Abstract
Recent reports on some methicillin-resistant Staphylococcus aureus (MRSA) with reduced susceptibility to vancomycin have been a major concern in Korea because of the widespread use of vancomycin due to a high prevalence of MRSA in the country. We describe a 45-year-old man with long-standing pelvic abscess due to MRSA. In spite of vancomycin and teicoplanin treatment for a long period of time, the patient died from MRSA sepsis. The blood culture isolate of MRSA exhibited reduced susceptibility to vancomycin (MIC, 8 μg/ml). This is the first report of a vancomycin-intermediate S. aureus case from Korea.
Case report.
A 45-year-old man from a Seoul suburb who had sigmoid colon cancer was admitted to Asan Medical Center in April 1997. The patient had signs of liver metastasis and bowel perforation on abdominopelvic computerized tomography and was discharged with a scheduling for chemotherapy after diagnostic workup. He was readmitted 10 days later due to fever, chills, and an abdominal mass; his blood culture grew Bacteroides uniformis, and fever continued spiking above 38°C in spite of antibiotic therapy with amikacin, ceftizoxime, and metronidazole for 2 weeks. He had a transverse loop colostomy at hospital day (HD) 19; a huge tumor mass was adhered to the abdominal wall in the sigmoid colon, but no evidence of a perforation of the sigmoid colon or abscesses was found during the operation. However, follow-up computerized tomography revealed clearly a pelvic abscess, for which a pigtail catheter was inserted at HD 30, and the purulent drainage grew methicillin-resistant Staphylococcus aureus (MRSA) and then MRSA and Escherichia coli on four separate occasions until HD 69. The MRSA isolates were resistant to ciprofloxacin, clindamycin, erythromycin, gentamicin, and tetracycline but susceptible to rifampin, cotrimoxazole, and vancomycin (MIC; ≤2 μg/ml) in MicroScan PosCombo 6 (Dade-Behring, West Sacramento, Calif.). The patient was treated with vancomycin (2 g per day) for 8 days, followed by teicoplanin (200 mg per day) for 30 days in combination with ciprofloxacin and metronidazole. C-reactive protein was 3.8 mg/dl, and leukocyte counts ranged from 11,800 to 38,200/μl with 82 to 98% neutrophils. When he was discharged at HD 94, fever had subsided for a week, C-reactive protein was 1.6 mg/dl, and the pigtail catheter was withdrawn. During that admission, he had received palliative chemotherapy with flucytosine-cisplatin and flucytosine-palladium regimens consecutively. However, 2 weeks later he was readmitted for the third time via the Emergency Department on 8 August 1997; he was septic and revealed an abdominal mass 20 cm in diameter. The blood culture grew MRSA, which showed the same antibiogram as previous isolates, but the vancomycin MIC was now 4 μg/ml by MicroScan. He was treated with vancomycin, ciprofloxacin, and metronidazole but expired 2 weeks later.
Laboratory investigation.
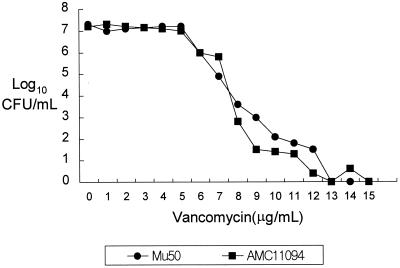
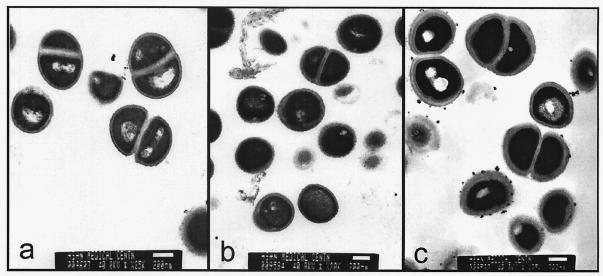
With the report of the first vancomycin-intermediate S. aureus (VISA) from Japan followed by similar reports from the United States (1, 4, 10, 11), we at Asan Medical Center (AMC) begun to look for VISA in clinical isolates. All S. aureus strains isolated during the 8-month period from January to August 1999 were screened for VISA and heterogeneous VISA using brain heart infusion agar containing 4 μg of vancomycin per ml (BHI-V4) (5). Ten microliters of each bacterial suspension (McFarland 0.5) was inoculated on BHI-V4 and incubated at 37°C for 48 h. Vancomycin-susceptible MRSA strain H1 and heterogeneous-VISA strain Mu3 were used as controls (5). Strains forming one or more colonies on BHI-V4 within 48 h of incubation were tested for MICs and population analysis for vancomycin (5). Of 4,483 S. aureus strains screened, 58 strains, all of which were MRSA, grew on BHI-V4, but no VISA strains were identified; the MIC for all was less than 4 μg/ml. Subsequently, we tested an additional 60 MRSA strains which had been isolated from blood cultures during 1997-1998 and stored at −70°C. One of those strains, AMC11094, grew confluently on BHI-V4 after 24 h of incubation. Susceptibility tests were performed by the National Committee for Clinical Laboratory Standards (NCCLS) broth microdilution method using cation-adjusted Mueller-Hinton broth (8). The MICs for strain AMC11094 were as follows: vancomycin, 8 μg/ml; teicoplanin, 16 μg/ml; oxacillin, >128 μg/ml; clinidamycin, >128 μg/ml; erythromycin, >128 μg/ml; ciprofloxacin, 64 μg/ml; rifampin, 0.006 μg/ml; gentamicin, 0.5 μg/ml; and quinupristin-dalfopristin, 0.5 μg/ml. The MICs of vancomycin and teicoplanin by E-test were also 8 and 16 μg/ml, respectively. A population analysis was performed by spreading 100 μl of the starting cell suspension (McFarland 0.5) and its serial diluents onto BHI agar plates containing 1 to 15 μg of vancomycin per ml in 1-μg/ml increments and counting the number of colonies after 24 h of incubation at 35°C (5); AMC11094 was indistinguishable from Mu50 (Fig. 1), the first VISA strain reported by Hiramatsu et al. (4). PCRs for the enterococcal vancomycin resistance genes vanA, vanB, vanC1, and vanC2/3 (7) were all negative. Transmission electron microscopy showed that the cell wall of AMC11094 was two or three times thicker than that of Mu3 (3, 9) and S. aureus ATCC 25923 (Fig. 2). In addition, pulsed-field gel electrophoresis (PFGE) of SmaI-restricted chromosomal DNA (M. N. Kim, J. S. Park, J. S. Jeong, J. S. Choi, and C. H. Pai, Abst. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. K-125, p. 538, 1998) revealed that AMC11094 differed from Mu50 and Mu3 by more than 10 bands (Fig. 3).
FIG. 1.
Population analysis for resistant subpopulations of AMC11094 and Mu50. The curves are comparable.
FIG. 2.
AMC11094 (c) shows rough, thick cell walls compared to ATCC25923 (a) and Mu3 (b). All of them were grown overnight on a blood agar plate. Bars, 200 nm.
FIG. 3.
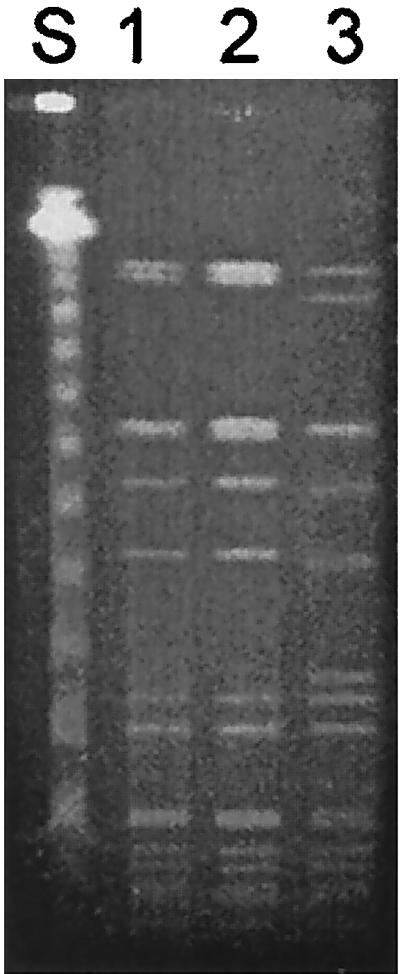
Patterns of SmaI-digested DNA of S. aureus isolates on PFGE. Lane S, phage lambda size standards; lane 1, Mu50 (Japanese VISA); lane 2, Mu3 (Japanese heterogenous-VISA); lane 3, AMC11094. Note that the PFGE pattern of AMC11094 differs from those of Mu50 and Mu3 by more than 10 bands.
Discussion.
As in the previous reports of VISA (3, 4, 10, 11), the patient had been treated with vancomycin and teicoplanin for a long time before the resistant strain emerged, and the resistance in AMC11094 was also associated with an increase in the cell wall, while an enterococcal vancomycin resistance gene was not detected. In PFGE analysis, AMC11094 was different from Mu50, as were U.S. isolates of VISA (11, 13). Although all isolates from the United States, Japan, and Korea have a thickened cell wall, which suggested sequestration of vancomycin as a common mechanism of resistance (3), they do not represent a single clone of MRSA that has disseminated. Those findings suggested that VISA has emerged by cellular modification under antibiotic pressure rather than by acquisition of a resistance gene from other strains, such as vancomycin-resistant enterococci, or by international transmission of resistant clones, as shown for drug-resistant Streptococcus pneumoniae (12).
When AMC11094 was first isolated from the patient's blood culture, the strain was considered susceptible to vancomycin because routine susceptibility testing showed a MIC of vancomycin of 4 μg/ml. In the U.S. VISA case, for which the MIC of vancomycin was 4 μg/ml by MicroScan but 8 μg/ml by NCCLS broth microdilution method (13). MRSA with a vancomycin MIC of 4 μg/ml by commercial susceptibility test should be investigated further, as the recent NCCLS guideline has recommended (9).
Vancomycin is one of the most frequently prescribed antimicrobial agents in tertiary-care hospitals in Korea (continuous quality improvement of antibiotic use, Asan Medical Center, 1998; unpublished data), because of a high prevalence of MRSA, especially in nosocomial infections (2, 6). The emergence of S. aureus with an intermediate resistance to vancomycin in a Korean university hospital is a warning to us that VISA may soon become a global problem unless antimicrobial agents are used more prudently. The clinical laboratory should identify and report strains with reduced susceptibility to vancomycin, including VISA, promptly for appropriate treatment of patients and start implementing infection control precautions to prevent the spread of resistance.
REFERENCES
- 1.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States. Morb Mortal Wkly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 2.Chong Y, Lee K, Park Y J, Jeon D S, Lee M H, Kim M Y, Chang C H, Kim E J, Lee N Y, Kim H S, Kang E S, Cho H C, Paik I K, Lee H S, Jang S J, Park A J, Cha Y J, Kang S H, Lee M H, Song W, Shin J H. Korean nationwide surveillance of antimicrobial resistance of bacteria in 1997. Yonsei Med J. 1998;39:569–577. doi: 10.3349/ymj.1998.39.6.569. [DOI] [PubMed] [Google Scholar]
- 3.Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum R S, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42:199–200. doi: 10.1093/jac/42.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 5.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 6.Kim M N, Jeong J S, Kim B C, Song J H, Pai C H. Comparison of antimicrobial susceptibility of nosocomial and community-acquired pathogens. Korean J Infect Dis. 1993;25:333–342. [Google Scholar]
- 7.Lee W G, Jung M K, Kwak Y S. Vancomycin-resistant enterococci: incidence, antimicrobial susceptibility, and resistance genotypes. Korean J Clin Pathol. 1998;18:51–56. [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 4th ed., 17, no. 2. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 5th ed., vol. 20, no. 2. Approved standard M7-A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 10.Sieradzki K, Roberts R B, Haber S W, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- 11.Smith T L, Pearson M L, Wilcox K R, Cruz C, Lancaster M V, Robinson-Dunn B, Tenover F C, Zervos M J, Band J D, White E, Jarvis W R. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 12.Song J, Lee N Y, Ichiyama S, Yoshida R, Hirakata Y, Fu W, Chonthaleong A, Aswapokee N, Chiu C, Lalitha M K, Thomas K, Perera J, Yee T T, Jamal F, Warsa U C, Vinh B X, Jacobs M R, Appelbaum P C, Pai C H the ANSORP Study Group. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Clin Infect Dis. 1999;28:1206–1211. doi: 10.1086/514783. [DOI] [PubMed] [Google Scholar]
- 13.Tenover F C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O'Hara C M C M, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]