Abstract
Rationale: The SYGMA (Symbicort Given as Needed in Mild Asthma) studies evaluated the efficacy and safety of as-needed budesonide (BUD)–formoterol (FORM) in patients whose asthma was uncontrolled on as-needed inhaled short-acting bronchodilators (subgroup 1) or controlled on inhaled corticosteroids (ICS) or leukotriene receptor antagonists (subgroup 2).
Objectives: To assess the influence of prestudy treatment in a post hoc analysis of the SYGMA studies.
Methods: In the SYGMA 1 (NCT022149199) and SYGMA 2 (NCT02224157) 52-week, double-blind, randomized, parallel-group studies, 6,735 patients with mild asthma were randomized to as-needed BUD–FORM, low-dose BUD + as-needed terbutaline (BUD maintenance), or as-needed terbutaline (SYGMA 1 only). Exacerbation rates and changes in symptom control and lung function were compared among treatments for both subgroups.
Results: In a pooled analysis of SYGMA 1 and 2, the annual severe exacerbation rate in subgroup 1 was significantly lower with as-needed BUD–FORM (0.08 [95% confidence interval (CI), 0.06–0.10]) than with BUD maintenance (0.10 [95% CI, 0.09–0.13]) (rate ratio [RR], 0.74 [95% CI, 0.56–0.98]; P = 0.03), and similar results were shown in subgroup 2 with BUD–FORM (0.12 [95% CI, 0.10–0.14]) and BUD maintenance (0.10 [95% CI, 0.09–0.13]) (RR, 1.10 [95% CI, 0.86–1.41]; P = 0.44). In SYGMA 1, the annual severe exacerbation rate in both subgroups was significantly lower with as-needed BUD–FORM than with as-needed terbutaline (subgroup 1: RR, 0.34 [95% CI, 0.20–0.58]; P < 0.001; subgroup 2: RR, 0.37 [95% CI, 0.25–0.54]; P < 0.001). The number needed to treat to prevent one severe exacerbation with as-needed BUD–FORM and BUD maintenance versus as-needed terbutaline were 20 and 34 in subgroup 1 and 13 and 12 in subgroup 2, respectively.
Conclusions: These findings suggest that, for patients with mild asthma currently receiving short-acting β2-agonists alone, as-needed low-dose ICS–FORM should be preferred over maintenance ICS as initial controller treatment. For patients whose asthma is controlled on maintenance low-dose ICS, as-needed BUD–FORM is an alternative to maintenance ICS without the need for daily treatment, and both of these options are safer than switching to short-acting β2-agonist–only treatment.
Keywords: as-needed, budesonide–formoterol, exacerbations, mild asthma
The majority of patients with asthma are considered to have “mild asthma” (1). However, patients with infrequent symptoms account for over 30% of severe asthma exacerbations requiring emergency department visits (1).
Historically, treatment of mild asthma in guidelines has focused on symptom relief with short-acting β2-agonists (SABA), with a controller maintenance treatment being added when symptoms become more frequent or patients have risk factors for exacerbations (2, 3). However, this two-step approach to “mild” asthma is a source of confusion for patients and their physicians; many patients who should be on regular inhaled corticosteroids (ICS) remain reliant on and overuse as-needed SABA (4, 5), whereas others who might qualify for a step-down option remain on ICS maintenance treatment, but this is often accompanied by faltering adherence (6–8). In an online survey of over 8,000 patients in Europe, 71% of patients treated with SABA alone did not have well-controlled asthma symptoms, and 26% had required oral corticosteroids (OCS) for an exacerbation in the previous year, indicating that they should be stepped up to receive an ICS-containing controller medication (9, 10). If a SABA is taken regularly without ICS, a paradoxical shortcoming is that it may reduce the bronchodilator response (11), increase airway inflammation (12), increase the allergic response (13), and increase airway hyperresponsiveness (8, 11, 14–16).
Four studies have evaluated an as-needed antiinflammatory reliever approach combining an ICS with a fast-onset, long-acting β2-agonist (formoterol [FORM]) in mild asthma (17–20). Together, these studies in almost 10,000 patients demonstrated that in mild asthma, as-needed, low-dose ICS–FORM treatment is superior to as-needed SABA treatment for both symptom control and prevention of exacerbations and is as effective for exacerbation prevention as (17, 18), or is more effective for exacerbation prevention than (19, 20), regular daily low-dose ICS plus as-needed SABA. This, together with other supportive evidence, led to the recommendation in the 2019 Global Initiative for Asthma (GINA) strategy that a low-dose ICS–FORM combination is the preferred reliever option for exacerbation prevention and symptom control in patients with mild asthma with symptoms occurring less than twice a month (step 1 treatment) and as an alternative to regular low-dose ICS treatment in step 2 (5, 21).
However, questions remain concerning the positioning of antiinflammatory reliever treatment for adults and adolescents with mild asthma (22). For example, for patients whose asthma is uncontrolled on SABA-only treatment, is as-needed ICS–FORM as effective as daily low-dose ICS, or should such patients, as some have proposed, first be stabilized on daily low-dose ICS? Second, for patients whose asthma is well controlled on regular low-dose ICS, is as-needed budesonide (BUD)–FORM an alternative and less demanding option, and is it safer than stepping down to as-needed SABA alone, as recommended in some current guidelines (2, 3)?
The SYGMA (Symbicort Given as Needed in Mild Asthma) studies (17, 18) included two prespecified subpopulations of patients: those whose asthma was uncontrolled on as-needed SABA alone (subgroup 1) and patients whose asthma was controlled on low-dose ICS or leukotriene receptor antagonists (LTRA) (subgroup 2). This post hoc analysis assessed the influence of prestudy treatment on clinical outcomes in the SYGMA studies, including the number needed to treat (NNT) for 1 year to prevent one exacerbation compared with as-needed SABA alone. The aim was to examine the potential of as-needed BUD–FORM as an alternative to regular low-dose ICS in patients with uncontrolled asthma on SABA alone and as an alternative approach to stepping down to SABA-only treatment in patients whose asthma is well controlled on low-dose ICS.
Methods
The SYGMA 1 (NCT022149199) (17) and SYGMA 2 (NCT02224157) (18) trials were 52-week, double-blind, randomized, multinational, parallel-group studies.
Trial Design and Patients
These have been previously described in detail (23); further details are provided in the online supplement. In brief, patients aged ⩾12 years with mild asthma were eligible if they were in need of GINA step 2 treatment, with their asthma being either uncontrolled on as-needed inhaled short-acting bronchodilators (subgroup 1) or being controlled on low-dose ICS or LTRA (subgroup 2). The baseline prebronchodilator forced expiratory volume in 1 second (FEV1) had to be ⩾60% predicted for subgroup 1 and ⩾80% predicted for subgroup 2. Eligible patients entered a 2- to 4-week run-in period on as-needed terbutaline 0.5 mg (Bricanyl Turbuhaler, AstraZeneca) to confirm their need for step 2 treatment as indicated by use of as-needed terbutaline on ⩾3 days during the last run-in week.
In SYGMA 1, patients were randomized to twice-daily placebo plus as-needed terbutaline 0.5 mg (delivered dose of 0.4 mg); twice-daily placebo plus as-needed BUD–FORM 200–6 μg (Symbicort Turbuhaler, AstraZeneca; delivered dose of 160–4.5 μg); or twice-daily BUD 200 μg (Pulmicort Turbuhaler, AstraZeneca) as maintenance plus as-needed terbutaline 0.5 mg (“BUD maintenance”). In SYGMA 2, patients were randomized to twice-daily placebo plus as-needed BUD–FORM 2006 μg or BUD maintenance. In SYGMA 1, open-label BUD could be added for moderate exacerbations or persistently poor asthma control.
Endpoints and Analysis
In a prespecified analysis of SYGMA 1 data, annualized severe exacerbation rates, time to the first severe exacerbation, and change from baseline in prebronchodilator FEV1 and asthma symptom control Asthma Control Questionnaire (5-item version) (ACQ-5) score (minimal clinically important difference of 0.5 units [23]) were evaluated between treatment arms for both subgroups; moderate or severe exacerbations were also assessed in the SYGMA 1 analysis (see details in the online supplement). In a post hoc analysis of SYGMA 1, change from baseline in the percentage of symptom-free days was also analyzed. Differences between treatment groups in the absolute number of symptom-free days were calculated by multiplying the estimated percentage of symptom-free days during the treatment period from the model by the exposure time for each group.
Severe exacerbations were defined according to American Thoracic Society/European Respiratory Society criteria as asthma worsening requiring the use of systemic corticosteroids for ⩾3 days or an inpatient hospitalization or emergency department visit requiring systemic corticosteroids (24). Moderate exacerbations were defined as a deterioration of asthma requiring a change in treatment (i.e., initiation of prescribed ICS treatment [inhaled BUD 200 μg twice daily]) to avoid progression or worsening to a severe exacerbation. The number of severe exacerbations and the number of moderate or severe exacerbations were analyzed by using a negative binomial model that included randomized treatment, prestudy asthma treatment, study, region, severe (or moderate or severe) exacerbations in the last 12 months (0, ⩾1), and the treatment–by–prestudy treatment interaction as factors. Change from baseline in the prebronchodilator FEV1% predicted by treatment was also analyzed (see details in the online supplement).
In a pooled post hoc analysis of SYGMA 1 and 2, similar analyses were conducted to assess the effect of prestudy treatment on efficacy outcomes by comparing the three treatment arms.
The NNT, defined as the number of patients that needed to be treated to have an additional patient free from a severe or moderate or severe exacerbation over 1 year, was compared for as-needed BUD–FORM and BUD maintenance versus as-needed terbutaline (the reference treatment) between subgroups in a post hoc analysis of SYGMA 1 data.
Adherence to maintenance treatment and the median daily ICS load were assessed separately for the highly controlled SYGMA 1 study and the more pragmatic SYGMA 2 study. The total daily ICS load for each patient included both the electronically recorded randomized treatment usage and the open-label ICS use collected via the electronic case report form.
Results
Patients
Of the 3,836 patients randomized in SYGMA 1 and with evaluable data, 1,706 (44%) had previously been taking bronchodilators alone (subgroup 1), and 2,130 (56%) had previously been treated with low-dose ICS or LTRA (subgroup 2). In SYGMA 2, of 4,176 patients randomized and with evaluable data, 1,934 (46%) and 2,242 (54%) were in subgroups 1 and 2, respectively. In the pooled population of SYGMA 1 and 2, 6,735 patients were included, of whom 3,075 (46%) were in subgroup 1 and 3,660 (54%) were in subgroup 2. Overall, 47.7% of patients had previously been treated with ICS, whereas 6.7% had previously been treated with LTRA.
Baseline demographics and clinical characteristics were generally similar between treatment arms in the pooled population and in SYGMA 1 and SYGMA 2 individually (Table 1; see Tables E1, E2, and E3 in the online supplement). However, some differences were seen by prestudy treatment, with lung function being lower and reversibility, time since asthma diagnosis, and the ACQ-5 score being higher in subgroup 1 than in subgroup 2.
Table 1.
Demographics and clinical characteristics according to prestudy treatment (pooled SYGMA 1 and 2)
| Overall |
Uncontrolled on BD (Subgroup 1) |
Well Controlled on ICS or LTRA (Subgroup 2) |
||||
|---|---|---|---|---|---|---|
| As-needed BUD–FORM (N = 3,366) | BUD Maintenance + As-needed Terbutaline (N = 3,369) | As-needed BUD–FORM (N = 1,524) | BUD Maintenance + As-needed Terbutaline (N = 1,551) | As-needed BUD–FORM (N = 1,842) | BUD Maintenance + As-needed Terbutaline (N = 1,818) | |
| Age, yr, mean (SD) | 40.7 (16.9) | 40.1 (17) | 39.5 (16.4) | 38.5 (16.5) | 41.7 (17.2) | 41.4 (17.2) |
| Female sex, n (%) | 2,085 (61.9) | 2,086 (61.9) | 920 (60.4) | 930 (60.0) | 1,165 (63.2) | 1,156 (63.6) |
| Pre-bronchodilator FEV1% predicted at study entry, mean (SD) | 85.96 (12.44) | 85.57 (12.26) | 79.28 (12.08) | 79.16 (12.27) | 91.48 (9.74) | 91.03 (9.24) |
| Pre-bronchodilator FEV1% predicted at baseline, mean (SD) | 84.33 (14.02) | 84.15 (13.93) | 80.27 (14.32) | 79.63 (14.26) | 87.68 (12.85) | 88.00 (12.40) |
| Reversibility at baseline, %, mean (SD) | 15.01 (12.00) | 15.00 (12.48) | 17.09 (12.63) | 17.45 (13.80) | 13.30 (11.16) | 12.93 (10.81) |
| Time since asthma diagnosis, yr, median (IQR) | 7.3 (2.6–15.5) | 6.9 (2.6–15.1) | 9.1 (3.3–19.0) | 9.1 (3.2–18.4) | 6.0 (2.3–12.9) | 5.7 (2.2–12.1) |
| Severe exacerbation in the last 12 mo, n (%) | ||||||
| 0 | 2,650 (78.7) | 2,668 (79.2) | 1,175 (77.1) | 1,192 (76.9) | 1,475 (80.1) | 1,476 (81.2) |
| 1 | 595 (17.7) | 574 (17.0) | 270 (17.7) | 285 (18.4) | 325 (17.6) | 289 (15.9) |
| ⩾2 | 121 (3.6) | 127 (3.8) | 79 (5.2) | 74 (4.7) | 42 (2.3) | 53 (2.9) |
| ACQ-5 score at study entry, mean (SD) | 1.53 (0.97) | 1.56 (0.99) | 1.77 (0.95) | 1.82 (0.96) | 1.34 (0.95) | 1.33 (0.95) |
| ACQ-5 score at baseline, mean (SD) | 1.53 (0.92) | 1.54 (0.92) | 1.61 (0.91) | 1.66 (0.90) | 1.47 (0.93) | 1.43 (0.93) |
Definition of abbreviations: ACQ-5 = Asthma Control Questionnaire (5-item version); BD = bronchodilator; BUD = budesonide; FEV1 = forced expiratory volume in 1 second; FORM = formoterol; ICS = inhaled corticosteroid; IQR = interquartile range; LTRA = leukotriene receptor antagonist; SABA = short-acting β2-agonist; SD = standard deviation; SYGMA = Symbicort Given as Needed in Mild Asthma.
“Uncontrolled on BD” indicates patients whose asthma was uncontrolled on as-needed short-acting BDs. The ACQ-5 consists of five questions concerning symptoms of asthma during the last week, each of which is scored on a range from 0 (no impairment) to 6 (maximum impairment); the minimal clinically important difference is 0.5 units. Study entry is defined as assessment at visit 2 (i.e., the start of run-in). Baseline is defined as the assessment at visit 3 (i.e., the end of 2–4 weeks’ run-in on SABA alone [randomization]).
Exacerbations
Comparison with BUD maintenance
In the overall pooled population, annual severe exacerbation rate with as-needed BUD–FORM (0.10 [95% confidence interval (CI), 0.08–0.11]) was similar to BUD maintenance (0.10 [95% CI, 0.09–0.12) (rate ratio [RR], 0.93 [95% CI, 0.77–1.11]; P = 0.41). For patients in subgroup 1, the annualized severe exacerbation rate was significantly lower (26%) with as-needed BUD–FORM (0.08 [95% CI, 0.06–0.10) than with BUD maintenance (0.10 [95% CI, 0.09–0.13) (RR, 0.74 [95% CI, 0.56–0.98], P = 0.03). For patients in subgroup 2, the annual severe exacerbation rate with as-needed BUD–FORM (0.12 [95% CI, 0.10–0.14]) was similar to that with BUD maintenance (0.10 [95% CI, 0.09–0.13]) (RR, 1.10 [95% CI, 0.86–1.41]; P = 0.44) (Table 2; Figure 1A). The difference by prestudy treatment was significant (interaction term P = 0.03).
Table 2.
Efficacy endpoints in the pooled analysis of SYGMA 1 and 2 by prestudy treatment
| Overall |
Uncontrolled on BD (Subgroup 1) |
Well Controlled on ICS or LTRA (Subgroup 2) |
||||
|---|---|---|---|---|---|---|
| As-needed BUD–FORM | BUD Maintenance + As-needed Terbutaline | As-needed BUD–FORM | BUD Maintenance + As-needed Terbutaline | As-needed BUD–FORM | BUD Maintenance + As-needed Terbutaline | |
| Severe exacerbations | ||||||
| N | 3,366 | 3,369 | 1,524 | 1,551 | 1,842 | 1,818 |
| Total number of severe exacerbations | 294 | 310 | 110 | 150 | 184 | 160 |
| Severe exacerbations, rate estimate (95% CI) | 0.10 (0.08 to 0.11) | 0.10 (0.09 to 0.12) | 0.08 (0.06 to 0.10) | 0.10 (0.09 to 0.13) | 0.12 (0.10 to 0.14) | 0.10 (0.09 to 0.13) |
| Rate ratio for BUD–FORM vs. comparator (95% CI)* | — | 0.93 (0.77 to 1.11) | — | 0.74 (0.56 to 0.98) | — | 1.10 (0.86 to 1.41) |
| P value | — | 0.41 | — | 0.03 | — | 0.44 |
| Number of patients with at least one event, n (%) | 248 (7.4) | 262 (7.8) | 95 (6.2) | 129 (8.3) | 153 (8.3) | 133 (7.3) |
| Time to first severe exacerbation, hazard ratio for BUD–FORM vs. comparator (95% CI)* | — | 0.94 (0.79 to 1.12) | — | 0.74 (0.56 to 0.96) | — | 1.13 (0.90 to 1.43) |
| P value | — | 0.46 | — | 0.02 | — | 0.30 |
| Asthma symptom control | ||||||
| N | 3,204 | 3,184 | 1,431 | 1,462 | 1,773 | 1,722 |
| ACQ-5 treatment, mean (SD) | 1.20 (0.79) | 1.08 (0.79) | 1.20 (0.78) | 1.14 (0.80) | 1.21 (0.80) | 1.03 (0.78) |
| Mean ACQ-5 change from baseline to treatment average (95% CI) | −0.34 (−0.36 to −0.32) | −0.47 (−0.49 to −0.44) | −0.37 (−0.40 to −0.34) | −0.45 (−0.48 to −0.42) | −0.32 (−0.35 to −0.28) | −0.48 (−0.51 to −0.45) |
| Difference (95% CI)* | — | 0.13 (0.10 to 0.16) | — | 0.08 (0.03 to 0.13) | — | 0.17 (0.12 to 0.21) |
| P value | — | P < 0.001 | — | P < 0.001 | — | P < 0.001 |
| Lung function | ||||||
| N | 3,257 | 3,242 | 1,465 | 1,486 | 1,792 | 1,756 |
| Pre-bronchodilator FEV1% predicted, change from baseline to treatment average (95% CI)* | 2.7 (2.4 to 3.1) | 4.0 (3.7 to 4.3) | 2.2 (1.7 to 2.7) | 3.4 (2.9 to 3.9) | 3.2 (2.8 to 3.7) | 4.6 (4.2 to 5.1) |
| Difference (95% CI)* | — | −1.3 (−1.7 to −0.8) | — | −1.1 (−1.8 to −0.4) | — | −1.4 (−2.0 to −0.8) |
| P value | — | P < 0.001 | — | P = 0.001 | — | P < 0.001 |
Definition of abbreviations: ACQ-5 = Asthma Control Questionnaire (5-item version); BD = bronchodilator; CI = confidence interval; BUD = budesonide; FEV1 = forced expiratory volume in 1 second; FORM = formoterol; ICS = inhaled corticosteroid; LTRA = leukotriene receptor antagonist; SD = standard deviation; SYGMA = Symbicort Given as Needed in Mild Asthma.
“Uncontrolled on BD” indicates patients whose asthma was uncontrolled on as-needed short-acting BDs. The severe exacerbation rate was analyzed by using a negative binomial model that included randomized treatment, prestudy treatment, severe exacerbations in the last 12 months (0, ⩾1), study, region, and the treatment–by–prestudy treatment interaction as covariates. The time to the first severe exacerbation was analyzed by means of a Cox regression model that included randomized treatment, prestudy asthma treatment, severe exacerbations in the last 12 months (0, ⩾1), study, region, and the treatment–by–prestudy treatment interaction as factors. ACQ-5 scores and the prebronchodilator FEV1 were analyzed by using an analysis of covariance model that included randomized treatment, prestudy asthma treatment, region, study, and the treatment–by–prestudy treatment interaction as factors. The minimal clinically important difference for the ACQ-5 is 0.5 units.
All comparisons are between as-needed BUD–FORM and the control (BUD maintenance plus as-needed terbutaline).
Figure 1.
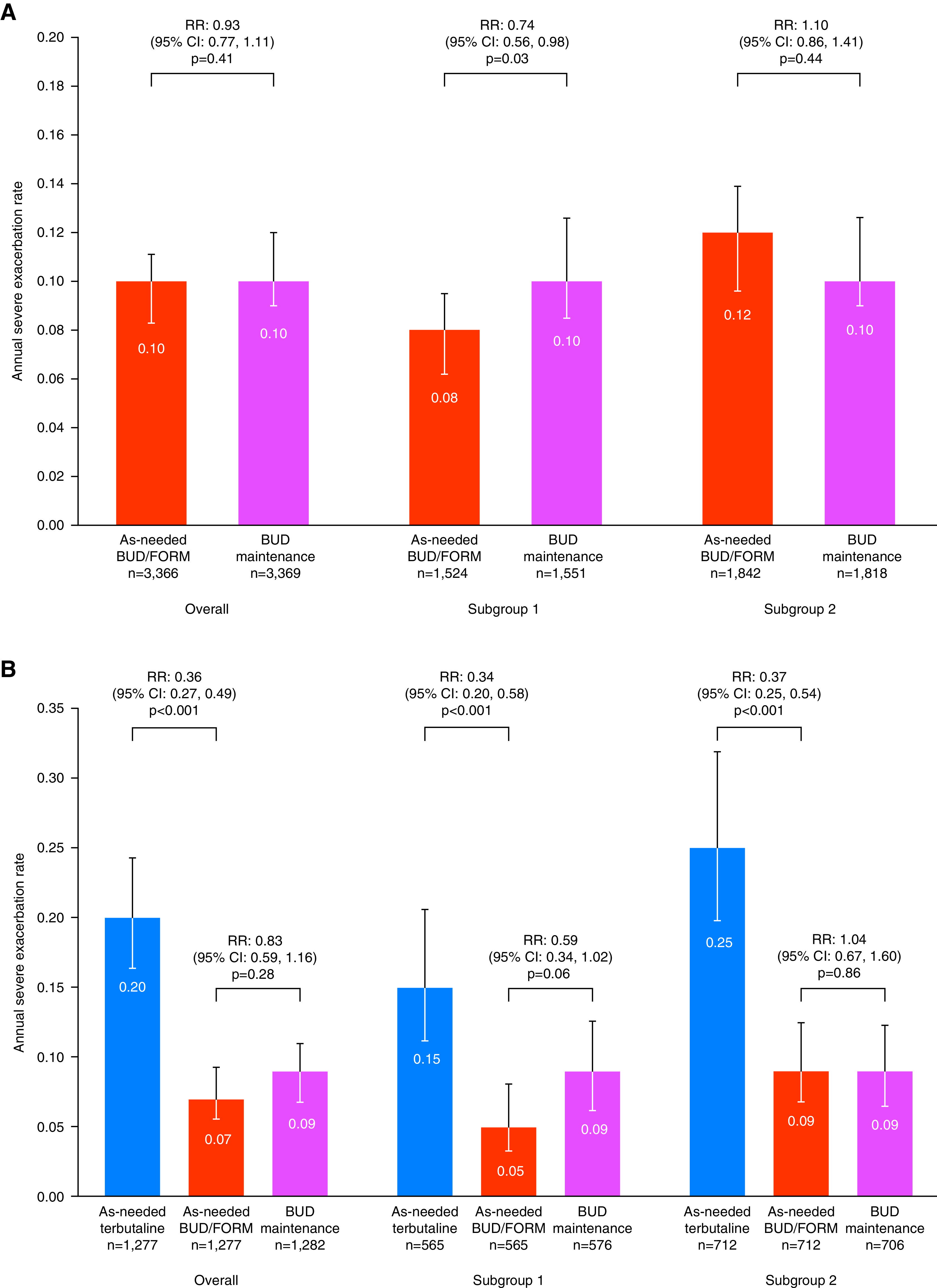
Severe exacerbation rate according to prestudy treatment in (A) the pooled analysis of SYGMA (Symbicort Given as Needed in Mild Asthma) 1 and SYGMA 2 and (B) SYGMA 1. Subgroup 1 includes patients with asthma uncontrolled on as-needed short-acting bronchodilators alone. Subgroup 2 includes patients with asthma controlled on inhaled corticosteroids or leukotriene receptor antagonists. BUD = budesonide; CI = confidence interval; FORM = formoterol; RR = rate ratio.
Likewise, in the analysis of time to the first severe exacerbation, there was no difference between treatment with as-needed BUD–FORM and BUD maintenance in the overall pooled population (hazard ratio [HR], 0.94 [95% CI, 0.79–1.12]; P = 0.46) (Table 2) or in subgroup 2 patients (HR, 1.13 [95% CI, 0.90–1.43], P = 0.30), but in subgroup 1 patients, as-needed BUD–FORM delayed the time to first severe exacerbation compared with BUD maintenance (HR, 0.74 [95% CI, 0.56–0.96], P = 0.02) (Table 2; Figure E1). The difference by prestudy treatment was significant (interaction term P = 0.02).
Comparison with as-needed terbutaline
In SYGMA 1, the annual rate of severe exacerbations was significantly lower with as-needed BUD–FORM than with as-needed terbutaline, regardless of prestudy treatment (subgroup 1: RR, 0.34 [95% CI, 0.20–0.58], P < 0.001; subgroup 2: RR, 0.37 [95% CI, 0.25–0.54], P < 0.001) (Table E4; Figure 1B). Similar findings were also observed for the rate of moderate or severe exacerbations (Table E4). Within the group randomized to as-needed terbutaline, the annual rate of severe exacerbations was significantly lower for those who had entered in subgroup 1 than for those who had entered subgroup 2 (0.60 [95% CI, 0.42–0.88], P = 0.008). A similar finding was observed for the annual rate of moderate or severe exacerbations between the two subgroups (0.68 [95% CI, 0.52–0.89]; P = 0.006).
In the analysis of time to first severe exacerbation in SYGMA 1, as-needed BUD–FORM reduced the risk of a severe exacerbation compared with as-needed terbutaline in both subgroup 1 patients (HR, 0.44 [95% CI, 0.27–0.72], P = 0.001) and subgroup 2 patients (HR, 0.43 [95% CI, 0.31–0.61]; P < 0.001) and was similar to BUD maintenance in both subgroups (Table E4). Similar findings were observed in the analysis of time to the first moderate or severe exacerbation (Table E4).
Symptom Control
Comparison with BUD maintenance
There were very small differences in mean ACQ-5 score improvements from baseline between as-needed BUD–FORM and BUD maintenance in both the overall pooled population (0.13 [95% CI, 0.10–0.16]; P < 0.001) and the two subgroups (subgroup 1: 0.08 [95% CI, 0.03–0.13]; P < 0.001; subgroup 2: 0.17 [95% CI, 0.12–0.21]; P < 0.001), with a significant effect of prestudy treatment (treatment–by–prestudy treatment interaction term P = 0.01) (Table 2). From the twice-daily electronic diary in SYGMA 1, small differences in improvements in mean percentage of symptom-free days from baseline were observed between as-needed BUD–FORM and BUD maintenance in subgroup 1 (−1.63 [95% CI, −4.34 to 1.08]; P = 0.24) and subgroup 2 (−3.94 [95% CI, −6.38 to −1.51]; P = 0.001) (Table E4). These changes equated to 6.1 fewer symptom-free days in subgroup 1 and 11.7 fewer symptom-free days in subgroup 2 over the study duration with as-needed BUD–FORM versus BUD maintenance.
Comparison with as-needed terbutaline
Similarly, small differences in improvements in mean ACQ-5 score from baseline were observed with as-needed BUD–FORM versus as-needed terbutaline in both prestudy treatment subgroups in SYGMA 1 (Table E4). In addition, small differences in improvements in mean percentage of symptom-free days from baseline were observed between as-needed BUD–FORM and as-needed terbutaline in subgroup 1 (2.79 [95% CI, 0.07–5.52]; P = 0.05) and subgroup 2 (2.63 [95% CI, 0.20–5.06]; P = 0.03) of SYGMA 1 (Table E4). These changes equated to 9.7 additional symptom-free days in subgroup 1 and 10.6 additional symptom-free days in subgroup 2 over the study duration with as-needed BUD–FORM versus as-needed terbutaline.
Lung Function
Comparison with BUD maintenance
Small differences in prebronchodilator FEV1% predicted change from baseline were observed between as-needed BUD–FORM and BUD maintenance in the pooled analysis in the overall population (−1.3 [95% CI, −1.7 to −0.8]; P < 0.001) and in both subgroups (subgroup 1: −1.1 [95% CI, −1.8 to −0.4]; P = 0.001; subgroup 2: −1.4 [95% CI, −2.0 to −0.8]; P < 0.001) (Table 2), and there was no significant effect of prestudy treatment (treatment–by–prestudy treatment interaction P = 0.606).
Comparison with as-needed terbutaline
Similarly, small differences in FEV1% predicted were observed between as-needed BUD–FORM and as-needed terbutaline in both prestudy treatment subgroups of SYGMA 1 (Table E4).
NNT
With as-needed terbutaline as the reference treatment, the NNT in subgroup 1 was lower for as-needed BUD–FORM than for BUD maintenance. For severe exacerbations, the NNTs were 20 (95% CI, 13–48) for as-needed BUD–FORM and 34 (95% CI, not done; see Table 3) for BUD maintenance. For moderate or severe exacerbations, the NNTs were 11 (95% CI, 8–19) and 20 (95% CI, 11–109) for as-needed BUD–FORM and BUD maintenance, respectively (Table 3).
Table 3.
NNT comparison for severe and moderate or severe exacerbations according to prestudy treatment (with as-needed terbutaline as reference treatment) from SYGMA 1
| Overall |
Uncontrolled on BD (Subgroup 1) |
Well Controlled on ICS or LTRA (Subgroup 2) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| As-needed Terbutaline (N = 1,277) | As-needed BUD–FORM (N = 1,277) | BUD Maintenance + As-needed Terbutaline (N = 1,282) | As-needed Terbutaline (N = 565) | As-needed BUD–FORM (N = 565) | BUD Maintenance + As-needed Terbutaline (N = 576) | As-needed Terbutaline (N = 712) | As-needed BUD–FORM (N = 712) | BUD Maintenance + As-needed Terbutaline (N = 706) | |
| Severe exacerbations | |||||||||
| Patients with ⩾1 exacerbation, n (%) | 152 (11.9) | 71 (5.6) | 78 (6.1) | 51 (9.0) | 23 (4.1) | 35 (6.1) | 101 (14.2) | 48 (6.7) | 43 (6.1) |
| ARR, % (95% CI) | — | 6.34 (4.17 to 8.52) | 5.82 (3.61 to 8.02) | — | 4.96 (2.09 to 7.83) | 2.95 (−0.11 to 6.01) | — | 7.44 (4.29 to 10.60) | 8.09 (4.98 to 11.21) |
| NNT (95% CI) | — | 16 (12 to 24) | 17 (12 to 28) | — | 20 (13 to 48) | 34 (ND) | — | 13 (9 to 23) | 12 (9 to 20) |
| Moderate or severe exacerbations | |||||||||
| Patients with ⩾1 exacerbation, n (%) | 274 (21.5) | 131 (10.3) | 143 (11.2) | 97 (17.2) | 46 (8.1) | 70 (12.2) | 177 (24.9) | 85 (11.9) | 73 (10.3) |
| ARR, % (95% CI) | — | 11.20 (8.40 to 14.00) | 10.30 (7.47 to 13.14) | — | 9.03 (5.19 to 12.87) | 5.02 (0.92 to 9.11) | — | 12.92 (8.95 to 16.89) | 14.52 (10.63 to 18.41) |
| NNT (95% CI) | — | 9 (7 to 12) | 10 (8 to 13) | — | 11 (8 to 19) | 20 (11 to 109) | — | 8 (6 to 11) | 7 (5 to 9) |
Definition of abbreviations: ARR = absolute risk reduction; BD = bronchodilator; CI = confidence interval; BUD = budesonide; FEV1 = forced expiratory volume in 1 second; FORM = formoterol; ICS = inhaled corticosteroid; LTRA = leukotriene receptor antagonist; ND = not done; NNT = number needed to treat; SYGMA = Symbicort Given as Needed in Mild Asthma.
“Uncontrolled on BD” indicates patients whose asthma was uncontrolled on as-needed short-acting BDs. The NNT was calculated by dividing 100 by the difference in the percentage response between the as-needed BUD–FORM and BUD maintenance treatment groups and the reference treatment group (as-needed terbutaline) by using the observed proportion of patients experiencing ⩾1 exacerbation, the total number of exacerbations, and the total exposure (years) during the 1-year study period. CIs for NNTs were calculated as Wald CIs, with the exception of when the ARR was not statistically significant between treatment groups. As the treatment effect approaches zero, the NNT approaches infinity (27).
In subgroup 2, patients had similar NNTs with as-needed BUD–FORM versus BUD maintenance. For severe exacerbations, the NNTs were 13 (95% CI, 9–23) for as-needed BUD–FORM and 12 (95% CI, 9–20) for BUD maintenance. For moderate or severe exacerbations, the NNTs were 8 (95% CI, 6–11) and 7 (95% CI, 5–9), respectively (Table 3).
Adherence to Maintenance Treatment
Overall adherence to maintenance treatment was greater in SYGMA 1 than in SYGMA 2 across both treatments (Table E5). Median adherence to maintenance treatment was generally similar between the treatment groups of SYGMA 1 and SYGMA 2, with slightly lower adherence observed in subgroup 1 patients than in subgroup 2 patients, both in SYGMA 1 (83–85% vs. 86–87%) and in SYGMA 2 (63–64% vs. 70–72%) (Table E5).
ICS Load
Overall, the median daily ICS load was higher in SYGMA 1 than in SYGMA 2 for both ICS-containing treatments, irrespective of prestudy treatment (Figure 2; Table E5), but was lower in the as-needed BUD–FORM treatment groups than in the BUD maintenance treatment groups in both subgroups of SYGMA 1 and 2 (Figure 2; Table E5).
Figure 2.
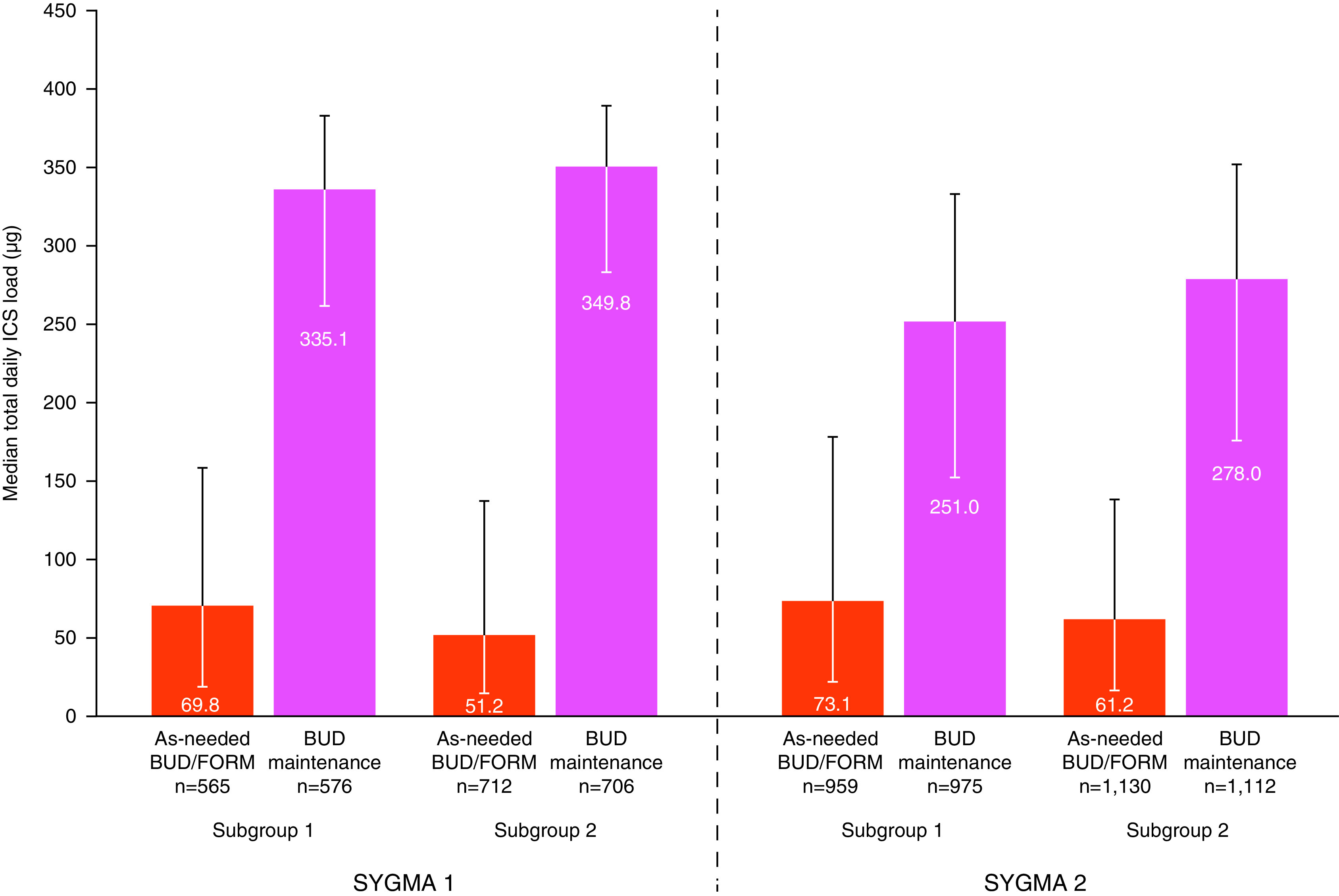
Median total daily metered inhaled corticosteroid (ICS) load, according to prestudy treatment in both SYGMA (Symbicort Given as Needed in Mild Asthma) 1 and SYGMA 2. Subgroup 1 includes patients with asthma uncontrolled on as-needed short-acting bronchodilators alone. Subgroup 2 includes patients with asthma controlled on ICS or leukotriene receptor antagonists. Error bars represent interquartile range. Note that the total ICS dose includes open-label ICS in SYGMA 1. BUD = budesonide; FORM = formoterol.
Adverse Events
There were no notable differences in adverse events between the pretreatment subgroups in either SYGMA 1 or SYGMA 2 (Table E6). In the pooled population, the proportion of patients experiencing adverse events potentially associated with any ICS or β2-agonist class effects was very low and was similar between treatment groups in subgroup 1 and subgroup 2 (Table E7).
Discussion
This post hoc analysis of pooled data from SYGMA 1 and 2 found that, in patients with mild asthma, as-needed BUD–FORM was associated with a significantly lower severe exacerbation rate (26% reduction) than BUD maintenance in patients previously receiving only as-needed SABA (subgroup 1), whereas in those whose asthma was well controlled on low-dose ICS or LTRA at entry (subgroup 2), there was no significant difference between these treatment arms. In addition, a significantly longer time to the first severe exacerbation compared with BUD maintenance was seen with as-needed BUD–FORM in subgroup 1, but not in subgroup 2, and although differences in symptom control and lung function over 12 months favored daily BUD maintenance treatment, these differences were small, and none approached thresholds for clinically important differences.
In subgroup 1 patients from SYGMA 1, as-needed BUD–FORM was associated with a large (66%) reduction in the severe exacerbation rate and an increase in the time to first severe exacerbation as compared with continuing on as-needed SABA. These observations are similar to findings in the smaller, open-label Novel Symbicort Turbuhaler Asthma Reliever Therapy (Novel START) study in which patients previously treated with as-needed SABA and randomized to as-needed BUD–FORM had fewer severe exacerbations than those randomized to twice-daily BUD (9 and 21 severe exacerbations, respectively; RR, 0.44 [95% CI, 0.20–0.96]) (19). In our study, the NNT to prevent one severe exacerbation in a year in subgroup 1 patients was 20 with as-needed BUD–FORM, at a total median BUD load of 69.8 μg/d, and 34 with twice-daily BUD maintenance, at an almost threefold higher total median load of BUD (335.1 μg/d). Both studies support the GINA 2019 recommendation that treatment with as-needed ICS–FORM is preferable to treatment with as-needed SABA alone (5).
In subgroup 2 patients, the NNTs to prevent one severe exacerbation in a year with as-needed BUD–FORM and twice-daily BUD maintenance were 12 and 13, respectively, and the total median BUD loads were 51.2 μg/d and 349.8 μg/d for as-needed BUD–FORM and twice-daily BUD maintenance, respectively. These findings support the GINA recommendation that treatment with as-needed low-dose ICS–FORM is an alternative option for adults and adolescents with satisfactory asthma control on regular low-dose ICS or LTRA (5).
Efficacy outcomes, such as the severe exacerbation rate and time to first severe exacerbation, were significantly improved with as-needed BUD–FORM versus BUD maintenance in patients in subgroup 1, but these differences were not significant in subgroup 2. The differences in efficacy outcomes observed between the two subgroups may possibly reflect a degree of self-selection by patients before the study, in that those who in the past had experienced an exacerbation or more symptoms after stopping prescribed ICS may have been more likely to continue treatment with ICS (and thereby be included in subgroup 2). This is supported by the observation that in subgroup 2 patients, the rate of severe exacerbations was substantially higher in those who were stepped down from low-dose ICS or LTRA to as-needed SABA than in patients whose asthma was uncontrolled on SABA (in subgroup 1) who were randomized to continue as-needed SABA (0.25 vs. 0.15, respectively; P = 0.008), despite severe exacerbations in the previous 12 months being more common in subgroup 1 than in subgroup 2. A further potential explanation for the difference in efficacy outcomes between the two subgroups is that subgroup 1 patients were accustomed to receiving only an as-needed medication, and their adherence with maintenance treatment was lower than that of those in subgroup 2, particularly in SYGMA 2, which included less intensive monitoring and no inhaler reminders.
It should be noted that the SYGMA results in patients previously on maintenance ICS differ from those of the PeRsonalised Asthma Combination Therapy: with Inhaled Corticosteroid And fast onset Long-acting beta agonist (PRACTICAL) study (n = 885), an open-label study in which 70% of patients were using low- to medium-dose ICS at entry but were not required to have well-controlled asthma (20). Despite this, the severe exacerbation rate in PRACTICAL was significantly lower in patients randomized to as-needed BUD–FORM than in patients randomized to maintenance ICS (relative rate, 0.69 [95% CI, 0.48–1.00]; P = 0.049), and there was no significant interaction for the severe exacerbation rate or end-of-study ACQ-5 score according to whether patients were or were not receiving ICS at baseline (20). Differences in the study design (i.e., the open-label design of PRACTICAL that allowed for patient behavior that mirrored usual clinical practice vs. the double-dummy design of the SYGMA studies), the above eligibility criteria, and the twice-daily adherence reminders for maintenance treatment in SYGMA 1 may explain the better results with as-needed BUD–FORM in PRACTICAL.
The large reduction in severe exacerbations with both of these low-dose ICS therapies compared with as-needed terbutaline supports the ongoing GINA recommendation that adults and adolescents with asthma should not stop ICS completely, even when symptoms are well controlled. This recommendation was based on studies summarized in a meta-analysis by Rank and colleagues (25), which reported an increased risk of a severe exacerbation if ICS were ceased entirely (relative risk, 2.35 [95% CI, 1.88–2.92]; P < 0.001) rather than being continued (25). In the pooled SYGMA analysis, 14% of patients stepping down from ICS or LTRA to as-needed SABA experienced a severe exacerbation within 12 months that required OCS, emergency department visit or hospitalization with systemic corticosteroid treatment. Given the evidence that as few as four lifetime courses of OCS significantly increase the risk of systemic OCS side effects (26), the present findings indicate a significant population-level opportunity to reduce OCS-related adverse effects in patients with mild asthma. Environmental triggers such as allergens, viral infections, and air pollution are highly variable over time, and the use of a reliever containing both ICS and FORM as soon as symptoms occur or increase may prevent both the need for daily, long-term ICS treatment and the need for courses of OCS in patients with mild asthma.
Strengths and Limitations
Strengths of this analysis are the large populations involved and their direct applicability to two common clinical scenarios: stepping up from as-needed SABA and stepping down from regular ICS. Small treatment effects on the FEV1 and ACQ-5 score were nominally statistically significant given that the study was overpowered for these endpoints, but both were well below degrees accepted as being clinically important. A limitation of the pooled analysis was its post hoc nature, but analysis by prestudy treatment group was prespecified for both SYGMA 1 and SYGMA 2. A limitation of the studies was that the double-blind design required for regulatory purposes meant that patients in all three treatment groups received a twice-daily “maintenance” inhaler, thus preventing an assessment of the impact of as-needed BUD–FORM on real-life factors such as adherence. Adherence to maintenance treatment was higher overall in SYGMA 1 than in SYGMA 2, reflecting the closer monitoring, twice-daily adherence reminders, and frequent study visits employed in SYGMA 1.
Conclusions
In conclusion, in this pooled analysis of SYGMA 1 and 2, the rate of severe exacerbations among patients whose asthma was uncontrolled on short-acting bronchodilators at study entry was significantly lower with as-needed BUD–FORM than with BUD maintenance, suggesting that as-needed low-dose ICS–FORM may be preferred over maintenance ICS as an initial controller treatment for patients with mild asthma currently receiving SABA alone. Among patients whose asthma was well controlled on ICS or LTRA at study entry, the similar rate of severe exacerbations after randomization to as-needed BUD–FORM versus BUD maintenance, with similar levels of symptom control, supports recent clinical recommendations for as-needed BUD–FORM as an alternative option for patients whose asthma is well controlled on low-dose ICS (5, 21). Furthermore, the marked reduction in risk of severe exacerbations with either ICS-containing strategy compared with as-needed SABA confirms clinical guidelines that adults and adolescents with asthma should not stop ICS completely, but may be better protected by switching to as-needed ICS–FORM than by reverting to SABA-only treatment.
Acknowledgments
Acknowledgment
The authors thank the healthcare providers, research staff, patients, and caregivers who participated in this trial and thank Samantha Blakemore of inScience Communications, Springer Healthcare Ltd., United Kingdom, for providing medical writing support.
Footnotes
Supported by AstraZeneca. Writing support was funded by AstraZeneca in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp3).
Author Contributions: E.D.B., P.M.O’B., J.M.F., P.J.B., and H.K.R. contributed to the study concept and design, data interpretation and writing, and review and approval of the final version to be published. J.Z. contributed to data interpretation and review and approval of the final version to be published. R.L., M.P., H.P., and V.A. contributed to data preparation, statistical analysis, data interpretation, and review and approval of the final version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Dusser D, Montani D, Chanez P, de Blic J, Delacourt C, Deschildre A, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy . 2007;62:591–604. doi: 10.1111/j.1398-9995.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 2.British Thoracic Society. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2019. https://www.sign.ac.uk/media/1773/sign158-updated.pdf [Google Scholar]
- 3.National Heart Lung and Blood Institute Bethesda, MD: National Heart, Lung, and Blood Institute; 2007https://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. [Google Scholar]
- 4. Reddel HK, Ampon RD, Sawyer SM, Peters MJ. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey. BMJ Open . 2017;7:e016688. doi: 10.1136/bmjopen-2017-016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reddel HK, FitzGerald JM, Bateman ED, Bacharier LB, Becker A, Brusselle G, et al. GINA 2019: a fundamental change in asthma management. Treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J . 2019;53:1901046. doi: 10.1183/13993003.01046-2019. [DOI] [PubMed] [Google Scholar]
- 6. Jónasson G, Carlsen KH, Sødal A, Jonasson C, Mowinckel P. Patient compliance in a clinical trial with inhaled budesonide in children with mild asthma. Eur Respir J . 1999;14:150–154. doi: 10.1034/j.1399-3003.1999.14a25.x. [DOI] [PubMed] [Google Scholar]
- 7. Shahidi N, Fitzgerald JM. Current recommendations for the treatment of mild asthma. J Asthma Allergy . 2010;3:169–176. doi: 10.2147/JAA.S14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J . 2017;50:1701103. doi: 10.1183/13993003.01103-2017. [DOI] [PubMed] [Google Scholar]
- 9. Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med . 2014;24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lacasse Y, Archibald H, Ernst P, Boulet LP. Patterns and determinants of compliance with inhaled steroids in adults with asthma. Can Respir J . 2005;12:211–217. doi: 10.1155/2005/375454. [DOI] [PubMed] [Google Scholar]
- 11. Bhagat R, Swystun VA, Cockcroft DW. Salbutamol-induced increased airway responsiveness to allergen and reduced protection versus methacholine: dose response. J Allergy Clin Immunol . 1996;97:47–52. doi: 10.1016/s0091-6749(96)70282-8. [DOI] [PubMed] [Google Scholar]
- 12. Aldridge RE, Hancox RJ, Robin Taylor D, Cowan JO, Winn MC, Frampton CM, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med . 2000;161:1459–1464. doi: 10.1164/ajrccm.161.5.9906052. [DOI] [PubMed] [Google Scholar]
- 13. Cockcroft DW, O’Byrne PM, Swystun VA, Bhagat R. Regular use of inhaled albuterol and the allergen-induced late asthmatic response. J Allergy Clin Immunol . 1995;96:44–49. doi: 10.1016/s0091-6749(95)70031-5. [DOI] [PubMed] [Google Scholar]
- 14. Reddel HK, Busse WW, Pedersen S, Tan WC, Chen Y-Z, Jorup C, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet . 2017;389:157–166. doi: 10.1016/S0140-6736(16)31399-X. [DOI] [PubMed] [Google Scholar]
- 15. Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, et al. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med . 1998;157:403–409. doi: 10.1164/ajrccm.157.2.96-08040. [DOI] [PubMed] [Google Scholar]
- 16. Wraight JM, Hancox RJ, Herbison GP, Cowan JO, Flannery EM, Taylor DR. Bronchodilator tolerance: the impact of increasing bronchoconstriction. Eur Respir J . 2003;21:810–815. doi: 10.1183/09031936.03.00067503. [DOI] [PubMed] [Google Scholar]
- 17. O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med . 2018;378:1865–1876. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 18. Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med . 2018;378:1877–1887. doi: 10.1056/NEJMoa1715275. [DOI] [PubMed] [Google Scholar]
- 19. Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, et al. Novel START Study Team. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med . 2019;380:2020–2030. doi: 10.1056/NEJMoa1901963. [DOI] [PubMed] [Google Scholar]
- 20. Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet . 2019;394:919–928. doi: 10.1016/S0140-6736(19)31948-8. [DOI] [PubMed] [Google Scholar]
- 21.Global Initiative for Asthma. Fontana, WI: Global Initiative for Asthma; 2020. www.ginasthma.org/reports/ [Google Scholar]
- 22. Bruce P, Hatter L, Beasley R. Anti-inflammatory reliever therapy in asthma: the evidence mounts but more is needed [editorial] Respirology . 2020;25:776–778. doi: 10.1111/resp.13889. [DOI] [PubMed] [Google Scholar]
- 23. Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med . 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 24. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med . 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 25.Rank MA, Hagan JB, Park MA, Podjasek JC, Samant SA, Volcheck GW, et al. The risk of asthma exacerbation after stopping low-dose inhaled corticosteroids: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;131:724–729. doi: 10.1016/j.jaci.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 26. Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy . 2018;11:193–204. doi: 10.2147/JAA.S176026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altman DG. Confidence intervals for the number needed to treat. BMJ . 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]


