Abstract
Immune therapeutic exosomes, derived exogenously from dendritic cells (DCs), the ‘directors’ of the immune response, are receiving favorable safety and tolerance profiles in phase I and II clinical trials for a growing number of inflammatory and neoplastic diseases. DC-derived exosomes (EXO), the focus of this review, can be custom tailored with immunoregulatory or immunostimulatory molecules for specific immune cell targeting. Moreover, the relative stability, small size and rapid uptake of EXO by recipient immune cells offer intriguing options for therapeutic purposes. This necessitates an in-depth understanding of mechanisms of EXO biogenesis, uptake and routing by recipient immune cells, as well as their in vivo biodistribution. Against this backdrop is recognition of endogenous exosomes, secreted by all cells, the molecular content of which is reflective of the metabolic state of these cells. In this regard, exosome biogenesis and secretion is regulated by cell stressors of chronic inflammation and tumorigenesis, including dysbiotic microbes, reactive oxygen species and DNA damage. Such cell stressors can promote premature senescence in young cells through the senescence associated secretory phenotype (SASP). Pathological exosomes of the SASP amplify inflammatory signaling in stressed cells in an autocrine fashion or promote inflammatory signaling to normal neighboring cells in paracrine, without the requirement of cell-to-cell contact. In summary, we review relevant lessons learned from the use of exogenous DC exosomes for immune therapy, as well as the pathogenic potential of endogenous DC exosomes.
Keywords: exosomes, dendritic cells, periodontitis, SASP, immune senescence, Porphyromonas gingivalis
1. Introduction
The overall goal of immune therapy is to restore homeostasis or health. Depending on the threat received, immune therapy can consist of various adjuvants or immune checkpoint inhibitors to stimulate tumor destruction or eliminate intracellular pathogens. It can also consist of immune suppressive factors for autoimmune disorders and unresolved chronic inflammatory conditions. The principal antigen presenting cells in the body that direct these pathways (and that are pliable for immune therapy), are dendritic cells (DCs). DCs patrol the peripheral tissues for danger signals from foreign antigens (Ag) and microbes. Internalization that ensues stimulates DC maturation. This entails upregulation chemokine receptors, accessory molecules and inflammatory cytokines needed to initiate an adaptive immune response in the lymphoid organs [1]. For example, mature DCs under the appropriate cytokine milieu direct Ag-specific cytotoxic T cell response for malignant cell destruction [2]. Mature DCs can also direct a T-helper cell 17 (Th17) response to eradicate invading bacteria, though these same effectors, when poorly regulated, can promote degenerative bone diseases [3,4,5]. Immature DCs (iDCs) in contrast can promote immune tolerance by induction of T cell anergy and regulatory T cell (Treg) responses to terminate inflammation [3,6,7,8,9,10] and attenuate inflammatory bone loss [11,12]. The unique capability of DCs to direct these responses has fueled immense interest in their use in immune therapy, for example, by using genetically modified DCs or DCs armed with immunosuppressive cytokines to suppress inflammatory disease in animals. The limitations of such therapy include the rarity of DCs and Treg in vivo and phenotypic instability of DCs [13]. Targeting proinflammatory cytokines using inhibitors of TNF and IL1 has shown therapeutic efficacy for treating inflammatory diseases. However, effects are short lived due to rapid clearance and proteolytic degradation in challenging inflammatory environments [14].
Exosomes (EXO), nano-sized extracellular vesicles (EVs) that originate in the endocytic pathway [15], were first discovered from sheep reticulocytes in 1987 [16]. Since then, the ubiquity of exosomes in all tissues and body fluids, including saliva, have been recognized. Intriguingly, EXO can retain sensitive cargo, including proteins, miRNA, mRNA, DNA and lipids, and transfer that cargo to distant sites in the body, where functions of target cells can be modified. This approach has garnered intense interest in the therapeutic potential of EXO [17,18,19,20,21,22,23]. Other than cargo preservation, EXO have many other features of an ideal delivery vehicle, including long circulation time, phenotypic stability and low levels of clearance and degradation. Moreover, EXO released from cells are endowed with surface adhesion molecules and other proteins that promote intercellular communication and uptake by recipient cells. These features are lacking in synthetic agents such as liposomes and other nanoparticles, which can also have toxicity issues [24,25,26,27,28]. EXO can be isolated from DCs, and their small size (30–150 nm) can optimize the delivery of their cargo to recipient cells. Moreover, DC EXO harbor unique proteins that shield them from attack by the complement system [29] and promote binding to tissue integrins [30]. DC EXO can also be loaded with cytokines or peptides using direct or indirect approaches [31,32,33]. EXO from immunostimulatory cargo-loaded mature DCs have been touted for anti-cancer benefits [34], while tolerogenic DC-derived EXO equipped with immunoregulatory cargo offer promise for treatment of autoimmune and inflammatory diseases [35]. The goal of this review is to summarize lessons learned from the use of exogenous DC EXO as immune therapy for chronic diseases, juxtaposed upon the pathogenic potential of endogenously induced DC EXO in infectious/inflammatory diseases.
2. Dendritic Cells (DCs): Directors of the Immune Response
DCs consist of multiple subsets, endowed with distinct pattern recognition receptor repertoires, that play distinct functions in the immune system. The phenotypic traits of these subsets, with commonalities and distinctions between mouse and human DC subsets, have been reviewed elsewhere [36]. This review will focus on conventional myeloid DCs, the most numerous DCs in blood and tissues of mice and humans, and which are most active in capture of bacteria. Commensal bacteria fail to activate DC maturation upon phagocytosis [37,38,39,40,41], resulting in an immature phenotype that induces bone protective T-regulatory cells (Tregs) to resolve inflammation [3,6,7,8,9,10]. However, prolonged exposure to dysbiotic bacteria triggers inflammatory signals that drive phenotypic instability, and loss of immune tolerance. DCs emerge in the periphery with hyperinflammatory or mature phenotype, involved in bone damaging Th-17 effector T cells [1,3,4,5,42,43].
Immature DCs internalize and process pathogen-associated antigens, stimulating upregulation of proteins needed for activation of efficient antigen presentation to T cells. These signals include MHCI/II molecules (Signal 1), which present processed antigen to T cell receptors (TCR), accessory molecules (e.g., CD86 and CD80) (Signal 2), which bind with CD28 on T cells to amplify signaling needed for optimum T cell activation. Signal 3 consists of cytokines and other soluble proteins that support T cell expansion. Expression of chemokine marker CCR7 by mature DCs guides them to lymph nodes for engagement with naïve T cells [44,45]. A single DC can contact approximately ~5000 T cells within an hour [46]. Free antigens may also travel to the lymph nodes and be presented to T cells by specialized DCs. DCs have the unique capacity to migrate to regional lymph nodes, unlike tissue resident macrophages and initiate clonal expansion of Ag-specific T cells [37].
Instructive in the design of immune therapeutic strategies is the capability of immature or tolerogenic DCs to obtund activation of TCR and CD28 signaling in T cells, resulting in T cell apoptosis or anergy (unresponsiveness) while promoting Treg differentiation. Of particular note is the minimal expression of signals 2 and 3, and high induction of TGFB1 and IL10 in tolerogenic DCs. TGFB1 promotes Treg differentiation while IL10 maintains Treg expansion and phenotypic stability. These cytokines can also inhibit other T and B effector cells and inhibit resident immune cells. In contrast, mature DCs secrete several proinflammatory mediators to promote immunostimulatory T effector cells including Th1, Th2 and Th17 while inhibiting Tregs [10,41]. TGFB1 and IL10 have autocrine functions on DCs, helping to generate and maintain tolerogenic DCs [41,47]. TGFB1 is essential for in vivo development of epidermal Langerhans DCs in mucosa, reported to maintain immune tolerance and mitigate inflammatory bone loss in inflammatory diseases [38,41]. The remarkable plasticity of DCs and other immune cells in an inflammatory environment has led to the development of strategies to reprogram these cells to resolve inflammation through induction of tolerogenic DCs and Tregs [14].
3. Exosomes (EXO): The Key to Sustained and Stable Immune Reprogramming
The discovery of EXO in the late 1980s led to the initial notion that they were waste products result from cellular damage [16]. EXO are derived from the internal vesicles of multivesicular bodies (MVBs) and are released into the extracellular environment by numerous cell types. EXO carry a variety of proteins [48,49,50,51], mRNAs, and small RNAs [51,52], and lipids [51,53,54,55,56] that can influence the phenotype and functions of adjacent cells. EXO can deliver this cargo to target recipient cells locally or at distant sites through release into the bloodstream. The bioactive cargo of EXO, released by all eukaryotic cells, reflects the function and phenotype of donor cells, with the advantage that direct cell-to-cell contact is not necessary for intercellular communication. This has obvious implications for therapeutics and has boosted EXO research in recent years [17,18,19,20,21,22,23].
4. General Characteristics of EXO
4.1. Size, Morphology and Physical Features
EXO are cup-shaped in scanning electron microscopy (SEM) images, which is an artifact of the fixation/contrast step that induces shrinking of subcellular structures. EXO prepared by Cryo-EM have a round shape. EXO are 30–150 nm in diameter and thus smaller than plasma membrane vesicles (200–1000 nm) [57]. Nanoparticle tracking analysis is used to confirm size distribution of isolated EVs, based on the Brownian motion of vesicles in suspension. Conventional flow cytometry (FC) cannot distinguish between vesicles that are <300 nm, though immunolabeled beads can be used to bind EV ligands, and label them with fluorochrome conjugated antibody to allow visualization by FC [58]. EXO light scatter is correlated to their size, geometry, and composition. EXO also can float in density gradients and have an equilibrium at density of 1.13–1.19 g/mL in sucrose. A large range of densities could reflect the heterogeneity of vesicles from small and large EVs. Subtypes of small EVs could also display different densities [59].
4.2. Composition (Protiens, Lipids and Nuclic Acid)
4.2.1. Proteins
EXO contain a specific subset of cellular proteins, some of which depend on the cell type that secretes them, whereas others are found in most EXO regardless of cell type. The latter include proteins from endosomes, the PM, and the cytosol. Proteins from the nucleus, mitochondria, endoplasmic reticulum, and golgi apparatus are deficient in EXO [51]. Two major domains are recognized:
Extracellular Membrane Bound Domain. This domain contains adhesion molecules tetraspanins, integrins, and MFGE8 (lactahedrin). Tetraspanins are highly expressed on EXO membranes and include CD81, CD82, CD9, and CD63. CD63 is more commonly associated in the smallest vesicles (<50 nm), while CD9 is more ubiquitous than other tetraspanins. They facilitate anchoring of multiple proteins and are important for efficient MHCII surface expression and antigen presentation on DCs. They mediate cell adhesion, motility, activation, and proliferation. Several integrins guide EXO biodistribution/trafficking and function. MFGE8 (lactahedrin) can act as an adhesion molecule that binds to phosphatidylserine (PS) exposed on the surface of apoptotic cells and integrins on phagocytic cells to facilitate phagocytosis and clearance of dead cells [48,49,50,51]. Antigen presentation and costimulatory molecules MHCI, MHCII, and CD86 are also in this domain, and commonly derived from antigen presenting cells [48,49,50,51]. Membrane transport/fusion Annexins, flotillins, RABs and ARFs are found here. Annexins act as scaffolding proteins to anchor other proteins to the cell membrane. They are involved in vesicle transport and sorting of endocytic vesicles to early endosomes and fusion of secretory vesicles to plasma membrane for export (endocytosis/exocytosis). Annexins can suppress phospholipases, inhibit arachidonic acid inflammatory metabolites, promote neutrophil detachment, and apoptosis for phagocytosis by macrophages and reduction in recruitment. Flotillins are components of lipid rafts and have a role in endocytosis, trafficking, and cell adhesion. RABs and ARFs are involved in vesicle formation, vesicle movement along actin, and tubulin networks and membrane fusion [48,49,50,51]. Other transmembrane proteins (e.g., LAMPs, TfR) are also described [51].
Intracellular Domain of EXO [48,49,50,51] contains ESCRT components (TSG101) and associated proteins (ALIX). TSG101 and ALIX in EXO play roles in multivesicular body (MVB) biogenesis, involving ubiquitin tagged proteins entering endosomes through the formation of intraluminal vesicles. Heat shock protein 70 and 90 are involved in protein folding, clearance of misfolded proteins and stabilization of proteosome and protect cell from stress, while signal transduction molecules include G proteins, syndican, and syntenin. Other cytosolic proteins have been described, such as histones, ribosomal proteins, and proteasome and cytoskeletal proteins, including actin, cofilin, moesin, tubulin, which are expressed. Enzymes such as elongation factors and glyceraldehyde 3-phosphate dehydrogenase are expressed in EXO.
4.2.2. B-Lipids
EXO are enriched with sphingomyelin, phosphatidylserine (PS), cholesterol, and ceramide. This specific lipid composition (e.g., sphingomyelin and cholesterol) resembles detergent-resistant subdomains of the PM called lipid rafts. This is supported by the presence of lipid raft–associated proteins, GPI-anchored proteins and flotillins and resistance of EXO to detergents. It is suggested that lipid rafts could be endocytosed from the plasma membrane (PM) and segregated into intraluminal vesicles (ILVs) during their formation at MVBs and eventually released in EXO [51,53,54,55,56].
4.2.3. Nucleic Acids
mRNAs, miRNAs, short noncoding RNAs and DNA have been identified in EXO cargo. A specific sequence within miRNA has been found to guide sorting to EXO through binding to sumoylated ribonucleoproteins. The protein, heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1), was shown to recognize and binds specific sequence motifs in miRNAs to facilitate their loading into exosomes. In addition, hnRNPA2B1 sumoylation regulate loading of EXO miRNAs [60]. Another study showed that the RNA binding protein SYNCRIP (synaptotagmin-binding cytoplasmic RNA-interacting protein) directly binds to specific EXO miRNAs sharing common motif [61]. ESCRT-II is an RNA binding complex and may also function to select RNA for incorporation into EXO [51,52]. EXO composition can be changed by modifications of in vitro culture conditions that mimic different extracellular environments or by altering the physiologic, differentiation or maturation state of the secreting cells. For instance, inflammatory signals (e.g., LPS, TNFα, IFNγ) strongly affect the protein and/or RNA composition of EVs released by dendritic, endothelial, or mesenchymal stem cells [51].
5. EXO Biogenesis
MVBs and their ILVs can be formed by both ESCRT-dependent and -independent mechanisms, which can lead to distinct EXO.
5.1. ESCRT-Dependent Mechanisms
Formation of MVBs and EXO is driven by the endosomal sorting complex required for transport (ESCRT), which is composed of four protein complexes (ESCRT-0, -I, -II and -III) with associated proteins (VPS4, VTA1, ALIX). The ESCRT-0 complex recognizes and sequesters ubiquitinated proteins in the endosomal membrane. ESCRT-0 consists of HRS (hepatocyte growth factor–regulated tyrosine kinase substrate) that recognizes the monoubiquitinated proteins and associates with STAM (signal transducing adaptor molecule). HRS recruits TSG101 of the ESCRT-I complex. ESCRT-I and -II is responsible for endosomal membrane deformation and inward budding with the sorted cargo into the endosomal lumen. ESCRT-I is involved in the recruitment of ESCRT-III via ESCRT-II or ALIX. ESCRT-III induces vesicle scission. The dissociation and recycling of the ESCRT machinery require interaction with the AAA-ATPase VPS4. ESCRT accessory protein Alix is involved in exosome biogenesis and exosomal sorting of syndecans through an interaction with syntenin, which is dependent on ESCRT-II, ESCRT-III, and VPS4 function. ALIX also can bind to transferrin receptors (TfR) and promote TfR sorting onto ILVs of MVBs. Some EV cargo proteins, which are not ubiquitinated, can be also selected. HSC70 chaperone can bind and allow sorting of soluble cytosolic proteins containing a KFERQ sequence to PS on the MVB outer membrane and formation of ILVs in a TSG101- and VPS4-dependent manner. HSC70 can also allow targeting of TfR to EXO. Ubiquitinated and KFERQ-containing proteins are abundant in EXO [49,51].
5.2. ESCRT-Independent Mechanisms
Studies show that some cargo is still sorted in EXO after knocking down ESCRT complex proteins through other mechanisms. Tetraspanins CD63, CD81, and CD9 are highly enriched in MVEs and have recently shown to be instrumental in formation ILVs and EXO independently of ESCRT [62,63,64]. Ubiquitinated MHCII (mutant form lacking ubiquitination site that cannot be recognized by ESCRT) can still be sorted to EXO in ESCRT independent manner. MHC class II molecules in EXO are associated with large protein complexes containing tetraspanins, which support their role in EXO biogenesis [51,65], including lipid metabolism enzymes. Neutral sphingomyelinase (nSMase) induces hydrolysis of sphingomyelin into ceramide in the limiting membrane of MVB and induce inward budding and formation of ILVs in an ESCRT-independent manner. NSMase can be inhibited by GW4869 to decrease EXO biogenesis. These observations are consistent with the presence of high concentrations of ceramide in EXO. Phospholipase D2 is another lipid enzyme that allows hydrolysis of phosphatidylcholine into phosphatidic acid and formation of ILVs [55]. A small integral membrane protein of lysosomes and late endosomes (SIMPLE) is secreted in association with EXO and has been shown to also regulate EXO biogenesis [66].
6. Mechanisms of EXO Secretion
6.1. Role of Rab GTPases in EXO Secretion
Rabs can be involved in vesicle budding, MVB mobility through interaction with the actin or tubulin cytoskeleton (with the help of motors such as myosins and kinesins), tethering, docking, and fusion to the membrane of an acceptor compartment or plasma membrane [67]. RAB11 mediate exocytosis of recycling endosomes [68]. RAB35 mediate exocytosis of early endosomes [69]. RAB27A/B mediate exocytosis of late endosomes [70]. RAB7 also mediate exocytosis of another population of late endosomes. Recycling of early endosomes fuse with the PM due to RAB11 or RAB35, respectively, to secret EXO mainly containing Wnt-associated, Proteolipid protein PLP, TfR, and flotillin. Late endosomes fuse with plasma membrane via RAB27 to release EXO rich in late endosomal proteins including CD63, ALIX, and TSG101. Late endosomes can also fuse with plasma membrane via RAB7 to release EXO rich in ALIX, syntenin-and syndican. Thus, different subtypes of endosomes can generate different population of EXO [51].
6.2. Potential Role for Other Molecules in Exosome Secretion
Activation of purinergic receptors by ATP, increases intracellular calcium and cytoskeleton remodeling, promoting EXO secretion. Neurotransmitters depolarize cells promoting EXO exocytosis. Lipopolysaccharide stimulation of T cells, B cell receptor signaling and DC maturation, as well as invasion of DCs by dysbiotic pathogen Porphyromonas gingivalis [71] as discussed below, induces release of EXO. Platelets produce more EXO when thrombin receptors are activated. EXO secretion is increased in peptide-loaded immature dendritic upon interaction with T cells. Diacyl glycerol kinase α (DGKα) in T cells has been shown to inhibit EXO secretion while citron kinase is involved in the exocytosis of late endosomal compartments [51].
6.3. Role for SNAREs and Other Components of the Fusion Machinery
SNARE proteins form complexes with SNAPs between two membrane compartments and mediate membrane docking and fusion. Ykt6, R SNARE, V0 subunit of V-ATPase, VAMP7, VAMP8, and SNAP-23 are possible mediators of Ca regulated fusion of MVBs with the PM. Different cell types may have distinct SNARE complexes that mediate the fusion of different subpopulations of MVBs within a single cell type. Thus, the downregulation of one SNARE protein might affect the secretion of only a particular subpopulation of EXO [51,72,73,74].
6.4. Distinct Populations of MVBs That Modify EXO Secretion
Different subpopulations of morphologically distinct multivesicular bodies (MVBs) based on the size and appearance of the ILVs are known. MVBs bearing early endosomal markers like RAB4 and RAB5A fuse with the PM for EXO release, more commonly than late endosomes. CD63 cholesterol-positive and CD63 lysobisphosphatidic acid (LBPA) negative MVBs are more committed to fusion with the plasma membrane for EXO release, while CD63 cholesterol-negative and CD63 LBPA- positive MVBs are fated for fusion with lysosomes and degradation. In immature DCs, ubiquitinated MHC class II molecules sorted into MVBs mainly are committed for lysosomal degradation. In the presence of antigen-specific T cells, DCs express MHC class II–CD9 complex containing MVBs that fuse with the PM to release EXO. Thus, some MVBs are destined for the degradation pathway, whereas others are fated for exocytosis [51].
7. EXO Fate and Mechanism of Uptake in Recipient Cells
In order to understand how EXO may alter physiological and biological functions in vivo, it is essential to study their biodistribution to target organs and cells, and how EXO interact with recipient cells.
7.1. Planktonic EXO
Planktonic or free-floating EXO can bind to extracellular matrix and interact with acceptor cells in situ, or they can redistribute through the lymphatics or blood stream and interact with distant cells, tissues, and organs. Circulation of EXO in body fluids is short lived, with ~30 min half-life in plasma after IV administration in lateral tail vein of mice. EXO are typically recovered in lung, liver, and spleen up to 4 h later. EXO can be sequestered by circulating monocyte/macrophages and DCs in the liver and spleen. Moreover, EXO express a variety of integrin and chemokines that can guide in vivo trafficking and tissue homing [75,76,77].
7.2. Exosome Cellular Recognition and Target Cell Specificity
Tetraspanins CD63, CD9, and CD81 are instrumental in EXO adhesion, motility, signal transduction and activation of acceptor cells. Differences in tetraspanin complexes influence target cell selection [78,79,80,81,82], spatial assembly of MHCII for antigen recognition and presentation to T cells and may affect the induced TCR signaling [83]. The interaction of ICAM-1 on mature DC-derived EXO with LFA-1 on T cells is critical for efficient naive T cell activation [84]. In addition, several other exosomal integrins have demonstrated roles in adhesion and EXO trafficking [51,75,85,86]. The level of MHC expression on isolated EXO is reflective of their expression on the parent cell. MHC class I molecules on EXO interact with ILT2, ILT4 inhibitory receptors on CD8 plus T cells and NK cells, which promote the inhibition or activation of these cells. EXO from DCs and B lymphocytes express MHC class II for antigen presentation and activation of CD4 T cells [87,88,89,90]. Moreover, EXO can bind and fuse with the plasma membrane of recipient cells, to which they transfer their cargo proteins and RNAs. The phenomenon is regulated by tetraspanin complexes and integrins [86,91].
7.3. Soluble and Juxtacrine Signaling by Exosome Surface Ligands
Soluble signaling involves cleavage of ligands from the EXO surface by proteases in the extracellular matrix while juxtracrine signaling requires the juxtaposition or interaction of exosomal surface bound ligands and receptors on target cells. EXO membrane bound FasL, TRAIL and TNF can be cleaved by metalloproteinases to form soluble cytokines. Soluble FasL and TRAIL, TNF and TGFB1 have a reduced activity when compared to that of the membrane-bound form of these ligands [86,92,93,94].
7.4. EXO Internalization
Several modes of EXO internalization have been proposed, depending on EXO size and the acceptor cell type. Large EVs or aggregates of small EVs like EXO mostly induce phagocytosis, whereas individual small EVs or EXO can be internalized by nonphagocytic processes, such as pinocytosis [86].
7.4.1. Phagocytosis
Phagocytosis of EXO requires opsonin receptors (FcR and complement receptors), scavenger receptors or Toll-like receptors. EXO can bind with opsonins and are internalized by the cell where they fuse with limiting membrane of endocytic compartments. Most EXO are sorted to endosomal rather than lysosomal pathways. Phagocytosis of EXO is dependent on the actin cytoskeleton, PI3K, and dynamin2. Phagocytosis can be blocked by using actin polymerization inhibitor as cytochalasin D [95].
7.4.2. Pinocytosis
Macropinocytosis. During macropinocytosis, plasma membrane protrusions are driven by polymerization of actin filaments forming an invagination for non-specific endocytosis of extracellular fluids and small particles. Phosphatidyl serine on the surface of EXO activates macropinocytosis. Macropinocytosis of EXO is dependent on Na+/H exchange, actin and PI3K, it can be inhibited using of Na+/H+ ion exchange inhibitors such as amiloride [96,97].
Micropinocytosis includes clathrin dependent endocytosis (ligand/receptor-mediated endocytosis) and clathrin independent endocytosis (lipid raft-mediated endocytosis). The former involves EXO ligand engagement with specific receptors on the cellular plasma membrane. It utilizes clathrin and adaptor protein 2 complexes which coat the membrane and induce invagination of the membrane into a vesicle. It is also dynamin dependent and can be inhibited by clathrin knock down using siRNAs [86,98]. The latter involves dynamin dependent and dynamin independent mechanisms, requiring cholesterol and sphingomyelin-rich microdomains in the plasma membrane. Dynamin dependent is mediated by caveolin 1) and/or RhoA kinase which can be inhibited by siRNA knock down of caveolin 1 or RhoA kinase respectively. Dynamin independent is divided into ARF6 or CDC42 GTPase and Flotilin-1 mediated. Studies showed knock down of flotillin as well as inhibitors or silencing the associated GTPases inhibits endocytosis by this mechanism [86,98].
8. Exogenously Produced DC EXO: Natural Nano-Delivery Systems for Inflammatory Diseases
The therapeutic advantages of EXO nano-delivery systems have been previously reviewed in detail [14,35,99,100,101,102,103,104,105]. DC-derived EXO mimic the biology of donor DCs generating considerable interest in their use as cell free therapeutic agents [106]. During interaction with cognate T cells, DCs promote the biogenesis of MVPs with MHC and CD9, carrying intraluminal vesicles to be exocytosed upon fusion with the plasma membrane [65,107]. Mature immune-stimulatory (mDCs EXO) or immature immune regulatory (imDCs EXO) can be isolated from mature or immature DCs, respectively. mDCs EXO loaded with specific peptides can elicit potent specific immune activation, resulting in tumor cell detection and eradication, and microbial elimination [22,108,109]. In clinical trials, autologous mDCs EXO vaccinated into cancer patients showed additional therapeutic effects without significant side effects [110,111]. EXO from DCs can be pulsed with bacterial antigen peptides for bacteria-free vaccines [112,113] or with Toxoplasma gondii anti-parasite immunity in mice [114].
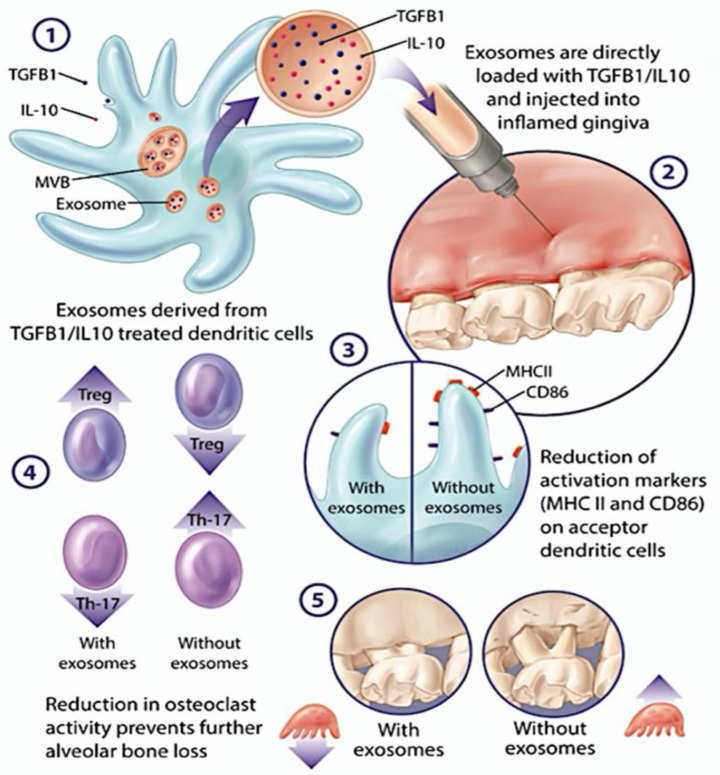
On the opposite side of the immune spectrum, immune tolerance can be induced by iDC EXO. DCs modified with anti-inflammatory molecules, including IL-10, IL4, TGFB, FasL, IDO, and CTLA4 have generated various immunoregulatory EXO subtypes for treatment of immune disorders [14,93,99,100,102,103,104,105]. DC lineage marker CD11c and low levels of antigen presenting MHCII and costimulatory molecules CD80, CD86 are a feature of EXO from immature or tolerogenic DCs. iDC EXO inhibit T cell activation and proliferation directly or indirectly by reprograming the biological function of acceptor DCs and promoting a regulatory/suppressive subset [14,93,103]. Delivery of iDC EXO by an intradermal route resulted in their interaction with acceptor CD11c+ cells in dermis and regional lymph nodes. Macrophages and CD11c+ DCs in liver and spleen were found to take up EXO injected intravenously [103]. iDCs EXO carrying tolerogenic molecules inhibit rheumatoid arthritis and joint destruction in animal models [14,102,103,104]. In an inflammatory bowel disease (IBD) model, TGF-β1 enriched iDCs EXO reversed the disease severity and clinical indices. This occurred through repression of Th17 responses and enhancement of T-regulatory cells (Tregs) [93]. Experimental autoimmune encephalomyelitis responded well to injection of iDCs EXO with membrane bound TGF-β1 [115]. iDC EXO can prolong the survival times of cardiac and liver transplantation [116,117] and improve cardiac function in mice post MI [118]. Our team recently found that immune-regulatory EXO (regDCs EXO) subsets, loaded with TGFb1 and IL10, were retained at the inflammatory sites of oral injection and were efficient in reprogramming the immune function of local acceptor DCs and CD4+ T cells, inducing tolerogenic DCs and Tregs in situ. Moreover, regDC EXO protected their therapeutic cargo against proteolytic degradation and abrogated inflammatory bone loss in experimental periodontitis [119] (Figure 1). The proteomic cargo and biodistribution characteristics of DC EXO were further examined. The protein cargo of regDC EXO was indeed complex, but dominated by tissue trafficking, cell binding, and immunoregulatory proteins were dominant, consistent with their functions [120]. Interestingly intravenous injection of regDCs EXO in mice led to predominant accumulation in the lungs. This generated interest in possible therapeutic application of regDC EXO for COVID-19. We showed that regDCs EXO inhibit the expression of ACE2, the SARS-CoV-2 target receptor, in respiratory tract epithelial cells (PBTECs) by a TGFb1 dependent mechanism. This generated speculation that regDC EXO may have efficacy in reversing harmful inflammatory lung responses in COVID-19, as reported [119]. Another approach is to block the SARS-CoV-2 entry point using regDC EXO therapy, reducing the infection severity [121]. Other suggested approaches involve EXO enrichment with antiviral drugs [122], decoy ACE2 [123], or the S-protein of the SARS-CoV-2, for COVID-19 infection [124].
Figure 1.
DC Exosome (Exo) Therapy for the Inflammatory Bone Disease Periodontitis. 1. TGFb-/IL-10 enriched exo (regDC exo) are released. 2. Exo injected into palatal gingiva interproximal at site of ligand induced PD. 3. Exo uptake by acceptor gingival DCs reduces DC maturation and alters cytokine profile; 4 and 5. Modulation of osteoclastogenic Th17 response by regDCexo.
EXO have been isolated from other immune cells for immune reprogramming, including Tregs [125,126,127] B lymphoblasts [128,129], natural killer cells [130] and mast cells [131]. EXO from macrophages were loaded with linezolid and vancomycin to inhibit intracellular infection by methicillin-resistant Staphylococcus aureus (MRSA) [132,133]. Other groups loaded EXO derived from muscle cells with various virus antigens to be used as a vaccine and induce specific cytotoxic T lymphocyte immunity [134]. In summary, DC EXO are preferable to whole immune cell-based therapy for inflammatory and infectious diseases for many reasons [14,35,99,100,101,102,103,104,105].
9. Endogenously Produced DC EXO: Diagnostic and Pathogenic Potential, Role in Immune Senescence
9.1. EXO Diagnostics
Endogenously produced EXO are found in the intracellular spaces, in tissues and body fluids. The proteins, lipids, and nucleic acids [135] in EXO are being studied for defining underlying diseases and conditions. Saliva EXO for example, are under intensive study as a non-invasive diagnostic of premalignant cancer lesions [136]. Salivary EXO from periodontitis patients is enriched in PDL-1 and miRNAs, hsa-miR-140-5p, hsa-miR-146a-5p, and hsa-miR-628-5p) compared with healthy controls [137,138]. EXO and their biologically active cargos offer diagnostic and prognostic information in chronic airway diseases [139], craniofacial disorders [140], renal diseases [141,142,143], neurodegenerative diseases [144], lipid metabolic diseases [145], and tumors [146].
9.2. EXO Pathogenesis
The potential pathogenic role of endogenous DC EXO cannot be overlooked since it can provide additional information about therapeutic applications. During HIV infection, DCs release HIV-bearing EXO that serve as a shuttle carrying HIV viral particles and transmitting infection to CD4T cells [147,148]. Fibronectin and galactin 3 may be instrumental in transmission of HIV infection to bystander T cells by DC EXO [149]. These viral-bearing DC EXO are more infectious and less efficiently eradicated than cell-free viral particles [150].
Involvement of DC EXO has been documented in chronic inflammatory diseases and allergic reactions [119,151,152,153]. We have recently shown the role of EXO derived from mature DCs in exacerbating an inflammatory response and alveolar bone loss in a murine model of periodontitis [119]. Further, ongoing unpublished work from our laboratory has revealed that EXO derived from DCs infected with the keystone oral pathogen P.gingivalis, aggravates inflammatory bone loss in mice. Mature DC EXO migrate to the spleen via CCR7 and induce inflammation in vivo [154] and exacerbate atherosclerosis through TNFa/Nf-KB-mediated stimulation of endothelial cells [155]. This study showed a significant increase in atherosclerotic lesions in APOE−/− mice upon 12-week injection of mature DC EXO [155]. Similarly, EXO secreted from atherogenic macrophages were found to promote atherosclerosis. Transfer of specific EXO miRNAs to recipient macrophages inhibits migration and promotes their entrapment in the blood vessel wall [156]. On the other hand, DC EXO have demonstrated a protective effect in myocardial infarction (MI) [157,158] and Ischemic reperfusion injury (IRI) [158], potentially through induction of Tregs. DC EXO contribute to the production of leukotrienes LT and other inflammatory mediators of arachidonic acid pathway [151,152]. Moreover, DC EXO can acquire and present the aeroallergen, Fel d1 inducing a Th2 response with the production of IL4 in PBMCs from individuals with cat allergy [153]. Additionally, donor DC EXO elicit an immune reaction and promote allograft rejection in cardiac transplantation in mice [159]. Serum EXO from HCV-infected patients contains HCV RNA and induces transmission of HCV to liver cells through viral receptor-independent mechanisms [160]. Serum EXO isolated from patients with tuberculosis were found to carry mycobacterial antigens [161]. EXO of intestinal lumen aspirates of inflammatory bowel disease IBD patients carry pro-inflammatory factors including TNF-α, IL6, and IL8 in levels higher than that of healthy controls. These EXO activate epithelial cells and macrophages to secrete IL-8 [162]. EXO purified from synovial fibroblast of rheumatoid arthritis patients contain TNF-α at the transmembrane domain that induces NF-κB activation and MMP-1 release in acceptor bystander cells [163]. DC EXO have been used as prognostic biomarkers for metastatic melanoma treated with Ipilimumab reflected by the level of CD86 expression [164].
9.3. Alteration of Exosome Secretion and Cargo Content in Infection
Pathogenic invasion of the host cell changes its metabolic activity and endocytic pathway utilization, which in turn affects EVs secretion [71,165]. An increase in the host cell secretion of EXO and associated protein was observed in Plasmodium, Rota virus, Chlamydia psittaci, and Mycobacterium bovis Bacille Calmette–Guerin (M.bovis BCG) infections [165,166,167,168,169]. Moreover, the concentration of secreted EVs in gingival crevicular fluid (GCF) was reported to increase in periodontitis patients compared to healthy/gingivitis subjects [170]. In line with these studies, our group has recently shown that P.gingivalis infection of host DCs leads to an increase in the number of secreted EXO [71], which was restored to base line levels with rapamycin treatment, a known inducer of autophagy [71], thus suggesting a potential role of autophagy in EXO secretion during infection. Autophagy inhibition promotes an increase in EXO secretion in response to accumulation of damaged proteins or organelles, whereas activation of autophagy diminishes EXO release due to the fusion of MVB and autophagosomes [71,171]. Other mechanisms for enhanced release of infection-mediated EXO have been discussed [165]. Ebola and HIV viral infections activate EXO biogenesis by increasing synthesis of CD63, apoptosis-linked-gene-2 product-interacting protein X (Alix) and endosomal sorting complex required for transport machinery-II (ESCRT) proteins [165,172,173,174]. In addition to an increase in the number of released EXO during infection, the host cell-derived EXO exhibit a distinct proteomic, lipid and nucleic acid profile. In this context, the microRNA (miRNA) content of DC EXO, is worth mentioning, as it results from a complex sorting process during cellular biogenesis and export [60]. MiRNAs are especially active in post transcriptional regulation of genes [175]. Recently, we have shown a change in miRNA content of EXO from DCs infected with P. gingivalis [71], contributing to inhibition of beclin-mediated autophagy [176], suppression of ULK1 and LC3I/II [177] (miR17-5p), and impediment of autophagy-dependent clearance of intracellular bacteria [178] (miR106B). Further, our study showed upregulation of miRNA 132-3p in Pg-induced DC EXO, consistent with aged bone-marrow derived DCs (BMDC). Infection-induced DC EXO often contain pathogen-related proteins [71,108,109] and virulence factors [71,179,180,181] that can affect the physiologic host response in multiple ways [165]. EXO can transmit infections to bystander cells [147,172], can evade the immune response [147,150], induce cell apoptosis [165,180,182] or promote immune suppression [113,165]. On the contrary, antigen-bearing EXO can activate the immune response and illicit an antigen specific T cells response [165,183]. Moreover, the efficacy of DC EXO, pulsed with Toxoplasma Gondii, Chlamydia psittaci, sporozoites-extracted- or tumor antigens to elicit an immune response has highlighted DC EXO as a strong candidate for cell free vaccines [108,109,169]. Worthy of note, is the ability of DC EXO to activate a humoral antibody response when challenged with Diphtheria Toxoid, or streptococcus pneumoniae [179,184]. The immunogenicity of EXO from other antigen presenting cells have been reported. For example, Mycobacterium tuberculosis or Mycobacterium bovis-infected macrophages secrete EXO bearing bacterial antigens to stimulate antigen specific immune responses [112,185].
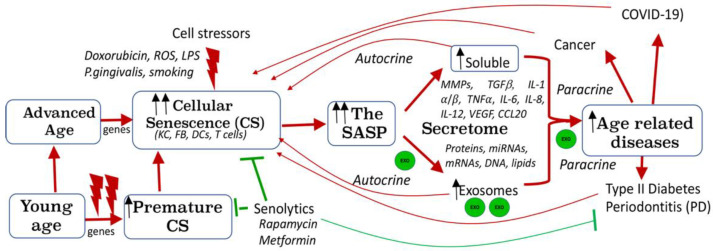
9.4. Role of DC EXO in Immune Senescence and Inflammaging
A well-established feature of physiological ageing is diminished immune function, or immune senescence, coupled with a low-grade chronic inflammation [186,187]. Senescence is regulated by several signaling pathways, most notably, Akt1 [188,189] and mTOR [190,191], with the latter inhibited by rapamycin [191]. Functional deficits are reported in senescent monocytes, macrophages (reviewed in [192]), and T cells (reviewed in [193]). Vulnerability of DCs to senescence was attributed to cellular stressors such as microbial infection [71]. The senescence associated secretory phenotype (SASP), a distinctive feature of senescence (Figure 2), consists of secreted exosomes (EXO) and inflammatory cytokines [194,195,196,197]. Mounting evidence indicates a critical role of EXO in immune senescence, inflammaging and age-related diseases (ARDs) [198]. Reports have shown that senescence leads to an increase in EXO secretion [199] (Figure 2). Recently, we have shown that exosomes derived from P.gingivalis-infected DC are increased in number, transmit immune senescence to bystander immune cells in vitro [71], and accelerate alveolar bone loss in a PD model in vivo (unpublished data). Further, P. gingivalis-induced DC EXO comprised SASP-related molecules, TNFa, IL1B and IL6, and Mfa1 fimbrial protein [71]. Prior published work from our group using human monocyte derived DCs (MoDCs) [200,201,202,203,204,205,206] has identified an important role for Pg minor Mfa1 fimbriae in invasion of MoDCs, activating mTOR (inhibiting autophagy) and hyper-phosphorylating Akt1 (inhibiting apoptosis), suggesting a role in premature immune senescence [189]. An increased role for Mfa1 fimbriae in vivo is suggested by its upregulation in Pg from dental plaques and blood DCs of humans with PD [207]. Activation of the inflammasome, implicated in periodontal inflammaging [208] reportedly orchestrates the SASP [194]. Microbial activation of host DCs triggers inflammasome activation [209] and secretion of pathogenic exosomes that contain mature IL-1B [71]. These pathogenic EXO transmit immune senescence to normal bystander cells, amplifying senescence in paracrine [71]. A study of immune senescence has yielded a wealth of knowledge about ARDs [193]. However, much work is required to better understand the role of EVs in senescence and age-related diseases.
Figure 2.
Cellular Senescence due to Advanced Age and Cell Stressors: Role of Exosomes. Advanced age and canonical (e.g., doxorubicin) and non-canonical (e.g., P.gingivalis) cell stressors can provoke cellular senescence (CS). Premature CS can occur by exposure to X irradiation, doxorubicin, reactive oxygen species (ROS), and microbial CS stressors. Senolytic agents (e.g., rapamycin, metformin) can remove senescence cells. CS profiling identifies elevated SA-β-Gal, p16 INK4A, pAkt-1, p53, p21Waf1/Clip1, and soluble and exosomal SASP. Exosomes (exo, green circles) can transmit senescence to young cells in paracrine. CS is implicated in many age-related diseases such as periodontitis, type II diabetes and COVID-19.
10. Extracellular vesicles (EV), DC EXO and Cancer
Several studies have shown the role of EVs in tumorigenesis. Tumor cells were found to secrete EVs that bear immunosuppressive molecules and disrupt differentiation, maturation, and function of DC [210]. Moreover, tumor EVs inactivate T lymphocytes or natural killer cells to suppress immune responses, promoting tumor progression [211]. At the site of the primary tumor, tumor cells secrete fibronectin-coated EXO at the leading edge of the cell that bind collagen fibrils extracellularly while interacting with integrins on cell membrane. This enhances cancer cell motility and migration by stabilizing cellular protrusions and facilitates amoeboid movement [212]. EXO-carrying multivesicular bodies are docked at cellular protrusion called invadopodia and secrete growth factors and matrix metalloproteinases containing EXO that enhance invadopodia stability, extracellular matrix (ECM) degradation, and invasion. [213,214]. On the other hand, tumor-derived EXO harbor tumor antigens that can activate DCs, stimulating antitumor specific cytotoxic immune response. This is more effective than that achieved by tumor cell lysates or soluble-free antigens when used as vaccines [215]. In addition, DCs EXO have been employed as a subcellular vaccine for cancer therapy in clinical trials [110,111,216] (Table 1). These include two phase I [110,111] and one phase II [216] clinical trials in end-stage cancer patients. The first phase I clinical trial utilized EXO isolated from autologous immature monocyte derived DCs (MoDCs). These DC EXO were loaded with both MHCI and MHCII melanoma associated antigen (MAGE) peptides. Four DC EXO vaccinations were administered at weekly intervals into 13 advanced non-small cell lung cancer (NSCLC) patients. Nine of these patients completed the therapy. Limited DTH reactivity against MAGE peptides, MAGE-specific T cell responses with increased natural killer cell (NK) lytic activity were observed. In addition, DC EXO therapy was found to be safe and well-tolerated [111]. These DCs EXO were also employed in the second phase I clinical trial once weekly for 4 weeks in 15 metastatic melanoma patients. NK cell functions were augmented and only grade 1 toxicity was observed [110]. Phase II clinical trial utilized EXO derived from IFN—stimulated autologous MoDCs. The EXO were loaded with both MHCI and MHCII restricted tumor antigens and administrated in non-small cell lung cancer in one-, two- and three- week intervals in a maintenance immunotherapy protocol. NK cell activity was also promoted in phase II clinical trial with limited T cell activity. Twenty two of the twenty-six patients completed the study. Grade 3 hepatotoxicity was found in one of the cases [216]. These phase I and II clinical trials showed the feasibility of DC EXO as anticancer vaccines, suggesting the safety and potentiality for other systemic diseases.
Table 1.
DC EXO Clinical Trials.
| Disease Type | Phase | n | DCs EXO | Doses | Outcome | Ref |
|---|---|---|---|---|---|---|
| Advanced Non-small cell lung cancer |
I | 13 (9 completed the study) | Autologous MoDCs derived EXO were loaded with MAGE peptides. |
4 vaccinations at weekly intervals. |
Limited T cell reactivity and DTH against MAGE peptides. Increased NK lytic activity. Safe, well tolerated activity. |
[186] |
| Metastatic Melanoma |
I | 15 | Autologous MoDCs derived EXO were loaded with MAGE peptides. |
4 vaccinations at weekly intervals. |
No MAGE-specific T cell response. No DTH response. NK cell activation. Safe, well tolerated. |
[187] |
| Advanced Non-small cell lung cancer |
II | 26 (22 completed the study) | EXO were isolated from IFN-stimulated autologous MoDCs and loaded with MHCI and MHCII restricted cancer antigens. |
Vaccination in 1, 2 and 3 week intervals in a maintenance immunotherapy protocol. |
Limited T cell activity. increased NK cell function. One patient had a grade-3 hepatotoxicity. |
[188] |
11. Conclusions
Exogenously produced exosomes from dendritic cells, custom tailored to activate, suppress or reprogram the immune system are under intensive study for a variety of infectious or inflammatory diseases, including periodontitis. The efficacy and safety of these therapeutic nanoparticles are predicated on further in-depth study of their biogenesis, biodistribution, and modes of cell-to-cell communication. Careful consideration must also be given to the diagnostic and pathologic significance of endogenously produced exosomes, secreted at inflammatory sites, into tissues and body fluids. The molecular cargo of such endogenous exosomes, including infectious agents, proteins, lipids, and nucleic acids, must be thoroughly studied to understand their role in disease, but also their potential for transmitting such cargo, in paracrine, to neighboring cells. This is particularly relevant to transmission and amplification of immune senescence between immune cells with or without cell-to-cell contact.
Author Contributions
Conceptualization, M.E.; R.E. and C.W.C.; writing—original draft preparation, M.E. and R.E.; writing—review and editing, C.W.C.; visualization, M.E. and C.W.C.; funding acquisition, C.W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Carlos and Marguerite Mason Trust grant and by NIH/NIDCR R01 DE029468 (CWC).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dudek A.M., Emartin S., Garg A.D., Eagostinis P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front. Immunol. 2013;4:438. doi: 10.3389/fimmu.2013.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin-Acevedo J.A., Soyano A.E., Dholaria B., Knutson K.L., Lou Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 2018;11:1–25. doi: 10.1186/s13045-017-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukasaki M., Komatsu N., Nagashima K., Nitta T., Pluemsakunthai W., Shukunami C., Iwakura Y., Nakashima T., Okamoto K., Takayanagi H. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-03147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komatsu N., Takayanagi H. Immune-bone interplay in the structural damage in rheumatoid arthritis. Clin. Exp. Immunol. 2018;194:1–8. doi: 10.1111/cei.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph S., Aduse-Opoku J., Hashim A., Hanski E., Streich R., Knowles S.C.L., Pedersen A.B., Wade W.G., Curtis M.A. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 2018:10. doi: 10.1126/scitranslmed.aat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S.-J., Nakagawa T., Kitamura H., Atsumi T., Kamon H., Sawa S.-I., Kamimura D., Ueda N., Iwakura Y., Ishihara K., et al. IL-6 Regulates In Vivo Dendritic Cell Differentiation through STAT3 Activation. J. Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 7.Horton C., Shanmugarajah K., Fairchild P.J. Harnessing the properties of dendritic cells in the pursuit of immunological tolerance. Biomed. J. 2017;40:80–93. doi: 10.1016/j.bj.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonuleit H., Schmitt E., Schuler G., Knop J., Enk A.H. Induction of Interleukin 10–Producing, Nonproliferating Cd4+ T Cells with Regulatory Properties by Repetitive Stimulation with Allogeneic Immature Human Dendritic Cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idoyaga J., Fiorese C., Zbytnuik L., Lubkin A., Miller J., Malissen B., Mucida D., Merad M., Steinman R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Investig. 2013;123:844–854. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa H., Matsumoto T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018;9:350. doi: 10.3389/fimmu.2018.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garlet G.P., Cardoso C.R.D.B., Mariano F.S., Claudino M., De Assis G.F., Campanelli A.P., Ávila-Campos M.J., Silva J.S. Regulatory T cells attenuate experimental periodontitis progression in mice. J. Clin. Periodontol. 2009;37:591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 12.Glowacki A.J., Yoshizawa S., Jhunjhunwala S., Vieira A.E., Garlet G.P., Sfeir C., Little S.R. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc. Natl. Acad. Sci. USA. 2013;110:18525–18530. doi: 10.1073/pnas.1302829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezzelarab M., Thomson A.W. Tolerogenic dendritic cells and their role in transplantation. Semin. Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S.-H., Lechman E., Bianco N., Menon R., Keravala A., Nash J., Mi Z., Watkins S., Gambotto A., Robbins P.D. Exosomes Derived from IL-10-Treated Dendritic Cells Can Suppress Inflammation and Collagen-Induced Arthritis. J. Immunol. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 15.Jakhar R., Crasta K. Exosomes as Emerging Pro-Tumorigenic Mediators of the Senescence-Associated Secretory Phenotype. Int. J. Mol. Sci. 2019;20:2547. doi: 10.3390/ijms20102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. doi: 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- 17.Keller S., Sanderson M., Stoeck A., Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Yang C., Kim S.-H., Bianco N.R., Robbins P.D. Tumor-Derived Exosomes Confer Antigen-Specific Immunosuppression in a Murine Delayed-Type Hypersensitivity Model. PLoS ONE. 2011;6:e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachenal G., Pernet-Gallay K., Chivet M., Hemming F.J., Belly A., Bodon G., Blot B., Haase G., Goldberg Y., Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Mallegol J., van Niel G., Heyman M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol. Dis. 2005;35:11–16. doi: 10.1016/j.bcmd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Guescini M., Genedani S., Stocchi V., Agnati L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 22.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 23.Skokos D., Le Panse S., Villa I., Rousselle J.-C., Peronet R., David B., Namane A., Mécheri S. Mast Cell-Dependent B and T Lymphocyte Activation Is Mediated by the Secretion of Immunologically Active Exosomes. J. Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 24.Fitts C.A., Ji N., Li Y., Tan C. Exploiting Exosomes in Cancer Liquid Biopsies and Drug Delivery. Adv. Healthc. Mater. 2019;8:1801268. doi: 10.1002/adhm.201801268. [DOI] [PubMed] [Google Scholar]
- 25.Barile L., Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson S., Nguyen J. Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J. Control. Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Liao W., Du Y., Zhang C., Pan F., Yao Y., Zhang T., Peng Q. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14. doi: 10.1016/j.actbio.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 28.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:6478. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clayton A., Harris C.L., Court J., Mason M.D., Morgan P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 2003;33:522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 30.Mentkowski K.I., Snitzer J.D., Rusnak S., Lang J.K. Therapeutic Potential of Engineered Extracellular Vesicles. AAPS J. 2018;20:1–17. doi: 10.1208/s12248-018-0211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A., et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qazi K.R., Gehrmann U., Domange Jordö E., Karlsson M.C.I., Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B cell–dependent mechanism. Blood. 2009;113:2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 33.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., Inskoe E., Piroyan A., Sokolsky M., Okolie O., et al. Development of exosome-encapsulated paclitaxel to overcome mdr in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segura E., Valladeau-Guilemond J., Donnadieu M.-H., Sastre-Garau X., Soumelis V., Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012;209:653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin W., Ouyang S., Li Y., Xiao B., Yang H. Immature Dendritic Cell-Derived Exosomes: A Promise Subcellular Vaccine for Autoimmunity. Inflammation. 2012;36:232–240. doi: 10.1007/s10753-012-9539-1. [DOI] [PubMed] [Google Scholar]
- 36.Macri C., Pang E.S., Patton T., O’Keeffe M. Dendritic cell subsets. Semin Cell Dev. Biol. 2018;84:11–21. doi: 10.1016/j.semcdb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Wilensky A., Segev H., Mizraji G., Shaul Y., Capucha T., Shacham M., Hovav A.-H. Dendritic cells and their role in periodontal disease. Oral Dis. 2013;20:119–126. doi: 10.1111/odi.12122. [DOI] [PubMed] [Google Scholar]
- 38.Capucha T., Koren N., Nassar M., Heyman O., Nir T., Levy M., Zilberman-Schapira G., Zelentova K., Eli-Berchoer L., Zenke M., et al. Sequential BMP7/TGF-β1 signaling and microbiota instruct mucosal Langerhans cell differentiation. J. Exp. Med. 2018;215:481–500. doi: 10.1084/jem.20171508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arizon M., Nudel I., Segev H., Mizraji G., Elnekave M., Furmanov K., Eli-Berchoer L., Clausen B., Shapira L., Wilensky A., et al. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proc. Natl. Acad. Sci. USA. 2012;109:7043–7048. doi: 10.1073/pnas.1116770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinbrink K., Graulich E., Kubsch S., Knop J., Enk A.H. CD4+ and CD8+ anergic T cells induced by interleukin-10–treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.V99.7.2468. [DOI] [PubMed] [Google Scholar]
- 41.Domogalla M.P., Rostan P.V., Raker V., Steinbrink K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017;8:1764. doi: 10.3389/fimmu.2017.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segura E., Touzot M., Bohineust A., Cappuccio A., Chiocchia G., Hosmalin A., Dalod M., Soumelis V., Amigorena S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 43.Rajendran M., Looney S., Singh N., Elashiry M., Meghil M.M., El-Awady A.R., Tawfik O., Susin C., Arce R.M., Cutler C.W. Systemic Antibiotic Therapy Reduces Circulating Inflammatory Dendritic Cells and Treg–Th17 Plasticity in Periodontitis. J. Immunol. 2019;202:2690–2699. doi: 10.4049/jimmunol.1900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jotwani R., Palucka A.K., Al-Quotub M., Nouri-Shirazi M., Kim J., Bell D., Banchereau J., Cutler C.W. Mature Dendritic Cells Infiltrate the T Cell-Rich Region of Oral Mucosa in Chronic Periodontitis: In Situ, In Vivo, and In Vitro Studies. J. Immunol. 2001;167:4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirrincione C., Pimpinelli N., Orlando L., Romagnoli P. Lamina Propria Dendritic Cells Express Activation Markers and Contact Lymphocytes in Chronic Periodontitis. J. Periodontol. 2002;73:45–52. doi: 10.1902/jop.2002.73.1.45. [DOI] [PubMed] [Google Scholar]
- 46.Miller M.J., Hejazi A.S., Wei S.H., Cahalan M.D., Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. USA. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Švajger U., Rožman P. Induction of Tolerogenic Dendritic Cells by Endogenous Biomolecules: An Update. Front. Immunol. 2018;9:2482. doi: 10.3389/fimmu.2018.02482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Théry C., Regnault A., Garin J., Wolfers J., Zitvogel L., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Molecular Characterization of Dendritic Cell-Derived Exosomes. J. Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Théry C., Boussac M., Véron P., Ricciardi-Castagnoli P., Raposo G., Garin J., Amigorena S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 50.Mathivanan S., Fahner C.J., Reid G., Simpson R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2011;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 52.Valadi H., Ekstrom K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 53.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J.-F., Kobayashi T., Salles J.-P., Perret B., Bonnerot C., et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 2004;380:161–171. doi: 10.1042/bj20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Llorente A., Skotland T., Sylvänne T., Kauhanen D., Róg T., Orlowski A., Vattulainen I., Ekroos K., Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta (BBA) -Mol. Cell Biol. Lipids. 2013;1831:1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 56.Wubbolts R., Leckie R.S., Veenhuizen P.T., Schwarzmann G., Möbius W., Hoernschemeyer J., Slot J.W., Geuze H.J., Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 57.Conde-Vancells J., Rodriguez-Suarez E., Embade N., Gil D., Matthiesen R., Valle M., Elortza F., Lu S.C., Mato J.M., Falcon-Perez J.M. Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J. Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 60.Villarroya-Beltri C., Gutierrez-Vazquez C., Sanchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santangelo L., Giurato G., Cicchini C., Montaldo C., Mancone C., Tarallo R., Battistelli C., Alonzi T., Weisz A., Tripodi M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016;17:799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 62.Verweij F.J., van Eijndhoven M.A., Hopmans E.S., Vendrig T., Wurdinger T., Cahir-McFarland E., Kieff E., Geerts D., van der Kant R., Neefjes J., et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. Embo. J. 2011;30:2115–2129. doi: 10.1038/emboj.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chairoungdua A., Smith D.L., Pochard P., Hull M., Caplan M.J. Exosome release of β-catenin: A novel mechanism that antagonizes Wnt signaling. J. Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez-Hernandez D., Gutiérrez-Vázquez C., Jorge I., López-Martín S., Ursa A., Sánchez-Madrid F., Vázquez J., Yáñez-Mó M. The Intracellular Interactome of Tetraspanin-enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buschow S.I., Nolte-‘t Hoen E.N.M.N., Van Niel G., Pols M.S., ten Broeke T.T., Lauwen M., Ossendorp F., Melief C.J.M., Raposo G., Wubbolts R., et al. MHC II in dendritic cells is targeted to lysosomes or t cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhu H., Guariglia S., Yu R.Y.L., Li W., Brancho D., Peinado H., Lyden D., Salzer J., Bennett C., Chow C.-W. Mutation of SIMPLE in Charcot–Marie–Tooth 1C alters production of exosomes. Mol. Biol. Cell. 2013;24:1619–1637. doi: 10.1091/mbc.e12-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 68.Savina A., Vidal M., Colombo M.I. The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- 69.Hsu C., Morohashi Y., Yoshimura S.-I., Manrique-Hoyos N., Jung S., Lauterbach M.A., Bakhti M., Grønborg M., Möbius W., Rhee J., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J. Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C.F., Schauer K., Hume A.N., Freitas R.P., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 71.Elsayed R., Elashiry M., Liu Y., El-Awady A., Hamrick M., Cutler C.W. Porphyromonas gingivalis Provokes Exosome Secretion and Paracrine Immune Senescence in Bystander Dendritic Cells. Front. Cell. Infect. Microbiol. 2021;11:669–989. doi: 10.3389/fcimb.2021.669989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross J.C., Chaudhary V., Bartscherer K., Boutros M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 73.Meiringer C.T.A., Auffarth K., Hou H., Ungermann C. Depalmitoylation of Ykt6 Prevents its Entry into the Multivesicular Body Pathway. Traffic. 2008;9:1510–1521. doi: 10.1111/j.1600-0854.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- 74.Fader C.M., Sánchez D.G., Mestre M.B., Colombo M.I. TI-VAMP/VAMP7 and VAMP3/cellubrevin: Two v-SNARE proteins involved in specific steps of the au-topha-gy/multivesicular body pathways. Biochim. Biophys. Acta. 2009;1793:1901–1916. doi: 10.1016/j.bbamcr.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Saunderson S., Dunn A.C., Crocker P., McLellan A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C., Ju S., Mu J., Zhang L., Steinman L., et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs from the Nasal Region to the Brain. Mol. Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen T., Guo J., Yang M., Zhu X., Cao X. Chemokine-Containing Exosomes Are Released from Heat-Stressed Tumor Cells via Lipid Raft-Dependent Pathway and Act as Efficient Tumor Vaccine. J. Immunol. 2011;186:2219–2228. doi: 10.4049/jimmunol.1002991. [DOI] [PubMed] [Google Scholar]
- 78.Maecker H.T., Todd S.C., Levy S. The tetraspanin superfamily: Molecular facilitators. FASEB J. 1997;11:428–442. doi: 10.1096/fasebj.11.6.9194523. [DOI] [PubMed] [Google Scholar]
- 79.Imai T., Kakizaki M., Nishimura M., Yoshie O. Molecular analyses of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J. Immunol. 1995;155:1229–1239. [PubMed] [Google Scholar]
- 80.Heijnen H.F., Schiel A.E., Fijnheer R., Geuze H.J., Sixma J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived from Exocytosis of Multivesicular Bodies and -Granules. Blood J. Am. Soc. Hematol. 1999;94:3791–3799. doi: 10.1182/blood.V94.11.3791.423a22. [DOI] [PubMed] [Google Scholar]
- 81.Morelli A.E., Larregina A.T., Shufesky W.J., Sullivan M.L.G., Stolz D.B., Papworth G.D., Zahorchak A.F., Logar A.J., Wang Z., Watkins S.C., et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 82.Zech D., Rana S., Büchler M.W., Zöller M. Tumor-exosomes and leukocyte activation: An ambivalent crosstalk. Cell Commun. Signal. 2012;10:37. doi: 10.1186/1478-811X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tai X.G., Toyooka K., Yashiro Y., Abe R., Park C.S., Hamaoka T., Kobayashi M., Neben S., Fujiwara H. CD9-mediated costimulation of TCR-triggered naive T cells leads to activation followed by apoptosis. J. Immunol. 1997;159:3799–3807. [PubMed] [Google Scholar]
- 84.Segura E., Nicco C., Lombard B., Véron P., Raposo G., Batteux F., Amigorena S., Théry C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 85.Christianson H.C., Svensson K.J., van Kuppevelt T.H., Li J.-P., Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKelvey K.J., Powell K.L., Ashton A.W., Morris J.M., McCracken S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 2015;4:7. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blanchard N., Lankar D., Faure F., Regnault A., Dumont C., Raposo G., Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. J. Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 88.Kshirsagar S., Alam S., Jasti S., Hodes H., Nauser T., Gilliam M., Billstrand C., Hunt J., Petroff M. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33:982–990. doi: 10.1016/j.placenta.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vincent-Schneider H., Stumptner-Cuvelette P., Lankar D., Pain S., Raposo G., Benaroch P., Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 2002;14:713–722. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 90.Mincheva-Nilsson L., Nagaeva O., Chen T., Stendahl U., Antsiferova J., Mogren I., Hernestål J., Baranov V. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on pe-ripheral blood mononuclear cells during human pregnancy: A possible novel immune escape mechanism for fetal survival. J. Immunol. 2006;176:3585–3592. doi: 10.4049/jimmunol.176.6.3585. [DOI] [PubMed] [Google Scholar]
- 91.del Conde I., Shrimpton C.N., Thiagarajan P., Lópezet J.A. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 92.Stenqvist A.-C., Nagaeva O., Baranov V., Mincheva-Nilsson L. Exosomes Secreted by Human Placenta Carry Functional Fas Ligand and TRAIL Molecules and Convey Apoptosis in Activated Immune Cells, Suggesting Exosome-Mediated Immune Privilege of the Fetus. J. Immunol. 2013;191:5515–5523. doi: 10.4049/jimmunol.1301885. [DOI] [PubMed] [Google Scholar]
- 93.Cai Z., Zhang W., Yang F., Yu L., Yu Z., Pan J., Wang L., Cao X., Wang J. Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2011;22:607–610. doi: 10.1038/cr.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taylor D.D., Akyol S., Gercel-Taylor C. Pregnancy-Associated Exosomes and Their Modulation of T Cell Signaling. J. Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 95.Feng D., Zhao W.-L., Ye Y.-Y., Bai X.-C., Liu R.-Q., Chang L.-F., Zhou Q., Sui S.-F. Cellular Internalization of Exosomes Occurs Through Phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 96.Fitzner D., Schnaars M., van Rossum D., Krishnamoorthy G., Dibaj P., Bakhti M., Regen T., Hanisch U.-K., Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 97.Tian T., Zhu Y.-L., Zhou Y.-Y., Liang G.-F., Wang Y.-Y., Hu F.-H., Xiao Z.-D. Exosome Uptake through Clathrin-mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery. J. Biol. Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mayor S., Pagano R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 99.Yang X., Meng S., Jiang H., Zhu C., Wu W. Exosomes Derived from Immature Bone Marrow Dendritic Cells Induce Tolerogenicity of Intestinal Transplantation in Rats. J. Surg. Res. 2011;171:826–832. doi: 10.1016/j.jss.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 100.Pêche H., Heslan M., Usal C., Amigorena S., Cuturi M.C. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503–1510. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 101.Bu N., Wu H.-Q., Zhang G.-L., Zhan S.-Q., Zhang R., Fan Q.-Y., Li Y.-L., Zhai Y.-F., Ren H.-W. Immature dendritic cell exosomes suppress experimental autoimmune myasthenia gravis. J. Neuroimmunol. 2015;285:71–75. doi: 10.1016/j.jneuroim.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 102.Bianco N.R., Kim S.H., Ruffner M., Robbins P.D. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009;60:380–389. doi: 10.1002/art.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim S.H., Bianco N.R., Shufesky W.J., Morelli A.E., Robbins P.D. Effective Treatment of Inflammatory Disease Models with Exosomes Derived from Dendritic Cells Genetically Modified to Express IL-4. J. Immunol. 2007;179:2242–2249. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 104.Kim S.H., Bianco N., Menon R., Lechman E., Shufesky W.J., Morelli A.E., Robbins P.D. Exosomes Derived from Genetically Modified DC Expressing FasL Are Anti-inflammatory and Immunosuppressive. Mol. Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 105.Yang X., Meng S., Jiang H., Chen T., Wu W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand. J. Gastroenterol. 2010;45:1168–1177. doi: 10.3109/00365521.2010.490596. [DOI] [PubMed] [Google Scholar]
- 106.Johansson S.M., Admyre C., Scheynius A., Gabrielsson S. Different types of in vitro generated human monocyte-derived dendritic cells release exosomes with distinct phenotypes. Immunology. 2008;123:491–499. doi: 10.1111/j.1365-2567.2007.02714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tamai K., Tanaka N., Nakano T., Kakazu E., Kondo Y., Inoue J., Shiina M., Fukushima K., Hoshino T., Sano K., et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem. Biophys. Res. Commun. 2010;399:384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 108.Aline F., Bout D., Amigorena S., Roingeard P., Dimier-Poisson I. Toxoplasma gondii Antigen-Pulsed-Dendritic Cell-Derived Exosomes Induce a Protective Immune Response against T. gondii Infection. Infect. Immun. 2004;72:4127–4137. doi: 10.1128/IAI.72.7.4127-4137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.del Cacho E., Gallego M., Lee S.H., Lillehoj H.S., Quilez J., Lillehoj E.P., Sánchez-Acedo C. Induction of Protective Immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina Infections Using Dendritic Cell-Derived Exosomes. Infect. Immun. 2012;80:1909–1916. doi: 10.1128/IAI.06413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Escudier B., Dorval T., Chaput N., André F., Caby M.-P., Novault S., Flament C., Leboulaire C., Borg C., Amigorena S., et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J. Transl. Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., Valente N., Shreeniwas R., Sutton M.A., Delcayre A., et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giri P.K., Schorey J.S. Exosomes Derived from M. Bovis BCG Infected Macrophages Activate Antigen-Specific CD4+ and CD8+ T Cells In Vitro and In Vivo. PLoS ONE. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Toro J., Herschlik L., Waldner C., Mongini C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic ap-plications. Front. Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beauvillain C., Ruiz S., Guiton R., Bout D., Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes. Infect. 2007;9:1614–1622. doi: 10.1016/j.micinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Yu L., Yang F., Jiang L., Chen Y., Wang K., Xu F., Wei Y., Cao X., Wang J., Cai Z. Exosomes with membrane-associated TGF-β1 from gene-modified dendritic cells inhibit murine EAE independently of MHC restriction. Eur. J. Immunol. 2013;43:2461–2472. doi: 10.1002/eji.201243295. [DOI] [PubMed] [Google Scholar]
- 116.Li X., Li J.-J., Yang J.-Y., Wang D.-S., Zhao W., Song W.-J., Li W.-M., Wang J.-F., Han W., Zhang Z.-C., et al. Tolerance Induction by Exosomes from Immature Dendritic Cells and Rapamycin in a Mouse Cardiac Allograft Model. PLoS ONE. 2012;7:e44045. doi: 10.1371/journal.pone.0044045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma B., Yang J.-Y., Song W.-J., Ding R., Zhang Z.-C., Ji H.-C., Zhang X., Wang J.-L., Yang X.-S., Tao K.-S., et al. Combining Exosomes Derived from Immature DCs with Donor Antigen-Specific Treg Cells Induces Tolerance in a Rat Liver Allograft Model. Sci. Rep. 2016;6:32971. doi: 10.1038/srep32971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xiong Y.-Y., Gong Z.-T., Tang R.-J., Yang Y.-J. The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction. Theranostics. 2021;11:1046–1058. doi: 10.7150/thno.53326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elashiry M., Elashiry M., Elsayed R., Rajendran M., Auersvald C., Zeitoun R., Rashid M.H., Ara R., Meghil M.M., Liu Y., et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J. Extracell. Vesicles. 2020;9:1795362. doi: 10.1080/20013078.2020.1795362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elashiry M., Elsayed R., Elashiry M.M., Rashid M.H., Ara R., Arbab A.S., Elawady A.R., Hamrick M., Liu Y., Zhi W., et al. Proteomic Characterization, Biodistribution, and Functional Studies of Immune-Therapeutic Exosomes: Implications for Inflammatory Lung Diseases. Front. Immunol. 2021;12:636222. doi: 10.3389/fimmu.2021.636222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D’Alessio F.R., Tsushima K., Aggarwal N.R., West E.E., Willett M.H., Britos M.F., Pipeling M.R., Brower R.G., Tuder R.M., McDyer J.F., et al. CD4 + CD25 + Foxp3 + Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Investig. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gupta S., Krishnakumar V., Sharma Y., Dinda A.K., Mohanty S. Mesenchymal stem cell derived exosomes: A nano platform for therapeutics and drug delivery in combating COVID-19. Stem Cell Rev. Rep. 2021;17:33–43. doi: 10.1007/s12015-020-10002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Inal J.M. Decoy ACE2-expressing extracellular vesicles that competitively bind SARS-CoV-2 as a possible COVID-19 therapy. Clin. Sci. 2020;134:1301–1304. doi: 10.1042/CS20200623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuate S., Cinatl J., Doerr H.W., Überla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Okoye I.S., Coomes S.M., Pelly V.S., Czieso S., Papayannopoulos V., Tolmachova T., Seabra M.C., Wilson M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smyth L.A., Ratnasothy K., Tsang J.Y.S., Boardman D., Warley A., Lechler R., Lombardi G. CD73 expression on extracellular vesicles derived from CD4 + CD25 + Foxp3 + T cells contributes to their regulatory function. Eur. J. Immunol. 2013;43:2430–2440. doi: 10.1002/eji.201242909. [DOI] [PubMed] [Google Scholar]
- 127.Clayton A., Al-Taei S., Webber J., Mason M.D., Tabi Z. Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 128.Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., Geuze H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y., Liu Y., Liu H., Tang W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:1–18. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lugini L., Cecchetti S., Huber V., Luciani F., Macchia G., Spadaro F., Paris L., Abalsamo L., Colone M., Molinari A., et al. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012;189:2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 131.Skokos D., Botros H.G., Demeure C., Morin J., Peronet R., Birkenmeier G., Boudaly S., Mécheri S. Mast Cell-Derived Exosomes Induce Phenotypic and Functional Maturation of Dendritic Cells and Elicit Specific Immune Responses In Vivo. J. Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 132.Yang X., Shi G., Guo J., Wang C., He Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2018;13:8095–8104. doi: 10.2147/IJN.S179380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang X., Xie B., Peng H., Shi G., Sreenivas B., Guo J., Wang C., He Y. Eradicating intracellular MRSA via targeted delivery of lysostaphin and vancomycin with mannose-modified exosomes. J. Control. Release. 2020;329:454–467. doi: 10.1016/j.jconrel.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 134.Anticoli S., Manfredi F., Chiozzini C., Arenaccio C., Olivetta E., Ferrantelli F., Capocefalo A., Falcone E., Ruggieri A., Federico M. An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity Against Viral Antigens. Biotechnol. J. 2018;13:e1700443. doi: 10.1002/biot.201700443. [DOI] [PubMed] [Google Scholar]
- 135.Simons M., Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 136.Nonaka T., Wong D.T. Saliva-Exosomics in Cancer: Molecular Characterization of Cancer-Derived Exosomes in Saliva. Viral Replication Enzym. Inhib. Part. B. 2017;42:125–151. doi: 10.1016/bs.enz.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu J., Lin Y., Xiong X., Li K., Yao Z., Dong H., Jiang Z., Yu D., Yeung S.-C.J., Zhang H. Detection of Exosomal PD-L1 RNA in Saliva of Patients with Periodontitis. Front. Genet. 2019;10:202. doi: 10.3389/fgene.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Han P., Bartold P.M., Salomon C., Ivanovski S. Salivary Small Extracellular Vesicles Associated miRNAs in Periodontal Status—A Pilot Study. Int. J. Mol. Sci. 2020;21:2809. doi: 10.3390/ijms21082809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lässer C., O’Neil S.E., Shelke G.V., Sihlbom C., Hansson S.F., Gho Y.S., Lundbäck B., Lötvall J. Exosomes in the nose induce immune cell trafficking and harbour an altered protein cargo in chronic airway inflammation. J. Transl. Med. 2016;14:181. doi: 10.1186/s12967-016-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xing X., Han S., Li Z., Li Z. Emerging role of exosomes in craniofacial and dental applications. Theranostics. 2020;10:8648–8664. doi: 10.7150/thno.48291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gonzalez-Calero L., Martin-Lorenzo M., Alvarez-Llamas G. Exosomes: A potential key target in cardio-renal syndrome. Front. Immunol. 2014;5:465. doi: 10.3389/fimmu.2014.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kishore R., Garikipati V.N.S., Gumpert A. Tiny Shuttles for Information Transfer: Exosomes in Cardiac Health and Disease. J. Cardiovasc. Transl. Res. 2016;9:169–175. doi: 10.1007/s12265-016-9682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Krause M., Samoylenko A., Vainio S.J. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front. Cell Dev. Biol. 2015;3:65. doi: 10.3389/fcell.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Howitt J., Hill A.F. Exosomes in the Pathology of Neurodegenerative Diseases. J. Biol. Chem. 2016;291:26589–26597. doi: 10.1074/jbc.R116.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Record M., Poirot M., Silvente-Poirot S. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie. 2014;96:67–74. doi: 10.1016/j.biochi.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 146.Li A., Zhang T., Zheng M., Liu Y., Chen Z. Exosomal proteins as potential markers of tumor diagnosis. J. Hematol. Oncol. 2017;10:1–9. doi: 10.1186/s13045-017-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chistiakov D.A., Grechko A.V., Orekhov A.N., Bobryshev Y.V. An immunoregulatory role of dendritic cell-derived exosomes versus HIV-1 infection: Take it easy but be warned. Ann. Transl. Med. 2017;5:362. doi: 10.21037/atm.2017.06.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Izquierdo-Useros N., Naranjo-Gomez M., Erkizia I., Puertas M.C., Borras F.E., Blanco J., Martinez-Picado J. HIV and Mature Dendritic Cells: Trojan Exosomes Riding the Trojan Horse? PLoS Pathog. 2010;6:e1000740. doi: 10.1371/journal.ppat.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kulkarni R., Prasad A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-14817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wiley R.D., Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cañas J.A., Sastre B., Rodrigo-Muñoz J.M., del Pozo V. Exosomes: A new approach to asthma pathology. Clin. Chim. Acta. 2019;495:139–147. doi: 10.1016/j.cca.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 152.Esser J., Gehrmann U., D’Alexandri F.L., Hidalgo-Estévez A.M., Wheelock C.E., Scheynius A., Gabrielsson S., Rådmark O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 2010;126:1032–1040.e4. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 153.Vallhov H., Gutzeit C., Hultenby K., Valenta R., Grönlund H., Scheynius A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy. 2015;70:1651–1655. doi: 10.1111/all.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wei G., Jie Y., Haibo L., Chaoneng W., Dong H., Jianbing Z., Junjie G., Leilei M., Hongtao S., Yunzeng Z., et al. Dendritic cells derived exosomes migration to spleen and induction of inflammation are regulated by CCR7. Sci. Rep. 2017;7:srep42996. doi: 10.1038/srep42996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao W., Liu H., Yuan J., Wu C., Huang D., Ma Y., Zhu J., Ma L., Guo J., Shi H., et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J. Cell Mol. Med. 2016;20:2318–2327. doi: 10.1111/jcmm.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nguyen M.-A., Karunakaran D., Geoffrion M., Cheng H.S., Tandoc K., Matic L.P., Hedin U., Maegdefessel L., Fish J.E., Rayner K.J. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler. Thromb. Vasc. Biol. 2018;38:49–63. doi: 10.1161/ATVBAHA.117.309795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu H., Gao W., Yuan J., Wu C., Yao K., Zhang L., Ma L., Zhu J., Zou Y., Ge J. Exosomes derived from dendritic cells improve cardiac function via activation of CD4+ T lymphocytes after myocardial infarction. J. Mol. Cell. Cardiol. 2016;91:123–133. doi: 10.1016/j.yjmcc.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 158.Wu R., Gao W., Yao K., Ge J. Roles of Exosomes Derived from Immune Cells in Cardiovascular Diseases. Front. Immunol. 2019;10:648. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu Q., Rojas-Canales D.M., DiVito S.J., Shufesky W.J., Stolz D.B., Erdos G., Sullivan M.L., Gibson G.A., Watkins S.C., Larregina A.T., et al. Donor dendritic cell–derived exosomes promote allograft-targeting immune response. J. Clin. Investig. 2016;126:2805–2820. doi: 10.1172/JCI84577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bukong T.N., Momen-Heravi F., Kodys K., Bala S., Szabo G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mehaffy C., Kruh-Garcia N.A., Graham B., Jarlsberg L.G., Willyerd C.E., Borisov A., Sterling T.R., Nahid P., Dobos K.M. Identification of Mycobacterium tuberculosis Peptides in Serum Extracellular Vesicles from Persons with Latent Tuberculosis Infection. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00393-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mitsuhashi S., Feldbrügge L., Csizmadia E., Mitsuhashi M., Robson S.C., Moss A.C. Luminal Extracellular Vesicles (EVs) in Inflammatory Bowel Disease (IBD) Exhibit Proinflammatory Effects on Epithelial Cells and Macrophages. Inflamm. Bowel Dis. 2016;22:1587–1595. doi: 10.1097/MIB.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang H.-G., Liu C., Su K., Yu S., Zhang L., Zhang S., Wang J., Cao X., Grizzle W., Kimberly R. A Membrane Form of TNF-α Presented by Exosomes Delays T Cell Activation-Induced Cell Death. J. Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 164.Stucci S., Tucci M., Ascierto P.A., Passarelli A., Mariaelena C., Madonna G., Ester S., Grimaldi A.M., Silvestris F. Dendritic cell-derived exosomes (Dex) are potential biomarkers of response to Ipilimumab in metastatic melanoma. J. Transl. Med. 2015;13:P15. doi: 10.1186/1479-5876-13-S1-P15. [DOI] [Google Scholar]
- 165.Zhang W., Jiang X., Bao J., Wang Y., Liu H., Tang L. Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions. Front. Immunol. 2018;9:90. doi: 10.3389/fimmu.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campos F.M., Franklin B.S., Teixeira-Carvalho A., Filho A.L., de Paula S.C., Fontes C.J., Brito C.F., Carvalho L.H. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar. J. 2010;9:327. doi: 10.1186/1475-2875-9-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barreto A., Rodríguez L.-S., Rojas O.L., Wolf M., Greenberg H.B., Franco M.A., Angel J. Membrane Vesicles Released by Intestinal Epithelial Cells Infected with Rotavirus Inhibit T-Cell Function. Viral Immunol. 2010;23:595–608. doi: 10.1089/vim.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh P.P., Smith V.L., Karakousis P.C., Schorey J.S. Exosomes Isolated from Mycobacteria-Infected Mice or Cultured Macrophages Can Recruit and Activate Immune Cells In Vitro and In Vivo. J. Immunol. 2012;189:777–785. doi: 10.4049/jimmunol.1103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Radomski N., Karger A., Franzke K., Liebler-Tenorio E., Jahnke R., Matthiesen S., Knittler M.R. Chlamydia psittaci -Infected Dendritic Cells Communicate with NK Cells via Exosomes to Activate Antibacterial Immunity. Infect. Immun. 2019;88:e00541-19. doi: 10.1128/IAI.00541-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chaparro Padilla A., Weber Aracena L., Realini Fuentes O., Albers Busquetts D., Hernandez Rios M., Ramirez Lobos V., Pascual La Rocca A., Nart Molina J., Beltran Varas V., Acuna-Gallardo S., et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral Dis. 2020;26:1318–1325. doi: 10.1111/odi.13338. [DOI] [PubMed] [Google Scholar]
- 171.Tian J., Popal M.S., Zhao Y., Liu Y., Chen K., Liu Y. Interplay between Exosomes and Autophagy in Cardiovascular Diseases: Novel Promising Target for Diagnostic and Therapeutic Application. Aging Dis. 2019;10:1302–1310. doi: 10.14336/AD.2018.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Narayanan A., Iordanskiy S., Das R., Van Duyne R., Santos S., Jaworski E., Guendel I., Sampey G., Dalby E., Iglesias-Ussel M., et al. Exosomes Derived from HIV-1-infected Cells Contain Trans-activation Response Element RNA. J. Biol. Chem. 2013;288:20014–20033. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pleet M.L., Mathiesen A., DeMarino C., Akpamagbo Y.A., Barclay R.A., Schwab A., Iordanskiy S., Sampey G.C., Lepene B., Ilinykh P.A., et al. Ebola VP40 in Exosomes Can Cause Immune Cell Dysfunction. Front. Microbiol. 2016;7:1765. doi: 10.3389/fmicb.2016.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sampey G.C., Saifuddin M., Schwab A., Barclay R., Punya S., Chung M.-C., Hakami R.M., Zadeh M.A., Lepene B., Klase Z.A., et al. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J. Biol. Chem. 2016;291:1251–1266. doi: 10.1074/jbc.M115.662171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stark A., Bushati N., Jan C.H., Kheradpour P., Hodges E., Brennecke J., Bartel D.P., Cohen S.M., Kellis M. A single Hox locus in Drosophila produces functional microRNAs from opposite DNA strands. Genes Dev. 2008;22:8–13. doi: 10.1101/gad.1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chatterjee A., Chattopadhyay D., Chakrabarti G. miR-17-5p Downregulation Contributes to Paclitaxel Resistance of Lung Cancer Cells through Altering Beclin1 Expression. PLoS ONE. 2014;9:e95716. doi: 10.1371/journal.pone.0095716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Duan X., Zhang T., Ding S., Wei J., Su C., Liu H., Xu G. microRNA-17-5p Modulates Bacille Calmette-Guerin Growth in RAW264.7 Cells by Targeting ULK1. PLoS ONE. 2015;10:e0138011. doi: 10.1371/journal.pone.0138011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu C., Chen J., Xu H., Zhou X., He Q., Li Y., Jiang G., Shan Y., Xue B., Zhao R., et al. MIR106B and MIR93 Prevent Removal of Bacteria from Epithelial Cells by Disrupting ATG16L1-Mediated Autophagy. Gastroenterology. 2014;146:188–199. doi: 10.1053/j.gastro.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Colino J., Snapper C.M. Dendritic Cell-Derived Exosomes Express a Streptococcus pneumoniae Capsular Polysaccharide Type 14 Cross-Reactive Antigen That Induces Protective Immunoglobulin Responses against Pneumococcal Infection in Mice. Infect. Immun. 2007;75:220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lenassi M., Cagney G., Liao M., Vaupotic T., Bartholomeeusen K., Cheng Y., Krogan N.J., Plemenitas A., Peterlin B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leone A.D., Rees A.J., Kain R. Dendritic cells and routing cargo into exosomes. Immunol. Cell Biol. 2018;96:683–693. doi: 10.1111/imcb.12170. [DOI] [PubMed] [Google Scholar]
- 182.Spencer N., Yeruva L. Role of bacterial infections in extracellular vesicles release and impact on immune response. Biomed. J. 2021;44:157–164. doi: 10.1016/j.bj.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Segura E., Guérin C., Hogg N., Amigorena S., Théry C. CD8 + Dendritic Cells Use LFA-1 to Capture MHC-Peptide Complexes from Exosomes In Vivo. J. Immunol. 2007;179:1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 184.Colino J., Snapper C.M. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen-specific IgG responses in naive recipients in the absence of free antigen. J. Immunol. 2006;177:3757–3762. doi: 10.4049/jimmunol.177.6.3757. [DOI] [PubMed] [Google Scholar]
- 185.Kruh-Garcia N., Wolfe L.M., Dobos K.M. Deciphering the role of exosomes in tuberculosis. Tuberculosis. 2015;95:26–30. doi: 10.1016/j.tube.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 186.Hazeldine J., Lord J.M. Innate immunesenescence: Underlying mechanisms and clinical relevance. Biogerontology. 2015;16:187–201. doi: 10.1007/s10522-014-9514-3. [DOI] [PubMed] [Google Scholar]
- 187.Ebersole J.L., Graves C.L., Gonzalez O.A., Dawson D., Morford L.A., Huja P.E., Hartsfield J.K., Jr., Huja S.S., Pandruvada S., Wallet S.M. Aging, inflammation, immunity and periodontal disease. Periodontology. 2016;72:54–75. doi: 10.1111/prd.12135. [DOI] [PubMed] [Google Scholar]
- 188.Maurelli R., Tinaburri L., Gangi F., Bondanza S., Severi A.L., Scarponi C., Albanesi C., Mesiti G., Guerra L., Capogrossi M.C., et al. The role of oncogenic Ras in human skin tumorigenesis depends on clonogenic potential of the founding keratinocytes. J. Cell Sci. 2016;129:jcs.176842–17. doi: 10.1242/jcs.176842. [DOI] [PubMed] [Google Scholar]
- 189.Nogueira V., Park Y., Chen C.-C., Xu P.-Z., Chen M.-L., Tonic I., Unterman T., Hay N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.An J., Quarles E., Mekvanich S., Kang A., Liu A., Santos D., Miller R.A., Rabinovitch P.S., Cox T.C., Kaeberlein M. Rapamycin treatment attenuates age-associated periodontitis in mice. GeroScience. 2017;39:457–463. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C., Raguz S., Acosta J.C., Innes A.J., Banito A., et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Linton P.-J., Thoman M.L. Immunosenescence in monocytes, macrophages, and dendritic cells: Lessons learned from the lung and heart. Immunol. Lett. 2014;162:290–297. doi: 10.1016/j.imlet.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Devarajan P., Jones M., Kugler-Umana O., Vong A.M., Xia J., Swain S.L. Pathogen Recognition by CD4 Effectors Drives Key Effector and Most Memory Cell Generation Against Respiratory Virus. Front. Immunol. 2018;9:596. doi: 10.3389/fimmu.2018.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.-W., Lasitschka F., Andrulis M., et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Naylor R.M., Baker D.J., Van Deursen J.M. Senescent Cells: A Novel Therapeutic Target for Aging and Age-Related Diseases. Clin. Pharmacol. Ther. 2013;93:105–116. doi: 10.1038/clpt.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mondal A.M., Horikawa I., Pine S.R., Fujita K., Morgan K.M., Vera E., Mazur S.J., Appella E., Vojtesek B., Blasco M.A., et al. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J. Clin. Investig. 2013;123:5247–5257. doi: 10.1172/JCI70355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Salminen A. Activation of immunosuppressive network in the aging process. Ageing Res. Rev. 2020;57:100998. doi: 10.1016/j.arr.2019.100998. [DOI] [PubMed] [Google Scholar]
- 198.Takasugi M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 2018;17:e12734. doi: 10.1111/acel.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lehmann B., Paine M.S., Brooks A.M., McCubrey J., Renegar R.H., Wang R., Terrian D.M. Senescence-Associated Exosome Release from Human Prostate Cancer Cells. Cancer Res. 2008;68:7864–7871. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zeituni A.E., Jotwani R., Carrion J., Cutler C.W. Targeting of DC-SIGN on Human Dendritic Cells by Minor Fimbriated Porphyromonas gingivalis Strains Elicits a Distinct Effector T Cell Response. J. Immunol. 2009;183:5694–5704. doi: 10.4049/jimmunol.0901030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zeituni A.E., McCaig W., Scisci E., Thanassi D.G., Cutler C.W. The Native 67-Kilodalton Minor Fimbria of Porphyromonas gingivalis Is a Novel Glycoprotein with DC-SIGN-Targeting Motifs. J. Bacteriol. 2010;192:4103–4110. doi: 10.1128/JB.00275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.El-Awady A.R., Arce R.M., Cutler C.W. Dendritic cells: Microbial clearance via autophagy and potential immunobiological consequences for periodontal disease. Periodontology 2000. 2015;69:160–180. doi: 10.1111/prd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Arjunan P., El-Awady A., Dannebaum R., Kunde-Ramamoorthy G., Cutler C. High-throughput sequencing reveals key genes and immune homeostatic pathways activated in myeloid dendritic cells by Porphyromonas gingivalis 381 and its fimbrial mutants. Mol. Oral Microbiol. 2015;31:78–93. doi: 10.1111/omi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.El-Awady A.R., Miles B., Scisci E., Kurago Z.B., Palani C.D., Arce R.M., Waller J.L., Genco C.A., Slocum C., Manning M., et al. Porphyromonas gingivalis Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk. PLoS Pathog. 2015;11:e1004647. doi: 10.1371/journal.ppat.1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Meghil M.M., Cutler C.W. Oral Microbes and Mucosal Dendritic Cells, “Spark and Flame” of Local and Distant Inflammatory Diseases. Int. J. Mol. Sci. 2020;21:1643. doi: 10.3390/ijms21051643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Meghil M., Tawfik O.K., Elashiry M., Rajendran M., Arce R.M., Fulton D.J., Schoenlein P.V., Cutler C.W. Disruption of Immune Homeostasis in Human Dendritic Cells via Regulation of Autophagy and Apoptosis by Porphyromonas gingivalis. Front. Immunol. 2019;10:2286. doi: 10.3389/fimmu.2019.02286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.El-Awady A., Rabelo M.D.S., Meghil M.M., Rajendran M., Elashiry M., Stadler A.F., Foz A.M., Susin C., Romito G.A., Arce R.M., et al. Polymicrobial synergy within oral biofilm promotes invasion of dendritic cells and survival of consortia members. NPJ Biofilms Microbiomes. 2019;5:1–12. doi: 10.1038/s41522-019-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qin Z., Gu X., Chen Y., Liu J., Hou C., Lin S., Hao N., Liang Y., Chen W., Meng H. Toll-like receptor 4 activates the NLRP3 inflammasome pathway and periodontal inflammaging by inhibiting Bmi-1 expression. Int. J. Mol. Med. 2020;47:137–150. doi: 10.3892/ijmm.2020.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jang H.-M., Park J.-Y., Lee Y.-J., Kang M.-J., Jo S.-G., Jeong Y.-J., Cho N.-P., Cho S.-D., Kim D.-J., Park J.-H. TLR2 and the NLRP3 inflammasome mediate IL-1β production in Prevotella nigrescens-infected dendritic cells. Int. J. Med. Sci. 2021;18:432–440. doi: 10.7150/ijms.47197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hosseini R., Asef-Kabiri L., Yousefi H., Sarvnaz H., Salehi M., Akbari M.E., Eskandari N. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol. Cancer. 2021;20:1–17. doi: 10.1186/s12943-021-01376-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Olejarz W., Dominiak A., Żołnierzak A., Kubiak-Tomaszewska G., Lorenc T. Tumor-Derived Exosomes in Immunosuppression and Immunotherapy. J. Immunol. Res. 2020;2020:1–11. doi: 10.1155/2020/6272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sung B.H., Ketova T., Hoshino D., Zijlstra A., Weaver A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hoshino D., Kirkbride K.C., Costello K., Clark E.S., Sinha S., Grega-Larson N., Tyska M.J., Weaver A.M. Exosome Secretion Is Enhanced by Invadopodia and Drives Invasive Behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yue S., Mu W., Erb U., Zöller M. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget. 2014;6:2366–2384. doi: 10.18632/oncotarget.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 216.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., Le Chevalier T., Livartoski A., Barlesi F., Laplanche A., et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. 2016;5:e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]