Abstract
Simple Summary
The aim of the present review is to compile and evaluate the literature data on paraneoplastic syndromes (PNS) associated with keratinocyte skin cancer (KSC). Forty relevant entries were assembled, reporting a total of 41 PNS cases associated with a KSC (34 male). No review paper compiling this topic was found. Six distinct PNS entities were identified, and malignancy associated hypercalcemia (MAH; 78%), anemia (10%) and Bazex syndrome (5%) were the most frequently reported among them. 85% of the PNS were reported in association with SCC, 10% with BCC, and the rest with adnexal tumors. The median age of the patients at the time of PNS diagnosis was 58 years (range: five–83 years). KSC predisposing conditions, as scars (22%) or hidradenitis suppurativa (20%), were reported in >70% of the PNS cases. In conclusion, PNS are rarely reported in association with KSC, possibly reflecting a limited capacity of KSC to provoke overt PNS.
Abstract
A variety of well-characterized cutaneous paraneoplastic syndromes (PNS) are diagnosed during internal malignancies; however, the spectrum of keratinocyte skin neoplasms (KSC) related to PNS is still obscure. The aim of the present review is to compile and evaluate the literature data on PNS associated with a keratinocyte skin neoplasm (KSC). Employing Pubmed, MEDLINE was searched for KSC-associated PNS reports. Forty relevant entries were assembled, reporting a total of 41 PNS cases associated with a KSC (34 male). No review paper compiling this topic was found. Six distinct PNS entities were identified, and malignancy associated hypercalcemia (MAH; 78%), anemia (10%) and Bazex syndrome (5%) were the most frequently reported among them. 85% of the PNS were reported in association with SCC, 10% with BCC, and the rest with adnexal tumors. The median age of the patients at the time of PNS diagnosis was 58 years (range: five–83 years). In most cases the PNS was diagnosed either concurrently or after the KSC diagnosis. KSC predisposing conditions, as scars (22%) or hidradenitis suppurativa (20%), were reported in >70% of the PNS cases. Most PNS resolved after KSC treatment. In conclusion, PNS of a rather limited spectrum of entities are reported in association with KSC. They also seem to be rare, possibly reflecting a limited capacity of KSC to provoke overt PNS.
Keywords: paraneoplasia, keratinocyte skin cancer, basal cell carcinoma, squamous cell carcinoma, adnexal skin tumours, hidradenitis suppurativa
1. Introduction
Paraneoplastic syndromes (PNS) are nonmetastatic tumor-associated findings caused by malignancies in remote body sites [1,2]. PNS are found in a considerable fraction of tumor patients and may primarily affect the neuromuscular/musculoskeletal, cardiovascular, cutaneous, hematologic, gastrointestinal, endocrine or renal systems [2]. Moreover, in some cases PNS may be even the first or most prominent manifestation of an underlying cancer, so their timely identification can guide the search for a still unrecognized malignancy. From a pathophysiologic point of view, most PNS are attributed to either endocrine phenomena (induced by tumor secreted biomolecules) or auto-immunity mediated immunological mechanisms [3,4]. Although the skin is a frequent target organ of PNS [5,6], PNS have been rather rarely reported in the course of skin neoplasms, even in relation to the biologically more aggressive ones, like malignant melanoma and Merkel cell carcinoma [7,8]. Herein, we compile the reported evidence of PNS associated with the growth of keratinocyte skin cancers (KSC). Our aim was to provide a typology of the spectrum of these PNS as an aid for the everyday clinical practice and as a basis for future comparative and mechanistic studies. To do so, we conducted a focused literature search and present our findings in the form of a research synthesis.
2. Materials and Methods
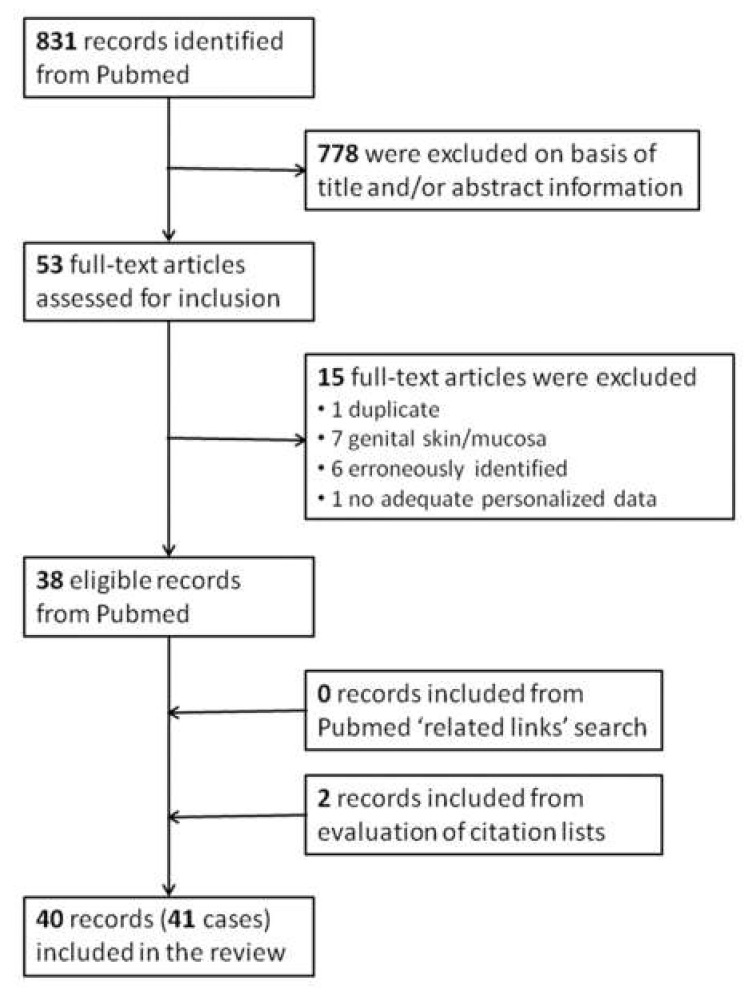
Only PNS attributed to KSC were included in this compilation, i.e., cutaneous squamous cell carcinomas (cSCC), basal cell carcinomas (BCC) and skin adnexal neoplasms. MEDLINE was searched using Pubmed (8 May 2021) for publications reporting PNS caused by KSC ({C}) with following search strategy: {C} = {A} AND {B}, with {A} = {[(basal cell carcinoma) OR (BCC)] OR [[(squamous cell carcinoma) OR (scc)] AND [(cutaneous) OR (skin)]] OR [keratinocyt* skin cancer] OR [(skin cancer) NOT (melano*)]} and {B} = {paraneopl*} OR {BN}, with {BN} = {B1} OR {B2} OR… OR {Bi} OR … OR {B43}. Bi = {text i} represents a paraneoplastic syndrome in text form, with Bi the ith of the 43 (“most widely accepted”) PNS (i = 1-43), listed in the review article by Bilynsky et al. [2]. All authors analyzed the assembled material independently for reports of PNS and KSC (including stage and histology) as the only malignant disease in the history of the patient and results were merged. By evaluating the title/abstract information of the initially returned 831 papers we localized 53 articles with potentially relevant content for full-text search (Figure 1). Of them, 15 were excluded (one was a duplicate publication, seven were cases of penile SCC, six were erroneously identified as reporting PNS in KSC and one reported no adequately personalized data) leaving to 39 papers reporting a total of 44 PNS cases associated with KSC [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. One paper ([47]) referred to five cases with relevant findings, however without any personalized data, and was excluded from this compilation, resulting in 38 papers reporting 39 relevant cases. An additional search of the selected papers for similar material in MEDLINE via the Pubmed option “related links” did not yield any additional relevant material. A search based on the references lists of the selected publications yielded two additional relevant non-MEDLINE included publications [48,49], leading to a total of 40 papers reporting 41 PNS cases associated with KSC. The KSC were classified as primary, locally relapsed (after definite therapy and no evidence of metastases) or metastatic (with locoregional or distant metastases). Core clinical data of the 41 identified PNS cases in patients with KSC are compiled in Table 1 and are summarized by descriptive statistics.
Figure 1.
Literature search: Flowchart of publicationsrelated to PNS cases in keratinocyte skin cancers selection process.
Table 1.
Characteristics of patients with a keratinocyte skin cancer associated with paraneoplastic syndromes.
| Ref a | Tumor | PNS b | Age/Sex | Localization | History | Recurrence/Metastases c | Timing of PNS d | Tumor/PNS Treatment | Resolution of PNS |
|---|---|---|---|---|---|---|---|---|---|
| [9] | SCC | Bazex Syndrome | 69/M | Axilla (metastasis) | SCC ipsilateral forearm | No/Yes | Before | Chemothe-rapy | Yes |
| [10] | SCC | Inflammatory arthralgias | 52/M | Skin (area N/A) | N/A | No/No | After | Surgery | Yes |
| [11] | SCC | Neuropathy | 50/M | Perineal | Hidradenitis suppurativa | No/No | Before | Surgery | Yes |
| [12] | SCC | Hypercalcemia | 67/M | Chest wall | Semi-comatose patient | No/No | Simultane-ously | Patient refused therapy | No |
| [13] | SCC | Hypercalcemia, leukocytosis | 58/M | Sole | Scar (accident) | No/Yes | After | Surgery, chemothe-rapy, radiation | Yes |
| [14] | SCC | Hypercalcemia | 82/M | Finger | Excision 2 years ago | Yes/Yes | After | Surgery, chemothe-rapy, radiation | No (patient died) |
| [15] | SCC | Bazex Syndrome | 54/M | Leg | Old scar | No/No | After | Surgery | Yes |
| [16] | SCC | Hypercalcemia | 38/M | Axilla | SCC on arm (burn scar area) | Yes/Yes | After | Surgery | Yes |
| [17] | SCC | Hypercalcemia, leukocytosis | 50/M | Sacral | Hidradenitis suppurativa | No/Yes | Simultane-ously | Chemo-therapy | No |
| [17] | SCC | Hypercalcemia | 60/M | Hand | N/A | No/Yes | After | Chem-otherapy | No |
| [18] | SCC | Hypercalcemia, leukocytosis | 20/M | Foot | Recessive dystrophic EB | Yes/Yes | Simultane-ously | Surgery, zolendro-nate | Yes |
| [19] | SCC | Hypercalcemia | 74/M | Back | Porokerato-sis Mibelli | Yes/Yes | After | Surgery | Yes |
| [20] | SCC | Hypercalcemia | 65/M | Buttock | Hidradenitis Suppurativa | Yes/Yes | After | Surgery, radiotherapy | Yes |
| [21] | SCC | Hypercalcemia | 72/M | Leg | Chronic venous ulcer | No/No | Simultane-ously | Surgery | No |
| [22] | SCC | Hypercalcemia | 45/M | Sacral | N/A | No/Yes | Simultane-ously | Cetuximab | No |
| [23] | SCC | Hypercalcemia | 59/M | Scapular area | ‘Pyoderma chronica’ | Yes/No | After | Chemo-therapy, radiation | Yes |
| [24] | SCC | Hypercalcemia | 65/F | Back | Lethargy | No/Yes | Simultane-ously | Chemo-therapy | Yes |
| [25] | SCC | Hypercalcemia | 80/F | Leg | Thrombo-phlebitis | No/No | Simultane-ously | Surgery | No (patient died) |
| [26] | SCC | Hypercalcemia | 17/M | Leg | Epidermo-lysis bullosa | Yes/Unknown | After | Denosumab | Yes |
| [27] | SCC | Hypercalcemia | 38/M | Back | Burn scar | No/No | Simultane-ously | Surgery | Yes |
| [28] | SCC | Hypercalcemia | 68/F | Arm | N/A | No/No | Simultane-ously | Surgery | Yes |
| [29] | BCC | Antiphospho-lipid syndrome | 64/F | Face | Chronic heart failure | No/No | After | Surgery | Yes |
| [30] | BCC | Pure red cell aplasia | 65/M | Shoulder | Hyper-tension | Yes/Yes | After | Surgery, radiatio,-chemo-therapy | No |
| [31] | TRC | Hypercalcemia | 42/M | Scalp | Burn scar | No/No | Simultane-ously | Surgery | Yes |
| [32] | PLM | Hypercalcemia | 32/M | Neck | N/A | No/No | Simultane-ously | Surgery | Yes |
| [33] | SCC | Pure Red Cell Aplasia | 80/M | Ear | Benign Prostate Hypertrophy | No/Unknown | Before | Surgery | No |
| [34] | SCC | Hypercalcemia leucocytosis | 5/F | Face | Xeroderma pigmento-sum | No/No | Simultane-ously | Chemo-therapy | Yes |
| [35] | SCC | Hypercalcemia | 83/M | Retro-Ear | N/A | No/Yes | Simultane-ously | Radio-therapy | Yes |
| [36] | SCC | Hypercalcemia | 35/M | Buttock | Hidradenitis suppurativa | No/No | Simultane-ously | Chemo-therapy | N/A |
| [37] | SCC | Hypercalcemia | 81/M | Chest wall | BCC | No/No | Simultane-ously | Surgery | Yes |
| [38] | SCC | Hypercalcemia | 50/M | Hip | Hidradenitis suppurativa | No/Yes | Simultane-ously | Surgery | Yes |
| [39] | SCC | Hypercalcemia, leukocytosis | 45/M | Thigh | Burn scar | Yes/Yes | Simultane-ously | Chemo-therapy | Yes |
| [40] | SCC | Hypercalcemia | 62/M | Groin | Psoriasis/chronic arsenic ingestion | No/No | After | Surgery | N/A |
| [41] | BCC | Anaemia | 50/M | Chest wall | N/A | N/A/No | After | Surgery | Yes |
| [42] | BCC | Anaemia | 76/F | Face | N/A | N/A | Simultane-ously | Radio-therapy | N/A |
| [43] | SCC | Hypercalcemia | 53/M | Back | N/A | N/A/Yes | Simultane-ously | Chemo-therapy | No (patient died) |
| [44] | SCC | Hypercalcemia | 68/M | Buttocks | Hidradenitis suppurativa | Yes/N/A | After | Chemo-therapy | No (patient died) |
| [45] | SCC | Hypercalcemia | 63/M | Buttocks | Hidradenitis suppurativa | N/A | Simultane-ously | Surgery | No (patient died) |
| [46] | SCC | Hypercalcemia | 51/M | Buttocks | Hidradenitis suppurativa | Yes/No | After | None specific | No (patient died) |
| [48] | SCC | Hypercalcemia | 28/F | Ischial tuberosity | Paraplegia/-chronic decubitus ulcer | No/Yes | Simultane-ously | Zolendronic acid | Yes |
| [49] | SCC | Hypercalcemia | 58/M | Leg | Lymph-edema | No/No | Simultane-ously | Surgery | Yes |
The background color highlights the data that refer to one case. a Ref: References list number. b Abreviations. F: female; KSC: Keratinocyte Skin Cancer; M: male; N/A: information not available; PLM: pilomatricoma; PNS:paraneoplastic syndrome; SCC: cutaneous Squamous Cell Carcinoma; TRC: trichilemmal carcinoma. c Recurrence: local relapse; metastasis: distant relapse. d Timing of PNS. before: PNS diagnosis precedes tumor diagnosis; simultaneous: PNS present at the time of tumor diagnosis; after: PNS diagnosis after tumor diagnosis.
3. Results
No review paper compiling the topic of KSC-associated PNS was found. Our literature search yielded only papers reporting solitary cases (40 papers reporting 41 PNS cases associated with KSC, Table 1). The core clinical features of the patients are compiled in Table 2 and stratified according to patients’ gender and KSC type in Tables S1 and S2 (Supplementary Materials), respectively. Of the 41 patients with KSC-associated PNS, 34 were male (83%) and only seven were (17%) female. Thirty-five PNS cases (85%) were associated with cSCC, four with BCC (10%; one of them with squamous metaplasia) and two (5% each) with other KSC (trichilemmal carcinoma and pilomatrixoma; compare Table S2, Supplementary Materials).
Table 2.
Paraneoplastic syndromes in patients with keratinocyte skin cancer. Compilation of the clinical features of all cases and separately for the different skin cancer types. No (%): Number of cases and % of corresponding cases with available information.
| Keratinocyte Skin Cancer (No) | Total (N = 41) | BCC a (N = 4) | SCC (N = 35) | Other b (N = 2) | |
|---|---|---|---|---|---|
| Sex, No (%) | Male | 34 (83) | 2 (50) | 30 (86) | 2 (100) |
| Female | 7 (17) | 2 (50) | 5 (14) | 0 (0) | |
| Localization c, No (%) | Head/neck | 7 (18) | 2 (50) | 3 (9) | 2 (100) |
| Trunk | 19 (47) | 2 (50) | 17 (50) | 0 | |
| Extremities | 14 (35) | 0 | 14 (41) | 0 | |
| N/A | 1 | 0 | 1 | 0 | |
| PNS, No (%) | MAH/HHM | 32 (78) | 0 (0) | 30 (85) | 2 (100) |
| Anemia | 4 (10) | 3 (75) | 1 (3) | 0 (0) | |
| Bazex syndrome | 2 (5) | 0 (0) | 2 (6) | 0 (0) | |
| Other i | 3 (7) | 1 (25) | 2 (6) | 0 (0) | |
| Timing of PNS d, No (%) | before | 3 (7) | 0 (0) | 3 (9) | 0 (0) |
| simultaneously | 22 (54) | 1 (25) | 19 (54) | 2 (100) | |
| after | 16 (39) | 3 (75) | 13 (37) | 0 (0) | |
| Primary e, No (%) | No | 21 (55) | 1 (33) | 20 (61) | 0 (0) |
| Yes | 17 (45) | 2 (67) | 13 (39) | 2 (100) | |
| N/A | 3 | 1 | 2 | 0 | |
| Recurrence f, No (%) | No | 27 (71) | 2 (67) | 23 (70) | 2 (100) |
| Yes | 11 (29) | 1 (33) | 10 (30) | 0 (0) | |
| N/A | 3 | 1 | 2 | 0 | |
| Metastases, No (%) | No | 20 (54) | 3 (75) | 15 (48) | 2 (100) |
| Yes | 17 (46) | 1 (25) | 16 (52) | 0 (0) | |
| N/A | 4 | 0 | 4 | 0 | |
| Predilection g, No (%) | No | 9 (28) | 2 (100) | 7 (24) | 0 (0) |
| Yes | 23 (72) | 0 | 22 (76) | 1 (100) | |
| N/A | 9 | 2 | 6 | 1 | |
| Genoderma-tosis j | 4 (17) | 0 (0) | 4 (18) | 0 (0) | |
| Acquired k | 19 (83) | 0 (0) | 18 (82) | 1 (100) | |
| Scar/Ulcus | 9 (48) | 0 (0) | 8 (44) | 1 (100) | |
| Hidradenitis suppurativa | 8 (42) | 0 (0) | 8 (44) | 0 (0) | |
| Other l,m | 2 (10) | 0 (0) | 2 (12) | 0 (0) | |
| Resolution h, No (%) | Yes | 25 (66) | 2 (67) | 21 (64) | 2 (100) |
| No | 13 (34) | 1 (33) | 12 (36) | 0 (0) | |
| N/A | 3 | 1 | 2 | 0 | |
The background color highlights the data that refer to one factor. a Abbreviations. BCC: basal cell carcinoma, SCC: cutaneous squamous cell carcinoma, N/A: not available, PNS: paraneoplastic syndrome, MAH: malignancy associated hypercalcemia, HHM: humoral hypercalcemia of malignancy. b One case of trichilemal carcinoma and one case of pilomatrixoma. c Localization: localization of the primary neoplasm. d Timing of PNS: time point of PNS diagnosis relative to the diagnosis of skin cancer. e Primary: primary tumor only. f Recurrence: local tumor recurrence after treatment. g Predilection: presence of a condition that predisposes to skin cancer development. h Resolution: resolution of paraneoplastic syndrome after skin cancer treatment. I Inflammatory arthralgias, neuropathy, antiphospholipid syndrome. j Genodermatoses: recessive dystrophic epidermolysis bullosa, porokeratosis Mibelli, xeroderma pigmentosum. k Acquired: acquired predilection. l Chronic arsenic intoxication, lymphedema. mItalics denote the subanalysis of cases with an acquired predilection.
Six distinct PNS entities (excluding variations) were reported (Table 2). Malignancy-associated hypercalcemia (MAH) was the most reported PNS (32/41 cases, 78% of all reported PNS cases: Table 2). MAH was primarily associated with cSCC: 30/35 cSCC cases (85%, Table 2) and 30/32 (94%, Table S2, Supplementary Materials) of the MAH cases. In two additional cases MAH was also described in patients with a rarer KSC variant, a pilomatrixoma and a trichilemmal carcinoma. It is worth noting that no cases of MAH were reported in association with a BCC. The core laboratory findings of the MAH cases are displayed in Table S3 (Supplementary Materials) and are compiled in Table 3. In five cases MAH was accompanied by leukocytosis (hypercalcemia-leukocytosis syndrome; Table S3, Supplementary Materials). Hypercalcemia was severe in most (19/32, 60%), mild in four and moderate in nine cases (Table 3). Parathormone related protein (PTHrP) was increased in most cases with available data (20/21 cases), while parathormone (PTH) was either decreased (15/28 cases) or within normal range (13/28 cases). Phosphorus and 1.25(OH)2D3 serum levels were in most cases either normal or decreased.
Table 3.
Malignancy associated hypercalcemia in KSC patients: Core demographic and laboratory findings. No (%): Number (% cases with available data).
| Demographics | ||
|---|---|---|
| Age, median [range], y | 58 [5–83] | |
| Sex, No (%) | Women | 5 (16) |
| Men | 27 (84) | |
| Tumor, No (%) | SCC | 30 (94) |
| Other | 2 (6) | |
| Laboratory findings | ||
| Hypercalcemia a , Νο (%) | mild | 4 (13) |
| moderate | 9 (28) | |
| severe | 19 (59) | |
| Phosphorus b , Νο (%) | decreased | 5 (25) |
| norm | 14 (70) | |
| increased | 1 (5) | |
| N/A | 12 (38) | |
| Parathormone b , PTH, Νο (%) | decreased | 15 (54) |
| norm | 13 (46) | |
| increased | 0 (0) | |
| N/A | 4 (12) | |
| Parathormone related protein, PTHrP b, Νο (%) | decreased | 0 (0) |
| norm | 1 (5) | |
| increased | 20 (95) | |
| N/A | 11 (34) | |
| Vitamin D3 b, No (%) | decreased | 5 (31) |
| norm | 10 (63) | |
| increased | 1 (6) | |
| N/A | 16 (50) |
The background color highlights the data that refer to one factor. a Mild: 10.7—11.9 mg/dL; moderate: 12.0–13.9 mg/dL; severe: ≥14 mg/dL. b Explanations. norm: within normal range; increased: above norm range; decreased: below norm range; N/A: value not available. Abbreviations. D3: 1,25 (OH)2 D3; KSC: Keratinocyte Skin Cancer; PLM: pilomatrixoma; PTH: parathormone; PTHrP: parathormone related protein; SCC: cutaneous Squamous Cell Carcinoma; TRC: trichilemmal carcinoma.
Other PNS included anemia in four patients (two cases of pure red cell aplasia), Bazex syndrome in two cSCC cases and three other syndromes (antiphospholipid syndrome, inflammatory arthralgias and neuropathy; Table 1 and Table 2). Notably, different KSC entities tended to associate with different PNS: KSC of squamous phenotype (cSCC and adnexal tumors) with MAH (32/32 MAH cases) and BCC with anemia (3/4 cases, Table S2, Supplementary Materials).
Considering the timing of PNS manifestation, PNS was diagnosed in most cases (38/41 patients) either concurrently or after the KSC (Table 2). A KSC predisposing condition, local or systemic in the context of a genodermatosis, was reported in >70% of the PNS cases with available information (23/32 cases; Table 2). In 4/23 cases with a predisposing factor (17%) the predisposing condition was a genodermatosis and in 19/23 cases (83%) some acquired condition (Table 2). Among the latter, scars (9 cases, 22%) and hidradenitis suppurativa (8 cases, 20%) were the most frequently associated conditions (Table 2). Finally, in most cases with available information, the PNS resolved after KSC treatment (25/38 cases, 66%, Table 2)
The median age of the patients at the time of PNS diagnosis was 58 years (range: five-83 years; Table 4). Overall, patients with any condition predisposing to KSC were younger at the time of PNS diagnosis (median age: 50.0, range five–74 years) compared to the rest (median age: 68.5, range 25–83 years; Table 4). This was particularly true for patients with a genodermatosis (compare Table 1).
Table 4.
Age of patients (Median [Range]) at the time of diagnosis of a keratinocyte skin cancer associated paraneoplastic syndrome, stratified according to patients’ gender.
| Factor | Total (N = 41) | Male (N = 34) | Female (N = 7) | |
|---|---|---|---|---|
| Tumor a | BCC (N = 4) | 64.5 [50–76] | 57.5 [50–64] | 70.0 [64–76] |
| SCC (N = 35) | 58.0 [5–83] | 58.0 [17–83] | 66.5 [05–80] | |
| Other b (N = 2) | 37.0 [32–42] * | 37.0 [32–42] * | - | |
| PNS | HHM all (N = 32) | 51.0 [5–83] | 58.0 [17–83] | 65.0 [5–80] |
| HHM without Leukocytosis (N = 27) | 60.0 [17–83] | 59.0 [17–83] | 66.5 [5–80] | |
| HHM with Leukocytosis (N = 5) | 45.0 [5–58] | 47.5 [20–58] | 5 * | |
| Other than HHM (N = 9) | 64.0 [50–80] | 54.0 [50–80] | 64 * | |
| Anemia (N = 4) | 70.5 [50–80] | 65.0 [50–80] | 76 * | |
| Bazex syndrome (N = 2) | 61.5 [54–69] * | 61.5 [54–69] * | - | |
| Other c (N = 3) | 52.0 [50–64] | 51.0 [50–52] | 64 * | |
| Localization d | Head/Neck (N = 7) | 64.0 [5–83] | 61.0 [32–83] | 64.0 [5–76] |
| Trunk (N = 20) | 56.0 [28–81] | 59.0 [28–81] | 28 * | |
| Extremities (N = 13) | 58.0 [17–82] | 56.0 [17–82] | 68.0 [65–68] | |
| N/A (N = 1) | 52 * | 52 * | - | |
| Predilection e | No (N = 9) | 74.5 [64–82] | 67.0 [65–82] | 65.0 [64–80] |
| Yes (N = 23) | 50.0 [5–74] | 52.5 [17–74] | 5 * | |
| N/A (N = 9) | 62.5 [32–83] | 52.0 [32–83] | 72.0 [68–76] | |
| Genodermatosis (N = 4) | 18.5 [5–74] | 20.0 [17–74] | 5 * | |
| Acquired f (N = 19) | 51.0 [28–72] | 58.0 [35–72] | 28 * | |
| Scar/ulcus (N = 9) | 45.0 [28–72] | 52.5 [38–72] | 28 * | |
| Hidradenitis suppurativa (N = 8) | 50.5 [35–68] | 50.0 [35–68] | - | |
| Other g (N = 2) | 60.0 [58–62] * | 69.0 [62–74] * | - | |
| Resolution h | No (N = 13) | 65.0 [45–82] | 63.0 [45–82] | 72.5 [65–80] |
| Yes (N = 25) | 52.0 [5–83] | 52.0 [17–83] | 46.0 [5–68] | |
| N/A (N = 3) | 62.5 [35–76] | 48.5 [35–62] * | 76 * |
The different colors separate rows referring to the different factor. Italics indicate data referring to subcategories of factors. a Abbreviations. BCC: basal cell carcinoma, SCC: cutaneous squamous cell carcinoma, HHM: humoral hypercalcemia of malignancy, PNS: paraneoplastic syndrome. b Trichilemmal carcinoma, pilomatrixoma. c Inflammatory arthralgias, neuropathy, antiphospholipid syndrome. d Localization: localization of the primary neoplasm. e Predilection: presence of a condition that predisposes to skin cancer development. f Acquired: acquired predilection. g Chronic arsenic intoxication, lymphedema. h Resolution: resolution of paraneoplastic syndrome after skin cancer treatment. * For <3 cases single values instead of ‘range’ are displayed.
4. Discussion
Malignancy associated hypercalcemia (MAH) [50,51,52] was the PNS most frequently reported in patients with KSC. Serum calcium levels are rigorously regulated in health (normal range: 8.2–10.6 mg/dL), and depending on the degree of hypercalcemia a plethora of clinical findings may result, ranging from non-specific symptoms like asthenia, nausea, and loss of appetite for mild hypercalcemia to more specific presentations for calcium levels >12.0 mg/dL (confusion, lethargy, coma) and finally to shock and death for extremely high serum calcium levels (>18.0 mg/dL) [50]. Immediate medical attention is necessary in these cases to reduce serum calcium levels, restore the glomerular filtration rate, and treat the underlying KSC to allow for the resolution of the crisis.
Almost all KSC-associated MAH cases presented with laboratory profiles consistent with the diagnosis of humoral hypercalcemia of malignancy (HHM), a PNS seen in up to 20% of cancer patients in the course of their disease [50,51,52]. HHM is caused by ectopic secretion of PTHrP, an immunologically distinct member of the PTH polypeptides hormone family [51] that acts through the PTH receptor to induce ubiquitous bone resorption and to reduce renal calcium clearance and phosphorus reabsorption. Increased PTHrP serum levels were also reported in most MAH cases (including two hypercalcemia-leukocytosis cases) with relevant information in the presently compiled material (Table 3). PTHrP is secreted by normal and malignant human keratinocytes and seems to act as a paracrine factor during normal growth and differentiation of the epidermis [53]. It is worth noting that the scarcity of reports identified herein seems to confirm the clinical experience that hypercalcemia is only occasionally diagnosed in association with SCC of epidermal origin, and this contrasts with the fact that SCC arising in extracutaneous sites accounts for a substantial fraction of all MAH cases [51,54]. This latter finding is in accordance with the conclusion of a former study that hypercalcemia is rare in patients with KSC [47]. Remarkably, although a cSCC complicating a severe hidradenitis suppurativa case is a rather exceptional event, ref. [10] a substantial fraction of the present PNS cases (8/41 cases), particularly MAH (7/41 or 17% of all cases), were observed within this patient setting. As MAH may develop on the background of concurrency with some other hypercalcemia-predisposing condition, like a granulomatous disease [50], it is worth inquiring whether a granulomatous hidradenitis suppurativa variant [55] underlies the development of this PNS.
With four cases, anemia was the second most frequent PNS reported in patients with KSC. Anemia is probably the most common systemic finding of patients with solid tumors [56]. It is caused by either a functional iron deficiency (anemia of inflammation or chronic disease) or by iron sequestration. It is worth noting that most anemia cases were found in association with advanced BCC. Chronic bleeding from the large erosive tumor surfaces of advanced BCC could be a plausible anemia explanation; however, in two of the four KSC-associated cases (two of three BCC associated cases) the etiology of anemia was the rather rare pure red cell aplasia syndrome [57].
Bazex syndrome (acrokeratosis paraneoplastica), a rare obligate cutaneous PNS, [58] was the third more frequently reported PNS in association with a KSC. It is worth noting that although most cases in the literature are related to SCC of upper aerodigestive mucosae, cases compiled here are associated with cSCC of the extremities: a lower leg and a forearm tumor with axillary lymph node metastases.
A limitation of this review is the restriction of the literature source to Medline inclusions. However, this has most probably not affected the above conclusions since the compiled data set is rather representative of the topic.
5. Conclusions
Overall, patients with cSCC seem to be at a much higher risk to develop a PNS compared to BCC patients. Particularly, hypercalcemia in the setting of malignancy, related to increased PTHrP serum levels, was the most frequently reported PNS. It was seen almost exclusively in association with a cSCC. Physicians who care for patients with KSC should be alerted for this condition and adequately monitor susceptible patients to prevent a hypercalcemic crisis, an emergency condition which is associated with severe neurologic symptoms, poor prognosis, and decreased survival.
Taken together, descriptions of cases of KSC-associated PNS are rather scarcely reported in the medical literature. This is in distinct contrast with the high incidence of KSC, at least among Caucasians. Future studies should inquire whether KSC-associated PNS are truly rare events or whether they are simply underrecognized and consequently underreported conditions. In this context it could be interesting to compare the epidemiology of PNS in KSC with that of the other, non-keratinocyte derived skin cancers and also with keratinocyte tumors that originate from skin-adjacent mucosal surfaces (oropharynx, external genitalia). Nevertheless, we would like to predict that with the wider availability and application of the emerging treatment modalities for advanced KSC, the need to differentiate between adverse events and PNS will probably lead to increasing numbers of PNS observations in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14010249/s1, Table S1: Paraneoplastic syndromes in patients with keratinocyte skin cancer: Compilation of the clinical features stratified according to patients’ gender, Table S2: Paraneoplastic syndromes in patients with keratinocyte skin cancer. Compilation of the clinical features stratified according to the different skin cancer types. No (%): Number of cases and distribution of the cases among the different cancer types (% of cases with available information), Table S3: Malignancy associated hypercalcemia (MAH) in KSC patients: Per patient laboratory findings of the N = 32 cases with MAH.
Author Contributions
Conceptualization, I.D.B.; methodology, C.V., C.T. and I.D.B.; writing—review and editing, C.V., C.T. and I.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hobbs C.B., Miller A.L. Review of Endocrine Syndromes Associated with Tumours of Non-Endocrine Origin. J. Clin. Pathol. 1966;19:119–127. doi: 10.1136/jcp.19.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilynsky B.T., Dzhus M.B., Litvinyak R.I. The Conceptual and Clinical Problems of Paraneoplastic Syndrome in Oncology and Internal Medicine. Exp. Oncol. 2015;37:82–88. doi: 10.31768/2312-8852.2015.37(2):82-86. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriadis G.K., Angelousi A., Weickert M.O., Randeva H.S., Kaltsas G., Grossman A. Paraneoplastic Endocrine Syndromes. Endocr.-Relat. Cancer. 2017;24:R173–R190. doi: 10.1530/ERC-17-0036. [DOI] [PubMed] [Google Scholar]
- 4.Maverakis E., Goodarzi H., Wehrli L.N., Ono Y., Garcia M.S. The Etiology of Paraneoplastic Autoimmunity. Clin. Rev. Allergy Immunol. 2012;42:135–144. doi: 10.1007/s12016-010-8248-5. [DOI] [PubMed] [Google Scholar]
- 5.Thiers B.H., Sahn R.E., Callen J.P. Cutaneous Manifestations of Internal Malignancy. CA Cancer J. Clin. 2009;59:73–98. doi: 10.3322/caac.20005. [DOI] [PubMed] [Google Scholar]
- 6.Ehst B.D., Minzer-Conzetti K., Swerdlin A., Devere T.S. Cutaneous Manifestations of Internal Malignancy. Curr. Probl. Surg. 2010;47:384–445. doi: 10.1067/j.cpsurg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Vyas R., Selph J., Gerstenblith M.R. Cutaneous Manifestations Associated with Melanoma. Semin. Oncol. 2016;43:384–389. doi: 10.1053/j.seminoncol.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Iyer J.G., Parvathaneni K., Bhatia S., Tarabadkar E.S., Blom A., Doumani R., McKenzie J., Asgari M.M., Nghiem P. Paraneoplastic Syndromes (PNS) Associated with Merkel Cell Carcinoma (MCC): A Case Series of 8 Patients Highlighting Different Clinical Manifestations. J. Am. Acad. Derm. 2016;75:541–547. doi: 10.1016/j.jaad.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vatandoust S., McKay B.P., McLeay W., Miliauskas J., Gordon L., Wesley J.A., Kichenadasse G. Acrokeratosis Paraneoplastica (Bazex Syndrome) Associated with Metastatic Cutaneous Squamous Cell Carcinoma. Intern. Med. J. 2016;46:119–120. doi: 10.1111/imj.12955. [DOI] [PubMed] [Google Scholar]
- 10.Hakkou J., Rostom S., Bahiri R., Hajjaj-Hassouni N. Paraneoplastic rheumatic syndromes: Report of eight cases and review of literature. Rheumatol. Int. 2012;32:1485–1489. doi: 10.1007/s00296-011-2252-9. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig L.B., Brett A.S., Lefaivre J.-F., Vandersteenhoven J.J. Hidradenitis Suppurativa Complicated by Squamous Cell Carcinoma and Paraneoplastic Neuropathy. Am. J. Med. Sci. 2005;329:150–152. doi: 10.1097/00000441-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Cisneros G., Lara L.F., Crock R., Whittier F.C. Humoral hypercalcemia of malignancy in squamous cell carcinoma of the skin: Parathyroid hormone-related protein as a cause. South Med. J. 2001;94:329–331. [PubMed] [Google Scholar]
- 13.Kato N., Yasukawa K., Onozuka T., Kimura K. Paraneoplastic syndromes of leukocytosis, thrombocytosis, and hypercalcemia associated with squamous cell carcinoma. J. Dermatol. 1999;26:352–358. doi: 10.1111/j.1346-8138.1999.tb03487.x. [DOI] [PubMed] [Google Scholar]
- 14.Mori H., Aoki K., Katayama I., Nishioka K., Umeda T. Humoral Hypercalcemia of Malignancy with Elevated Plasma PTHrP, TNF Alpha and IL-6 in Cutaneous Squamous Cell Carcinoma. J. Derm. 1996;23:460–462. doi: 10.1111/j.1346-8138.1996.tb04055.x. [DOI] [PubMed] [Google Scholar]
- 15.Hara M., Hunayama M., Aiba S., Suetake T., Watanabe M., Tanaka M., Tagami H. Acrokeratosis paraneoplastica (Bazex syndrome) associated with primary cutaneous squamous cell carcinoma of the lower leg, vitiligo and alopecia areata. Br. J. Dermatol. 1995;133:121–124. doi: 10.1111/j.1365-2133.1995.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 16.Gerner R.E., Moore G.E. Burn Scar Carcinoma and Hypercalcemia. Ann. Surg. 1974;180:95–97. doi: 10.1097/00000658-197407000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben Said B., Maitre S., Perrot J.-L., Labeille B., Cambazard F. Syndrome paranéoplasique hypercalcémie–hyperleucocytose au cours des carcinomes épidermoïdes cutanés. À propos de deux observations. La Rev. De Méd. Interne. 2010;31:309–311. doi: 10.1016/j.revmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Miura K., Umegaki N., Kitaoka T., Kubota T., Namba N., Etani Y., Hirai H., Kogaki S., Nakajima S., Takahashi Y., et al. A male patient with humoral hypercalcemia of malignancy (hhm) with leukocytosis caused by cutaneous squamous cell carcinoma resulting from recessive dystrophic epidermolysis bullosa. Clin. Pediatr. Endocrinol. 2011;20:65–71. doi: 10.1297/cpe.20.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawai T., Hayakawa H., Danno K., Miyauchi H., Uehara M. Squamous cell carcinoma arising from giant porokeratosis: A case with extensive metastasis and hypercalcemia. J. Am. Acad. Dermatol. 1996;34:507–509. doi: 10.1016/S0190-9622(96)90459-4. [DOI] [PubMed] [Google Scholar]
- 20.Miquel J., Adamski H., Faujour G. Hypercalcémie aiguë et carcinomes épidermoïdes multiples compliquant une hidradénite suppurée. Presse Med. 2009;38:1177–1180. doi: 10.1016/j.lpm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Crespo M., Sopeña B., Orloff J.J., CameselleTeijeiro J.F., Dann P., Andrade M.A., Freire M., de la Fuente J., Martinez-Vazquez C. Immunohistochemical detection of parathyroid hormone-related protein in a cutaneous squamous cell carcinoma causing humoral hypercalcemia of malignancy. Arch Pathol Lab Med. 1999;123:725–730. doi: 10.5858/1999-123-0725-IDOPHR. [DOI] [PubMed] [Google Scholar]
- 22.O’Malley J.T., Schoppe C., Husain S., Grossman M.E. Squamous cell carcinoma (Marjolin’s ulcer) arising in a sacral decubitus ulcer resulting in humoral hypercalcemia of malignancy. Case Rep. Med. 2014;2014:715809. doi: 10.1155/2014/715809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito Y., Iwasaki T., Wakamatsu K., Hashimoto M., Iizuka H. Hypercalcemia associated with squamous cell carcinoma arising in pyoderma chronica. J. Dermatol. 2009;36:555–556. doi: 10.1111/j.1346-8138.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaur M.R., Marsden J.R., Nelson H.M. Humoral hypercalcaemia of malignancy associated with primary cutaneous squamous cell carcinoma. Clin. Exp. Dermatol. 2007;32:237–238. doi: 10.1111/j.1365-2230.2006.02316.x. [DOI] [PubMed] [Google Scholar]
- 25.Loche F., Bennet A., Bazex J., Thouvenin M.D. Hypercalcaemia of malignancy associated with invasive cutaneous squamous cell carcinoma. Br. J. Dermatol. 1999;141:577–579. doi: 10.1046/j.1365-2133.1999.03066.x. [DOI] [PubMed] [Google Scholar]
- 26.Giri D., Ramakrishnan R., Hayden J., Brook L., Das U., Mughal M.Z., Selby P., Dharmaraj P., Senniappan S. Denosumab therapy for refractory hypercalcemia secondary to squamous cell carcinoma of skin in epidermolysis bullosa. World J. Oncol. 2015;6:345–348. doi: 10.14740/wjon907w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sungur N., Kilinç H., Uysal C., Ortak T., Sensöz O. A gigantic squamous cell carcinoma with hypercalcemia arising in an old burn scar. Ann. Plast. Surg. 2001;47:351–352. doi: 10.1097/00000637-200109000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Marino M.T., Asp A.A., Budayer A.A., Marsden J.S., Strewler G.J. Hypercalcaemia and Elevated Levels of Parathyroid Hormone-Related Protein in Cutaneous Squamous/Basal Cell Carcinoma. J. Intern. Med. 1993;233:205–207. doi: 10.1111/j.1365-2796.1993.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 29.Funauchi M., Yamagata T., Sugiyama M., Ikoma S., Sakaguchi M., Kinoshita K., Kawata A. A Case of Antiphospholipid Antibody Syndrome That Manifested in the Course of Basal Cell Carcinoma. Mod. Rheumatol. 2007;17:153–155. doi: 10.3109/s10165-006-0550-y. [DOI] [PubMed] [Google Scholar]
- 30.Carneiro B.A., Watkin W.G., Mehta U.K., Brockstein B.E. Metastatic basal cell carcinoma: Complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24:396–400. doi: 10.1080/07357900600705474. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda T., Tsuru K., Hayashi K., Ichihashi M., Ueda M. Hypercalcemia of malignancy associated with trichilemmal carcinoma in burn scar. Acta Derm Venereol. 2000;80:396–397. [PubMed] [Google Scholar]
- 32.Yamauchi M., Yotsuyanagi T., Saito T., Ikeda K., Urushidate S., Higuma Y. Three Cases of Giant Pilomatrixoma--Considerations for Diagnosis and Treatment of Giant Skin Tumours with Abundant Inner Calcification Present on the Upper Body. J. Plast. Reconstr. Aesthet. Surg. 2010;63:e519–e524. doi: 10.1016/j.bjps.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Guthrie T.H., Jr., Thornton R.M. Pure red cell aplasia obscured by a diagnosis of carcinoma. South Med. J. 1983;76:532–534. doi: 10.1097/00007611-198304000-00037. [DOI] [PubMed] [Google Scholar]
- 34.Emir S., Hacısalihoğlu Ş., Özyörük D., Kaçar D., Erdem A., Karakuş E. Squamous cell carcinoma associated with xeroderma pigmentosum: An unusual presentation with a tremendously huge mass over the face and paraneoplastic hypercalcemia-hyperleukocytosis. Turk J. Pediatr. 2017;59:711–714. doi: 10.24953/turkjped.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Vlachostergios P.J., Balmiki R.L. Bone metastases and hypercalcaemia from cutaneous squamous cell carcinoma. BMJ Case Rep. 2014;2014:bcr2014204947. doi: 10.1136/bcr-2014-204947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparks M.K., Kuhlman D.S., Prieto A., Callen J.P. Hypercalcemia in association with cutaneous squamous cell carcinoma. Occurrence as a late complication of hidradenitis suppurativa. Arch Dermatol. 1985;121:243–246. doi: 10.1001/archderm.1985.01660020101028. [DOI] [PubMed] [Google Scholar]
- 37.Picascia D.D., Caro W.A. Cutaneous Squamous Cell Carcinoma and Hypercalcemia. J. Am. Acad. Derm. 1987;17:347–351. doi: 10.1016/S0190-9622(87)70210-2. [DOI] [PubMed] [Google Scholar]
- 38.Welsh D.A., Powers J.S. Elevated parathyroid hormone-related protein and hypercalcemia in a patient with cutaneous squamous cell carcinoma complicating hidradenitis suppurativa. South Med. J. 1993;86:1403–1404. doi: 10.1097/00007611-199312000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Hayakawa Y., Ishizaki H., Tanabe S., Kimura A., Yamamichi N. Squamous cell carcinoma with hypercalcemia and leukocytosis. Dermatologica. 1986;172:169–172. doi: 10.1159/000249324. [DOI] [PubMed] [Google Scholar]
- 40.Southwick G.J., Schwartz R.A. Arsenically Associated Cutaneous Squamous Cell Carcinoma with Hypercalcemia. J. Surg. Oncol. 1979;12:115–118. doi: 10.1002/jso.2930120204. [DOI] [PubMed] [Google Scholar]
- 41.Clements W.D., Ritchie A.J., Kinley J.G. Basal cell carcinoma presenting with profound anaemia. Ulster Med. J. 1991;60:243–245. [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins J., Hull S.M. Profound anaemia secondary to an ulcerated basal cell carcinoma. Clin. Exp. Dermatol. 1996;21:389–390. doi: 10.1111/j.1365-2230.1996.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 43.Reynaud-Mendel B., Robert C., Flageul B., de Vernejoul M.C., Verola O., Dubertret L. Malignant hypercalcemia induced by a parathyroid hormone-related protein secreted by a cutaneous squamous cell carcinoma. Arch Dermatol. 1997;133:113. doi: 10.1001/archderm.1997.03890370125030. [DOI] [PubMed] [Google Scholar]
- 44.Constantinou C., Widom K., Desantis J., Obmann M. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am. Surg. 2008;74:1177–1181. doi: 10.1177/000313480807401209. [DOI] [PubMed] [Google Scholar]
- 45.Pitch M.A., Bryan D.J., McMillan J., Chavez L., Hammes S.R., Scott G., Mercurio M.G., Somers K.E. A Fatal Case of Parathyroid Hormone-Related Peptide (PTHrP)-Producing Squamous Cell Carcinoma Arising in the Context of Long-Standing Hidradenitis Suppurativa. JAAD Case Rep. 2018;4:426–428. doi: 10.1016/j.jdcr.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makoui C., Fishburne C. Hypercalcemia in squamous cell carcinoma of the skin. JAMA. 1978;239:1882–1883. doi: 10.1001/jama.1978.03280450054026. [DOI] [PubMed] [Google Scholar]
- 47.Nicolae I., Schipor S. PTH-Independent Hypercalcaemia and Non-Melanoma Skin Cancer. J. Eur. Acad. Derm. Venereol. 2010;24:449–452. doi: 10.1111/j.1468-3083.2009.03441.x. [DOI] [PubMed] [Google Scholar]
- 48.Ramadas P., Bansal N., Krishnan P., Caza T., Manocha D. Hypercalcemia as the first diagnostic clue of a malignant decubitus ulcer. Austin Intern. Med. 2016;1:1003. [Google Scholar]
- 49.Saeed M.A., George G.A., Lajara S., Perkins B., Knight C.M. A Rare Case of Hypercalcemic Crisis Secondary to a Squamous Cell Carcinoma Arising from a Nonhealing Ulcer. AACE Clin. Case Rep. 2017;3:303–306. doi: 10.4158/EP161485.CR. [DOI] [Google Scholar]
- 50.Goltzman D. Approach to Hypercalcemia. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., Dungan K., Grossman A., Hershman J.M., et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 51.Zagzag J., Hu M.I., Fisher S.B., Perrier N.D. Hypercalcemia and Cancer: Differential Diagnosis and Treatment. CA Cancer J. Clin. 2018;68:377–386. doi: 10.3322/caac.21489. [DOI] [PubMed] [Google Scholar]
- 52.Stewart A.F. Clinical Practice. Hypercalcemia Associated with Cancer. N. Engl. J. Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 53.Weckmann M.T., Gröne A., Capen C.C., Rosol T.J. Regulation of Parathyroid Hormone-Related Protein Secretion and MRNA Expression in Normal Human Keratinocytes and a Squamous Carcinoma Cell Line. Exp. Cell Res. 1997;232:79–89. doi: 10.1006/excr.1997.3481. [DOI] [PubMed] [Google Scholar]
- 54.Burt M.E., Brennan M.F. Incidence of Hypercalcemia and Malignant Neoplasm. Arch. Surg. 1980;115:704–707. doi: 10.1001/archsurg.1980.01380060012004. [DOI] [PubMed] [Google Scholar]
- 55.Attanoos R.L., Appleton M.A., Hughes L.E., Ansell I.D., Douglas-Jones A.G., Williams G.T. Granulomatous Hidradenitis Suppurativa and Cutaneous Crohn’s Disease. Histopathology. 1993;23:111–115. doi: 10.1111/j.1365-2559.1993.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 56.Gaspar B.L., Sharma P., Das R. Anemia in Malignancies: Pathogenetic and Diagnostic Considerations. Hematology. 2015;20:18–25. doi: 10.1179/1607845414Y.0000000161. [DOI] [PubMed] [Google Scholar]
- 57.Means R.T. Pure Red Cell Aplasia. Blood. 2016;128:2504–2509. doi: 10.1182/blood-2016-05-717140. [DOI] [PubMed] [Google Scholar]
- 58.Räßler F., Goetze S., Elsner P. Acrokeratosis Paraneoplastica (Bazex Syndrome)—A Systematic Review on Risk Factors, Diagnosis, Prognosis and Management. J. Eur. Acad. Derm. Venereol. 2017;31:1119–1136. doi: 10.1111/jdv.14199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.