Zanon F., Facchin E. Effects of induced endometritis on the life-span of corpora lutea in pseudopregnant rabbits and incidence of spontaneous uterine infections related to fertility of breeding does. Theriogenology. 1999;52:1123–1132. doi: 10.1016/S0093-691X(99)00204-6. [DOI] [PubMed] [Google Scholar]
- 29.Boiti C., Guelfi G., Zerani M., Zampini D., Brecchia G., Gobbetti A. Expression patterns of cytokines, p53 and nitric oxide synthase isoenzymes in corpora lutea of pseudopregnant rabbits during spontaneous luteolysis. Reproduction. 2004;127:229–238. doi: 10.1530/rep.1.00083. [DOI] [PubMed] [Google Scholar]
- 30.Collodel G., Moretti E., Brecchia G., Kuželová L., Arruda J., Mourvaki E., Castellini C. Cytokines release and oxidative status in semen samples from rabbits treated with bacterial lipopolysaccharide. Theriogenology. 2015;83:1233–1240. doi: 10.1016/j.theriogenology.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Maertens L., Moermans R., De Groote G. The influence of the dietary energy content on the performances of post-partum breeding does. J. Appl. Rabbit Res. 1988;11:60–67. [Google Scholar]
- 32.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Lozupone C.A., Turnbaugh P.J., Fierer N., Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher R.A., Corbet A.S., Williams C.B. The Relation Between the Number of Species and the Number of Individuals in a Random Sample of an Animal Population. J. Anim. Ecol. 1943;12:42. doi: 10.2307/1411. [DOI] [Google Scholar]
- 34.Chao A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984;11:265–270. [Google Scholar]
- 35.Smith B., Wilson J.B. A Consumer’s Guide to Evenness Indices. Oikos. 1996;76:70. doi: 10.2307/3545749. [DOI] [Google Scholar]
- 36.Bray J.R., Curtis J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957;27:325–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 37.Paulson J.N., Colin Stine O., Bravo H.C., Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods. 2013;10:1200–1202. doi: 10.1038/nmeth.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biscarini F., Palazzo F., Castellani F., Masetti G., Grotta L., Cichelli A., Martino G. Rumen microbiome in dairy calves fed copper and grape-pomace dietary supplementations: Composition and predicted functional profile. PLoS ONE. 2018;13:e0205670. doi: 10.1371/journal.pone.0205670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pẽa A.G., Goodrich J.K., Gordon J.I., et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wickham H. Ggplot2 Elegant Graphics for Data Analysis. Springer; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 41.Team R.D.C. A Language and Environment for Statistical Computing. Volume 2 R Foundation for Statistical Computing; Vienna, Austria: 2018. [Google Scholar]
- 42.Pryce J.D. A modification of the Barker-Summerson method for the determination of lactic acid. Analyst. 1969;94:1151–1152. doi: 10.1039/an9699401151. [DOI] [PubMed] [Google Scholar]
- 43.Patton C.J., Crouch S.R. Spectrophotometric and Kinetics Investigation of the Berthelot Reaction for the Determination of Ammonia. Anal. Chem. 1977;49:469. doi: 10.1021/ac50011a034. [DOI] [Google Scholar]
- 44.Xu Z., Knight R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 2015;113:S1–S5. doi: 10.1017/S0007114514004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abecia L., Rodríguez-Romero N., Yañez-Ruiz D.R., Fondevila M. Biodiversity and fermentative activity of caecal microbial communities in wild and farm rabbits from Spain. Anaerobe. 2012;18:344–349. doi: 10.1016/j.anaerobe.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Arrazuria R., Pérez V., Molina E., Juste R.A., Khafipour E., Elguezabal N. Diet induced changes in the microbiota and cell composition of rabbit gut associated lymphoid tissue (GALT) Sci. Rep. 2018;8:14103. doi: 10.1038/s41598-018-32484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abecia L., Fondevila M., Balcells J., Edwards J.E., Newbold C.J., McEwan N.R. Molecular profiling of bacterial species in the rabbit caecum. FEMS Microbiol. Lett. 2005;244:111–115. doi: 10.1016/j.femsle.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Monteils V., Cauquil L., Combes S., Godon J.J., Gidenne T. Potential core species and satellite species in the bacterial community within the rabbit caecum. FEMS Microbiol. Ecol. 2008;66:620–629. doi: 10.1111/j.1574-6941.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crowley E.J., King J.M., Wilkinson T., Worgan H.J., Huson K.M., Rose M.T., McEwan N.R. Comparison of the microbial population in rabbits and guinea pigs by next generation sequencing. PLoS ONE. 2017;12:e0165779. doi: 10.1371/journal.pone.0165779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao W., Wang Y., Liu S., Huang J., Zhai Z., He C., Ding J., Wang J., Wang H., Fan W., et al. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS ONE. 2015;10:e0117441. doi: 10.1371/journal.pone.0117441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velasco-Galilea M., Piles M., Viñas M., Rafel O., González-Rodríguez O., Guivernau M., Sánchez J.P. Rabbit microbiota changes throughout the intestinal tract. Front. Microbiol. 2018;9:2144. doi: 10.3389/fmicb.2018.02144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horz H.P., Conrads G. The discussion goes on: What is the role of Euryarchaeota in humans? Archaea. 2010;2010:967271. doi: 10.1155/2010/967271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrow A.L., Lagomarcino A.J., Schibler K.R., Taft D.H., Yu Z., Wang B., Altaye M., Wagner M., Gevers D., Ward D.V., et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1:13. doi: 10.1186/2049-2618-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Combes S., Gidenne T., Cauquil L., Bouchez O., Fortun-Lamothe L. Coprophagous behavior of rabbit pups affects implantation of cecal microbiota and health status. J. Anim. Sci. 2014;92:652–665. doi: 10.2527/jas.2013-6394. [DOI] [PubMed] [Google Scholar]
- 55.Gosalbes M.J., Durbán A., Pignatelli M., Abellan J.J., Jiménez-Hernández N., Pérez-Cobas A.E., Latorre A., Moya A. Metatranscriptomic approach to analyze the functional human gut microbiota. PLoS ONE. 2011;6:e17447. doi: 10.1371/journal.pone.0017447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Y., Wang C., Li F. Impact of dietary fiber/starch ratio in shaping caecal microbiota in rabbits. Can. J. Microbiol. 2015;61:771–784. doi: 10.1139/cjm-2015-0201. [DOI] [PubMed] [Google Scholar]
- 57.Zeng X., Gao X., Peng Y., Wu Q., Zhu J., Tan C., Xia G., You C., Xu R., Pan S., et al. Higher Risk of Stroke Is Correlated with Increased Opportunistic Pathogen Load and Reduced Levels of Butyrate-Producing Bacteria in the Gut. Front. Cell. Infect. Microbiol. 2019;9:4. doi: 10.3389/fcimb.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu B., Tsen H.Y. Lactobacillus cells in the rabbit digestive tract and the factors affecting their distribution. J. Appl. Bacteriol. 1993;75:269–275. doi: 10.1111/j.1365-2672.1993.tb02776.x. [DOI] [PubMed] [Google Scholar]
- 59.Cui H.X., Xu X.R. Comparing the effect of intestinal bacteria from rabbit, pig, and chicken on inflammatory response in cultured rabbit crypt and villus. Can. J. Microbiol. 2019;65:59–67. doi: 10.1139/cjm-2017-0757. [DOI] [PubMed] [Google Scholar]
- 60.Kawashima T., Kosaka A., Yan H., Guo Z., Uchiyama R., Fukui R., Kaneko D., Kumagai Y., You D.J., Carreras J., et al. Double-Stranded RNA of Intestinal Commensal but Not Pathogenic Bacteria Triggers Production of Protective Interferon-β. Immunity. 2013;38:1187–1197. doi: 10.1016/j.immuni.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 61.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 62.Mariat D., Firmesse O., Levenez F., Guimarǎes V.D., Sokol H., Doré J., Corthier G., Furet J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jami E., White B.A., Mizrahi I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE. 2014;9:e85423. doi: 10.1371/journal.pone.0085423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeoman C.J., White B.A. Gastrointestinal tract microbiota and probiotics in production animals. Annu. Rev. Anim. Biosci. 2014;2:469–486. doi: 10.1146/annurev-animal-022513-114149. [DOI] [PubMed] [Google Scholar]
- 65.Fassarella M., Blaak E.E., Penders J., Nauta A., Smidt H., Zoetendal E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70:595–605. doi: 10.1136/gutjnl-2020-321747. [DOI] [PubMed] [Google Scholar]
- 66.Marounek M., Březina P., Baran M. Fermentation of carbohydrates and yield of microbial protein in mixed cultures of rabbit caecal microorganisms. Arch. Anim. Nutr. 2000;53:241–252. doi: 10.1080/17450390009381950. [DOI] [PubMed] [Google Scholar]
- 67.Vernay M. Origin and utilization of volatile fatty acids and lactate in the rabbit: Influence of the faecal excretion pattern. Br. J. Nutr. 1987;57:371–381. doi: 10.1079/BJN19870045. [DOI] [PubMed] [Google Scholar]
- 68.De Blas E., Gidenne T. Digestion of starch and sugars. In: De Blas E., Wiseman J., editors. The Nutrition of the Rabbit. CABI Publishing; Wallingford, UK: 1998. pp. 17–38. [Google Scholar]
- 69.Priyadarshini M., Kotlo K.U., Dudeja P.K., Layden B.T. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr. Physiol. 2018;8:1065–1090. doi: 10.1002/cphy.c170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soomro A.H., Masud T., Anwaar K. Role of Lactic Acid Bacteria (LAB) in food preservation and human health—A review. Pak. J. Nutr. 2002;1:20–24. [Google Scholar]
- 71.Lavefve L., Howard L.R., Carbonero F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020;11:45–65. doi: 10.1039/C9FO01634A. [DOI] [PubMed] [Google Scholar]
- 72.Nardi G.M., De Farias Januário A.G., Freire C.G., Megiolaro F., Schneider K., Perazzoli M.R.A., Do Nascimento S.R., Gon A.C., Mariano L.N.B., Wagner G., et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacogn. Res. 2016;8:S42–S49. doi: 10.4103/0974-8490.178642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article and Supplementary materials. Further information is available upon request from the corresponding author.