Abstract
Objective:
Develop unifying definitions and paradigms for data-driven methods to augment postoperative resource intensity decisions.
Summary Background Data:
Postoperative level-of-care assignments and frequency of vital sign and laboratory measurements (i.e., resource intensity) should align with patient acuity. Effective, data-driven decision-support platforms could improve value of care for millions of patients annually, but their development is hindered by the lack of salient definitions and paradigms.
Methods:
Embase, PubMed, and Web of Science were searched for articles describing patient acuity and resource intensity after inpatient surgery. Study quality was assessed using validated tools. Thirty-five studies were included and assimilated according to PRISMA guidelines.
Results:
Perioperative patient acuity is accurately represented by combinations of demographic, physiologic, and hospital-system variables as input features in models that capture complex, non-linear relationships. Intraoperative physiologic data enriches these representations. Triaging high-acuity patients to low-intensity care is associated with increased risk for mortality; triaging low-acuity patients to ICUs has low value and imparts harm when other, valid requests for ICU admission are denied due to resource limitations, increasing their risk for unrecognized decompensation and failure-to-rescue. Providing high-intensity care for low-acuity patients may also confer harm through unnecessary testing and subsequent treatment of incidental findings, but there is insufficient evidence to evaluate this hypothesis. Compared with data-driven models, clinicians exhibit volatile performance in predicting complications and making postoperative resource intensity decisions.
Conclusions:
To optimize value, postoperative resource intensity decisions should align with precise, data-driven patient acuity assessments augmented by models that accurately represent complex, non-linear relationships among risk factors.
Mini-Abstract
Postoperative level-of-care assignments and frequency of vital sign and laboratory measurements (i.e., resource intensity) should align with patient acuity. Triaging high-acuity patients to low-intensity care increases risk for mortality; triaging low-acuity patients to ICUs has low value and imparts harm when other, valid requests for ICU admission are denied. Clinicians’ resource intensity decisions should be augmented by machine learning models integrated with clinical workflows.
Introduction
More than 15 million major, inpatient surgeries are performed each year in the United States. Complications occur in as many as 32% of these surgeries, increasing cost by approximately $11,000 per major complication.1–3 According to surgeons, judgement errors are the most common cause of major complications.4 When surgeons rely on hypothetical-deductive reasoning and heuristics to make time-sensitive decisions, judgement errors are expected and extend beyond the operating room.5, 6 Immediately after major surgery, risk for adverse events and death (patient acuity) should align with level-of-care assignments and the frequency of vital sign and laboratory measurements (resource intensity). When high-acuity patients receive low-intensity care, postoperative complications can progress to critical illness and cardiac arrest.7–11 Providing high-intensity care to low-acuity patients increases costs and may impart harm from unnecessary treatments.12–15
Most data regarding resource intensity decisions describe medical or mixed medical-surgical populations, limiting their generalizability to surgical patients: a population uniquely vulnerable to postoperative hemorrhage, respiratory failure, opioid-related adverse drug events, and hospital-acquired sepsis.16–18 Surgical literature is rife with prediction models that forecast postoperative outcomes, but lack of workflow integration impairs clinical adaptation, especially when busy clinicians face time constraints.19 Furthermore, associations between predictions and resource intensity decisions are rarely reported.19 Surgeons can easily infer relevant associations for extreme scenarios, but for common, daily resource intensity decisions, the absence of data-driven decision-making yields high variability and poor outcomes.5, 6 Finally, there are no consensus definitions for level-of-care that apply at national or international levels, which hinders systematic, hypothesis-driven investigation.
Toward the goal of developing data-driven methods to augment postoperative resource intensity decisions, this review distills existing surgical literature to develop validated, unifying definitions and paradigms. Given the substantial methodologic heterogeneity of relevant studies, a scoping review was performed to systematically map and critically evaluate available evidence. Major themes, supported by high-quality data, were united in proposing a conceptual framework to align postoperative resource intensity with patient acuity.
Methods
Embase, PubMed, and Web of Science databases were searched from their inception to September 22, 2020 for relevant articles (see figure, Supplemental Digital Content 1) published in English. Titles and abstracts were searched for specific terms and keywords to identify articles describing major, inpatient surgery and concepts relating to patient acuity and resource intensity. Reviews, editorials, letters, and conference abstracts were excluded by screening criteria. Seventy articles were identified. The article search filter could not identify case reports, case series, and other articles featuring low grade evidence; these article types were excluded by study quality assessments. Study quality was independently rated by two investigators using quality assessment tools specific to the design of the study in question (available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed 9/22/2020). Studies rated “poor” or deemed not relevant to review objectives were recommended for exclusion. There were disagreements between the two investigators regarding the exclusion of six articles; disagreements were resolved via adjudication by a third investigator (see table, Supplemental Digital Content 2). Cohen’s kappa statistic summarizing interrater agreement regarding article inclusion was 0.802 (observed agreement = 0.914, expected agreement = 0.566), suggesting that agreement between reviewers was substantial.20, 21 Fifty-one articles were excluded. Works cited by the remaining 19 articles were reviewed to identify other relevant articles, using the same inclusion and exclusion criteria. Another 16 articles were included in this manner. In total, 35 total studies were included and assimilated into relevant categories according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines (see table, Supplemental Digital Content 3).
Results
A conceptual framework for aligning patient acuity and resource intensity is proposed in Figure 1. This framework is applied to illustrate major findings from each study in Figure 2. The design, population, analytic approach, and major findings for each study are listed in the Supplemental Digital Content 4 table and described in detail with context below.
Figure 1:
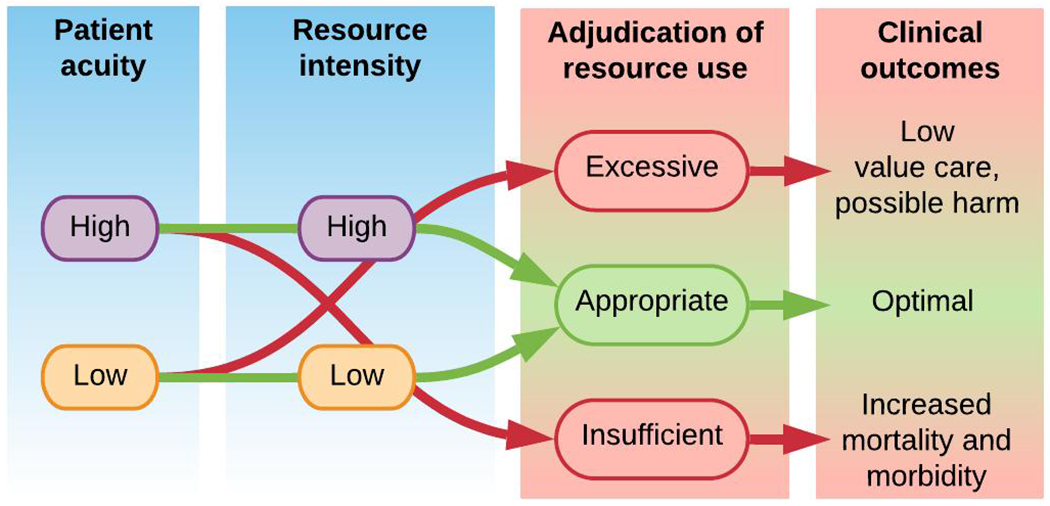
Conceptual framework for aligning patient acuity with resource intensity.
Figure 2: Published literature describes postoperative patient acuity and resource intensity, but rarely establishes alignment between them.
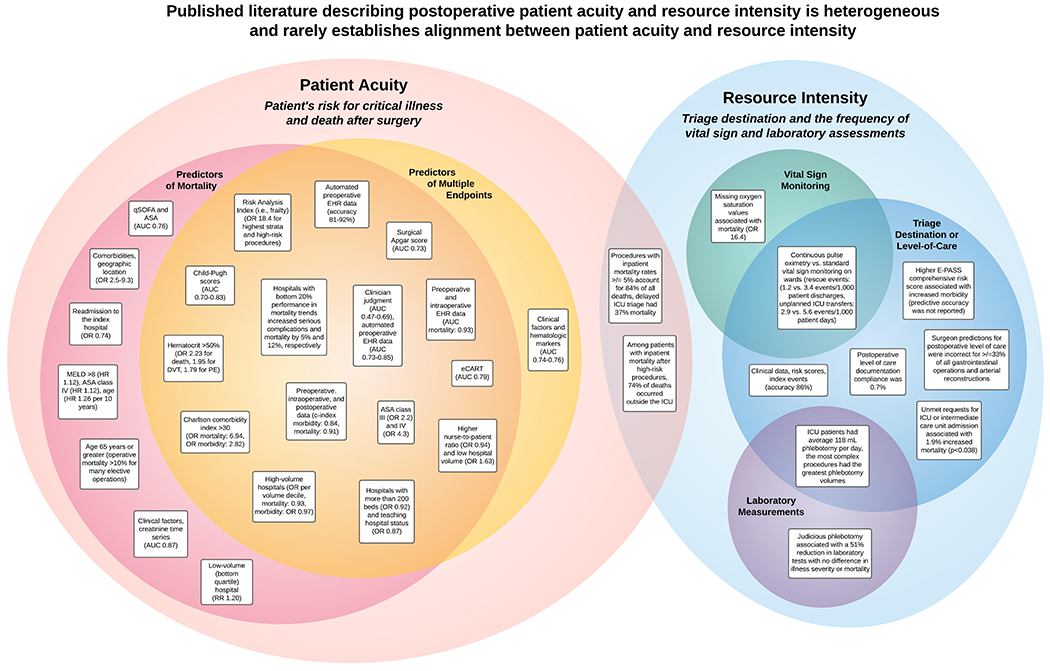
ASA: American Society of Anesthesiologists physical status classification system, AUC: area under the receiver operating characteristic curve, DVT: deep vein thrombosis, eCART: Electronic-Cardiac Arrest Risk Triage, E-PASS: Estimation of Physiologic Ability and Surgical Stress, EWS: early warning score, HR: hazard ratio, ICU: intensive care unit, MELD: Model for End-Stage Liver Disease, OR: odds ratio, PE: pulmonary embolism, qSOFA: quick sequential organ failure assessment score, RR: relative risk.
Classifying Patient Acuity
Risk for Mortality
Seven studies classified patient acuity according to risk for mortality (see table, Supplemental Digital Content 4). All were retrospective, with population sizes ranging from 825 to 1.2 million patients, and a median of 46 thousand patients per study. These studies demonstrated that risk for death after major, inpatient surgery was associated with, or predicted by, several factors: advanced age,22, 23 severe co-morbidities,24 mixed subjective and objective patient classification models (i.e., American Society of Anesthesiologists [ASA] physical status classification),23, 25 laboratory or vital sign abnormalities,26 organ dysfunction and failure,23, 25 surgery performed at low-volume hospitals,26 and readmission to a different hospital.27 Collectively, findings from included studies demonstrated that postoperative patient acuity can be accurately represented by risk for mortality, with the strongest associations—and most accurate predictions—obtained when incorporating multiple demographic, physiologic, and hospital characteristic variables.
Several other key findings emerged from articles that classify patient acuity by risk for mortality. Regarding the optimization of physiologic input features, Korenkevych et al.28 demonstrated that probabilistic modeling of non-linear creatinine time-series can improve model discrimination in predicting 90-day mortality (AUROC 0.87) compared with using static input features. Regarding hospital and health care delivery factors, Brooke et al.27 found that when managing post-discharge complications, the odds of death were lowest for patients being readmitted to the index hospital (OR 0.74). The authors note that readmission to the index hospital may represent a surrogate for geographic location, access to health care resources, and care fragmentation in which data and knowledge regarding the patient’s care is not shared between institutions. These variables have known, significant effects on patient outcomes.24 Finally, readmission to a different hospital that is closer to home often equates to receiving care at a low-volume hospital; a risk factor for postoperative mortality (RR 1.20 vs. the top volume quartile of hospitals), as identified by Forte et al.26 Therefore, continuously resampled physiologic variables and careful interpretation of hospital and health care delivery factors have the potential to optimize patient mortality predictions and acuity assessments after major surgery.
Risk for Multiple Complication Endpoints
Sixteen studies classified patient acuity according to risk for multiple complication endpoints (see table, Supplemental Digital Content 4). Two had prospective, observational designs; the rest were retrospective. Population sizes ranged from 150 patients to 1.4 million inpatient surgeries, with a median of 42,533 inpatient surgeries per study. Compared with studies analyzing risk for mortality, these 16 studies had an expanded list of factors that include: laboratory values,29, 30 early warning scores,31 organ dysfunction and failure,32 comorbidities,33 frailty,34 mixed subjective and objective patient classification models (i.e., ASA physical status classification),35 combinations of clinical, demographic, and administrative variables,36–39 hospital volume,40–42 and system factors that potentiate failure-to-rescue.43 Associations and predictions were strongest when incorporating multiple demographic, physiologic, and hospital-level variables.
Several other key findings emerged from articles that classify patient acuity by risk for complications. Bartkowiak et al.31 and Bertsimas et al.44 each compared predictive performance across multiple analytic methods; in both studies, methods with greatest discrimination used larger input feature sets and had greater ability to capture complex, non-linear relationships among input variables (AUROC 0.79-0.91). Several studies found it advantageous to incorporate both preoperative and intraoperative variables.36–39 In a prospective, observational study of 150 patients undergoing inpatient surgery, Brennan et al.37 reported that discrimination in predicting postoperative complications was significantly greater for a machine learning model using automated electronic health record data (AUROC 0.73-0.85) than for clinicians, whose predictions were slightly better than chance (AUROC 0.47-0.69).
Hospital characteristics had significant associations with failure-to-rescue (i.e., death after a complication). Analyzing more than 1.8 million total surgeries, Eggli et al.41 and Silber et al.42 each found that higher hospital volume was associated with lower incidence of failure-to-rescue. The importance of failure-to-rescue as a quality metric was substantiated by Fry et al.43 in a large, multicenter study demonstrating that hospitals with improving mortality rates achieved better outcomes primarily by decreasing the incidence of failure-to-rescue, not by decreasing the incidence of complications themselves. A critically ill, postoperative patient admitted to a high-performance hospital seems to have a lower risk for death than a similar patient admitted to low-performance hospital; therefore, hospital characteristics should be considered in models that represent and classify patient acuity.
Classifying Resource Intensity
Level-of-Care Assignments
Six studies classified resource intensity according to postoperative level-of-care assignments (see table, Supplemental Digital Content 4). Two of these studies had prospective, observational designs; four were retrospective. Population sizes ranged from 20 to 2,000 patients, with median a of 256 patients per study. Findings of these studies include: a substantial proportion of decompensation events occur after transfer out of the ICU at a median of two days after surgery;16 unmet requests for postoperative ICU care are associated with increased mortality (3.1% vs. 1.2%);45 surgeon predictions of postoperative level-of-care requirements are highly variable and incorrect in approximately one third of all cases;46, 47 and machine learning techniques can predict the need for ICU resources after major surgery with 86% accuracy.47 Overall, postoperative level-of-care assignments were highly variable, often suboptimal, and likely to the detriment of patients. However, the reported associations between level-of-care assignments and outcomes do not imply causality. Therefore, it remains unknown whether a patient who is triaged to an ICU and does well received excessive care (poor decision) or appropriate care that prevented complications (good decision).
Several other key findings emerged from articles that classify resource intensity by postoperative triage assignments. Guidelines for appropriate ICU admission lack objectivity, rendering them difficult to apply in quantitative research.48, 49 Systematic, hypothesis-driven investigation is particularly difficult in the absence of adequate documentation in electronic health records. In one study of patients undergoing major surgery, level-of-care documentation met quality metrics for 0.7% of the study population.50 As an alternative to adequate level-of-care documentation, Wang et al.47 proposed a list of index events that suggest appropriate ICU admission, though some events generate tautology; it remains plausible that patients with uneventful ICU stays simply received appropriate ICU-level care that prevented index events. However, the list of index events allowed the authors to perform decision tree modeling to predict the need for intensive care resources after major surgery. This method outperformed standard clinical practice (86% vs. 37% accuracy), suggesting that modeling risk for ICU admission on index events has the potential to augment clinical decision-making regarding postoperative level-of-care.
Frequency of Vital Sign Monitoring
Two studies assessed associations between postoperative vital sign monitoring and postoperative complications (see table, Supplemental Digital Content 4). Kyriacos et al.51 performed a retrospective review of 11 patients who died on hospital wards after surgery, matching each decedent with 4 survivors that had similar characteristics, thus forming a control cohort. Among the 11 patients who died on surgical wards, 55% had missing oxygen saturation records, compared with 7% among survivors. Taenzer et al.52 prospectively investigated the impact of routine continuous pulse oximetry among 2,841 surgical ward patients, integrated with a system for managing etiologies of respiratory dysfunction. During the intervention period, all patients had continuous pulse oximetry. Rapid response teams were activated by a respiratory rate of less than 8 or greater than 30 breaths per minute or blood oxygen saturation less than 90% while receiving supplemental oxygen. The frequency of rescue events decreased from 3.4 to 1.2 per 1,000 patient discharges (p=0.01); the frequency of ICU transfers decreased from 5.6 to 2.9 per 1,000 patient days (p=0.02) after routine continuous pulse oximetry. In two control units using standard monitoring during the same period, rescue events and ICU transfers were unchanged. These studies suggest that infrequent postoperative oxygen saturation measurements are associated with increased risk for rescue events and death after surgery, and that vital sign frequency is an important aspect of postoperative resource intensity. Although there is a lack of level 1 evidence supporting routine continuous postoperative monitoring, it remains plausible that maintaining a certain baseline level of continuous monitoring for all patients may preserve intensive care resources for critically ill patients by decreasing the incidence of unplanned ICU transfers from wards.53, 54
Frequency of Laboratory Measurements
Associations among frequency of laboratory measurements, patient outcomes, and level-of-care assignments are less clear. Two studies assessed these associations (see table, Supplemental Digital Content 4). In a retrospective review of 1,894 patients undergoing cardiac surgery, Koch et al. 55 found that patients who underwent complex procedures had the greatest postoperative phlebotomy volumes. Median phlebotomy volume for an ICU patient was 116 mL per day, compared with approximately 18 mL per day on hospital wards. The reported median phlebotomy volumes for cardiac surgical ICU patients was substantially greater than volumes reported for mixed medical-surgical populations, which has been estimated at 40 mL per day.56 Ko et al.57 implemented a daily checklist and staff education to promote judicious phlebotomy and assessed the efficacy of this program in a retrospective review of approximately 5,465 patient days in a surgical ICU. The program was associated with a 51% reduction in laboratory tests with no difference in patient illness severity or mortality, indicating that a substantial proportion of laboratory measurements performed for surgical ICU patients were not associated with outcomes and may have been unnecessary. Together, these studies suggest that although laboratory measurement frequency corresponds to operative complexity and postoperative level-of-care assignments, there is no evidence demonstrating that laboratory frequency affects clinical outcomes across low- or high-patient acuity or resource intensity.
Aligning Patient Acuity with Resource Intensity
Two studies classified both patient acuity and resource intensity after inpatient surgery. Pearse et al.7 defined high-risk procedures as those with mortality rate 5% or greater (timeframe not specified). This definition allows identification of high-acuity patients via procedural codes. In an analysis of 4.1 million hospital admissions involving a general surgical procedure in the United Kingdom, high-risk procedures accounted for 13% of all procedures and 84% of all postoperative deaths. Less than 15% of the high-risk cohort was admitted to the ICU postoperatively. Among patients who were admitted to the ICU after initial triage to a general ward, mortality was 37%. In a similar analysis using the same high-risk procedure definition, Jhangji et al.8 reported that high-risk patients had 12% overall in-hospital mortality; among patients who died, only 49% were admitted to an ICU at any time and 74% of deaths occurred outside the ICU. These findings demonstrate that in a single large health care system, postoperative patient acuity and resource intensity are often misaligned, and insufficient resource intensity is associated with increased in-hospital mortality.
Beyond simple classification of patient acuity and resource intensity, it may be possible to align these elements by predicting risk for postoperative ICU admission. This approach introduces some bias by incorporating triage decisions in outcome modeling, but offers the potential advantage of accounting for real-world, inherent variability in decision-making. Risk for postoperative ICU admission has been modeled accurately by Kongkaewpaisan et al.58 for patients undergoing emergency surgery, by Sobol et al.59 for patients undergoing major intraabdominal surgery, and by Glass et al.60 for patients undergoing non-trauma surgery at a Veterans Affairs hospital. Further investigation is needed to determine whether such models improve triage decisions and patient outcomes.
Recommendations
Collectively, results from this review suggest that postoperative patient acuity should be classified using a combination of demographic, physiologic, and hospital system variables as inputs for models that accurately represent complex, non-linear relationships among risk factors for morbidity and mortality. Intraoperative physiologic data enriches these representations and should be used to update patient acuity assessments immediately after surgery. High-acuity patients benefit from close surveillance with continuous vital sign monitoring and the immediate availability of critical care resources and personnel for preventing and treating organ dysfunction and postoperative complications. Low-acuity patients garner little or no clinical benefit from high-intensity care, but can displace critically ill patients to hospital wards, increasing their risk for unrecognized decompensation and failure-to-rescue. To promote high-value care after major surgery, clinicians’ resource intensity decisions should be augmented by data-driven patient acuity classifications that are integrated with clinical workflows, as illustrated in Figure 3.
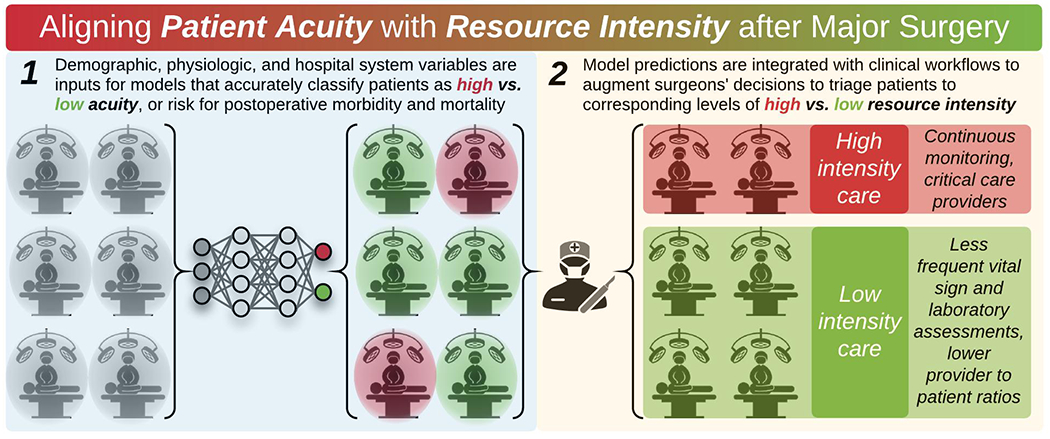
After major surgery, clinicians’ resource intensity decisions should be augmented by data-driven patient acuity classifications.
Discussion
This review found substantial evidence that postoperative patient acuity is most accurately classified by combinations of demographic, physiologic, and hospital system variables. Classifications were most accurate when using machine learning models that accurately represent complex, non-linear relationships among inputs. Several studies support the hypothesis that data-driven methods to augment postoperative resource intensity decisions are enriched by data representing intraoperative physiologic changes. There is a paucity of literature classifying postoperative resource intensity, most of which focuses on level-of-care assignments. These classifications are limited by the lack of consensus definitions or standards for level-of-care that apply at the national or international level, much less the inter-institutional level. Clinicians exhibited volatile performance in predicting complications and making postoperative resource intensity decisions. In two direct comparisons between clinicians and machine learning algorithms, the algorithms performed best.37, 47 Suboptimal postoperative triage was associated with low-value care and adverse outcomes, especially when high-acuity patients were triaged to wards. This is consistent with a Delphi Consensus in which there was 100% agreement that inadequate staffing levels threaten surgical ward safety.61 Conversely, triaging low-acuity patients to ICUs may impart population-level harm through increased systemic costs or the occupation of limited beds that are better suited for higher-acuity patients. ICU bed stewardship mimics antibiotic stewardship in that excessive treatment of one patient can harm another. Although it seems plausible that providing high-intensity care for a low-acuity patient may confer harm through unnecessary testing and subsequent treatment of incidental findings, there is insufficient evidence to support this hypothesis. Regardless, excessive resource intensity is expensive; surgical ICU admission costs range from $2,000 to more than $10,000 per day.14, 15 The weight of evidence suggests that mismatched patient acuity and resource intensity leads to decreased value and increased morbidity and mortality.
The present review focuses on major, inpatient surgery; similar themes and concepts emerge for outpatient surgery and mixed analyses of inpatient and outpatient surgery. This is fortunate because unexpected hospital admission after ambulatory surgery is not rare. The National Surgical Quality Improvement Program Surgical Risk Calculator is the most prominent and well-validated method for predicting postoperative complications and death.62, 63 It is constructed from massive volumes of patient-level input variables—including procedure type, demographics, and physiology—and conveys risks through regression cutoff values. Yet, the ability of regression coefficients to represent complex, non-linear associations among correlated, interacting, and nested input variables is questionable, especially when applied to atypical patient presentations and non-elective operations.64–67 In such instances, it may be advantageous to leverage analytic techniques that learn from data rather than conforming to rules and static variable thresholds. For example, Bertsimas et al.44 developed a machine learning model to predict morbidity and mortality after emergency surgery. This method achieved predictive discrimination that was greater than that of the ACS NSQIP calculator, though both methods were effective (AUROC 0.92 vs. 0.90). While existing methods for measuring and predicting patient acuity are strong, methods for classifying resource intensity are comparatively weak.
Previous work describing alignment between patient acuity and resource intensity has focused primarily on medical and medical-surgical populations. Moorman et al.68 have demonstrated that potentially catastrophic respiratory, hemorrhagic, and sepsis events are often preceded by physiological signatures of adaptive regulation. Multivariable regression can predict these events up to 24 hours in advance with reasonable accuracy (AUROC 0.61-0.88).69 In predicting more general adverse events in mixed medical-surgical populations, other high-performing methods include eCART70 and the Rothman Index,71 which leverage multi-source, patient-level variables. Predictive accuracy is further enhanced when physiologic data are represented by continuous, time-series inputs to deep learning models.72 Medical and mixed medical-surgical literature features a relative paucity of evidence regarding resource intensity, most of which supports the postoperative resource intensity concepts presented in Figure 1. For example, routine chest radiographs increase the probability of false-positive results thus leading to potentially harmful treatments; false alarms from continuous vital sign monitoring beget alarm fatigue; and denied requests for ICU admission are associated with increased mortality.73–77 Literature from medical populations corroborates findings from surgical literature regarding patient acuity assessments, despite fundamental differences in the etiologies of adverse events between medical and surgical patients.
This study was limited by heterogeneity in available literature regarding study populations and methods for classifying patient acuity and resource intensity. The article screening and inclusion criteria sought to establish balance between homogeneity of study populations and generalizability to all patients undergoing major, inpatient surgery. This approach, while optimal for addressing review objectives, introduces selection bias because all patients and operations represented in the main analysis were subject to one, common resource intensity decision: hospital admission rather than discharge home. In the absence of trials randomizing postoperative patients to admission or discharge, this element of selection bias was unavoidable. The diffuse methods for classifying patient acuity and resource intensity precluded a pooled analysis of results and strengthened the rationale for performing this scoping review. Finally, this review does not include intermediate care and high-dependency units, which offer greater patient surveillance compared with general wards, but use fewer overall resources compared with ICUs. These intermediate units, in combination with initial postoperative observation in a post-anesthesia care unit, have been associated with decreased use of ICU resources without significantly increasing risk for postoperative morbidity and mortality.78, 79 This approach introduces a third layer for triage decisions and warrants further investigation in prospective studies. Future research should also quantify potential gains in mortality, morbidity, and health care costs for appropriate alignment of patient acuity with postoperative resource intensity. Associations between patient acuity and resource intensity should be investigated with causal inference methods to determine whether a patient who is triaged to an ICU, and has favorable outcomes, received excessive care that consumed resources unnecessarily versus appropriate care that prevented complications. To ensure generalizability of results and determine whether hospital characteristics (e.g., ICU bed capacity) affect postoperative triage patterns and outcomes, future research should incorporate multiple practice settings (i.e., high- and low-volume academic and non-academic hospitals) and patient populations (i.e., urban and rural areas with varied race and ethnicity distributions). Finally, developing international consensus definitions for level-of-care would promote systematic, hypothesis-driven investigation that is generalizable across practice settings.
Conclusions
Postoperative patient acuity can be classified using a combination of demographic, physiologic, and hospital system variables in models that accurately represent complex, non-linear relationships among risk factors for morbidity and mortality. Among the few studies classifying postoperative resource intensity, the predominant representation is level-of-care assignment. Triaging high-acuity patients to hospital wards is associated with increased risk for mortality; further studies are needed to determine whether triaging low-acuity patients to ICUs imparts harm to other patients via unwarranted occupation of ICU beds. Clinicians exhibited volatile performance in estimating both risk for complications and assigning postoperative resource intensity, while machine learning algorithms consistently demonstrated superior performance. Future research should quantify potential gains in mortality, morbidity, and health care costs for appropriate alignment of patient acuity with postoperative resource intensity and develop data-driven platforms that augment clinicians’ decisions.
Supplementary Material
Acknowledgements
The authors declare no conflicts of interest. TJL was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under Award Number K23GM140268. PTJ was supported by R01GM114290 from the NIGMS and R01AG121647 from the National Institute on Aging (NIA). PR was supported by National Science Foundation CAREER award 1750192, P30AG028740 and R01AG05533 from the NIA, 1R21EB027344 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and R01GM-110240 from the NIGMS. AB was supported by R01GM110240 from the NIGMS and 1R21EB027344 from the NIBIB. This work was supported in part by the National Center for Advancing Translational Sciences and Clinical and Translational Sciences Award to the University of Florida UL1TR000064. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Elixhauser A, Andrews RM. Profile of inpatient operating room procedures in US hospitals in 2007. Arch Surg 2010; 145(12):1201–8. [DOI] [PubMed] [Google Scholar]
- 2.Healey MA, Shackford SR, Osler TM, et al. Complications in surgical patients. Arch Surg 2002; 137(5):611–7; discussion 617-8. [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199(4):531–7. [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg 2010; 251(6):995–1000. [DOI] [PubMed] [Google Scholar]
- 5.Wolf FM, Gruppen LD, Billi JE. Differential diagnosis and the competing-hypotheses heuristic. A practical approach to judgment under uncertainty and Bayesian probability. JAMA 1985; 253(19):2858–62. [PubMed] [Google Scholar]
- 6.Bekker HL. Making choices without deliberating. Science 2006; 312(5779):1472; author reply 1472. [DOI] [PubMed] [Google Scholar]
- 7.Pearse RM, Harrison DA, James P, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care 2006; 10(3):R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhanji S, Thomas B, Ely A, et al. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia 2008; 63(7):695–700. [DOI] [PubMed] [Google Scholar]
- 9.Skogvoll E, Isern E, Sangolt GK, et al. In-hospital cardiopulmonary resuscitation. 5 years’ incidence and survival according to the Utstein template. Acta Anaesthesiol Scand 1999; 43(2):177–84. [DOI] [PubMed] [Google Scholar]
- 10.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med 2011; 39(11):2401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perman SM, Stanton E, Soar J, et al. Location of In-Hospital Cardiac Arrest in the United States-Variability in Event Rate and Outcomes. J Am Heart Assoc 2016; 5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levinson W, Kallewaard M, Bhatia RS, et al. ‘Choosing Wisely’: a growing international campaign. BMJ Qual Saf 2015; 24(2):167–74. [DOI] [PubMed] [Google Scholar]
- 13.Emanuel EJ, Fuchs VR. The perfect storm of overutilization. JAMA 2008; 299(23):2789–91. [DOI] [PubMed] [Google Scholar]
- 14.Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med 2005; 33(6):1266–71. [DOI] [PubMed] [Google Scholar]
- 15.Gershengorn HB, Garland A, Gong MN. Patterns of Daily Costs Differ for Medical and Surgical Intensive Care Unit Patients. Ann Am Thorac Soc 2015; 12(12):1831–6. [DOI] [PubMed] [Google Scholar]
- 16.Helling TS, Martin LC, Martin M, et al. Failure events in transition of care for surgical patients. J Am Coll Surg 2014; 218(4):723–31. [DOI] [PubMed] [Google Scholar]
- 17.Calcaterra SL, Yamashita TE, Min SJ, et al. Opioid Prescribing at Hospital Discharge Contributes to Chronic Opioid Use. J Gen Intern Med 2016; 31(5):478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun EC, Darnall BD, Baker LC, et al. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med 2016; 176(9):1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeds IL, Rosenblum AJ, Wise PE, et al. Eye of the beholder: Risk calculators and barriers to adoption in surgical trainees. Surgery 2018; 164(5):1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vries H, Elliott MN, Kanouse DE, et al. Using pooled kappa to summarize interrater agreement across many items. Field Methods 2008; 20(3):272–282. [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33(1):159–74. [PubMed] [Google Scholar]
- 22.Finlayson EV, Birkmeyer JD. Operative mortality with elective surgery in older adults. Effective clinical practice : ECP 2001; 4(4):172–177. [PubMed] [Google Scholar]
- 23.Teh SH, Nagorney DM, Stevens SR, et al. Risk Factors for Mortality After Surgery in Patients With Cirrhosis. Gastroenterology 2007; 132(4):1261–1269. [DOI] [PubMed] [Google Scholar]
- 24.Pan Y, Wang W, Wang J, et al. Incidence and Risk Factors of in-hospital mortality from AKI after non-cardiovascular operation: A nationwide Survey in China. Scientific reports 2017; 7(1):13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh TK, Jeon YT, Do SH, et al. Pre-operative assessment of 30-day mortality risk after major surgery: the role of the quick sequential organ failure assessment: A retrospective observational study. European Journal of Anaesthesiology 2019; 36(9):688–694. [DOI] [PubMed] [Google Scholar]
- 26.Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: Does provider volume matter? Journal of Bone and Joint Surgery - Series A 2010; 92(4):799–806. [DOI] [PubMed] [Google Scholar]
- 27.Brooke BS, Goodney PP, Kraiss LW, et al. Readmission destination and risk of mortality after major surgery: an observational cohort study. The Lancet 2015; 386(9996):884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, et al. The pattern of longitudinal change in serum creatinine and 90-day mortality after major surgery. Annals of Surgery 2016; 263(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musallam KM, Porter JB, Sfeir PM, et al. Raised haematocrit concentration and the risk of death and vascular complications after major surgery. British Journal of Surgery 2013; 100(8):1030–1036. [DOI] [PubMed] [Google Scholar]
- 30.van Koeverden ID, den Ruijter HM, Scholtes VPW, et al. A single preoperative blood test predicts postoperative sepsis and pneumonia after coronary bypass or open aneurysm surgery. European Journal of Clinical Investigation 2019; 49(3). [DOI] [PubMed] [Google Scholar]
- 31.Bartkowiak B, Snyder AM, Benjamin A, et al. Validating the Electronic Cardiac Arrest Risk Triage (eCART) Score for Risk Stratification of Surgical Inpatients in the Postoperative Setting: Retrospective Cohort Study. Ann Surg 2019; 269(6):1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim TH, Um SH, Yim SY, et al. The risk of perioperative adverse events in patients with chronic liver disease. Liver International 2015; 35(3):713–723. [DOI] [PubMed] [Google Scholar]
- 33.Nouraei SAR, Middleton SE, Hudovsky A, et al. A national analysis of the outcome of major head and neck cancer surgery: Implications for surgeon-level data publication. Clinical Otolaryngology 2013; 38(6):502–511. [DOI] [PubMed] [Google Scholar]
- 34.Shah R, Attwood K, Arya S, et al. Association of frailty with failure to rescue after low-risk and high-risk inpatient surgery. JAMA Surgery 2018; 153(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolters U, Wolf T, Stutzer H, et al. ASA classification and perioperative variables as predictors of postoperative outcome. British Journal of Anaesthesia 1996; 77(2):217–222. [DOI] [PubMed] [Google Scholar]
- 36.Bihorac A, Ozrazgat-Baslanti T, Ebadi A, et al. MySurgeryRisk: Development and Validation of a Machine-learning Risk Algorithm for Major Complications and Death After Surgery. Annals of surgery 2019; 269(4):652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brennan M, Puri S, Ozrazgat-Baslanti T, et al. Comparing clinical judgment with the MySurgeryRisk algorithm for preoperative risk assessment: A pilot usability study. Surgery 2019; 165(5):1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datta S, Loftus TJ, Ruppert MM, et al. Added Value of Intraoperative Data for Predicting Postoperative Complications: The MySurgeryRisk PostOp Extension. Journal of Surgical Research 2020; 254:350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gawande AA, Kwaan MR, Regenbogen SE, et al. An Apgar score for surgery. J Am Coll Surg 2007; 204(2):201–8. [DOI] [PubMed] [Google Scholar]
- 40.Arkin N, Lee PH, McDonald K, et al. Association of Nurse-to-Patient Ratio with mortality and preventable complications following aortic valve replacement. J Card Surg 2014; 29(2):141–8. [DOI] [PubMed] [Google Scholar]
- 41.Eggli Y, Halfon P, Meylan D, et al. Surgical safety and hospital volume across a wide range of interventions. Medical Care 2010; 48(11):962–971. [DOI] [PubMed] [Google Scholar]
- 42.Silber JH, Romano PS, Rosen AK, et al. Failure-to-rescue: comparing definitions to measure quality of care. Med Care 2007; 45(10):918–25. [DOI] [PubMed] [Google Scholar]
- 43.Fry BT, Smith ME, Thumma JR, et al. Ten-year Trends in Surgical Mortality, Complications, and Failure to Rescue in Medicare Beneficiaries. Ann Surg 2019. [DOI] [PubMed] [Google Scholar]
- 44.Bertsimas D, Dunn J, Velmahos GC, et al. Surgical Risk Is Not Linear: Derivation and Validation of a Novel, User-friendly, and Machine-learning-based Predictive OpTimal Trees in Emergency Surgery Risk (POTTER) Calculator. Ann Surg 2018; 268(4):574–583. [DOI] [PubMed] [Google Scholar]
- 45.Turner M, McFarlane HJ, Krukowski ZH. Prospective study of high dependency care requirements and provision. Journal of the Royal College of Surgeons of Edinburgh 1999; 44(1):19–23. [PubMed] [Google Scholar]
- 46.Ruckley CV, Ferguson JBP, Cuthbertson C. Surgical decision making. British Journal of Surgery 1981; 68(12):837–839. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Carrano FM, Fisichella PM, et al. A Quest for Optimization of Postoperative Triage after Major Surgery. Journal of Laparoendoscopic and Advanced Surgical Techniques 2019; 29(2):203–205. [DOI] [PubMed] [Google Scholar]
- 48.Nates JL, Nunnally M, Kleinpell R, et al. ICU Admission, Discharge, and Triage Guidelines: A Framework to Enhance Clinical Operations, Development of Institutional Policies, and Further Research. Critical Care Medicine 2016; 44(8):1553–1602. [DOI] [PubMed] [Google Scholar]
- 49.Ramos JGR, Perondi B, Dias RD, et al. Development of an algorithm to aid triage decisions for intensive care unit admission: a clinical vignette and retrospective cohort study. Critical Care 2016; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergman S, Martelli V, Monette M, et al. Identification of quality of care deficiencies in elderly surgical patients by measuring adherence to process-based quality indicators. Journal of the American College of Surgeons 2013; 217(5):858–866. [DOI] [PubMed] [Google Scholar]
- 51.Kyriacos U, Jelsma J, Jordan S. Record Review to Explore the Adequacy of Post-Operative Vital Signs Monitoring Using a Local Modified Early Warning Score (Mews) Chart to Evaluate Outcomes. Plos One 2014; 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taenzer AH, Pyke JB, McGrath SP, et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010; 112(2):282–7. [DOI] [PubMed] [Google Scholar]
- 53.Watkinson PJ, Barber VS, Price JD, et al. A randomised controlled trial of the effect of continuous electronic physiological monitoring on the adverse event rate in high risk medical and surgical patients. Anaesthesia 2006; 61(11):1031–9. [DOI] [PubMed] [Google Scholar]
- 54.Loftus TJ, Tighe PJ, Filiberto AC, et al. Opportunities for machine learning to improve surgical ward safety. Am J Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koch CG, Reineks EZ, Tang AS, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg 2015; 99(3):779–84. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen BV, Bota DP, Melot C, et al. Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Crit Care Med 2003; 31(2):406–10. [DOI] [PubMed] [Google Scholar]
- 57.Ko A, Murry JS, Hoang DM, et al. High-value care in the surgical intensive care unit: effect on ancillary resources. J Surg Res 2016; 202(2):455–60. [DOI] [PubMed] [Google Scholar]
- 58.Kongkaewpaisan N, Lee JM, Eid AI, et al. Can the emergency surgery score (ESS) be used as a triage tool predicting the postoperative need for an ICU admission? American Journal of Surgery 2019; 217(1):24–28. [DOI] [PubMed] [Google Scholar]
- 59.Sobol JB, Gershengorn HB, Wunsch H, et al. The Surgical Apgar Score Is Strongly Associated with Intensive Care Unit Admission After High-Risk Intraabdominal Surgery. Anesthesia and Analgesia 2013; 117(2):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glass NE, Pinna A, Masi A, et al. The Surgical Apgar Score Predicts Postoperative ICU Admission. Journal of Gastrointestinal Surgery 2015; 19(3):445–450. [DOI] [PubMed] [Google Scholar]
- 61.Hassen YAM, Johnston MJ, Singh P, et al. Key Components of the Safe Surgical Ward: International Delphi Consensus Study to Identify Factors for Quality Assessment and Service Improvement. Ann Surg 2018. [DOI] [PubMed] [Google Scholar]
- 62.Hollenbeak CS, Boltz MM, Wang L, et al. Cost-effectiveness of the National Surgical Quality Improvement Program. Ann Surg 2011; 254(4):619–24. [DOI] [PubMed] [Google Scholar]
- 63.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217(5):833–42 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark DE, Fitzgerald TL, Dibbins AW. Procedure-based postoperative risk prediction using NSQIP data. J Surg Res 2018; 221:322–327. [DOI] [PubMed] [Google Scholar]
- 65.Lubitz AL, Chan E, Zarif D, et al. American College of Surgeons NSQIP Risk Calculator Accuracy for Emergent and Elective Colorectal Operations. J Am Coll Surg 2017; 225(5):601–611. [DOI] [PubMed] [Google Scholar]
- 66.Cohen ME, Liu Y, Ko CY, et al. An Examination of American College of Surgeons NSQIP Surgical Risk Calculator Accuracy. J Am Coll Surg 2017; 224(5):787–795 e1. [DOI] [PubMed] [Google Scholar]
- 67.Hyde LZ, Valizadeh N, Al-Mazrou AM, et al. ACS-NSQIP risk calculator predicts cohort but not individual risk of complication following colorectal resection. Am J Surg 2019; 218(1):131–135. [DOI] [PubMed] [Google Scholar]
- 68.Moorman JR, Rusin CE, Lee H, et al. Predictive monitoring for early detection of subacute potentially catastrophic illnesses in critical care. Conf Proc IEEE Eng Med Biol Soc 2011; 2011:5515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moss TJ, Lake DE, Calland JF, et al. Signatures of Subacute Potentially Catastrophic Illness in the ICU: Model Development and Validation. Critical Care Medicine 2016; 44(9):1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Green M, Lander H, Snyder A, et al. Comparison of the Between the Flags calling criteria to the MEWS, NEWS and the electronic Cardiac Arrest Risk Triage (eCART) score for the identification of deteriorating ward patients. Resuscitation 2018; 123:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothman MJ, Rothman SI, Beals Jt. Development and validation of a continuous measure of patient condition using the Electronic Medical Record. J Biomed Inform 2013; 46(5):837–48. [DOI] [PubMed] [Google Scholar]
- 72.Shickel B, Loftus TJ, Adhikari L, et al. DeepSOFA: A Continuous Acuity Score for Critically Ill Patients using Clinically Interpretable Deep Learning. Sci Rep 2019; 9(1):1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zampieri FG, Einav S. When will less monitoring and diagnostic testing benefit the patient more? Intensive Care Medicine 2019; 45(10):1447–1450. [DOI] [PubMed] [Google Scholar]
- 74.Ranasinghe T, Freeman WD. ‘ICU vampirism’ - time for judicious blood draws in critically ill patients. British Journal of Haematology 2014; 164(2):302–303. [DOI] [PubMed] [Google Scholar]
- 75.Oba Y, Zaza T. Abandoning Daily Routine Chest Radiography in the Intensive Care Unit: Meta-Analysis. Radiology 2010; 255(2):386–395. [DOI] [PubMed] [Google Scholar]
- 76.Jerison HJ, Pickett RM. Vigilance: The Importance of the Elicited Observing Rate. Science 1964; 143(3609):970–1. [DOI] [PubMed] [Google Scholar]
- 77.Iapichino G, Corbella D, Minelli C, et al. Reasons for refusal of admission to intensive care and impact on mortality. Intensive Care Medicine 2010; 36(10):1772–1779. [DOI] [PubMed] [Google Scholar]
- 78.Dahm P, Tuttle-Newhall JE, Nimjee SM, et al. Indications for admission to the surgical intensive care unit after radical cystectomy and urinary diversion. Journal of Urology 2001; 166(1):189–193. [PubMed] [Google Scholar]
- 79.Schweizer A, Khatchatoutrian G, Hohn L, et al. Opening of a new postanesthesia care unit: Impact on critical care utilization and complications following major vascular and thoracic surgery. Journal of Clinical Anesthesia 2002; 14(7):486–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


