Abstract
Schistosomiasis is one of the snail-borne diseases responsible for the second-highest burden of diseases among neglected tropical diseases. The use of mass drug administration to the populations most at risk is a backbone of the strategy to prevent and control schistosomiasis transmission. However, it offers no protection against re-infection, and humans are often re-exposed when they return to water bodies where snails release cercariae. Surveys on cercarial infection in snails could provide better insights on human disease risk. Hence, in this study, we investigated cercarial infection in snails and also determined the epidemiology of Schistosoma mansoni among fishermen at Ethiopian Rift Valley lakes. Freshwater snails were collected from the shorelines of Ethiopian Rift Valley lakes for examination of cercarial infection during 2020. Environmental data on water quality variables and physical characteristics of snail habitats were collected. Stool samples were collected from fishermen and the Kato-Katz technique was applied for the quantification of Schistosoma mansoni eggs. A malacological survey indicated that six morphologically distinguishable types of cercariae were found in snails. Infected snails with cercaria were more likely present in habitats with high five-day biological oxygen demand and low dissolved oxygen. The overall prevalence of Schistosoma mansoni infection among the fishermen at Ethiopian Rift Valley lakes was found to be 21.5%. This indicates that fishermen at Ethiopian Rift Valley lakes are one of the groups of people harboring schistosome cercariae which are potentially responsible for the transmission of schistosomiasis to lakeshore communities who have contact with lake water. Therefore, complementary medical treatment, public health interventions, environmental management and snail reduction are needed to control the transmission of schistosomiasis.
Keywords: freshwater snails, cercarial infection, Schistosoma mansoni, fishermen, Ethiopian Rift Valley lakes
1. Introduction
Snail-borne diseases form an important share of parasitic diseases that pose risks to human health and cause major socioeconomic problems [1]. Schistosomiasis and fascioliasis are two of the most common snail-borne diseases worldwide which are widespread in many tropical and sub-tropical countries. The medical and economic burden of these diseases are often neglected which is why they are included in the list of the neglected tropical diseases (NTDs) [2]. Schistosomiasis is responsible for the second highest disease burden among NTDs [3], with almost 93% of the 230 million infected people worldwide living in African regions [4]. Fascioliasis is another important snail-borne disease that affects livestock and humans throughout the world [5]. Traditionally regarded as a disease of livestock, fascioliasis is now recognized as important emerging zoonotic disease of humans [6]. An estimated 2.4 million people are infected worldwide while 180 million people are at risk of infection [7]. Ethiopia is one of those countries with the highest number of cases of human schistosomiasis where 38.3 million people are either infected or live in schistosomiasis endemic areas [8]. Both diseases, schistosomiasis and fascioliasis, share similarities in their life cycle, of which the most prominent feature is the infection of specific freshwater snails that act as intermediate hosts [9,10,11]. Many species of freshwater snails belonging to the genera Biomphalaria, Bulinus and Oncomelania are intermediate hosts of different trematode parasites of medical and veterinary importance [2]. In Ethiopia, snails of the genus Biomphalaria (B. pfeifferi and B. sudanica) Bulinus (Bu. abyssinicus and Bu. africanus) are intermediate hosts of S. mansoni and S. heamatobium, respectively [12]. Snails of the genus Lymnaea (L. natalensis and L. truncatula) are intermediate hosts of Fasciola parasites [13,14].
The transmission cycle of snail-borne diseases starts when urine or feces containing parasites are deposited in freshwater bodies and the hatched miracidia infect the snail intermediate hosts [15]. In the snails, the miracidium develops into a mother sporocyst. In the schistosomes, the sporocyst develops into the second generation sporocysts, after which in the infective larvae, cercariae are formed. In some hermaphroditic trematodes (e.g., liver flukes), the mother sporocyst develops into rediae which produce cercariae [15]. Once the cercariae are released into the water, they either penetrate the skin of the definitive host (e.g., schistosomes) or are ingested after encysting as metacercariae in or on edible plants or animals. After entering the definitive host, the schistosome larvae mature into adult worms in the blood vessels of the liver, intestine and bladder. The worms lay thousands of eggs that causes damage as they grow through tissues and consequently, infection occurs accordingly [16]. The cycle perpetuates when infected human/animal defecate or urinate into freshwater sources [15]. The populations that are at risk of schistosomiasis include school-aged children and adults in endemic areas and people with occupations that put them in direct contact with potentially infectious waters, such as fishermen, farmers, irrigation workers and women fetch/transport water for domestic use [4,17].
The World Health Organization (WHO) guided schistosomiasis prevention and control strategies depending on mass drug administration (MDA) [18,19]. Similarly, following the London declaration on NTDs in 2012 [20], the Federal Ministry of Health (FMoH) of Ethiopia developed a national master plan for combating the country’s most common NTDs to attain a transmission break plan by 2025 [21]. For schistosomes, the target is to control morbidity by means of MDA of Praziquantel to the population at risk. The MDA strategies are effective in reducing morbidity associated with schistosomes by decreasing the worm burden and the intensity of infection [15]. Although MDA is the backbone to break the transmission of schistosomiasis, the prevalence of the disease remains very high in many countries [22,23]. Mass drug administration offers no protection against re-infection, and humans are often re-exposed when they return to water bodies with snail releasing cercariae [24,25]. Hence, MDA is not effective as sole component to limit the transmission of schistosomiasis in high prevalence regions [26,27]. Snail control has appeared to be a more effective strategy to reduce the transmission of snail-borne diseases in several countries [28,29,30]. However, snail control efforts that make considerable reductions in density of snail intermediate hosts at water-access points can sometimes fail if infected snails remain [31]. In recent times, it has been shown that information on cercarial infection in freshwater snail intermediate hosts could provide a better prediction of human disease risk rather than investigating snail population size alone [32,33,34].
Therefore, this study aimed to (i) investigate cercarial infection in freshwater snails and (ii) determine the epidemiology of S. mansoni infection among fishermen, an important group being at risk, at Ethiopian Rift Valley lakes and its associated risk factors. The findings of this study can be used to provide information on the importance of a holistic approach to support the prevention and control of schistosomiasis.
2. Materials and Methods
2.1. Study Area
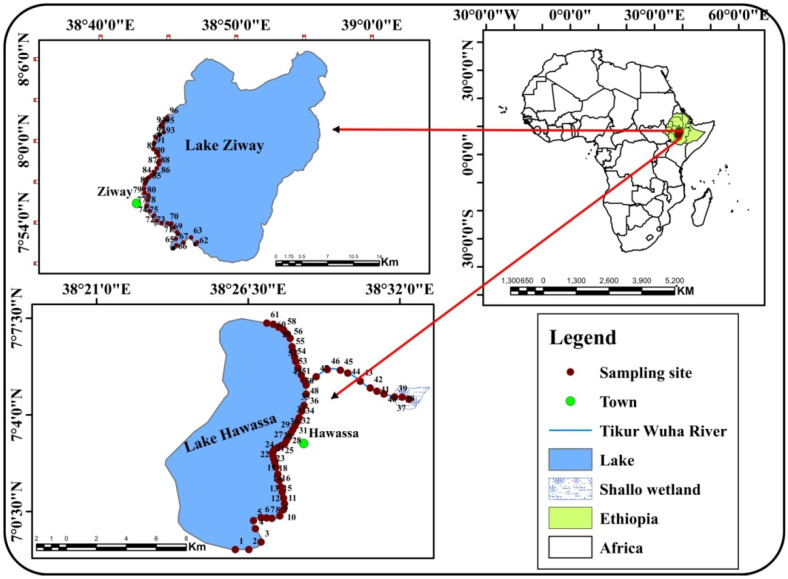
The study was conducted on Ethiopian Rift Valley lakes, namely Lake Hawassa and Lake Ziway which are situated in central Ethiopia (Figure 1). Lake Hawassa is located in the tropical rainy climate zone at a distance of 271 km from Addis Ababa, the capital city. It is situated between latitudes 06°0.97′ N and 07°0.23′ N and longitudes 38°0.37′ E and 38°0.47′ E at an elevation of 1685 m above sea level, covering a total area of km2, with an average depth of 11 m [35,36]. Lake Ziway is located in the warm temperate rainy climate zone at a distance of about 160 km from Addis Ababa. It has an open water area of 434 km2, with an average depth of 4 m. It is situated between latitudes 07°0.85′ N and 08°0.01′ N and longitudes 38°0.72′ E and 38°0.83′ E at an elevation of 1636 m above sea level [37,38]. Ethiopian Rift Valley lakes provide social, economic and ecological benefits for the local communities in the area. In spite of these benefits, there is a high probability of acquiring snail-borne diseases as there is frequent human-water contact for purposes such as domestic use, irrigation, livestock watering, fishing, recreation and alike. Fishing is an off-season activity dominant in both zones through which fishing communities get in contact with water possibly contaminated with trematode parasites.
Figure 1.
Map of the study area showing locations of sampling sites. The map was constructed using the geographic information system (GIS) software ArcGIS 10.7.
2.2. Sampling Site Selection
Shorelines of the Ethiopian Rift Valley lakes and Tikur Wuha River (the tributary of Lake Hawassa) where there was evidence of human-water contact activities were selected as sampling sites for data collection. Data on environmental factors (water quality variables and snail physical habitat characteristics) and cercarial infections of freshwater snail intermediate hosts were collected from each sampling site. Data collection was carried out during the dry (March) and wet (November) seasons in 2020 at Lake Hawassa, whereas only during the wet season (November) in 2020 at Lake Ziway. During the dry season, data collection at Lake Ziway could not be carried out due to interruption by the worldwide COVID-19 pandemic.
2.3. Environmental Variables
Physico-chemical water quality variables including pH, water temperature, dissolved oxygen and electrical conductivity were measured onsite using a portable Multiprobe Meter (HQ40d Single-Input Multi-parameter Digital Meter, Hach Company, Loveland, CO, USA). Turbidity was measured onsite using a turbidity meter (Wag-WT3020; Halma PLC Company, Amersham, UK). Chlorophyll-a was measured onsite using a hand-held fluorometer (Aqua Fluor; Turner Designs, San Jose, CA, USA). A water sample (2000 mL) was taken from each sampling site in polyethylene bottles and transported to the laboratory using an ice cooler box for analysis of other water quality variables. In the laboratory, a water sample (250 mL) was filtered through a 45 µm filter paper and then analyzed for concentrations of total hardness and ions such as calcium, magnesium and chloride. An unfiltered water sample was used for the determination of total suspended solids, and five-day biological oxygen demand (BOD5). These analyses were carried out according to the standard methods for the examination of water and wastewater [39].
The percentage of macrophyte (emergent, submerged and floating) cover was visually estimated at each sampling site [40]. The percentage of the macrophyte cover was categorized into five groups: very low (<10%); low (10–35%); moderate (35–65%); high (65–90%); and very high (>90%) [41]. Canopy cover was estimated visually based on the percentage of shade [42]. Water depth was measured using a graduated stick calibrated in centimeters. Transparency of water was determined with a Secchi disk 30 cm in diameter attached to a calibrated cord. Ambient temperature was measured using a mercury-in-glass thermometer (THL-210-050T; Vintage Gallenkamp Griffin, England). The type of substrate was carefully assessed by observation and classified into detritus, silt, sand, gravel, cobble, boulder or bedrock [43]. The presence or absence of anthropogenic activities taking place at each sampling site was recorded following direct observations (Figure 2). The common anthropogenic activities recorded were: fishing, farming/cultivation, washing/bathing, swimming/playing in the water, open defecation/urination, livestock watering and water abstraction for irrigation and industry.
Figure 2.
Pictures taken at sampling sites in the study area: Lake Hawassa (a,b); Lake Ziway (c); and Tikur Wuha River (d). Data were collected at Lake Hawassa and Tikur Wuha River during both the dry (15–22 March) and wet (13–30 November) seasons in 2020, but only during the wet season (13–30 November) at Lake Ziway.
A hand-held global positioning system (GPS) instrument (GPS 72H; Garmin Ltd., Olathe, KS, USA) was used to record altitude and coordinates (latitude and longitude) at each sampling site.
2.4. Snail Collection and Examination of Cercarial Infection
Freshwater snails were collected using long-handheld scoops [44]. Two experienced and well-trained persons carried out scooping for 30 min. The scoop was pushed through vegetation; the biomphalarid snails were picked out of the scoops by hand using gloves and placed in plastic containers containing water and vegetation from the same habitat to transport them to the laboratory. In the laboratory, the biomphalarid snails were morphologically identified to species level using the standard identification keys [45].
The biomphalarid snail species were examined for cercarial infections by the natural shedding method following procedures used in previous studies [34,44,46]. The snails were rinsed with aerated (dechlorinated) tap water to remove the mud from their shells and placed individually in beakers containing 10 mL aerated tap water and exposed to sunlight for 1–4 h to induce shedding cercariae. The time for cercariae shedding was carefully selected to coincide with the early peak shedding time (mid-day). The water in the bottle was then checked frequently for cercariae shedding with a hand lens. If any beaker confirmed the presence of cercaria, a sample of water was transferred to slides using a dropper and stained with iodine solution and covered by a cover slip for cercariae identification. Shed cercariae were morphologically identified to genus level with a light microscope (100×) (TK-C921BEG; Victor Company of Japan Limited, T2 Tokyo, Japan) and identification keys [47]. Snails that did not shed on the first exposure were re-exposed to sunlight for the cercariae shedding every day for another consecutive seven days. During this course of time, the snails were fed lettuce in the containers containing aerated water which was replaced daily. The genus of the cercariae released by each snail was recorded. Pictures of the cercariae were taken by digital camera fitting the eye lens of the microscope (SM-G920F).
2.5. Mapping Spatial Distribution of Sampling Sites
The type of land use/land cover at each sampling site in a 10 m stretch starting from the lakeshore and moving outwards was assessed [48] and then checked with the map templates of land use/land cover types of the study area computed from satellite images. The Sentinel-2 images of the study area were downloaded from the United States Geological Survey website (https://earthexplorer.usgs.gov; accessed on 25 March 2020) from which land use/land cover types were computed. Sentinel-2 images with spatial resolution of 10 m were used to assess land use/land cover of the study area through earth resource data analysis system (ERDAS) 2015 image processing software. Images used dated from the dry season of 2020. The catchment landscapes (land use/land cover types) adjacent to sampling sites were classified into five categories, including built-up, farmland, marshy land, wetland or water body based on standard guidelines [48]. A confusion matrix was employed to assess the classification accuracy. Accuracy of the classified land use/land cover maps were assessed using a combination of overall accuracy, producer’s accuracy, user’s accuracy, errors of commission and omission [49] and kappa coefficient [50].
Maps of the study area and each land use/land cover types were mapped and visually digitized using the satellite image in the geographic information system (GIS) software packages ERDAS 2015, ArcGIS 10.7 and validated by ground truth points.
2.6. Parasitological Survey and Assessment of Risk Factors
The parasitological survey was carried out to determine the prevalence and infection intensity of S. mansoni among fishermen at Ethiopian Rift Valley lakes. Despite the fact that the malacological survey included all cercarial infections in biomphalarid snails, the parasitological survey focused on S. mansoni, a severe intestinal infection in Ethiopia. Based on the WHO monitoring guidelines [51], stool samples were collected from 200 fishermen at each Lake. Fishermen who were members of fishermen associations and had no history of taking Praziquantel (anthelmintic) in the past 6 months were considered as possible participants of the study. They were purposively selected based on their availability at the landing place.
A unique identification number was given to each participant. Each participant was provided with a labeled stool cup with an applicator stick for a stool sample after orienting them on how to provide a sufficient stool sample. Collected stool samples were processed using a Kato–Katz technique [52]. For quantification of S. mansoni eggs the samples were examined using a light microscope (TK-C921BEG; Victor Company of Japan Limited, T2 Tokyo, Japan). The egg of S. mansoni in the Kato slides was counted and multiplied by 24 to convert eggs per gram of stool (EPG). The intensity of infection was classified as light (1–99 EPG), moderate (100–399 EPG), and heavy infections (≥400 EPG) [53].
A questionnaire was used to assess the practices of the fishermen to prevent and control schistosomiasis transmission.
2.7. Ethical Approval and Consent to Participate
Ethical clearance was obtained from the institutional review boards (IRBs) of the Institute of Health Sciences, Jimma University. A formal letter was written to all concerned bodies and permission was secured at all levels. The objectives of the study were explained to the study participants and written consents were obtained. The confidentiality of the information was assured, and the respondent’s privacy was maintained. Participants with positive results in the microscopic examination test for S. mansoni were referred to the nearby health facility for treatment.
2.8. Data Analysis
Data analyses were carried out using R software (Version 3.5.2) [54]. The prevalence of cercarial infection in snails was determined as a percentage, by dividing the number of snails that shed cercariae by the total number of snails examined and multiplying the outcome by 100. Shapiro–Wilk normality tests for normality and homogeneity of variance showed that data were not normally distributed. Hence, a non-parametric Kruskal–Wallis test was performed to test whether significant differences in the number of infected snails existed among physical characteristics of the snail habitats (i.e., category of macrophyte cover and substrate type). A Wilcoxon post-hoc multiple comparison test was performed to identify significantly different pairs. The post-hoc test was Bonferroni corrected.
Logistic regression analysis was used to identify the factors that significantly influence the occurrence (presence/absence) of infected snails. Spearman’s rank-order correlation was used to determine associations between the number of infected snails and environmental (physico-chemical) variables.
Prevalence and intensity of S. mansoni infection were reported in percent and mean egg count, respectively. Risk factors associated with S. mansoni infection among the fishermen were analyzed by bivariate logistic regression followed by a multiple logistic regression model. The magnitude of association was measured through odds ratio at the 95% confidence interval. p-values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Cercarial Infection in Snails
A total of 169 biomphalarid snails were collected from 61 sampling sites on the shorelines of Lake Hawassa during both dry and wet seasons, while 88 biomphalarid snails were collected from 35 sampling sites on the shorelines of Lake Ziway during the wet season. Overall, a total of 257 snails were collected from the total of 96 sampling sites on the shorelines of Ethiopian Rift Valley lakes, 78 snails were infected which accounted for an infection prevalence of 30.5% (pooling all cercariae identified).Infected snails were encountered at 20 sampling sites out of the total number of 96 sampling sites (20.8%). During the wet season, a higher prevalence of cercarial infection in snails (49.2%) was encountered at Lake Hawassa compared to Lake Ziway (36.7%). The prevalence of different types of cercarial infection in snails and the number of infected snails are summarized in Table 1.
Table 1.
Prevalence of cercarial infection in biomphalarid snails collected from the shorelines of Ethiopian Rift Valley lakes (BAD = Brevifurcate-apharyngeate distome cercariae, Echis = Echinostome cercariae, Xior = Ornatae xiphidiocercariae, Gymn = Gymnocephalous, Amph = Amphistome, Meta = Metacercariae).
| Study Area | Season | Snail Species | Infection Prevalence with a Type of Cercaria (%) | |||||
|---|---|---|---|---|---|---|---|---|
| BAD | Echis | Xior | Gymn | Amph | Meta | |||
| Lake Hawassa | Wet | B. pfeifferi | 9 | 30 | 7 | 2 | 12 | 5 |
| B. sudanica | 0 | 0 | 0 | 0 | 13 | 0 | ||
| Dry | B. pfeifferi | 0 | 0 | 0 | 0 | 0 | 0 | |
| B. sudanica | 6 | 4 | 1 | 2 | 3 | 0 | ||
| Lake Ziway | Wet | B. pfeifferi | 4 | 16 | 4 | 6 | 12 | 0 |
| B. sudanica | 0 | 14 | 0 | 0 | 5 | 0 | ||
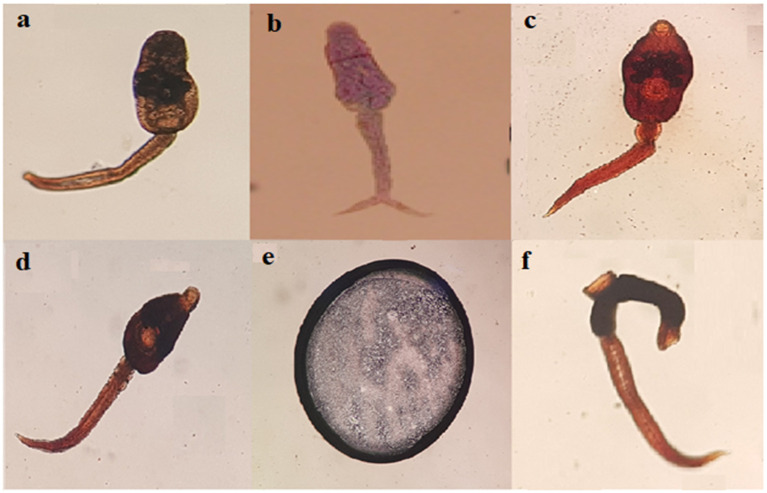
Collectively, six morphologically distinguishable types of cercariae were found in biomphalarid snails. These are amphistomes, brevifurcate-apharyngeate distome (BAD), echinostome, gymnocephalous, metacercariae, and ornatae xiphidiocercariae (Figure 3).
Figure 3.
Microscopic images of different types of cercariae that shed by biomphalarid snails: (a) Amphistome cercariae; (b) BAD cercariae; (c) Echinostome cercariae; (d) Gymnocephalous cercariae; (e) Metacercariae; (f) Ornatae xiphidiocercariae. All cercariae identified to the genus level were 2 mm in size.
Concurrent infections, with more than one type of cercaria, were observed in a single biomphalarid snail. With the exception of metacercariae which was only found in B. pfeifferi, the other five types of cercariae were found in both B. pfeifferi and B. sudanica. The highest infection prevalence was recorded for BAD cercariae in biomphalarid snails collected from Lake Hawassa (37.5%) during dry season, whereas the highest infection prevalence was recorded for echinostome cercariae (30.2%) during the wet season. Echinostomes were the most prevalent cercariae released by biomphalarid snails collected from Lake Ziway (16.6%) during the wet season.
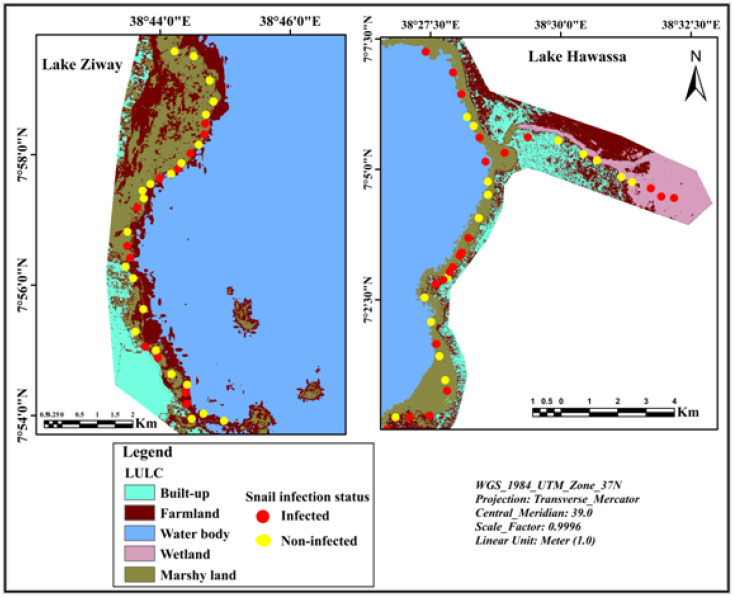
3.2. Spatial Distribution of Infected Snails
The spatial mapping of sampling sites indicated that infected snails were distributed in habitats surrounded by all land use/land cover types found in the study area. However, the presence of infected snails was most frequently collected from sampling sites located adjacent to farmland (Figure 4).
Figure 4.
Mapping of hotspots of infected snails in relation to land use/land cover types on the shorelines of Ethiopian Rift Valley lakes.
3.3. Factors Affecting the Occurrence of Infected Snails
Descriptive statistics of the environmental variables determined at snail collection sites are given in Table 2. The output of the logistic regression analysis revealed that snails living in water with low dissolved oxygen and high BOD5 were more likely infected (Table 3). The number of infected snails was negatively associated with dissolved oxygen and water transparency, but positively associated with water turbidity (all p < 0.05).
Table 2.
Descriptive statistics for environmental variables used to assess the occurrence of infected snails at Ethiopian Rift Valley lakes (TSS = total suspended solids, NTU = nephelometric turbidity unit, SD = standard deviation).
| Environmental Variable | Unit | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| pH | - | 9 | 1 | 6 | 10 |
| Turbidity | NTU | 20 | 31 | 2 | 247 |
| Dissolved oxygen | mg/L | 5 | 3 | 0.5 | 17 |
| Chlorophyll-a | µg/L | 25 | 13 | 11 | 76 |
| Electrical conductivity | µs/cm | 564 | 243 | 71 | 940 |
| BOD5 | mg/L | 26 | 40 | 0.3 | 184 |
| TSS | mg/L | 43 | 31 | 5.2 | 136 |
| Total hardness | mg/L | 68 | 22 | 24 | 120 |
| Calcium ion | mg/L | 49 | 18 | 16 | 100 |
| Magnesium ion | mg/L | 19 | 8 | 0 | 36 |
| Chloride ion | mg/L | 29 | 9 | 11 | 48 |
| Water depth | m | 0.6 | 0.3 | 0.2 | 2 |
| Water transparency | m | 0.3 | 0.1 | 0 | 0.6 |
| Water temperature | °C | 24 | 3 | 19 | 30 |
| Ambient temperature | °C | 26 | 2 | 20 | 31 |
| Canopy cover | % | 16 | 21 | 0 | 100 |
Table 3.
Output of the logistic regression model to predict the occurrence of infected snails.
| Variable | Estimate | Std. Error | z Value | Pr (>|z|) |
|---|---|---|---|---|
| Dissolved oxygen | −0.29322 | 0.11558 | −2.537 | 0.0112 * |
| BOD5 | 0.011696 | 0.005558 | 2.104 | 0.0354 * |
* Significant association (p < 0.05).
3.4. Schistosoma Mansoni Infection and Associated Risk Factors
The result of the parasitological survey indicated that the overall prevalence of S. mansoni among the fishermen at Ethiopian Rift Valley lakes was found to be 21.5% (86/400). Comparing the two lakes, S. mansoni infection was more prevalent among the fishermen at Lake Hawassa, 31% (62/200) than those at Lake Ziway, 12% (24/200). The overall infection intensities recorded for S. mansoni were categorized as light, moderate and heavy among the fishermen at the Ethiopian Rift Valley lakes (Table 4). The majority of overall infections were categorized as light with EPG ranging from 24 to 2112. Specifically, the distribution of infection intensities of S. mansoni was fairly even among fishermen at Lake Hawassa with one-third of the fishermen having light, one-third having moderate and one-third having heavy signs of infection, whereas most of the infection intensities were categorized as light among fishermen at Lake Ziway.
Table 4.
Distribution of the infection intensities of S. mansoni among fishermen at Ethiopian Rift Valley lakes.
| Study Area | Infection Intensity of S. mansoni, n (%) | ||
|---|---|---|---|
| Light | Moderate | Heavy | |
| Lake Hawassa | 22 (35) | 19 (31) | 21 (34) |
| Lake Ziway | 17 (71) | 4 (17) | 3 (13) |
| Both lakes | 39 (45) | 23 (27) | 24 (28) |
n, the number of participants; % = percentage of participants categorized by the type of infection intensity.
Multiple logistic regression analysis indicated that the odds of infection by S. mansoni was significantly associated with the age of the fishermen, habit to defecate in the shorelines of lakes, using water from the lakes for domestic purposes and the type of activity to which the fishermen are engaged (Table 5). The odds of infection by S. mansoni were 79% less among fishermen aged between 18 and 27 years compared to fishermen aged 38 years and above (AOR = 0.21; 95% CI: 0.07–0.64). Likewise, the odds of infection among fishermen aged between 28 and 37 years were 63% less compared to fishermen aged 38 years and above (AOR = 0.37; 95% CI: 0.13–0.96).
Table 5.
Multiple logistic regression analysis of factors associated with S. mansoni infection among the fishermen at Ethiopian Rift Valley lakes (n = number participants tested positive/negative for S. mansoni, % = percentage of participants tested positive/negative for S. mansoni).
| Risk Factor | Category | S. mansoni Infection Status | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|---|
| Positive, n (%) |
Negative, n (%) |
||||
| Age group (years) | 18–27 | 19 (15) | 104 (85) | 0.40 (0.17–0.95) * | 0.21 (0.07–0.64) * |
| 28–37 | 57 (24) | 185 (76) | 0.67 (0.31–1.46) | 0.38 (0.13–0.96) * | |
| 38 and above | 11 (31) | 24 (69) | 1 | 1 | |
| Level of education | No formal education | 11 (27) | 30 (73) | 1.1 (0.35–3.45) | 1.77 (0.45–7.19) |
| Primary education | 70 (21) | 265 (79) | 0.79 (0.30–2.1) | 0.97 (0.32–2.97) | |
| Secondary education and above | 6 (25) | 18 (75) | 1 | 1 | |
| Residence | Urban | 46 (22) | 160 (78) | 1.07 (0.67–1.74) | 1.29 (0.73–2.27) |
| Rural | 41 (21) | 153 (79) | 1 | 1 | |
| Type of activity | Fishing | 58 (27) | 160 (73) | 1.91 (1.16–3.15) * | 2.24 (1.29–3.92) * |
| Fish processing | 29 (16)) | 153 (84) | 1 | 1 | |
| Swimming/bathing in lake | Yes | 75 (22) | 264 (78) | 1.16 (0.59–2.29) | 1.08 (0.49–2.38) |
| No | 12 (20) | 49 (80) | 1 | 1 | |
| Open defecation/urination in lake | Yes | 32 (32) | 68 (68) | 2.10 (1.26–3.50) * | 2.37 (1.35–4.16) * |
| No | 55 (18) | 245 (82) | 1 | 1 | |
| Using water from lake for domestic purposes | Yes | 13 (16) | 70 (84) | 0.61 (0.32–1.14) | 0.33 (0.14–0.76) * |
| No | 74 (23) | 243 (77) | 1 | 1 | |
| Boiling water before drinking | Yes | 3 (19) | 13 (81) | 0.84 (0.23–2.96) | 0.67 (0.18–2.51) |
| No | 84 (22) | 300 (78) | 1 | 1 | |
| Defecating in bush | Yes | 59 (21) | 220 (79) | 0.89 (0.53–1.49) | 0.79 (0.42–1.48) |
| No | 28 (23) | 93 (77) | 1 | 1 | |
Abbreviations: COR, crude odds ratio; AOR, adjusted odds ratio; CI confidence interval (an AOR has been adjusted to account for other predictor variables in a model). * Significant association (p < 0.05).
The odds of infection by S. mansoni were 2.37 times higher among fishermen with the habit to defecate in the shorelines of lakes compared to the counter parts (AOR = 2.37; 95% CI: 1.37–4.16). The odds of infection by S. mansoni were 2.24 times higher among fishermen who engaged in fishing activity compared to those who engaged in fish processing (AOR = 2.24; 95% CI: 1.28–3.91).
On the contrary, fishermen who used water from the lakes for domestic purposes were 67% less likely to acquire S. mansoni infection compared to those who did not use water from the lakes for the same purposes (AOR = 0.33; 95% CI: 0.14–0.75).
4. Discussion
This study is one of the few studies investigating the infection prevalence of cercariae in freshwater snails. In this study, the malacological survey revealed that six morphologically distinguishable types of cercariae are shed by biomphalarid snails which accounted to an infection prevalence of 30.5%. This finding is higher than the infection prevalence of cercariae in snails reported from Omo Gibe river basin, southwest Ethiopia (3.6%) [44], Chitwan district, central Nepal (3.5%) [55], and Kavre, Nepal (1.7%) [56]. Previous research has reported that cercarial infection in freshwater snails could be due to contamination of the water bodies by feces of human beings, aquatic birds, and domestic and/or wild animals being present in the catchment area [57,58].
Previous studies have demonstrated that cercarial infection in freshwater snails is associated with water quality variables and physical characteristics of snail habitats [34,44,59]. In this study, snails living in water with high BOD5 and low dissolved oxygen were more likely infected. In addition, the number of infected snails was positively associated with water turbidity, but negatively associated with dissolved oxygen and transparency of the water. This could be related to contamination of water with human feces and the presence of organic matter that snails feed upon. Well-fed snails tend to produce more parasites [60,61]. Consequently, humans and animals that drink lake water or come into contact with cercariae-infested water are at risk of infection. At the time of data collection in this study, infected snails were collected from lakeshores where anthropogenic activities (i.e., open defecation, washing and bathing, swimming and children playing in the water, and others) were present. Similarly, it has been documented that water pollution caused by people also promotes the occurrence of snails being infected with cercariae [44,62]. Open defecation in and around water bodies, fields, forests, bushes, or other open spaces is common practice in low-and middle-income countries [44,63,64]. These practices may result in the release of Schistosoma eggs into water bodies where they hatch and release miracidia, which enter into snail hosts and release cercariae [26]. Infection with cercariae occurs when humans are exposed to water bodies infested with cercariae released by snail intermediate hosts [65].
In this study, the presence of BAD cercariae (schistosome cercariae) in biomphalarid snails is a potentially important digenean of medical importance which could be linked to the transmission of S. mansoni infection among fishermen in the study area. Biomphalarid snails can serve as an intermediate hosts for the S. mansoni parasite in Ethiopia [66,67]. A parasitological survey carried out in this study showed that the overall prevalence of S. mansoni among fishermen at Ethiopian Rift Valley lakes was found to be 21.5%. This finding is higher than reports on the prevalence of S. mansoni among fishermen from different African countries, such as Burkina Faso (16.35%) [61] and Zambia (12.6%) [68], but lower than the prevalence reported from Ethiopia (29.2%) [69] and Egypt (26.6%) [70]. This variation might be due to the differences in environmental factors that favor the distribution of snail intermediate hosts, frequency of human-water contact, endemicity in the area, and others. Moreover, differences in personal and environmental sanitation levels might be responsible for the variation of S. mansoni infection from place to place. In this study, defecation on the shorelines of lakes was found to be a risk factor associated with S. mansoni infection among fishermen. Overall, the fishermen at Ethiopian Rift Valley lakes are one of the groups of people at risk of S. mansoni infection and that might be responsible for the transmission of schistosomiasis to other segments of the lakeshore community. However, fishermen using lake water for domestic purposes were less likely infected with S. mansoni which is probably due to the effect of household water treatment (i.e., boiling, chlorination) and/or poor report by fishermen on the practices to prevent and control schistosomiasis transmission. Unless appropriate measures are taken to protect the lakes from pollution, the range of suitable habitats for snail intermediate hosts can further extend and threaten the health of nearby residents.
In principle, individual protection from schistosomiasis infection can be achieved by avoiding contact with water infested with schistosome cercariae. However, for people living in areas of Ethiopian Rift Valley lakes, water-human contact is often unavoidable as their daily lives are dependent on the lakes. Therefore, the Ethiopian Rift Valley lakes should be protected from disturbance by anthropogenic activities in order to control schistosomiasis in a sustainable way. For instance, there is an urgent need to establish a buffer zone to reduce pollutants entering Lake Ziway. In the case of Lake Hawassa, there is a good start to establish a buffer zone to retain the pollutants from urban runoff, but it should be expanded to other areas surrounding the lake to reduce pollutants originating from agricultural areas.
In addition to protecting the lakes from pollution, there should be public toilets available in strategic places around the lakes so that people especially fishermen and farmers can defecate safely during occupational activities on the field as well as when they are not in their houses. There is also a need for behavioral change, because even if toilets are available, people still need to be convinced to cease open defecation. Health workers and local authorities should give health education on the health problems associated with open defecation and lake pollution. Several countries succeeded in eliminating schistosomiasis as a public health problem through integrated intervention tools [71,72].
Although this study has shed some light on cercarial infections in Ethiopia, it also had some limitations. This study focused only on biomphalarid snails for the examination of cercarial infection, further study is suggested to investigate cercarial infection of other freshwater snails (i.e., lymnaeid snails). The presence of BAD cercariae in snails does not constitute robust epidemiological information unless schistosome cercariae are precisely identified to species level by molecular techniques. Hence, molecular techniques are useful to differentiate human schistosome cercariae from non-human schistosomes or cryptic cercariae (such as a bird or wildlife parasites) shed by the same snail species. In addition, molecular techniques are required to determine pre-patent stages of cercarial infection of snails to improve the detection of snail infectivity. A more integrated analysis on fisheries and ecosystem management in the context of sustainability as indicated by Forio and Goethals [73] and Gebremedhin et al. [74] would be useful to tackle these and other major needs of freshwater ecosystems [75].
5. Conclusions
This study revealed the presence of six morphologically distinguishable types of cercariae in biomphalarid snails collected from Ethiopian Rift Valley lakes. According to a parasitological survey, fishermen and people frequently visiting the lake water are at highest risk of S. mansoni infection where infected snails are present in the environment. Therefore, there is a need to apply medical treatment accompanied by public health interventions, environmental management and snail control to reduce the transmission of schistosomiasis and avoid re-infection with trematode cercariae in such settings. There is also a need to promote health education to increase awareness of the fishermen and the community in the area on the practices of the prevention and control of snail-borne diseases. Furthermore, enforcement of existing environmental, fisheries and public health laws along lakeshore inhabitants is essential to tackle problems in an integrated manner.
Acknowledgments
We wish to acknowledge the VLIR-UOS Network Ethiopia Programme and the Global Minds Fund of Ghent University for the financial and logistic support. Our gratitude goes to Bruno Levecke and Veerle Devriendt for their support during the research activity. We are also grateful to laboratory technicians at the Department of Environmental Health Science and Technology, Jimma University, for their technical support with field and laboratory activities. Technical staffs of the Laboratory Environmental Health Science of Hawassa University and Batu Fishery and other Aquatic Life Research Center are highly acknowledged in assisting in laboratory activities. Finally, we are grateful to study participants.
Author Contributions
B.K.O., P.L.M.G., S.T.M. and P.B. participated in design of the study and drafted the manuscript. B.K.O., S.T.M., B.M., W.K. and M.A. involved in field and laboratory activities. A.A. reviewed the manuscript. G.D. digitalized spatial distribution of cercarial infections in freshwater snails. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Flemish Interuniversity Council (VLIR-UOS Network Ethiopia Programme) (Grant ET2017NET039A103).
Institutional Review Board Statement
Ethical clearance was obtained from IRBs of the Institute of Health Sciences, Jimma University.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study after explaining the aims of the study.
Data Availability Statement
The dataset generated and/or analyzed during the present study is available from the corresponding author.
Conflicts of Interest
The authors declare that no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu X.T., Gu Q.Y., Limpanont Y., Song L.G., Wu Z.D., Okanurak K., Lv Z.Y. Snail-borne parasitic diseases: An update on global epidemiological distribution, transmission interruption and control methods. Infect. Dis. Poverty. 2018;7:28. doi: 10.1186/s40249-018-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation: Executive Summary. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- 3.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Schistosomiasis. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 15 July 2021)]. Available online: https://www.who.int/en/news-room/factsheets/detail/schistosomiasis. [Google Scholar]
- 5.Dida G.O., Gelder F.B., Anyona D.N., Matano A.S., Abuom P.O., Adoka S.O., Ouma C., Kanangire C.K., Owuor P.O., Ofulla A.V. Distribution and abundance of schistosomiasis and fascioliasis host snails along the Mara River in Kenya and Tanzania. Infect. Ecol. Epidemiol. 2014;1:24281. doi: 10.3402/iee.v4.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson M.W., Dalton J.P. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2763–2776. doi: 10.1098/rstb.2009.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degheidy N.S., Al-Malki J.S. Epidemiological studies of fasciolosis in human and animals at Taif, Saudi Arabia. World Appl. Sci. J. 2012;19:1099–1104. doi: 10.5829/idosi.wasj.2012.19.08.6660. [DOI] [Google Scholar]
- 8.Arancha A., Anegagrie M., Zewdie D., Benito A. Schistosoma mansoni in a rural community of Ethiopia; Proceedings of the 27th European Congress of Clinical Microbiology and Infectious Diseases; Veinna, Austria. 22–25 April 2017. [Google Scholar]
- 9.Gordy M.A., Kish L., Tarrabain M., Hanington P.C. A comprehensive survey of larval digenean trematodes and their snail hosts in central Alberta, Canada. Parasit. Res. 2016;115:3867–3880. doi: 10.1007/s00436-016-5152-9. [DOI] [PubMed] [Google Scholar]
- 10.Krauth S.J., Wandel N., Traoré S.I., Vounatsou P., Hattendorf J., Achi L.Y., McNeill K., N’Goran E.K., Utzinger J. Distribution of intermediate host snails of schistosomiasis and fascioliasis in relation to environmental factors during the dry season in the Tchologo region, Côte d’Ivoire. Adv. Water Resour. 2017;108:386–396. doi: 10.1016/j.advwatres.2016.11.016. [DOI] [Google Scholar]
- 11.Loker E.S. Research on the Molluscan Intermediate Hosts for Schistosomiasis: What Are the Priorities? Presented to the Scientific Working Group on Schistosomiasis. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 12.Kloos H., Souza C.D., Gazzinelli A., Soares Filho B.S., Temba P.D.C., Bethony J. The distribution of Biomphalaria spp. in different habitats in relation to physical, biological, water contact and cognitive factors in a rural area in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz. 2001;96:57–66. doi: 10.1590/S0074-02762001000900008. [DOI] [PubMed] [Google Scholar]
- 13.Aliyu A.A., Ajogi I.A., Ajanusi O.J., Reuben R.C. Epidemiological studies of Fasciola gigantica in cattle in Zaria, Nigeria using coprology and serology. J. Public Health Epidemiol. 2014;6:85–91. doi: 10.5897/JPHE2013.0535. [DOI] [Google Scholar]
- 14.Ayalneh B., Bogale B., Dagnachew S. Review on Ovine fasciolosis in Ethiopia. Acta Parasitol. Glob. 2018;9:7–14. doi: 10.5829/idosi.apg.2018.07.14. [DOI] [Google Scholar]
- 15.WHO . Integrating Neglected Tropical Diseases into Global Health and Development: Fourth WHO Report on Neglected Tropical Diseases. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 16.Brown D.S. Freshwater Snails of Africa and Their Medical Importance. CRC Press; Boca Raton, FL, USA: 2002. [Google Scholar]
- 17.Ekpo U.F., Laja-Deile A., Oluwole A.S., Sam-Wobo S.O., Mafiana C.F. Urinary schistosomiasis among preschool children in a rural community near Abeokuta, Nigeria. Parasit. Vectors. 2010;3:58. doi: 10.1186/1756-3305-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker M., Allen T. Does mass drug administration for the integrated treatment of neglected tropical diseases really work? Assessing evidence for the control of schistosomiasis and soil-transmitted helminths in Uganda. Health Res. Policy Syst. 2011;9:3. doi: 10.1186/1478-4505-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster J.P., Molyneux D.H., Hotez P.J., Fenwick A. The contribution of mass drug administration to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130434. doi: 10.1098/rstb.2013.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . Uniting to Combat Neglected Tropical Diseases. London: Declaration on Neglected Tropical Diseases. World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- 21.FMoH . National Master Plan for Neglected Tropical Disease (2013–2015) Federal Ministry of Health of Ethiopia; Addis Ababa, Ethiopia: 2012. [Google Scholar]
- 22.Hotez P. Mass drug administration and integrated control for the world’s high-prevalence neglected tropical diseases. J. Clin. Pharm. Ther. 2009;85:659–664. doi: 10.1038/clpt.2009.16. [DOI] [PubMed] [Google Scholar]
- 23.Hotez P.J., Kamath A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed A.M., El Tash L.A., Mohamed E.Y., Adam I. High levels of Schistosoma mansoni infections among school children in central Sudan one year after treatment with praziquantel. J. Helminthol. 2012;86:228–232. doi: 10.1017/S0022149X11000290. [DOI] [PubMed] [Google Scholar]
- 25.Melman S.D., Steinauer M.L., Cunningham C., Kubatko L.S., Mwangi I.N., Wynn N.B., Mutuku M.W., Karanja D.M., Colley D.G., Black C.L. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evan Secor W. Water-based interventions for schistosomiasis control. Pathog. Glob. Health. 2014;108:246–254. doi: 10.1179/2047773214Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King C.H., Sutherland L.J., Bertsch D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Negl. Trop. Dis. 2015;9:e0004290. doi: 10.1371/journal.pntd.0004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray D.J., McManus D.P., Li Y., Williams G.M., Bergquist R., Ross A.G. Schistosomiasis elimination: Lessons from the past guide the future. Lancet Infect. Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 29.King C.H., Bertsch D. Historical perspective: Snail control to prevent schistosomiasis. PLoS Negl. Trop. Dis. 2015;9:e0003657. doi: 10.1371/journal.pntd.0003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokolow S.H., Wood C.L., Jones I.J., Swartz S.J., Lopez M., Hsieh M.H., Lafferty K.D., Kuris A.M., Rickards C., De Leo G.A. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl. Trop. Dis. 2016;10:e0004794. doi: 10.1371/journal.pntd.0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu K.Y. Trials of ecological and chemical measures for the control of Schistosoma haematobium transmission in a Volta Lake village. Bull. World Health Organ. 1978;56:313. [PMC free article] [PubMed] [Google Scholar]
- 32.Angelo T., Shahada F., Kassuku A., Mazigo H., Kariuki C., Gouvras A. Population abundance and disease transmission potential of snail intermediate hosts of human schistosomiasis in fishing communities of Mwanza region, north-western, Tanzania. Int. J. Sci. Res. 2014;3:1230–1236. [Google Scholar]
- 33.Kazibwe F., Makanga B., Rubaire-Akiiki C., Ouma J., Kariuki C., Kabatereine N.B., Booth M., Vennervald B.J., Sturrock R.F., Stothard J.R. Ecology of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert, Western Uganda: Snail distributions, infection with schistosomes and temporal associations with environmental dynamics. Hydrobiologia. 2006;568:433–444. doi: 10.1007/s10750-006-0224-y. [DOI] [Google Scholar]
- 34.Opisa S., Odiere M.R., Jura W.G., Karanja D.M., Mwinzi P.N. Malacological survey and geographical distribution of vector snails for schistosomiasis within informal settlements of Kisumu City, western Kenya. Parasit. Vectors. 2011;4:226. doi: 10.1186/1756-3305-4-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawassa City: Natural Resource Management and Environmental Protection Authority, Hawassa, Ethiopia. 2015. Unpublished report.
- 36.Worako A.W. Evaluation of the water quality status of Lake Hawassa by using water quality index, Southern Ethiopia. J. Water Res. Environ. Res. 2015;7:58–65. doi: 10.5897/ijwree2014.0528. [DOI] [Google Scholar]
- 37.Desta H., Lemma B., Stellmacher T., Gebremariam E. Water use and management of Lake Ziway and its watershed, Ethiopia: The perception of experts vis-à-vis the latest state of research. Environ. Dev. Sustain. 2020;22:3621–3640. doi: 10.1007/s10668-019-00359-8. [DOI] [Google Scholar]
- 38.Mengesha G., Mamo Y., Bekele K.S. Effects of Land-use on Birds Diversity in and around Lake Zeway, Ethiopia. J. Sci. Dev. 2014;2:5–22. [Google Scholar]
- 39.Mereta S.T., Bedewi J., Yewhalaw D., Mandefro B., Abdie Y., Tegegne D., Birke W., Mulat W.L., Kloos H. Environmental determinants of distribution of freshwater snails and trematode infection in the Omo Gibe River Basin, southwest Ethiopia. Infect. Dis. Poverty. 2019;8:93. doi: 10.1186/s40249-019-0604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itagaki H., Suzuki N., Ito Y., Hara T., Wonde T. Study on the Ethiopian freshwater molluscs, especially on identification, distribution and ecology of vector snails of human schistosomiasis. Jpn. J. Trop. Med. Hyg. 1975;3:107–134. doi: 10.2149/tmh1973.3.107. [DOI] [Google Scholar]
- 41.Mutuku M.W., Laidemitt M.R., Mwangi I.N., Otiato F.O., Agola E.L., Steinauer M.L., Ochanda H., Kamel B., Loker E.S., Mkoji G.M. Persistent hotspots of Schistosoma mansoni transmission in the Kenyan waters of Lake Victoria: A search for snail-related answers beneath the waves. BioRxiv. 2018:394031. doi: 10.1101/394031. [DOI] [Google Scholar]
- 42.Frandsen F., Christensen N.O. Introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance Taxonomic key. Acta Trop. 1984;41:181–202. doi: 10.5169/seals-313293. [DOI] [PubMed] [Google Scholar]
- 43.APHA. AWWA. WPCF . Standard Methods for the Examination of Water and Wastewater. 19th ed. American Public Health Association; Washington, DC, USA: 1995. [Google Scholar]
- 44.Baldwin D.S., Nielsen D.L., Bowen T., Williams J. Recommended Methods for Monitoring Floodplains and Wetlands. Murray-Darling Basin Commission; Canberra, Australia: 2005. [Google Scholar]
- 45.Parsons M., Ransom G., Thoms M., Norris R. Australian River Assessment System: AusRivAS Physical and Chemical Assessment Module. Environmental Australia; Canberra, Australia: 2001. p. 47. [Google Scholar]
- 46.Posa M.R.C., Sodhi N.S. Effects of anthropogenic land use on forest birds and butterflies in Subic Bay, Philippines. Biol. Conserv. 2006;129:256–270. doi: 10.1016/j.biocon.2005.10.041. [DOI] [Google Scholar]
- 47.McCreadie J.W., Adler P.H. The roles of abiotic factors, dispersal, and species interactions in structuring stream assemblages of black flies (Diptera: Simuliidae) Aquat. Biosyst. 2012;8:14. doi: 10.1186/2046-9063-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.FAO . Global Forest Resource Assessment. FAO Forestry Paper 163. Food and Agriculture Organization of the United Nations; Rome, Italy: 2010. [Google Scholar]
- 49.Jensen P., Krogsgaard M.R., Christiansen J., Brændstrup O., Johansen A., Olsen J. Observer variability in the assessment of type and dysplasia of colorectal adenomas, analyzed using kappa statistics. Dis. Colon Rectum. 1995;38:195–198. doi: 10.1007/BF02052450. [DOI] [PubMed] [Google Scholar]
- 50.Cohen J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 51.Montresor A., Crompton D.W., Hall A., Bundy D.A., Savioli L., WHO . Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level: A Guide for Managers of Control Programmes. World Health Organization; Geneva, Switzerland: 1998. [Google Scholar]
- 52.Katz N., Chaves A., Pellegrino J.A. simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 53.WHO Expert Committee . Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. World Health Organization; Geneva, Switzerland: 2002. p. 912. (WHO Technical Report Series). [PubMed] [Google Scholar]
- 54.Venables W., Smith D.M. An Introduction to R: Notes on R, A Programming Environment for Data Analysis and Graphics. R Development; Viena, Austria: 2008. [Google Scholar]
- 55.Devkota R., Budha P.B., Gupta R. Trematode cercariae infections in freshwater snails of Chitwan district, central Nepal. J. Himal. Sci. 2011;7:9–14. doi: 10.3126/hjs.v7i9.2183. [DOI] [Google Scholar]
- 56.Pandey K. Master’s Dissertation. Tribhuvan University; Kathmandu, Nepal: 2001. Prevalence of Fasciolosis in Buffaloes in Relation to Fasciola Larvae Infection in Lymnaea Snails in Dev Bhumi Baluwa VDC of Kavre District. [Google Scholar]
- 57.Grimes J.E., Croll D., Harrison W.E., Utzinger J., Freeman M.C., Templeton M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasit. Vectors. 2015;8:156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hechinger R.F., Lafferty K.D. Host diversity begets parasite diversity: Bird final hosts and trematodes in snail intermediate hosts. Proc. R. Soc. B. 2005;272:1059–1066. doi: 10.1098/rspb.2005.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siama A., Saotoing P., Nloga A.M. Malacological survey and dynamic of Lymnaeanatalensis population intermediate host of Fasciola gigantica in the Douvar dam freshwater of Farth Nord region Cameroon. J. Entomol. Zool. Stud. 2020;8:1213–1221. [Google Scholar]
- 60.De Troyer N., Mereta S.T., Goethals P.L., Boets P. Water quality assessment of streams and wetlands in a fast growing east African city. Water. 2016;8:123. doi: 10.3390/w8040123. [DOI] [Google Scholar]
- 61.Zongo D., Kabré B.G., Poda J.N., Dianou D. Schistosomiasis among farmers and fisherman in the West part of Burkina Faso (West Africa) J. Biol. Sci. 2008;8:482–485. doi: 10.3923/jbs.2008.482.485. [DOI] [Google Scholar]
- 62.Ayanda O.I. Prevalence of snail vectors of schistosomiasis and their infection rates in two localities within Ahmadu Bello University (ABU) Campus, Zaria, Kaduna State, Nigeria. J. Cell Anim.Biol. 2009;3:58–61. doi: 10.5897/JCAB.9000122. [DOI] [Google Scholar]
- 63.Soboksa N.E., Yimam G.N. Assessment of household level sanitation practice of mothers’ and associated factors in Gedeo Zone, South Ethiopia. Am. J. Public Health Res. 2017;5:43–49. doi: 10.12691/ajphr-5-2-3. [DOI] [Google Scholar]
- 64.Ayalew A.M., Mekonnen W.T., Abaya S.W., Mekonnen Z.A. Assessment of diarrhea and its associated factors in under-five children among open defecation and open defecation-free rural settings of Dangla District, Northwest Ethiopia. J. Environ. Public Health. 2018;2018:4271915. doi: 10.1155/2018/4271915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 66.Alebie G., Erko B., Aemero M., Petros B. Epidemiological study on Schistosoma mansoni infection in Sanja area, Amhara region, Ethiopia. Parasit. Vectors. 2014;7:15. doi: 10.1186/1756-3305-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tadege B., Shimelis T. Infections with Schistosoma mansoni and geohelminths among school children dwelling along the shore of the Lake Hawassa, southern Ethiopia. PLoS ONE. 2017;12:e0181547. doi: 10.1371/journal.pone.0181547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chimbari M.J., Dhlomo E., Mwadiwa E., Mubila L. Transmission of schistosomiasis in Kariba, Zimbabwe, and a cross-sectional comparison of schistosomiasis prevalences and intensities in the town with those in Siavonga in Zambia. Ann. Trop. Med. Parasit. 2003;97:605–616. doi: 10.1179/000349803225001508. [DOI] [PubMed] [Google Scholar]
- 69.Menjetta T., Dana D., Debalke S. Schistosoma mansoni infection and risk factors among fishermen at Lake Hawassa, Southern Ethiopia. BioRxiv. 2018;51:817–826. doi: 10.1017/S0021932019000075. [DOI] [PubMed] [Google Scholar]
- 70.Taman A., El-Tantawy N., Besheer T., Taman S., Helal R. Schistosoma mansoni infection in a fishermen community, the Lake Manzala region-Egypt. Asian Pac. J. Trop. Dis. 2014;4:463–468. doi: 10.1016/S2222-1808(14)60607-1. [DOI] [Google Scholar]
- 71.Campbell S.J., Biritwum N.-K., Woods G., Velleman Y., Fleming F., Stothard J.R. Tailoring water, sanitation, and hygiene (WASH) targets for soil-transmitted helminthiasis and schistosomiasis control. Trends Parasitol. 2018;34:53–63. doi: 10.1016/j.pt.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Rollinson D., Knopp S., Levitz S., Stothard J.R., Tchuenté L.A., Garba A. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 73.Forio M.A., Goethals P.L. An integrated approach of multi-community monitoring and assessment of aquatic ecosystems to support sustainable development. Sustainability. 2020;12:5603. doi: 10.3390/su12145603. [DOI] [Google Scholar]
- 74.Gebremedhin S., Bruneel S., Getahun A., Anteneh W.P., Goethals P. Scientific Methods to Understand Fish Population Dynamics and Support Sustainable Fisheries Management. Water. 2021;13:574. doi: 10.3390/w13040574. [DOI] [Google Scholar]
- 75.Maasri A., Jähnig S.C., Adamescu M.C., Adrian R., Baigun C., Baird D.J., Worischka S. A global agenda for advancing freshwater biodiversity research. Ecol. Let. 2021 doi: 10.1111/ele.13931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and/or analyzed during the present study is available from the corresponding author.