Abstract
Background
Tiotropium and ipratropium bromide are both recognised treatments in the management of people with stable chronic obstructive pulmonary disease (COPD). There are new studies which have compared tiotropium with ipratropium bromide, making an update necessary.
Objectives
To compare the relative effects of tiotropium to ipratropium bromide on markers of quality of life, exacerbations, symptoms, lung function and serious adverse events in patients with COPD using available randomised controlled trial (RCT) data.
Search methods
We identified RCTs from the Cochrane Airways Group Specialised Register of trials (CAGR) and ClinicalTrials.gov up to August 2015.
Selection criteria
We included parallel group RCTs of 12 weeks duration or longer comparing treatment with tiotropium with ipratropium bromide for patients with stable COPD.
Data collection and analysis
Two review authors independently assessed studies for inclusion and then extracted data on study quality and outcome results. We contacted trial sponsors for additional information. We analysed the data using Cochrane Review Manager.
Main results
This review included two studies of good methodological quality that enrolled 1073 participants with COPD. The studies used a similar design and inclusion criteria and were of at least 12 weeks duration; the participants had a mean forced expiratory volume in one second (FEV1) of 40% predicted value at baseline. One study used tiotropium via the HandiHaler (18 µg) for 12 months and the other via the Respimat device (5 µg and 10 µg) for 12 weeks. In general, the treatment groups were well matched at baseline but not all outcomes were reported for both studies. Overall the risk of bias across the included RCTs was low.
For primary outcomes this review found that at the three months trough (the lowest level measured before treatment) FEV1 significantly increased with tiotropium compared to ipratropium bromide (mean difference (MD) 109 mL; 95% confidence interval (CI) 81 to 137, moderate quality evidence, I2 = 62%). There were fewer people experiencing one or more non‐fatal serious adverse events on tiotropium compared to ipratropium (odds ratio (OR) 0.5; 95% CI 0.34 to 0.73, high quality evidence). This represents an absolute reduction in risk from 176 to 97 per 1000 people over three to 12 months. Concerning disease specific adverse events, the tiotropium group were also less likely to experience a COPD‐related serious adverse event when compared to ipratropium bromide (OR 0.59; 95% CI 0.41 to 0.85, moderate quality evidence).
For secondary outcomes, both studies reported fewer hospital admissions in the tiotropium group (OR 0.34; 95% CI 0.15 to 0.70, moderate quality evidence); as well as fewer patients experiencing one or more exacerbations leading to hospitalisation in the people on tiotropium in both studies (OR 0.56; 95% CI 0.31 to 0.99, moderate quality evidence). There was no significant difference in mortality between the treatments (OR 1.39; 95% CI 0.44 to 4.39, moderate quality evidence). One study measured quality of life using the St George's Respiratory Questionnaire (SGRQ); the mean SGRQ score at 52 weeks was lower in the tiotropium group than the ipratropium group (lower on the scale is favourable) (MD ‐3.30; 95% CI ‐5.63 to ‐0.97, moderate quality evidence). There were fewer participants suffering one of more exacerbations in the tiotropium arm (OR 0.71; 95% CI 0.52 to 0.95, high quality evidence) and there was also a reported difference in the mean number of exacerbations per person per year which reached statistical significance (MD ‐0.23; 95% CI ‐0.39 to ‐0.07, P = 0.006, moderate quality evidence). From the 1073 participants there were significantly fewer withdrawals from the tiotropium group (OR 0.58; 95% CI 0.41 to 0.83, high quality evidence).
Authors' conclusions
This review shows that tiotropium treatment, when compared with ipratropium bromide, was associated with improved lung function, fewer hospital admissions (including those for exacerbations of COPD), fewer exacerbations of COPD and improved quality of life. There were both fewer serious adverse events and disease specific events in the tiotropium group, but no significant difference in deaths with ipratropium bromide when compared to tiotropium. Thus, tiotropium appears to be a reasonable choice (instead of ipratropium bromide) for patients with stable COPD, as proposed in guidelines. A recent large double‐blind trial of the two delivery devices found no substantial difference in mortality using 2.5 µg or 5 µg of tiotropium via Respimat in comparison to 18 µg via Handihaler.
Plain language summary
Tiotropium versus ipratropium bromide in the management of COPD
Background
Chronic obstructive pulmonary disease (COPD) is a lung disease that includes the conditions chronic bronchitis and emphysema. COPD is mainly caused by smoking or inhaling dust, which leads to blockage or narrowing of the airways. The symptoms include breathlessness and a chronic cough. Tiotropium is an inhaled medication, taken once a day, to help widen the airways (bronchodilator) and is used in the management of COPD. Ipratropium bromide is also a bronchodilator but has a shorter duration of action and has to be taken several times a day.
What did we find?
We found two studies including 1073 participants that compared the long‐term effectiveness and side effects of tiotropium compared to ipratropium bromide. One trial was 12 weeks long and one was a year long. The people included in the studies had moderate to severe COPD (average forced expiratory volume in one second (FEV1) was 40% the predicted value).
Compared to ipratropium bromide, tiotropium treatment led to improved lung function, fewer COPD exacerbations, fewer hospital admissions (including those for exacerbations of COPD) and improved quality of life. Tiotropium appears to be safer with fewer adverse events, but there was no significant difference in deaths with ipratropium bromide when compared to tiotropium.
Quality of the evidence
Overall the evidence was of moderate to high quality. Tiotrpium is available in two different inhalers, Respimat and Handihaler. A recent large double‐blind trial of the two delivery devices found no substantial difference in mortality using 2.5 µg or 5 µg of tiotropium via Respimat in comparison to 18 µg via Handihaler.
Conclusions
Based on this review, tiotropium has more benefits than ipratropium bromide for people with stable moderate to severe COPD.
The review was current as of August 2015.
Summary of findings
Summary of findings for the main comparison. Tiotropium versus ipratropium for chronic obstructive pulmonary disease.
| Tiotropium versus ipratropium for chronic obstructive pulmonary disease | ||||||
| Patient or population: chronic obstructive pulmonary disease (COPD). Participants in included studies had an average baseline FEV1 of around 40% predicted and are regarded as having moderate to severe COPD Settings: community Intervention: tiotropium versus ipratropium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ipratropium | Tiotropium | |||||
|
Change in baseline trough FEV1 Follow‐up: 12 weeks |
The mean drop in trough FEV1 was 20 to 30 mL in the ipratropium group | The mean change from baseline in trough FEV1 in the tiotropium group was 109 mL better (80 to 137 better) | 1073 (2) | Moderate1 | MCID = 150 mL | |
|
All‐cause serious adverse events Follow‐up: 12 weeks to 12 months |
176 per 1000 |
97 per 1000 (68 to 135) |
OR 0.50 (0.34 to 0.73) | 1073 (2) | High | |
|
Hospital admissions (all‐cause) Follow‐up: 12 weeks |
84 per 1000 | 30 per 1000 (14 to 65) | OR 0.34 (0.15 to 0.76) | 538 (1) | Moderate2 | There were also fewer hospitalisation due to exacerbations in the tiotropium group OR 0.56 (95% CI 0.31 to 0.99) |
|
Mortality (all‐cause) Follow‐up: 12 weeks to 12 months |
11 per 1000 | 15 per 1000 (5 to 47) | OR 1.39 (0.44 to 4.39) | 1073 (2) | Moderate3 | |
|
Quality of life (measured with the SGRQ). The SGRQ scale runs from 0 to 100 and lower on the scale indicates a better quality of life. Follow‐up: 12 months |
The mean SGRQ score was 44 (SD 13) on ipratropium | The mean SGRQ in the tiotropium group was 3.3 better (0.97 to 5.63 better) | 535 (1) | Moderate2 | MCID = 4 points | |
|
Patients with one or more exacerbations Follow‐up: 12 weeks to 12 months |
297 per 1000 | 231 per 1000 (180 to 286) | OR 0.71 (0.52 to 0.95) | 1073 (2) | High | |
|
Withdrawals Follow‐up: 12 weeks to 12 months |
193 per 1000 | 122 per 1000 (89 to 166) | OR 0.58 (0.41 to 0.83) | 1073 (2) | High | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FEV1: forced expiratory volume in one second; OR: Odds ratio; MCID: minimal clinically important difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Heterogeneity present between the trial results (I2 = 62%)
2. Results from a single paper only, so heterogeneity could not be assessed
3. The number of participants and/or events were low, leading to wide CIs and imprecision in the result
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is characterised by airflow obstruction in the lungs. The airflow obstruction is usually progressive, not fully reversible, and does not change markedly over several months. Symptoms include progressive breathlessness on exertion and frequent lower respiratory tract infections. The disease is predominantly caused by smoking (NICE 2010). An estimated three million people are affected by COPD in the UK. About 900,000 have been diagnosed with COPD and an estimated two million people have COPD which remains undiagnosed (Healthcare Commission 2006). COPD is usually a progressive disease and lung function can be expected to worsen over time, even with the best available care (GOLD 2015). Management of the disease includes reduction of risk factors, pharmacological treatments, education, pulmonary rehabilitation and exercise programmes (Karner 2011b).
Description of the intervention
Medications for COPD are used to decrease symptoms or complications, or both. Bronchodilator medications are central to the management of COPD (GOLD 2015). Beta2‐agonists act directly on bronchial smooth muscle to cause bronchodilation whereas anticholinergics act by inhibiting resting bronchomotor tone. As well as improving breathlessness through their direct bronchodilator effects, both classes of drugs also appear to work by reducing hyperinflation (NICE 2010). Long‐acting bronchodilators are used to prevent and reduce symptoms in COPD. Inhaled anticholinergic agents, such as tiotropium, have been shown to improve symptoms, reduce exacerbations and improve quality of life in patients with COPD (Ogale 2010); they can be used alone or in combination with inhaled corticosteroids and long‐acting beta2‐agonists (LABA). Both of these inhaled therapies can be used alone or in combination (Karner 2011; Karner 2011a; Karner 2011b; Karner 2012).
Tiotropium
Tiotropium is a long‐acting (lasting for 24 hours) anticholinergic bronchodilator used in the management of COPD. Continuous treatment with tiotropium significantly reduces the number of exacerbations and the risk of hospitalisation due to exacerbations compared with placebo (Barr 2005; Karner 2012). Treatment trials in COPD show a greater benefit in symptom control and lung function obtained from tiotropium compared with either short‐acting anticholinergics (ipratropium) or LABA (Sears 2008). Tiotropium is intended as a maintenance treatment for COPD and is not indicated for the initial treatment of acute exacerbations. In the UK, a tiotropium inhaler (either HandiHaler (18 µg) or Respimat (5 µg)) may be introduced for once daily use for patients with frequent exacerbations, exertional breathlessness, or both, and with a diagnosis of COPD regardless of their lung function (NICE 2010).
Ipratropium bromide
The short‐acting (lasting six to eight hours) anticholinergic agent ipratropium bromide is used in both the long‐term and acute management of patients with COPD. In trials, it has been suggested to have equal or superior efficacy compared with LABA (Salpeter 2006). According to the British National Formulary (BNF) the optimal dosing is 20 to 40 µg three to four times a day.
How the intervention might work
Tiotropium
Tiotropium is a long‐acting anticholinergic agent which blocks the action of the neurotransmitter acetylcholine. It has similar affinity for the M1 to M5 subtypes of muscarinic (M) receptors. Activation of M3 receptors on airway smooth muscle leads to bronchoconstriction through stimulation of phospholipase C, which generates the formation of diacylglycol and inositol triphosphate. These in turn stimulate a number of intracellular signalling cascades leading to changes in intracellular calcium homeostasis and bronchoconstriction (Lubinski 2004).
Tiotropium exhibits pharmacological effects through the inhibition of these M3 receptors. Prevention of methacholine‐induced bronchoconstriction is dose dependent and lasts longer than 24 hours. The bronchodilation following inhalation of tiotropium is predominantly site specific.
Ipratropium bromide
Ipratropium bromide is a short‐acting anticholinergic, with effects lasting six to eight hours. It is a non‐selective muscarinic antagonist and therefore blocks M2 receptors as well as M1 and M3 receptors in airway smooth muscle and prevents the increases in intracellular concentrations of cyclic guanosine monophosphate (cyclic GMP). M2 receptors at cholinergic nerve endings inhibit the release of acetylcholine and therefore act as inhibitory feedback receptors. Inhibition of these receptors with ipratropium bromide results in increased acetylcholine release in the airways, which may overcome the blockade of other muscarinic receptors in the muscle (Barnes 2000).
Why it is important to do this review
Both tiotropium and ipratropium bromide are separately recommended for the treatment of COPD (NICE 2010; GOLD 2015). They both work by blocking M3 receptors. The long‐acting anticholinergic tiotropium has pharmacokinetic selectivity for M3 and M1 receptors, whereas the short‐acting drug also blocks M2 receptors, thought to be less important in COPD. Tiotropium has a much longer duration of action compared with ipratropium bromide (GOLD 2015). It has been suggested that the use of tiotropium may be more cost‐effective (NICE 2010). The review is necessary to examine the potential benefit of tiotropium compared with ipratropium bromide.
This review forms part of a suite of reviews comparing tiotropium with placebo, ipratropium, and long‐acting beta2‐agonists for the treatment of COPD (including Welsh 2010; Karner 2011; Karner 2011a; Karner 2011b).
Objectives
To compare the relative effects of tiotropium to ipratropium bromide on markers of quality of life, exacerbations, symptoms, lung function and serious adverse events in patients with COPD using available randomised controlled trial data.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of at least 12 weeks duration and with a parallel group design, both open label studies and blinded studies.
Types of participants
We included adult patients with a diagnosis of COPD as defined by an external set of diagnostic criteria (for example from the Global Initiative for Chronic Obstructive Lung Disease (GOLD), American Thoracic Society (ATS), British Thoracic Society (BTS) and the Thoracic Society of Australia and New Zealand (TSANZ)). Both reversible COPD (greater than 15% improvement in the forced expiratory volume in one second (FEV1) after a single dose of short or long‐acting bronchodilator) and non‐reversible COPD were included.
Types of interventions
We included patients randomised to receive tiotropium or ipratropium bromide. Participants were allowed inhaled steroids and other co‐medications provided they were not part of the randomised treatment.
Types of outcome measures
Primary outcomes
Trough forced expiratory volume in one second (FEV1) at three months
All‐cause non‐fatal serious adverse events (SAEs)
Disease specific serious adverse events, if independently adjudicated
We chose all‐cause SAEs in addition to disease specific SAEs because disease specific adverse events are at higher risk of ascertainment bias.
Secondary outcomes
Hospital admissions, all‐cause and due to exacerbations
Mortality, all‐cause
Quality of life (measured with a validated scale e.g. St George's Respiratory Questionnaire, Chronic Respiratory Disease Questionnaire)
Disease specific mortality, if independently adjudicated
Exacerbations, requiring short burst oral corticosteroids or antibiotics, or both
Symptoms (measures of breathlessness ‐ Baseline Dyspnea Index (BDI) and Transition Dyspnea Index (TDI))
Withdrawals
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1). All records in the Specialised Register coded as 'COPD' were searched using the following terms:
(tiotropium or Spiriva or "Ba 679 BR") AND (ipratropium or Atrovent or n‐isopropylatropine).
A search of ClinicalTrials.gov was also conducted using the same terms. All databases were searched from their inception with no restriction on language of publication. The previously published version of the review included searches up to November 2012. The search period for this update is November 2012 to August 2015.
Searching other resources
Reference lists of all primary studies and review articles were checked for additional references. Authors of identified trials were contacted and asked to identify other published and unpublished studies. Manufacturers and experts in the field were also contacted.
Data collection and analysis
Selection of studies
Two review authors (LC, MI) independently assessed all the potential studies identified as a result of the search strategy for inclusion in the review. Disagreement was resolved by discussion and a third person (JW) was arbiter.
Data extraction and management
Two review authors (LC, MI) independently extracted information from each study for the following characteristics.
Design (design, total study duration and run‐in, number of study centres and location, withdrawals, date of study).
Participants (number (N), mean age, age range, gender, COPD severity, diagnostic criteria, baseline lung function, co‐morbidities, concomitant medication, smoking history, inclusion criteria, exclusion criteria).
Interventions (run‐in, intervention treatment and inhaler type, control treatment and inhaler type).
Outcomes (primary and secondary outcomes specified and collected, time points reported).
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Any disagreement was resolved by discussion or by involving a third assessor. We assessed the risk of bias according to the following domains:
allocation sequence generation;
concealment of allocation;
blinding of participants and investigators;
incomplete outcome data;
selective outcome reporting.
Other sources of bias were noted. Each potential source of bias was graded as high, low, or unclear risk of bias.
Measures of treatment effect
Dichotomous data
We analysed dichotomous data variables (such as mortality and withdrawals) using odds ratios. If count data were not available as the number of participants experiencing an event, we analysed the data as continuous, time‐to‐event or rate ratios, depending on how the data were reported. This includes the outcomes: hospital admissions, exacerbations, serious adverse events and side effects.
Continuous data
We analysed continuous outcome data (such as FEV1 and quality of life) as fixed‐effect model mean differences when the same scale was used, and as standardised mean differences when different scales were employed in different studies. Mean difference based on change from baseline was preferred over mean difference based on absolute values.
If data were not available for the same time point in all studies, the closest time points were used. Alternatively, end of study was used as the time of analysis for all studies. We used an intention‐to‐treat (ITT) analysis of outcomes from all randomised participants for primary analyses, where possible.
Unit of analysis issues
We analysed dichotomous data using participants as the unit of analysis (rather than events) to avoid counting the same participant more than once. Where trials included two active treatment arms, we split the participants in the comparison arm to avoid double counting.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data, where possible.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity we planned to explored it by pre‐specified subgroup analysis.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact the study authors asking them to provide the missing outcome data. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Data synthesis
We combined dichotomous data using Mantel‐Haenzsel odds ratios with 95% confidence intervals, with a fixed‐effect model. Where events were rare, we employed the Peto odds ratio (since this does not require a continuity correction for zero cells).
Where treatment effects were reported as a mean difference with 95% confidence interval or exact P value, we calculated the standard error, entered it with the mean difference and combined the results using a fixed‐effect model Generic Inverse Variance (GIV) in RevMan 5.
Rate ratios and hazard ratios were combined using a fixed‐effect model GIV.
Numbers needed to treat were calculated from the pooled odds ratio and its confidence interval, and applied to appropriate levels of baseline risk.
We have presented the findings of our primary outcomes in a summary of findings table.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Tiotropium formulation or delivery device formulation.
Severity at baseline.
Concomitant medication.
Smoking status.
Co‐morbidity (i.e. vascular diseases, cardiac diseases, metabolic disease etc.).
Open and double‐blind trial design.
Sensitivity analysis
We planned to assess the sensitivity of our primary outcomes to degree of bias by comparing overall results with those exclusively from trials assessed as being at low risk of bias. We also planned to compare the results from the fixed‐effect model with results from the random‐effects model.
Results
Description of studies
Results of the search
The original search in November 2012 identified a total of 76 references We identified seven references for further appraisal. Two studies were included in the systematic review. The updated search in August 2015 identified a further 15 references. The full‐text of 3 trial reports were assessed for eligibility, but none were judged to be eligible for inclusion. (Figure 1).
1.

study flow diagram.
Included studies
Two separate studies that met the inclusion criteria were included (1073 participants), studying the effects of tiotropium compared with ipratropium bromide (Vincken 2002; Voshaar 2008).
Interventions
One study used the tiotropium HandiHaler 18 µg device for 12 months (Vincken 2002) and the other used both tiotropium 5 µg and 10 µg with the Respimat (soft mist) device for 12 weeks (Voshaar 2008). Both studies used an ipratropium bromide metered dose inhaler (MDI). Voshaar 2008 was technically two separate, identical RCTs, but the results were reported together. When results from these trials were meta‐analysed, we halved the number of participants in the control group to avoid double counting. Voshaar 2008 also included a placebo arm, which was not eligible for this review.
Participant characteristics
Inclusion criteria were similar for both of the studies with participants required to be 40 years or over and with a smoking history of equal to or greater than 10 pack years. A clinical diagnosis of COPD according to ATS guidance was used in Vincken 2002 and spirometric criteria were specified in Voshaar 2008 (FEV1 of ≤ 65% predicted and a FEV1/FVC ratio of ≤ 70%). The mean smoking history was 34 pack years in Vincken 2002 and 51 pack years in Voshaar 2008. COPD severity was described as moderate‐severe in both studies. The study participants had a mean age of 65 years and were predominately male. Theophylline, inhaled steroids and oral steroids (prednisolone) up to and including a dose of 10 mg (if the dose was stable for at least six weeks) were allowed in both studies. Inhaled beta2‐agonists and other anticholinergic drugs (not used in the study) were disallowed. We felt that the baseline data were well balanced between the treatment and control groups.
Not all outcomes were reported in the study reports and we obtained some information from the manufacturer.
Both studies were sponsored by Boehringer Ingelheim (BI) (the manufacturer of tiotropium). For further details see Characteristics of included studies.
Excluded studies
We excluded five studies after obtaining the full study reports. Studies were excluded due to be being less than 12 weeks in duration, including an ipratropium and albuterol combination, and not being conducted as an RCT. For further information see Characteristics of excluded studies.
Risk of bias in included studies
Full details of our 'Risk of bias' judgments can be found in Characteristics of included studies and in the summary graph in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation and allocation concealment were sufficiently described for Voshaar 2008. Allocation concealment was not described in Vincken 2002, but because the trial was run by a manufacturer it is safe to assume that the allocation sequence was protected (although we did not request this information from BI).
Blinding
Double‐blinding of participants and study personnel was described in both studies.
Incomplete outcome data
The withdrawal rates were relatively low for both studies.
Selective reporting
All specified outcomes were reported. Data on adverse events were gained from BI for both studies.
Other potential sources of bias
None identified
Effects of interventions
See: Table 1
SeeTable 1.
We had planned several subgroup analyses. Although we subgrouped by tiotropium inhaler type and dose, due to possible differences in the dose received, we did not perform any further subgroup analysis as there were insufficient data to allow this.
Primary outcome: forced expiratory volume in one second (FEV1) at three months
Combining the two studies on 1073 participants, trough FEV1 values at three months significantly increased with tiotropium compared to ipratropium bromide (mean difference (MD) 109 mL; 95% confidence interval (CI) 81 to 137, moderate quality evidence; Analysis 1.1). However, there was a high level of heterogeneity (I2 = 62%). In addition one study of 535 participants reported change in baseline trough FEV1 at 12 months (Vincken 2002). At 12 months, the trough FEV1 was 120 mL above baseline for patients on tiotropium and the trough FEV1 declined by 30 mL in participants on ipratropium (MD 150 mL; 95%CI 111 to 190; Analysis 1.2).
1.1. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 1 Change in baseline trough FEV1 at 3 months.
1.2. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 2 Change in baseline trough FEV1 at 12 months.
Primary outcome: all‐cause non‐fatal serious adverse events
Both included studies reported serious adverse events (1073 participants). There were fewer people experiencing one or more non‐fatal serious adverse events on tiotropium compared to ipratropium (odds ratio (OR) 0.50; 95% CI 0.34 to 0.73; Analysis 1.3). Specific details on cardiovascular events were not presented and at 12 weeks the Voshaar 2008 trial may be too short to assess long‐term cardiovascular risk.
1.3. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 3 Patients with at least one serious adverse event.
Primary outcome: disease specific serious adverse events
One study on 535 participants reported on serious adverse events related to COPD (Vincken 2002). There were fewer disease specific adverse events (described as COPD exacerbations) in the tiotropium group (OR 0.59; 95% CI 0.41 to 0.85, moderate quality evidence; Analysis 1.4).
1.4. Analysis.
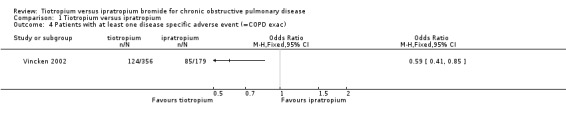
Comparison 1 Tiotropium versus ipratropium, Outcome 4 Patients with at least one disease specific adverse event (=COPD exac).
Secondary outcome: hospital admissions, all‐cause and due to exacerbations
There were fewer people experiencing one or more hospital admissions in the intervention group in Voshaar 2008 (OR 0.34; 95% CI 0.15 to 0.70, moderate quality evidence; Analysis 1.5). We were not able to obtain data for hospital admissions from Vincken 2002.
1.5. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 5 Patients with at least one hospital admission (all cause).
There were also fewer patients experiencing one or more exacerbations leading to hospitalisation in people on tiotropium (OR 0.56; 95% CI 0.31 to 0.99; Analysis 1.6); we were able to obtain data from both studies on this outcome.
1.6. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 6 Patients with at least one exacerbation requiring hospitalisation.
Secondary outcomes: mortality, all‐cause
All‐cause mortality was recorded in both studies (1073 participants). Overall, there was no statistically significant difference in the number of deaths between tiotropium and ipratropium (OR 1.39; 95% CI 0.44 to 4.39, moderate quality evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 7 All cause mortality.
Secondary outcomes: quality of life (QoL)
One study on 535 participants measured QoL using the SGRQ (Vincken 2002). The mean QoL score on the SGRQ at 52 weeks was lower in the tiotropium group than in the ipratropium group (lower on the scale is favourable) (MD ‐3.30; 95%CI ‐5.63 to ‐0.97, moderate quality evidence; Analysis 1.8). Four units difference is regarded as clinically significant.
1.8. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 8 SGRQ.
Secondary outcomes: disease specific mortality
Neither study reported disease specific mortality.
Secondary outcomes: exacerbations requiring short burst oral corticosteroids or antibiotics, or both
Both studies reported the number of people experiencing one or more COPD exacerbations but it was not clear how the exacerbations were defined. There were fewer participants suffering one or more exacerbations in the tiotropium arm (OR 0.71; 95% CI 0.52 to 0.95, high quality evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 10 Patients with one or more exacerbations.
Vincken 2002 also reported the difference in the mean number of exacerbations per person per year and this reached statistical significance (MD ‐0.23; 95% CI ‐0.39 to ‐0.07; P = 0.006; Analysis 1.11).
1.11. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 11 Mean number of exacerbations per patient per year.
Secondary outcomes: symptoms
One trial on 535 participants reported the baseline dyspnoea index (BDI) and transition dyspnoea index (TDI) (Vincken 2002). BDI was not significantly different for the treatments (MD ‐0.28; 95% CI ‐0.74 to 0.18; Analysis 1.12) but TDI did show a statistically significant difference in favour of tiotropium (MD 0.90; 95% CI 0.39 to 1.41; Analysis 1.13).
1.12. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 12 BDI.
1.13. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 13 TDI.
Secondary outcomes: withdrawals
Both studies reported withdrawals from their trials. From the 1073 participants there were significantly fewer withdrawals from the tiotropium group (OR 0.58; 95% CI 0.41 to 0.83, high quality evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 9 Withdrawals.
Discussion
Summary of main results
This systematic review set out to investigate the medium to long‐term (three months or longer) effects of tiotropium when compared to ipratropium bromide. We identified two eligible randomised, parallel group, placebo‐controlled trials with a total of 1073 participants and a mean duration of seven months. Both studies were sponsored by Boehringer Ingelheim (the manufacturer of tiotropium) and both studies had participants with a mean FEV1 of 40% predicted at baseline. The studies were of high methodical quality with relatively low withdrawal rates, which were balanced between treatment arms. We found that, compared to ipratropium bromide, treatment with tiotropium led to a significant increase in trough FEV1 at three months. On one hand this is not surprising given that tiotropium is a long‐acting bronchodilator and ipratropium is not, however trough FEV1 is the best measure for long‐acting bronchodilators because other outcome benefits may be conferred due to improvements in FEV1 over the course of 24 hours. Significantly more adverse events and disease specific events were seen in the ipratropium group (although events remain low for both groups). Of the secondary outcomes, tiotropium showed fewer hospital admissions due to exacerbations of COPD and exacerbation of COPD not requiring hospitalisation. There was no significant difference in all‐cause mortality between tiotropium and ipratropium, although disease specific mortality was not recorded. Quality of life was better in the tiotropium group but this did not reach the threshold for clinical significance, which is four units on the SGRQ. Whilst there was an effect seen in symptom indices using TDI, this was not seen using BDI. There were fewer withdrawals in the intervention arm.
Overall completeness and applicability of evidence
Tiotropium has been on the market for several years. It was approved in Europe in 2002 and the United States in 2004. To date, numerous trials on tiotropium have been completed. Despite this there have only been two good quality studies comparing tiotropium and ipratropium. Both trials are described in this review and are of high methodological standard, giving good evidence regarding the relative risks and benefits of tiotropium treatment. Ideally further research should be done.
The increase in trough FEV1 at 12 weeks for tiotropium compared to ipratropium would suggest that tiotropium may have advantages over ipratropium bromide, and that it would be suitable for those patients diagnosed with COPD who have exertional breathlessness, frequent infections and worsening lung function.
This review does not show any significant difference in mortality with the use of tiotropium in comparison with ipratropium (although cardiovascular risk was not looked at specifically, and Voshaar 2008 only presented 12 weeks of data). In addition a systematic review, including 19,545 randomised patients in studies of four weeks or longer, showed that tiotropium was associated with a reduction in the risk of serious cardiovascular events (Celli 2010). When we compared the two trials (Vincken 2002; Voshaar 2008) withdrawal rates where lower in Voshaar 2008 (which used the soft mist inhaler) when compared to Vincken 2002 (dry powder inhaler) and the mortality rates showed no significant difference between the tiotropium dry powder and soft mist inhaler. Based on the limited data available from the two studies included in this review we cannot recommend one inhaler over the other.
Recently, a large, industry‐supported prospective randomised, double blind study has found no significant increase in the risk of death or major cardiovascular adverse events with soft mist (Respimat 2.5 µg or 5 µg) compared with dry powder (Handihaler 18 µg) delivery devices for tiotropium (Wise 2013). Over 17,000 participants were followed for a mean period of 2.3 years. The study included patients with a history of cardiac disease and stable heart failure, but patients were not included in the study if they had a recent heart attack, serious arrhythmia, cancer therapy or drug and alcohol abuse. The trial did not include a placebo group, so we cannot assess whether tiotropium provided a benefit on overall mortality. All three treatment arms had a similar effect on reducing exacerbations. The trial followed up vital status for over 99% of the people who were randomised, but was subject to withdrawal rates of 21‐23% in all arms. Nevertheless the Wise 2013 trial provides the least biased evidence currently available, and has allayed some of the concerns of differences in mortality between the delivery devices (at the doses used and in the population studied).
A further limitation is that the participants in both trials had a mean FEV1 of 40% predicted, and many were already taking inhaled corticosteroids. This means that the findings are applicable to patients with moderate to severe COPD.
The review supports that fewer hospital admissions and COPD exacerbations occur with tiotropium when compared to ipratropium in the patient population recruited to the two included studies. Since serious adverse events are largely defined by admission to hospital, it is not possible to separate out the beneficial effects of tiotropium in reducing exacerbations from the assessment of all‐cause serious adverse events. The disease specific serious adverse event data provided by the manufacturers were descried as related to COPD exacerbations.
Quality of the evidence
The studies included in this review were of high methodological quality. Both were sponsored by Boehringer Ingelheim and were conducted with similar protocols and definitions.
Potential biases in the review process
We performed comprehensive searches to identify relevant studies. We contacted Boehringer Ingelheim to supply missing data. They supplied the majority of the missing data requested, where available.
Agreements and disagreements with other studies or reviews
Our results are comparable with other reviews looking at tiotropium versus ipratropium bromide for COPD.
A systematic review by Yohannes 2011 looked at the effectiveness of tiotropium versus placebo, ipratropium or LABA. The review had similar selection criteria to our review and identified the same two studies. The review reported comparable results with improved quality of life (OR 2.03; 95% CI 1.34 to 3.07; P = 0.001), improved symptoms using TDI (OR 2.10; 95% CI 1.28 to 3.44; P = 0.003), a non‐significant reduction in hospitalisations related to exacerbations (OR 0.59; 95% CI 0.32 to 1.09; P = 0.09) and overall exacerbations of COPD (OR 0.64; 95% CI 0.44 to 0.92; P = 0.02). They also showed no statistically significant difference in the number of patients experiencing a serious adverse event (OR 1.06; 95% CI 0.97 to 1.17).
Celli 2010 is a safety review of Boehringer Ingelheim‐sponsored tiotropium trials (19,545 patients). The review only included studies comparing tiotropium to placebo and therefore did not include any studies from this review. The pooled result showed a significant decrease in both fatal (RR 0.88; 95% CI 0.77 to 1.00) and serious adverse events (RR 0.94; 95% CI 0.89 to 1.00), including fatal events with tiotropium. Meta‐analysis of the cardiovascular data from these trials showed tiotropium to be associated with a reduction in major cardiovascular events (RR 0.83; 95% CI 0.71 to 0.98) and fatal cardiovascular events (RR 0.77; 95% CI 0.60 to 0.98) when compared to placebo. The cardiovascular composite endpoint included fatal events in the system organ classes cardiac and vascular disorders combined with myocardial infarction (fatal and non‐fatal), stroke (fatal and non‐fatal), and the preferred terms sudden death, sudden cardiac death, and cardiac death.
Kesten 2009 is another safety review of Boehringer Ingelheim‐sponsored tiotropium trials but only covered trials using the dry powder inhaler compared to placebo. It included 24 trials with a minimum of four weeks duration. None were included in our review. Presenting the data as a risk difference per 100 patient‐years at risk showed a significantly lowered risk of mortality with tiotropium compared to placebo (RD ‐0.63; 95% CI ‐1.14 to ‐0.12) (Kesten 2009). Kesten 2009 found a statistically significant decrease in serious adverse events (RD ‐1.41; 95% CI ‐2.81 to ‐0.00) using tiotropium dry powder inhaler.
Two recent meta‐analyses and systematic reviews by Singh et al (Singh 2008; Singh 2013) have looked at the cardiovascular risk with anticholinergic drugs. Singh 2013 found that people with known rhythm and cardiac disorders at baseline had a higher risk of cardiac death (RR 8.6; 95% CI 1.1 to 67.2).
A further meta‐analysis (Dong 2013) with the fixed‐effect model indicated that the tiotropium soft mist inhaler was associated with a universally increased risk of overall death compared with placebo (OR 1.51; 95% CI 1.06 to 2.19), the tiotropium HandiHaler (OR 1.65; 95% CI 1.13 to 2.43), LABA (OR 1.63; 95% CI 1.10 to 2.44) and LABA‐inhaled corticosteroid (ICS) (OR 1.90; 95% CI 1.28 to 2.86). The risk was more evident for cardiovascular death, in patients with severe COPD, and at a higher daily dose.
Another systematic review has looked at direct comparisons between the soft mist inhaler and other inhaler devices (Ram 2011). It found three studies looking at the difference between tiotropium via the soft mist inhaler and tiotropium via the dry powder inhaler. These were short‐term (three to four weeks) cross‐over or parallel group trials. They showed no statistically significant difference between soft mist and dry powder inhalers in the risk of exacerbation (715 patients, RR 0.94; 95% CI 0.58 to 1.54). The results of a large head‐to‐head study comparing the dry powder and the soft mist inhaler for tiotropium have now been reported (Wise 2013) and have not confirmed any important difference in mortality between the soft mist and dry powder inhalers.
Authors' conclusions
Implications for practice.
Compared with ipratropium bromide, tiotropium treatment was associated with an improvement in lung function. It did not appear to increase numbers of serious adverse events. Improvement in COPD patients' quality of life was seen along with a reduction in the risk of exacerbations, including exacerbations leading to hospitalisation.
This review confirms the recommendations for the use of tiotropium in place of ipratropium bromide in the management of patients with stable COPD, but acknowledges that evidence is scare and further research is advised. A recent large double‐blind trial of the two delivery devices found no substantial difference in mortality using 2.5 µg or 5 µg of tiotropium via Respimat in comparison to 18 µg via Handihaler.
Implications for research.
Because of the high and often uneven withdrawal rates in COPD trials, new trials should follow up the vital status of all participants, even if they have withdrawn from the study.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2015 | New search has been performed | New literature search run. No new included studies. |
| 18 August 2015 | New citation required and conclusions have changed | Added information about head‐to‐head trial of tiotropium respimat versus tiotropium handihaler. |
Notes
None
Acknowledgements
We are grateful to Elizabeth Stovold for help in designing our search strategy. We thank Emma Welsh, Managing Editor at Cochrane Airways for screening the literature search for the updated review and amending the text throughout. We thank Chris Cates for providing wording on the Wise 2013 head‐to‐head trial of tiotropium respimat vs handihaler.
We are deeply grateful to Dr Emma Welsh and Chris Cates for all their help and support whilst producing this review.
Boehringer Ingleheim has been helpful clarifying and supplying additional information about studies it has sponsored.
Chris Cates was the Editor for this review and commented critically on the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. Tiotropium versus ipratropium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in baseline trough FEV1 at 3 months | 2 | Mean Difference (Fixed, 95% CI) | 108.87 [80.37, 137.37] | |
| 1.1 Tiotropium Handihaler | 1 | Mean Difference (Fixed, 95% CI) | 142.0 [100.42, 183.58] | |
| 1.2 Tiotropium Respimat 5 | 1 | Mean Difference (Fixed, 95% CI) | 64.0 [8.63, 119.37] | |
| 1.3 Tiotropium Respimat 10 | 1 | Mean Difference (Fixed, 95% CI) | 95.0 [39.63, 150.37] | |
| 2 Change in baseline trough FEV1 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Tiotropium18 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Patients with at least one serious adverse event | 2 | 1073 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.34, 0.73] |
| 3.1 Tiotropium18 | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.85] |
| 3.2 Tiotropium5 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.79] |
| 3.3 Tiotropium10 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.20, 1.34] |
| 4 Patients with at least one disease specific adverse event (=COPD exac) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Patients with at least one hospital admission (all cause) | 1 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.76] |
| 5.1 Tiotropium5 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.79] |
| 5.2 Tiotropium10 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.17, 1.30] |
| 6 Patients with at least one exacerbation requiring hospitalisation | 2 | 1073 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.31, 0.99] |
| 6.1 Tiotropium5 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.03, 7.95] |
| 6.2 Tiotropium18 | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.09] |
| 6.3 Tiotropium10 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.02, 2.72] |
| 7 All cause mortality | 2 | 1073 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.44, 4.39] |
| 7.1 Tiotropium18 | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.41, 5.69] |
| 7.2 Tiotropium5+10 | 1 | 538 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.09, 10.98] |
| 8 SGRQ | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Withdrawals | 2 | 1073 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.41, 0.83] |
| 9.1 Tiotropium18 | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.05] |
| 9.2 Tiotropium5 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 1.03] |
| 9.3 Tiotropium10 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.05] |
| 10 Patients with one or more exacerbations | 2 | 1073 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.95] |
| 10.1 Tiotropium18 | 1 | 535 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.92] |
| 10.2 Tiotropium5 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.30, 1.47] |
| 10.3 Tiotropium10 | 1 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.52, 2.26] |
| 11 Mean number of exacerbations per patient per year | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 12 BDI | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 TDI | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Change from baseline in total SGRQ score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
1.14. Analysis.

Comparison 1 Tiotropium versus ipratropium, Outcome 14 Change from baseline in total SGRQ score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Vincken 2002.
| Methods | Randomised, double‐blind, double‐dummy, parallel group study (2 separate RCTs reported as one study) in 29 centres in the Netherlands (85%) and Belgium (15%) between October 1996 and June 1998. 12 months | |
| Participants | Participants: n= 356 (tiotropium), n= 179 (ipratropium bromide) Baseline characteristics: mean age (tiotropium 63.6, ipratropium bromide 64.5: gender (tiotropium 84% male, ipratropium bromide 86% male: mean % predicted FEV1 (tiotropium 41.9, ipratropium bromide 39.4): mean smoking pack year history (tiotropium 34.3, ipratropium bromide 33.2). Mean baseline FEV1 40% predicted Diagnostic criteria: as inclusion criteria COPD severity: moderate to severe Inclusion criteria: clinical diagnosis of COPD according to ATS criteria and stable airways obstruction with FEV1 of ≤ 65% predicted and a FEV1/FVC ratio of ≤ 70%. They had to be at least 40 years of age and all had to be current or previous smoker (≥ 10 pack years) Exclusion criteria: patients were excluded if they had a history of asthma, allergic rhinitis, atopy, or an increased total blood eosinophil count, a significant disease other than COPD. In addition, patients were excluded if they were on oxygen therapy or had a recent upper respiratory tract infection |
|
| Interventions | Run‐in period: 2‐week run‐in period, ITT Intervenions: 1st group: tiotropium 18 mcg once daily (Spiriva HandiHaler taken between 8 am and 10 am) and ipratropium matched placebo four times daily. Two thirds of patients in this group 2nd group: tiotropium matched placebo once daily and ipratropium 40 mcg four times daily (Atrovent taken between 8am and 10am firstly, lunch, dinner and when going to bed). One third of patients in this group Concominat medication: patients were permitted salbutamol MDI (100 mcg per actuation) as needed for acute symptom relief. Other beta2‐agonists (long‐ and short‐acting) and inhaled anticholinergic medications (other than study drugs) were not permitted. Concomitant use of theophylline, inhaled steroids and oral steroids (at a dose of ≤ 10 mg/day prednisolone or equivalent) was allowed if the dosage was stable for ≥ 6 weeks before screening |
|
| Outcomes | Collected outcomes: Spirometry was conducted in the outpatient clinic at screening and on day 1 (randomisation) as well as after 1, 7, 13, 26, 39 and 52 weeks of therapy. Testing was performed 1h before drug administration, immediately before drug administration, and at 30, 60, 120 and 180 min after dosing (and at 240, 300 and 360 min on day 1 and at 1, 7 and 13 weeks). The highest FEV1 and FVC from three technically adequate measurements were retained. PEFR were self‐measured by patients twice daily (upon arising and at bedtime) using a Mini‐Wright Peak Flow Meter (Clement Clarke International, Harlow, UK). Patients were requested to record the best of three efforts. Patients also recorded the number of puffs of 'as needed' salbutamol used. Dyspnoea was evaluated using the BDI and the TDI. HRQOL was determined using the SGRQ and the SF‐36. No primary endpoint identified Reported outcomes: Trough FEV1 and FVC at 1 year FEV1 at day 1, and after 1 and 52 weeks FVC at day 1 and after 1, 7, 13, 26, 39 and 52 weeks of therapy Baseline morning and evening PEFR and comparable values throughout the 1 year period Mean BDI and TDI on all test days Mean SGRQ and SF‐36 on all test days COPD exacerbations and adverse events Concomitant use of medication during the treatment period |
|
| Notes | Safety assessments included a complete blood count, biochemistry, urinalysis and electrocardiogram on entry and upon completion of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each patient within each centre was randomly assigned to one of the two treatment groups with two thirds of the patients assigned to the tiotropium group and one third to the ipratropium group. Boehringer Ingelheim Pharma KG generated the randomisation list using the internal programme BEA 01 running on a HP 1000 computer. The randomised list was based on a block size of three |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The double‐dummy feature prevented both investigators and patients from differentiating active drug from placebo. Both arms used both inhaler devices and therefore were completely blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | When a patient could not continue in the study because of deterioration of COPD, the missing efficacy data were estimated using the least favourable data observed prior to withdrawal from the study. For patients who missed study visits for other reasons, missing data were estimated using the patient's last observed data. For patients who did not complete all the pulmonary function measurements on a specific pulmonary function test day, linear interpolation was used to estimate random, missing, or middle spirometric measurements. Likewise, the minimum observed spirometric measurements on a specific test day were used to estimate values at the end of profiles that were missing because rescue medication was taken. Finally, the last available spirometric measurements were used to estimate values at the end of the profiles that were missing for reasons unrelated to the patient's treatment response The withdrawal rates were relatively low and were balanced between arms (tiotropium 18 mcg 10% and ipratropium 11%) |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
Voshaar 2008.
| Methods | Randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled, parallel group studies in 39 centres across Germany, Italy, South Africa and Switzerland and in 25 centres across the USA and Canada from November 2002 to December 2003. 2 identical 12 week trials | |
| Participants | Participants: n= 180 (tiotropium 5 mcg), n=180 (tiotropium 10 mcg), n= 178 (ipratropium bromide 36 mcg) Baseline characteristics: mean age (tiotropium 5 mcg 64, tiotropium 10 mcg 64, ipratropium bromide 65: gender (tiotropium 5 mcg 69% male, tiotropium 10 mcg 72% male, ipratropium bromide 67% male: mean %predicted FEV1 (tiotropium mcg 40, tiotropium 10 mcg 39, ipratropium bromide 41): mean smoking pack year history (tiotropium 5 mcg 52, tiotropium 10 mcg 53 ipratropium bromide 48). Mean baseline FEV1 40% predicted Diagnostic criteria: as inclusion criteria COPD severity: moderate to severe Inclusion criteria: clinical diagnosis of COPD according to ATS criteria and stable airways obstruction with FEV1 of ≤ 65% predicted and a FEV1/FVC ratio of ≤ 70%. They had to be at least 40 years of age and all had to be current or previous smokers (≥10 pack years) Exclusion criteria: patients were excluded if they had a history of asthma, allergic rhinitis, atopy, or an increased total blood eosinophil count, a significant disease other than COPD. In addition, patients were excluded if they were on oxygen therapy or had a recent upper respiratory tract infection |
|
| Interventions | Run‐in period: 2 week run‐in period Interventions: Group 1: tiotropium 5 mcg delivered via SPIRIVA Respimat SMI once daily and placebo delivered via pMDI four times daily Group 2: tiotropium 10 mcg delivered via SPIRIVA Respimat SMI once daily and placebo delivered via pMDI four times daily Group 3: ipratropium bromide 36 mcg, delivered via pMDI four times daily and 2 inhalations of placebo Respimat Group 4: placebo delivered via pMDI four times daily and via Respimats SMI once daily Concomitant medication: rescue medication (salbutamol pMDI) was permitted as needed during the study. Oral corticosteroids (equivalent of < 10 mg prednisone per day), orally inhaled corticosteroids, theophylline and mucolytics were allowed if stabilized for at least 6 weeks prior to and throughout the study. Oral beta2‐adrenergics and other investigational drugs were not allowed for at least 1 month prior to run‐in. Cromolyn sodium and nedocromil sodium were not allowed for at least 3 months prior to run‐in. Anticholinergics, inhaled beta2‐adrenergics other than salbutamol or fixed combination inhalers were also not allowed during the treatment period. |
|
| Outcomes | Collected outcomes: The primary endpoint was the change in trough (i.e. morning pre‐dose) FEV1 after 12 weeks of treatment Secondary spirometry endpoints included FVC, PEFR and the number of patients achieving a 15% increase above baseline (i.e. pre‐dose on test Day 1) in FEV1. Spirometry measures were performed (75) min pre‐dose and up to 6 h post‐dose. PEFR was recorded in patient diary cards. Other secondary endpoints included the weekly mean number of occasions per day that rescue medication (salbutamol) was used; the severity of COPD symptoms (i.e., wheezing, shortness of breath, coughing and tightness of chest), which was based on the physician’s assessment of the patient’s condition during the week prior to a clinic visit, and was rated from 0 (not present) to 3 (severe); the physician’s global evaluation of the patient’s condition, which was rated on an 8‐point scale from poor (1–2) to excellent. Reported outcomes: Trough FEV1 Post‐dose FEV1 AUC 0‐6 h Post‐dose FEV1 peak 0‐6 h FVC PEFR Rescue medication use COPD symptoms Safety assessments ≥15% increase in FEV1 above baseline from 2 h of dosing |
|
| Notes | Safety was assessed by adverse events, vital signs, 12‐lead ECG, routine laboratory tests and physical examination. Funding BI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by BI using a validated system, which involved a pseudo‐random number generator so that the resulting treatment sequence was both reproducible and non‐predictable |
| Allocation concealment (selection bias) | Low risk | All investigational medication for each patient was identified by a unique medication number. Each eligible patient was assigned the lowest medication number available to the investigator at the time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The double‐dummy feature prevented both investigators and patients from differentiating active drug from placebo, despite the different inhaler devices, which could otherwise not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In all studies, a selection of standard respiratory endpoints like pulmonary function, SGRQ, TDI, treadmill, exacerbations, etc. were used. Outcome assessors remained blinded with regard to the treatment assignments up to database lock |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The withdrawal rates were relatively low (tiotropium 5 mcg 8.9%, tiotropium 10 mcg 10%, and placebo 12.2%) |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
ATS: American Thoracic Society; AUC: area under the curve; BDI: Baseline Dyspnea Index; COPD: chronic obstructive pulmonary disease; ECG: electrocardiogram; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HRQOL: health related quality of life; ITT: intention tro treat; MDI: metered dose inhaler; PEFR: peak expiratory flow rate; pMDI: pressurised metered dose inhaler; RCT: randomised controlled trials; SF‐36 Medical Outcomes Study 36‐Item Short‐Form Health Survey; SGRQ: St George's Respiratory Questionnaire; SMI: soft mist inhaler; TDI: Transition Dyspnea Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kerwin 2013 | Crossover |
| Kim 2005 | Less than 12 weeks duration |
| NCT02172443 | Less than 12 weeks duration |
| NCT02172469 | Less than 12 weeks duration |
| Niewoehner 2009 | Combined ipratropium and albuterol |
| Serby 2002 | Not an RCT |
| Wang 2007 | Less than 12 weeks duration |
| Zheng 2006 | Less than 12 weeks duration |
Contributions of authors
LC initiated the protocol. LC and MI drafted the protocol. LC wrote the review.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Other.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
LC and MI: none known.
JW has received funding from Boehringer Ingleheim and Pfizer to provide an educational session in primary care on the management of respiratory conditions.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Vincken 2002 {published data only}
- Oostenbrink JB, Rutten‐van Molken MP, Al MJ, Noord JA, Vincken W. One‐year cost‐effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. European Respiratory Journal 2004;23(2):241‐9. [DOI] [PubMed] [Google Scholar]
- Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJG. A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. Thorax 2000;55(4):289‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincken W, Noord JA, Greefhorst A, Bantje TA, Kesten S, Korducki L, et al. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. European Respiratory Journal 2002;19(2):209‐16. [DOI] [PubMed] [Google Scholar]
Voshaar 2008 {published data only}
- 205.251. SPIRIVA ® – Pulmonary Disease, Chronic Obstructive (Clinical trial 205.251). http://trials.boehringer‐ingelheim.com/trial_results/clinical_trials_overview/205/205_251.html (accessed 2 September 2015).
- 205.252. SPIRIVA ® – Pulmonary Disease, Chronic Obstructive. http://trials.boehringer‐ingelheim.com/trial_results/clinical_trials_overview/205/205_252.html (accessed 2 September 2015).
- Voshaar T, Lapidus R, Maleki‐Yazdi R, Timmer W, Rubin E, Lowe L, et al. A randomized study of tiotropium Respimats Soft MistTM Inhaler vs. ipratropium pMDI in COPD. Respiratory Medicine 2008;102:32‐41. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kerwin 2013 {unpublished data only}
- Kerwin EM, Fogarty C, Dunn K, Singh D, Tutuncu A. Cardiovascular safety of nebulized glycopyrrolate (SUN‐101) compared with tiotropium, ipratropium and placebo in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187:A1483. [Google Scholar]
Kim 2005 {published data only}
- Kim SJ, Kim MS, Lee SH, Kim YK, Moon HS, Park SH, et al. A comparison of tiotropium 18mug, once daily and ipratropium 40mug, 4 times daily in a double‐blind, double‐dummy, efficacy and safety study in adults with chronic obstructive pulmonary disease. Tuberculosis and Respiratory Diseases 2005;58(5):498‐506. [Google Scholar]
NCT02172443 {unpublished data only}
- NCT02172443. Comparison of 18 mcg of tiotropium inhalation capsules and atrovent metered dose inhaler (2 puffs of 20 mcg) in a double blind, double dummy, efficacy and safety study in adults with chronic obstructive pulmonary disease (COPD). https://clinicaltrials.gov/ct2/show/NCT02172443 (accessed 1 September 2015).
NCT02172469 {unpublished data only}
- NCT02172469. A comparison of 18 µg of tiotropium inhalation capsules and Atrovent® metered dose inhaler (2 puffs of 20 µg, 4 times daily) in a double‐blind, double‐dummy, efficacy and safety study in adults with chronic obstructive pulmonary disease (COPD). https://clinicaltrials.gov/ct2/show/study/NCT02172469 (accessed 1 September 2015).
Niewoehner 2009 {published data only}
- Niewoehner DE, Lapidus R, Cote C, Sharafkhaneh A, Plautz M, Johnson P, et al. Therapeutic conversion of the combination of ipratropium and albuterol to tiotropium in patients with chronic obstructive pulmonary disease. Pulmonary Pharmacology and Therapeutics 2009;22(6):587‐92. [DOI] [PubMed] [Google Scholar]
Serby 2002 {published data only}
- Serby CW, Schwartzstein RM, Jones PW, Ries AL, Killian KJ. Tiotropium: 1‐Yr studies versus placebo/ipratropium. European Respiratory Review 2002;12(82):40‐2. [Google Scholar]
Wang 2007 {published data only}
- Wang HY, Xiao Y, Shi LL, Gong Q, Wen ZG. Study on efficacy and safety of tiotropium powder in patients with stable COPD. Chinese Journal of New Drugs 2007;16(14):1119‐22. [Google Scholar]
Zheng 2006 {published data only}
- Zheng JP, Kang J, Cai BQ, Zhou X, Cao ZL, Bai CX, et al. Comparison of tiotropium inhalation capsules and ipratropium metered dose inhaler in a randomized, double‐blind, double‐dummy, efficacy and safety study in patients with chronic obstructive pulmonary disease. Chinese Journal of Tuberculosis and Respiratory Diseases 2006;29(6):363‐7. [PubMed] [Google Scholar]
Additional references
Barnes 2000
- Barnes P. The pharmacological properties of tiotropium. Chest 2000;117:63S. [DOI] [PubMed] [Google Scholar]
Barr 2005
- Barr RG, Bourbeau J, Camargo Carlos A. Tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF
- British National Formulary. www.bnf.org (accessed 1 November 2011).
Celli 2010
- Celli B, Decramer M, Leimer I, Vogel U, Kesten S, Tashkin DP. Cardiovascular safety of tiotropium in patients with COPD. Chest 2010;137(1):20‐30. [DOI] [PubMed] [Google Scholar]
Dong 2013
- Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta‐analysis of randomised controlled trials. Thorax 2013;68(1):48‐56. [DOI] [PubMed] [Google Scholar]
GOLD 2015
- GOLD. From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Apr2.pdf (accessed 2 September 2015).
Healthcare Commission 2006
- Great Britain. Commission for Healthcare Audit and Inspection. Clearing the air: A national study of chronic obstructive pulmonary disease. Healthcare Commission. The Healthcare Commission (Jun. 2006), 2006. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Karner 2011
- Karner C, Cates CJ. The added effect of inhaled corticosteroids to tiotropium and long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009039] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karner 2011a
- Karner C, Cates CJ. Combination inhaled steroid and long‐acting beta2‐agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD008532.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karner 2011b
- Karner C, Cates CJ. Long‐acting beta2‐agonist in addition to tiotropium versus either tiotropium or long‐acting beta2‐agonist alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008989] [DOI] [Google Scholar]
Karner 2012
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009285.pub2] [DOI] [PubMed] [Google Scholar]
Kesten 2009
- Kesten S, Celli B, Decramer M, Leimer I, Tashkin D. Tiotropium HandiHaler® in the treatment of COPD: A safety review. International Journal of Chronic Obstructive Pulmonary Disease 2009;4:397‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lubinski 2004
- Lubinski W. Tiotropium as a controller of bronchoconstriction. Polski Merkuriusz Lekarski 2004;16:75‐76, 78. [PubMed] [Google Scholar]
NICE 2010
- NICE. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update). https://www.nice.org.uk/guidance/cg101 (accessed 2 September 2015).
Ogale 2010
- Ogale SS, Lee TA, Au DH, Boudreau DM, Sullivan SD. Cardiovascular events associated with ipratropium bromide in COPD. Chest 2010;137(1):13‐9. [0012‐3692] [DOI] [PubMed] [Google Scholar]
Ram 2011
- Ram FS. Tiotropium mist inhaler for COPD increases risk of mortality compared with placebo. Evidence Based Medicine 2011;16(6):189‐90. [DOI] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salpeter 2006
- Salpeter R, Buckley N, Salpeter E. Meta‐analysis: Anticholinergics, but not beta agonists, reduce severe exacerbations and respiratory mortality in COPD. Journal of General Internal Medicine 2006;21(10):1011‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sears 2008
- Sears M, Hamilton M. Long‐acting bronchodilators in COPD. Chest 2008;133(5):1057‐8. [DOI] [PubMed] [Google Scholar]
Singh 2008
- Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. JAMA 2008;300(12):1439‐50. [DOI] [PubMed] [Google Scholar]
Singh 2013
- Singh S, Loke YK, Enright P, Furberg CD. Pro‐arrhythmic and pro‐ischaemic effects of inhaled anticholinergic medications. Thorax 2013;68(1):114‐6. [DOI] [PubMed] [Google Scholar]
Welsh 2010
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007891.pub2] [DOI] [PubMed] [Google Scholar]
Wise 2013
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. Tiotropium respimat inhaler and the risk of death in COPD. New England Journal of Medicine 2013;369(16):1491‐501. [DOI] [PubMed] [Google Scholar]
Yohannes 2011
- Yohannes AW, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: A meta‐analysis of clinically relevant outcomes. Respiratory Care 2011;56(4):477‐87. [DOI] [PubMed] [Google Scholar]


