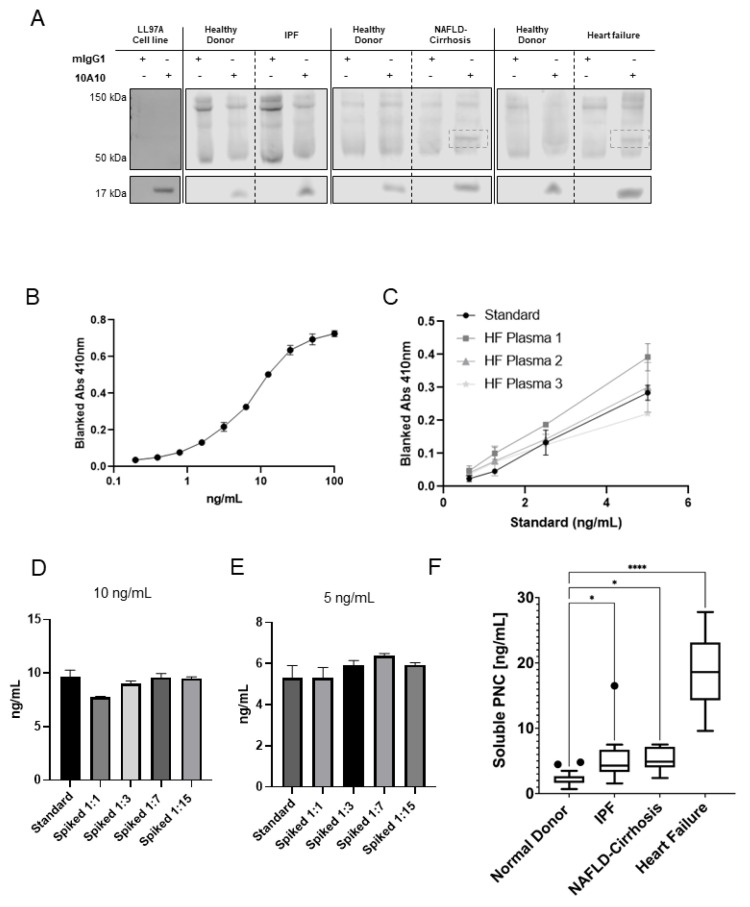
Figure 4.
Feasibility and development of a PNC ELISA. (A) Soluble PNC product was immunoprecipitated from LL29 conditioned media and patient plasma from pooled healthy donors, IPF patients, NAFLD-Cirrhosis patients and cardiomyopathy patients using m-α-PNC mAb 10A10 compared to mouse IgG1 isotype control (mIgG1) and immunoblotted. (B) Recombinant prodomain analyte was serially diluted in duplicate starting at 100 ng/mL in standard diluent and measured by ELISA to determine the range of the standard. (C) Linearity of endogenous analyte was measured. Plasma from patients with heart failure was serially diluted 1:3, 1:7, 1:15, 1:31 in standard diluent and sPNC was measured by ELISA in duplicate. All linear regression r2 values were calculated to be within acceptable range (r2 ≥ 0.99). (D,E) Healthy donor plasma was diluted in standard diluent to indicated dilutions (1:1, 1:3, 1:7, 1:15) then spiked with recombinant prodomain analyte at 10 ng/mL (D) and 5 ng/mL (E) and analyzed by ELISA. Recovery of analyte was quantified and compared to back calculation of the standard. All dilutions were within the consensus range of 20 percent ± the standard back calculations at concentrations 10 ng/mL and 5 ng/mL. (F) Plasma was assayed for sPNC from healthy controls (n = 26), patients with IPF (n = 9), NAFLD with cirrhosis (n = 12), and cardiomyopathy (n = 9). Ordinary one-way ANOVA analysis with Dunnett’s multiple comparisons test was performed to determine significance (* p ≤ 0.05, **** p ≤ 0.0001).