Abstract
Simple Summary
Rye and rapeseed meal can be alternative feed components for weaner piglets instead of wheat and soybean meal. Both components can help to meet current challenges in pig nutrition, such as increasingly dry weather conditions and the high amount of imported soybean. Since they contain more and differently composed fiber, effects on digestive physiology and intestinal microbiota might help to maintain gut health and prevent post-weaning diarrhea. This study shows that despite a similar composition of the large intestinal microbiota, the higher amount and solubility of complex carbohydrates from rye lead to a higher fermentative activity compared to wheat, which is considered a beneficial effect. The high amount of insoluble dietary fiber in rapeseed-based diets lowered bacterial metabolic activity and caused a shift toward insoluble fiber degrading bacteria.
Abstract
This study aimed to investigate the effect of fiber-rich rye and rapeseed meal (RSM) compared to wheat and soybean meal (SBM) on fiber digestibility and the composition and metabolic activity of intestinal microbiota. At weaning, 88 piglets were allocated to four feeding groups: wheat/SBM, wheat/RSM, rye/SBM, and rye/RSM. Dietary inclusion level was 48% for rye and wheat, 25% for SBM, and 30% for RSM. Piglets were euthanized after 33 days for collection of digesta and feces. Samples were analyzed for dry matter and non-starch-polysaccharide (NSP) digestibility, bacterial metabolites, and relative abundance of microbiota. Rye-based diets had higher concentrations of soluble NSP than wheat-based diets. RSM-diets were higher in insoluble NSP compared to SBM. Rye-fed piglets showed a higher colonic and fecal digestibility of NSP (p < 0.001, p = 0.001, respectively). RSM-fed piglets showed a lower colonic and fecal digestibility of NSP than SBM-fed piglets (p < 0.001). Rye increased jejunal and colonic concentration of short-chain fatty acids (SCFA) compared to wheat (p < 0.001, p = 0.016, respectively). RSM-fed pigs showed a lower jejunal concentration of SCFA (p = 0.001) than SBM-fed pigs. Relative abundance of Firmicutes was higher (p = 0.039) and of Proteobacteria lower (p = 0.002) in rye-fed pigs compared to wheat. RSM reduced Firmicutes and increased Actinobacteria (jejunum, colon, feces: p < 0.050), jejunal Proteobacteria (p = 0.019) and colonic Bacteroidetes (p = 0.014). Despite a similar composition of the colonic microbiota, the higher amount and solubility of NSP from rye resulted in an increased fermentative activity compared to wheat. The high amount of insoluble dietary fiber in RSM-based diets reduced bacterial metabolic activity and caused a shift toward insoluble fiber degrading bacteria. Further research should focus on host–microbiota interaction to improve feeding concepts with a targeted use of dietary fiber.
Keywords: cereal, dietary fiber, microbiome, nutrient digestibility, pigs, protein source, rapeseed, rye, soybean, wheat
1. Introduction
An important approach to stabilize gut health of weaner pigs is the optimization of intestinal microbial colonization in the sense of intestinal eubiosis and beneficial bacterial metabolic activity [1]. Here, the inclusion of dietary fiber is a promising strategy. Dietary fiber influences digestion and fermentation processes and many sources of dietary fiber were shown to increase the potentially beneficial microbiota, to reduce pathogens, and to improve intestinal barrier function [2,3,4,5]. Non-starch-polysaccharides (NSP) are the major component of dietary fiber. They are not digestible in the small intestine but are fermented in the upper and lower intestinal tract by the resident microbiota [6]. Fermentation products of intestinal bacteria can have different impact on the microbial community and on the host. Proteolytic bacterial activities produce potentially harmful metabolites such as ammonia and other degradation products [7]. Beneficial metabolites are short-chain fatty acids (SCFA) and lactic acid. They are products of substrate fermentation and may hamper the growth of pathogens and provide energy for beneficial bacteria [8]. Acetic, propionic, and butyric acid are the predominant forms of SCFA. After absorption, they serve as an energy substrate for the pig [9]. Moreover, butyric acid is the main energy source of the colonic epithelial cells [10]. Most intestinal bacteria preferably ferment carbohydrates including NSP, which implies that diets containing high amounts of complex structured NSP might improve gut health by promoting growth of fermentative bacteria and providing beneficial metabolites to the host. Dietary fiber may also influence the integrity of the epithelial mucus layer and on mucus production [11]. N-acetylneuraminic acid is a sialic acid present in mucin molecules and can be considered a marker for mucus [12], but data on its intestinal concentration in pigs fed different fiber sources are scarce.
Rye and rapeseed meal (RSM) are fiber-rich feed components and interesting alternatives to wheat and soybean meal (SBM). Rye is not used much in pig nutrition due to the idea of low palatability, its high content of fiber and its susceptibility to the infection with Claviceps purpurea [13]. However, recent studies investigating the feeding of rye instead of wheat at dietary inclusion up to 69% showed no reduction of feed intake and growth performance [14,15]. This might be related to the use SCFA from large intestinal fiber fermentation as an energy substrate [16]. Moreover, increasing pollen fertility and selection of resistance genes lowered the risk of ergot infections in rye [17]. Generally, wheat and rye have a similar concentration of carbohydrates, but rye has a higher concentration of total dietary fiber (TDF) and fermentable fractions [16]. The content of total NSP, soluble NSP (sNSP), arabinoxylans (AX) and β-glucans is higher in rye compared to wheat [18,19]. Thus, rye increases intestinal butyrate production compared to wheat [20,21] and helps to prevent Salmonella infection in weaner pigs [15]. Moreover, rye might be effective against enterotoxigenic E. coli (ETEC) colonization [5] and promote the growth of Bifidobacterium and Prevotella spp., both important lactic acid-producing bacteria. Lactic acid is considered to inhibit growth of pathogens [22]. A high abundance of proteolytic Clostridium can impair gut health and might be reduced by increasing dietary AX and β-glucans [23]. Consequently, replacing wheat by rye might induce a favourable shift in the large intestinal microbiome by providing more readily fermentable substrate and thereby promoting the production of useful metabolites.
Next to SBM, RSM is the second most important protein meal in pig nutrition in Europe. RSM and SBM contain similar amounts of NSP, but RSM has less soluble NSP and more lignin, total, and soluble AX [18]. Insoluble fiber helps to maintain normal gut function but might decrease feed intake and nutrient digestibility [9]. Very little research has been done on the fermentability of RSM carbohydrates. However, some studies showed that RSM might increase relative abundance of SCFA producing genera in the hindgut and decrease major proteolytic bacteria compared to SBM [24,25].
Providing non-digestible fiber substrates using rye and RSM in piglet diets could support gut health. Knowledge of the effects of feeding rye in combination with RSM is scarce. For the present study, we hypothesized that rye and RSM would increase microbial fiber fermentation and SCFA production in the large intestine compared to wheat and SBM and cause a shift in the microbial community toward potentially beneficial bacteria. The aim of this study was to investigate the effect of diets containing either rye or wheat as a cereal combined with either RSM or SBM as a protein meal on fiber digestibility, on the mucus marker N-acetylneuraminic acid, as well as on composition and metabolic activity of intestinal microbiota.
2. Materials and Methods
2.1. Animals and Diets
The animal study was described in detail previously [14]. In brief, 88 piglets (German Landrace, bred at the Institute of Animal Nutrition, Freie Universität Berlin, 8.3 ± 1.1 kg body weight) were weaned at day 28 of life and randomly allocated to four feeding groups in a 2 × 2 factorial design with wheat/SBM (W-SBM), wheat/RSM (W-RSM), rye/SBM (R-SBM), and rye/RSM (R-RSM). Piglets were housed with two animals per pen and 11 pens per treatment. The pen was used as statistical unit (n = 11). The health status was monitored daily by controlling general condition, feed intake, and fecal consistency of the piglets. Water and feed were supplied ad libitum during the experimental period of 33 days. The diets were formulated to meet or exceed the recommendations for piglet nutrition [26] and calculated to be isonitrogenous. Wheat (Triticum aestivum) or hybrid rye (Secale cereale) were included as cereals at 48 and 25% of SBM or 30% of RSM as protein rich ingredient. The average concentration of crude protein in the complete diet was 220 g/kg. Crude fiber varied among the groups with 22 g/kg in W-SBM, 52 g/kg in W-RSM, 18 g/kg in R-SBM, and 48 g/kg in R-RSM. The exact feed formulation, results of the nutrient analysis, and digestibility determinations are available elsewhere [14].
2.2. Sampling
One piglet per pen was dissected after 33 days of trial duration. Pigs were chosen to achieve balanced numbers of males and females in each treatment group. After anaesthesia by 20 mg of ketamine hydrochloride (Ursotamin, 10%; Serumwerk Bernburg AG, Bernburg, Germany) and 2 mg of xylazine (Xylazin, 2%, Serumwerk Bernburg AG, Bernburg, Germany) per kg body weight, pigs were euthanized with an intracardial injection of tetracaine hydrochloride, mebezonium iodide, and embutramide (T61; Intervet, Unterschleißheim, Germany). The gastrointestinal tract was removed and digesta samples were collected from jejunum, ileum, colon ascendens, and rectum. From each intestinal segment, one subset of digesta samples was stored at −20 °C for analysis of dry matter (DM), fiber and N-acetylneuraminic acid. Another subset was snap frozen with liquid nitrogen and stored at −80 °C for analysis of bacterial metabolites and 16S rRNA sequencing.
2.3. Determination of Digesta Content and Apparent Digestibility of DM and NSP
Digesta samples from jejunum, colon ascendens, and feces were dried in an oven at 103 °C overnight to determine the DM content. Colonic and fecal samples were lyophilized and total and insoluble NSP content was measured as described for NSP in the diets [14]. To determine apparent digestibility of DM and NSP, titanium dioxide content was determined as described before [27] in lyophilized and grinded (0.5 mm particle size) digesta, feces, and in the diets. Apparent digestibility of DM and NSP was calculated as follows [28]:
| Digestibility (%) = 100 − (TiO2 in feed (%))/(TiO2 in digesta/feces (%)) × (Nutrient in digesta/feces (%))/(Nutrient in feed (%)) × 100 |
Apparent digestibility determined in feces was further considered as apparent total tract digestibility (ATTD).
2.4. Analysis of N-Acetylneuraminic Acid as Marker of Intestinal Mucus Production
Lyophilized and grinded samples of ileal digesta were hydrolyzed with acetic acid (2 mol/L). After centrifugation, the supernatant was analyzed for N-acetylneuraminic acid via HPIC with an amperometrically pulsed detector cell (Thermo Fisher Scientific, Waltham, MA, USA) using a commercial standard of N-acetylneuraminic acid as reference substance (Sigma–Aldrich, Taufkirchen, Germany).
2.5. Analysis of Bacterial Metabolites
In jejunal and colonic digesta samples and feces, concentration of SCFA, d- and l-lactate, and ammonia was analyzed as described before [29]. Briefly, SCFA were determined via gas chromatography (Agilent Technologies 6890N, autosampler G2614A, and injection tower G2613A; Network GC Systems, Böblingen, Germany) using caproic acid as an internal standard. d- and l-lactate were measured by HPLC (Agilent 1100; Agilent Technologies, Böblingen, Germany). Ammonia was analyzed colorimetrically using the Berthelot reaction in microtitration plates at 620 nm in a Tecan Sunrise microplate reader (Tecan Austria GmbH, Grödig, Austria).
2.6. DNA Extraction and 16S rRNA Sequencing
To extract total DNA from 0.25 g of jejunal and colonic digesta and feces, a commercial extraction kit (QIAamp PowerFaecal Pro DNA Kit, Qiagen, Hilden, Germany) was used in accordance with the manufacturer’s instructions with an additional lysis step at 65 °C. Homogenization was carried out using FastPrep-24TM 5G (M.P. Biomedicals LLC, Santa Ana, CA, USA) at 6 m/s for 10 min (4 times 5 × 30 s and 15 s pause time). DNA extracts were stored at −30 °C until further analysis. Extracts were subjected to amplicon sequencing using an Illumina NextSeq500 sequencer (LGC, Berlin, Germany) with two 150–base pair reads. After demultiplexing, BBMerge tool [30] was used for combining paired reads. Resulting 16S rDNA sequences were analyzed using QIIME2 pipeline [31] and the SILVA SSU database [32]. Quality control and determination of sequence counts were performed using the DADA2 [33]. Further details were described previously [34]. Indices of bacterial diversity (Richness, Shannon index, and Evenness) were calculated from ASV level data. Principal component analysis of 16S rRNA data was carried out using the online software ClustVis [35].
2.7. Statistical Analysis
Statistical analyses were carried out in SPSS 26.0 (IBM, Chicago, IL, USA). The distribution of data was tested by Kolmogorov–Smirnov test. Normally distributed data were analyzed by 2-factorial ANOVA with cereal (CER) and protein meal (PM) as fixed factors. Group differences were assessed by post hoc Tukey test. p-values below 0.05 were considered significant. Pearson‘s correlation was analyzed between colonic Bacteroidetes and average daily gain (ADG) and between jejunal Proteobacteria and apparent ileal digestibility (AID) of crude protein and total amino acids.
3. Results
Results of growth performance and fecal score were described in detail previously [14]. In brief, average daily gain, average daily feed intake, feed conversion ratio and final body weight were not influenced by the feeding of rye compared to wheat. RSM in comparison to SBM reduced average daily gain and average daily feed intake in the overall trial period. Fecal score was within the physiological range throughout the trial.
3.1. NSP Concentration in Diets
The four experimental diets contained more iNSP (insoluble non-starch-polysaccharides, 76.9–93.0 g/kg) than sNSP (12.8–34.6 g/kg) with glucose (27.0–48.2 g/kg), xylose (24.2–30.6 g/kg), and arabinose (18.2–30.5 g/kg) being the predominant sugars in the NSP fractions (Table 1). The two rye-based diets had higher concentrations of soluble dietary fiber (SDF) and sNSP than the wheat-based diets (47 and 118% more, respectively). RSM-diets were higher in iNSP and the A/X-ratio was higher compared to SBM-diets (15 and 35% more, respectively).
Table 1.
Analyzed content of DM and dietary fiber of the experimental diets.
| Item, g/kg (as-Fed Basis) | Diet | |||
|---|---|---|---|---|
| W-SBM | W-RSM | R-SBM | R-RSM | |
| DM | 931 | 937 | 931 | 928 |
| NDF | 154 | 153 | 163 | 141 |
| ADF | 26.3 | 74.1 | 23.0 | 70.5 |
| ADL | 2.2 | 28.2 | 4.3 | 24.9 |
| NSP | ||||
| Total | 89.6 (12.8) 1 | 102 (14.2) | 104 (24.2) | 128 (34.6) |
| Fucose | 0.86 (0.03) | 0.82 (0.02) | 0.99 (0.01) | 0.91 (0.08) |
| Rhamnose | 0.79 (0.07) | 0.81 (0.09) | 0.76 (0.11) | 1.00 (0.13) |
| Arabinose | 18.2 (2.02) | 25.1 (2.82) | 21.3 (4.46) | 30.5 (7.67) |
| Galactose | 13.1 (2.13) | 8.20 (1.82) | 14.1 (2.38) | 7.72 (0.90) |
| Glucose | 27.0 (0.86) | 38.0 (2.50) | 30.7 (5.35) | 48.2 (11.8) |
| Mannose | 3.43 (2.17) | 2.83 (1.90) | 4.81 (4.03) | 5.21 (3.22) |
| Xylose | 24.2 (5.18) | 23.9 (4.92) | 27.5 (7.13) | 30.6 (10.8) |
| Galacturonic acid | 1.90 (0.26) | 1.71 (0.08) | 4.00 (0.65) | 3.39 (0.08) |
| Glucuronic acid | 0.18 (0.06) | 0.18 (0.05) | 0.20 (0.05) | 0.14 (0.03) |
| Total AX 2 | 42.4 (7.2) | 49.0 (7.7) | 48.9 (11.6) | 61.1 (18.5) |
| Ratio A/X 3 | 0.75 | 1.05 | 0.77 | 1.00 |
| TDF | 135 | 164 | 152 | 188 |
| SDF 4 | 27.6 | 27.1 | 37.1 | 42.4 |
| IDF | 108 | 137 | 115 | 146 |
W-SBM, wheat/soybean meal; W-RSM, wheat/rapeseed meal; R-SBM, rye/soybean meal; R-RSM, rye/rapeseed meal; DM, dry matter; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; NSP, non-starch-polysaccharides; AX, arabinoxylans; TDF: total dietary fiber; SDF, soluble dietary fiber; IDF, insoluble dietary fiber. 1 Values in parentheses are concentration of soluble fraction: total concentration of each component–concentration of the respective insoluble fraction. 2 Arabinose + xylose. 3 A/X: Arabinose/Xylose. 4 SDF, soluble dietary fiber: TDF–IDF.
3.2. Apparent Digestibility of DM and NSP
Fecal DM digestibility was lower in rye-fed piglets than pigs receiving wheat-based diets (p = 0.035, Table 2). DM digestibility was lower in RSM-pigs than in SBM-pigs in the jejunum, colon and feces (each p < 0.001).
Table 2.
Effects of the experimental diets on apparent digestibility of DM and NSP in weaned piglets 1.
| Digestibility, % | Diet | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| W-SBM | W-RSM | R-SBM | R-RSM | SEM | CER | PM | CER * PM | |
| Jejunum | ||||||||
| DM | 73.3 | 53.1 | 70.6 | 57.4 | 1.9 | 0.787 | <0.001 | 0.246 |
| Colon ascendens | ||||||||
| DM | 86.7 | 80.0 | 85.4 | 78.4 | 0.8 | 0.224 | <0.001 | 0.925 |
| NSP | ||||||||
| Total | 70.4 | 64.5 | 77.4 | 71.1 | 1.0 | <0.001 | <0.001 | 0.903 |
| Fucose | 81.8 | 67.8 | 79.0 | 64.4 | 1.7 | 0.241 | <0.001 | 0.890 |
| Rhamnose | 55.0 | 26.5 | 53.9 | 37.8 | 4.7 | 0.573 | 0.018 | 0.495 |
| Arabinose | 69.5 | 72.9 | 74.2 | 75.3 | 0.7 | 0.011 | 0.097 | 0.375 |
| Galactose | 92.0 | 70.1 | 89.5 | 64.1 | 2.1 | 0.043 | <0.001 | 0.394 |
| Glucose | 62.0 | 61.3 | 75.3 | 72.1 | 1.2 | <0.001 | 0.222 | 0.448 |
| Mannose | 74.8 a | 48.8 b | 78.7 a | 67.6 a | 2.3 | 0.001 | <0.001 | 0.020 |
| Xylose | 70.0 | 71.1 | 76.9 | 76.7 | 0.9 | <0.001 | 0.757 | 0.639 |
| Galacturonic acid | 83.1 a | −29.7 c | 91.7 a | 31.3 b | 7.6 | <0.001 | <0.001 | <0.001 |
| Glucuronic acid | 47.3 b | 40.6 b | 72.3 a | 42.0 b | 2.6 | 0.001 | <0.001 | 0.002 |
| Soluble NSP 2 | 70.7 | 71.4 | 86.3 | 88.2 | 1.8 | <0.001 | 0.624 | 0.821 |
| Insoluble NSP | 70.4 | 63.4 | 74.8 | 64.7 | 1.1 | 0.116 | <0.001 | 0.405 |
| Total AX | 69.8 | 72.1 | 75.7 | 76.0 | 0.8 | 0.001 | 0.370 | 0.467 |
| Soluble AX 3 | 81.9 | 88.3 | 94.5 | 96.2 | 1.5 | <0.001 | 0.130 | 0.362 |
| Insoluble AX | 67.3 | 69.0 | 69.9 | 67.2 | 0.8 | 0.807 | 0.783 | 0.189 |
| Ratio A/X 4 | 0.82 | 1.05 | 0.94 | 1.14 | 0.02 | <0.001 | <0.001 | 0.640 |
| Feces 5 | ||||||||
| DM | 87.7 | 80.7 | 87.1 | 77.8 | 0.7 | 0.035 | <0.001 | 0.160 |
| NSP | ||||||||
| Total | 65.3 | 59.5 | 74.8 | 62.5 | 1.2 | 0.001 | <0.001 | 0.073 |
| Fucose | 80.9 | 64.0 | 74.0 | 56.7 | 1.9 | 0.011 | <0.001 | 0.948 |
| Rhamnose | 41.5 | 1.0 | 25.4 | 0.5 | 3.7 | 0.129 | <0.001 | 0.155 |
| Arabinose | 60.5 b | 68.1 a | 67.7 a | 67.1 a,b | 1.0 | 0.096 | 0.058 | 0.027 |
| Galactose | 90.6 a | 67.6 b | 87.4 a | 51.3 c | 2.5 | <0.001 | <0.001 | <0.001 |
| Glucose | 51.1 b | 51.2 b | 71.4 a | 59.4 b | 1.7 | <0.001 | 0.018 | 0.017 |
| Mannose | 83.7 a | 57.4 c | 87.1 a | 74.3 b | 2.0 | <0.001 | <0.001 | 0.002 |
| Xylose | 66.7 | 69.2 | 73.4 | 70.3 | 1.0 | 0.040 | 0.865 | 0.131 |
| Galacturonic acid | 85.5 a | −45.2 c | 93.4 a | 11.7 b | 9.1 | <0.001 | <0.001 | <0.001 |
| Glucuronic acid | 30.8 b | 29.9 b | 66.3 a | 28.5 b | 2.9 | <0.001 | <0.001 | <0.001 |
| Soluble | 71.4 | 54.9 | 85.8 | 79.7 | 2.5 | <0.001 | 0.004 | 0.162 |
| Insoluble | 64.1 b | 59.9 bc | 71.2 a | 55.7 c | 1.2 | 0.413 | <0.001 | 0.003 |
| Total AX | 64.0 | 68.7 | 70.9 | 68.7 | 0.9 | 0.060 | 0.508 | 0.061 |
| Soluble AX | 81.5 | 81.9 | 90.9 | 93.2 | 1.8 | 0.005 | 0.686 | 0.779 |
| Insoluble AX | 60.4 a,b | 66.2 a | 64.7 a,b | 58.0 b | 1.0 | 0.282 | 0.788 | 0.001 |
| Ratio A/X | 0.95 | 1.18 | 1.01 | 1.19 | 0.02 | 0.143 | <0.001 | 0.367 |
W-SBM, wheat/soybean meal; W-RSM, wheat/rapeseed meal; R-SBM, rye/soybean meal; R-RSM, rye/rapeseed meal; SEM, standard error of the mean; CER, cereal; PM, protein meal; DM, dry matter; NSP, non-starch-polysaccharides; AX, arabinoxylans. 1 Data are presented as means (n = 11); p-values indicate effects of the factors cereal (CER), protein meal (PM) and their interaction (CER * PM). 2 Soluble NSP: total NSP–insoluble NSP. 3 Soluble AX, total AX–insoluble AX. 4 A/X, ratio of concentration of arabinose and xylose in digesta or feces. 5 R-SBM: n = 10 (lack of collectable feces). a, b, c Values within a row with different superscripts differ significantly at p ≤ 0.05 (Tukey test).
Colonic NSP digestibility (70.7–88.2%) was higher than fecal NSP digestibility (59.5–74.8%) across the four feeding groups. Both sites showed a higher digestibility of soluble than of insoluble NSP and AX. Rye-fed piglets showed a higher colonic and fecal digestibility of NSP, sNSP, glucose and soluble AX (colon: each p < 0.001; feces: p = 0.001, p < 0.001, p < 0.001, p = 0.005, respectively). Compared to SBM, RSM-fed piglets showed a lower colonic and fecal digestibility of NSP and iNSP (each p < 0.001). Fecal digestibility of iNSP was highest in R-SBM-fed pigs and lowest in R-RSM-fed pigs (p < 0.001). Fecal digestibility of insoluble AX was highest in W-RSM and lowest in R-RSM-fed piglets (p = 0.009).
3.3. Concentration of N-Acetylneuraminic Acid in the Ileum Digesta
Ileal digesta concentration of N-acetylneuraminic acid and ratio of N-acetylneuraminic acid to titanium dioxide were measured to estimate mucus production in the small intestine. Nor concentrations neither the ratio were affected by the dietary treatments (p > 0.05, Table 3).
Table 3.
Effects of the experimental diets on N-acetylneuraminic acid concentration in ileal digesta 1.
| Item | Diet | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| W-SBM | W-RSM | R-SBM | R-RSM | SEM | CER | PM | CER * PM | |
| Neu5Ac, g/kg DM | 1.15 | 1.41 | 1.25 | 1.47 | 0.11 | 0.702 | 0.294 | 0.932 |
| Ratio Neu5Ac/TiO2 | 11.7 | 21.3 | 14.1 | 21.6 | 2.3 | 0.761 | 0.070 | 0.813 |
W-SBM, wheat/soybean meal; W-RSM, wheat/rapeseed meal; R-SBM, rye/soybean meal; R-RSM, rye/rapeseed meal; SEM, standard error of the mean; CER, cereal; PM, protein meal; Neu5Ac, N-acetylneuraminic acid; DM, dry matter. 1 Data are presented as means (W-SBM, W-RSM, R-SBM: n = 7; R-RSM: n = 6, lack of collectable digesta); p-values indicate effects of the factors cereal (CER), protein meal (PM) and their interaction (CER * PM).
3.4. Bacterial Metabolites
Concentration of SCFA was highest in colonic digesta, followed by feces and jejunal digesta (Table 4). Acetic acid was the predominant fraction of SCFA followed by butyric acid in jejunal digesta and by propionic acid in colonic digesta and feces. In comparison to wheat, rye increased concentration of SCFA (p < 0.001), acetic acid (p < 0.001), and propionic acid (p = 0.024) in the jejunum and of SCFA (p = 0.016), acetic acid (p = 0.014), propionic acid (p = 0.034), and i-butyric acid (p = 0.035) in the colon. RSM-fed pigs showed a lower concentration of SCFA (p = 0.001), acetic acid (p = 0.002), and n-butyric acid (p = 0.010) in the jejunum and a lower colonic concentration of acetic acid (p = 0.049), i-butyric acid (p = 0.001), n-butyric acid (p = 0.041), and i-valeric acid (p = 0.002) than SBM-pigs.
Table 4.
Effects of the experimental diets on bacterial metabolites in digesta and feces of weaned piglets 1.
| Concentration, µmol/g DM | Diet | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| W-SBM | W-RSM | R-SBM | R-RSM | SEM | CER | PM | CER * PM | |
| Jejunum | ||||||||
| SCFA | 89.6 | 30.8 | 192 | 104 | 13.6 | <0.001 | 0.001 | 0.511 |
| Acetic acid | 79.0 | 27.8 | 172 | 96.1 | 12.1 | <0.001 | 0.002 | 0.517 |
| Propionic acid | 0.47 | 0.68 | 3.60 | 1.26 | 0.43 | 0.024 | 0.188 | 0.115 |
| i-butyric acid | 1.33 | 0.94 | 1.42 | 1.33 | 0.11 | 0.288 | 0.286 | 0.509 |
| n-butyric acid | 8.43 | 1.12 | 14.0 | 5.51 | 1.58 | 0.097 | 0.010 | 0.845 |
| i-valeric acid | 0.08 | 0.00 | 0.27 | 0.03 | 0.05 | 0.195 | 0.072 | 0.353 |
| n-valeric acid | 0.24 | 0.28 | 0.36 | 0.28 | 0.03 | 0.351 | 0.709 | 0.342 |
| Ammonia | 37.9 | 29.7 | 53.7 | 37.8 | 3.4 | 0.073 | 0.070 | 0.551 |
| l-lactate | 177 | 213 | 303 | 133 | 32 | 0.717 | 0.295 | 0.112 |
| d-lactate | 5.64 | 9.83 | 8.92 | 7.15 | 2.04 | 0.944 | 0.775 | 0.483 |
| Colon ascendens | ||||||||
| SCFA | 990 | 922 | 1316 | 1040 | 48 | 0.016 | 0.057 | 0.244 |
| Acetic acid | 536 | 489 | 743 | 566 | 30 | 0.014 | 0.049 | 0.243 |
| Propionic acid | 251 | 236 | 314 | 280 | 12 | 0.034 | 0.323 | 0.706 |
| i-butyric acid | 11.0 | 9.56 | 14.2 | 9.78 | 0.48 | 0.036 | 0.001 | 0.064 |
| n-butyric acid | 153 | 141 | 191 | 143 | 7 | 0.172 | 0.041 | 0.197 |
| i-valeric acid | 10.9 | 8.58 | 13.9 | 7.61 | 0.74 | 0.430 | 0.002 | 0.133 |
| n-valeric acid | 28.9 | 37.0 | 39.9 | 33.7 | 2.3 | 0.402 | 0.836 | 0.124 |
| Ammonia | 51.5 | 52.9 | 65.5 | 41.5 | 3.6 | 0.849 | 0.116 | 0.078 |
| l-lactate | 22.4 | 34.1 | 40.8 | 28.4 | 3.9 | 0.425 | 0.965 | 0.133 |
| d-lactate | 7.69 | 11.0 | 9.17 | 7.80 | 1.31 | 0.754 | 0.723 | 0.390 |
| Feces 2 | ||||||||
| SCFA | 592 | 529 | 688 | 571 | 34 | 0.308 | 0.187 | 0.694 |
| Acetic acid | 283 | 257 | 358 | 291 | 17 | 0.112 | 0.171 | 0.537 |
| Propionic acid | 154 | 133 | 163 | 140 | 9 | 0.642 | 0.236 | 0.972 |
| i-butyric acid | 15.1 | 15.9 | 21.6 | 15.6 | 1.0 | 0.106 | 0.168 | 0.079 |
| n-butyric acid | 97.1 | 80.8 | 93.2 | 81.3 | 6.5 | 0.898 | 0.296 | 0.870 |
| i-valeric acid | 21.0 | 21.5 | 31.0 | 21.5 | 1.4 | 0.065 | 0.094 | 0.063 |
| n-valeric acid | 22.7 | 20.8 | 21.9 | 22.0 | 1.3 | 0.943 | 0.755 | 0.711 |
| Ammonia | 78.4 | 84.5 | 86.0 | 78.1 | 5.6 | 0.958 | 0.939 | 0.552 |
| l-lactate | 12.0 | 19.2 | 12.9 | 15.5 | 2.0 | 0.714 | 0.223 | 0.562 |
| d-lactate | 6.42 | 11.7 | 6.45 | 10.0 | 1.45 | 0.778 | 0.135 | 0.770 |
DM, dry matter; W-SBM, wheat/soybean meal; W-RSM, wheat/rapeseed meal; R-SBM, rye/soybean meal; R-RSM, rye/rapeseed meal; SEM, standard error of the mean; CER, cereal; PM, protein meal; SCFA, short-chain fatty acids; 1 Data are presented as means (n = 11); p-values indicate effects of the factors cereal (CER), protein meal (PM), and their interaction (CER * PM). 2 R-SBM: n = 10 (lack of collectable feces).
In caudal direction, ammonia increased, and l-lactate decreased. Ammonia, d-, and l-lactate were not affected by the dietary treatments (p > 0.05).
3.5. Microbial Diversity Indices
Microbial richness was reduced in jejunal digesta of piglets fed rye compared to wheat (p = 0.047; Table 5). Shannon index and evenness were not affected (p > 0.05).
Table 5.
Effects of the experimental diets on ecological indices of the intestinal microbiota of weaned piglets 1.
| Diet | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| W-SBM | W-RSM | R-SBM | R-RSM | SEM | CER | PM | CER * PM | |
| Jejunum 2 | ||||||||
| Richness | 42.3 | 72.8 | 37.2 | 38.9 | 5.1 | 0.047 | 0.098 | 0.138 |
| Shannon Index | 1.53 | 1.94 | 1.61 | 1.65 | 0.08 | 0.522 | 0.165 | 0.247 |
| Evenness | 0.420 | 0.475 | 0.448 | 0.462 | 0.011 | 0.781 | 0.223 | 0.475 |
| Colon ascendens | ||||||||
| Richness | 186 | 202 | 203 | 190 | 6 | 0.811 | 0.899 | 0.246 |
| Shannon Index | 3.46 | 3.65 | 3.69 | 3.88 | 0.06 | 0.055 | 0.112 | 0.957 |
| Evenness | 0.666 | 0.688 | 0.699 | 0.743 | 0.010 | 0.060 | 0.149 | 0.633 |
| Feces | ||||||||
| Richness | 159 | 177 | 192 | 182 | 6 | 0.163 | 0.748 | 0.286 |
| Shannon Index | 3.20 | 3.38 | 3.35 | 3.74 | 0.09 | 0.176 | 0.128 | 0.558 |
| Evenness | 0.633 | 0.652 | 0.639 | 0.720 | 0.023 | 0.213 | 0.096 | 0.300 |
W-SBM, wheat/soybean meal; W-RSM, wheat/rapeseed meal; R-SBM, rye/soybean meal; R-RSM, rye/rapeseed meal; SEM, standard error of the mean; CER, cereal; PM, protein meal. 1 Data are presented as means (n = 11); p-values indicate effects of the factors cereal (CER), protein meal (PM), and their interaction (CER * PM). 2 W-RSM: n = 10 (DNA-extract not amplifiable).
3.6. Relative Abundance of Bacterial Phyla, Order, and Genera
Most abundant phyla in jejunum, colon, and feces were Firmicutes and Bacteroidetes (Table 6). In rye-fed pigs, jejunal Firmicutes were higher and Proteobacteria lower than in wheat-fed pigs (p = 0.039, p = 0.002, respectively). Compared to SBM, RSM reduced Firmicutes, and increased Actinobacteria at the three sampling sites (p < 0.050). RSM increased Proteobacteria in the jejunum and Bacteroidetes in the colon (p = 0.019, p = 0.014, respectively). Correlation (r) between the relative abundance of jejunal Proteobacteria and AID of crude protein was 0.119 (p = 0.484) and with AID of total amino acids it was 0.097 (p = 0.605). Correlation (r) between colonic Bacteroidetes and ADG of days 0–33 was −0.106 (p = 0.498) and of days 28–33 −0.302 (p = 0.049). In the jejunum, W-RSM-fed pigs showed a lower relative abundance of Firmicutes compared to the other three groups (p = 0.002).
Table 6.
Effects of the experimental diets on the relative abundance (%) of bacterial phyla 1.
| Diet | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| W-SBM | W-RSM | R-SBM | R-RSM | SEM | CER | PM | CER * PM | |
| Jejunum 2 | ||||||||
| Actinobacteria | 0.550 | 5.40 | 1.36 | 2.72 | 0.673 | 0.467 | 0.019 | 0.177 |
| Bacteroidetes | 0.064 | 3.11 | 0.089 | 0.174 | 0.686 | 0.292 | 0.258 | 0.284 |
| Firmicutes | 98.4 a | 88.7 b | 98.2 a | 96.7 a | 1.1 | 0.039 | 0.004 | 0.032 |
| Proteobacteria | 0.749 | 2.60 | 0.131 | 0.407 | 0.255 | 0.002 | 0.019 | 0.077 |
| Colon ascendens | ||||||||
| Actinobacteria | 0.344 | 2.61 | 1.44 | 3.00 | 0.435 | 0.380 | 0.029 | 0.678 |
| Bacteroidetes | 17.5 | 23.4 | 18.2 | 26.1 | 1.4 | 0.540 | 0.014 | 0.713 |
| Firmicutes | 80.0 | 72.3 | 79.1 | 68.2 | 1.7 | 0.423 | 0.005 | 0.607 |
| Proteobacteria | 1.82 | 1.00 | 0.702 | 2.10 | 0.358 | 0.989 | 0.688 | 0.131 |
| Spirochaetes | 0.202 | 0.082 | 0.350 | 0.133 | 0.044 | 0.245 | 0.053 | 0.569 |
| Feces | ||||||||
| Actinobacteria | 0.749 | 3.61 | 2.08 | 4.86 | 0.516 | 0.182 | 0.005 | 0.965 |
| Bacteroidetes | 10.6 | 12.3 | 11.0 | 16.3 | 1.1 | 0.322 | 0.123 | 0.415 |
| Firmicutes | 86.7 | 82.4 | 85.6 | 76.9 | 1.3 | 0.184 | 0.012 | 0.377 |
| Proteobacteria | 0.961 | 1.19 | 0.247 | 1.35 | 0.221 | 0.533 | 0.137 | 0.323 |
| Spirochaetes | 0.671 | 0.098 | 0.387 | 0.179 | 0.145 | 0.730 | 0.189 | 0.537 |
| Tenericutes | 0.061 | 0.056 | 0.049 | 0.058 | 0.012 | 0.844 | 0.956 | 0.783 |
W-SBM, wheat/soybean meal; W-RSM, wheat/rapeseed meal; R-SBM, rye/soybean meal; R-RSM, rye/rapeseed meal; SEM, standard error of the mean; CER, cereal; PM, protein meal. 1 Data are presented as means (n = 11); p-values indicate effects of the factors cereal (CER), protein meal (PM) and their interaction (CER * PM). 2 W-RSM: n = 10 (DNA-extract not amplifiable). a, b Values within a row with different superscripts differ significantly at p ≤ 0.05 (Tukey test).
At the genus level, rye increased relative abundance of Clostridium sensu stricto 1 in the jejunum (p = 0.005) and Terrisporobacter in the jejunum and feces (p = 0.018, p = 0.004, respectively). Compared to SBM, RSM increased Bifidobacterium spp. in the jejunum (p = 0.046) and the genera Prevotella 9, Blautia, and Syntrophococcus in feces (p = 0.022, p = 0.039, p = 0.024, respectively). RSM decreased Clostridium sensu stricto 1 in the three sampling sites (p < 0.05), Terrisporobacter in jejunum and feces (p = 0.033, p = 0.004, respectively), and unknown Ruminococcaceae and Christensenellaceae R-7 group in feces (p = 0.042, p = 0.031, respectively). Results at the order level are displayed in the Supplementary Materials.
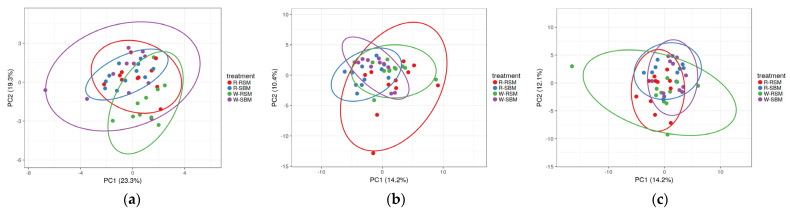
Principal component analysis showed higher variabilities of the relative abundance of bacterial genera in jejunal digesta (≤23.3%) than in colonic digesta and feces (≤14.2%; Figure 1). Clusters of the experimental diets were overlapping to a high extent.
Figure 1.
PCA showing the effect of the experimental diets on the relative abundance (%) of bacterial genera in digesta of jejunum (a), colon ascendens (b), and feces (c) in weaned piglets; R-SBM, rye/soybean meal; R-RSM, rye/rapeseed meal; W-SBM, wheat/soybean meal; W-RSM, wheat/rapeseed meal. Principal component analysis was performed with ClustVis.
4. Discussion
4.1. Fiber Composition of the Experimental Diets
The use of fiber-rich feed components might help to stabilize the intestinal milieu during the post-weaning period. As rye contains more SDF and sNSP [18,36] the rye-based diets used in the current study were characterized by higher concentrations of soluble fiber fractions than the wheat-based diets. Similarly, the high concentrations of iNSP in RSM [18] resulted in a higher concentration of iNSP in the RSM-based diets compared to the SBM-based ones.
AX are the predominant fraction of cereal NSP and consist of a xylan backbone substituted by arabinose to varying degrees [18]. The degree of substitution can be described by the A/X-ratio, which is similar in rye and wheat [18,20], but higher in RSM compared to SBM [18]. Readily fermentable and more soluble AX are usually more substituted and characterized by a higher A/X-ratio [37]. However, solubility and fermentability of AX are reduced with increasing cross-linkages to lignin, ferulic acid or other polysaccharides and by a higher molecular weight of AX [18,38]. As discussed below, intestinal microbiota was shaped differently by the RSM-based diets in the present study compared to SBM. This might be related to the higher dietary and intestinal A/X-ratio of RSM. However, the high content of insoluble dietary fiber (IDF) and acid detergent lignin (ADL) in the RSM-based diets indicating a high degree of lignification might have reduced fermentability, as indicated by the reduced concentration of SCFA in RSM-fed pigs. Therefore, A/X-ratio could not be used as an indicator of degradability of AX in the current study.
4.2. Apparent Digestibility of NSP
The apparent colonic and total tract digestibility of total and soluble NSP in rye-fed pigs was higher compared to pigs receiving wheat-based diets. A higher ATTD of TDF and arabinose in rye-fed pigs was associated with an increased microbial fermentation in the large intestine [16]. In rye compared to wheat a higher fermentative activity might be related to the higher solubility of rye AX, the higher content of β-glucans and the higher total amount of dietary fiber [16,37]. Therefore, the digestibility of NSP in the current study might have been caused by a higher uptake of fermentable fiber with the rye-based diets, including more sNSP and soluble AX. Moreover, wheat AX might be less degradable, because of cross-linkages to other polysaccharides such as cellulose [37]. Cellulose is a major plant cell wall component, which is highly insoluble, poorly fermentable, and has a higher concentration in wheat than in rye [18]. The increased concentrations of digesta SCFA and the higher relative abundance of major plant cell wall-degrading bacterial species in the jejunum indicate an enhanced bacterial fiber degradation and can further explain the higher digestibility of NSP in rye-fed pigs of the present study.
With respect to NSP digestibility in RSM-fed pigs, values for colonic and total tract digestibility of total and iNSP was lower than in SBM-fed pigs. Similarly, ATTD of neutral detergent fiber (NDF), acid detergent fiber (ADF), carbohydrates, and dietary fiber was lower in other studies investigating the feeding of RSM instead of SBM to pigs [39,40]. However, one of these studies did not show a different ATTD of NSP and cellulose between RSM and SBM [39]. Microbial fiber fermentation and metabolic activity might be reduced as RSM is more lignified and insoluble than SBM [18]. This is reflected in the greater amount of IDF and iNSP in RSM-based diets of the current study and the lower SCFA concentration in jejunum, colon, and feces of RSM-fed pigs compared to SBM.
Surprisingly, the present values for colonic digestibility of NSP were higher than ATTD of NSP. In contrast, other studies show an increasing digestibility of NSP and AX in caudal direction [16,38]. However, the values of NSP digestibility were numerically in the same range, and furthermore, the determination of digestibility in feces is susceptible to interference. Bacteria may act as “non-dietary interfering substance” in NSP analytical procedures resulting in overestimated values of NSP concentration that are higher than the true diet derived NSP concentration [41]. Considering the higher number of bacteria in feces compared to colonic digesta and that in pigs and humans bacterial mass represents 40–50% of fecal DM [42,43], the interfering effect would be more pronounced with respect to ATTD of NSP compared to colonic digestibility. Additionally, some bacteria produce exopolysaccharides such as colonic acid (Enterobacteria) or dextran (Lactobacillales), which might have contributed to increased fecal concentration of NSP [44].
4.3. Bacterial Metabolites and Composition of the Microbiome
In the current study, compared to wheat, rye-fed pigs had a higher concentration of SCFA in the jejunum and colon. As shown in a study comparing the feeding of an AX-rich diet (65% rye-flakes) with a diet based on wheat flour, complex cereal polysaccharides such as AX are not degraded enzymatically in the small intestine and might therefore promote the growth of SCFA-producing bacteria in the distal parts of the intestine [38]. Another study focused on the feeding of wheat and rye breads to pigs, demonstrating that increased SCFA-production in the small intestine might be related to the more soluble fraction of dietary fiber, which is readily fermentable, whilst insoluble fractions would be degraded more distally [37]. Moreover, rye might cause a higher bacterial production of butyrate due to the structure of AX and the lower content of cellulose [45]. Therefore, the high concentration of TDF and SDF in the rye-based diets of this study may have provided more substrate for growth of SCFA-producing bacteria. Positive effects of SCFA derived from fermentation of dietary fiber on gut and animal health have been widely reviewed, including the use of acetate as an energy source and butyrate as the main fuel for colonocytes and its efficiency against possible pathogens [7,46]. In the present study, only data on digesta concentration of SCFA are available, but SCFA are absorbed rapidly via the gut wall [47]. Rye-derived SCFA might be of systemic use as peripheral blood concentration of SCFA was increased by an AX-rich diet based on rye-flakes compared to a wheat flour-based diet [48]. Despite the increased colonic concentration of SCFA and especially butyrate in the current study, no changes of the microbial community were determined in colonic digesta. This might be related to cross-feeding of AX-degrading bacteria, e.g., between Bifidobacteria and butyrate-producing bacteria, and to an increased abundance of the phosphotransferase system (kl02060) regulating carbohydrate uptake into bacterial cells [22]. Nevertheless, it is likely that rye compared to wheat enhanced the production of bacterial metabolites by providing a higher amount of easier fermentable substrate without a shift in the microbial community.
Compared to SBM-fed pigs, concentration of SCFA was lower in RSM-fed pigs in the jejunum and tendentially in the colon which indicates a lower metabolic activity of the resident microbiota. In contrast, previous studies showed an equal level of SCFA between RSM and SBM [24,25,28]. However, compared to these studies, the inclusion level of RSM and consequently the content of lignin and IDF was higher in the present study. A high degree of lignification may hamper enzymatic as well as microbial fiber degradation in RSM-fed pigs [39]. In combination with the lower digestibility of NSP and DM, this could explain the lower SCFA compared to SBM-fed pigs. Additionally, SCFA might have been absorbed rapidly or used by other microbiota in the sense of cross-feeding [49].
In accordance with a recent meta-analysis that identified Firmicutes, Proteobacteria, and Bacteroidetes as the most abundant phyla of the core microbiome in pigs [50], Firmicutes and Bacteroidetes were the predominant phyla of the pigs in the current study.
In rye-fed piglets of this study, composition of the microbial community was only different to wheat-fed pigs in the jejunum. This was unexpected, because according to other studies, the major impact of rye on the microbial community is expected in the proximal parts of the large intestine [16,23,38]. A higher relative abundance of Firmicutes in the small intestine might be explained by the higher content of sNSP in the rye-based diets, since more insoluble substrate would be degraded further distally [7]. The phylum Firmicutes contains many plant cell wall-degrading species including Clostridium sensu stricto 1 and Terrisporobacter. These two genera are known to ferment complex indigestible plant polysaccharides such as hemicellulose and cellulose [51]. Therefore, the fiber provided by the rye-based diets might have served as a substrate and stimulated Firmicutes’ proliferation. Firmicutes contains many butyrate-producers [52], therefore an increased abundance could be considered as a positive effect of the feeding of rye. However, SCFA are absorbed primarily in the caecum and proximal colon, and might not utilized by the host in the same extent in the jejunum [47].
Proteobacteria had a lower jejunal relative abundance in rye-fed pigs of the current study. Many putative pathogens such as E. coli and Salmonella belong to Proteobacteria and a lower presence of Proteobacteria was associated with an increased intestinal barrier function and a higher anti-inflammatory capacity of the local immune system [53].
In contrast to an increased concentration of SCFA in jejunal digesta, the analysis of microbial diversity in rye-fed pigs showed a lower richness compared to wheat. It is possible that the increased production of metabolites might be driven by only a few selected genera of Firmicutes which were more abundant in the rye-fed pigs.
With respect to effects of RSM on the relative abundance of microbiota compared to SBM, the present study resulted in a reduced abundance of Firmicutes at the three sampling sites. As mentioned above, Firmicutes contains many fiber-degrading species and is known to produce SCFA. The lower NSP and DM digestibility together in RSM-fed pigs compared to SBM indicate that the fiber provided by the RSM-based diets was not used as a suitable substrate for fermentation by microbiota, especially fiber-degrading Firmicutes. Consequently, the metabolic activity and growth of microbiota was lower as illustrated by the lower concentration of SCFA and the lower relative abundance of Firmicutes. In line with this, compared to alfalfa meal, wheat bran and pure cellulose containing a higher amount of IDF also reduced the relative abundance of Firmicutes in large intestinal mucosa of suckling pigs [54]. However, another study investigating the feeding of RSM instead of SBM showed an increase of Firmicutes, although the lower inclusion level and lower content of IDF might have prevented an inverse result [55]. A higher ratio of Firmicutes:Bacteroidetes was associated with a reduction of the incidence of diarrhea and infections [55]. Nonetheless, previously published results of the present study showed that the fecal score of the pigs was in a physiological range throughout the trial [14]. Within Firmicutes, the predominant genera Clostridium sensu stricto 1 and Terrisporobacter were also decreased by RSM in the current study. This might explain the reduced concentration of SCFA in RSM-fed pigs because another study showed a strong correlation between the relative abundance of these genera with the production of large amounts of metabolites from plant fiber [51].
In line with another study investigating the feeding of RSM [25], RSM increased the relative abundance of Actinobacteria along the gastro-intestinal tract in the current study. In contrast, another study did not show differing values of abundance of Actinobacteria in RSM- or SBM-fed pigs [55]. Actinobacteria efficiently use hemicellulose and cellulose [52], both mainly insoluble fiber fractions. Despite RSM and SBM containing similar amounts of cellulose [18] the higher inclusion of RSM compared to SBM and the higher content of IDF in RSM-diets might have promoted growth of Actinobacteria in RSM-fed pigs. In the jejunum, Bifidobacterium, a genus belonging to Actinobacteria, was also increased by the feeding of RSM. Bifidobacterium is considered to improve gut health [7,56] and was increased by the feeding of cellulose in another study [57].
As mentioned above, an increased relative abundance of Proteobacteria may be a risk for animal health. Nevertheless, the pigs of both RSM and SBM groups were in a good condition throughout the trial. Proteobacteria contains many proteolytic genera [58]. RSM may lead to a lower AID of protein and amino acids than SBM [59] which also was demonstrated in this study [14]. However, there was no correlation detected between AID of crude protein and total amino acids and the jejunal relative abundance of Proteobacteria. It is likely that the protein was not available as a substrate for growth of Proteobacteria, because IDF in RSM caused an encapsulation in the rigid cell wall and an increased digesta passage rate [39].
In RSM-fed pigs, the increased relative abundance of Bacteroidetes is most likely related to the higher content of IDF than in SBM-based diets. Bacteroidetes as the second most abundant phylum of the gut microbiota in pigs [50] was also increased by resistant starch in humans [60] and by corn bran in pigs [61], both sources of IDF. In contrast, a study investigating the feeding of RSM instead of SBM to pigs showed a lower abundance of Bacteroidetes which was related to the high pectin content [25]. An increased abundance of Bacteroidetes is associated with weight loss in humans, mice [62], and pigs [63]. The negative correlation between abundance of Bacteroidetes and ADG in the last six days of the trial might suggest that the shift towards more colonic Bacteroidetes was connected to the reduced weight gain in RSM-fed pigs.
5. Conclusions
In conclusion, compared to wheat, the higher amount and solubility of NSP from rye resulted in an increased degradation and fermentation of NSP and in a higher metabolic activity of intestinal microbiota. However, relative abundance of large intestinal microbiota was not different between pigs fed rye and wheat. RSM caused a lower bacterial metabolic activity than SBM. The higher fiber content of RSM-based diets was expected to increase fermentation, but 30% inclusion of RSM might have provided an excess of IDF. Still, RSM lead to a lower abundance of common fiber degraders of the predominant phylum Firmicutes and an increased abundance of IDF degrading Actinobacteria and Bacteroidetes. Further research is needed to better understand host–microbiota interaction and to improve feeding concepts with a targeted use of dietary fiber.
Acknowledgments
The authors thank the technical staff of the Institute of Animal Nutrition at the Freie Universität Berlin for excellent support during the animal experiment and laboratory analyses. The authors are grateful to KWS Lochow GmbH, Bergen, Germany for providing the hybrid rye used in the present study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani12010109/s1, Table S1: Effects of the experimental diets on the relative abundance (%) of bacterial orders, Table S2: Effects of the experimental diets on the relative abundance (%) of bacterial genera.
Author Contributions
Conceptualization, J.Z.; methodology, J.Z.; formal analysis, C.E.; investigation, C.E.; resources, J.Z. and C.E.; data curation, J.Z. and C.E.; writing—original draft preparation, C.E.; writing—review and editing, J.Z. and A.G.W.; validation, J.Z.; visualization, C.E.; supervision, J.Z.; project administration, J.Z. and C.E.; funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry of Food and Agriculture (Bundesministerium für Ernährung und Landwirtschaft) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (Bundesantstalt für Landwirtschaft und Ernährung) under the innovation support program, Grant number 281B101316. The publication of this article was funded by Freie Universität Berlin.
Institutional Review Board Statement
This study was performed in accordance with the German ethical and animal care guidelines and approved by the State Office of Health and Social Affairs ‘Landesamt für Gesundheit und Soziales Berlin’ (LAGeSo, Berlin, Germany, registration number T 0063/19).
Data Availability Statement
Data supporting reported results is contained within the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Molist F., van Oostrum M., Perez J.F., Mateos G.G., Nyachoti C.M., van der Aar P.J. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim. Feed Sci. Technol. 2014;189:1–10. doi: 10.1016/j.anifeedsci.2013.12.013. [DOI] [Google Scholar]
- 2.Uerlings J., Schroyen M., Bautil A., Courtin C., Richel A., Sureda E.A., Bruggeman G., Tanghe S., Willems E., Bindelle J., et al. In vitro prebiotic potential of agricultural by-products on intestinal fermentation, gut barrier and inflammatory status of piglets. Br. J. Nutr. 2020;123:293–307. doi: 10.1017/S0007114519002873. [DOI] [PubMed] [Google Scholar]
- 3.Wiese M. The potential of pectin to impact pig nutrition and health: Feeding the animal and its microbiome. FEMS Microbiol. Lett. 2019;366:fnz029. doi: 10.1093/femsle/fnz029. [DOI] [PubMed] [Google Scholar]
- 4.Pieper R., Jha R., Rossnagel B., Van Kessel A.G., Souffrant W.B., Leterme P. Effect of barley and oat cultivars with different carbohydrate compositions on the intestinal bacterial communities in weaned piglets. FEMS Microbiol. Ecol. 2008;66:556–566. doi: 10.1111/j.1574-6941.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y.A., Gonzalez-Ortiz G., Sola-Oriol D., Lopez-Colom P., Martin-Orue S.M. Screening of the ability of natural feed ingredients commonly used in pig diets to interfere with the attachment of ETEC K88 (F4) to intestinal epithelial cells. Anim. Feed Sci. Technol. 2018;242:111–119. doi: 10.1016/j.anifeedsci.2018.06.005. [DOI] [Google Scholar]
- 6.Bach Knudsen K.E., Laerke H.N., Ingerslev A.K., Hedemann M.S., Nielsen T.S., Theil P.K. Carbohydrates in pig nutrition—Recent advances. J. Anim. Sci. 2016;94:1–11. doi: 10.2527/jas.2015-9785. [DOI] [Google Scholar]
- 7.Jha R., Fouhse J.M., Tiwari U.P., Li L.G., Willing B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019;6:48. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiwari U.P., Singh A.K., Jha R. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: A review. Anim. Nutr. 2019;5:217–226. doi: 10.1016/j.aninu.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agyekum A.K., Nyachoti C.M. Nutritional and Metabolic Consequences of Feeding High-Fiber Diets to Swine: A Review. Engineering. 2017;3:716–725. doi: 10.1016/J.ENG.2017.03.010. [DOI] [Google Scholar]
- 10.Bach Knudsen K.E., Serena A., Canibe N., Juntunen K.S. New insight into butyrate metabolism. Proc. Nutr. Soc. 2003;62:81–86. doi: 10.1079/PNS2002212. [DOI] [PubMed] [Google Scholar]
- 11.Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003;108:95–117. doi: 10.1016/S0377-8401(03)00163-9. [DOI] [Google Scholar]
- 12.Miner-Williams W., Moughan P.J., Fuller M.F. Methods for Mucin Analysis: A Comparative Study. J. Agric. Food. Chem. 2009;57:6029–6035. doi: 10.1021/jf901036r. [DOI] [PubMed] [Google Scholar]
- 13.Kamphues J., Hartung C., Wilke V., Grone R. Übersichten zur Tierernährung. Volume 2 DLG-Verlags-GmbH; Frankfurt am Main, Germany: 2019. Rye: Renaissance of a Traditional Grain Type in Animal Feeding? [Google Scholar]
- 14.Ellner C., Martínez-Vallespín B., Saliu E.M., Zentek J., Röhe I. Effects of cereal and protein source on performance, apparent ileal protein digestibility and intestinal characteristics in weaner piglets. Arch. Anim. Nutr. 2021;75:263–277. doi: 10.1080/1745039X.2021.1958647. [DOI] [PubMed] [Google Scholar]
- 15.Chuppava B., Wilke V., Hartung C.B., El-Wahab A.A., Grone R., von Felde A., Kamphues J., Visscher C. Effect of a High Proportion of Rye in Compound Feed for Reduction of Salmonella Typhimurium in Experimentally Infected Young Pigs. Microorganisms. 2020;8:1629. doi: 10.3390/microorganisms8111629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGhee M.L., Stein H.H. The apparent ileal digestibility and the apparent total tract digestibility of carbohydrates and energy in hybrid rye are different from some other cereal grains when fed to growing pigs. J. Anim. Sci. 2020;98:1–10. doi: 10.1093/jas/skaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miedaner T., Geiger H.H. Biology, Genetics, and Management of Ergot (Claviceps spp.) in Rye, Sorghum, and Pearl Millet. Toxins. 2015;7:659–678. doi: 10.3390/toxins7030659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach Knudsen K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014;93:2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- 19.Rodehutscord M., Ruckert C., Maurer H.P., Schenkel H., Schipprack W., Bach Knudsen K.E., Schollenberger M., Laux M., Eklund M., Siegert W., et al. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 2016;70:87–107. doi: 10.1080/1745039X.2015.1133111. [DOI] [PubMed] [Google Scholar]
- 20.Bach Knudsen K.E., Laerke H.N. Rye Arabinoxylans: Molecular Structure, Physicochemical Properties and Physiological Effects in the Gastrointestinal Tract. Cereal Chem. 2010;87:353–362. doi: 10.1094/CCHEM-87-4-0353. [DOI] [Google Scholar]
- 21.Bach Knudsen K.E., Jorgensen H., Theil P.K. Changes in short-chain fatty acid plasma profile incurred by dietary fiber composition. J. Anim. Sci. 2016;94:476–479. doi: 10.2527/jas.2015-9786. [DOI] [Google Scholar]
- 22.Burbach K., Strang E.J.P., Mosenthin R., Camarinha-Silva A., Seifert J. Porcine intestinal microbiota is shaped by diet composition based on rye or triticale. J. Appl. Microbiol. 2017;123:1571–1583. doi: 10.1111/jam.13595. [DOI] [PubMed] [Google Scholar]
- 23.Gorham J.B., Kang S., Williams B.A., Grant L.J., McSweeney C.S., Gidley M.J., Mikkelsen D. Addition of arabinoxylan and mixed linkage glucans in porcine diets affects the large intestinal bacterial populations. Eur. J. Nutr. 2017;56:2193–2206. doi: 10.1007/s00394-016-1263-4. [DOI] [PubMed] [Google Scholar]
- 24.Umu O.C.O., Mydland L.T., Overland M., Press C.M., Sorum H. Rapeseed-based diet modulates the imputed functions of gut microbiome in growing-finishing pigs. Sci. Rep. 2020;10:9372. doi: 10.1038/s41598-020-66364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umu O.C.O., Fauske A.K., Akesson C.P., de Nanclares M.P., Sorby R., Press C.M., Overland M., Sorum H. Gut microbiota profiling in Norwegian weaner pigs reveals potentially beneficial effects of a high-fiber rapeseed diet. PLoS ONE. 2018;13:e0209439. doi: 10.1371/journal.pone.0209439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GfE . Empfehlungen zur Energie- und Nährstoffversorgung von Schweinen. 1st ed. DLG-Verlag; Frankfurt am Main, Germany: 2006. pp. 1–247. [Google Scholar]
- 27.Myers W.D., Ludden P.A., Nayigihugu V., Hess B.W. Technical note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 2004;82:179–183. doi: 10.2527/2004.821179x. [DOI] [PubMed] [Google Scholar]
- 28.Mejicanos G.A., Gonzalez-Ortiz G., Nyachoti C.M. Effect of dietary supplementation of xylanase in a wheat-based diet containing canola meal on growth performance, nutrient digestibility, organ weight, and short-chain fatty acid concentration in digesta when fed to weaned pigs. J. Anim. Sci. 2020;98:1–13. doi: 10.1093/jas/skaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kröger S., Vahjen W., Zentek J. Influence of lignocellulose and low or high levels of sugar beet pulp on nutrient digestibility and the fecal microbiota in dogs. J. Anim. Sci. 2017;95:1598–1605. doi: 10.2527/jas.2016.0873. [DOI] [PubMed] [Google Scholar]
- 30.Bushnell B., Rood J., Singer E. BBMerge—Accurate paired shotgun read merging via overlap. PLoS ONE. 2017;12:e0185056. doi: 10.1371/journal.pone.0185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz P., Parfrey L.W., Yarza P., Gerken J., Pruesse E., Quast C., Schweer T., Peplies J., Ludwig W., Glöckner F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014;42:D643–D648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren H., Vahjen W., Dadi T., Saliu E.M., Boroojeni F.G., Zentek J. Synergistic Effects of Probiotics and Phytobiotics on the Intestinal Microbiota in Young Broiler Chicken. Microorganisms. 2019;7:684. doi: 10.3390/microorganisms7120684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metsalu T., Vilo J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein H.H., Lagos L.V., Casas G.A. Nutritional value of feed ingredients of plant origin fed to pigs. Anim. Feed Sci. Technol. 2016;218:33–69. doi: 10.1016/j.anifeedsci.2016.05.003. [DOI] [Google Scholar]
- 37.Le Gall M., Serena A., Jorgensen H., Theil P.K., Bach Knudsen K.E. The role of whole-wheat grain and wheat and rye ingredients on the digestion and fermentation processes in the gut--a model experiment with pigs. Br. J. Nutr. 2009;102:1590–1600. doi: 10.1017/S0007114509990924. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen T.S., Laerke H.N., Theil P.K., Sorensen J.F., Saarinen M., Forssten S., Bach Knudsen K.E. Diets high in resistant starch and arabinoxylan modulate digestion processes and SCFA pool size in the large intestine and faecal microbial composition in pigs. Br. J. Nutr. 2014;112:1837–1849. doi: 10.1017/S000711451400302X. [DOI] [PubMed] [Google Scholar]
- 39.Perez de Nanclares M., Marcussen C., Tauson A.H., Hansen J.O., Kjos N.P., Mydland L.T., Bach Knudsen K.E., Overland M. Increasing levels of rapeseed expeller meal in diets for pigs: Effects on protein and energy metabolism. Animal. 2019;13:273–282. doi: 10.1017/S1751731118000988. [DOI] [PubMed] [Google Scholar]
- 40.Huang C.F., Li P., Ma X.K., Jaworski N.W., Stein H.H., Lai C.H., Zhao J.B., Zhang S.A. Methodology effects on determining the energy concentration and the apparent total tract digestibility of components in diets fed to growing pigs. Asian Australas. J. Anim. Sci. 2018;31:1315–1324. doi: 10.5713/ajas.17.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montoya C.A., Henare S.J., Rutherfurd S.M., Moughan P.J. Potential misinterpretation of the nutritional value of dietary fiber: Correcting fiber digestibility values for nondietary gut-interfering material. Nutr. Rev. 2016;74:517–533. doi: 10.1093/nutrit/nuw014. [DOI] [PubMed] [Google Scholar]
- 42.Achour L., Nancey S., Moussata D., Graber I., Messing B., Flourie B. Faecal bacterial mass and energetic losses in healthy humans and patients with a short bowel syndrome. Eur. J. Nutr. 2007;61:233–238. doi: 10.1038/sj.ejcn.1602496. [DOI] [PubMed] [Google Scholar]
- 43.Marien C. Ph.D. Thesis. University of Kassel; Kassel, Germany: 2011. Effects of Tubers of the Jerusalem Artichoke (Helianthus tuberosus) and Potatoes (Solanum tuberosum) on the Intestinal Microbiota of Pigs and Evaluation of a Procedure for Quantification of Microbial Mass in Pig Faeces. [Google Scholar]
- 44.Schmid J., Sieber V., Rehm B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015;6:496. doi: 10.3389/fmicb.2015.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bach Knudsen K.E., Serena A., Kjaer A.K.B., Jorgensen H., Engberg R. Rye bread enhances the production and plasma concentration of butyrate but not the plasma concentrations of glucose and insulin in pigs. J. Nutr. 2005;135:1696–1704. doi: 10.1093/jn/135.7.1696. [DOI] [PubMed] [Google Scholar]
- 46.Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann J., Hermes R., Breves G. Transepithelial transport and intraepithelial metabolism of short-chain fatty acids (SCFA) in the porcine proximal colon are influenced by SCFA concentration and luminal pH. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011;158:169–176. doi: 10.1016/j.cbpa.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Ingerslev A.K., Theil P.K., Hedemann M.S., Laerke H.N., Bach Knudsen K.E. Resistant starch and arabinoxylan augment SCFA absorption, but affect postprandial glucose and insulin responses differently. Br. J. Nutr. 2014;111:1564–1576. doi: 10.1017/S0007114513004066. [DOI] [PubMed] [Google Scholar]
- 49.Den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holman D.B., Brunelle B.W., Trachsel J., Allen H.K. Meta-analysis To Define a Core Microbiota in the Swine Gut. Msystems. 2017;2:e00004-17. doi: 10.1128/mSystems.00004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H., Ma L.T., Li Z.Q., Yin J., Tan B., Chen J.S., Jiang Q., Ma X.K. Evolution of the Gut Microbiota and Its Fermentation Characteristics of Ningxiang Pigs at the Young Stage. Animals. 2021;11:638. doi: 10.3390/ani11030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long C., de Vries S., Venema K. Differently Pre-treated Rapeseed Meals Affect in vitro Swine Gut Microbiota Composition. Front. Microbiol. 2020;11:570985. doi: 10.3389/fmicb.2020.570985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humphrey B., Zhao J., Faris R. Review: Link between intestinal immunity and practical approaches to swine nutrition. Animal. 2019;13:2736–2744. doi: 10.1017/S1751731119001861. [DOI] [PubMed] [Google Scholar]
- 54.Mu C., Zhang L., He X., Smidt H., Zhu W. Dietary fibres modulate the composition and activity of butyrate-producing bacteria in the large intestine of suckling piglets. Antonie Van Leeuwenhoek. 2017;110:687–696. doi: 10.1007/s10482-017-0836-4. [DOI] [PubMed] [Google Scholar]
- 55.Hong J., Ndou S.P., Adams S., Scaria J., Woyengo T.A. Canola meal in nursery pig diets: Growth performance and gut health. J. Anim. Sci. 2020;98:skaa338. doi: 10.1093/jas/skaa338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heo J.M., Opapeju F.O., Pluske J.R., Kim J.C., Hampson D.J., Nyachoti C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 57.Owusu-Asiedu A., Patience J.F., Laarveld B., Van Kessel A.G., Simmins P.H., Zijlstra R.T. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 2006;84:843–852. doi: 10.2527/2006.844843x. [DOI] [PubMed] [Google Scholar]
- 58.Pieper R., Tudela C.V., Taciak M., Bindelle J., Perez J.F., Zentek J. Health relevance of intestinal protein fermentation in young pigs. Anim. Health Res. Rev. 2016;17:137–147. doi: 10.1017/S1466252316000141. [DOI] [PubMed] [Google Scholar]
- 59.Mejicanos G., Sanjayan N., Kim I.H., Nyachoti C.M. Recent advances in canola meal utilization in swine nutrition. J. Anim. Sci. Technol. 2016;58:7. doi: 10.1186/s40781-016-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martínez I., Kim J., Duffy P.R., Schlegel V.L., Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J.B., Bai Y., Tao S.Y., Zhang G., Wang J.J., Liu L., Zhang S. Fiber-rich foods affected gut bacterial community and short-chain fatty acids production in pig model. J. Funct. Foods. 2019;57:266–274. doi: 10.1016/j.jff.2019.04.009. [DOI] [Google Scholar]
- 62.Lee J.W. Ph.D. Thesis. South Dakota State University; Brookings, SD, USA: 2019. Optimization of Canola Co-Product Utilization in Swine. [Google Scholar]
- 63.Guevarra R.B., Lee J.H., Lee S.H., Seok M.J., Kim D.W., Kang B.N., Johnson T.J., Isaacson R.E., Kim H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019;10:1. doi: 10.1186/s40104-018-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting reported results is contained within the article and the Supplementary Materials.