Abstract
A patient with AIDS, treated with highly active antiretroviral therapy and trimethoprim-sulfamethoxazole, presented with confusion, a hemifield defect, and a mass lesion in the right occipital lobe. A brain biopsy confirmed granulomatous amebic encephalitis (GAE) due to Acanthamoeba castellanii. The patient was treated with fluconazole and sulfadiazine, and the lesion was surgically excised. This is the first case of AIDS-associated GAE responding favorably to therapy. The existence of a solitary brain lesion, absence of other sites of infection, and intense cellular response in spite of a very low CD4 count conditioned the favorable outcome. We review and discuss the diagnostic microbiologic options for the laboratory diagnosis of infections due to free-living amebae.
CASE REPORT
The patient, a 33-year-old male Spanish ex-intravenous drug user who was human immunodeficiency virus positive since 1984, was managed at the Infectious Disease Unit of the Complexo Hospitalario of Pontevedra since 1993. He denied homosexual contacts and had not been exposed to contaminated freshwater or ocean water. He was maintained on highly active antiretroviral therapy (HAART) with didanosine (200 mg twice a day [BID]), estavudine (40 mg [BID]), and saquinavir (600 mg three times a day) since July 1997, in addition to trimethoprim-sulfamethoxazole (TMP-SMX) (160 and 800 mg per day, respectively). A laboratory examination in October 1997, revealed CD4 counts of 82/mm3 and a viral load of less than 200 copies/ml. He was hepatitis B virus positive in immune phase and hepatitis C virus positive.
The patient was admitted to the hospital in late January 1998 presenting with a 2-day history of headache, confusion, and a left hemifield defect. A cranial computed tomography scan revealed a contrast-enhancing mass in the right occipital lobe. Toxoplasmosis was suspected, and sulfadiazine (500 mg four times a day) and pyrimethamine (50 mg once a day) were added to the treatment regimen. However, his condition worsened with fever, malaise, headache, and meningeal signs. One week after admission, he developed a generalized tonic-clonic seizure with increase in mass effect and contrast enhancement. Phenytoin was begun, and the seizures did not recur.
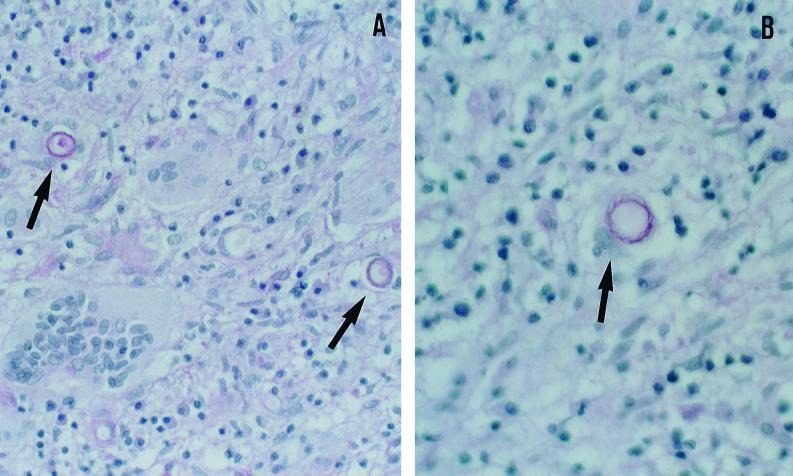
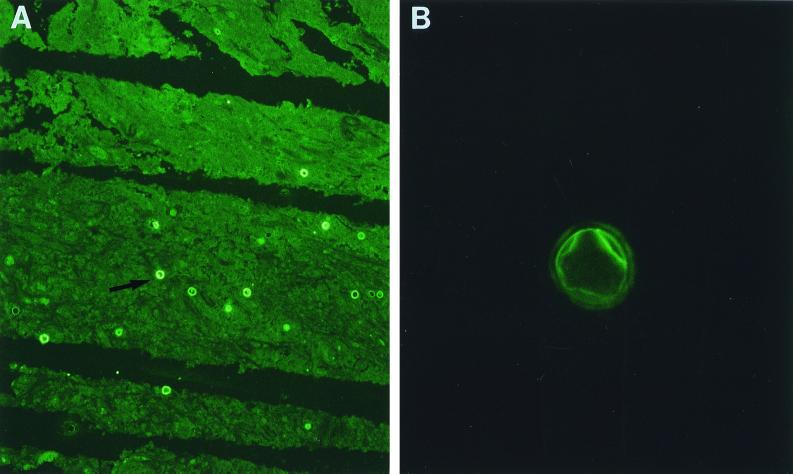
A biopsy specimen was obtained from the right parieto-occipital region, which exhibited on microscopic examination granulomatous changes with multinucleated giant cells, histiocytes and inflammatory cells, necrotizing vasculitis, and inflammatory perivascular infiltrate. In the hematoxylin-eosin and periodic acid-Schiff (PAS)-stained sections, multiple double-walled cyst-like structures measuring 18 to 25 μm in diameter were seen (Fig. 1). The histopathologic findings and morphologic appearance of the agent were consistent with those of either Acanthamoeba spp. or Balamuthia mandrillaris. Since the biopsy specimen was fixed in formalin and embedded in paraffin, culture studies were not possible. Six weeks after the onset of symptoms, a right parieto-occipital craniotomy was performed and the mass was excised. Multiple widespread foci of necrosis were found in the brain tissue. The mass was divided into two portions. One portion was processed for culture and the other was processed for histopathology. Cultures were initiated by inoculating small pieces of the tissue onto nonnutrient agar plates covered with Escherichia coli and incubated in room air at 30 and 37°C (13). After 48 h of incubation at 37°C many motile trophozoites consistent with those of Acanthamoeba were seen, and these trophozoites differentiated into the characteristic double-walled cysts beginning from the 6th day of incubation. They were subsequently identified, based on the cyst morphology, as Acanthamoeba group II. A number of special strains, including PAS, trichrome, hematoxylin-eosin, and Gomori's methenamine silver, performed on the formalin-fixed and paraffin-embedded tissue sections revealed cysts but no trophozoites. An indirect immunofluorescence (IIF) test was also performed on a number of tissue sections by first treating them with a 1:100 dilution of rabbit antiserum made against B. mandrillaris, Naegleria fowleri, and several species of Acanthamoeba (e.g., A. castellanii, A. culbertsoni, A. polyphaga, A. rhysodes, and A. healyi) for 30 min at 37°C. After three washes in phosphate-buffered saline the sections were exposed to a 1:100 dilution of a goat anti-rabbit immunoglobulin conjugated with fluorescein isothiocyanate, incubated, and washed as described elsewhere (8, 12–14, 25, 29). The organisms in those sections that were reacted only with the various anti-Acanthamoeba sera fluoresced but showed no fluorescence with the other sera (Fig. 2). The intensity of fluorescence varied, from bright (4+) fluorescence seen with the A. castellanii serum to dull (1+) fluorescence seen with the A. healyi serum. Based on the cyst morphology (wrinkled ectocyst and stellate endocyst) and the fluorescence patterns, the ameba was tentatively identified as A. castellanii.
FIG. 1.
Histopathological examination of the brain biopsy specimen demonstrating Acanthamoeba cysts (arrows). PAS stain. (A) Magnification, ×200; (B) magnification, ×400.
FIG. 2.
Immunofluorescence patterns of A. Castellani. (A) Magnification, ×100; (B) magnification, ×650. (Courtesy of G. S. Visvesvara [Centers for Disease Control and Prevention].)
After the surgical procedure fluconazole (200 mg BID) was added to the pyrimethamine regimen. The postoperative course was favorable, and the patient, 22 months postsurgery, has a left homonymous hemianopsia but his general state of health is excellent.
Discussion.
Granulomatous amebic encephalitis (GAE) is a rare opportunistic infection caused by the pathogenic free-living amebae of the genus Acanthamoeba or the recently discovered genus Balamuthia. Acanthamoeba usually causes infection in immunocompromised individuals, leading almost always to death, and hence most cases are diagnosed only at the postmortem. We report a patient with AIDS presenting with acute encephalopathy, a left hemifield defect, and a contrast-enhancing mass lesion in the right occipital lobe. Although his general condition improved with sulfadiazine and pyrimethamine therapy, the mass lesion increased in size, and a brain biopsy confirmed GAE. Fluconazole at a high dose was instituted, in addition to sulfadiazine, and the lesion was surgically excised with a favorable outcome.
Three genera of free-living amebae, Naegleria, Acanthamoeba, and Balamuthia have been known to cause brain infection. N. fowleri causes an acute, fulminant infection known as primary amebic meningoencephalitis in previously healthy individuals who come in contact with contaminated freshwater 1 to 2 weeks prior to infection. It is important to distinguish GAE from primary amebic meningoencephalitis since the latter may respond to early aggressive treatment with amphotericin B in combination with other drugs (22) while there is no accepted therapy for GAE.
Although 169 (103 due to Acanthamoeba and 66 due to Balamuthia) cases of GAE have been reported from around the world (19) this is the first documented case from Spain. There are several pathologically confirmed cases of GAE due to Acanthamoeba in patients with AIDS (3, 8, 12, 13, 21, 29, 30), all occurring in males. Six of these were homosexuals, and all died in less than a month after the onset of neurological symptoms.
Acanthamoeba spp. are ubiquitous organisms and can be found in almost all types of ecological niches (19), including the ocean waters of northwest of Spain (1, 16). Most authors believe that Acanthamoeba is inhaled, producing nasal or sinus infection, and that it then, via hematogenous dissemination infects the lungs and skin (18). Skin, in some cases, may be a primary portal of entry (25). Brain involvement is usually a later manifestation of disseminated disease. Our patient did not have any clinical or radiological manifestations implicating eye (14), urinary, skin, or upper or lower respiratory tract infection.
In AIDS-associated GAE, the chronic inflammatory and granulomatous reaction, usually seen in the pathological examination of immunocompetent patients, may be absent or minimal, and trophozoites are usually present. These findings are interpreted as an impairment of cellular immune response to the parasite (13, 30). However, our patient presented with granulomatous changes and an intense inflammatory response consisting of mononuclear cells, abundant cysts, and no trophozoites, indicating a good capacity of the host defensive mechanisms or activity of the antimicrobials. The parasite tends to encyst under adverse environmental conditions (19) such as food source depletion and accumulation of nitrogenous waste (25) and probably also in the presence of antimicrobials. Although cysts are probably infectious, only trophozoites invade host tissue (9, 10). In our patient, surgical excision may have been effective in eliminating the cyst-containing tissue.
The existence of a solitary brain lesion, the absence of other sites of infection, and the presence of intense cellular response in spite of very low CD4 count probably influenced the favorable outcome and may be related to the following. (i) These may be related to the serotype of the Acanthamoeba. Different strains of the same species differ in pathogenicity and virulence (17, 19, 27), antimicrobial sensitivities, and tendency to cause GAE without other manifestations (26). The patient described herein had an infection with A. castellanii, a group II Acanthamoeba species which has been isolated from a few AIDS-related, pathologically confirmed GAE cases. (ii) They may be related to previous treatment with TMP-SMX. Most patients with confirmed GAE had previous Pneumocystis carinii infection, and it is probable that prophylactic treatment with TMP-SMX was instituted. However, none of the reports discuss either the treatment or its compliance. Sulfa drugs have antiamebic properties, and one report indicates clinical response to these drugs (5). Treatment with TMP-SMX may also have confined the amebae to a solitary lesion in our patient. (iii) They may be related to treatment with HAART. In AIDS patients with GAE, the immune state is usually one of severe suppression, with a CD4 count of <164 cells/ml (25). Our patient was treated with HAART, with the evidence of decreasing viral load, and although he was in a state of relatively severe immunosuppression at disease onset, this was improving, as reflected in the increasing CD4 count. HAART is reported to curb various opportunistic AIDS-associated disorders (4, 6, 24).
There is no effective treatment for GAE to date, and to our knowledge most of the immunocompromised patients with GAE have died. Factors contributing to the poor response to treatment include delay in diagnosis of this condition, its rarity, poor general or neurological condition at presentation, low penetration of diverse antiparasitic drugs into the cerebrospinal fluid, and the ability of Acanthamoeba to form cysts, as cysts are highly resistant to diverse stimuli (2, 15). Various drugs may be potentially beneficial to patients with Acanthamoeba infection, including sulfadiazine, fluconazole, pentamidine, and itraconazole (5, 7, 11, 20, 23, 26, 28, 29).
Diagnosis of infections due to N. fowleri is relatively easy because of the smaller size (8 to 11 μm) of the amebae and the absence of cysts in the brain tissue. Diagnosis of infections caused by Acanthamoeba and Balamuthia is, however, problematic. It is sometimes, but not always, possible to discriminate Balamuthia amebae from Acanthamoeba based on the nuclear morphology. The nucleus of Balamuthia may at times possess two or three nucleoli, whereas Acanthamoeba trophozoites do not have multiple nucleoli. Since Acanthamoeba and Balamuthia trophozoites in fixed tissue are more or less of the same size (18 to 30 μm) and cysts, if present, appear to be similar under bright-field microscopy, it is therefore necessary to perform either electron microscopy or an IIF test to identify the causal agent. Ultrastructurally, the cyst wall of Balamuthia has three layers whereas that of Acanthamoeba has only two. Since Acanthamoeba and Balamuthia are antigenically distinct, amebae in tissue sections can easily be identified by IIF using rabbit antisera made against the two amebae.
Both N. fowleri and Acanthamoeba spp. can be easily grown on nonnutrient agar covered with bacteria such as E. coli or Enterobacter aerogenes. Balamuthia, however, will not grow on agar plates covered with bacteria but can be grown on mammalian cell lines. Acanthamoeba spp. and Naegleria spp. can also be inoculated onto many types of mammalian cell cultures. A positive culture can facilitate identification because of the characteristic morphology of the cultured amebae (13). Culture, however, takes time and may not always yield positive results. A method for identification of these amebae using molecular probes is still under development.
This is the first report of an AIDS patient with GAE who responded favorably to surgery and medical treatment with sulfadiazine and fluconazole. This disease should always be considered in the differential diagnosis of mass lesions in AIDS, and a cerebral biopsy should be done as soon as the condition is suspected.
REFERENCES
- 1.Arias Fernandez C, Paniagua Crespo E. Avances en parasitologia: protozoologia. La Coruña, Spain: Universidad Santiago de Compostela; 1992. pp. 143–162. [Google Scholar]
- 2.Bottone E J. Free-living amebas of the genera Acanthamoeba and Naegleria: an overview and basic microbiological correlates. Mt Sinai J Med. 1993;60:260–270. [PubMed] [Google Scholar]
- 3.Calore E E, Cavaliere M J, Calore N M P. Cerebral amebiasis in the acquired immunodeficiency syndrome. Acta Neurol Belg. 1997;97:248–250. [PubMed] [Google Scholar]
- 4.Carr A, Marriott D, Field A, Vasak E, Cooper D A. Treatment of HIV-1 associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–261. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 5.Cleland P G, Lawande R V, Onyemelukwe G, Whittle H C. Chronic amebic meningoencephalitis. Arch Neurol. 1982;39:56–57. doi: 10.1001/archneur.1982.00510130058016. [DOI] [PubMed] [Google Scholar]
- 6.Clifford D B, Yiannoutsos C, Glicksman M, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology. 1999;52:623–625. doi: 10.1212/wnl.52.3.623. [DOI] [PubMed] [Google Scholar]
- 7.Culbertson C G, Holmes D H, Overton W M. Hartmannella castellanii (Acanthamoeba sp): Preliminary report on experimental chemotherapy. Am J Clin Pathol. 1965;43:361–364. doi: 10.1093/ajcp/43.4.361. [DOI] [PubMed] [Google Scholar]
- 8.Di Gregorio C, Rivasi F, Mongiardo N, De Rienzo B, Wallace S, Visvesvara G S. Acanthamoeba meninoencephalitis in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1992;116:1363–1365. [PubMed] [Google Scholar]
- 9.Ferrante A. Immunity to Acanthamoeba. Rev Infect Dis. 1991;13(Suppl. 5):S403–409. doi: 10.1093/clind/13.supplement_5.s403. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante A. Free-living amoebae: pathogenicity and immunity. Parasite Immunol. 1991;13:31–47. doi: 10.1111/j.1365-3024.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 11.Friedland L R, Raphael S A, Deutsch E S, et al. Disseminated Acanthamoeba infection in a child with symptomatic human immunodeficiency virus infection. Pediatr Infect Dis J. 1992;11:404–407. doi: 10.1097/00006454-199205000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Gardner H A R, Martinez A J, Visvesvara G S, Sotrel A. Granulomatous amebic encephalitis in an AIDS patient. Neurology. 1991;41:1993–1995. doi: 10.1212/wnl.41.12.1993. [DOI] [PubMed] [Google Scholar]
- 13.Gordon S M, Steinberg J P, DuPuis M H, Kozarsky P E, Nickerson J F, Visvesvara G S. Culture isolation of Acanthamoeba species and leptomyxid amebas from patients with amebic meningoencephalitis, including two patients with AIDS. Clin Infect Dis. 1992;15:1024–1030. doi: 10.1093/clind/15.6.1024. [DOI] [PubMed] [Google Scholar]
- 14.Jones D B, Visvesvara G S, Robinson N M. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc United Kingdom. 1975;25:221–232. [PubMed] [Google Scholar]
- 15.Khunkitti W, Lloyd D, Furr J R, Russel A D. Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility. J Infect. 1998;36:43–38. doi: 10.1016/s0163-4453(98)93054-7. [DOI] [PubMed] [Google Scholar]
- 16.Lloves M, Lores B, Pascual S, Arias C, Paniagua E. Isolation of Acanthamoeba spp. in intensive aquaculture areas of Vigo estuary (NM, Spain) Sci Mar. 1996;60:549–551. [Google Scholar]
- 17.Martinez A J. Free-living amebas: natural history, prevention, diagnosis, pathology, and treatment of disease. Boca Raton, Fla: CRC Press; 1985. [Google Scholar]
- 18.Martinez A J, Markowitz S M, Duma R J. Experimental pneumonitis and encephalitis caused by Acanthamoeba in mice: pathogenesis and ultrastructural features. J Infect Dis. 1975;131:692–699. doi: 10.1093/infdis/131.6.692. [DOI] [PubMed] [Google Scholar]
- 19.Martinez A J, Visvesvara G S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7:583–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakawa G J, McCalmont T, Altman J, et al. Disseminated acanthamebiasis in patients with AIDS. A report of five cases and a review of the literature. Arch Dermatol. 1995;131:1291–1296. [PubMed] [Google Scholar]
- 21.Robinson G, Wilson S E, Williams R A. Surgery in patients with acquired immunodeficiency syndrome. Arch Surg. 1987;122:170–175. doi: 10.1001/archsurg.1987.01400140052006. [DOI] [PubMed] [Google Scholar]
- 22.Seidel J S, Harmatz P, Visvesvara G S, Cohen A, Edwards J, Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306:346–348. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- 23.Selby D M, Rakusan T A, Markle B M, Visvesvara G S. Amebic osteomyelitis in a child with acquired immunodeficiency syndrome: a case report. Pediatr Pathol Lab Med. 1998;18:89–95. [PubMed] [Google Scholar]
- 24.Sepkowitz K A. Effect of HAART on natural history of AIDS-related opportunistic disorders. Lancet. 1998;351:228–230. doi: 10.1016/S0140-6736(05)78279-9. [DOI] [PubMed] [Google Scholar]
- 25.Sison J P, Kemper C A, Loveless M, McShane D, Visvesvara G S, Deresinski S C. Disseminated Acanthamoeba infection in patients with AIDS: case reports and review. Clin Infect Dis. 1995;20:1207–1216. doi: 10.1093/clinids/20.5.1207. [DOI] [PubMed] [Google Scholar]
- 26.Slater C A, Sickel J Z, Visvesvara G S, Pabico R C, Gaspari A A. Successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N Engl J Med. 1994;331:85–87. doi: 10.1056/NEJM199407143310204. [DOI] [PubMed] [Google Scholar]
- 27.Szenasi Z, Endo Y, Yagita K, Nagy E. Isolation, identification and increasing importance of “free-living” amoebae causing human disease. J Med Microbiol. 1998;47:5–16. doi: 10.1099/00222615-47-1-5. [DOI] [PubMed] [Google Scholar]
- 28.Tachikawa T, Ishibashi Y, Takazawa S, Miyanaga Y, Watanabe R. Entire course of Acanthamoeba keratitis in a patient. Folia Ophthalmol Jpn. 1995;46:1035–1040. [Google Scholar]
- 29.Tan B, Weldon-Linne C M, Rhone D P, Penning C L, Visvesvara G S. Acanthamoeba infection presenting as skin lesions in patients with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1993;117:1043–1046. [PubMed] [Google Scholar]
- 30.Wiley C A, Safrin R E, Davis C E, et al. Acanthamoeba meningoencephalitis in a patient with AIDS. J Infect Dis. 1987;155:130–133. doi: 10.1093/infdis/155.1.130. [DOI] [PubMed] [Google Scholar]