Abstract
The supraspinal pathophysiology of the painful neuropathy induced by paclitaxel, a chemotherapeutic agent, is not well understood. The γ-aminobutyric acid (GABA) neurotransmitter system has been implicated in the pathogenesis of neuropathic pain. Gene expression of GABAergic system molecules was examined in the anterior cingulate cortex (ACC) of mice brains, by real-time PCR, during paclitaxel-induced neuropathic pain, because this area is involved in pain perception and modulation that might contribute to neuropathic pain. Paclitaxel treatment resulted in thermal hyperalgesia and in increased GABA transporter-1 (GAT-1) mRNA expression, but not that of other GABA transporters or GABAergic enzymes in the ACC compared to vehicle treatment. Among the 18 GABAA receptor subunits analyzed, only β2, β3, δ, and γ2 had increased mRNA levels, and for the GABAB receptor subunit, only GABAB2 had increased mRNA levels in the ACC of paclitaxel-treated mice, whereas the rest of the GABA receptor subunits were not altered. The mRNA expression of GABAA receptor subunits α6, θ, π, ρ1, ρ2, and ρ3 were not detected in the ACC. In conclusion, these data show that during paclitaxel-induced neuropathic pain there is significant increase in GAT-1 expression in the ACC. GAT-1 is the main transporter of GABA from the synapse, and thus its increased expression possibly results in less GABA at the synapse and dysregulation of the GABAergic system. GAT-1 is a potential therapeutic target for managing paclitaxel-induced neuropathic pain.
Key words: Chemotherapy-induced neuropathic pain, Paclitaxel, Anterior cingulate cortex (ACC), γ-Aminobutyric acid (GABA), GABA transporter, GABA receptor
INTRODUCTION
Pain is a complex experience involving sensory, affective, cognitive, and emotional components processed in neural networks that span from the periphery to the brain (1). Recent studies suggest that areas of the cerebral cortex such as the anterior cingulate cortex (ACC), the insular cortex, ventrolateral cortex, and motor cortex are involved in pain modulation (1). The ACC and other prefrontal cortex areas might be involved in either the sensory experience of the heightened pain perception or the modulatory circuits that contribute to the hyperalgesia (2). Neurophysiological and molecular changes have been observed in these areas in conditions of chronic pain or neuropathic pain (2–5).
Imaging and neurophysiology studies have shown increased activity in the ACC in patients and animal models of neuropathic pain (5–9). The major inhibitory neurotransmitter γ-aminobutyric acid (GABA) has a regulatory control on the levels of neuronal excitability (10–12). Thus, reduced GABAergic activity can result in increased neuronal activity in the ACC associated with neuropathic pain. There are no studies that report evaluation of molecular or electrophysiological changes in the GABAergic system in the ACC of animal models of neuropathic pain. However, administration of GABA into the ACC of rats with neuropathic pain has been shown to attenuate place escape/avoidance behavior associated with noxious mechanical stimulus (13), suggesting a possible role or changes of the GABAergic system in the ACC during neuropathic pain.
The GABAergic system consists of GABA, which is synthesized from glutamate via the activity of glutamate decarboxylase (GAD), of which there are two isoforms, GAD65 and GAD67 (14,15). GABA is catabolized by the enzyme GABA transaminase (GABA-T). GABAergic neurons secrete GABA into the synaptic cleft where it binds to GABA receptors on postsynaptic neurons to inhibit the activity of these neurons. GABA receptors are classified into the ionotropic GABAA receptors and the metabotropic GABAB receptors. There are 19 GABAA receptor subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π, and ρ1–3) and two principle GABAB receptor subunits GABAB1 and GABAB2 (16–18). GABA is removed from the postsynaptic cleft by GABA transporters (GATs). There are four GABA transporters GAT-1–4 of which the most abundant in the brain is GAT-1 (19–21).
In this study, the gene expression of molecules of the GABAergic system in the ACC was evaluated in mice treated with paclitaxel, at a time point when these mice have paclitaxel-induced thermal hyperalgesia. Paclitaxel is a chemotherapeutic agent whose use is limited by the development of dose-limiting painful peripheral neuropathy (22,23). Various studies have examined the inflammatory changes that occur during paclitaxel-induced neuropathic pain (PINP) in the CNS (24–26), but none have examined changes in the GABAergic neurotransmitter system in the cortex.
MATERIALS AND METHODS
Animals
Female BALB/c mice (8 to 12 weeks old; 20–30 g, n = 40) were used and were supplied by the breeding unit at the Health Sciences Center, Kuwait University, Kuwait, and were kept in temperature-controlled (24 ± 1°C) rooms with food and water ad libitum. All experiments were performed during the same period of the day (8:00 AM to 4:00 PM) to exclude diurnal variations in pharmacological effects. The animals were handled in compliance with Animal Resources Centre of the Kuwait University Health Sciences Center guidelines and the European Communities Council Directive 86/609 for the care of laboratory animals and ethical guidelines for research in experimental pain with conscious animals (27). All procedures were approved by the Ethical Committee for the Use of Laboratory Animals in Teaching and in Research, Health Sciences Centre, Kuwait University.
Administration of Paclitaxel
Paclitaxel (Tocris, Bristol, UK) was dissolved in a solution made up of 50% Cremophor EL and 50% absolute ethanol to a concentration of 6 mg/ml and stored at −20°C for a maximum of 14 days. The 6-mg/ml paclitaxel solution was then diluted in normal saline (NaCl 0.9%) to a final concentration of 0.2 mg/ml just before administration. The vehicle for paclitaxel was diluted at the time of injection with normal saline in the same proportion as the paclitaxel solution. Paclitaxel 2 mg/kg or its vehicle was administered to mice intraperitoneally (IP) in a volume of 10 ml/kg, once per day for 5 consecutive days; the cumulative dose of paclitaxel was 10 mg/kg. This treatment regimen has been reported to produce painful neuropathy and thermal hyperalgesia in mice (25,28).
Assessment of Thermal Nociception
Reaction latencies to the hot plate test were measured, as described previously (25), before (baseline latency) and at day 7 after the first injection of paclitaxel. Briefly, mice were individually placed on a hot plate (Panlab SL, Barcelona, Spain) with the temperature adjusted to 55 ± 1°C. The time to the first sign of nociception, paw licking, flinching, or jump response to avoid the heat was recorded, and the animal immediately removed from the hot plate. A cutoff period of 20 s was maintained to avoid damage to the paws.
ACC Tissue Preparation
On day 7 post-first administration of paclitaxel, mice were anesthetized with isoflurane and sacrificed by decapitation. Brains were dissected, and 1-mm coronal sections obtained at the level of the lateral ventricles using a mouse brain slicer (Zivic Instruments, Pittsburgh, PA, USA). Brain slices were placed on a Petri dish in ice, and the ACC was punched out using a 1000 Micron Tissue Biopsy Punch (Zivic Instruments), placed in a solution of 1 ml TRIzol (Invitrogen, Carlsbad, CA, USA) plus 10 µl β-mercaptoethanol (Merck, Hoenbrunn, Germany), snap frozen in liquid nitrogen, and kept at −70°C prior to mRNA extraction.
Gene Expression Analysis by Real-Time PCR
Gene transcripts of GAD65, GAD67, GABA-T, mGAT1, mGAT2 (BGT-1), mGAT3, mGAT4, GABAA receptor subunits (α1, α2, α3, α4, α5, α6, β1, β2, β3, γ1, γ2, δ, ε, θ, π, ρ1, ρ2, and ρ3), and GABAB receptor subunits (GABAB1 and GABAB2) were quantified in the ACC dissected out from mice sacrificed at 7 days after first administration of vehicle or paclitaxel by real-time PCR. Total RNA was extracted from the fresh frozen ACC using the RNeasy Kit (Qiagen GmbH), following the manufacturer’s instructions for small amounts of tissue and reverse-transcribed as described previously (29). The mRNA levels were quantified on an ABI Prism® 7500 sequence detection system (Applied Biosystems) as previously described (30). The primer sequences that were used, listed in Table 1, were ordered from Invitrogen (Life Technologies). Threshold cycle (Ct) values for all cDNA samples were obtained, and the amount of mRNA of the individual animal sample (n = 10–20 per group) was normalized to cyclophilin (ΔCt). The relative amount of target gene transcripts was calculated using the 2−ΔΔCt method, as described previously (31). These values were then used to calculate the mean and standard error of the relative expression of the target gene mRNA in the brain of drug- and vehicle-treated mice.
Table 1.
PCR Primer Sequences of Cyclophilin and GABAergic System Molecules
| Gene | Polarity | |
|---|---|---|
| Sense Sequence 5′ to 3′ |
Antisense Sequence 5′ to 3′ |
|
| Cyclophilin | GCTTTTCGCCGCTTGCT | CTCGTCATCGGCCGTGAT |
| GAD65 | GCTGGAACCACCGTGTATGG | TCCACGTGCATCCAGATCTTAT |
| GAD67 | TCCACCATCAACGGCATTAA | AGCGGCAGGTGTTGGATAAC |
| GABA-T | GGGGTCATGGCCTTCTTGTT | AGTGGTCCATCATAATCAAATTCAA |
| mGAT1 | TAACAACAACAGCCCATCCA | GGAGTAACCCTGCTCCATGA |
| mGAT2 (BGT-1) | GGTCCCTGAGGAAGGAGAGAT | GGGGATGAAGAAAGCTCCACC |
| mGAT3 | CCTCCATGATCTGCATTCCT | CCAAATACCCCCTTTCGTCT |
| mGAT4 | TTTGGTCTTCCCCTTTTCCT | AAGACTCCACTCAACCCCCT |
| GABAA α1 | TGCTGGACGGTTATGACAAT | GAAACTGGTCCGAAACTGGT |
| GABAA α2 | ACAACCTTGAGCATCAGTGC | AATTCACGGTTGCAAATTCA |
| GABAA α3 | GACAGTCCTGCTGAGACCAA | ATAGCTGATTCCCGGTTCAC |
| GABAA α4 | AGAACTCAAAGGACGAGAAATTGT | TTCACTTCTGTAACAGGACCCC |
| GABAA α5 | TCCATTGCACACAACATGAC | GCAGAGATTGTCAGACGCAT |
| GABAA α6 | GGTGACCGGGCATCCCAGTGA | TGTTACAGCACCCCCAAATCCTGGC |
| GABAA β1 | GGTTTGTTGTGCACACAGCTCC | ATGCTGGCGACATCGATCCGC |
| GABAA β2 | AGCTGCTAATGCCAACAATG | GTCCCATTACTGCTTCGGAT |
| GABAA β3 | CAAAGCCATCGACATGTACC | CTTCTCCGCAAGCTTCTTCT |
| GABAA γ1 | ATCCACTCTCATTCCCATGAACAGC | ACAGAAAAAGCTAGTACAGTCTTTGC |
| GABAA γ2 | TGGTCACCGAATGTGTTTCT | TACTTTGCCTTGCAGGTTTG |
| GABAA δ | TCAAATCGGCTGGCCAGTTCCC | GCACGGCTGCCTGGCTAATCC |
| GABAA ε | ACTGCGCCCTGGCATTGGAG | AGGCCCGAGGCTGTTGACAA |
| GABAA θ | GCTGGAGGTGGAGAGCTATGGCT | CCCCAGGTACGTGTACTGAGGGA |
| GABAA π | TCGGTGGTGACCCAGTTCGGAT | TCTGTCCAACGCTGCCGGAG |
| GABAA ρ1 | CCATCTAGGAAAGGCAGCAG | GAGCTTCGTCTCAGGATTGG |
| GABAA ρ2 | GCTGCCTGTTGCATCATAGA | ATACAAATGGCTTGGCTTGG |
| GABAA ρ3 | CAACTCAACAGGAGGGGAAA | TCCACATCAGTCTCGCTGTC |
| GABAB1 | ACGTCACCTCGGAAGGTTG | CACAGGCAGGAAATTGATGGC |
| GABAB2 | CAGCAAGCGTTCGGGTGTA | GTCTTGGCGATGACCCAGAT |
Statistical Analyses
Statistical analyses were performed using Mann–Whitney U-test. The differences were considered significant at p < 0.05. The results in the text and figures are expressed as the means ± SEM.
RESULTS
Paclitaxel-Induced Thermal Hyperalgesia
Paclitaxel produced a significant reduction in response latency time to thermal stimuli (thermal hyperalgesia) on day 7 after first drug administration compared to the baseline latency (pretreatment values) and vehicle-only-treated animals in the hot plate test, as previously described (data not shown) (25).
Expression of Transcripts of Enzymes Involved in the Biosynthesis and Catabolism of GABA in the ACC at 7 Days After Paclitaxel Administration
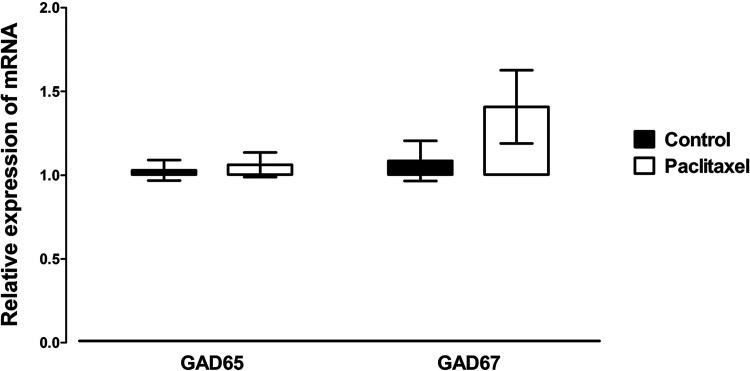
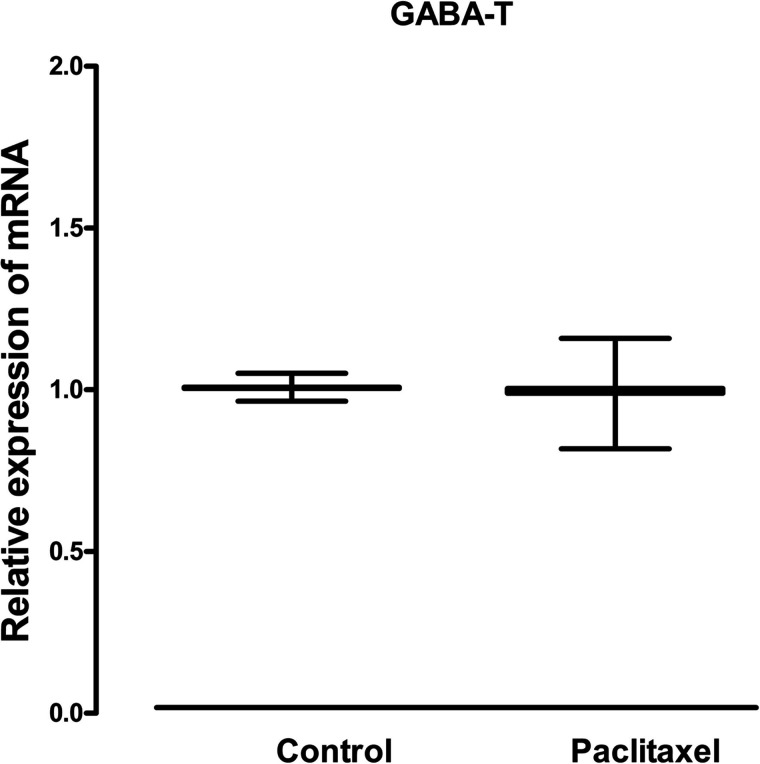
Treatment with paclitaxel did not significantly alter the mRNA expression of either the two enzymes involved in the biosynthesis of GABA GAD65 and GAD67 (Fig. 1) or the enzyme involved in its catabolism GABA-T (Fig. 2) in the ACC of mice compared to treatment with vehicle.
Figure 1.
Effects of treatment with paclitaxel on relative GAD65 and GAD67 mRNA expression in the ACC of BALB/c mice on day 7 after first administration of the drug or its vehicle. Each point represents the mean ± SEM of the values obtained from 14–20 animals. No statistically significant differences were observed between paclitaxel- and vehicle-treated animals.
Figure 2.
Effects of treatment with paclitaxel on relative GABA-T mRNA expression in the ACC of BALB/c mice on day 7 after first administration of the drug or its vehicle. Each point represents the mean ± SEM of the values obtained from 10–12 animals. No statistically significant differences were observed between paclitaxel- and vehicle-treated animals.
Expression of Transcripts of GABA Transporters in the ACC at 7 Days After Paclitaxel Administration
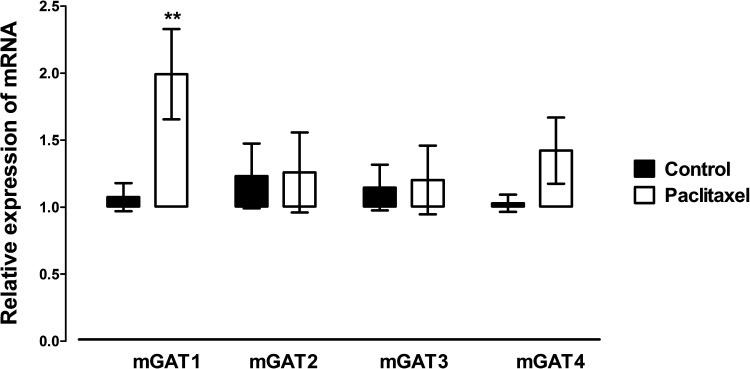
Of the four GATs quantified, mGAT1, mGAT2 (BGT-1), mGAT3, and mGAT4, only mGAT1 was significantly altered (p = 0.0076) in the ACC by treatment with paclitaxel compared to treatment with vehicle (Fig. 3). mGAT1 mRNA expression was increased in paclitaxel-treated mice by almost twofold compared to vehicle-treated controls (Fig. 3).
Figure 3.
Effects of treatment with paclitaxel on relative mouse GATs (mGAT) 1, mGAT2, mGAT3, and mGAT4 mRNA expression in the ACC of BALB/c mice on day 7 after first administration of the drug or its vehicle. Each point represents the mean ± SEM of the values obtained from 10–16 animals. **p < 0.01 compared to vehicle-treated control mice.
Expression of Transcripts of GABAA Receptor Subunits in the ACC at 7 Days After Paclitaxel Administration
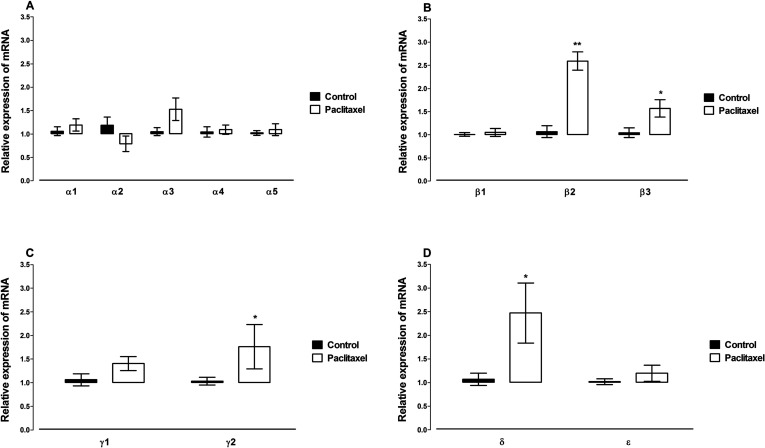
The mRNA expression of 18 GABAA receptor subunits, α1, α2, α3, α4, α5, α6, β1, β2, β3, γ1, γ2, δ, ε, θ, π, ρ1, ρ2, and ρ3, was quantified in the ACC. The mRNA expression of six GABAA receptor subunits, α6, θ, π, ρ1, ρ2, and ρ3, in the ACC was not detected by real-time PCR. Treatment with paclitaxel did not significantly alter the mRNA expression of the GABAA receptor α subunits α1, α2, α3, α4, and α5 (Fig. 4A). However, the paclitaxel treatment significantly increased the expression of the GABAA receptors β2 (p = 0.0003), β3 (p = 0.04), γ2 (p = 0.04), and δ (p = 0.02) subunits, but not β1, γ1, or ε (p > 0.05), compared to vehicle-treated controls (Fig. 4B–D).
Figure 4.
Effects of treatment with paclitaxel on relative GABAA receptor subunits (A) α subunits α1, α2, α3, α4, α5, (B) β subunits β1, β2, β3, (C) γ subunits γ1, γ2, (D) other subunits δ and ε mRNA expression in the ACC of BALB/c mice on day 7 after first administration of the drug or its vehicle. Each point represents the mean ± SEM of the values obtained from 10–20 animals. *p < 0.05, **p < 0.01 compared to vehicle-treated control mice.
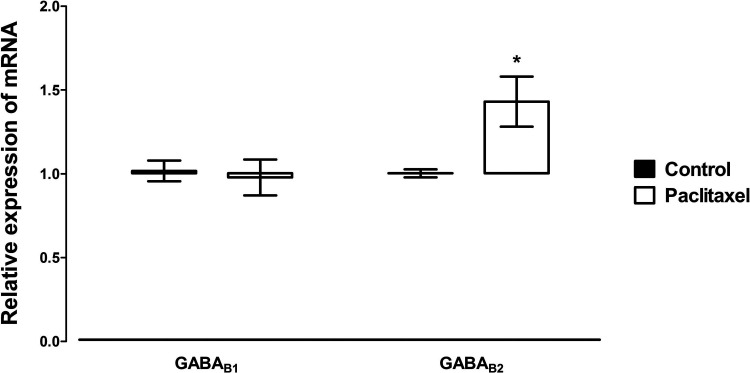
Expression of Transcripts of GABAB Receptor Subunits in the ACC at 7 Days After Paclitaxel Administration
Treatment with paclitaxel significantly increased the expression of the GABAB receptor subunit GABAB2 (p = 0.02), but not GABAB1 (p > 0.05), compared to vehicle-treated controls (Fig. 5).
Figure 5.
Effects of treatment with paclitaxel on relative GABAB receptor subunits GABAB1 and GABAB2 mRNA expression in the ACC of BALB/c mice on day 7 after first administration of the drug or its vehicle. Each point represents the mean ± SEM of the values obtained from 10–12 animals. *p < 0.05 compared to vehicle-treated control mice.
DISCUSSION
This is the first study to report altered gene expression of the GABAergic system in the ACC of an animal model of neuropathic pain. The results show that there was increased GAT-1; GABAA receptor subunits β2, β3, δ, and γ2; and GABAB receptor subunit GABAB2 mRNA expression in the ACC of paclitaxel-treated mice, whereas the rest of the molecules of the GABAergic system were either not altered or not detected, at a time point when these animals exhibited paclitaxel-induced thermal hyperalgesia.
The ACC and other prefrontal cortex areas are involved in pain perception and modulation (1–3,5). During neuropathic pain, altered expression of molecules from various neurotransmitter systems, such as the dopaminergic and the muscarinergic systems, have been observed in the ACC (32,33). These data suggest that there are molecular changes that might contribute to the increased activity observed in the ACC of patients and animal models of neuropathic pain using imaging and neurophysiology techniques (5–9).
GABA is the major inhibitory neurotransmitter in the CNS and has a regulatory control on the levels of neuronal excitability (10–12). GABA, the enzymes involved in its synthesis and catabolism, its receptors, and the transporters that terminate its activity in the synaptic cleft are all expressed in the ACC (34–37). However, to my knowledge, molecular changes of this essential GABAergic system in the ACC during neuropathic pain have not been reported as yet.
Changes in both GABA and GAD expression have been observed in the spinal cord of animal models of nerve or spinal cord injury and neuropathic pain (38–40). Eaton et al. observed an increased number of cells immunostaining for GAD67 but less GABA immunostaining (38). On the other hand, Moore et al. observed a reduction in both GABA and GAD65 expression, and Meisner et al. observed reduced immunostaining for both GAD65 and GAD67 (39,40). Lee et al. did not observe any changes in the expression of either transcripts or protein of GAD65 and GAD67 in the spinal cord after nerve injury at a time when the animals had developed neuropathic pain (41). There are no studies published that have evaluated the expression of these enzymes supraspinally in animal models of neuropathic pain. In the current study, there were no changes in GAD65 and GAD67 expression in the ACC of mice with PINP.
Vigabatrin, which is an inhibitor of GABA-T, has been shown to have analgesic activity against neuropathic pain (42). However, the expression of GABA-T at the spinal cord or supraspinal sites during neuropathic pain has not been reported. Similar to GAD enzymes, no changes in GABA-T expression were observed in the ACC of mice with PINP.
In animal models of nerve or spinal cord injury and neuropathic pain, GAT-1 expression was found to be decreased (39,43,44) or increased (45) in the spinal cord. Lee et al. did not observe any changes in the expression of GAT-1 and GAT-3 in the spinal cord after nerve injury, at a time when the animals had developed neuropathic pain (41). One study evaluated the expression of GATs in a supraspinal site, the gracile nucleus in the brain stem, in a nerve injury animal model of neuropathic pain and observed an increase in the expression of GAT-1, with no change in GAT-3 expression (46). However, both GAT-1 and GAT-3 were not altered in the cuneate nucleus of the brain stem (46). In the present study, among the four GATs, only GAT-1 expression was significantly increased in the ACC of mice with PINP. GAT-1 is the most abundantly expressed GAT and is responsible for most of the GABA uptake in the brain (19,20,47). The association of GAT-1 with pain has also been shown in transgenic animal models where overexpression of GAT-1 results in hyperalgesia (48). The GAT-1 overexpression was observed in the various areas in the brain including the ACC of these transgenic mice (48). Mice with deficiency in GAT-1 caused by genetic knockout had hypoalgesia (49). Moreover, administration of NO-711, a GAT-1 inhibitor, has been shown to decrease pain in an animal model of neuropathic pain induced by sciatic nerve ligation (45). Thus, it is plausible to suggest that the upregulation of GAT-1 expression in the ACC of mice with PINP contributes to the pathophysiology of the hyperalgesia observed in these mice and is a potential therapeutic candidate.
Administration of GABA receptor agonists peripherally, spinally, or supraspinally has been reported to attenuate or modulate neuropathic pain in animal models (13,41,50–54). The expression of GABA receptors is altered in the dorsal root ganglia and spinal cord during neuropathic pain (55–59). In the current study, among the GABA receptor subunits, only the expression of GABAA receptor β2, β3, γ2, and δ subunits and GABAB receptor GABAB2 subunit were significantly increased in the ACC of mice with PINP. The GABAA receptor α subunits were not altered, which is in agreement with Okabe et al.’s study where they did not observe any change in the expression of GABAA α1 receptor subunit in the brain during neuropathic pain (60). In the current study, the mRNA expression of six GABAA receptor subunits, α6, θ, π, ρ1, ρ2, and ρ3, was not detected in the ACC. The expression of GABAA α6 receptor subunit is restricted, and π subunit has not been observed in the brain (18). Thus, the present data confirm that some of the GABAA receptor subunits are not expressed in the ACC and give new information on GABA receptor subunits differentially expressed in the ACC during neuropathic pain.
In conclusion, this study shows for the first time that during PINP there is significant increase in GAT-1 mRNA expression in the ACC. The increased expression of GAT-1 possibly results in less GABA at the synapse and dysregulation of the GABAergic system and the resultant increase in the expression of some GABA receptor subunits in the ACC. Thus, GAT-1 is a potential therapeutic target for managing PINP and chemotherapy-induced neuropathic pain in general.
ACKNOWLEDGMENTS
This study was supported by grants PT01/09 and SRUL02/13 from Kuwait University Research Sector. I am grateful to Dr. Subramanian S. Parvathy and Ms. Salini Somani for their technical assistance and to the staff from the Animal Resources Centre, HSC, Kuwait University, for their support.
Footnotes
The author declares no conflict of interest.
REFERENCES
- 1. Xie YF, Huo FQ, Tang JS. Cerebral cortex modulation of pain. Acta Pharmacol Sin 2009; 30:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage 2009; 47:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain 2006; 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 2009; 141:52–59. [DOI] [PubMed] [Google Scholar]
- 5. Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 2008; 28:7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao XY, Xu H, Wu LJ, Li XY, Chen T, Zhuo M. Characterization of intrinsic properties of cingulate pyramidal neurons in adult mice after nerve injury. Mol Pain 2009; 5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moisset X, Villain N, Ducreux D, Serrie A, Cunin G, Valade D, et al. Functional brain imaging of trigeminal neuralgia. Eur J Pain 2011; 15:124–131. [DOI] [PubMed] [Google Scholar]
- 8. Tseng MT, Chiang MC, Chao CC, Tseng WY, Hsieh ST. fMRI evidence of degeneration-induced neuropathic pain in diabetes: Enhanced limbic and striatal activations. Hum Brain Mapp 2013; 34:2733–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, et al. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 2006; 26:8923–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 2003; 6:484–490. [DOI] [PubMed] [Google Scholar]
- 11. Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: Modulating gain and maintaining the tone. Trends Neurosci 2004; 27:262–269. [DOI] [PubMed] [Google Scholar]
- 12. Whittington MA, Lambert JD, Little HJ. Increased NMDA receptor and calcium channel activity underlying ethanol withdrawal hyperexcitability. Alcohol 1995; 30:105–114. [PubMed] [Google Scholar]
- 13. LaGraize SC, Fuchs PN. GABAA but not GABAB receptors in the rostral anterior cingulate cortex selectively modulate pain-induced escape/avoidance behavior. Exp Neurol 2007; 204:182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron 1991; 7:91–100. [DOI] [PubMed] [Google Scholar]
- 15. Pinal CS, Tobin AJ. Uniqueness and redundancy in GABA production. Perspect Dev Neurobiol 1998; 5:109–118. [PubMed] [Google Scholar]
- 16. Gassmann M, Bettler B. Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat Rev Neurosci 2012; 13:380–394. [DOI] [PubMed] [Google Scholar]
- 17. Olsen RW, Sieghart W. GABA A receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology 2009; 56:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uusi-Oukari M, Korpi ER. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol Rev 2010; 62:97–135. [DOI] [PubMed] [Google Scholar]
- 19. Borden LA. GABA transporter heterogeneity: Pharmacology and cellular localization. Neurochem Int 1996; 29:335–356. [DOI] [PubMed] [Google Scholar]
- 20. Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: Localization, development and pathological implications. Brain Res Brain Res Rev 2004; 45:196–212. [DOI] [PubMed] [Google Scholar]
- 21. Madsen KK, White HS, Schousboe A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther 2010; 125:394–401. [DOI] [PubMed] [Google Scholar]
- 22. Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol 2006; 72:151–169. [PubMed] [Google Scholar]
- 23. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur J Cancer 2008; 44:1507–1515. [DOI] [PubMed] [Google Scholar]
- 24. Burgos E, Gomez-Nicola D, Pascual D, Martin MI, Nieto-Sampedro M, Goicoechea C. Cannabinoid agonist WIN 55,212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur J Pharmacol 2012. [DOI] [PubMed] [Google Scholar]
- 25. Parvathy SS, Masocha W. Matrix metalloproteinase inhibitor COL-3 prevents the development of paclitaxel-induced hyperalgesia in mice. Med Princ Pract 2013; 22:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang H, Yoon SY, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain 2012; 13:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16:109–110. [DOI] [PubMed] [Google Scholar]
- 28. Nieto FR, Entrena JM, Cendan CM, Pozo ED, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain 2008; 137:520–531. [DOI] [PubMed] [Google Scholar]
- 29. Masocha W, Robertson B, Rottenberg ME, Mhlanga J, Sorokin L, Kristensson K. Cerebral vessel laminins and IFN-gamma define Trypanosoma brucei brucei penetration of the blood-brain barrier. J Clin Invest 2004; 114:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masocha W. Systemic lipopolysaccharide (LPS)-induced microglial activation results in different temporal reduction of CD200 and CD200 receptor gene expression in the brain. J Neuroimmunol 2009; 214:78–82. [DOI] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 32. Ortega-Legaspi JM, de Gortari P, Garduno-Gutierrez R, Amaya MI, Leon-Olea M, Coffeen U, et al. Expression of the dopaminergic D1 and D2 receptors in the anterior cingulate cortex in a model of neuropathic pain. Mol Pain 2011; 7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ortega-Legaspi JM, Leon-Olea M, de Gortari P, Amaya MI, Coffeen U, Simon-Arceo K, et al. Expression of muscarinic M1 and M2 receptors in the anterior cingulate cortex associated with neuropathic pain. Eur J Pain 2010; 14:901–910. [DOI] [PubMed] [Google Scholar]
- 34. Long Z, Medlock C, Dzemidzic M, Shin YW, Goddard AW, Dydak U. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry 2013; 44:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 2008; 508:906–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: Evaluation of the four-region neurobiological model. Hum Brain Mapp 2009; 30:2336–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res 2009; 43:970–977. [DOI] [PubMed] [Google Scholar]
- 38. Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat 1998; 16:57–72. [DOI] [PubMed] [Google Scholar]
- 39. Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma 2010; 27:729–737. [DOI] [PubMed] [Google Scholar]
- 40. Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci 2002; 22:6724–6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee J, Back SK, Lim EJ, Cho GC, Kim MA, Kim HJ, et al. Are spinal GABAergic elements related to the manifestation of neuropathic pain in rat? Korean J Physiol Pharmacol 2010; 14:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alves ND, de Castro-Costa CM, de Carvalho AM, Santos FJ, Silveira DG. Possible analgesic effect of vigabatrin in animal experimental chronic neuropathic pain. Arq Neuropsiquiatr 1999; 57:916–920. [DOI] [PubMed] [Google Scholar]
- 43. Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain 2003; 105:347–353. [DOI] [PubMed] [Google Scholar]
- 44. Shih A, Miletic V, Miletic G, Smith LJ. Midazolam administration reverses thermal hyperalgesia and prevents gamma-aminobutyric acid transporter loss in a rodent model of neuropathic pain. Anesth Analg 2008; 106:1296–1302, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Daemen MA, Hoogland G, Cijntje JM, Spincemaille GH. Upregulation of the GABA-transporter GAT-1 in the spinal cord contributes to pain behaviour in experimental neuropathy. Neurosci Lett 2008; 444:112–115. [DOI] [PubMed] [Google Scholar]
- 46. Gosselin RD, Bebber D, Decosterd I. Upregulation of the GABA transporter GAT-1 in the gracile nucleus in the spared nerve injury model of neuropathic pain. Neurosci Lett 2010; 480:132–137. [DOI] [PubMed] [Google Scholar]
- 47. Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: Differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol 2003; 90:2690–2701. [DOI] [PubMed] [Google Scholar]
- 48. Hu JH, Yang N, Ma YH, Zhou XG, Jiang J, Duan SH, et al. Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice. J Neurosci Res 2003; 73:565–572. [DOI] [PubMed] [Google Scholar]
- 49. Xu YF, Cai YQ, Cai GQ, Jiang J, Sheng ZJ, Wang ZG, et al. Hypoalgesia in mice lacking GABA transporter subtype 1. J Neurosci Res 2008; 86:465–470. [DOI] [PubMed] [Google Scholar]
- 50. Besson M, Daali Y, Di Lio A, Dayer P, Zeilhofer HU, Desmeules J. Antihyperalgesic effect of the GABA(A) ligand clobazam in a neuropathic pain model in mice: A pharmacokinetic-pharmacodynamic study. Basic Clin Pharmacol Toxicol 2013; 112:192–197. [DOI] [PubMed] [Google Scholar]
- 51. Hwang JH, Hwang KS, Kim JU, Choi IC, Park PH, Han SM. The interaction between intrathecal neostigmine and GABA receptor agonists in rats with nerve ligation Injury. Anesth Analg 2001; 93:1297–1303. [DOI] [PubMed] [Google Scholar]
- 52. Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology 2002; 96:1161–1167. [DOI] [PubMed] [Google Scholar]
- 53. Patel S, Naeem S, Kesingland A, Froestl W, Capogna M, Urban L, et al. The effects of GABA(B) agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain 2001; 90:217–226. [DOI] [PubMed] [Google Scholar]
- 54. Smith GD, Harrison SM, Birch PJ, Elliott PJ, Malcangio M, Bowery NG. Increased sensitivity to the antinociceptive activity of (+/-)-baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology 1994; 33:1103–1108. [DOI] [PubMed] [Google Scholar]
- 55. Engle MP, Merrill MA, Marquez De Prado B, Hammond DL. Spinal nerve ligation decreases gamma-aminobutyric acidB receptors on specific populations of immunohistochemically identified neurons in L5 dorsal root ganglion of the rat. J Comp Neurol 2012; 520:1663–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain 1998; 78:13–26. [DOI] [PubMed] [Google Scholar]
- 57. Wang XL, Zhang Q, Zhang YZ, Liu YT, Dong R, Wang QJ, et al. Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett 2011; 490:112–115. [DOI] [PubMed] [Google Scholar]
- 58. Wu J, Xu Y, Pu S, Jiang W, Du D. p38/MAPK inhibitor modulates the expression of dorsal horn GABA(B) receptors in the spinal nerve ligation model of neuropathic pain. Neuroimmunomodulation 2011; 18:150–155. [DOI] [PubMed] [Google Scholar]
- 59. Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA 2002; 99:8360–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Okabe T, Sato C, Matsumoto K, Ozawa H, Sakamoto A. Electroconvulsive stimulation (ECS) increases the expression of neuropeptide Y (NPY) in rat brains in a model of neuropathic pain: A quantitative real-time polymerase chain reaction (RT-PCR) study. Pain Med 2009; 10:1460–1467. [DOI] [PubMed] [Google Scholar]