Abstract
Tooth extraction is a routine surgical procedure in dental treatment. As a wound healing process after tooth extraction, a saddle-shaped residual ridge forms due to bone formation in the extraction socket and localized bone resorption on the external surface of the jawbone. The residual ridge is subjected to continuous bone resorption with substantial differences among individuals. In some cases, it results in excessive bone atrophy, which complicates dental restorative treatment. This unique oral wound healing process may be influenced by factors that are specific to oral tissue. HIF expression is different in oral wound healing compared to that of skin wounds. The objective of this study was to examine a genetic association between SNP of the HIF-1α gene, which is known to have high genetic diversity, and the residual ridge resorption (RRR). Two hundred and two Korean subjects (70.80 ± 9.40 years) with partially or completely edentulous mandible were recruited, and edentulous mandibular bone height was measured following the protocol of the American College of Prosthodontists. The HIF-1α allele was directly sequenced in 24 subjects resulting in the variants over 5% frequency in 95% likelihood, whereas tag-SNPs were selected to perform analysis for the remaining population. Student’s t-test and ANOVA were used for statistical analysis to examine the association between the SNPs and the RRR. Four novel variants were discovered, and a minor allele of rs11549467 was associated with the RRR of the subjects (p = 0.028). rs11549467 increases HIF-1α transactivity, enhancing angiogenesis and increasing new vessel formation. Thus, rs11549467 may play an important role in the disturbed bone remodeling balance resulting in RRR. Results of this study may be useful in developing novel genetic diagnostic tests and identifying Koreans susceptible to developing excessive jawbone atrophy after dental extraction. Most importantly, early screening using genetic information will rescue susceptible patients from the vulnerable situation of excessive jawbone atrophy where no effective prosthetic treatment is available.
Key words: Single nucleotide polymorphism (SNP), HIF-1α, Residual ridge resorption (RRR), Edentulous mandible, Atrophy
INTRODUCTION
According to Statistics Korea, there were 5,193,000 senior adults aged 65 years and older in 2008, which is 10.7% of the total Korean population. This number is predicted to be increased to 14.3% in 2018 and 20.8% in 2026 (1). Data from 2006 implied that 53% of Korean senior adults aged 65 years and older had discomfort in masticatory function due to edentulism (2). With the increasing life expectancy of the elderly population, the prosthetic treatment of tooth loss, which is common in old age, has been developed to provide functional satisfaction. A complete denture is often used for treating edentulous patients, and the maintenance of the residual ridge after tooth extraction is essential for the successful outcome of complete denture treatment. The wound healing process after tooth extraction involves bone formation inside the socket and bone resorption on the outer surface of the alveolar bone, consequently forming a saddle-shaped residual ridge in the edentulous jaw (3,4). In most cases, the alveolar bone undergoes bone resorption most rapidly in the first 6 months, and the resorption activity continues at a slower rate. However, in some cases, active bone resorption continues after the healing of wounds, resulting in excessive bone atrophy (5). Previous studies concerning the resorption of the alveolar ridge have been mostly focused on a gypsum model or radiological structural change in the residual alveolar ridge (3). Many inquiring attempts were made to investigate the pathogenesis of resorption of the persistent residual alveolar ridge, but they were not able to show any significant results. Interestingly, the quantitative comparison of the persisting alveolar ridge resorption shows huge differences among individuals under similar circumstances, suggesting feasible genetic association (6).
After tooth extraction, bone tissues in the residual ridge become hypoxic, the most prominent event among the changes in osseous tissue, by reduced mechanical loading from the teeth through periodontal ligament. Hypoxia inducible factor (HIF-1), a transcriptional complex, plays an important role in mammalians’ cellular and systemic oxygen homeostasis. It is a master regulator of oxygen-regulated gene expression, and 60 HIF-1 target genes are known. HIF-1 effectors are involved in angiogenesis, vascular tone, epithelial homeostasis, and extracellular matrix metabolism, which is very critical for the wound healing process after teeth extraction (7–9). Unlike skin wounds, an oral wound exhibits a unique healing process possibly contributed by highly vascularized oral mucosa. Both oral mucosal and dermal wounds proceed through hemostasis, inflammation, proliferation, and remodeling of the collagen matrix (7,8), but wound healing in the oral mucosa is clinically distinguished from dermal healing in terms of both its rapidity and lack of scar formation (9). Recent study on different HIF-1α expression in skin and oral wounds suggests a significant difference in HIF-1α expression in the wound healing process might be responsible for a different rate of healing in skin and oral mucosal wounds. The study implies that skin injury induces significantly higher expression of HIF-1α and its signaling pathway than does tongue injury, which may partially explain the more robust angiogenesis seen in skin versus oral mucosal wounds (10). Hence, this particular phenomenon in oral mucosa would be responsible for the long-term consequence of wound healing manifesting the residual ridge resorption (RRR) (Fig. 1).
Figure 1.
Formation of epithelium relatively thick with elongated rete pegs after tooth extraction.
In this study, we used the marker SNPs of HIF-1α to perform SNP association analysis in patients who were edentulous in the posterior area of the mandible for at least 2 years and had denture treatments after tooth extraction, and subsequently, we selected a marker SNP of HIF-1α related to RRR. By using the selected marker as a gene indicator that predicts residual ridge loss after tooth extraction, we hope to prevent the vulnerable situation where no prosthodontic treatment is available due to severe jaw atrophy.
MATERIALS AND METHODS
Ethics Statement
The Institutional Review Board of Yonsei University College of Dentistry and Kyung Hee University Dental Hospital (Yonsei IRB No. 2-2010-0022, KHUSD IRB No. 2011-01) approved all research concerning human subjects or human data. Clinical investigation was followed as stated in the Declaration of Helsinki. Before taking part in this study, participants were provided with the agreement form, and then written consent was obtained.
Study Population
The study population consisted of 202 unrelated Korean individuals, 81 men and 121 women, who were recruited between January 2011 and February 2013 from Yonsei University Dental Hospital and Kyung Hee University Dental Hospital. Subjects, 70–80, were either partially or completely edentulous for at least 2 years, and an oral examination was performed to confirm the condition of subjects. Subjects should not have known systemic conditions that could affect bone conditions and history of bone transplantation.
Measurement of Mandibular Residual Ridge Height
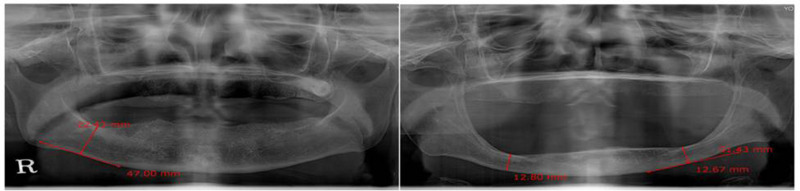
Mandibular residual ridge height was measured similar to that reported by a previous study (11). The lowest height of the edentulous mandible was measured following the protocol of the American College of Prosthodontics (ACP) (12) (Fig. 2).
Figure 2.
Characterization of mandibular ridge. The lowest residual height (arrow) of the edentulous mandible was determined by panoramic dental radiographs. The figure on the right shows severe atrophy of the edentulous mandible.
Genomic DNA Extraction
A 2-ml saliva sample from each subject was collected using an OG-500 tube that had 2 ml of DNA-preserving solution (Cat. #OG-500; DNA Genotek, Ottawa, Ontario, Canada). After collection of saliva, the lid part of the OG-500 tube was closed to mix the saliva sample with the DNA-preserving solution. A total volume of 4 ml sample was stored at 25°C until further analysis. DNA extractions and further analysis were performed by DNA Link Inc., Seoul, South Korea.
DNA Direct Sequencing
It has been suggested that 24 DNA samples should be used to evaluate SNP association with a frequency above the 5% α level in 95% likelihood (13). From the DNA of the first 24 patients, HIF-1α was divided into 15 fragments, and each fragment was amplified by polymerase chain reaction. A final volume of 10 µl consisted of 10 ng of DNA, 0.5 µM of each primer pair, 0.25 mM dNTPs, 3 mM MgCl2, 1 µl 1× reaction buffer, and 0.25 U Taq DNA polymerase (Intron Biotechnology, Seongnam-Si, Gyeonggi-do, Korea). The PCR conditions used were as follows: initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60–65°C for 30 s, initial extension at 72°C for 30–60 s, and final extension at 72°C for 10 min. For the remaining steps, we followed the methods used in our previous study (11).
SNP Genotyping-SNaPshot Assay/Direct Sequencing
The Tagger function within HaploView was used to assign tag SNPs. From the whole gene sequencing, a total of six tag SNPs were selected (ss526883730, rs11549465, rs11549467, rs4902080, rs2057482, ss526883733). All of the tag SNPs were genotyped similar to methods used in our previous study (11).
Statistical Analysis
Data analysis was completed using the statistics software SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA) and R, an open source programming language. The Hardy–Weinberg equilibrium (HWE) test was used to ensure the quality of genotyping. Pairwise linkage disequilibrium was estimated by calculating D′ and r 2 using the HaploView program. Owing to a small sample size, D′ and r 2 were calculated for SNPs with MAF > 0.1%. Values of p < 0.05 were considered statistically significant.
The association of SNPs with the mandibular jawbone atrophy was evaluated using Student’s t-test for dominant and recessive groups and ANOVA for the codominant group (11). Moreover, statistical analysis was performed to find the relationship between the ages, the time since tooth extraction of the patients, and the height of mandible. Linear regression was used to verify the independence among the three factors.
RESULTS
Mandibular Residual Ridge Characterization
The total study population consisted of 121 females and 81 males with average age of 70.80 ± 9.40 years. Panoramic dental radiographs were provided from all subjects, using which the bone height of the lowest vertical height of the edentulous mandible was measured following the protocol of the ACP (12) (Fig. 2). The mean of mandibular bone height was 15.17 (n = 202) and varied from 8.52 mm to 35.08 mm (Fig. 3).
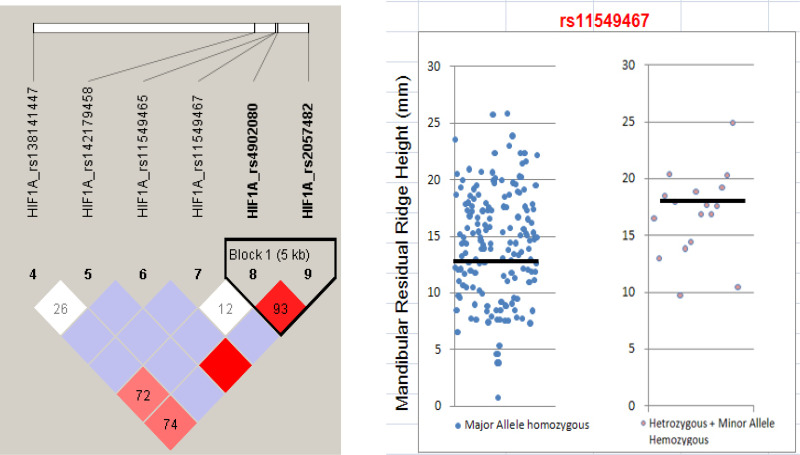
Figure 3.
Haplo-block of six tag SNPs B. Mandibular residual ridge height and SNP rs11549467 (p < 0.05).
Polymorphism Discovery and Taq-SNP Selection
A total of nine variants were identified via direct sequencing on a Korean population (n = 24). Out of nine variants, four were found to be novel: two in the promoter region (ss526883730, ss526883723), one in intron 9 (ss526883728), and one in exon 9 (ss526883733) (Table 1). Among the variants that were discovered, six SNPs with MAF > 5% (ss526883730, rs11549465, rs11549467, rs4902080, rs10147275, rs2057482) were selected for constructing the haplotype and linkage disequilibrium block. For HIF-1α, two different types of haplotype blocks were constructed using the HaploView program version 4.2 (www.broadinstitute.org/haploview/haploview). Haplotype 1 was constructed by six HIF-1α SNPs (rs138141447–rs142179458–rs11549465–rs11549467–rs4902080–rs2057482), and haplotype 2 was constructed by 2 HIF-1α SNPs (rs4902080–rs2057483) (Fig. 3). No statistical significance was shown from either type 1 or 2 haplotype with RRR (Tables 2 and 3). By pairwise tagging, five tag SNPs from six common variants in the HIF-1α gene (ss526883730, rs11549465, rs11549467, rs4902080, rs2057482) were chosen to capture the common variations within this gene (r 2 > 0.80). To our analysis, an additional SNP (MAF > 1%) in exon 9 (ss526883733) was selected as a tag SNP because it is a novel nonsynonymous variant that substitutes G to A resulting in 349Asp to Asn. Therefore, a total of six tag SNPs (ss526883730, rs11549465, rs11549467, rs4902080, rs2057482, ss526883733) were selected for genotyping and further analysis. The genotype frequency and heterozygosity of each of the six SNPs studied are shown in Table 4. Genotype distributions of all SNPs were in HWE.
Table 1.
Genetic Variants Discovered in HIF-1α Gene by Direct Sequencing (n = 24)
| Region | rs Number | SNP name | Position | Variation | Allele frequency | HWE |
|---|---|---|---|---|---|---|
| Promoter | ss526883730 | –1779A/–* | chr14:62160744 | A/–(del) | A:– = 0.833:0.167 | 1 |
| Promoter | ss526883723 | –578C/T | chr14:62161945 | C/T | C:T = 0.979:0.021 | 1 |
| Exon9 | ss526883733 | D349N* | chr14:62203623 | G/A | G:A = 0.979:0.021 | 1 |
| Intron9 | ss526883728 | IVS9+84G/A | chr14:62203911 | G/A | G:A = 0.979:0.021 | 1 |
| Exon12 | rs11549465 | P582S* | chr14:62207557 | C/T | C:T = 0.896:0.104 | 0.4113 |
| Exon12 | rs11549467 | T588A* | chr14:62207575 | A/G | G:A = 0.917:0.083 | 1 |
| Intron12 | rs4902080 | IVS12+99C/T* | chr14:62208005 | C/T | C:T = 0.875:0.125 | 0.5961 |
| Intron14 | rs10147275 | IVS14–99G/T | chr14:62213553 | G/T | G:T = 0.771:0.229 | 0.6587 |
| 3’UTR | rs2057482 | 2526C/T* | chr14:62213848 | C/T | C:T = 0.771:0.229 | 0.6587 |
Selected tag SNPs.
Table 2.
Frequencies of HIF-1α Haplotype 1
| Haplotype | Frequency |
|---|---|
| A-G-C-G-C-C | 0.71484 |
| --G-C-G-T-T | 0.11758 |
| A-G-T-G-C-T | 0.05328 |
| A-G-C-A-C-C | 0.03223 |
| --G-C-G-C-C | 0.03017 |
| A-G-C-G-T-T | 0.02524 |
| A-G-C-A-T-C | 0.00870 |
| A-A-C-G-C-C | 0.00401 |
| --G-T-G-C-T | 0.00382 |
| --A-C-G-C-C | 0.00353 |
| A-G-C-G-C-T | 0.00253 |
| A-G-C-A-T-T | 0.00181 |
| --G-C-A-C-C | 0.00124 |
| --G-C-A-T-T | 0.00090 |
| A-G-T-A-C-T | 0.00011 |
Table 3.
Frequencies of HIF-1α Haplotype 2
| Haplotype | Frequency |
|---|---|
| C-C | 0.78501 |
| T-T | 0.14611 |
| C-T | 0.06076 |
| T-C | 0.00812 |
Table 4.
Frequencies of HIF-1α Polymorphisms in 202 Koreans
| Position | Region | A.A Change | SNP Number | Genotype | Frequency | Heterozygosity | HWE | |||
|---|---|---|---|---|---|---|---|---|---|---|
| chr14:62160744 | Promoter | – | ss526883730 | AA | A- | – | N | 0.1571 | 0.243 | 1 |
| 135 | 52 | 4 | 191 | |||||||
| chr14:62203623 | Exon9 | D349N | ss526883733 | GG | GA | AA | N | 0.0076 | 0.020 | 1 |
| 195 | 3 | 0 | 198 | |||||||
| chr14:62207557 | Exon12 | P582S | rs11549465 | CC | CT | TT | N | 0.0575 | 0.101 | 0.1254 |
| 179 | 19 | 2 | 200 | |||||||
| chr14:62207575 | Exon12 | T588A | rs11549467 | GG | GA | AA | N | 0.0450 | 0.114 | 1 |
| 182 | 18 | 0 | 200 | |||||||
| chr14:62208005 | Intron12 | – | rs4902080 | CC | CT | TT | N | 0.1542 | 0.227 | 1 |
| 143 | 54 | 4 | 201 | |||||||
| chr14:62213848 | 3 UTR | – | rs2057482 | CC | CT | TT | N | 0.2060 | 0.311 | 0.665 |
| 124 | 68 | 7 | 199 | |||||||
Association of HIF-1α SNPs and Residual Ridge Resorption
Association of HIF-1α SNPs and RRR in total subjects is listed in Table 5. The results from the statistical analysis of tag SNPs showed that rs11549467 was associated with the risk of RRR in dominant and codominant models (p = 0.028) (Fig. 3). Results from linear regression showed that age, length of year after tooth extraction, and the height of mandible are independent (p = 0.785).
Table 5.
Association of HIF-1α Polymorphisms in 202 Koreans
| SNP Number |
Additive p Value |
Dominant p Value |
Recessive p Value |
|
|---|---|---|---|---|
| chr14:62160744 | ss526883730 | 0.840 | 0.640 | 0.647 |
| chr14:62203623 | ss526883733 | 0.847 | 0.847 | – |
| chr14:62207557 | rs11549465 | 0.281 | 0.153 | 0.800 |
| chr14:62207575 | rs11549467 | 0.028 * | 0.028 * | – |
| chr14:62208005 | rs4902080 | 0.822 | 0.554 | 0.740 |
| chr14:62213848 | rs2057482 | 0.902 | 0.650 | 0.914 |
p < 0.05.
DISCUSSION
Although genetic information and its usage have been an important part of health care for many years, it has not been a common factor in dentistry. In fact, dentists rarely get genetic training to diagnose patients in advance of actual dental treatment. Currently, PerioPredict from Interluekin Genetics, Inc., leads genetic testing in the dental industry, detecting susceptibility to periodontitis and tooth loss in patients for both research and medical purposes. PerioPredict was based on the study that the University of Michigan carried out to explore tooth loss outcomes based on the frequency of preventive periodontal care and the role of three primary risk factors: smoking, diabetes, and personal genetics. The findings from this study suggested patients with more risk factors were susceptible to periodontitis, and the most prevalent risk factor was genetic status (14). Health care costs in many countries appear unsustainable (15), and a substantial proportion of those costs may arise from missed chances to prevent chronic disease, such as peri-implantitis and tooth loss in dentistry (16). Hence, patient stratification through genetics would be essential in saving medical costs in the near future. For example, employers in the US would be able to save 4–6% for better dental care through patient stratification methods, such as PerioPredict from Interluekin Genetics, Inc. (17).
In prosthetic treatment, preservation of the residual ridge is essential for a successful outcome. Edentulous patients with severe RRR might suffer from poorly fitting, loose dentures and even implant failure in severe cases (18,19). If complications are repeated, edentulous patients would face a situation of excessive jawbone atrophy with no other treatment option in the end. Therefore, a genetic screening process before prosthetic treatment is imperative in dentistry. To find more genetic variants that might be associated with the residual resorption of the mandible in the Korean population, more subjects were recruited and added to the study population of the previous study for the current study. Many patients registered to the School of Dentistry at Yonsei University and Kyung-Hee University had already given out samples, and hence recruiting more subjects with age and dental condition criteria was one of the challenging factors in this study. Active recruitment and effort accomplished more than 200 subjects.
After tooth extraction, bone tissues in the residual ridge become hypoxic by reduced mechanical loading, which is delivered by periodontal ligament. Bone tissues in the residual ridge face a new situation that requires glycolytic ATP generation due to hypoxia, which is very expensive from an energetic viewpoint. Therefore, new blood vessel formation is inevitable to increase oxygen delivery to the bone tissue. HIF is the fundamental hypoxia-response protein, and over 70 genes have been identified to be HIF dependent. Also, disuse atrophy is observed when normally developed bone decreases in size as a result of reduced mechanical load (20). The load is diminished dramatically as a consequence of dental extraction, and therefore it is thought that the continuing pattern of alveolar bone resorption is related to this change. The reduced partial pressure of oxygen is the most prominent event among the changes in osseous tissue from the reduced mechanical load. It has been reported that osteocytes act as a mediator that responds to the mechanical stimulus in tissue and signal other regulatory factors depending on the physical changes in the external environment (20,21). The loss of mechanical loading, or disuse, rapidly precipitates locally mediated bone resorption. The increase in bone resorption, followed by an increased number of osteocytes in accordance with the oxygen level, may be correlated with the effect of HIF-1, a master regulator of oxygen-regulated gene expression. HIF-1 is known to have a high genetic diversity due to genetic variation. Therefore, we hypothesize that genetic variation of HIF-1 will result in individual differences in bone resorption in the mandible.
Current study results from the statistical analysis of tag SNPs in the Korean population showed that rs11549467 was associated with the risk of RRR in dominant and codominant models (p = 0.028). A588T (rs11549467) is one of the two common missense mutations encoding threonine with P582S (rs11549465), which are both related to increased transactivation capacity of HIF-1α. The target genes of HIF-1 encode angiogenesis, erythropoiesis, energy metabolism, vasomotor function, and apoptotic/proliferative responses of cells, which are relevant to pathogenesis of cancer. The variant forms increase numbers of microvessels compared with homozygous alleles and may be associated with increased expression levels of HIF-1α-regulated genes, which enhance angiogenesis (22).
Several studies showed association between HIF-1α and the wound healing process in various conditions. In wound healing, phagocytosis of apoptotic neutrophils by macrophages is an important event in the release of soluble factors including TGF-β1, which plays a major role in regulating formation and remodeling of granulation tissue. Overall, the effects of hypoxia on macrophages are likely to be important in accelerating wound healing (23). As hypoxia in the early wounds increases, macrophage-derived factors are likely to be increased and provide additional stimuli for attracting macrophages and mesenchymal cells such as fibroblasts and endothelial cells. Described hypoxia-induced macrophage-derived soluble factors include TGF-α, TGF-β1, VEGF, FGF, PDGF, TNF-α, IL-1, and IL-8 (24–28). This mixture of growth factors and cytokines can have an additive effect on early wound healing events such as reepithelialization and granulation tissue formation. Oral wounds and skin wounds differ in the short-term period of the wound healing process due to different levels of hypoxia experienced in both types of wounds leading to different expression of HIF-1α. Hence, we focused on HIF-1α and its SNP associated with residual ridge height in this study.
Wound healing after tooth extraction further leads to RRR at a different rate among individuals. A critical stimulus for normal wound healing is relative hypoxia (29), and an impaired reaction to hypoxia could contribute to impaired wound healing as well. Under hypoxic conditions, HIF-1α is stabilized against degradation and transactivates and upregulates a series of genes that enable cells to adapt to reduced oxygen availability (30). After tooth extraction, an oral wound experiences a hypoxic condition, and hypoxic tissue appears as acidic due to the increase of anaerobic metabolism and reduced vascular perfusion. A small pH reduction simulates the resorptive activity of osteoclasts, and bone remodeling occurs in oral wounds. Chen et al. examined levels of hypoxia and HIF-1α in mucosal and skin wounds and looked at how HIF-1α is regulated under conditions of stress and demonstrated that skin wounds, but not mucosal wounds, exhibit a marked elevation of HIF-1α (10). It has been repeatedly reported that skin wounds express higher HIF-1α and VEGF expression compared to that of oral wounds despite accelerated epithelial closure (10,31,32). According to these studies, healing rate decreases when HIF-1α expression increases.
Many other factors influencing RRR were investigated to interpret our result. While the depth of the extraction sockets and the original size of the mandible are known to influence resorption, it has been shown that neither factor is associated with the amount of subsequent resorption in longitudinal studies. The preextraction bony support of the teeth and the amount of subsequent bone loss showed no association (33). The development of periodontal disease is in close relationship with the microbial and host interactions. Junctional epithelium is the linkage of the gingiva and the teeth. It is highly permeable and functions as a pathway that disappears upon tooth removal (5). Studies have suggested that denture wears are both the cause (33) and the means to prevent RRR. However, bone loss is observed whether dentures are implemented or not (34). It has been cited that the rate of bone loss reduction over time is due to the resorption of ridge, which occurs by losing contact with the base of the denture.
CONCLUSION
Patient stratification is necessary in decreasing the medical costs in the dentistry field as well as in providing appropriate medical treatment to patients. Results of this study may be useful in developing novel genetic diagnostic tests and identifying Koreans susceptible to developing excessive jawbone atrophy after dental extraction, which will eventually help in decreasing medical costs. Screening through the genetic method will prevent susceptible patients from getting unnecessary treatment, which can lead patients with no other effective prosthetic treatment.
The data of this study are retrospective. Therefore, the result of the genetic association should be repeatedly proven prospectively through an independent cohort group to be used in clinical studies and to be confirmed. When predicting the prognosis of the alveolar ridge after extracting the teeth, rs11549467 may be used as marker SNPs to set a treatment plan accordingly so that medical expenses can be reduced.
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (7-2011-0259).
REFERENCES
- 1. Elderly Statistics. Korea: Statistics Korea; 2009. [Google Scholar]
- 2. Research on the actual condition of the people oral health 2006. Seoul, Korea: Ministry for Health, Welfare and Family Affairs; 2006. [Google Scholar]
- 3. Jahangiri L, Devlin H, Ting K, Nishimura I. Current perspectives in residual ridge remodeling and its clinical implications: A review. J Prosthet Dent 1998; 802:224–237. [DOI] [PubMed] [Google Scholar]
- 4. Van der Weijden F, Dell’Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J Clin Periodontol 2009; 3612:1048–1058. [DOI] [PubMed] [Google Scholar]
- 5. Kingsmill VJ. Post-extraction remodeling of the adult mandible. Crit Rev Oral Biol Med 1999; 103:384 404. [DOI] [PubMed] [Google Scholar]
- 6. Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: A mixed-longitudinal study covering 25 years. 1972. J Prosthet Dent 2003; 895:427–435. [DOI] [PubMed] [Google Scholar]
- 7. Sciubba JJ, Waterhouse JP, Meyer J. A fine structural comparison of the healing of incisional wounds of mucosa and skin. J Oral Pathol 1978; 74:214–227. [DOI] [PubMed] [Google Scholar]
- 8. Walsh LJ, L’Estrange PR, Seymour GJ. High magnification in situ viewing of wound healing in oral mucosa. Aust Dent J 1996; 412:75–79. [DOI] [PubMed] [Google Scholar]
- 9. Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995; 108(Pt 3):985–1002. [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Gajendrareddy PK, DiPietro LA. Differential expression of HIF-1alpha in skin and mucosal wounds. J Dent Res 2012; 919:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim JH, Oh MY, Paek J, Lee J. Association between FGFR1OP2/wit3.0 polymorphisms and residual ridge resorption of mandible in Korean population. PLoS One 2012; 78:e42734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGarry TJ, Nimmo A, Skiba JF, Ahlstrom RH, Smith CR, Koumjian JH. Classification system for complete edentulism. The American College of Prosthodontics. J Prosthodont 1999; 81:27–39. [DOI] [PubMed] [Google Scholar]
- 13. Kruglyak L, Nickerson DA. Variation is the spice of life. Nat Genet 2001; 273:234–236. [DOI] [PubMed] [Google Scholar]
- 14. Giannobile WV, Braun TM, Caplis AK, Doucette-Stamm L, Duff GW, Kornman KS. Patient stratification for preventive care in dentistry. J Dent Res 2013; 928:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keehan SP, Sisko AM, Truffer CJ, Poisal JA, Cuckler GA, Madison AJ, et al. National health spending projections through 2020: Economic recovery and reform drive faster spending growth. Health Aff (Millwood) 2011; 308:1594–1605. [DOI] [PubMed] [Google Scholar]
- 16. The healthcare imperative: Lowering costs and improving outcomes: Workshop series summary. Washington, DC: National Academy of Sciences; 2010. [PubMed] [Google Scholar]
- 17. Improving preventive care in dentistry utilizing genetic testing. In 5th Annual Consumer Genetics Conference. Boston, MA; 2013. [Google Scholar]
- 18. Slagter AP, Olthoff LW, Bosman F, Steen WH. Masticatory ability, denture quality, and oral conditions in edentulous subjects. J Prosthet Dent 1992; 682:299–307. [DOI] [PubMed] [Google Scholar]
- 19. Wolff A, Gadre A, Begleiter A, Moskona D, Cardash H. Correlation between patient satisfaction with complete dentures and denture quality, oral condition, and flow rate of submandibular/sublingual salivary glands. Int J Prosthodont 2003; 161:45–48. [PubMed] [Google Scholar]
- 20. Skerry TM, Bitensky L, Chayen J, Lanyon LE. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res 1989; 45:783–788. [DOI] [PubMed] [Google Scholar]
- 21. Dodd JS, Raleigh JA, Gross TS. Osteocyte hypoxia: A novel mechanotransduction pathway. Am J Physiol, 1999; 2773(Pt 1):C598–602. [DOI] [PubMed] [Google Scholar]
- 22. Tanimoto K, Yoshiga K, Eguchi H, Kaneyasu M, Ukon K, Kumazaki T, et al. Hypoxia-inducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis 2003; 2411:1779–1783. [DOI] [PubMed] [Google Scholar]
- 23. Lokmic Z, Musyoka J, Hewitson TD, Darby IA. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int Rev Cell Mol Biol 2012; 296:139–185. [DOI] [PubMed] [Google Scholar]
- 24. Albina JE, Henry WL Jr., Mastrofrancesco B, Martin BA, Reichner JS. Macrophage activation by culture in an anoxic environment. J Immunol 1995; 1559:4391–4396. [PubMed] [Google Scholar]
- 25. Hempel SL, Monick MM, Hunninghake GW. Effect of hypoxia on release of IL-1 and TNF by human alveolar macrophages. Am J Respir Cell Mol Biol 1996; 142:170–176. [DOI] [PubMed] [Google Scholar]
- 26. Jackson IL, Chen L, Batinic-Haberle I, Vujaskovic Z. Superoxide dismutase mimetic reduces hypoxia-induced O2*-, TGF-beta, and VEGF production by macrophages. Free Radic Res 2007; 411:8–14. [DOI] [PubMed] [Google Scholar]
- 27. Kuwabara K, Ogawa S, Matsumoto M, Koga S, Clauss M, Pinsky DJ, et al. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci USA 1995; 9210:4606–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scannell G, Waxman K, Kaml GJ, Ioli G, Gatanaga T, Yamaoto R, et al. Hypoxia induces a human macrophage cell line to release tumor necrosis factor-alpha and its soluble receptors in vitro. J Surg Res 1993; 544:281–285. [DOI] [PubMed] [Google Scholar]
- 29. Tandara AA, Mustoe TA. Oxygen in wound healing More than a nutrient. World J Surg 2004; 283:294–300. [DOI] [PubMed] [Google Scholar]
- 30. Semenza GL. Life with oxygen. Science 2007; 3185847:62–64. [DOI] [PubMed] [Google Scholar]
- 31. Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. Distinct patterns of angiogenesis in oral and skin wounds. J Dent Res 2005; 844:309–314. [DOI] [PubMed] [Google Scholar]
- 32. Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res 2003; 828:621–626. [DOI] [PubMed] [Google Scholar]
- 33. Carlsson GE, Persson G. Morphologic changes of the mandible after extraction and wearing of dentures. A longitudinal, clinical, and x-ray cephalometric study covering 5 years. Odontol Rev 1967; 181:27–54. [PubMed] [Google Scholar]
- 34. Campbell RL. A comparative study of the resorption of the alveolar ridges in denture-wearers and non-denture-wearers. J Am Dent Assoc 1960; 60:143–153. [DOI] [PubMed] [Google Scholar]