Abstract
Hepatocellular carcinoma is an emerging worldwide health threat that has few curative treatment options and poor overall survival. Progressive hepatic fibrosis is a common pathway for all forms of chronic liver disease and is closely linked epidemiologically to hepatocellular carcinoma risk. However, the molecular events that predispose a fibrotic liver to cancer development remain elusive. Nonetheless, a permissive hepatic microenvironment provides fertile soil for transition of damaged hepatocytes into hepatocellular carcinoma. Key predisposing features include alterations in the extracellular matrix, bidirectional signaling pathways between parenchymal and nonparenchymal cells, and immune dysfunction. Emerging research into the contributions of autophagy, tumor-associated fibroblasts, and hepatocellular carcinoma progenitor cells to this dangerous milieu also provides new mechanistic underpinnings to explain the contribution of fibrosis to cancer. As effective antifibrotic therapies are developed, these approaches could attenuate the rising surge of hepatocellular carcinoma associated with chronic liver disease.
Keywords: Hepatocellular carcinoma (HCC), Liver fibrosis, Hepatic stellate cells (HSCs), Extracellular matrix (ECM)
INTRODUCTION
Fibrosis contributes significantly to chronic diseases of the heart, lungs, liver, kidneys, bone marrow, skin, joints, and bowel and is implicated in at least one third of all deaths in the industrialized world (1). Fibrosis is a process in which fibrogenic cells produce excessive amounts of extracellular matrix (ECM) in response to acute injury with the initial intent to protect parenchymal cells and maintain liver function. However, chronic injury often leads instead to the replacement of parenchymal cells with fibrotic scar tissue and eventual organ dysfunction (2).
Liver fibrosis is present in virtually all patients with chronic liver injury, regardless of the etiology. While the ability of the liver to regenerate may attenuate some of the injury and associated deposition of ECM, the majority of patients with chronic liver disease will ultimately progress over decades to advanced fibrosis or cirrhosis, leading to complications that include encephalopathy, ascites, variceal bleeding, synthetic dysfunction, and hepatocellular carcinoma (3).
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide with over 700,000 cases diagnosed in 2008, and it is the third leading cause of cancer death (4). Traditionally considered a disease of Asia and Sub-Saharan Africa where hepatitis B is endemic, HCC incidence is rising in Western countries driven by the aging of cohorts infected with hepatitis C up to four decades ago, and the increasing prevalence of fatty liver disease associated with obesity [NAFLD/NASH and the metabolic syndrome (5)]. The overall survival of HCC remains poor, and progress is urgently needed in defining new pathways of disease, diagnostic markers, and targets for therapy. Patients frequently present at advanced stages, relapse rates are high, and palliative management is often the only treatment option (6).
Up to 90% of cases of HCC arise in the setting of advanced fibrosis or cirrhosis (7). Despite this well-known link, there are few unifying pathways that mechanistically link liver fibrosis with the development of HCC. Specifically, is hepatocellular injury leading to fibrosis directly responsible for concurrent development of HCC, or is fibrosis an innocent bystander in HCC development that occurs concurrently but unrelated to changes that culminate in HCC?
The link between fibrosis and cancer is an emerging area of inquiry. Tumor stroma is the supporting architecture of a tumor and is typically rich in ECM (2). Central to this ECM are fibrogenic cells, which are increasingly implicated in all stages of tumor development (8). Despite many similarities between fibrosis pathways across organs, fibrosis is more strongly linked to epithelial cancer in liver than in other organs. One reason may be the liver’s unique regenerative capacity, which enables fibrosis to persist for decades while maintaining normal function, whereas in tissues like lung, idiopathic pulmonary fibrosis leads to pulmonary failure in a short interval before cancers develop to the same extent (9). However, sustained hepatocyte regeneration and liver progenitor cell expansion in the setting of chronic liver injury may also promote accumulation of oncogene activation in hepatocytes (10), thereby promoting the development of cancer. Hepatocarcinogenesis is multifactorial, yet fibrosis contributes to a susceptible tumor microenvironment in liver, combined with concurrent tissue responses including angiogenesis, chronic inflammation, and oxidant stress, among others (11).
HEPATIC FIBROSIS
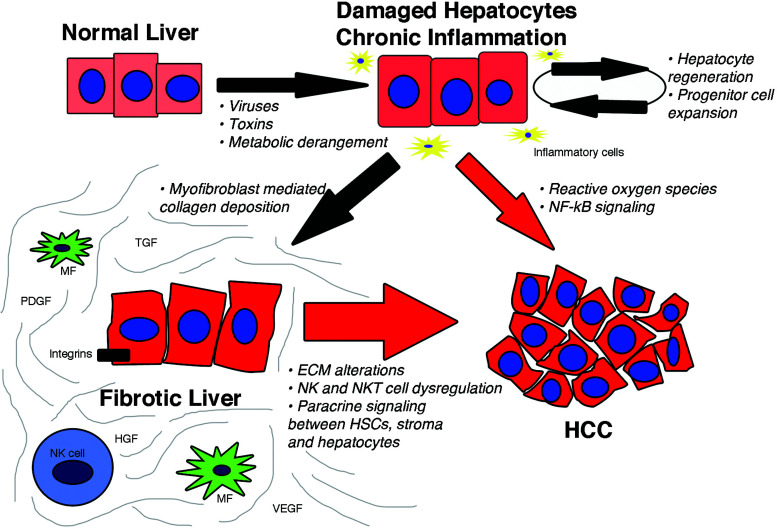
Our understanding of the response of the liver to injury has expanded significantly in recent years (Fig. 1). Viewed initially as a protective mechanism, fibrosis eventually can lead to significant organ dysfunction over decades [reviewed in (2)]. Central to this understanding has been the characterization of the nonparenchymal hepatic stellate cell (HSC) (12), which is the major source of ECM in the liver, following its activation into proliferative, fibrogenic, contractile myofibroblasts. More recently, evidence has implicated HSCs in immunoregulation, regeneration, and hepatic development as well [reviewed in (12)]. Specifically, HSCs, which reside in the perisinusoidal space of Disse, are activated following liver injury and coordinate the repair response, primarily by secreting interstitial, fibrillar collagens, especially types I and III. If the injury is acute, excess ECM is eventually degraded, and the sinusoidal microenvironment returns to normal. If the injury is chronic, for example, in chronic hepatitis B or C, the ongoing deposition of ECM may eventually lead to replacement of the normal parenchyma with scar matrix, distorting the liver architecture and vasculature and impairing its differentiated function.
Figure 1.
The progression from normal hepatocytes to hepatocellular carcinoma. Chronic injury from viruses, toxins, or metabolic derangements leads to hepatocyte damage that provokes inflammation, increasing cell turnover, and progenitor cell expansion. Cell turnover and progenitor expansion, combined with reactive oxygen species and enhanced NF-κB signaling may progress directly to HCC. Activated myofibroblasts in the setting of chronic injury increase deposition of ECM components leading to a fibrotic microenvironment, which promotes HCC development. The red arrows indicate hepatocarcinogenic steps, and their size reflects relative contributions to HCC development; most cases occur in the setting of advanced fibrosis or cirrhosis. Abbreviations: MF, myofibroblast; NK, natural killer; HCC, hepatocellular carcinoma; TGF, transforming growth factor; HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor.
Significant alterations in the hepatic microenvironment resulting from fibrosis are shared among all causes of chronic liver disease. Understanding these alterations helps clarify how they enhance the susceptibility to developing HCC in progressive fibrotic disease and raise the prospect that successful antifibrotic therapy could attenuate its risk.
HEPATOCARCINOGENIC MECHANISMS LINKED TO HEPATIC FIBROSIS
Oxidative Stress
Oxidative stress (OS) is a general term used to describe increased production of reactive oxygen species (ROS) or decreased production of antioxidants, creating an imbalance in the prooxidant/antioxidant state in cells and subsequently leading to cell damage (13). Hepatocytes produce ROS in response to injury, which in turn act on HSCs to promote fibrosis development. OS has been implicated in all etiologies of chronic liver disease and plays a significant dual role in both the initiation and perpetuation of liver fibrosis via its effect on HSCs. OS also directly promotes cancer independent of the presence of fibrosis by provoking DNA damage and altering intracellular signaling, as described below (13,14).
In chronic alcohol exposure, hepatocytes generate excessive ROS via mitochondria, the NADPH oxidase membrane complex (NOX), and CYP2E1 induction, resulting in increased production of collagen with subsequent ECM remodeling by HSCs and periportal fibroblasts (15). ROS liberated by NOX are central to this fibrogenic response. In murine models of liver fibrosis, HSC NOX mRNA expression is induced, along with NOX-mediated fibrogenic and mitogenic signaling by transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor (PDGF), respectively (16). Furthermore, in transgenic murine models with deletion of specific NOX proteins, fibrosis is attenuated in response to liver injury (16). In addition to these profibrotic changes, excessive ROS lead to DNA damage, mitochondrial damage, increased nuclear factor-κB (NF-κB)-mediated inflammation and a reduction in antioxidant defenses, which collectively amplify injury, fibrosis, and the propensity to develop hepatocellular carcinoma especially in alcoholic and nonalcoholic steatohepatitis (14,15).
Hepatic ROS are increased in patients with chronic hepatitis C, combined with reduced levels of antioxidants such as glutathione and antioxidant defense systems including manganese superoxide dismutase (17). These abnormalities in redox balance are due to the direct effects of hepatitis C virus proteins on mitochondria, NOX, intracellular calcium metabolism, and induction of CYP2E1. ROS also stimulate TGF-β1 signaling and increase fibromodulin production in hepatocytes, leading to HSC proliferation and migration, with increased fibrosis (17). As in alcoholic liver disease, ROS generated in chronic hepatitis C are hepatocarcinogenic primarily through induction of DNA mutations, promotion of angiogenesis via elevated prostaglandins and vascular endothelial growth factor (VEGF) signaling, and reduced effectiveness of DNA repair systems (17,18).
Oxidative stress is considered pivotal in provoking the transition from simple nonalcoholic fatty liver (NAFLD) to the more clinically significant nonalcoholic steatosis (NASH) (19). Increased free fatty acids (FFA) in NAFLD liberate increased ROS via hepatocyte mitochondria and peroxisomes. Subsequent elevated ROS-mediated hepatocyte lipid peroxidation not only promotes cell death but also activates HSCs, which drive the inflammatory and fibrotic response, and Kupffer cells, which further stimulate HSC activation (20).
Several OS-associated pathways and molecules in NASH are hepatocarcinogenic, including NF-κB, which has been proposed as a common link between liver fibrosis, injury, and HCC (21). NF-κB is a highly conserved and tightly regulated signaling pathway central to liver survival and homeostasis, and Kupffer cell and HSCs responses, which are activated following hepatocellular injury (21). The IκB kinase (Iκκ) complex is essential for the activation of NF-κB. Transgenic mice with genetic deletion of a regulatory subunit of Iκκ, known as NF-κB essential modulator (NEMO/Iκκ), spontaneously develop a chronic hepatitis resembling human NASH followed by HCC, highlighting the central role that NF-κB plays in fatty liver and HCC (22). Studies linking OS to HCC will have wide translational implications as OS pathways are common to all models of chronic liver disease, raising interest in the use of antioxidants to treat NASH (20); encouraging results may translate into studies assessing antioxidants in the prevention of HCC.
Extracellular Matrix Alterations
The ECM is not a static mechanical support structure, but rather a dynamic and interactive component of the liver’s architecture involved beyond providing simple cellular support. Several alterations in the ECM during progressive fibrosis predispose to the development of HCC.
The hallmark of hepatic fibrosis is an imbalance between ECM deposition and degradation that favors net matrix accumulation. Both acute and chronic injury activates HSCs, leading to qualitative and quantitative changes in the ECM. Accumulation of collagenous and noncollagenous ECM is controlled in part by matrix metalloproteinases (MMPs) (which degrade substrates) and tissue inhibitors of metalloproteinases (TIMPs) (which inhibit MMPs). Enhanced collagen deposition increases the stiffness of the ECM, which in turn promotes ongoing HSC activation and thus more collagen deposition in a positive feedback loop (3).
Several different MMPs in liver act upon a range of substrates including collagen and other ECM components, including proteoglycans, laminin, and fibronectin. TIMP concentrations and the balance between MMPs and TIMPs determine the net effect on ECM homeostasis; high MMP/TIMP ratios lead to net degradation of ECM, while increasing TIMP concentrations favor MMP inhibition and an increase in ECM quantity (3,23). HSCs are the major source of MMPs and TIMPs, and their activity is controlled by cytokines central to the fibrotic response to injury such as TGF-β1 and TNF-α (12,23). In addition, dendritic cells and macrophage subsets may also provide critically important MMPs during fibrosis regression (3).
MMPs and TIMPs are important in progression of hepatocytes from dysplasia to the development of poorly differentiated HCC. In the early stages of HCC development, hepatocytes acquire a phenotype that may lead to MMP-1 production in the setting of cirrhosis, which promotes proliferation, invasion through portal tracts, and fibrous tissue. Subsequently, well-differentiated hepatoma cells may express MT1-MMP, MMP-2, and MMP-9, which promote stromal invasion (24). Studies clarifying the roles of MMPs and TIMPs have been pursued using a model of woodchuck hepatitis virus (WHV)-related HCC, which resembles human HBV. Levels of MMP-1, MMP-2, MMP-9, and neutrophil gelatinase-associated lipocalin (which forms a complex with MMP-9) are elevated in HCC tissue of WHV-infected mice compared to adjacent nontumoral tissue as well as compared to liver tissue of WHV-naive mice. Furthermore, when an inhibitor of mammalian MMPs was introduced into the model, levels of MMP-2 and MMP-9 were reduced in WHV-positive mice, but the effect on development of HCC was not assessed (25). Several other groups are currently investigating drug therapies targeting MMPs and TIMPs in HCC.
A key feature of the chronically injured liver is increasing stiffness, which may favor the growth of HCC cells. A recent meta-analysis of 17 trials including 7,058 patients demonstrated that increasing liver stiffness as measured by transient elastography correlates with the risk of HCC development (26). In culture, when human HCC cell lines are grown upon polyacrylamide gels designed to mimic increasing degrees of matrix stiffness, their proliferative index (assessed by Ki-67 staining) is 12 times higher than for cells grown on a soft gel (27). Furthermore, the STAT3 pathway is regulated by matrix stiffness. When HCC cells are exposed to hepatocyte growth factor (HGF), STAT3 activation is greater in cells on stiff gels compared to soft gels (27). Interestingly, STAT3 has been uncovered as a key component of TGF-β-mediated induction of connective tissue growth factor (CTGF), which is fibrogenic, in an immortalized rat HSC cell line (28), further linking fibrosis and HCC development.
Paracrine Crosstalk Between Hepatic Stellate Cells, Hepatocytes, and the Extracellular Matrix
In addition to increased matrix stiffness, there are significant alterations in the signaling pathways and effector molecules accompanying the activation of stromal cells, which are likely derived in part from HSCs. The ECM sequesters important growth factors and cytokines that contribute to paracrine interactions in a bidirectional fashion influencing cellular function in HCC pathogenesis. These functions include angiogenesis, modulating HSC proliferation and activation, tumor cell survival, and differentiation (3).
Important proliferative, angiogenic, and regenerative cytokines secreted by HSCs contribute to a carcinogenic milieu. These include transforming growth factors (TGF-α, TGF-β), platelet-derived growth factors (PDGF-B and PDGF-C), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and interleukin-6 (IL-6) (11). Mice with liver-specific overexpression of PDGF-C develop extensive fibrosis followed by epithelial transition to neoplasia (29,30). Moreover, levels of expression of its receptor (PDGFRα) are lower in the tumor than surrounding tissue (30), suggesting that PDGF-C is more important in modulating the microenvironment to promote neoplasia development in a paracrine fashion than in providing direct tumorigenic stimulation to hepatocytes or liver progenitor cells.
Many ECM–cell interactions are transmitted via transmembrane adhesion molecules, in particular, integrins. This family of mechanoreceptors binds growth factors and components of the ECM and transmits cell–cell signals as well as bidirectional signals between the ECM and hepatocytes. When integrins bind to growth factors or ECM ligands, such as collagen, laminin, and fibronectin, they form focal adhesions and activate specific intracellular effector pathways, including protein kinase C, talin, paxillin, and the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinases (MAPK) pathways (31). Integrins are central to the fibrogenic response (31) and also influence survival, differentiation, and proliferation, which are dysregulated in cancer cells (32). Integrin expression patterns are altered in human HCC, potentially mediated by selective pressure from both tumoral and peritumoral stromal cells. Their contribution to the transition of normal or dysplastic hepatocytes to HCC within a fibrotic stroma is unclear; however, integrins promote established HCC by their actions on cell proliferation, adhesion, invasion, migration, and apoptosis (33). There are currently integrin antagonists in preclinical studies for HCC treatment (33).
Natural Killer and Natural Killer T-Cells
Natural killer (NK) cells account for up to 40% of lymphocytes of the liver. They contribute to a range of hepatic disorders, including alcoholic liver disease, NASH, chronic viral infections, autoimmune disease, cancer, and fibrogenesis (34). NK cells attenuate liver fibrosis development, particularly in the early phases of fibrosis by inducing apoptosis of HSCs. Natural killer T-cells (NKT) may in fact be fibrogenic, although this pathway has not been fully elucidated. In the late stages of advanced fibrosis and cirrhosis, NK cell function is reduced, which promotes ongoing ECM deposition and in turn increases the risk of HCC (3,34). NK cells are also vital for immune surveillance to remove tumor cells, but as fibrosis progresses, NK-mediated tumor killing is diminished due to phagocytosis of NK cells (based on culture studies) (35), inability of the NK cells to achieve target cell contact due to increased fibrosis, which reduces their infiltrative capacity (36), and switching of ligands and receptors in dysplastic lives, allowing preneoplastic hepatocytes to escape immune recognition and hence NK cell-mediated clearance (37). These NK cell mechanisms are not necessarily fibrosis dependent; rather, they exist within a milieu of chronic disease (e.g., chronic viral infection), promoting both parenchymal liver abnormalities (e.g., genomic abnormalities by cellular injury-induced ROS) and fibrosis, eventually leading to HCC development in some cases.
EMERGING AND FUTURE AREAS OF INTEREST
Tumor-Associated Fibroblasts
Tumor stroma and tumor-associated fibroblasts (TAFs) are emerging areas of cancer research. TAFs are the most abundant cell type of the tumor stroma and proliferate in response to cytokines and mitogens, including HGF. In the context of fibrosis and HCC, a contribution of TAFs to tumor initiation has been proposed (38). In HCC, they are the major source of collagen in tumor stroma and promote angiogenesis, tumor growth, migration, and reduce antitumor immunity (11). The origins of TAFs and their relationship to HSCs in HCC are not clear, but TAFs could derive from resident HSCs. Moreover, the two cells share many similarities in growth hormone, cytokine, and mitogen expression profiles.
Autophagy
Autophagy has a role in both liver fibrosis and HCC development (39). Autophagy in HSCs amplifies hepatic fibrosis and loss of the autophagy protein ATG7 in cultured murine HSCs and in HSC-specific ATG7 knockout mice attenuates the fibrotic response (40). This loss of autophagy may limit the hydrolysis of FFAs, depriving stellate cells of a key energy source, thereby attenuating activation and fibrosis development (40).
Autophagy in hepatocytes, in contrast to HSCs, may be generally beneficial. For example, transgenic mice with hepatocyte rather than HSC-specific ATG7 knockdown spontaneously develop chronic hepatitis, fibrosis, and multiple benign hepatic adenomas (41). In cancer, autophagy has been described as a “double-edged sword” based on its activity in hepatocytes. On the one hand, it protects from the development of tumors by removing damaged cellular components. On the other hand, once a tumor has developed, the cancerous cells use autophagy to promote cellular growth and survival by providing necessary metabolic and nutrient support (39). In human HCC liver tissue, ATG7 is upregulated relative to surrounding tissue, likely as a protective response to allow tumor cells to survive cellular stress (42). MicroRNA-375 (miR-375) acts in human HCC cell lines to inhibit ATG7 activity, highlighting the possible therapeutic benefit of microRNAs in HCC (42).
Gene Signatures in Fibrosis and HCC
Recent advances in genomic profiling using high-throughput or next-generation sequencing has facilitated the analysis of molecular alterations in both tumor tissue and the surrounding stroma across the entire genome. The discovery of new oncogenes from these approaches is likely to translate into significant advances in the clinical management of HCC from surveillance and diagnosis to prognosis prediction and refined therapies (43). These techniques will advance our understanding of the effect of permissive microenvironment on HCC development. For example, genomic profiling of 6,100 genes from 106 patients in both tumor tissue and surrounding tumor stroma identified gene signatures that correlate with overall survival. Specifically, the gene signature of the surrounding tissue strongly correlates with overall survival, while the signature of the tumor tissue itself does not (44). While subsequent studies have shown the gene signature of HCC tumors to correlate with outcomes [reviewed in (43)], the findings nonetheless underscore the important role of tumor stroma in HCC development and outcomes.
Proteomics provides an accurate way to assess the broad range of ECM alterations in HCC (e.g., the integrin family) and thus allows for targeted assessment of candidate causative molecules. Proteomic analysis of PDGF-C transgenic and Pten null mice identified a series of integrins and collagens (including six types that had not been previously identified in the liver) in mice with steatosis, fibrosis, and HCC (45).
Publicly available HCC gene expression data from The Cancer Genome Atlas (TCGA) has facilitated dissemination of changes in the ECM transcriptome. This analysis identified several collagens and proteoglycans upregulated in HCC stroma, as well as a decrease in activity of the insulin-like growth factor-binding protein family (46). The RNA sequencing used in this analysis accurately quantifies changes in transcript variants, which arise as a consequence of alternate splicing events, a common feature in ECM regulation. Several genes previously uncharacterized in the setting of cancer were identified as having differential expression in HCC stroma (46).
HCC Progenitor Cells Within a Permissive Microenvironment
Malignant tumors are thought to arise in some cases from a single progenitor cell, which, through genetic alterations, has developed growth and survival advantages. The search for HCC progenitor cells (HcPCs) has been linked to a permissive microenvironment within the damaged and fibrotic liver (47). In one study, isolated progenitor cells from livers of BL/6 mice 3 to 5 months after receiving the procarcinogenic diethyl nitrosamine (DEN) only led to tumor development in the livers of syngeneic mice after splenic injection in which the recipients had been treated with the fibrotic hepatotoxin CCl4, but did not lead to tumor development in mice that were untreated. This model suggests that premalignant HcPCs will only transition to malignancy when placed within an environment of chronic damage and fibrosis (47), further reinforcing the dependence on permissive tumor environment.
CONCLUSIONS AND DISCUSSION
Growing evidence mechanistically links the development of HCC to a permissive fibrotic microenvironment. However, much of the data are associative rather than causal, and the paucity of animal models that accurately reflect the human liver’s response to injury (besides the PDGF-C transgenic mouse model) is hampering efforts to conclusively link the two. On the other hand, fibrosis and cirrhosis are not the only prerequisites for HCC development. Several etiologies of liver disease are directly hepatocarcinogenic, including hepatitis C and hepatitis B (18), NASH (21), and alcohol (14).
The paradigm that HCC typically arises after the development of advanced liver fibrosis is not sufficient to definitively link the two at a mechanistic level. Consider that the 5-year cumulative incidence of HCC development in Japanese patients with hepatitis C cirrhosis reaches 30%, whereas HCC rates for patients with advanced primary biliary cirrhosis are as low as 4% (48). The example of OS as both a stimulus for the initiation and perpetuation of fibrosis, and directly stimulating carcinogenesis through DNA damage (among other pathways), provides a useful framework for considering how to untangle the complex interrelationship between disease (e.g., chronic viral infection), tissue response (development of fibrosis), and development of complications (cirrhosis and HCC).
Several barriers undermine the development of a unifying theory linking fibrosis with HCC development. First is the lack of adequate experimental models. Fibrosis represents a spectrum of quantitative and qualitative abnormalities of the ECM and of parenchymal and nonparenchymal cells that translate to mild fibrosis through to cirrhosis in a clinical context. Similarly, HCC develops along a pathway of molecular and genomic abnormalities that progress from normal hepatocytes to dysplasia, then to poorly differentiated tumors and eventually metastatic carcinoma. Experimental work struggles to translate this knowledge of human fibrosis and HCC into reproducible, representative models. Current mice models of HCC development often use a single intraperitoneal injection of DEN at day 15. HCC typically develops in these animals after 9 to 12 months, but the liver parenchyma exhibits little or no fibrosis. Weekly injections of DEN in rats may provide an accurate reflection of human fibrosis and HCC development. In a study assessing the effectiveness of erlotinib, an epidermal growth factor receptor inhibitor, rats were injected weekly with a low dose of DEN for 8 weeks to assess the drug’s impact on fibrogenesis and HCC prevention. After 18 weeks, the animals developed advanced fibrosis and cirrhosis. Control animals not receiving erlotinib developed a median of 20.4 ± 5.5 HCC tumors compared to a median of 5.0 ± 2.2 and 10.3 ± 3.8 tumors in the 2 mg/kg and 0.5 mg/kg erlotinib dose groups, respectively (p < 0.01 for both groups), demonstrating the efficacy of this regimen (compared to a single injection of DEN) in mimicking the development of fibrosis followed by HCC as seen in humans (49).
HCC develops within an abnormal, permissive microenvironment brought on by chronic injury; fibrosis is just one component that may be difficult to separate from other hepatocarcinogenic insults. These pathways, including angiogenesis, chronic inflammation, and oxidative stress, frequently share common signaling and effector molecules. In order to accurately gauge the effect of fibrosis on HCC development, it is imperative to functionally separate fibrosis from the various other changes within a damaged liver using experimental models. Importantly, specific pathways may only contribute to cancer development within a limited interval along the carcinogenic spectrum.
These findings have implications for developing antifibrotic therapy to attenuate the risk of HCC. Early antifibrotic clinical trials are testing both new molecules and established drugs (50); assessing their potential role in reducing the risk of HCC development will be an important component of their evaluation.
ACKNOWLEDGMENT
M.W. is supported by the Sir Charles Gairdner Hospital Edith Marston Cancer Ph.D. Scholarship.
REFERENCES
- 1. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 2013;304:C216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425–56. [DOI] [PubMed] [Google Scholar]
- 4. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 5. White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10:1342–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- 7. Chiesa R, Donato F, Tagger A, Favret M, Ribero ML, Nardi G, et al. Etiology of hepatocellular carcinoma in Italian patients with and without cirrhosis. Cancer Epidemiol Biomarkers Prev 2000;9:213–6. [PubMed] [Google Scholar]
- 8. Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochim Biophys Acta 2013;1832:1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fell CD. Idiopathic pulmonary fibrosis: Phenotypes and comorbidities. Clin Chest Med 2012;33:51–7. [DOI] [PubMed] [Google Scholar]
- 10. Shiraha H, Yamamoto K, Namba M. Human hepatocyte carcinogenesis (review). Int J Oncol 2013;42:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013;144:512–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanchez-Valle V, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr Med Chem 2012;19:4850–60. [DOI] [PubMed] [Google Scholar]
- 14. Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006;387:349–60. [DOI] [PubMed] [Google Scholar]
- 15. Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: Updated experimental and clinical evidence. J Dig Dis 2012;13:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paik YH, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA. Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses 2013;5:439–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer 2013;13:123–35. [DOI] [PubMed] [Google Scholar]
- 19. Day CP, James OF. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998;114:842–5. [DOI] [PubMed] [Google Scholar]
- 20. Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 2012;52:59–69. [DOI] [PubMed] [Google Scholar]
- 21. Luedde T, Schwabe RF. NF-kappaB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011;8:108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007;11:119–32. [DOI] [PubMed] [Google Scholar]
- 23. Consolo M, Amoroso A, Spandidos DA, Mazzarino MC. Matrix metalloproteinases and their inhibitors as markers of inflammation and fibrosis in chronic liver disease (review). Int J Mol Med 2009;24:143–52. [DOI] [PubMed] [Google Scholar]
- 24. Okazaki I. Novel cancer-targeting agents/application strategies developed from MMP Science. Anticancer Agents Med Chem. in press. [PubMed] [Google Scholar]
- 25. Ochoa-Callejero L, Toshkov I, Menne S, Martinez A. Expression of matrix metalloproteinases and their inhibitors in the woodchuck model of hepatocellular carcinoma. J Med Virol 2013;85:1127–38. [DOI] [PubMed] [Google Scholar]
- 26. Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11(12):1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011;53:1192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, Liu H, Meyer C, Li J, Nadalin S, Konigsrainer A, et al. Transforming growth factor-beta (TGF-beta)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem 2013;288:30708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci USA 2005;102:3389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright JH, Johnson MM, Shimizu-Albergine M, Bauer RL, Hayes BJ, Surapisitchat J, et al. Paracrine activation of hepatic stellate cells in platelet-derived growth factor C transgenic mice: Evidence for stromal induction of hepatocellular carcinoma. Int J Cancer 2014;134(4):778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patsenker E, Stickel F. Role of integrins in fibrosing liver diseases. Am J Physiol Gastrointest Liver Physiol 2011;301:G425–34. [DOI] [PubMed] [Google Scholar]
- 32. Hehlgans S, Haase M, Cordes N. Signalling via integrins: Implications for cell survival and anticancer strategies. Biochim Biophys Acta 2007;1775:163–80. [DOI] [PubMed] [Google Scholar]
- 33. Wu Y, Qiao X, Qiao S, Yu L. Targeting integrins in hepatocellular carcinoma. Expert Opin Ther Targets 2011;15:421–37. [DOI] [PubMed] [Google Scholar]
- 34. Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology 2013;57:1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, et al. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology 2008;48:963–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, et al. NK cells and the tumour microenvironment: Implications for NK-cell function and anti-tumour activity. Trends Immunol 2003;24:603–9. [DOI] [PubMed] [Google Scholar]
- 37. Coulouarn C, Factor VM, Conner EA, Thorgeirsson SS. Genomic modeling of tumor onset and progression in a mouse model of aggressive human liver cancer. Carcinogenesis 2011;32:1434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pietras K, Ostman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp Cell Res 2010;316:1324–31. [DOI] [PubMed] [Google Scholar]
- 39. Czaja MJ, Ding WX, Donohue TM, Friedman SL, Kim JS, Komatsu M, et al. Functions of autophagy in normal and diseased liver. Autophagy 2013;9:1131–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology 2012;142:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev 2011;25:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang Y, Yan W, He X, Zhang L, Li C, Huang H, et al. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology 2012;143:177–87. [DOI] [PubMed] [Google Scholar]
- 43. Hoshida Y, Moeini A, Alsinet C, Kojima K, Villanueva A. Gene signatures in the management of hepatocellular carcinoma. Semin Oncol 2012;39:473–85. [DOI] [PubMed] [Google Scholar]
- 44. Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008;359:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lai KK, Shang S, Lohia N, Booth GC, Masse DJ, Fausto N, et al. Extracellular matrix dynamics in hepatocarcinogenesis: A comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLoS Genet 2011;7:e1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duncan MB. Extracellular matrix transcriptome dynamics in hepatocellular carcinoma. Matrix Biol 2013; 32(7–8):393–8. [DOI] [PubMed] [Google Scholar]
- 47. He G, Dhar D, Nakagawa H, Font-Burgada J, Ogata H, Jiang Y, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell 2013;155:384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004;127:S35–50. [DOI] [PubMed] [Google Scholar]
- 49. Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, et al. EGFR inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest 2013;123:1887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]