Abstract
Background
Pancreatic cancer remains one of the five leading causes of cancer deaths in industrialised nations. For adenocarcinomas in the head of the gland and premalignant lesions, partial pancreaticoduodenectomy represents the standard treatment for resectable tumours. The gastro‐ or duodenojejunostomy after partial pancreaticoduodenectomy can be reestablished via either an antecolic or retrocolic route. The debate about the more favourable technique for bowel reconstruction is ongoing.
Objectives
To compare the effectiveness and safety of antecolic and retrocolic gastro‐ or duodenojejunostomy after partial pancreaticoduodenectomy.
Search methods
In this updated version, we conducted a systematic literature search up to 6 July 2021 to identify all randomised controlled trials (RCTs) in the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library 2021, Issue 6, MEDLINE (1946 to 6 July 2021), and Embase (1974 to 6 July 2021). We applied no language restrictions. We handsearched reference lists of identified trials to identify further relevant trials, and searched the trial registries clinicaltrials.govand World Health Organization International Clinical Trials Registry Platform for ongoing trials.
Selection criteria
We considered all RCTs comparing antecolic with retrocolic reconstruction of bowel continuity after partial pancreaticoduodenectomy for any given indication to be eligible.
Data collection and analysis
Two review authors independently screened the identified references and extracted data from the included trials. The same two review authors independently assessed risk of bias of included trials, according to standard Cochrane methodology. We used a random‐effects model to pool the results of the individual trials in a meta‐analysis. We used odds ratios (OR) to compare binary outcomes and mean differences (MD) for continuous outcomes.
Main results
Of a total of 287 citations identified by the systematic literature search, we included eight randomised controlled trials (reported in 11 publications), with a total of 818 participants. There was high risk of bias in all of the trials in regard to blinding of participants and/or outcome assessors and unclear risk for selective reporting in six of the trials.
There was little or no difference in the frequency of delayed gastric emptying (OR 0.67; 95% confidence interval (CI) 0.41 to 1.09; eight trials, 818 participants, low‐certainty evidence) with relevant heterogeneity between trials (I2=40%).
There was little or no difference in postoperative mortality (risk difference (RD) ‐0.00; 95% CI ‐0.02 to 0.01; eight trials, 818 participants, high‐certainty evidence); postoperative pancreatic fistula (OR 1.01; 95% CI 0.73 to 1.40; eight trials, 818 participants, low‐certainty evidence); postoperative haemorrhage (OR 0.87; 95% CI 0.47 to 1.59; six trials, 742 participants, low‐certainty evidence); intra‐abdominal abscess (OR 1.11; 95% CI 0.71 to 1.74; seven trials, 788 participants, low‐certainty evidence); bile leakage (OR 0.82; 95% CI 0.35 to 1.91; seven trials, 606 participants, low‐certainty evidence); reoperation rate (OR 0.68; 95% CI 0.34 to 1.36; five trials, 682 participants, low‐certainty evidence); and length of hospital stay (MD ‐0.21; 95% CI ‐1.41 to 0.99; eight trials, 818 participants, low‐certainty evidence).
Only one trial reported quality of life, on a subgroup of 73 participants, also without a relevant difference between the two groups at any time point. The overall certainty of the evidence was low to moderate, due to some degree of heterogeneity, inconsistency and risk of bias in the included trials.
Authors' conclusions
There was low‐ to moderate‐certainty evidence suggesting that antecolic reconstruction after partial pancreaticoduodenectomy results in little to no difference in morbidity, mortality, length of hospital stay, or quality of life. Due to heterogeneity in definitions of the endpoints between trials, and differences in postoperative management, future research should be based on clearly defined endpoints and standardised perioperative management, to potentially elucidate differences between these two procedures. Novel strategies should be evaluated for prophylaxis and treatment of common complications, such as delayed gastric emptying.
Plain language summary
What are the benefits and risks of bowel reconstruction routes after partial surgical removal of the pancreas and duodenum (first part of the small intestine)?
Key messages
‐ Antecolic bowel reconstruction may not reduce delayed gastric emptying after partial surgical removal of the pancreatic head and duodenum.
‐ Our results do not suggest any relevant differences between both techniques in other morbidity, mortality, length of hospital stay, and quality of life.
Background
The pancreas is a digestive gland situated in the upper abdomen, which is also vital to normal control of blood sugar. Pancreatic cancer is one of the leading causes of cancer death in industrialised nations. The standard surgical treatment for cancer of the head of the gland and precancerous abnormalities is partial removal of the pancreas, together with the attached duodenum, known as a pancreaticoduodenectomy. Removal of the duodenum requires the restoration of the digestive pathway from the stomach to the rest of the gut. This can be accomplished by joining it to the jejunum (second part of the small intestine) either in front of (antecolic) or behind (retrocolic) the overlying large intestine (transverse colon).
What did we want to find out?
We wanted to find out whether one of the above‐mentioned two routes of reconstruction provides a benefit to the patient by reducing delayed gastric emptying (emptying of the stomach after ingestion of food); postoperative mortality (death); and other complications, such as pancreatic fistula (leakage of pancreatic juice), reoperation, perioperative measures (before, during, and after the operation), or length of hospital stay; and improving quality of life. Delayed gastric emptying was the primary outcome of this review because it is one of the most frequent complications after a pancreaticoduodenectomy; it can make it difficult to take anything by mouth and interferes with the patient’s quality of life, often resulting in a prolonged hospital stay and delay of further treatment.
What did we do?
We searched for studies that compared antecolic with retrocolic reconstruction in patients undergoing partial removal of the pancreas together with the duodenum. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We included eight randomised controlled trials (reported in 11 publications), reporting data on a total of 818 adult participants, who underwent pancreaticoduodenectomy for any pancreatic disease.
Main results
We did not identify relevant differences in delayed gastric emptying; postoperative mortality; postoperative pancreatic fistula, or other complications; reoperations; or length of hospital stay. Quality of life, only reported for a subset of participants in one trial, did not differ between the two groups. Our results do not suggest any relevant differences between antecolic and retrocolic reconstruction of the gastro‐ or duodenojejunostomy after partial pancreaticoduodenectomy.
What are the limitations of the evidence?
Our confidence in the results is limited because the results from the studies varied widely, and most studies involved only small numbers of people. Most studies used methods likely to introduce errors. Therefore, the results should be interpreted in the light of these limitations.
How up to date is this evidence?
This review updates our previous review. The evidence is current to July 2021.
Summary of findings
Summary of findings 1. Summary of findings.
| Delayed gastric emptying following antecolic versus retrocolic reconstruction after partial pancreatoduodenectomy | ||||||
|
Patient or population: adults with any pancreatic diagnosis leading to an indication for elective (classical, pylorus‐preserving, pylorus‐resecting) partial pancreatoduodenectomy Settings: Inpatient treatment in Europe and Asia Intervention: bowel continuity via an antecolic reconstruction Comparison: bowel continuity via a retrocolic reconstruction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Retrocolic reconstruction | Antecolic reconstruction | |||||
| Delayed gastric emptying (all definitions) | 350 per 1000 | 265 per 1000 (180 to 370) | OR 0.67 (0.41 to 1.09) | 818 participants (8 trials) | ⊕⊕⊝⊝ low1 | |
| Mortality (within 30 days or 'in‐hospital') | 31 per 1000 | 31 per 1000 | RD ‐0.00 (‐0.02 to 0.01) | 818 participants (8 trials) |
⊕⊕⊕⊕ high3 | |
| Pancreatic fistula | 268 per 1000 | 269 per 1000 (181 to 339) | OR 1.01(0.73 to 1.40) | 818 participants (8 trials) | ⊕⊕⊝⊝ low1,2 | |
| Postoperative haemorrhage | 67 per 1000 | 59 per 1000 (33 to 102) | OR 0.87 (0.47 to 1.59) | 742 participants (6 trials) | ⊕⊕⊝⊝ low1,2 | |
| Intra‐abdominal fluid collection, abscess | 144 per 1000 | 157 per 1000 (107 to 226) | OR 1.11 (0.71 to 1.74) | 788 participants (7 trials) | ⊕⊕⊝⊝ low1,2 | |
| Reoperations | 66 per 1000 | 46 per 1000 (23 to 88) | OR 0.68 (0.34 to 1.36) | 682 participants (5 trials) | ⊕⊕⊝⊝ low1,2 | |
| Length of postoperative hospital stay (days) | The mean length of postoperative hospital stay in the control groups ranged from 9 to 48 days | The mean length of postoperative hospital stay in the intervention groups ranged from 9 to 36 days | MD ‐0.21 (‐1.41 to 0.99) | 818 participants (8 trials) | ⊕⊕⊝⊝ low1,2 | |
| Quality of life (EQ‐5D, EORTC QLQ‐C30 and PAN26, GIQLI at 2, 4 and 12 weeks after surgery) | No relevant differences in the EQ‐5D(TM) questionnaire, the 'European Organization for Research and Treatment of Cancer" QLQ‐C30 and PAN26 questionnaires and the 'Gastrointestinal Quality of Life Index' at 2, 4 and 12 weeks after the operation. | No relevant differences in the EQ‐5D(TM) questionnaire, the 'European Organization for Research and Treatment of Cancer" QLQ‐C30 and PAN26 questionnaires and the 'Gastrointestinal Quality of Life Index' at 2, 4 and 12 weeks after the operation. | no quantitative synthesis | 73 participants (1 trial) | ⊕⊝⊝⊝ very low1,2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; OR: Odds Ratio; RD: Risk difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for the GRADE domain 'risk of bias'. Explanation: There was lack of blinding in most trials, unclear risk for selective reporting and other bias.
2Downgraded one level for the GRADE domain 'imprecision'. Explanation: Confidence interval crosses the "no difference" margin and does not rule out a relevant effect.
3Evidence was not downgraded, because mortality is a 'hard' endpoint that is unlikely to be influenced by the risk of bias of the underlying studies.
4Downgraded one level for the GRADE domain 'imprecision'. Explanation: Quality of life was only evaluated in a subset of patients of one trial and due to the small sample of this subset, the optimal information size is not met.
Background
Pancreatic cancer is the fourth leading cause of cancer death in Europe and third in the USA. The global age‐standardised incidence rate of pancreatic cancer is estimated to be around 5.7 per 100,000 men and 4.1 per 100,000 women, with substantial differences between highly‐developed countries and less‐developed countries (GLOBOCAN 2020). While mortality rates for other types of cancer are predicted to fall, the mortality rate for pancreatic ductal adenocarcinoma has been rising in recent years (Malvezzi 2014). The majority of pancreatic ductal adenocarcinomas are located in the head of the gland, and if the lesion is resectable, radical surgery usually followed by adjuvant chemotherapy is still the treatment of choice (Conroy 2018; Shaib 2007). Partial pancreaticoduodenectomy (pPD) is the standard treatment for tumours of the pancreatic head, benign precursor lesions such as intraductal papillary mucinous neoplasia, or chronic pancreatitis that require a resective surgical approach (Schnelldorfer 2008). Due to improvements in imaging techniques and their increased use in health checks, an increasing number of asymptomatic lesions are discovered incidentally, leading to higher rates of pancreatic surgery (Plichta 2015; Spinelli 2004). Due to surgical expertise and standardised perioperative management, pancreatic surgery, which is technically challenging, can be performed in specialised centres with mortality rates of less than 5% (Büchler 2003; de Wilde 2012; McPhee 2007). However, morbidity, consisting typically of postoperative pancreatic fistula, post‐pancreatectomy haemorrhage, biliary leakage, intra‐abdominal abscess, and delayed gastric emptying, remains at a high level, up to 60% (Stojadinovic 2003).
A glossary of terms is provided in Appendix 1.
Description of the condition
One of the most frequent complications after pPD, with rates between 14% to 61% reported in the literature, is delayed gastric emptying (Kim 2005). Delayed gastric emptying impairs oral intake, interferes with the patient’s quality of life, and often results in prolonged hospitalisation and delay of further treatment (e.g. necessary adjuvant chemotherapy). Several modifications of surgical techniques have been used in an attempt to reduce the frequency of delayed gastric emptying, for instance, pylorus‐resecting versus pylorus‐preserving pPD, since the pylorus might have an influence on delayed gastric emptying, acting as a gatekeeper of the gastric outlet (Hackert 2018; Yang 2014). Billroth‐II versus Roux‐en‐Y reconstruction have also been compared, since the gastroenteric passage could be influenced by the passing of biliopancreatic juice (Shimoda 2013). Different perioperative treatment strategies have also been used to reduce the impact of delayed gastric emptying, e.g. erythromycin is supposed to improve delayed gastric emptying by acting as a motilin‐agonist (Yeo 1993), while somatostatin analogues decrease gastric motility in healthy patients (Kollmar 2008). The large variations in rates of delayed gastric emptying in different studies are attributable to the use of different definitions and assessment of delayed gastric emptying. In 2007, the International Study Group of Pancreatic Surgery (ISGPS) developed a consensus definition to provide an objective assessment of this condition, and comparability of future trial results (Wente 2007a). There is a reasonable assumption that the method of bowel reconstruction after pPD (antecolic versus retrocolic) has an impact on delayed gastric emptying.
Description of the intervention
The aetiology of delayed gastric emptying has been attributed to multiple factors, including other intra‐abdominal complications, e.g. intra‐abdominal abscess or postoperative pancreatic fistula. Nevertheless, not all cases of delayed gastric emptying are related to other complications, and the route of reconstruction might be another influencing factor. Bowel continuity after pPD can either be established via an antecolic route (i.e. a jejunal loop is brought up anterior to the transverse colon for the gastroenteric anastomosis), or via a retrocolic route through a mesocolic window (i.e. a jejunal loop is brought up posterior to the transverse colon for the gastroenteric anastomosis). As suggested by several randomised and non‐randomised studies, the route of reconstruction and the angulation of the stomach and duodenum might influence the occurrence of delayed gastric emptying (Hartel 2005; Nikfarjam 2009; Tani 2006).
How the intervention might work
Several explanations have been provided for the potential difference between antecolic and retrocolic reconstruction: the mobility of the descending jejunal loop might be different, which supposedly decreases the risk of torsion or angulation. The different anatomic position between the gastroenteric anastomosis and the pancreaticojejunostomy might also have an effect on the gastrointestinal passage, e.g. caused by a small pancreaticojejunal anastomotic leak or a transient mild postoperative pancreatitis (Hartel 2005). Furthermore, the angulation of the stomach can be different between the two techniques, thus influencing the gastrointestinal passage.
Why it is important to do this review
Since delayed gastric emptying is still a frequent and clinically relevant problem after pPD, and the optimal reconstruction method to reduce its occurrence is still under debate; a recent systematic review including randomised and non‐randomised studies addressed this issue (Bell 2015). However, the analysis of the retrospective studies might introduce substantial bias in the light of a sufficient number of randomised controlled trials. Furthermore, one of the included trials (Chijiiwa 2009), represents a preliminary report on the same set of patients of another included trial (Imamura 2014), which is not addressed in the review. Therefore, a systematic review with clear methodology, that compares these two reconstruction techniques appears both feasible and important. The combined analysis of evidence from randomised controlled trials might be able to provide a conclusive answer to the ongoing debate.
Objectives
To compare the effectiveness and safety of antecolic and retrocolic gastro‐/duodenojejunostomy after partial pancreaticoduodenectomy (pPD).
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs) in this review. We did not apply any restrictions to the language of the original report.
Types of participants
We included all RCTs considering adults with any pancreatic diagnosis (e.g. pancreatic carcinoma, chronic pancreatitis, cystic neoplasms of the pancreas, etc) leading to an indication for elective pPD.
Types of interventions
We only included trials that compared antecolic reconstruction with retrocolic reconstruction of bowel continuity after pPD. In cases of insufficient description of the surgical procedures involved, we contacted the trial authors for more details, to enable us to decide about including the trial.
Types of outcome measures
Primary outcomes
Delayed gastric emptying (preferably defined according to the International Study Group of Pancreatic Surgery (ISGPS) definition (Wente 2007a)).
Secondary outcomes
Clinically relevant delayed gastric emptying (ISGPS grade B/C).
Postoperative mortality (30‐day and in‐hospital mortality).
Postoperative pancreatic fistula (preferably defined according to the International Study Group of Pancreatic Fistula (ISGPF) definition (Bassi 2005)).
Postoperative haemorrhage (preferably defined according to the ISGPS definition (Wente 2007b)).
Bile leakage.
Intra‐abdominal fluid collection or abscess.
Reoperation rate.
Duration of operation.
Intraoperative blood loss.
Length of hospital stay.
Time to nasogastric tube (NGT) removal (in days) after surgery.
Quality of life (considering all aspects e.g. physical and emotional quality of life, ability to eat, pain level, etc.).
Most of the outcomes we chose represent the major complications specific to pancreatic surgery. Duration of operation and length of hospital stay have been chosen as indirect measures of hospital costs. Quality of life represents the most patient‐relevant outcome.
Reporting of all outcomes listed here was not an inclusion criteria for the review. If a trial did not provide data on the primary outcome, we contacted the trial authors for clarification.
Search methods for identification of studies
Electronic searches
We conducted a systematic literature search to identify all published and unpublished RCTs that examined antecolic or retrocolic reconstruction after pPD. We designed the literature search to identify potential trials in all languages. We did not find any non‐English language papers that were relevant for the review.
We searched the following electronic databases for identification of potential trials. In the previous published version, search was performed on 29 September 2015. In this updated version, search was performed on 6 July 2021.
Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2021, issue 5; Appendix 2).
MEDLINE (1946 to 06 July 2021; Appendix 3).
Embase (1974to 06 July 2021; Appendix 4).
We also searched the clinical trials registries, ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform on 6 July 2021, to identify potential trials in the field that were unpublished or ongoing.
Searching other resources
We checked the reference lists of all primary studies and relevant review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished trials. Furthermore, we asked experts in the field for further relevant trials.
Data collection and analysis
Selection of studies
We identified and excluded duplicates and multiple reports of the same trial, so that each trial, rather than each report, was the unit of interest in the review. Two review authors (FJH, RK) independently screened titles and abstracts of all potential studies, which were identified as a result of the search, and coded them as 'retrieve' (eligible or potentially eligible or unclear), or 'do not retrieve'. We retrieved the full text of the trial reports and publications deemed eligible, potentially eligible, or unclear, and the same two review authors independently screened the full text, identified trials for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. They resolved any disagreements through discussion, or if required, in consultation with a third review author (MKD or PP). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and Characteristics of excluded studies table.
Data extraction and management
We used a standard data collection form, which had been piloted on at least one trial in the review, to gather trial characteristics and outcome data. Two review authors (FJH, RK) extracted these trial characteristics from included trials.
Methods: trial design, total duration of the trial, number of trial centres and location, trial setting, sample size calculation, withdrawals, publication date.
Participants: sample size, mean age, gender, diagnosis (underlying disease), inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant medications, excluded medications, technical details of intervention.
Outcomes: primary and secondary outcomes specified and collected, time points reported, exact definitions of outcomes reported.
Notes: funding of trial, notable conflicts of interest of trial authors.
Two review authors (FJH, RK) independently extracted outcome data from included trials. If outcome data were reported in an unusable way, we noted this in the Characteristics of included studies table. We resolved all disagreements by consensus, or by involving a third person (MKD or PP). One review author (FJH) copied the data from the data collection form into the Review Manager file (RevMan 2014). We double‐checked that the data were entered correctly by comparing the trial reports with data in the systematic review. A second review author spot‐checked trial characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (FJH, RK) independently assessed risk of bias for each trial, using the original Cochrane risk of bias tool outlined in the former version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), because the first version of this review was created before the introduction of RoB 2.0 tool. The review authors resolved any disagreement by discussion or by involving a third review author (MKD or PP). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. baseline imbalance, funding bias, etc).
We graded each potential source of bias as high, low, or unclear, and we presented a quote from the trial report together with a justification for the judgement in the risk of bias table. We summarised the risk of bias judgements across trials for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
When considering treatment effects, we took the risk of bias for the trials that contributed to that outcome into account.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol, and reported any deviations in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We used risk difference (RD) as the summary measure for the outcome 'mortality' instead of ORs, because of the computational problem in meta‐analyses concerning trials with zero events in both groups. We ensured that higher scores for continuous outcomes had the same meaning for the particular outcome, we explained the direction to the reader, and we reported where the directions were reversed, if this was necessary. All analyses were conducted with RevMan 2014.
We only performed meta‐analyses where this was meaningful, i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
A common way that trialists indicate when they have skewed data is by reporting medians and interquartile ranges. When this was encountered, we used the methods described by Higgins 2021, Hozo 2005, or Wan 2014, to calculate means and standard deviations (SDs). The decision to conduct quantitative synthesis with these data was based upon individual decisions for each outcome and are explained in the review.
Unit of analysis issues
When data were presented in different forms across included trials, or they were difficult to categorise, we dichotomised these data, if possible, for the purpose of analysis. We did not find any RCTs with non‐standard designs.
Dealing with missing data
We contacted investigators or trial sponsors in order to verify key trial characteristics and obtain missing numerical outcome data, where possible (e.g. when a trial was identified as abstract only).
Assessment of heterogeneity
We used I² statistics to measure statistical heterogeneity among the trials in each analysis. An I2 of < 25% was considered as no relevant heterogeneity, 25% to 50% as moderate heterogeneity and > 50% as substantial heterogeneity.
We explored clinical heterogeneity by assessing differences in baseline data, definitions of outcome parameters, and operative or perioperative management, or both. We discussed clinical heterogeneity in the appropriate sections. In the case of substantial clinical heterogeneity we did not perform meta‐analysis.
Assessment of reporting biases
We contacted trial authors to ask them to provide missing outcome data, if this was necessary.
We created a funnel plot and examined it for asymmetry to explore possible publication bias.
Data synthesis
Whenever sufficient data for a specific outcome were provided, and it made clinical and statistical sense to pool the results, we performed a meta‐analysis using a random‐effects model (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We had planned to perform the following subgroup analyses, if sufficient data were available.
Antecolic versus retrocolic reconstruction after pylorus‐preserving pPD.
Antecolic versus retrocolic reconstruction after classical pPD (Whipple's procedure).
Antecolic versus retrocolic reconstruction for different underlying diagnoses (e.g. pancreatic adenocarcinoma, chronic pancreatitis, etc).
Antecolic versus retrocolic reconstruction with single‐loop reconstruction.
Antecolic versus retrocolic reconstruction with Roux‐en‐Y reconstruction.
We used the following outcome in subgroup analysis.
Delayed gastric emptying.
Sensitivity analysis
We conducted the following sensitivity analysis, defined a priori, to assess the robustness of our conclusions:
only trials applying the ISGPS definition;
excluding studies that presented medians and (interquartile) ranges or standard errors instead of means and standard deviations; and
fiixed‐effect meta‐analysis for the primary outcome: delayed gastric emptying.
Reaching conclusions
The conclusions from this systematic review are based only on findings from the quantitative or narrative synthesis of included trials for this review. We avoided making recommendations for practice and the Implications for research section gives the reader a clear sense of where the focus of any future research in the area should be, and what the remaining uncertainties are.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the following outcomes: delayed gastric emptying, postoperative mortality, postoperative pancreatic fistula, postoperative haemorrhage, intra‐abdominal fluid collection/abscess, reoperation rate, length of hospital stay and quality of life. These outcome parameters have been chosen because they represent the most important outcomes from a clinical point of view and could potentially be influenced by the different interventions. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it related to the trials that contributed data to the meta‐analyses for the prespecified outcomes. We used the methods and recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), and GRADEproGDT software (GRADEproGDT 2015). We justified all decisions to down‐ or up‐grade the certainty of the evidence in footnotes, and provided comments to aid the reader's understanding of the review, where necessary. If there was any additional outcome information that we were unable to incorporate into the meta‐analyses, we considered and noted this in the comments. We stated if it supported or contradicted the information from the meta‐analyses.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
In total, we identified 216 titles and abstracts with the systematic literature search in 2015 and an additional 82 with the search in 2021. After accounting for the exclusion process described below, the quantitative data of eight trials reported in 11 publications, with 818 analysed patients comparing antecolic (407 patients) and retrocolic (411 patients) reconstruction were found suitable for this review (Eshuis 2014; Gangavatiker 2011; Imamura 2014; Kakaei 2019; Kurahara 2011; Tamandl 2014; Tani 2006; Toyama 2021). No ongoing trials were identified through the search of the trial registry ClinicalTrials.gov andWorld Health Organization International Clinical Trials Registry Platform.
Included studies
Trials showed obvious discrepancy in sample sizes (range from 30 (Kakaei 2019), to 246 (Eshuis 2014)), but intra‐ and inter‐study population baseline characteristics revealed comparability.
One trial did not report a sample size calculation (Kakaei 2019), and one trial reported sample size calculation based on a non‐inferiority margin of 10% with a power of 80% and a one‐sided α of 2.5% resulting in a planned sample size of 108 patients per group (Toyama 2021). All other included trials described a sample size calculation for superiority with a power of 80% and an α of 5%, but the underlying assumed incidence of delayed gastric emptying varied in both the retrocolic (30% to 50%) and the antecolic groups (10% to 15%), resulting in planned sample sizes of 91 to 20 patients per group (Eshuis 2014; Tamandl 2014. respectively). Three trials conducted planned interim analyses (Eshuis 2014; Kurahara 2011; Tani 2006). Investigators terminated two trials at that point due to significant inter‐group differences and ethical factors (Kurahara 2011; Tani 2006), whereas investigators in another trial increased the sample size from 91 to 126 patients per arm (Eshuis 2014). In the trial by Tamandl 2014, the recruitment of patients did not reach the planned sample size calculation.
Statistical analysis was performed according to the intention‐to‐treat principle in two trials (Eshuis 2014; Toyama 2021), and per‐protocol in another (Tamandl 2014); the other trial reports did not describe the statistical analysis models used.
We observed different technical approaches for the surgical intervention: two trials included only pylorus‐resecting Whipple's procedure (Kakaei 2019; Kurahara 2011), three trials included only pylorus‐preserving Whipple's procedure (Imamura 2014; Tamandl 2014; Tani 2006), two trials included both, classical and pylorus‐preserving Whipple's procedure (Eshuis 2014; Gangavatiker 2011), and one trial included subtotal stomach‐preserving and pylorus‐preserving Whipple's procedure (Toyama 2021). Reconstruction was performed by pancreaticojejunostomy in seven trials and pancreaticogastrostomy in one trial (Kurahara 2011). All included trials described single‐loop reconstruction.
Different standards were applied for removing the nasogastric tube (NGT): In one trial, the NGT was removed in the operating room and was reinserted only if necessary (Tamandl 2014). In three trials, it was removed routinely if the gastric amount was below 500 mL per day (Imamura 2014; Kurahara 2011; Tani 2006). In one trial, the NGT was removed on or before the third postoperative day, or when daily output had fallen below 300 mL (Eshuis 2014). The NGT was removed if the output was less than 200 mL on two consecutive days in one trial (Gangavatiker 2011). In another trial, the NGT was removed after 48 hours if there was no bleeding and the output was less than 50 ml per six hours (Kakaei 2019). In the last trial, the NGT was routinely removed on the first postoperative day if the output was less than 250 mL (Toyama 2021).
Excluded studies
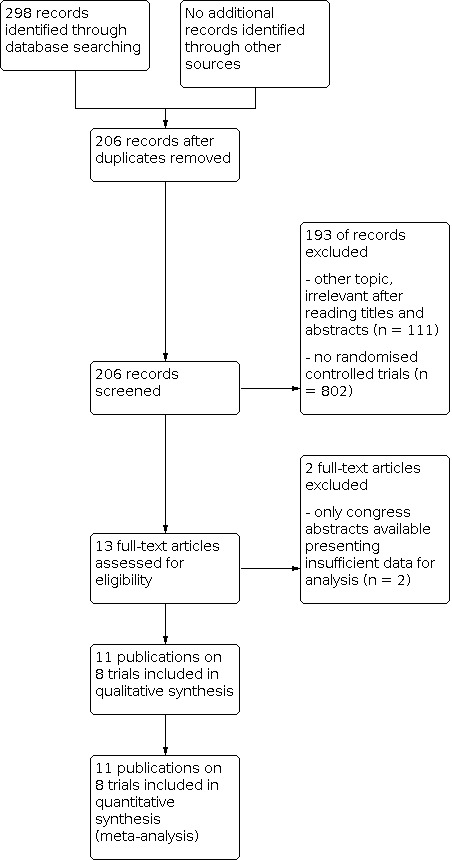
From the total of 298 abstracts, we excluded 92 because they were duplicates. Of the remaining 206 abstracts, 193 were excluded (111 covered other topics and 82 were not randomised controlled trial (RCTs)). The study selection process is displayed in Figure 1.
1.
Study flow diagram.
Thirteen publications were potentially eligible (Chijiiwa 2009 [reference listed under Imamura 2014; Chijiiwa 2012 [reference listed under Imamura 2014]; Eshuis 2012 [reference listed under Eshuis 2014]; Eshuis 2014; Gangavatiker 2011; Imamura 2014; Kakaei 2019; Kurahara 2011; Rebala 2012; Siripong 2012; Tamandl 2014; Tani 2006; Toyama 2021). The trial by Imamura 2014 presented the final results of both the preliminary report by Chijiiwa 2009 and Chijiwa's congress abstract Chijiiwa 2012, with overlapping patient samples across all three reports. Thus, we extracted only the data from Imamura 2014. Equally the congress abstract of Eshuis 2012 reported on the same trial population as the final report (Eshuis 2014) of which the data were finally extracted. We could not include the data of the trials by Rebala 2012 and Siripong 2012 in the quantitative analysis, since only congress abstracts were available and the presented data were not sufficient to estimate odds ratios for delayed gastric emptying. We contacted the trial authors for further information but did not get an answer from the authors of Rebala 2012; the authors of Siripong 2012 could not provide more data because the final results had not yet been published.
Risk of bias in included studies
Our assessment of risk of bias revealed a heterogenous picture of the design of the included trials.
We assessed publication bias by creating a funnel plot for the primary endpoint delayed gastric emptying (Figure 2). We only inspected the funnel plot for potential publication bias; we applied no formal tests since we included fewer than 10 trials in the analyses. There is some asymmetry in the funnel plot caused by the lack of smaller trials favouring retrocolic reconstruction.
2.

Funnel plot of comparison: 1 antecolic vs. retrocolic, outcome: 1.1 delayed gastric emptying (all definitions).
A summary of our risk of bias assessment is given in Figure 3 and Figure 4.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Random sequence generation
The randomisation process was adequately described in five trials as computer‐generated (Eshuis 2014; Gangavatiker 2011; Kakaei 2019; Tani 2006; Toyama 2021); in one trial, a non‐random approach was used (Tamandl 2014); the remaining two trials did not adequately specify the process of random sequence generation (Imamura 2014; Kurahara 2011).
Allocation concealment
Four trials described that allocation concealment was maintained by consecutive, sealed envelopes (Gangavatiker 2011; Imamura 2014; Kakaei 2019; Kurahara 2011); central randomisation was performed intraoperatively in two trials (Eshuis 2014; Toyama 2021). Thus, selection bias seemed to be low in seven trials. In one trial, the method of allocation concealment was not sufficiently described and thus judges as unclear risk of bias (Tani 2006). In the last trial with the non‐random approach (allocation to group according to birth date), allocation concealment was obviously not sufficient (Tamandl 2014).
Blinding
In one trial, blinding of patients and outcome assessors was described in the protocol, but not in the paper. Blinding of patients was confirmed by the authors when we asked them directly. The authors wrote that "the treating physicians were usually not aware of the route of gastroenteric anastomosis, but formally, there was no blinding" (Eshuis 2014). One trial reported that all perioperative clinical data were gathered by a person, who was completely blinded to the allocation for the follow‐up period of 30 days (Kakaei 2019). However, in this trial it was not stated that patients or other trial personnel was blinded. In another trial, patients and statisticians were blinded to treatment allocation, but it was not mentioned whether outcome assessment was blinded (Toyama 2021). None of the other trials accomplished blinding, although blinding of patients or outcome assessors would have been possible in this setting.
Incomplete outcome data
Two trials did not provide any information about length of follow‐up (Gangavatiker 2011; Kurahara 2011). Follow‐up varied in the other trials from 30 days after surgery (Kakaei 2019), to one year after surgery (Imamura 2014). In general, delayed gastric emptying appears in the first couple of days after the operation, so a long‐term follow‐up might not be critical; therefore, differences in follow‐up are not important to evaluate attrition bias in this trial. Dropouts occurred in some trials when allocation to the planned trial arm was not possible intraoperatively because of feasibility or anatomical reasons (Tamandl 2014), after randomisation when two patients in each group suffered severe sepsis (Imamura 2014), or after randomisation, because two patients in the antecolic group required prolonged intubation and intensive care unit stay. In one trial, three patients died in the antecolic group and one in the retrocolic group (Gangavatiker 2011). In the trial by Tamandl 2014, attrition bias was judged as unclear, since data for some endpoints of four patients (two in each group) were not reported due to early discharge of the patients. However, the patient flow was transparently reported in a flow chart. All other trials reported data on dropouts sufficiently, so they could be judged as low risk.
Selective reporting
A published protocol with pre‐specified endpoints was only available for one trial; outcomes in the protocol and in the published report were compared and the risk of selective reporting was declared as low (Eshuis 2014). One trial was registered in an openly accessible clinical trial registry and sufficient information on the prespecified endpoints were available, so the risk for selective reporting was judged as low (Tamandl 2014). Another trial was also registered in an openly accesible trial registry, but the definition of the outcome 'delayed gastric emptying' was changed during the course of the trial (Imamura 2014). Thus, this trial was judged to be at unclear risk of bias. One trial was registered in an openly accessible trial registry, but only information on the primary endpoint was provided. Thus, the risk of selective reporting was judged as unclear (Toyama 2021). For the other trials no protocol and no registry information was available. Therefore, outcomes listed in the methods section of the article were compared with the results reported in the results section, and risk for reporting bias was judged as unclear.
Other potential sources of bias
In the trial by Eshuis 2014, the NGT management (described above) was a potential source of other bias with unclear risk, since it interfered with the definition of the primary endpoint 'delayed gastric emptying according to the ISGPS definition'. The retrospective use of the ISGPS definition of delayed gastric emptying represented another potential source of other bias in the trial by Gangavatiker 2011 and Tamandl 2014.
Effects of interventions
See: Table 1
Primary outcome
Delayed gastric emptying
All eight trials (N = 818; antecolic reconstruction (AC) = 407; retrocolic (RC) = 411) reported delayed gastric emptying; there was little or no difference between the antecolic and retrocolic groups in the meta‐analysis (odds ratio (OR) 0.67; 95% confidence interval (CI) 0.41 to 1.09; P = 0.11; I² = 40%; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 1: Delayed gastric emptying (all definitions)
Five trials used the International Study Group of Pancreatic Surgery (ISGPS) definition of delayed gastric emptying prospectively, whereas Gangavatiker 2011 and Tamandl 2014 applied this definition retrospectively, after they had first applied the Johns Hopkins Criteria (Yeo 1993). Tani 2006 defined delayed gastric emptying in a different way: (1) prolonged aspiration of 500 mL per day from a nasogastric tube left in place for 10 days (delayed gastric emptying (DGE10)), (2) need for reinsertion of a nasogastric tube, (3) failure of unlimited oral intake by the 14th postoperative day (DGE14).
We performed a sensitivity analysis that only included trials that assessed delayed gastric emptying according to the ISGPS definition. There was little or no difference between the AC group (101/321 participants; 31%) and the RC group (118/329; 36%) when only trials with the ISGPS definition were included (OR 0.75; 95% CI 0.49 to 1.14; P = 0.18; I² = 14%; 5 trials; Analysis 1.2). There was also no relevant difference seen in the rate of clinically relevant delayed gastric emptying (ISGPS Grade B/C) between the AC and the RC groups (OR 0.71; 95% CI 0.42 to 1.20; P = 0.20; I² = 19%; 5 trials; N = 650; AC = 321; RC = 329; Analysis 1.3).
1.2. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 2: Delayed gastric emptying (ISGPS definition)
1.3. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 3: Clinically relevant delayed gastric emptying (ISGPS grade B/C)
We performed a subgroup analysis for delayed gastric emptying that only included trials in which pylorus‐preserving partial pancreaticoduodenectomy (pPD) was performed (Imamura 2014; Kakaei 2019; Tamandl 2014; Tani 2006). There was no relevant difference between the AC and RC groups (OR 0.43; 95% CI 0.17 to 1.07; P = 0.07, I² = 33%; analysis not shown).
We could not perform the other planned subgroup analyses. No trials reported only classical Whipple's procedure and in the trials reporting both, there were no individual data for those subgroups reported (Eshuis 2014; Gangavatiker 2011). All included trials used single‐loop reconstruction, therefore, we could not compare single‐loop versus Roux‐en‐Y reconstruction. We could not perform the subgroup analysis for underlying diagnoses, since the trials did not provide these individual patient data.
Fixed‐effect sensitivity meta‐analysis also showed a small or no different overall effect estimate for delayed gastric emptying between the two reconstruction techniques (OR 0.74; 95% CI 0.53 to 1.02; P = 0.06; I² = 40%; 8 trials; analysis not shown).
We observed a statistical heterogeneity of 14% to 40% in the I² statistic of the analyses on delayed gastric emptying; besides the different endpoint definitions, one reason might be that different drugs were administered, such as, octreotide and erythromycin. Somatostatin was not administered perioperative in most trials (Imamura 2014; Kurahara 2011; Tamandl 2014; Tani 2006; Toyama 2021); in others, it was administered for a soft pancreas or a non‐dilated pancreatic duct (Eshuis 2014; Gangavatiker 2011; Kakaei 2019). Prokinetics were not applied routinely in most trials (Imamura 2014; Kurahara 2011; Tamandl 2014; Tani 2006; Toyama 2021), and on demand in the others (Eshuis 2014; Gangavatiker 2011). One trial did not provide information on the use of prokinetics (Kakaei 2019).
Secondary outcomes
Postoperative mortality
Mortality was analysed using risk differences (RD) because in two trials both groups had zero events, which leads to a computational error when summarising these results as odds ratios (Kurahara 2011; Toyama 2021). Mortality rates were provided by all trials and showed similar ranges in the AC group (0% to 9.4% (Kurahara 2011; Tani 2006 and Gangavatiker 2011, respectively)) and the RC group (0% to 6.6% (Kurahara 2011 and Kakaei 2019, respectively)), with a mortality across all trials of 5.7%. There were no differences in mortality between the two groups (risk difference (RD) ‐0.00; 95% CI ‐0.02 to 0.01; P = 0.85; I² = 0%; high‐certainty evidence; Analysis 1.4; 8 trials; 818 participants).
1.4. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 4: Postoperative mortality
Postoperative pancreatic fistula
All eight trials reported fistula rates (N = 818; AC = 108/407; RC = 110/411). The fistula rate showed no relevant difference between groups (OR 1.01; 95% CI 0.73 to 1.40; P = 0.94; I² = 0%; low‐certainty evidence; Analysis 1.5). There was also no relevant difference in a subgroup of trials in which the ISGPS definition for pancreatic fistula was prospectively applied (OR 1.05; 95% CI 0.75 to 1.49; P = 0.77; I² = 0%; 4 trials; N = 620; AC = 99/306; RC = 99/314; Analysis 1.6). There was also little or no difference between AC and RC reconstruction in the same four trials in the clinically relevant (Grade B/C) postoperative pancreatic fistula rate (OR 1.00; 95% CI 0.66 to 1.53; P = 1.00; I² = 0%; Analysis 1.7).
1.5. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 5: Postoperative pancreatic fistula
1.6. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 6: Postoperative pancreatic fistula ISGPF definition
1.7. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 7: Clinically relevant postoperative pancreatic fistula ( ISGPF grade B/C)
Postoperative haemorrhage
Six trials addressed postoperative haemorrhage (N = 742; AC = 22/368; RC = 25/374). The pooled result was not relevantly different (OR 0.87; 95% CI 0.47 to 1.59; P = 0.65; I² = 0%; low‐certainty evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 8: Postoperative hemorrhage
Bile leakage
Bile leakage was reported by seven of eight included trials (N = 606; AC = 12/304; RC = 13/302). The risk of bile leakage did not differ substantially between the two groups (OR 0.82; 95% CI 0.35 to 1.91; P = 0.65; I² = 0%; Analysis 1.9), with an overall occurrence of 4.1% in both groups.
1.9. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 9: Bile leakage
Intra‐abdominal fluid collection or abscess
Rates of intra‐abdominal fluid collection or abscess were reported by seven trials (N = 788; AC = 62/392; RC = 57/396). There was little or no difference between groups (OR 1.11; 95% CI 0.71 to 1.74; P = 0.65; I² = 9%; low‐certainty evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 10: Intra‐abdominal fluid collection/abscess
Reoperation rate
Five trials reported this outcome (N = 682; AC = 14/334; RC = 23/348). There was little or no difference between groups (OR 0.68; 95% CI 0.34 to 1.36; P = 0.28; I² = 0%; Analysis 1.11).
1.11. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 11: Reoperation rate
Duration of operation
Sufficient information on duration of the operation was provided in all eight included trials (N = 822; AC = 409; RC = 413; 4 additional patients from the trial by Tamandl 2014, for which only intraoperative data were available). Mean operating time (in minutes) showed no substantial difference between the groups (MD ‐4.77; 95% CI ‐15.85 to 6.31; P = 0.40; I² = 1%; Analysis 1.12). Mean operating time (± standard deviation) varied from 270 ± 35.4 to 564 ± 124.8 minutes in the AC group (Kakaei 2019 and Toyama 2021, respectively); and 279.6 ± 46.2 to 605 ± 150.3 minutes in the RC group (Kakaei 2019 and Kurahara 2011, respectively).
1.12. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 12: Duration of operation
Intraoperative blood loss
Intraoperative blood loss was reported in seven trials (N = 758; AC = 373; RC = 385) and showed little or no difference between the AC and RC groups (MD 5.21; 95% CI ‐52.85 to 63.28; P = 0.86; I² = 0%; Analysis 1.13). The mean intraoperative blood loss ranged from a minimum of 466 ± 147 mL in the AC group of the trial by Kakaei 2019 to a maximum of 1621 ± 758 mL in the RC group of the trial by Kurahara 2011.
1.13. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 13: Intraoperative blood loss
Length of postoperative hospital stay
All eight included trials reported length of hospital stay (in days; N = 818; AC = 407; RC = 411). Both groups showed similar results (MD ‐0.21; 95% CI ‐1.41 to 0.99; P = 0.73; I² = 22%; low‐certainty evidence; Analysis 1.14) with a mean length of hospital stay (± standard deviation) between 8.9 ± 1.5 and 36 ± 22.25 days in the AC group (Kakaei 2019 and Imamura 2014, respectively) and 8.9 ± 1.5 and 47.7 ± 37.7 days in the RC group (Kakaei 2019 and Tani 2006, respectively). These large differences might be explained by the variations in discharge policies between the different countries and hospitals and the different periods of trial conduct.
1.14. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 14: Length of hospital stay
The sensitivity analysis excluding trials that reported medians and (interquartile) ranges or standard errors instead of means and standard deviations did not change the overall result or the direction of the meta‐analysis (MD ‐3.85; 95% CI ‐10.64 to 2.94; P = 0.27; I² = 72%; analysis not shown).
Time to nasogastric tube (NGT) removal (in days) after surgery
The length of time before the NGT was removed postoperatively was reported in all eight trials (N = 818; AC = 407; RC = 411) and showed little or no difference between the groups (MD 0.07; 95% CI ‐0.30 to 0.44; P = 0.71; I² = 34%; Analysis 1.15). However, the mean duration varied widely, from one to five days in the AC group and 1 to 19 days in the RC group.
1.15. Analysis.

Comparison 1: antecolic vs. retrocolic, Outcome 15: Length of postoperative NGT
As mentioned above, different standards were applied to the removal of the nasogastric tube, which might explain the moderate heterogeneity in the I² statistic. Intertrial comparability cannot be guaranteed for this outcome.
Again, the sensitivity analysis excluding trials that reported medians and (interquartile) ranges or standard errors instead of means and standard deviations did not change the overall result of the meta‐analysis (MD ‐1.24; 95% CI ‐4.47 to 1.99; P = 0.45; I² = 69%; analysis not shown).
Quality of life
None of the main trial publications reported data on quality of life after surgery. However, quality of life data for a subset of the patients in the trial by Eshuis 2014, more specifically those who were recruited at the initiating centre of this trial, were reported in a separate report (Eshuis 2015 [reference listed under Eshuis 2014]; AC = 35; RC = 38). Quality of life was assessed by the EQ‐5D TM questionnaire, the European Organization for Research and Treatment of Cancer QLQ‐C30 and QLQ‐PAN26 questionnaires, and the Gastrointestinal Quality of Life Index preoperatively, and at two, four, and 12 weeks after surgery. No differences in quality of life between the AC and RC groups were assessed at any time point. However, patients with delayed gastric emptying had worse scores compared to patients without delayed gastric emptying at two weeks after surgery.
Discussion
Summary of main results
Over the last few years, there have been controversial discussions about reconstruction of bowel continuity after partial pancreaticoduodenectomy (pPD) via an antecolic or retrocolic route, and its potential influence on the incidence of delayed gastric emptying. Several randomised controlled trials (RCTs) and non‐randomised studies that examined reconstruction after classical, pylorus‐preserving and pylorus‐resecting Whipple have been performed, but results were inconclusive. Likewise, results of previously published meta‐analyses about the best type of reconstruction showed conflicting evidence (Ramia 2013; Su 2012; Zhou 2015). Thus, an updated meta‐analysis was needed to include the results of the most recent RCTs, which had not been included in previous meta‐analyses. The qualitative and quantitative summary of existing results in this systematic review and meta‐analysis showed no evidence of superiority of one procedure over the other as indicated in the Table 1 for the main outcome parameters. Furthermore, clinically relevant delayed gastric emptying (defined as grade B/C according to the International Study Group of Pancreatic Surgery (ISGPS) definition) and a subgroup analysis, including only trials that used the ISGPS definition, resulted in no relevant difference between the two groups either.
The type of reconstruction (antecolic (AC) versus retrocolic (RC)) also had no impact on mortality, further morbidity, length of hospital stay, or quality of life after pPD.
Overall completeness and applicability of evidence
The eight included trials, that could be identified by the systematic literature search, provided comprehensive data on all of our predefined outcome parameters except of quality of life. Quality of life was only reported on a subset of patients from one trial (Eshuis 2014), and thus, the evidence for this outcome is by far weaker than for the other outcome parameters. Since the trials were conducted in different international regions, the results are applicable to a large population of patients undergoing pPD. Furthermore, we performed a subgroup analysis for pylorus‐preserving pPD and could not find a relevant difference for delayed gastric emptying in this subgroup, which strengthens the evidence for this subgroup. Equally, the sensitivity analyses did not change the overall results of our meta‐analyses for the primary outcome parameter.
Period and frequency of follow‐up was not described sufficiently in several trials and differed between the trials, but for the primary endpoint, delayed gastric emptying, the immediate postoperative period seems to be the most relevant, so that a severe attrition bias concerning delayed gastric emptying did not have to be suspected. However, long‐term differences between the two procedures may have been missed due to the short follow‐up in most of the trials. For instance, Imamura 2014 showed that postoperative weight gain was quicker, and the retrocolic group recovered almost back to their preoperative weight after one year, whereas weight loss was prolonged in the antecolic group. On the other hand, in the most recent trial by Toyama 2021 there were no clinically relevant differences in postoperative nutritional status during the six‐month follow‐up. Nevertheless, such long‐term differences might be crucial in the discussion about the optimal reconstruction technique and should be addressed in future trials.
Our findings are in line with the two largest multicentre randomised controlled trials (RCTs), in which the incidence of delayed gastric emptying was examined according to the type of bowel reconstruction (Eshuis 2014; Toyama 2021). In both trials, neither the overall delayed gastric emptying (Eshuis 2014: AC versus RC: 61% versus 60%, P = 0.89; Toyama 2021: AC versus RC: 12.6% versus 15.6%, P = 0.058), nor the clinically relevant delayed gastric emptying (Eshuis 2014: AC versus RC: 34% versus 36%; Toyama 2021: AC versus RC: 9.7% versus 12.9%) differed between the two groups, but the absolute rates differed substantially between the two trials. Since these trials had the largest sample sizes, they also achieved the highest weight in the meta‐analysis. Thus, both trials had a strong influence on the results of our meta‐analysis. One explanation for the substantial differences of the rates of delayed gastric emptying between the two trials is the nasogastric tube (NGT) management policy in the trial by Eshuis 2014. In this trial all patients received a NGT postoperatively for a median of three days, leading to high rates in both trial groups, because of the interference with the ISGPS definition, which is based on the presence of a NGT after postoperative day three. Furthermore, the high rate of octreotide prophylaxis, i.e. approximately two thirds of patients received octreotide, might have contributed to the high rates of delayed gastric emptying, because octreotide reduces gastrointestinal motility (Kollmar 2008).
Several reports have shown that routine nasogastric drainage after pPD is not necessary and routine postoperative NGT does not represent the current standard in many centres that specialise in pancreatic surgery (Choi 2011; Fisher 2011; Robertson 2012). Therefore, the impact of the reconstruction method might be blurred by the excessive rates of delayed gastric emptying in the largest trial by Eshuis 2014. This presumption is corroborated by the fact that the results of analysis on delayed gastric emptying in trials using the ISGPS definition turned to statistical significance, when the trial by Eshuis 2014 was excluded from the analysis (4 trials: odds ratio (OR) 0.57; 95% confidence interval (CI) 0.33 to 0.97; P = 0.04, I2 = 0%; analysis not shown).
Since all of the meta‐analyses of delayed gastric emptying (overall delayed gastric emptying, subgroup ISGPS definition of delayed gastric emptying, and clinically relevant delayed gastric emptying) showed lower rates in the AC route, the current meta‐analysis might still be underpowered to demonstrate a potential difference. However, with the increasing sample size during the current update the statistical power and precision are also increasing, and still no relevant difference in delayed gastric emptying after antecolic and retrocolic reconstruction can be suggested.
Quality of the evidence
We included eight trials from different countries and continents with a total of 818 participants in this review. Interstudy results were inconsistent. On one hand, there were obvious sources of potential bias in the included trials, e.g. missing blinding of outcome assessors and patients, in part small sample sizes with two trials being stopped at interim analyses, and an unclear risk of reporting bias in several trials. On the other hand, this systematic review and meta‐analysis has been performed with a clear methodology, thus creating a higher level of evidence through pooling of data from all available RCTs on this issue. With the additional information of the data included in the current update, the overall certainty of the evidence according to the GRADE criteria was still only low for the main endpoints, because it had to be downgraded due to the above‐mentioned potential risk of bias and some imprecision of the results with confidence intervals crossing the 'no difference' border and margins that cannot rule out a relevant effect (Table 1). Regarding mortality, the certainty of the evidence according to GRADE is high, because the potential risk of bias is very unlikely to influence this hard endpoint and the results were more precise than for the other outcome parameters.
Potential biases in the review process
Well‐conducted systematic reviews can allow generalisation of scientific findings and increase power and precision in estimating effects and risks (Mulrow 1994). They allow for a more objective appraisal of the evidence, which may lead to resolution of uncertainty and disagreement (Egger 1997). Obviously, the quality of a systematic review is directly depending on the quality of the reviewed studies. In spite of the sources of clinical and methodological heterogeneity already mentioned, inclusion criteria and baseline population characteristics were adequately comparable between included trials.
Even though a comprehensive and systematic literature search of three large databases was conducted and reference lists of relevant publications were screened, some relevant trials might have been missed by the search. Concerning the data of the included trials, most publications presented sufficient information on the prespecified endpoints and further relevant information was achieved by contacting the respective authors. Data accuracy was secured by double checks by two independent reviewers.
For the continuous outcomes, some trials only reported medians and (interquartile) ranges or standard errors instead of means and standard deviations. Means and standard deviations were calculated by the methods described by Higgins 2021, Hozo 2005 and Wan 2014. Since reporting of medians and (interquartile) ranges in primary reports is suspicious of skewed data, there might remain some bias in the calculated means and standard deviations.
A further potential source of bias in the review process that has to be mentioned is the variation of the definition of delayed gastric emptying as the internationally accepted and scaled ISGPS definition was published in 2007 (Wente 2007a), when some of the included trials had already been started. The use of different definitions represent one restriction of the performed meta‐analysis, since it has been shown that applying different definitions of delayed gastric emptying on the same population introduces a large variation in the incidence of delayed gastric emptying, from 5.9% to 14.7% (Butturini 2006). Five trials prospectively used the definition of the most widely accepted consensus definition of the ISGPS, based on the duration of postoperative (naso)gastric drainage and return to solid food (Eshuis 2014; Imamura 2014; Kakaei 2019; Kurahara 2011; Toyama 2021). Two trials retrospectively applied this ISGPS definition (Gangavatiker 2011; Tamandl 2014), after they had first applied the Johns Hopkins criteria (Yeo 1993); another trial used a third definition of delayed gastric emptying similar to the Johns Hopkins criteria (Tani 2006). This heterogeneous definition of the primary endpoint may have caused some bias and the substantial heterogeneity in the meta‐analyses of delayed gastric emptying. However, a subgroup analysis was performed that only included trials primarily using the ISGPS definition prospectively. Moreover, perioperative management, in terms of the use, duration and indication of nasogastric tubes, influences the occurrence of delayed gastric emptying; in this case, inter‐study heterogeneity is obvious. Furthermore, prokinetics and somatostatin were applied as part of various standardised or non‐standardised therapeutic concepts in the different trials. Due to the lack of reporting of indication, duration, and dosage of these medications, an inter‐study heterogeneity must be suspected, which might decrease the external validity of our results.
Agreements and disagreements with other studies or reviews
One previously published meta‐analysis based on two RCTs and three non‐randomised studies favoured an AC reconstruction (Su 2012). Another meta‐analysis including four RCTs and seven non‐randomised studies came to the conclusion that the route of gastroenteric reconstruction with a benefit in delayed gastric emptying cannot currently be determined (Ramia 2013). Both studies were published before five RCTs, including the three largest RCTs, addressing this question were available (Eshuis 2014; Imamura 2014; Kakaei 2019; Tamandl 2014; Toyama 2021). Two meta‐analyses on this issue were published in 2015 (Bell 2015; Zhou 2015). One of them included four non‐randomised studies (Bell 2015). Neither of them included the RCT by Kurahara 2011, both of them included the preliminary report of Chijiiwa 2009 and the final results of Imamura 2014 with their overlapping patient samples, which was not addressed in the review. The review including only RCTs concluded that the route of gastroenteric reconstruction had no impact on delayed gastric emptying, thus, our results agreed (Zhou 2015). On the other hand, Bell 2015 concluded that AC reconstruction was associated with a lower incidence of delayed gastric emptying, which might be because they included non‐randomised studies.
Since the first version of the current Cochrane Review was published, four other meta‐analyses on this topic (Imamura 2016; Joliat 2016; Qian 2016; Qiu 2019) and one network meta‐analysis on various techniques of gastro‐enteric reconstruction methods (Kamarajah 2020) were published. Two of these meta‐analysis included both, RCTs and non‐randomised studies and led to a result in favour of the AC route in the analysis of all study types, but no relevant difference in the subset of RCTs (Imamura 2016; Qiu 2019). In general, it is recommended that RCTs and non‐randomised studies should not be pooled together in a meta‐analysis, since this can lead to biased results. Both other meta‐analysis, of which one only included five RCTs, did not show any differences between AC and RC reconstruction (Joliat 2016; Qian 2016). The network meta‐analysis by Kamarajah 2020 led to the conclusion that AC Billroth‐II reconstruction is associated with the lowest rates of delayed gastric emptying. However, there were no noteworthy differences in the pairwise comparisons in line with the findings of our current meta‐analysis.
None of the previous systematic reviews and meta‐analysis included the two most recent trials (Kakaei 2019; Toyama 2021), which are now included in this updated version.
In summary, the results of previously published meta‐analyses are conflicting and their results must be interpreted with caution, due to the inclusion of non‐randomised trials and other potential sources of bias in the review process.
Authors' conclusions
Implications for practice.
In summary, the current evidence suggests that antecolic (AC) reconstruction after partial pancreaticoduodenectomy (pPD) results in little to no relevant difference in delayed gastric emptying and overall morbidity, and probably does not reduce mortality. Delayed gastric emptying remains a burdensome and frequent complication after pPD, with its causative factors remaining still largely unknown. As shown by Qu and colleagues in a systematic review and meta‐analysis of the risk factors for delayed gastric emptying, preoperative diabetes, pancreatic fistulas, and postoperative complications were predictive risk factors for delayed gastric emptying (Qu 2013). As clinically relevant delayed gastric emptying often occurs in patients also suffering from other intra‐abdominal complications, such as postoperative pancreatic fistula, bile leakage, and abscess, prevention of these complications is necessary to reduce the incidence of delayed gastric emptying. Currently, gastroenteric reconstruction after pPD might be routed according to the individual hospital standard and preference of the surgeon.
Implications for research.
Concerning the difference of 6% in the occurrence of delayed gastric emptying favouring AC reconstruction, the previous trials and even this strictly conducted meta‐analysis might have been underpowered to demonstrate a benefit. Furthermore, the differences in nasogastric tube (NGT) management might have influenced the results of this meta‐analysis. Therefore, a large‐scale randomised controlled trial (RCT) with clearly defined NGT management, according to modern treatment protocols, could potentially elucidate the impact of antecolic versus retrocolic reconstruction. However, with the growing certainty of the evidence with the additional trials that have been included in this update and the relatively small difference, such a trial would need to include several thousand patients, which is hardly achievable in a pancreatic surgical trial. Thus, future trials should rather focus on long‐term follow‐up and patient‐relevant outcomes such as quality of life, to elucidate potential differences between these two procedures, which might have been missed by previous trials.
Furthermore, different surgical approaches to reduce the rates of delayed gastric emptying after PD should be assessed in future RCTs. One surgical variation that has been discussed in this respect is single‐loop versus dual‐loop reconstruction after pancreaticoduodenectomy (PD). The results of a recent meta‐analysis did not show a benefit in terms of delayed gastric emptying or other postoperative complications; however, it did find a prolonged operation time with dual‐loop reconstruction (Klaiber 2015). Another aspect, which might have an impact on delayed gastric emptying is pylorus‐resection versus pylorus‐preservation. In 2018, the results of the PROPP trial and a meta‐analysis on this issue were published (Hackert 2018; Klaiber 2018). But neither the trial, nor the meta‐analysis of RCTs could corroborate the expectations of reduced delayed gastric emptying by pylorus‐resection as suggested by former studies.
What's new
| Date | Event | Description |
|---|---|---|
| 12 July 2021 | New citation required but conclusions have not changed | Two trials with an additional 244 patients have been identified within an updated literature search in July 2021 and have been included in the updated version of the review. The inclusion of these trials did not change the direction or clinical implication of the findings or the certainty of the evidence. |
| 12 July 2021 | New search has been performed | Two additional trials with an additional 244 patients have been identified within an updated literature search in July 2021 and have been included in the updated version of the review. The inclusion of these trials did not change the direction or clinical implication of the findings or the certainty of the evidence. |
History
Protocol first published: Issue 9, 2015 Review first published: Issue 9, 2016
Acknowledgements
We thank Dr. Cathy (Yuhong) Yuan for helping to construct, run, and update the systematic literature search. We thank Heather Maxwell for copy‐editing the review. We acknowledge the help and support of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Review Group (now the Cochrane Gut Group).
The methods section of this review is based on a standard template used by the Cochrane Upper Gastrointestinal and Pancreatic Diseases Review Group (now Cochrane Gut).
Appendices
Appendix 1. Glossary of terms
Abscess: A collection of pus that has built up within the body.
Adenocarcinoma: A type of cancer that can occur in several parts of the body with a glandular origin.
Adjuvant chemotherapy: Chemotherapy given after, and in addition to, resection of the tumour.
Anastomosis: Connection between two organs (e.g. stomach and small intestine), created by surgery.
Anterior: Situated in front of something.
Asymptomatic: Showing no clinical signs of disease or condition.
Benign: Tumours lacking the ability to invade neighbouring tissue or to metastasise.
Biliary: Anatomical term related to the bile secretion and ducts.
Chemotherapy: Medication administered to treat cancer.
Duodenum: The first part of the small intestine, connecting the stomach to the jejunum.
Erythromycin: An antibiotic increasing the motility of the intestine.
Etiology: Factors causing an illness.
Gastric: Related to the stomach.
Gastro‐/duodenojejunostomy: Surgical connection between the stomach or duodenum and the small intestine.
Gastroenteric: Connection between the stomach and the small intestine.
Haemorrhage: Bleeding.
Intra‐abdominal: Within the abdomen.
Intraductal: Within a duct.
Jejunum: Second part of the small intestine, connecting the duodenum to the ileum.
Mesocolic window: Artificial pathway through the mesocolon.
Mesocolon: Fatty tissue carrying the blood vessels that supply the colon.
Morbidity: Consequences of a procedure impairing the subject's health.
Mortality: A measure of the number of deaths in a given population.
Motilin‐agonist: Drug acting similar to an intestinal hormone, which promotes bowel movements.
Neoplasia: Tumour.
Pancreatic fistula: Opening between pancreas and another organ or space, allowing leakage of pancreatic secretions from the pancreatic gland.
Pancreaticoduodenectomy: Partial surgical removal of the pancreas, with the attached duodenum.
Pancreaticojejunal: Referring to the pancreatic remnant and the second part of the small intestine.
Pancreatitis: Inflammation of the pancreas.
Perioperative: In direct temporary context to surgery (before, during, and after).
Posterior: Situated behind something.
Premalignant: Precancerous.
Prokinetic: Stimulating the movements of the the oesophagus, stomach, and intestines.
Prophylaxis: Prevention.
Pylorus: Circular muscle building the connection between the stomach and the small intestine.
Resectable: Able to be removed by surgery.
Somatostatin analogues: Proteins that slow down the production of hormones, the emptying of stomach and bowel, and the release of hormones from the pancreas.
Torsion: Twisting of a structure (e.g. intestine).
Transverse colon: The middle part of the colon.
Appendix 2. CENTRAL search strategy (ovid)
exp pancreaticoduodenectomy/
exp pancreaticojejunostomy/
pancreatectomy/
(pancreaticoduodenectom* or pancreatoduodenectom* or duodenopancreatectom* or pancreatectom* or hemipancreatectom* or pancreaticojejunostom* or pancreatojejunostom*).tw,kw.
(pancreas* adj5 (duodenectom* or jejunostom*)).tw,kw.
(pancrea* adj3 (PPD or surger* or surgical or operat* or resect* or remov* or recis*)).tw,kw.
(pancreas* adj5 PPPD).tw,kw.
Whipple.tw,kw.
(Pylorus adj5 Preserv*).tw,kw.
(pancreatogastrostom* or (pancreas* adj5 gastrostom*)).tw,kw.
or/1‐9
(antecolic or retrocolic or mesocolic).tw,kw.
11 and 12
Appendix 3. MEDLINE search strategy (ovid)
exp pancreaticoduodenectomy/
exp pancreaticojejunostomy/
pancreatectomy/
(pancreaticoduodenectom* or pancreatoduodenectom* or duodenopancreatectom* or pancreatectom* or hemipancreatectom* or pancreaticojejunostom* or pancreatojejunostom*).tw,kw.
(pancreas* adj5 (duodenectom* or jejunostom*)).tw,kw.
(pancrea* adj3 (PPD or surger* or surgical or operat* or resect* or remov* or recis*)).tw,kw.
(pancreas* adj5 PPPD).tw,kw.
Whipple.tw,kw.
(Pylorus adj5 Preserv*).tw,kw.
(pancreatogastrostom* or (pancreas* adj5 gastrostom*)).tw,kw.
or/1‐9
(antecolic or retrocolic or mesocolic).tw,kw.
11 and 12
exp animals/ not humans/
13 not 14
Appendix 4. Embase search strategy
exp pancreaticoduodenectomy/
exp pancreaticojejunostomy/
exp pancreatectomy/
(pancreaticoduodenectom* or pancreatoduodenectom* or duodenopancreatectom* or pancreatectom* or hemipancreatectom* or pancreaticojejunostom* or pancreatojejunostom*).tw,kw.
(pancreas* adj5 (duodenectom* or jejunostom*)).tw,kw.
(pancrea* adj3 (PPD or surger* or surgical or operat* or resect* or remov* or recis*)).tw,kw.
(pancreas* adj5 PPPD).tw,kw.
Whipple.tw,kw.
(Pylorus adj5 Preserv*).tw,kw.
(pancreatogastrostom* or (pancreas* adj5 gastrostom*)).tw,kw.
or/1‐10
(antecolic or retrocolic or mesocolic).tw,kw.
11 and 12
exp animal/ not human/
13 not 14
Data and analyses
Comparison 1. antecolic vs. retrocolic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Delayed gastric emptying (all definitions) | 8 | 818 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.41, 1.09] |
| 1.2 Delayed gastric emptying (ISGPS definition) | 5 | 650 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.49, 1.14] |
| 1.3 Clinically relevant delayed gastric emptying (ISGPS grade B/C) | 5 | 650 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.20] |
| 1.4 Postoperative mortality | 8 | 818 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 1.5 Postoperative pancreatic fistula | 8 | 818 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.73, 1.40] |
| 1.6 Postoperative pancreatic fistula ISGPF definition | 4 | 620 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.75, 1.49] |
| 1.7 Clinically relevant postoperative pancreatic fistula ( ISGPF grade B/C) | 4 | 620 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.53] |
| 1.8 Postoperative hemorrhage | 6 | 742 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.47, 1.59] |
| 1.9 Bile leakage | 7 | 606 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.35, 1.91] |
| 1.10 Intra‐abdominal fluid collection/abscess | 7 | 788 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.71, 1.74] |
| 1.11 Reoperation rate | 5 | 682 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.34, 1.36] |
| 1.12 Duration of operation | 8 | 822 | Mean Difference (IV, Random, 95% CI) | ‐4.77 [‐15.85, 6.31] |
| 1.13 Intraoperative blood loss | 7 | 758 | Mean Difference (IV, Random, 95% CI) | 5.21 [‐52.85, 63.28] |
| 1.14 Length of hospital stay | 8 | 818 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐1.41, 0.99] |
| 1.15 Length of postoperative NGT | 8 | 818 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.30, 0.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Eshuis 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): 6 (3 AC; 3 RC), due to total pancreatectomy Losses to follow‐up: no Intention‐to‐treat analysis: yes Description of sample size calculation: yes Duration of trial: 2009 to 2011 |
|
| Participants | Number: 246 (AC: 121; RC: 125) Age: 65.4± 9.0 (mean in the AC group); 65.2± 10.3 (mean in the RC group) Sex ratio (M/F): 2.2 (AC), 1.2 (RC) Inclusion criteria: Patients ≥ 18 years scheduled to undergo PD Exclusion criteria: other surgical procedures besides PD Disease type: pancreatic adenocarcinoma (AC: 49; RC: 34), ampullary adenocarcinoma (AC: 20; RC: 21), distal bile duct adenocarcinoma (AC: 13; RC: 21), duodenal adenocarcinoma (AC: 6; RC: 2), other (pre)malignant lesions (AC: 8; RC: 10), chronic pancreatitis (AC: 2; RC: 7) other benign lesions (AC: 2; RC: 3) Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction (AC): the anastomosis was positioned anterior to the transverse colon. No adjunctive measures to enhance gastric emptying, such as pyloric stretching, were taken.
Retrocolic reconstruction (RC): the duodenal stump (or distal stomach) was brought down through a separate opening in the transverse mesocolon, at the left side of the middle colic artery. It was anastomosed end‐to‐side to the jejunum. The stomach was fixed to the mesocolon, to prevent herniation of the loop, thereby ensuring that the gastroenteric anastomosis was not positioned in the same abdominal compartment as the pancreaticojejunostomy and hepaticojejunostomy. Administration of prokinetics: only as needed Administration of somatostatin: for the indication of soft pancreatic tissue or a non‐dilated pancreatic duct. Standard management of NGT: All patients received an NGT. The NGT had to be removed before or on the third POD, or when daily output had fallen below 300 mL. |
|
| Outcomes | Primary outcome: postoperative incidence of delayed gastric emptying, according to the ISGPS consensus definition, and incidence of 'primary' delayed gastric emptying (delayed gastric emptying occurring in the absence of other intra‐abdominal complications). Secondary outcomes: morbidity, mortality, length of hospital stay, pancreatic fistula, haemorrhage, bile leakage, chylous ascites, intra‐abdominal abscess, wound infection, nonsurgical complications (pneumonia, other pulmonary complications, myocardial infarction, other cardiac complications, urinary tract infections, cerebrovascular accidents). Re‐laparotomies for any cause and hospital readmissions within 30 days of discharge | |
| Notes | Country: the Netherlands The authors declared no conflicts of interest. No financial support had been taken. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "computer‐generated random‐numbers, blocked randomisation stratified to trial centre" |
| Allocation concealment (selection bias) | Low risk | quote: "central randomisation performed intraoperatively" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | quote: "treating physicians were not blinded for the treatment allocation" comment: patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | comment: no blinding of outcome assessors described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | comment: missing outcome data balanced in numbers across intervention groups, with same reasons for missing data across groups, transparently described in the patient flow chart. |
| Selective reporting (reporting bias) | Low risk | comment: trial registered at the Dutch primary register for clinical trials, trial protocol with pre‐specified endpoints available |
| Other bias | Unclear risk | Standard NGT management with potential interference to ISGPS definition of DGE. |
Gangavatiker 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): 4 died (3 AC; 1 RC) Losses to follow‐up: no Intention‐to‐treat analysis: not stated Description of sample size calculation: yes Duration of trial: September 2006 to November 2008 |
|
| Participants | Number: 72 ‐ 4 (AC: 35 ‐ 3; RC: 37 ‐ 1) Age: 52.8± 11.6 (mean in the AC group); 50.8± 10.6 (mean in the RC group) Sex ratio (M/F): 2.2 (AC); 2.6 (RC) Inclusion criteria: patients < 70 years with a good performance status who underwent a classical Whipple’s PD or a PPPD Exclusion criteria: patients with metastatic and locally advanced disease, peptic ulcer disease, gastric outlet obstruction, tumour infiltration into the stomach, previous gastric surgery, and poorly controlled diabetes mellitus (long standing history of diabetes with poor glycaemic control (HbA1c > 7.5%) or systemic complications of diabetes) Disease type: carcinoma (periampullary, duodenal, and pancreatic head), neuroendocrine tumours, or chronic pancreatitis Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction: the jejunal loop was brought up anterior to the transverse colon and anastomosed to the duodenum or stomach
Retrocolic reconstruction: the jejunal loop was anastomosed to the duodenum or stomach through a separate mesocolic window on the left of the middle colic vessels. Administration of prokinetics: only as needed Administration of somatostatin: for the indication of soft pancreas and small ducts Standard management of NGT: The NGT was removed if the output was < 200 mL on two consecutive days. |
|
| Outcomes | Primary outcome: delayed gastric emptying, defined by the Johns Hopkins criteria; ISGPS criteria were applied retrospectively Secondary outcome: postoperative NGT, pancreatic fistula, mortality, haemorrhage, abscess, operation time, reoperation rate, length of hospital stay |
|
| Notes | Country: India The authors declared no conflicts of interest. No funding declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "computer‐generated random‐numbers" |
| Allocation concealment (selection bias) | Low risk | quote: "sealed envelope technique" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | comment: blinding not described, probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | comment: blinding not described, probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | comment: transparent patient flow chart available, 3 patients died in the AC group, 1 patient died in the RC group; otherwise, all patients completed the trial and there were no further losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | no trial registration, no published protocol |
| Other bias | Unclear risk | ISGPS criteria applied retrospectively |
Imamura 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): 4 (2 AC; 2 RC) Losses to follow‐up: 45 patients (26 AC; 19 RC) lost to follow‐up after 1 year Intention‐to‐treat analysis: not stated Description of sample size calculation: yes Duration of trial: March 2005 to July 2011 |
|
| Participants | Number: 120‐4 patients (58 AC; 58 RC) Age: 70.0 (36 to 86, median in the AC group); 69.0 (46 to 86, median in the RC group) Sex ratio (M/F): 1.6 (AC); 1.2 (RC) Inclusion criteria: PPPD Exclusion criteria: patients who underwent pancreaticoduodenectomy with gastric resection, subtotal stomach‐preserving PD (SSPPD), additional hepatic resection and total pancreatectomy, old cerebral infarction, post spinal cord injury state, haemodialysis for chronic renal failure Disease type: pancreatic cancer (17 AC; 16 RC), bile duct cancer (17 AC; 20 RC), ampullary carcinoma (4 AC; 9 RC), duodenal cancer (2 AC; 0 RC), cystic tumour (IPMN/MCN, 11 AC; 6 RC), chronic pancreatitis (2 AC; 16 RC), benign bile duct disease (2 AC; 2 RC), others (3 AC; 2 RC) Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction: the stomach and duodenum were brought down in a straight vertical manner anterior to the transverse colon. The anastomosis was performed at the caudal to the transverse colon. Retrocolic reconstruction: the left side of the transverse mesocolon (to the left of the middle colic vessels) was opened, and the stomach and duodenum were brought down in a straight, vertical manner. The retrocolic duodenojejunostomy was performed at the caudal side of the transverse mesocolon, and the gastric antrum was fixed to the transverse mesocolon with several sutures. A Braun anastomosis was added in both reconstruction procedures. Administration of prokinetics: no Administration of somatostatin: no Standard management of NGT: the NGT was routinely removed on POD 1 if the gastric amount was below 500 mL. If the patients vomited persistently, it was reinserted. |
|
| Outcomes | Primary outcome: incidence of delayed gastric emptying defined by ISGPS criteria Secondary outcome: postoperative complications besides delayed gastric emptying, evaluation of gastric emptying and nutritional status for 1 year after surgery |
|
| Notes | Country: Japan No conflicts of interest declared. Funded by the Japanese Ministry of Education, Culture, Sports, Science and Technology. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quote: "equal numbers of envelopes for antecolic and retrocolic were sequentially prepared" comment: sequence generation not described |
| Allocation concealment (selection bias) | Low risk | quote: "sequentially prepared envelopes in a blinded fashion" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | no blinding performed as described in trial registry |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | no blinding performed as described in trial registry |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | comment: sufficient reporting of dropouts and losses in flow chart with reasons for exclusion |
| Selective reporting (reporting bias) | Unclear risk | comment: trial registered, National Clinical Database (University Hospital Medical Information Network Clinical Trials Registry as UMIN000001712),with sufficient information on pre‐specified endpoints. Definition of delayed gastric emptying changed during the trial (trial started 2005, ISGPS definition published 2007). |
| Other bias | Low risk | No other source of bias. |
Kakaei 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): none Losses to follow‐up: none Intention‐to‐treat analysis: not stated Description of sample size calculation: no Duration of trial: April 2016 to March 2017 |
|
| Participants | Number: 30 (AC: 15; RC: 15) Age: 53.6 ± 9.8 (mean in the AC group); 52.9 ± 8.8 (mean in the RC group) Sex ratio (M/F): 1.5 (AC); 2 (RC) Inclusion criteria: 18‐75 years of age, operable (i.e. resectable) periampullary malignancy, candidate for PPPD Exclusion criteria: previous upper abdominal surgery, general conditions not suitable for a Whipple procedure (e.g. heart, renal failure), liver cirrhosis, Disease type: pancreatic cancer (8 AC; 10 RC), bile duct cancer (4 AC; 2 RC), duodenal cancer (2 AC; 2 RC), undefinable (1 AC; 1 RC) Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction (AC): a duodenojejunostomy 2 cm distal to the pylorus in an end to side fashion in 2 layers by 2‐0 or 3‐0 PDS was fashioned 20 cm to 30 cm distal to previous site of Treitz ligament in antecolic position. Retrocolic reconstruction (RC): a duodenojejunostomy 2 cm distal to the pylorus in an end to side fashion in 2 layers by 2‐0 or 3‐0 PDS, 5 cm to 10 cm after the choledochojejunostomy in retrocolic position. Administration of prokinetics: not stated Administration of somatostatin: quote: "subcutaneous octreotide continued for 48‐72 hours after the operation in high risk pancreatic anastomosis (patients with very small ducts or fragile pancreatic tissue)" Standard management of NGT: NGT was removed after 48 hours if no bleeding was seen and when its excretion was less than 50 mL per 6 hours |
|
| Outcomes | No primary outcome defined. Outcomes include delayed gastric emptying (ISGPS definition), pancreatic fistula, gastric leakage, biliary leakage, postoperative bleeding requiring reoperation, wound infection, intraabdominal abscess, deep vein thrombosis and other systemic complications, mortality and length of hospital stay. |
|
| Notes | Country: Iran The authors declared no conflict of interest. The study was funded by Tabriz University of Medical Science (internal funding) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "randomly assigned ... by Randlist(R) software" |
| Allocation concealment (selection bias) | Low risk | quote: "sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | comment: no blinding of participants and personnel described. Thus, probably not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | quote: "data were collected by the same person ... who was completely blinded to the type of the procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | comment: no flow chart, but information on all patients within the trial given in text. No losses to follow‐up, one patient died in each group. |
| Selective reporting (reporting bias) | Unclear risk | no trial registration, no published protocol |
| Other bias | Low risk | No other source of bias. |
Kurahara 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): none Losses to follow‐up: none Intention‐to‐treat analysis: not stated Description of sample size calculation: yes Duration of trial: May 2007 to June 2010 |
|
| Participants | Number: 46 patients (24 AC; 22 RC) Age: 67.6 ±11.6 (mean in the AC group); 62.3 ±12.6 (mean in the RC group) Sex ratio (M/F): 2.0 (AC); 2.7 (RC) Inclusion criteria: SSPPD with pancreatogastrostomy for pancreatic head and periampullary tumours Exclusion criteria: classic PD or PPPD, pancreaticojejunostomy reconstruction, distant metastasis, or local unresectable tumours Disease type: pancreatic cancer (10 AC; 10 RC), bile duct cancer (7 AC; 4 RC), neuroendocrine tumour (3 AC; 2 RC), ampullary cancer (0 AC; 2 RC) duodenal cancer (1 AC; 1 RC) Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction performed in the following sequence: end‐to‐side pancreatogastric anastomosis, end‐to‐side choledochojejunostomy, and end‐to‐side gastrojejunostomy by the antecolic route
Retrocolic reconstruction performed in the following sequence: end‐to‐side pancreatogastric anastomosis using an internal stent, end‐to‐side gastrojejunostomy, and end‐to‐side choledochojejunostomy using an internal stent Administration of prokinetics: not stated Administration of somatostatin: not stated Standard management of NGT: The NGT was removed when drainage was < 500 mL/day, but it was reinserted in case of vomiting or nausea. |
|
| Outcomes | primary outcome: delayed gastric emptying (ISGPS definition) secondary outcome: rate of other postoperative complications, time‐to‐full resumption of diet, surgical mortality, readmission date, and length of postoperative hospital stay (LOS) |
|
| Notes | A 'Billroth‐1'‐type reconstruction was compared to a 'Billroth‐2'‐type reconstruction Trial was terminated after planned interim analysis because of obviously lower delayed gastric emptying in AC group than in RC group. COI and sources of funding not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | comment: sequence generation not reported sufficiently |
| Allocation concealment (selection bias) | Low risk | quote: "consecutive sealed envelopes containing random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | comment: no blinding described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | comment: no blinding described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | comment: all patients completed the trial and there were no losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | comment: no trial registration; no protocol available |
| Other bias | Low risk | No other source of bias. |
Tamandl 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): 5 (2 AC; 3 RC, allocated treatment was not performed, due to surgeon's preference) Losses to follow up: 4 due to early discharge (2 AC; 2 RC) Intention‐to‐treat analyses: no; per‐protocol analysis used Description of sample size calculation: yes Duration of trial: April 2007 to November 2009 |
|
| Participants | Number: 71 (38 AC; 33 RC) Age: 67.1 (55.7 to 75.3 median in the AC group); 65.4 (55.6 to 70.6 median in the RC group) Sex ratio (M/F): 0.9 (AC); 0.75 (RC) Inclusion criteria: adults between the ages of 18 and 90 years undergoing PPPD for cancer of either the pancreatic head, uncinate process, ampulla or distal common bile duct, or with a radiographically suspicious solid or cystic tumour requiring pancreaticoduodenectomy Exclusion criteria: distant metastases; locally unresectable tumours (arterial involvement or involvement of the portal vein or superior mesenteric vein); invasion of the stomach; prior surgical resection of the stomach or duodenum. Disease type: ductal adenocarcinoma (AC: 20; RC: 13), distal cholangiocarcinoma (AC: 1; RC: 1), ampullary cancer (AC: 4; RC: 5), IPMN (AC: 2; RC: 0), SCN/MCN (AC: 1; RC: 1), chronic pancreatitis (AC: 5; RC: 7), other (AC: 3; RC: 1) Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction: antecolic end‐to‐side duodenojejunostomy
Retrocolic reconstruction: retrocolic end‐to‐side duodenojejunostomy Administration of prokinetics: not given on a routine basis Administration of somatostatin: not reported Standard management of NGT: The NGT was removed in the operating room and was reinserted only in case of repeated vomiting or abdominal distension, or the inability to ingest food. |
|
| Outcomes | Primary outcome: delayed gastric emptying as defined by clinical criteria on POD 10. Delayed gastric emptying is defined as the NGT remaining in place beyond POD 10 and one of the following criteria: (1) emesis after NGT removal; (2) NGT reinsertion; (3) failure to progress with the diet; (4) use of prokinetics after POD 10. If the NGT was removed before day 10, two of the previous criteria had to be fulfilled to qualify for delayed gastric emptying. Secondary outcome: gastric emptying determined by the paracetamol absorption test; the kinetics of intestinal peptides (GLP‐1 and PYY) after ingestion of a test meal; postoperative length of hospital stay; morbidity and mortality. | |
| Notes | Country: Austria The authors did not disclose any potential conflict of interest. Funding not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | quote: "patients with an uneven birth date (e.g. February 1) were allocated to the antecolic arm. Patients who had an even birth date were treated with retrocolic reconstruction." comment: non‐random approach |
| Allocation concealment (selection bias) | High risk | comment: obvious allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | comment: no blinding performed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | comment: no blinding performed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | comment: transparent patient flow chart: 2 AC; 5 RC patients excluded intraoperatively due to technical feasibility or anatomical reasons; 2 AC; 2 RC patients excluded from analysis due to early discharge, before POD 10 (leaving 36 in the AC group and 28 in the RC group) |
| Selective reporting (reporting bias) | Low risk | comment: trial registry |
| Other bias | Unclear risk | comment: ISGPS definition only applied retrospectively |
Tani 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): none Losses to follow‐up: none Intention‐to‐treat analysis: not stated Description of sample size calculation: yes Duration of trial: May 2002 to April 2004 |
|
| Participants | Number: 40 (20 AC; 20 RC) Age: 63.1 ± 9.21 (mean in the AC group); 66.7 ± 12.2 (mean in the RC group) Sex ratio (M/F): 1.2 (AC); 1.0 (RC) Inclusion criteria: PPPD for periampullary and bile duct lesions Exclusion criteria: peptic ulcer, tumour infiltration in the stomach, metastasis into lymph nodes of the pre‐pylorus Disease type: pancreatic cancer (AC: 4; RC: 10), bile duct cancer (AC: 10; RC: 2), ampullary cancer (AC: 2; RC: 4), IPMN (AC: 2; RC: 3), solid‐pseudopapillary tumour (AC: 1; RC: 0), pancreatitis (AC: 1; RC: 1) Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction
Retrocolic reconstruction Administration of prokinetics: not given Administration of somatostatin: not given Standard management of NGT: The time for removal of the NGT was determined when drainage was < 500 mL/day. |
|
| Outcomes | Primary outcome: delayed gastric emptying defined as: (1) prolonged aspiration of 500 mL/day from a nasogastric tube left in place for 10 days (DGE10), (2) need for reinsertion of a nasogastric tube, or (3) failure of unlimited oral intake by the 14th POD (DGE14). Percentage of oral intake of solid foods was defined as the ratio between actual intake and provided diet. Secondary outcome: mortality and morbidity, including pancreatic fistula, intra‐abdominal haemorrhage, and intra‐abdominal abscess | |
| Notes | Trial was terminated after the planned interim analysis, since the delayed gastric emptying rate was significantly lower in the antecolic group than in the retrocolic group Country: Japan No COI statement. Funding not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "computer‐generated random‐number pattern" |
| Allocation concealment (selection bias) | Unclear risk | comment: allocation concealment not described; intraoperative randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | comment: no blinding described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | comment: no blinding described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | comment: all patients completed the trial and there were no losses to follow‐up; one patient died in the RC group due to gastrointestinal bleeding. |
| Selective reporting (reporting bias) | Unclear risk | comment: trial not registered; no published protocol |
| Other bias | Low risk | No other source of bias |
Toyama 2021.
| Study characteristics | ||
| Methods | Randomised controlled trial Exclusion after randomisation (total and per group): 2 (AC), due to prolonged intubation and ICU stay Losses to follow‐up: none Intention‐to‐treat analysis: yes Description of sample size calculation: yes Duration of trial: August 2011 to June 2016 |
|
| Participants | Number: 214 (105 AC; 109 RC) Age: 45.6% ≥70 years (in the AC group); 48.6% ≥70 years (in the RC group) Sex ratio (M/F): 1.7 (AC); 1.5 (RC) Inclusion criteria: SSPPD or PPPD for pancreatic head tumours, periampullary tumours, or other pancreatic mass, ≥20 and <80 years of age, Eastern Cooperative Oncology Group grade ≤1 Exclusion criteria: total pancreatectomy, combined liver resection, pancreatogastrostomy, history of gastric surgery Disease type: pancreatic cancer (AC: 47; RC: 38), bile duct cancer (AC: 24; RC: 21), ampullary cancer (AC: 16; RC: 18), IPMN (AC: 8; RC: 21), others (AC: 8; RC: 11) Equivalence of baseline characteristics: yes |
|
| Interventions | Antecolic reconstruction (AC): 2‐layered, side‐to‐side gastroenterostomy or end‐to‐side duodenojejunostomy positioned anterior to the transverse colon.
Retrocolic reconstruction (RC): 2‐layered, side‐to‐side gastroenterostomy or end‐to‐side duodenojejunostomy performed on the caudal side of the transverse mesocolon after the stomach was brought down vertically through a transmesocolic opening left of the middle colic vessels (similar to Imamura et al. 2014). The stomach was fixed to the mesocolon to prevent postoperative herniation. Administration of prokinetics: prophylactic administration not allowed Administration of somatostatin: prophylactic administration not allowed Standard management of NGT: NGT was routinely removed on postoperative day 1, if drainage volume was <2 50 mL |
|
| Outcomes | Primary outcome: delayed gastric emptying (ISGSPS definition) Secondary outcomes: 90‐day mortality, length of hospital stay, postoperative pancreatic fistula, post‐pancreatectomy haemorrhage, intra‐abdominal abscess, wound infection, reoperation, readmission, postoperative nutritional status | |
| Notes | Country: Japan The authors declare no conflicts of interest. No information on funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | quote: "computer‐generated random number pattern" |
| Allocation concealment (selection bias) | Low risk | quote: "randomization was carried out at the trial coordinating center, using the minimization method." comment: i.e. central randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | quote: "Both patients and statisticians were blinded to treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | comment: no information on blinding of outcome assessment in manuscript. Stated as 'open label' in trial registry |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | comment: flow chart with reasons for exclusion of any dropouts |
| Selective reporting (reporting bias) | Unclear risk | comment: trial registered (NCT01460550 & UMIN000005827), but only primary outcome defined in registry and no sufficient information on secondary endpoints |
| Other bias | Low risk | No other source of bias. |
AC = antecolic reconstruction; DGE = delayed gastric emptying; ICU = intensive care unit; IPMN = intraductal papillary mucinous neoplasm; ISGPS = International Study Group of Pancreatic Surgery; MCN = mucinous cystic neoplasm; NGT = nasogastric tube; POD = postoperative day; PD = pancreaticoduodenectomy; PPPD = pylorus preserving pancreaticoduodenectomy; RC = retrocolic reconstruction; SSPPD = subtotal stomach‐preserving PD.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Rebala 2012 | Congress abstract only. Insufficient data available for inclusion in review. Authors contacted but no answer. |
| Siripong 2012 | Congress abstract only. Insufficient data available for inclusion in review. Authors contacted but no data available, since final results have not yet been published. |
Differences between protocol and review
The results for mortality have been pooled by using the risk difference as the summary measure instead of the odds ratio. This decision was made due to the well‐known computational problems in meta‐analyses concerning trials with zero events in both groups.
Clinically relevant delayed gastric emptying (ISGPS grade B/C) was included as a secondary outcome to assess potential differences between the two procedures.
The planned subgroup analyses for patients undergoing classical Whipple's procedure, single‐loop reconstruction, Roux‐en‐Y reconstruction and for underlying diseases could not be conducted, because sufficient data were not available.
During the most recent update of this review, the World Health Organization International Clinical Trials Registry Platform was searched in addition to the trial registry www.clinicaltrials.gov.
In the initial protocol, substantial heterogeneity was defined as I2 > 75%. During the current update, substantial heterogeneity was defined as I2 > 50%, moderate heterogeneity as 25% to 50% and < 25% was defined as no relevant heterogeneity.
The sensitivity analysis excluding studies with less than the average number of positive judgements in the risk of bias assessment was not performed anymore in the current update.
Contributions of authors
Conceiving the review: FJH*, RK*, PP Designing the review: FJH*, RK*, MKD Co‐ordinating the review: MKD, MWB Designing search strategies: FJH*, RK*, MKD Writing the review: FJH*, RK*, PP, MKD Providing general advice on the review: MKD, AU, MWB Performing previous work that was the foundation of the current study: FJH*, MKD, AU, MWB
* Both authors contributed equally to this work.
Sources of support
Internal sources
-
Department of General, Visceral, and Transplantation Surgery, University of Heidelberg, Germany
General support.
External sources
-
None, Other
No external sources of support
Declarations of interest
FH: none known. RK: none known. MKD: none known. MWB: none known. AU: none known. PP: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Eshuis 2014 {published data only}
- Eshuis W, Van Eijck C, Coene PP, De Hingh I, Karsten T, Bonsing B, et al. Antecolic versus retrocolic route of the gastroenteric anastomosis after pancreatoduodenectomy-arco-trial. In: HPB : the Official Journal of the International Hepato Pancreato Biliary Association. Vol. 14(Supplement S2). 2012:257. [DOI: ]
- Eshuis WJ, Bree K, Sprangers MA, Bennink RJ, Van Gulik TM, Busch OR, et al. Gastric emptying and quality of life after pancreatoduodenectomy with retrocolic or antecolic gastroenteric anastomosis. British Journal of Surgery 2015;102(9):1123-32. [DOI] [PubMed] [Google Scholar]
- Eshuis WJ, Van Eijck CH, Gerhards MF, Coene PP, Hingh IH, Karsten TM, et al. Antecolic versus retrocolic route of the gastroenteric anastomosis after pancreatoduodenectomy: a randomized controlled trial. Annals of Surgery 2014;259(1):45-51. [DOI: 10.1097/SLA.0b013e3182a6f529] [DOI] [PubMed] [Google Scholar]
Gangavatiker 2011 {published data only}
- Gangavatiker R, Pal S, Javed A, Dash NR, Sahni P, Chattopadhyay TK. Effect of antecolic or retrocolic reconstruction of the gastro/duodenojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled trial. Journal of Gastrointestinal Surgery 2011;15(5):843-52. [DOI: 10.1007/s11605-011-1480-3] [DOI] [PubMed] [Google Scholar]
Imamura 2014 {published data only}
- Chijiiwa K, Imamura N, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. Prospective randomized controlled study of gastric emptying assessed by (13)C-acetate breath test after pylorus-preserving pancreaticoduodenectomy: comparison between antecolic and vertical retrocolic duodenojejunostomy. Journal of Hepato-biliary-pancreatic Surgery 2009;16(1):49-55. [DOI] [PubMed] [Google Scholar]
- Chijiiwa K. Prospective randomized controlled trial of gastric emptying after pylorus-preserving pancreaticoduodenectomy: Comparison between antecolic and vertical retrocolic duodenojejunostomy. In: HPB: the Official Journal of the International Hepato-Pancreato-Biliary Association. Vol. 14(Supplement S2). 2012:111. [DOI: 10.1111/j.1477-2574.2012.00511.x] [DOI] [PMC free article] [PubMed]
- Imamura N, Chijiiwa K, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. Prospective randomized clinical trial of a change in gastric emptying and nutritional status after a pylorus-preserving pancreaticoduodenectomy: comparison between an antecolic and a vertical retrocolic duodenojejunostomy. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2014;16(4):384-94. [DOI: 10.1111/hpb.12153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kakaei 2019 {published data only}
- Kakaei F, Fakhri MA, Azizi A, Kermani TA, Tarvirdizade K, Sanei B. Effects of antecolic versus retrocolic duodenojejunostomy on delayed gastric emptying after pyloric preserving pancreaticoduodenectomy in patients with periampullary tumors. Asian Journal of Surgery 2019;42(11):963-8. [DOI] [PubMed] [Google Scholar]
Kurahara 2011 {published data only}
- Kurahara H, Shinchi H, Maemura K, Mataki Y, Iino S, Sakoda M, et al. Delayed gastric emptying after pancreatoduodenectomy. Journal of Surgical Research 2011;171(2):187-92. [DOI] [PubMed] [Google Scholar]
Tamandl 2014 {published data only}
- Tamandl D, Sahora K, Prucker J, Schmid R, Holst JJ, Miholic J, et al. Impact of the reconstruction method on delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: a prospective randomized study. World Journal of Surgery 2014;38(2):465-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tani 2006 {published data only}
- Tani M, Terasawa H, Kawai M, Ina S, Hirono S, Uchiyama K, et al. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Annals of Surgery 2006;243(3):316-20. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toyama 2021 {published data only}
- Toyama H, Matsumoto I, Mizumoto T, Fujita H, Tsuchida S, Kanbara Y, et al. Influence of the retrocolic versus antecolic route for alimentary tract reconstruction on delayed gastric emptying after pancreatoduodenectomy: a multicenter, noninferiority randomized controlled trial. Annals of Surgery 2021;6:935-44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Rebala 2012 {published data only}
- Rebala P, Rao Guduru V, Patil S, Kumaresan K, Rao Balmoori U, Dama R, et al. Effect of supracolic versus infracolic gastrojejunostomy on gastric emptying in patients undergoing Whipples pancreaticoduodenectomy - A prospective randomized control trial. In: HPB : the Official Journal of the International Hepato Pancreato Biliary Association. Vol. 14(Supplement S2). 2012:8-9.
Siripong 2012 {published data only}
- Siripong A, Chung M, Mascarenhas C. Effect of antecolic versus retrocolic reconstruction of gastrojejunostomy on delayed gastric emptying following classic whipple procedure: a prospective randomized trial. Annals of Surgical Oncology 2012;19:S153-4. [Google Scholar]
Additional references
Bassi 2005
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138(1):8-13. [DOI] [PubMed] [Google Scholar]
Bell 2015
- Bell R, Pandanaboyana S, Shah N, Bartlett A, Windsor JA, Smith AM. Meta-analysis of antecolic versus retrocolic gastric reconstruction after a pylorus-preserving pancreatoduodenectomy. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2015;17(3):202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Büchler 2003
- Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Archives of Surgery 2003;138(12):1310-4. [DOI] [PubMed] [Google Scholar]
Butturini 2006
- Butturini G, Marcucci S, Molinari E, Mascetta G, Landoni L, Crippa S, et al. Complications after pancreaticoduodenectomy: the problem of current definitions. Journal of Hepato-biliary-pancreatic Surgery 2006;13(3):207-11. [DOI] [PubMed] [Google Scholar]
Chijiiwa 2009
- Chijiiwa K, Imamura N, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. Prospective randomized controlled study of gastric emptying assessed by (13)C-acetate breath test after pylorus-preserving pancreaticoduodenectomy: comparison between antecolic and vertical retrocolic duodenojejunostomy. Journal of Hepato-biliary-pancreatic Surgery 2009;16(1):49-55. [DOI] [PubMed] [Google Scholar]
Chijiiwa 2012
- Chijiiwa K. Prospective randomized controlled trial of gastric emptying after pylorus-preserving pancreaticoduodenectomy: comparison between antecolic and vertical retrocolic duodenojejunostomy. In: HPB: the Official Journal of the International Hepato-Pancreato-Biliary Association. Vol. 14(Supplement S2). 2012:111. [DOI: 10.1111/j.1477-2574.2012.00511.x] [DOI] [PMC free article] [PubMed]
Choi 2011
- Choi YY, Kim J, Seo D, Choi D, Kim MJ, Kim JH, et al. Is routine nasogastric tube insertion necessary in pancreaticoduodenectomy? Journal of the Korean Surgical Society 2011;81(4):257-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conroy 2018
- Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al, Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. New England Journal of Medicine 2018;379(25):2395-406. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
de Wilde 2012
- Wilde RF, Besselink MG, Van der Tweel I, Hingh IH, Van Eijck CH, Dejong CH, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. British Journal of Surgery 2012;99(3):404-10. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD. Meta-analysis. Potentials and promise. BMJ 1997;315(7119):1371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eshuis 2012
- Eshuis W, Van Eijck C, Coene PP, De Hingh I, Karsten T, Bonsing B, et al. Antecolic versus retrocolic route of the gastroenteric anastomosis after pancreatoduodenectomy-arco-trial. In: HPB : the Official Journal of the International Hepato Pancreato Biliary Association. Vol. 14(Supplement S2). 2012:257. [DOI: ]
Eshuis 2015
- Eshuis WJ, Bree K, Sprangers MA, Bennink RJ, Van Gulik TM, Busch OR, et al. Gastric emptying and quality of life after pancreatoduodenectomy with retrocolic or antecolic gastroenteric anastomosis. British Journal of Surgery 2015;102(9):1123-32. [DOI] [PubMed] [Google Scholar]
Fisher 2011
- Fisher WE, Hodges SE, Cruz G, Artinyan A, Silberfein EJ, Ahern CH, et al. Routine nasogastric suction may be unnecessary after a pancreatic resection. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2011;13(11):792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
GLOBOCAN 2020
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today.. International Agency for Research on Cancer. Lyon, France.Available from: https://gco.iarc.fr/today accessed 07 March 2021.
GRADEproGDT 2015 [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool. Available from: gradepro.org. Hamilton, ON: McMaster University, developed by GRADE Working Group and Evidence Prime, Inc, 2015.
Hackert 2018
- Hackert T, Probst P, Knebel P, Doerr-Harim C, Bruckner T, Klaiber U, et al. Pylorus resection does not reduce delayed gastric emptying after partial pancreatoduodenectomy: a blinded randomized controlled trial (PROPP study, DRKS00004191). Annals of Surgery 2018;267(6):1021-7. [DOI] [PubMed] [Google Scholar]
Hartel 2005
- Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Müller MW, et al. Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Archives of Surgery 2005;140(11):1094-9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration. Available from: handbook.cochrane.org 2011.
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021 2021:Available from www.training.cochrane.org/handbook.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imamura 2016
- Imamura M, Kimura Y, Ito T, Kyuno T, Nobuoka T, Mizuguchi T, Hirata K. Effects of antecolic versus retrocolic reconstruction for gastro/duodenojejunostomy on delayed gastric emptying after pancreatoduodenectomy: a systematic review and meta-analysis. Journal of Surgical Research 2016;200(1):147-57. [DOI] [PubMed] [Google Scholar]
Joliat 2016
- Joliat GR, Labgaa I, Demartines N, Schäfer M, Allemann P. Effect of Antecolic versus Retrocolic Gastroenteric Reconstruction after Pancreaticoduodenectomy on Delayed Gastric Emptying: A Meta-Analysis of Six Randomized Controlled Trials. Digestive Surgery 2016 ;33(1):15-25. [DOI] [PubMed] [Google Scholar]
Kamarajah 2020
- Kamarajah SK, Bundred JR, Alessandri G, Robinson SM, Wilson CH, French JJ, et al. systematic review and network-meta-analysis of gastro-enteric reconstruction techniques following pancreatoduodenectomy to reduce delayed gastric emptying. World Journal of Surgery 2020;44(7):2314-22. [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim DK, Hindenburg AA, Sharma SK, Suk CH, Gress FG, Staszewski H, et al. Is pylorospasm a cause of delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? Annals of Surgical Oncology 2005;12(3):222-7. [DOI] [PubMed] [Google Scholar]
Klaiber 2015
- Klaiber U, Probst P, Knebel P, Contin P, Diener MK, Büchler MW, et al. Meta-analysis of complication rates for single-loop versus dual-loop (Roux-en-Y) with isolated pancreaticojejunostomy reconstruction after pancreaticoduodenectomy. British Journal of Surgery 2015;102(4):331-40. [DOI] [PubMed] [Google Scholar]
Klaiber 2018
- Klaiber U, Probst P, Strobel O, Michalski CW, Dörr-Harim C, Diener MK, et al. Meta-analysis of delayed gastric emptying after pylorus-preserving versus pylorus-resecting pancreatoduodenectomy. British Journal of Surgery 2018;105(4):339-49. [DOI] [PubMed] [Google Scholar]
Kollmar 2008
- Kollmar O, Moussavian MR, Richter S, Roi P, Maurer CA, Schilling MK. Prophylactic octreotide and delayed gastric emptying after pancreaticoduodenectomy: results of a prospective randomized double-blinded placebo-controlled trial. European Journal of Surgical Oncology 2008;34(8):868-75. [DOI] [PubMed] [Google Scholar]
Malvezzi 2014
- Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Annals of Oncology 2014;25(8):1650-6. [DOI] [PubMed] [Google Scholar]
McPhee 2007
- McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Annals of Surgery 2007;246(2):246-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Mulrow 1994
- Mulrow CD. Rationale for systematic reviews. BMJ 1994;309(6954):597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikfarjam 2009
- Nikfarjam M, Kimchi ET, Gusani NJ, Shah SM, Sehmbey M, Shereef S, et al. A reduction in delayed gastric emptying by classic pancreaticoduodenectomy with an antecolic gastrojejunal anastomosis and a retrogastric omental patch. Journal of Gastrointestinal Surgery 2009;13(9):1674-82. [DOI] [PubMed] [Google Scholar]
Plichta 2015
- Plichta JK, Brosius JA, Pappas SG, Abood GJ, Aranha GV. The changing spectrum of surgically treated cystic neoplasms of the pancreas. HPB Surgery 2015;2015 March 30 [Epub ahead of print]:7 pages. [DOI: 10.1155/2015/791704] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qian 2016
- Qian D, Lu Z, Jackson R, Wu J, Liu X, Cai B, et al. Effect of antecolic or retrocolic route of gastroenteric anastomosis on delayed gastric emptying after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Pancreatology 2016;16(1):142-50. [DOI] [PubMed] [Google Scholar]
Qiu 2019
- Qiu J, Li M, Du C. Antecolic reconstruction is associated with a lower incidence of delayed gastric emptying compared to retrocolic technique after Whipple or pylorus-preserving pancreaticoduodenectomy. Medicine (Baltimore) 2019;98(34):e16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qu 2013
- Qu H, Sun GR, Zhou SQ, He QS. Clinical risk factors of delayed gastric emptying in patients after pancreaticoduodenectomy: a systematic review and meta-analysis. European Journal of Surgical Oncology 2013;39(3):213-23. [DOI] [PubMed] [Google Scholar]
Ramia 2013
- Ramia JM, la Plaza R, Quiñones JE, Veguillas P, Adel F, García-Parreño J. Gastroenteric reconstruction route after pancreaticoduodenectomy: antecolic versus retrocolic. Cirugia Espanola 2013;91(4):211-6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Robertson 2012
- Robertson N, Gallacher PJ, Peel N, Garden OJ, Duxbury M, Lassen K, et al. Implementation of an enhanced recovery programme following pancreaticoduodenectomy. HPB: the Official Journal of the International Hepato Pancreato Biliary Association 2012;14(10):700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnelldorfer 2008
- Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Annals of Surgery 2008;247(3):456-62. [DOI] [PubMed] [Google Scholar]
Shaib 2007
- Shaib Y, Davila J, Naumann C, El-Serag H. The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. population-based study. American Journal of Gastroenterology 2007;102(7):1377-82. [DOI] [PubMed] [Google Scholar]
Shimoda 2013
- Shimoda M, Kubota K, Katoh M, Kita J. Effect of billroth II or Roux-en-Y reconstruction for the gastrojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled study. Annals of Surgery 2013;257(5):938-42. [DOI] [PubMed] [Google Scholar]
Spinelli 2004
- Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, et al. Cystic pancreatic neoplasms: observe or operate. Annals of Surgery 2004;239(5):651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stojadinovic 2003
- Stojadinovic A, Brooks A, Hoos A, Jaques DP, Conlon KC, Brennan MF. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. Journal of the American College of Surgeons 2003;196(6):954-64. [DOI] [PubMed] [Google Scholar]
Su 2012
- Su AP, Cao SS, Zhang Y, Zhang ZD, Hu WM, Tian BL. Does antecolic reconstruction for duodenojejunostomy improve delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy? A systematic review and meta-analysis. World Journal of Gastroenterology 2012;18(43):6315-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standarddeviation from the sample size, median, rangeand/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wente 2007a
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142(5):761-8. [DOI] [PubMed] [Google Scholar]
Wente 2007b
- Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142(1):20-5. [DOI] [PubMed] [Google Scholar]
Yang 2014
- Yang C, Wu HS, Chen XL, Wang CY, Gou SM, Xiao J, et al. Pylorus-preserving versus pylorus-resecting pancreaticoduodenectomy for periampullary and pancreatic carcinoma: a meta-analysis. PLOS One 2014;9(3):e90316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeo 1993
- Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Annals of Surgery 1993;218(3):229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2015
- Zhou Y, Lin J, Wu L, Li B, Li H. Effect of antecolic or retrocolic reconstruction of the gastro/duodenojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a meta-analysis. BMC Gastroenterology 2015;15:68. [DOI: 10.1186/s12876-015-0300-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hüttner 2015
- Hüttner FJ, Klotz R, Diener MK, Büchler MW, Ulrich A. Antecolic versus retrocolic reconstruction for prevention of delayed gastric emptying after partial pancreaticoduodenectomy. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011862. [DOI: 10.1002/14651858.CD011862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hüttner 2016
- Hüttner FJ, Klotz R, Ulrich A, Büchler MW, Diener MK. Antecolic versus retrocolic reconstruction after partial pancreaticoduodenectomy. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD011862. [DOI: 10.1002/14651858.CD011862.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


